Abstract
BACKGROUND
Leukoreduction of blood components was implemented to reduce transfusion‐associated risks. The detection level for residual white blood cells (rWBCs) required to demonstrate leukoreduction was originally considered too low for hematology analyzers. Developments enabling cell counts in body fluids have, however, renewed interest in rWBC counting. An assessment of Sysmex XN hematology analyzers with software offering automated rWBC enumeration intended for use on blood components was performed.
STUDY DESIGN AND METHODS
Performance characteristics were determined using platelet, red blood cell (RBC), and plasma samples spiked with WBCs. Subsequently, components (platelets, n = 1367; and plasma, n = 80) were tested and results compared with flow cytometry, to monitor leukoreduction efficiency to a level of less than 1 × 106/unit. Components identified by flow cytometry as having poor leukoreduction, exceeding this limit, were also tested (platelets, n = 3; and RBCs, n = 10).
RESULTS
Linearity studies up to 32 WBCs/μL showed good correlation between observed and expected results (R2 > 0.9996). Precision analysis gave an average limit of quantitation of 2 WBCs/μL with coefficients of variation less than 20%. Average carryover was 0.1%. Plain sample tubes were a source of aberrant results with routine components. Using ethylenediaminetetraacetic acid tubes the analyzer gave results greater than 1 × 106/unit in 2.7% of cases compared with 1.4% by flow cytometry, but overall results were within specification, with more than 90% of components having rWBC values below the limit. All incidences of poor leukoreduction, with flow cytometry results greater than 13 rWBCs/μL were correctly identified, with an excellent correlation between results (R2 = 0.9818).
CONCLUSION
The analyzer demonstrated acceptable performance characteristics for enumeration of rWBCs; consequently, additional multisite evaluations are warranted.
Short abstract
ABBREVIATIONS
- BB
Blood Bank (mode)
- BC
buffy coat
- CV
coefficient of variation
- hsA
high‐sensitivity analysis
- LOD
limit of detection
- LOQ
limit of quantitation
- NHSBT
NHS Blood and Transplant
- PCs
platelet concentrates
- QA
quality assessment
- QC
quality control
- QM
quality monitoring
- RCCs
red blood cell concentrates
- rWBCs
residual white blood cells
Universal leukoreduction of the blood supply was implemented both as a precautionary measure against the potential risk of variant Creutzfeld‐Jacob disease transmission and for other potential benefits, such as reduction in febrile reactions and human leukocyte antigen alloimmunization. Application of leukoreduction technology required introduction of methodology for quality monitoring (QM) of blood components to ensure compliance with specifications for residual white blood cells (rWBCs) on an ongoing basis, which for the United Kingdom are that more than 90% of components should contain less than 1 × 106 rWBCs and more than 99% of components should contain less than 5 × 106 rWBCs per component, with 95% confidence.1 In the United States, the AABB requires that components contain less than 5 × 106 rWBCs in greater than 95% of components.2
Initially, there was widespread variance in methodology used for rWBC enumeration, although all used nucleotide‐binding dyes to identify WBCs. Techniques included manual microscopy with a Nageotte hemocytometer, microvolume fluorimetry, and flow cytometry. Currently, flow cytometry with commercial reagents has become the most widely used technique for rWBC enumeration, with results being incorporated into statistical process control systems designed to detect potentially poorly performing processes.3, 4 Improvements in hardware, software, and QM procedures have continued and led to new semiautomated systems.5, 6, 7
The potential benefits of being able to use a routine hematology analyzer rather than flow cytometry for rWBC enumeration have previously been recognized; a single instrument could be used for enumeration of rWBCs as well as other parameters such as platelet count and hemoglobin content. However, the detection level required for rWBC enumeration was below the limits of hematology analyzers. However, the latest generation of analyzers is able to count low levels of cells in body fluids and has renewed interest in rWBC counting.8
The Sysmex XN series (Sysmex Japan) of analyzers include an option for low‐level cell counting in body fluids that has been reported as giving results comparable with microscopy and having a lower limit of detection of 5 WBCs/μL.9 This success prompted development of high‐sensitivity analysis (hsA) in which the sample analysis volume was increased. This hsA mode was found to have lower limits of quantitation of 2 WBCs/μL.10 The ability to detect rWBCs around 2 cells/μL by hematology analyzers would bring them within the critical region to detect leukoreduction failures, which in early flow cytometry studies was described as 5 to 30 cells/μL depending on component volume.11 Data from NHS Blood and Transplant (NHSBT) currently suggests that detection of 1 to 25 cells/μL is required to monitor leukoreduction performance and detect failures. Additionally, detection of 2 cells/μL would provide comparability with flow cytometry, which is reported to give acceptable performance levels of 1 to 3 cells/μL.12
Sysmex have further developed the hsA to generate the Blood Bank (BB) mode. The BB mode maintained high analysis volumes but also modified the gating strategy for optimal detection of nucleated WBCs. The aim of the current study was to evaluate the performance characteristics of BB mode for rWBC enumeration and assess the potential impact of using the analyzer in a routine blood component manufacturing environment.
MATERIALS AND METHODS
Early studies of potential rWBC counting technology noted that although a technique may perform well with specifically prepared spiked and fixed samples it may fail to reach satisfactory performance with samples from routinely prepared leukoreduced components.11, 12 Hence, assessment of the Sysmex BB mode was performed in two parts. The first focused on performance characteristics such as linearity, precision, and limits of quantitation using spiked samples, followed by using the analyzer in parallel with flow cytometry in a routine component manufacturing environment to determine the effect of using the BB mode for process control purposes to identify the incidence of components that met the two UK guideline limits.
Blood components
Components were manufactured and stored according to UK guidelines.1 RBC concentrates (RCCs), plasma, and pooled buffy coat (BC)‐derived platelet concentrates (PCs) suspended in platelet additive solution were processed from whole blood donations and leukoreduced with standard NHSBT procedures. Apheresis machines (Trima Accel, Terumo) were used to generate both leukoreduced plasma and single‐donor PCs suspended in plasma.
Hematology analyzers
Cell counts on nonleukoreduced samples were obtained using routine clinical analysis modes on XN‐series analyzers (Sysmex UK). These were either based at the Component Development Laboratory (CDL) at NHSBT Cambridge using an XN‐1000 analyzer (XN20 format) or in the QM department at NHSBT Manchester (QM‐Manchester) using an XN‐2000 analyzer (dual XN10 format).
Testing of specifically produced leukoreduced samples to determine performance characteristics of BB analysis such as linearity, precision, and carryover were performed on the XN‐1000 analyzer. All samples were collected into plain tubes without anticoagulant (Greiner Bio‐One), tested within 10 hours of preparation, and mixed by manual inversion a maximum of eight times immediately before testing. Validation with standard leukoreduced components utilized the BB mode on both XN systems. Early in the study, a development version of the BB mode on the XN‐1000 was used to test previously identified leukoreduction failures.
The BB mode uses fluorescence flow cytometry and impedance measurements to count WBCs, RBCs, and platelets in blood components. For flow cytometry, the forward scatter light (size of cells), side scatter light (inner complexity of cells), and fluorescence intensity (DNA/RNA content) are combined to identify and cluster each cell.
Quality control (QC) of the analyzers was performed with three levels of XN‐Check material (Sysmex UK).
Flow cytometry
At CDL determination of rWBC levels was performed using a flow cytometer (FACSCalibur, BD Biosciences) with QC performed with Calibrite beads, (BD Biosciences). QM‐Manchester used Navios analyzers with QC performed using Flow‐Check Pro and Flow‐Set Pro beads (Beckman Coulter). Both sites used WBC enumeration kits (Leucocount, BD Biosciences) for staining rWBCs before flow cytometric analysis. At QM‐Manchester, flow cytometry results were recorded in a QM database, with results of 1 WBC/μL or less being recorded as 1 WBC/μL.
Linearity studies
Leukoreduced blood components for linearity studies were double filtered with an additional leukoreduction process before spiking with whole blood, using an Autostop BC filter for PCs (Haemonetics), PLAS4 (Macopharma) for plasma, and LRCD2 (Macopharma) for RCCs. Five units of PCs (four apheresis derived, and one BC derived), four RCC units, and four whole blood–derived plasma units were tested. Units were spiked with ABO‐compatible whole blood to generate calculated concentrations of 32, 16, 8, 4, 2, 1, 0.5, and 0.3 WBCs/μL, with the nonspiked material used as a zero‐level sample.
Precision, limit of quantitation, and carryover
Three PCs (two BC derived and one apheresis), three RCCs, and three plasma samples with calculated concentrations of 32, 16, and 1 WBCs/μL were tested on 10 occasions to assess within‐run precision, with the mean concentration and coefficient of variation (CV%) being calculated.
Within NHSBT, cellular components form the greatest percentage of samples tested for rWBC enumeration. The limit of quantitation (LOQ) was therefore determined using one BC‐derived PC, one apheresis PC, and two RCCs. Components were spiked to give calculated WBC concentrations of 16, 8, 4, 2, and 1 cell/μL and repeat tested (n = 10). The LOQ was defined as the concentration at which the CV% was equivalent to 20%.10, 13
Carryover with use of three PCs (two BC derived and one apheresis), three RCCs, and three plasma samples was assessed according to the appropriate guidelines.14 Briefly, a high sample of 32 cells/μL (H) was tested three times followed by aliquots of the corresponding nonspiked components (L) and the percentage carryover calculated with the formula (L1‐L3) / (H3‐L3) × 100.
Limit of detection
Limit of detection (LOD) was assessed by testing one BC‐derived PC, one apheresis PC, and two RCCs spiked to give calculated concentrations of 4, 2, and 1 WBCs/μL and tested to give 20 values for each component at each cell concentration. The mean and 95% confidence intervals (CIs) were calculated and compared with the corresponding values for nonspiked components (n = 20). The LOD was defined as the concentration at which the 95% CI results did not overlap with that of the nonspiked samples.15
Validation with standard components produced in a routine manufacturing department
For QM purposes, manufactured components were routinely sampled into two tube types. These spent minimally 10 minutes on a roller‐mixer prior to testing by either flow cytometry or on a hematology analyzer (LH780, Beckman Coulter). Approximately 1 to 1.5 mL of components were placed into either an ethylenediaminetetraacetic acid tube (Greiner) to measure platelet counts, or into plain tubes (Greiner) for measuring various RBC parameters and rWBC counting. Having been processed for QM, ethylenediaminetetraacetic acid and plain samples with sufficient volume were manually inverted twice and placed on the XN‐2000 analyzer, for subsequent automatic mixing before rWBC enumeration.
The samples tested were EDTA tubes: apheresis PC (n = 1821), BC‐PC (n = 127), RCC (n = 13), apheresis plasma (n = 187); and plain tubes: apheresis PC (n = 1305), BC‐PC (n = 127), RCC (n = 14), and apheresis plasma (n = 94). All testing was completed within 10 hours of component sampling. Use of these nonpaired samples enabled the range of rWBC results obtained with the BB mode to be determined and any potential issues associated with sample type identified.
Where both flow cytometry and BB mode results were available for the same component, these were reviewed and converted to cells/unit to identify the effect of source data on identifying those components that met the less than 1 × 106 and less than 5 × 106 rWBC guidelines.
The highly efficient nature of the leukoreduction process results in infrequent failures. Consequently, the number seen within one manufacturing facility makes it difficult to test the ability of new systems to detect levels of rWBC representing LD failures. Therefore, early in the study, any NHSBT manufacturing site that identified leukoreduction failures sent them to CDL for testing on the XN‐1000, with a development version of the software. Components were resampled at CDL and the rWBC results compared with the original flow cytometry values determined by routine QM. Components were not retested by flow cytometry, as the manufacturer's guidelines that testing of samples should be performed within 48 hours of leukoreduction could not be met. At QM‐Manchester, for leukoreduction failures occurring during routine testing, a single sample was tested by both BB mode and flow cytometry, within these time guidelines.
Statistical analysis
Data were analyzed with computer software (Prism version 5, GraphPad Software Inc). The BB mode results from plain and ethylenediaminetetraacetic acid tubes were analyzed with a two‐tailed Mann–Whitney U test at 95% CI. The correlation between flow cytometry and BB mode results was analyzed using linear regression; p values less than 0.05 were considered statistically significant.
RESULTS
Linearity
For PCs, RCCs, and plasma components, there was an excellent linear correlation between the expected and observed WBC counts from the BB mode, with R2 0.9996 or greater for all cases over the full range of concentrations tested (Fig. 1A through C). Observed values from the BB mode sometimes differed from the expected values. For PCs at an expected result of 2 WBCs/μL, the average observed result was 8.5% higher at 2.17, while at concentrations greater than 2 WBCs/μL the observed results ranged between approximately 3% lower to 4% higher than expected. For plasma components, the observed results were 0.3% to 6% lower than expected. For RCCs, an average difference of 24.7% lower than expected was observed (Table 1).
Figure 1.
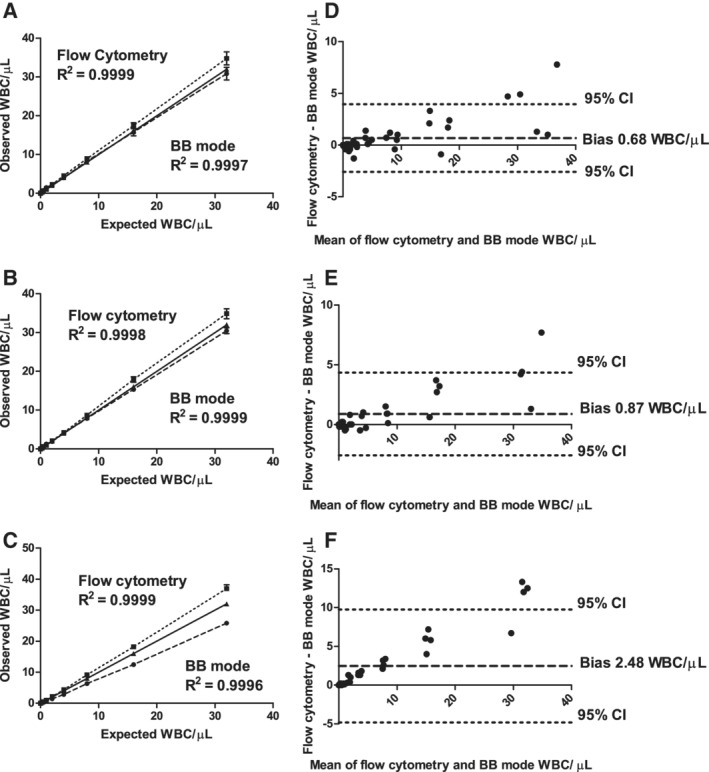
Linearity studies. Linear regression of observed versus expected rWBC results. (A) Platelets, (B) plasma, and (C) RCCs, by flow cytometry and the Sysmex XN‐1000 BB mode. For all linearity graphs; the dotted line represents the flow cytometric data, the solid line the line of equivalence and the dashed line the Sysmex BB mode data. Results from Bland‐Altman analysis comparing flow cytometry and BB mode data for (D) platelets, (E) plasma, and (F) RCCs.
Table 1.
Linearity results from both flow cytometry and Sysmex BB mode using RCC samples (n = 4)
| Expected WBC/μL | Flow cytometry average values | BB mode average values | ||
|---|---|---|---|---|
| Observed WBC/μL | % difference* | Observed WBC/μL | % difference* | |
| 32 | 36.9 | +15.4 | 25.8 | −19.4 |
| 16 | 18.2 | +13.7 | 12.4 | −22.3 |
| 8 | 9.1 | +13.8 | 6.3 | −21.3 |
| 4 | 4.3 | +8.5 | 2.8 | −29.4 |
| 2 | 2.1 | +5.4 | 1.4 | −31.3 |
Percent difference between expected and observed values.
For flow cytometry, the agreement between expected and observed counts gave R2 0.9998 or greater for all cases over the full range of concentrations (Fig. 1A through C). In the range of 2 to 32 WBCs/μL, unlike the BB mode, the observed values for flow cytometry were always greater than the expected values. In RCC, for example, the flow cytometry results were 5.4% to 15.4% higher than expected (Table 1).
Comparison of flow cytometry and BB mode results using Bland‐Altman analysis reiterated the pattern seen in the linearity analysis, with flow cytometry giving higher biases in all components, the highest being 2.48 WBCs/μL in RCC (Fig. 1D through F).
Precision, LOQ, carryover, and LOD
Precision testing showed that at 32 WBCs/μL, CVs for all components were 5.6% or less, which increased to a maximum of 28.1% at 1 WBC/μL (Table 2). LOQ analysis showed that although the average CVs for PC and RCC samples were greater than 20% at 1 WBC/μL, at 2 WBCs/μL the average CV% was less than 20%, thus giving an overall mean LOQ of 2 WBCs/μL (Table 2).
Table 2.
Precision analysis results of Sysmex BB mode with PC, RCC, and plasma samples
| Expected WBC | Buffy coat platelets* | Apheresis platelets* | RCC* | Plasma* | ||||
|---|---|---|---|---|---|---|---|---|
| Observed† | CV‡ | Observed | CV | Observed | CV | Observed | CV | |
| 32 | 32.3 | 4.9 | 30.4 | 5.1 | 24.4 | 5.6 | 30.2 | 4.5 |
| 16 | 15.9 | 5.0 | 14.8 | 6.9 | 13.1 | 5.5 | 15.7 | 5.8 |
| 8 | 8.0 | 8.6 | 6.7 | 9.4 | 7.5 | 8.9 | …§ | … |
| 4 | 4.2 | 12.9 | 3.5 | 16.0 | 3.7 | 10.9 | … | … |
| 2 | 2.0 | 18.4 | 1.9 | 10.1 | 2.1 | 20.7 | … | … |
| 1 | 1.2 | 26.6 | 1.3 | 19.5 | 0.8 | 24.1 | 1.2 | 28.1 |
Initial precision testing carried out at 32, 16, and 1 WBCs/μL, n = 3 for all components. Additional LOQ testing done at 16, 8, 4, 2, and 1 WBCs/μL, n = 2 for PC and RCC. To determine the observed count and CV each component was tested 10 times.
Average WBCs/μL.
Not tested.
Average CV%.
CV = coefficient of variation; LOQ = limit of quantitation; PC = platelet concentrate; RCC = red blood cell concentrate.
The carryover assessment process for PCs, RCCs, and plasma showed a range across the three components of 0.04% to 0.22%, with an average value of 0.1%.
Using 1 BC, 1 apheresis PC, and 2 RCCs, the 95% CI values for the nonspiked samples ranged from 0.00 to 0.06 WBC/μL, which did not overlap with the range of 0.86 to 1.36 for 1 WBC/μL. Nor did any of the 95%CI values for samples containing 1, 2, and 4 WBCs/μL overlap with each other, thereby indicating an LOD of 1 WBC/μL.
Validation using standard components produced in a routine manufacturing department
Effect of sample tube type
Across the different components, results were available from 1540 plain and 2148 ethylenediaminetetraacetic acid tubes, with the majority from PCs. It was evident that with some PCs, especially apheresis platelets, results from some plain tubes gave very high rWBC values (median, 2.5; range, 0.2‐1012.6; n = 1305), suggesting a large number of LD failures. Although the median result of 0.9 rWBC/μL obtained from PCs with ethylenediaminetetraacetic acid tubes was similar, the range was smaller (0‐23.2, n = 1821) and significantly different from those obtained with plain tubes (p < 0.0001; Fig. 2). Similarly, in apheresis plasma, although the median values and ranges were very similar (plain tubes, 1.5; range, 0‐11.9; n = 94; ethylenediaminetetraacetic acid, 0.8; range, 0‐13.6; n = 187), the measurements were significantly different (p < 0.0001; Fig. 2).
Figure 2.
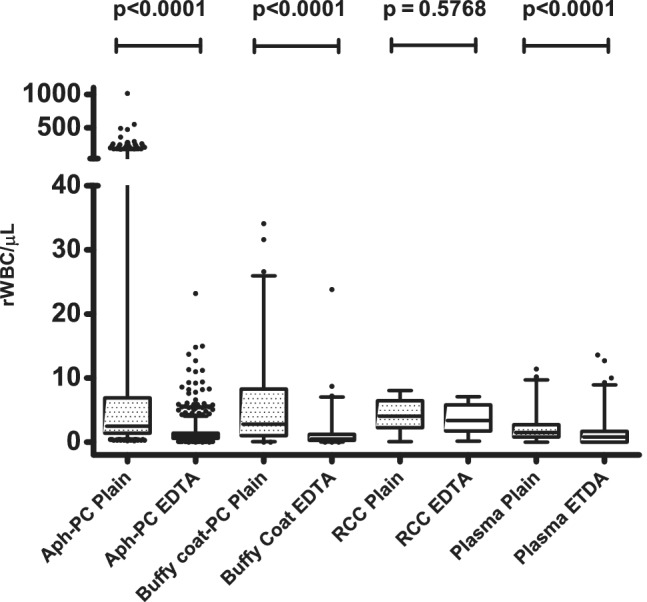
BB mode results from plain and ethylenediaminetetraacetic acid (EDTA) tubes. The range of BB mode results from plain (spotted boxes) and ethylenediaminetetraacetic acid tubes (clear boxes) taken from apheresis PCs (plain, n = 1305; ethylenediaminetetraacetic acid, n = 1821), BC‐derived PCs (plain, n = 127; ethylenediaminetetraacetic acid, n = 127), RCCs (plain, n = 14; ethylenediaminetetraacetic acid, n = 13), and apheresis plasma (plain, n = 94; ethylenediaminetetraacetic acid, n = 187). The boxes represent the 25th and 75th percentile values with the horizontal bar corresponding to the median value, the whiskers the 2.5th to 97.5th percentile ranges, and black full circles the outliers.
Although the range of results from plain (n = 14) and ethylenediaminetetraacetic acid (n = 13) tubes from RCCs were not significantly different from each other (p = 0.5768; Fig. 2), the value of this observation is limited, as it is derived from a relatively small number of samples.
A review of components with leukoreduction failures showed that the BB software multidimensional gating strategy clearly identified WBCs and debris in different regions (Fig. 3A). For successful leukoreduction, with an ethylenediaminetetraacetic acid tube, the BB software detected some particles, but correctly identified them as debris (Fig. 3B). A plain tube from the same component, however, showed some particles in the WBC region along with debris, leading to an incorrect high rWBC result (Fig. 3C).
Figure 3.
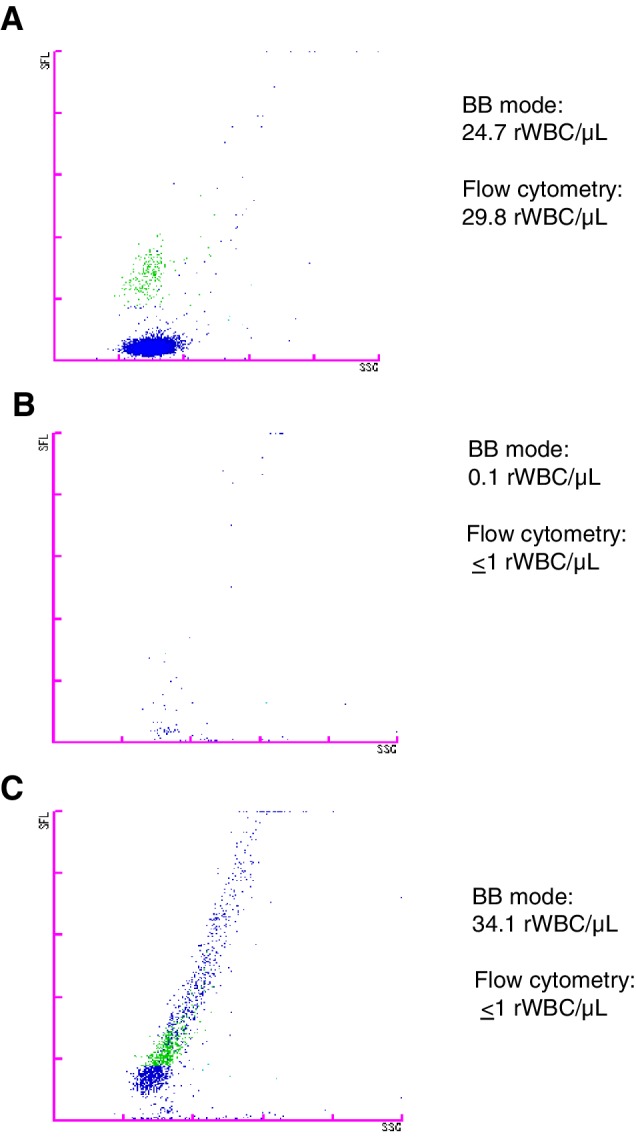
Illustration of BB mode analysis plots. Example of BB mode scatter plot results from PCs. (A) A leukoreduction failure, with high rWBC count identified by both BB mode and flow cytometry. The BB mode software identified lymphocytes and monocytes (green), which are separated from debris (blue) by multidimensional gating. (B) Successful leukoreduction identified by flow cytometry showing results from the BB mode ethylenediaminetetraacetic acid sample. While some signals appeared on the scatter plot, they were correctly classified as debris by the BB mode software. (C) The same component as in (B) but showing the BB mode result from a plain sample tube. Some particles appear in the WBC region along with debris and are incorrectly classified, leading to a false high result. [Color figure can be viewed at http://wileyonlinelibrary.com]
Comparison of BB mode and flow cytometry results for successful leukoreduction components
Components that were identified by flow cytometry as having less than 5 × 106 rWBCs/unit were used to compare flow cytometry and BB mode data. Results were used only where there were paired samples, with the flow cytometry taken from plain tubes and BB results from ethylenediaminetetraacetic acid tubes. This enabled comparison of data from 1367 PCs (apheresis, 1274; BC derived, 93) and 80 apheresis plasma components. There were no paired RCC samples.
BB mode results were available as direct outputs from the analyzer; however, flow cytometry results were derived from the QM database where measurements of 1 WBC/μL or less were recorded as 1 WBC/μL. This meant that for all 1447 samples, the BB mode results of 1 WBC/μL or less could not be compared with their corresponding quantitative flow cytometry results. Figure 4A illustrates an apparent linear relationship between the actual BB mode results and flow cytometry values for apheresis PCs, even though values of 1 WBC/μL or less had been recorded as 1 WBC/μL.
Figure 4.
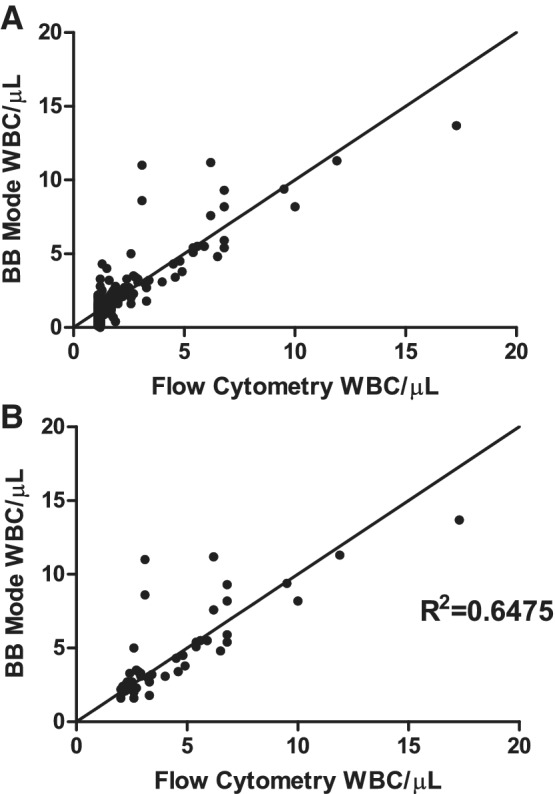
Flow cytometry and BB results from apheresis PCs with successful leukoreduction. Correlation between flow cytometry results using plain tubes and BB mode results with ethylenediaminetetraacetic acid tubes from apheresis PCs. (A) All samples (n = 1274); and (B) samples with BB mode results values >2 WBCs/μL (n = 48).
This combination of a lack of actual flow cytometry results of 1 WBC/μL or less combined with the 2 WBCs/μL LOQ for the BB mode means that there is little practical value in statistical correlation analysis of results below 2 WBCs/μL. For samples with BB mode values of 2 WBCs/μL or greater (n = 48), there was a moderate correlation with flow cytometry results (R2 = 0.6475; Fig. 4B).
As an alternative means of assessing the data, results with the BB mode were combined with the volume of each individual component (obtained from NHSBT QM database) to obtain a value for the rWBC/unit of component. Consequently, the percentage of components meeting the less than 1 × 106 rWBC guideline were compared with analogous flow cytometry results.
This approach showed that BB data resulted in slightly more components exceeding the less than 1 × 106 rWBC limit. For apheresis PCs, the BB mode identified 29 units as having values greater than this limit, compared with 20 identified by flow cytometry (Table 3). Sixteen units were identified as having values greater than 1 × 106 rWBC/unit by both techniques. For BC‐derived PCs, all were within specification with flow cytometry data, with 5 above the limit with BB mode, although the highest result was only 1.3 × 106/unit (Table 3). The same pattern was seen with apheresis plasma components, with only the BB mode giving high results, 1.8 × 106/unit or less (Table 3). Notably, though, the guideline target of more than 90% of components containing less than 1 × 106 rWBC was met with use of data from both sources (Table 3).
Table 3.
Components classified according to the <1 × 106 rWBC/unit guidelines
| Apheresis PCs (n = 1274) | Buffy coat PCs (n = 93) | Apheresis plasma (n = 80) | ||||
|---|---|---|---|---|---|---|
| Flow cytometry | BB mode | Flow cytometry | BB mode | Flow cytometry | BB mode | |
| Number with ≥1 × 106 rWBC/unit | 20 | 29 | 0 | 5 | 0 | 5 |
| rWBC/unit range* | 1.02‐4.14 | 1.008‐3.288 | N/A | 1.0791‐1.3366 | N/A | 1.0656‐1.7794 |
| Pass rate (%)† | 98.43 | 97.72 | 100 | 94.62 | 100 | 93.75 |
Range of results in units with rWBC ≥1 × 106/unit, N/A = no data available as no units were observed at this level.
Percentage of components meeting the <1 × 106 rWBC/unit guideline.
PCs = platelet concentrates.
Comparison of BB mode and flow cytometry results for components failing leukoreduction
Of the components that were 5 × 106 rWBCs/unit or greater, there were 10 RCCs and 3 PCs that had flow cytometric rWBC values greater than 13.4 cells/μL and less than 350 cells/μL and therefore within the manufacturer's stated linear range and similar to that used in another comparative study.5
Eight RCCs and one PC were sampled retrospectively and tested on the XN‐1000 between 1 and 11 days after the original flow cytometry tests; of these components, seven RCCs and one PC were measured using the development BB software, while the remaining RCC was measured using the BB mode. Four components were tested at the time of manufacture with the BB mode on the XN‐2000. All leukoreduction failures were clearly identified by the BB mode, with an excellent correlation with flow cytometry (R2 = 0.9818; Fig. 5A). Consistent with the linearity studies, there was a small underestimation by XN analyzer compared with flow cytometry results, giving a negative bias of 5.22 WBCs/μL (Fig. 5B).
Figure 5.
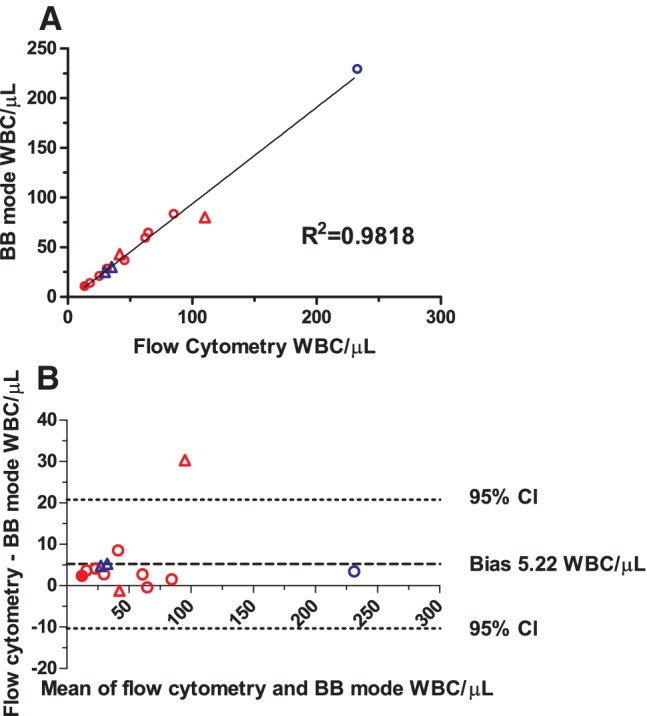
Flow cytometry and BB results from RCCs and PCs with poor leukoreduction. Comparison of rWBC results from the BB mode and flow cytometry for leukoreduction failures. RCCs are shown as red symbols and PCs as blue symbols. Circles represent data derived from samples tested retrospectively on the XN‐1000 using either the BB mode development software (open circles) or using the BB mode (closed circle), while triangles are samples tested at the time of manufacture with the XN = 2000 and BB mode software. (A) Regression analysis showing RCCs (n = 10) and PCs (n = 3); and (B) Bland–Altman analysis of the same data, showing the slight negative bias for the overall BB mode results. CI = confidence interval. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
Implementation of leukoreduction for blood components required development of a QM process with results from technologies able to count rWBCs.3, 4, 12 Recent developments in automated hematology analyzers have enabled counting of low levels of WBCs in body fluids9, 10 and lead to renewed interest in the use of these analyzers for counting rWBCs.8
A study of the CSF analytical mode on an ADVIA‐2120 hematology analyzer for rWBC counting found that both the preanalytical variation associated with manual sample preparation, and the need for a modified software gating strategy probably contributed to performance failure.8 These issues did not apply to the Sysmex XN series analyzers, as they did not require any preanalytical sample preparation, and software development for the BB mode had already incorporated modifications in the gating strategy.
We carried out the first evaluation of the BB mode using both leukoreduced blood components spiked with known concentrations of WBC and routine leukoreduced components. Linearity assessment using platelet, plasma, and RBC components showed excellent concordance with flow cytometry. There were good correlations between expected and observed results, although there was a tendency for the BB mode to underestimate WBC concentrations, most notably with RCCs. Conversely, flow cytometry tended to overestimate WBC concentrations.
Precision and LOQ analyses showed acceptable results, comparable with those previously ascribed to flow cytometry. For example, with use of RCC at 16 WBCs/μL, the CV for BB mode results was 5.5%; this compared favorably to that reported by Cardigan et al.,12 with reported intra‐assay CV of 6% with Leucocount reagents and a FACSCalibur flow cytometer for RBC products at 15 to 20 WBCs/μL. The same study reported a CV of 8% for WBC at the same concentration in platelets,12 which again compared well with our observed BB mode average CV for PCs of 6.0%. For the range of 2 to 4 WBCs/μL in PCs, the BB mode CV was 14.4%, which is slightly higher than the 9% for 3.3 WBCs/μL reported by Dijkstra‐Tiekstra.16
The average LOQ with the BB mode of 2 WBCs/μL agreed with the value described with the Sysmex hsA mode for CSF analysis,10 but slightly above the value implied in another study with the Leucocount‐FACSCalibur combination where CVs less than 20% were still observed at the level of 1 WBC/μL in platelet components.16
When testing manufactured components, the type of sample used for BB analysis was found to be a factor influencing the quality of results, with PCs and plain tubes without anticoagulant sometimes giving aberrant results due to unidentified events appearing in the analysis plots and counted as WBC. The cause and nature of these events has yet to be determined. As a significant difference between results obtained with ethylenediaminetetraacetic acid and plain tubes was also seen with plasma components, it is possible that this phenomenon was applicable to all components, but unfortunately there were too few RCC samples to reliably assess the incidence of aberrant results in this component.
Testing the samples by the BB mode after being used for routine QM processes may have contributed to handling‐induced artifacts. This possibility is supported by the absence of aberrant WBC values in performance characterization assays where samples were prepared in plain tubes, but were only mixed immediately prior to testing. Atypical patterns of results have previously been described in flow cytometric rWBC assays and ascribed to being possibly damaged cells, autofluorescence debris, fragmented nuclei, or DNA.17, 18
Although use of ethylenediaminetetraacetic acid tubes appeared to diminish the issue of aberrant events being counted as WBCs, we cannot be certain that they completely resolved the problem. Such events may have been detected as WBCs and contributed to the discrepancy between results from manufactured components that had undergone successful leukoreduction where, overall, BB mode results gave a higher estimation of leukoreduction failures at the 1 × 106 WBC/unit level compared with flow cytometry. However, in both the linearity studies and in leukoreduction failures with 5 × 106 rWBC/unit or greater, the BB mode gave a slight underestimation of rWBCs compared with flow cytometry.
From blood component QM and stock management points of view, in routine leukoreduced components, the overestimation of rWBCs by BB mode would not have resulted in components being discarded, as levels above the 5 × 106 WBCs/unit disposal limit were not observed. Furthermore, overestimation of rWBCs could be seen as a safer option compared with underestimation, which has the potential to allow leukoreduction failures to enter the blood supply chain. Notably, all components identified as leukoreduction failures by flow cytometry were also clearly identified by BB mode.
Operationally, use of the Sysmex XN for rWBC counting offers the potential to automate, simplify, and rationalize QM procedures. One ethylenediaminetetraacetic acid sample could be used for both rWBC enumeration and routine hematologic QM. In addition, all requirements for preanalytical manipulation, such as pipetting of samples and staining reagents, would be eliminated, as would potential sample transposition and transcription errors. An estimate of processing times for a daily workload of approximately 100 tests within QM‐Manchester suggested that by moving from an analysis system of hematology analyzer and flow cytometer to a single‐platform hematology analyzer system would reduce processing time by approximately 3 hours, primarily by removing the need for manual sample preparation before flow cytometry. Furthermore, management processes linked to equipment solely used for rWBC counting would be removed.
Implementation of new quantitative technology requires consideration of both internal and external quality assessment (QA) and the availability of QM material. A laboratory could consider extending its analyzer QC procedures to incorporate suitable material such as use of a 1 in 200 dilution of whole blood with a known WBC count.12 Participation in external QA schemes1 could require interaction with QA providers such as previously occurred for flow cytometry.11
As this study was a single organization's assessment, the true value of the system will be realized only by additional multisite national and international evaluations to identify optimal processes, such as occurred with flow cytometry and other technologies.12, 19
Multisite testing will provide the opportunity to further assess preanalytical variables such as sample type, mixing, time between sample acquisition, and testing. Such studies may help understand the currently observed discrepancies between patterns of results seen in artificially spiked and in routinely manufactured components. It could also provide further information on the reproducibility and potential relevance of the underestimation of rWBCs, as seen in the current RCC linearity studies. Should this observation become identified as a consistent negative bias in routinely manufactured components, options for dealing with this issue could be considered, which might include use of correction factors as previously described.15, 20
Overall, this first report into the use of Sysmex XN‐series hematology analyzers that use the BB analytical mode for enumeration of rWBCs indicates that the system has acceptable performance characteristics with both artificially spiked samples and routine leukoreduced blood components, successfully identifying leukoreduction failures initially detected by flow cytometry. These findings support continued studies into the role of hematology analyzers in rWBC enumeration to optimize testing processes.
CONFLICTS OF INTEREST
RAB, CC, LW, EA, NA, HS, SP, SFG, and RC have disclosed no conflicts of interest. AS, JS, and JL are employees of Sysmex.
ACKNOWLEDGMENTS
We acknowledge the NHSBT Cambridge donor clinic and NHSBT manufacturing facilities for the supply of samples and blood components and thank all the staff at NHSBT CDL and the Manufacturing and Development team for their support.
Sources of support: This study was funded by NHS Blood and Transplant. In addition, Sysmex provided financial support for studies performed in the Component Development Laboratory, and for funding for staff, reagents and loan of the XN‐2000 during the NHS Blood and Transplant Manchester section of the study. Sysmex also contributed the publication costs.
REFERENCES
- 1. MacLennan S, editors. Guidelines for the Blood Transfusion Services in the United Kingdom. 8th ed. London: TSO; 2013. p. 399. [Google Scholar]
- 2. American Association of Blood Banks, Standards Program Committee . Blood Bank/Transfusion Service Standards Program Unit In: Standards for blood banks and transfusion services; 2008. Bethesda, MD 20814: American Association of Blood Banks (AABB). [Google Scholar]
- 3. Dumont LJ, Dzik WH, Rebulla P, et al. Practical guidelines for process validation and process control of white cell‐reduced blood components: report of the Biomedical Excellence for Safer Transfusion (BEST) Working Party of the International Society of Blood Transfusion (ISBT). Transfusion 1996;36:11‐20. [DOI] [PubMed] [Google Scholar]
- 4. Beckman N, Cardigan R, Wallington T, et al. Value of central analysis of leucocyte depletion quality control data within the National Blood Service, England. Vox Sang 2002;83:110‐8. [DOI] [PubMed] [Google Scholar]
- 5. Zeng Y, Dabay M, George V, et al. Comparison of flow cytometric methods for the enumeration of residual leucocytes in leucoreduced blood products: a multicenter study. Cytometry A 2018;93:420‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bae SY, Lee CH, Kim JS, et al. Portable microscopic cell counter for the determination of residual leucocytes in blood components. Vox Sang 2007;92:64‐8. [DOI] [PubMed] [Google Scholar]
- 7. Strobel J, Antos U, Zimmermann R, et al. Comparison of a new microscopic system for the measurement of residual leucocytes in apheresis platelets with flow cytometry and manual counting. Vox Sang 2014;107:233‐8. [DOI] [PubMed] [Google Scholar]
- 8. Petersson A, Ekblom K. Methods for counting residual leukocytes in leukocyte‐depleted plasma‐a comparison between a routine hematology instrument, the Nageotte chamber, flow cytometry, and a fluorescent microscopy analyzer. Transfusion 2017;57:1192‐8. [DOI] [PubMed] [Google Scholar]
- 9. Fleming C, Brouwer R, Lindemans J, et al. Validation of the body fluid module on the new Sysmex XN‐1000 for counting blood cells in cerebrospinal fluid and other body fluids. Clin Chem Lab Med 2012;50:1791‐8. [DOI] [PubMed] [Google Scholar]
- 10. Fleming C, Russcher H, Brouwer R, et al. Evaluation of Sysmex XN‐1000 high‐sensitive analysis (hsA) research mode for counting and differentiating cells in cerebrospinal fluid. Am J Clin Pathol 2016;145:299‐307. [DOI] [PubMed] [Google Scholar]
- 11. Barnett D, Goodfellow K, Ginnever J, et al. Low level leucocyte counting: a critical variable in the validation of leucodepleted blood transfusion components as highlighted by an external quality assessment study. Clin Lab Haematol 2001;23:43‐51. [DOI] [PubMed] [Google Scholar]
- 12. Cardigan R, Phipps A, Seghatchian J, et al. The development of a national standardized approach for the enumeration of residual leucocytes in blood components. Vox Sang 2002;83:100‐9. [DOI] [PubMed] [Google Scholar]
- 13. Tholen D. Protocols for determination of limits of detection and limits of quantitation. Approved guideline [Internet]. 2004. Available from: https://ci.nii.ac.jp/naid/10030014457/en/
- 14. International Council for Standardization in Haematology, Writing Group , Briggs C, Culp N, et al. ICSH guidelines for the evaluation of blood cell analysers including those used for differential leucocyte and reticulocyte counting. Int J Lab Hematol 2014;36:613‐27. [DOI] [PubMed] [Google Scholar]
- 15. Dzik WH. Leukocyte counting during process control of leukoreduced blood components. Vox Sang 2000;78(Suppl 2):223‐6. [PubMed] [Google Scholar]
- 16. Dijkstra‐Tiekstra MJ, Schrijver JG, van der Meer PF, et al. Crossover study of three methods for low‐level white blood cell counting on two types of flow cytometer. Cytometry B Clin Cytom 2003;54:39‐45. [DOI] [PubMed] [Google Scholar]
- 17. Janatpour K, Paglieroni TG, Schuller L, et al. Interpretation of atypical patterns encountered when using a flow cytometry‐based method to detect residual leukocytes in leukoreduced red blood cell components. Cytometry 2002;50:254‐60. [DOI] [PubMed] [Google Scholar]
- 18. Bashir S, Cardigan R. The origin and identification of unknown events associated with low‐level leucocyte counting by flow cytometry. Vox Sang 2003;85:190‐8. [DOI] [PubMed] [Google Scholar]
- 19. Dzik S, Moroff G, Dumont L. A multicenter study evaluating three methods for counting residual WBCs in WBC‐reduced blood components: Nageotte hemocytometry, flow cytometry, and microfluorometry. Transfusion 2000;40:513‐20. [DOI] [PubMed] [Google Scholar]
- 20. Dzik S. Principles of counting low numbers of leukocytes in leukoreduced blood components. Transfus Med Rev 1997;11:44‐55. [DOI] [PubMed] [Google Scholar]


