Abstract
Background and Aim
In Japan, risk stratification after baseline colonoscopy is not widely accepted. We investigated the findings of baseline colonoscopies at 17 community practices and evaluated the risk of the incidence of advanced neoplasia over a 5‐year period.
Methods
This retrospective cohort study enrolled 3115 subjects over 40 years of age who underwent baseline colonoscopies and had at least one repeated colonoscopy within 5 years. Each group was classified based on the endoscopic findings of the baseline colonoscopy: no neoplasia/diminutive polyp <5 mm (N/D); small adenoma <10 mm; advanced adenoma; invasive cancer, respectively. We examined the incidence of advanced neoplasia during these 5 years and investigated the relationship between the surveillance colonoscopy and newly detected advanced neoplasia.
Results
The small adenoma group did not show any significant increased risk as compared to the N/D group (hazard ratio [HR]: 0.799. 95% CI 0.442–1.443). There was a significantly increased risk in the advanced adenoma and invasive cancer groups (HR: 4.996, 95% CI 2.940–8.491, HR: 3.737, 95% CI 1.309–10.666). Cancer incidences during the study period were 0.18% in the N/D group, and 1.9% in the invasive cancer group, respectively. Undergoing surveillance colonoscopies twice within 5 years decreased the risk of advanced neoplasia.
Conclusions
There was a close relationship between the endoscopic findings of baseline colonoscopies and subsequent advanced neoplasia development. Risk stratification for advanced neoplasia based on the baseline findings can serve as a useful index for determining the optimal interval and frequency of colonoscopies over a 5‐year period.
Keywords: advanced neoplasia, colorectal cancer, invasive cancer, risk stratification, surveillance colonoscopy
Introduction
In Japan, colorectal cancer (CRC) is the third‐leading cause of cancer mortality among men, and the leading cause among women, with the prevalence of CRC also shown to be gradually rising within the overall population.1 Colonoscopy has generally been accepted as a useful tool for colorectal cancer screening and surveillance.2, 3, 4 In addition, as shown by the National Polyp Study, endoscopic resection of a colonic neoplasm is associated with a reduced risk of CRC.2, 5, 6, 7 In particular, number of adenomas, adenoma size, and histology detected at initial colonoscopy are important risk factors for subsequent advanced neoplasia (AN) as well as CRC.5, 8 Furthermore, results of large‐scale clinical trials have been used to establish optimal surveillance guidelines after baseline colonoscopy.8, 9, 10 However, in Asia, there are no established guidelines or recommendations for CRC screening and surveillance colonoscopy.11
Although colonoscopy is popularly practiced as a routine examination within community practices in Japan, it is not widely used as a screening program. The main purpose of the present study was to examine the incidence of newly detected neoplasia after baseline colonoscopy and investigate the surveillance times and intervals based on real‐world data obtained from community practices in Japan.
Methods
Setting and study participants
We designed a 5‐year multicenter retrospective cohort study to investigate the risk for AN after baseline colonoscopy. The study protocol was approved by the Institutional Review Board of Kumamoto University (Accession No. 1127). Informed consent was obtained at each practice. This study was conducted at 17 Japanese community practices in Kumamoto, Japan. All participating endoscopists were fellows certified by the Japan Gastroenterological Endoscopy Society. Asymptomatic subjects with excellent/good bowel preparation12 who underwent the first colonoscopy in their lifetime (baseline colonoscopy) between January 2004 and December 2006 were recruited to this study. After their baseline colonoscopy, we excluded subjects who had inflammatory bowel disease (IBD), familial adenomatous polyposis (FAP), subjects who were less than 40 years of age and subjects who had incomplete colonoscopy. We also excluded subjects who had no follow‐up colonoscopy during the study period. After the exclusions, subjects who underwent surveillance colonoscopy at least once after the baseline colonoscopy by June 2011 were enrolled in this study.
When neoplastic lesions were detected, endoscopic resection was carried out in accordance with the procedures of each practice. Basically, polyps larger than 5 mm were resected using either polypectomy or endoscopic mucosal resection. However, diminutive <5 mm polyps were left unresected in accordance with the decision of the endoscopists.13 These unresected diminutive polyps were not counted as part of the baseline findings in this study. Characteristics of the resected neoplasias (size, number and histology) found at baseline colonoscopy were analyzed on the basis of endoscopic reports. Data were collected by two or more authors at each institution for this study. In cases with insufficient records, neoplasias were directly reviewed by two authors (T.S., O.S.) using the endoscopic images stored at each practice.
According to US guidelines,10 subjects were classified into four groups based on the most advanced histological lesion found during the baseline colonoscopy in accordance with the following criteria: no neoplasia present or any unresected diminutive <5 mm polyps (no neoplasia/diminutive polyp group: N/D group); any small <10 mm adenomas present (small adenoma group); and tubular adenomas ≥10 mm, adenomas with villous histology, high‐grade dysplasia (HGD) including intramucosal carcinoma (advanced adenoma group); and invasive cancer (invasive cancer group).3, 10 AN includes advanced adenoma and invasive cancer. Subjects with hyperplastic polyps in the distal colon that were detected during baseline colonoscopy were included in the no‐neoplasia group14 as they are not generally considered to be premalignant lesions15 as has been indicated in the US guidelines.10 Literature on interval cancer and post‐colonoscopy colorectal cancer (PCCRC) lack agreement on terminology and methodology. In the present study, PCCRC was defined as a colorectal cancer diagnosed during the study period after the baseline colonoscopy, as previously described.16
Primary endpoint was a new detection of AN (advanced adenoma or invasive cancer). In the first step, we initially calculated hazard ratio (HR) for AN incidence during the 5‐year observation period. Second, we then examined the relationship between the times of surveillance colonoscopies and the incidence of AN during the 5‐year period. Third, we investigated the incidence of AN according to the difference of time from the baseline colonoscopy to the first surveillance colonoscopy.
Statistical analysis
Risk of AN development was compared among the four groups at baseline. Kaplan‐Meier curve was described among four groups and compared by using log‐rank test. HR was estimated using Cox proportional hazards model. Estimates of HR were calculated along with the 95% confidence intervals (CI) after adjustments were made for age and gender. The N/D group was used as the reference group for the evaluation of HR for the other groups. Continuous data were analyzed by Student's t‐test, whereas categorical data were analyzed by Fisher's exact test. All statistical analyses were carried out using the Statistical Package for the Social Sciences version 17.0. All P values were two‐sided, with statistical significance considered to be P < .05.
Results
Enrolment of the subjects in the present study
A total of 8815 consecutive participants underwent their baseline colonoscopies at the 17 community practices. Of this initial group, we excluded 33 IBD patients, two FAP patients, 211 subjects who were less than 40 years of age and seven subjects as a result of incomplete colonoscopy. We also excluded 5447 subjects who had no follow‐up colonoscopy. Eventually, 3115 subjects who had undergone at least one repeat colonoscopy as of June 2011 were enrolled in this analysis as shown in Figure 1.
Figure 1.
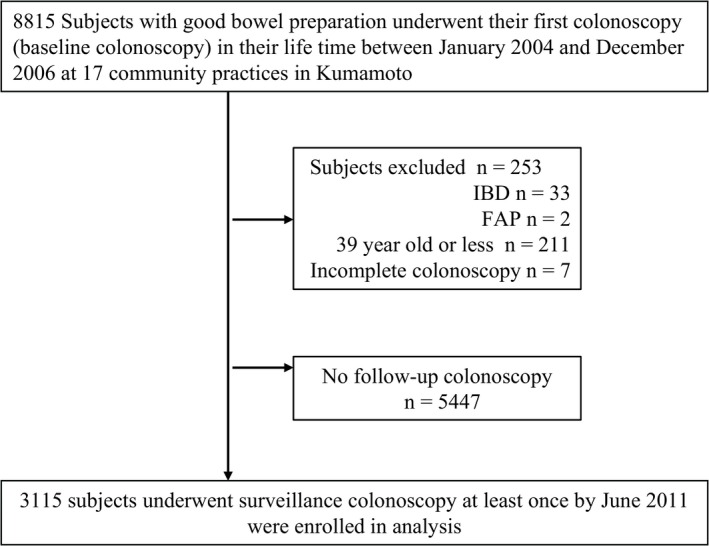
Study enrolment protocol. FAP, familial adenomatous polyposis; IBD, inflammatory bowel disease.
Characteristics of the study population and baseline colonoscopy findings
Table 1 presents the characteristics of the study subjects. Out of 3115 subjects, 1596 (51%) were males and 1519 (49%) were females. Subjects between the ages of 60 and 69 years were the most common in each gender. By institution, 2525 (81%) subjects underwent their baseline colonoscopies at 13 private clinics, and 590 (19%) subjects underwent their baseline colonoscopies at four medical health centers. Table 2 shows the baseline colonoscopy findings. The N/D group comprised 1621/3115 (52%) subjects. In contrast, there were 1494/3115 (48.0%) subjects who had small adenomas, advanced adenomas or invasive cancers. These subjects comprised the small adenoma group: 1147/3115 (36.8%), the advanced adenoma group: 296/3115 (9.5%) and the invasive cancer group: 51/3115 (1.6%).
Table 1.
Characteristics of study subjects
| Age (years) | Male | Female | Total |
|---|---|---|---|
| No. of subjects | |||
| 40–49 | 248 | 210 | 458 |
| 50–59 | 486 | 411 | 897 |
| 60–69 | 495 | 515 | 1010 |
| 70–79 | 335 | 346 | 681 |
| >80 | 32 | 37 | 69 |
| Institution | |||
| 13 private clinics | 1201 | 1324 | 2525 |
| 4 medical health centers | 395 | 195 | 590 |
| Total | 1596 (51%) | 1519 (49%) | 3115 |
Table 2.
Baseline colonoscopy findings
| Baseline finding | Male (%) | Female (%) | Total (%) |
|---|---|---|---|
| No neoplasia/diminutive (<5 mm) polyp (N/D) | 692 (43.4) | 929 (61.2) | 1621 (52.0) |
| Small (<10 mm) adenoma | 694 (43.5) | 453 (29.8) | 1147 (36.8) |
| Advanced adenoma | 210 (13.2) | 116 (7.6) | 296 (9.5) |
| Adenoma ≥ 10 mm | 94 (5.6) | 53 (3.5) | 147 (4.7) |
| Villous adenoma | 23 (1.4) | 14 (0.9) | 37 (1.2) |
| High‐grade dysplasia | 63 (3.9) | 49 (3.2) | 112 (3.6) |
| Invasive cancer | 30 (1.9) | 21 (1.4) | 51 (1.6) |
| Total | 1596 | 1519 | 3115 |
Surveillance results based on baseline colonoscopy findings
Table 3 presents the surveillance results and HR of AN incidence during the 5‐year period after baseline colonoscopy. A total of 81 (81/3115, 2.6%) AN, including nine (9/3115, 0.29%) invasive cancers, were newly detected. Among the N/D group, 28 (28/1621, 1.7%) developed AN, which included three (3/1621, 0.18%) invasive cancers. Among the small adenoma group, 19 (19/1147, 1.7%) developed AN, which included one invasive cancer (1/1147, 0.08%). When the subjects were subclassified according to the number of small adenomas, there was no significant increase in the risk for subsequent AN observed in either of the subgroups (HR, 0.798; 95% CI: 0.424–1.503, HR, 0.765; 95% CI: 0.264 2.210, respectively).
Table 3.
Relative risk of advanced neoplasia within 5 years based on baseline findings
| Baseline finding | Subjects, n | Advanced neoplasia, n (%) | Hazard ratio | 95% CI | Hazard ratio | 95% CI | Invasive cancer (PCCRC) n (%) |
|---|---|---|---|---|---|---|---|
| No neoplasia/diminutive (<5 mm) polyps (N/D) | 1621 | 28 (1.7) | 1.00 | — | 1.00 | — | 3 (0.18) |
| Small (<10 mm) adenoma | 1147 | 19 (1.7) | 0.799 | 0.442–1.443 | — | — | 1 (0.08) |
| 1 or 2 | 929 | 15 (1.6) | — | — | 0.798 | 0.424–1.503 | 0 |
| 3 and more | 218 | 4 (1.8) | — | — | 0.765 | 0.264–2.210 | 1 (0.46) |
| Advanced adenoma | 296 | 30 (10.1) | 4.996 | 2.940–8.491 | — | — | 4 (1.4) |
| Adenoma ≥10 | 147 | 17 (11.6) | — | — | 6.251 | 3.386–11.539 | 2 (1.4) |
| Villous adenoma | 37 | 2 (5.4) | — | — | 1.060 | 0.142–7.900 | 0 |
| High‐grade dysplasia | 112 | 11 (9.8) | — | — | 4.951 | 2.490–9.846 | 2 (1.8) |
| Invasive cancer | 51 | 4 (7.8) | 3.737 | 1.309–10.666 | 3.716 | 1.301–10.610 | 1 (1.9) |
| Female | 1519 | 45 (3.0) | 0.875 | 0.557–1.375 | 0.859 | 0.546–1.350 | 6 (0.4) |
| Age (1 year) | — | — | 1.024 | 1.001–1.048 | 1.026 | 1.003–1.050 | — |
| Total | 3115 | 81 (2.6) | — | — | — | — | 9 (0.29) |
PCCRC, post‐colonoscopy colorectal cancer.
In contrast, among the advanced adenoma group, 30 (30/296, 10.1%) developed AN, including four (4/296, 1.4%) with invasive cancers. These patients had a statistically significantly increased risk compared to those in the N/D group (HR, 4.996; 95% CI: 2.940–8.491). The invasive cancer group also showed a similar trend (HR, 3.737; 95% CI: 1.309–10.666). The incidence of AN, stratified by the baseline finding, was significantly higher among the advanced adenoma group and invasive cancer group than among the N/D group (log‐rank test P < 0.001) as shown in Figure 2. In terms of PCCRC, the incidence among the invasive cancer group was more than 10‐fold higher than that found among the N/D group (1.9% vs 0.18%, respectively), as shown in Table 3.
Figure 2.
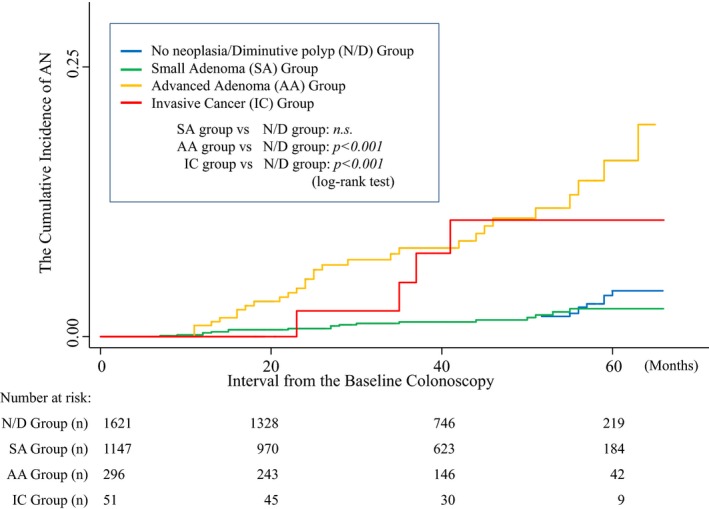
Incidence of advanced neoplasia (AN) in each group by baseline endoscopic finding. Incidence of advanced neoplasia was significantly higher among the advanced adenoma group and invasive cancer group than among the N/D group (log‐rank test P < 0.001).
Advanced neoplasia during surveillance colonoscopy in accordance with time of colonoscopy
Table 4 shows the incidence of AN in accordance with the times of the surveillance colonoscopy during the 5‐year period. Average number of surveillance colonoscopies was 1.9 during the 5‐year period in the N/D group, 2.0 in the small adenoma group, 2.2 in the advanced adenoma group, and 2.5 in the invasive cancer group.
Table 4.
Advanced neoplasia and invasive cancer at surveillance colonoscopy based on time of colonoscopy
| Baseline finding | No. of advanced neoplasias/no. of invasive cancers | |||
|---|---|---|---|---|
| No. of surveillance colonoscopies (N) | ||||
| Once (3115) | Twice (1639) | Three times (831) | Four times or more (420) | |
| No neoplasia/diminutive (<5 mm) polyps (N/D) (1621) | 16/1 (1621) | 8/2 (813) | 4/0 (392) | 0/0 (212) |
| Incidence of AN, IC (%) | 0.58%, 0.06% | 0.56%, 0.14% | 0.56%, 0% | 0%, 0% |
| Small (<10 mm) adenoma (1147) | 14/1 (1147) | 5/0 (610) | 0/0 (316) | 0/0 (145) |
| 1 or 2 (929) | 12/0 (929) | 3/0 (487) | 0/0 (256) | 0/0 (116) |
| >3 (218) | 2/1 (218) | 2/0 (123) | 0/0 (60) | 0/0 (29) |
| Incidence of AN, IC (%) | 1.1%, 0.09% | 0.82%, 0% | 0%, 0% | 0%, 0% |
| Advanced adenoma (296) | 20/3 (296) | 8/1 (184) | 1/0 (100) | 1/0 (49) |
| Adenoma ≥10 mm (147) | 14/1 (147) | 3/1 (83) | 0/0 (44) | 1/0 (21) |
| Villous adenoma (37) | 0/0 (37) | 0/0 (23) | 0/0 (13) | 0/0 (3) |
| High‐grade dysplasia (112) | 6/2 (112) | 5/0 (78) | 1/0 (43) | 0/0 (25) |
| Incidence of AN, IC (%) | 6.8%, 1.01% | 4.3%, 0.54% | 1.0%, 0% | 2.0%, 0% |
| Invasive cancer (51) | 3/1 (51) | 1/0 (32) | 0/0 (21) | 0/0 (14) |
| Incidence of AN, IC (%) | 5.9%, 2.0%, | 3.1%, 0% | 0%, 0% | 0%, 0% |
AN, advanced neoplasia; IC, invasive cancer.
For the first and second surveillances, the incidence of AN in the N/D group and small adenoma group was very low (0.58%, 0.56% and 1.1%, 0.82%, respectively). In contrast, the incidence of AN in the advanced adenoma or invasive cancer group was much higher compared with the N/D group (6.8%, 4.3% and 5.9%, 3.1%, respectively). However, in the third surveillance, the incidence of AN in the advanced adenoma group (1.0%) or invasive cancer group (0%) was almost equivalent to that for the N/D group (0.56%). Our study also found that there were no invasive cancers after the third surveillance in all groups.
Relationship between advanced neoplasia incidence and the interval to first surveillance colonoscopy
Figure 3 shows that the incidence of AN was dependent on the interval to first surveillance colonoscopy. We divided the intervals into five terms as follows: 1st term (0–12 months), 2nd term (13–24 months), 3rd term (25–36 months), 4th term (37–48 months), and 5th term (49–66 months). In the N/D or small adenoma group, the incidence of AN consistently remained at a low level in spite of the interval time for each term (0.6–2.5% and 0–2.3%, respectively). However, in the advanced adenoma group, the incidence of AN remained high from the second to the fifth term (7.5%, 10.4%, 10.5%, 9.1%, respectively). This was particularly the case in the invasive cancer group, with the incidence rapidly increasing over 20% from that seen in the third and fourth terms (20%, 28.5%, respectively).
Figure 3.
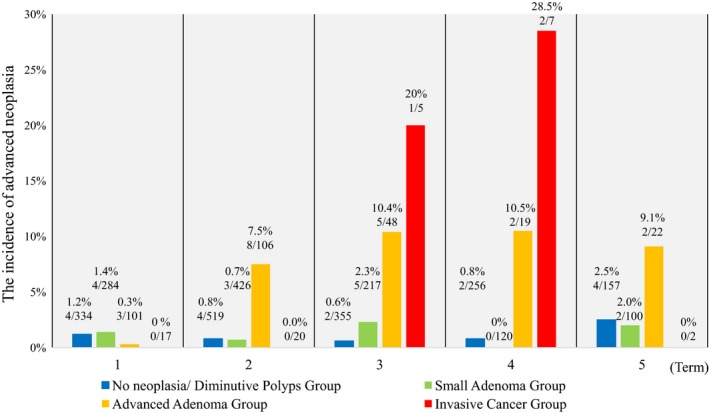
Incidence of advanced neoplasia (AN) at the first surveillance colonoscopy in each term. We divided interval time for surveillance colonoscopy into five terms as follows; 1st term (0–12 months), 2nd term (13–24 months), 3rd term (25–36 months), 4th term (37–48 months), and 5th term (49–66 months). In the N/D or small adenoma group, the incidence of AN consistently remained low despite the time interval for each term (0.6–2.5% and 0–2.3%, respectively). On the contrary, in the advanced adenoma group, the incidence of AN remained high from the second to the fifth term (7.5%, 10.4%, 10.5%, 9.1%, respectively).
Discussion
The Japanese National Health Insurance System enables all participants to easily undergo a colonoscopy, as the cost of a colonoscopy in Japan is much lower than that found in Western countries. However, the Japanese system has led to an increased number of colonoscopies being carried out and, in fact, more than are actually necessary in many cases. Therefore, risk stratification based on the endoscopic findings of baseline colonoscopy might be an urgent issue in Japan, from the viewpoint of securing medical resources.
The present observations showed that the endoscopic findings for baseline colonoscopy could be used to stratify the risk of AN including invasive cancer (Table 3). Our findings showed that the N/D group developed AN (28/1621,1.7%), including invasive cancer (3/1621, 0.18%), during the observation period. These findings are supported by previous reports from other Asian and Western countries which have shown the incidence of AN during surveillance of subjects without any polyps at baseline colonoscopy being between 1.3% and 2.4% over a period of 5 years.3, 4, 14, 17
As shown in Table 3, when the small adenoma group was compared with the N/D group, the subsequent incidence of AN within 5 years was approximately similar (1.7%, 1.7%, respectively). Furthermore, our study indicated that the presence of more than three small adenomas at baseline did not increase the risk of subsequent AN within a period of 5 years (HR, 0.765; 95% CI: 0.264 2.210) in contrast to previous studies.9, 10
In contrast, the incidence of AN in the advanced adenoma group or invasive cancer group was much higher (10.1%, 7.8%, respectively), indicating a significant relationship between endoscopic findings at baseline colonoscopy and risk of subsequent AN.
Although the number of polyps is a well‐known risk factor for AN and regarded as one of the stratification factors in the US guidelines,10 when taking the current findings together, we could emphasize size of polyp and histological grade of malignancy rather than number of polyps from the viewpoint of subsequent AN development. In addition, we suggest that any diminutive polyps and any small adenomas with appropriate intervention are not risk factors for subsequent AN development limited to 5 years.
We also investigated time of surveillance colonoscopy (Table 4), and the time to first surveillance (Figure 3). For the N/D and small adenoma groups, the incidence of AN after baseline colonoscopy remained unchanged regardless of the number of surveillance colonoscopies carried out within the following 5‐year period (range: 0–1.1%). The incidence of AN remained at a consistently low level for each of the interval terms for the first surveillance. Taken together, these findings indicate that first surveillance interval within 5 or more years might be acceptable for the N/D or small adenoma groups.
In contrast, the incidence of AN at the first and the second surveillance colonoscopies remained high in the advanced adenoma and invasive cancer groups (first: 6.8%, second: 4.3%; and first: 5.9%, second: 3.1%, respectively) (Table 4). We also confirmed that two surveillance colonoscopies contributed to a reduction in the risk of advanced adenoma and invasive cancer, which is compatible with a previous study showing that undergoing two or more colonoscopies within 5 years was associated with a 67% and a 52% reduced risk of AN based on low risk (no neoplasia/diminutive polyps or 1–2 cumulative adenomas <10 mm) or increased risk (advanced adenoma and >3 cumulative adenomas ≥10 mm) patients, respectively.18
With regard to the time interval to first surveillance, there was a higher incidence of AN in the advanced adenoma group after the second term (Figure 3). In addition, there was also a higher incidence of AN for the invasive cancer group after the third term. Thus, in these groups, higher risks were likely after 2–3 years. These results led us to speculate that subjects with AN at baseline colonoscopy should undergo their first surveillance colonoscopy within 2–3 years, with at least two surveillance colonoscopies carried out within the following 5 years.
Post‐colonoscopy colorectal cancer was detected among all groups in this study. It has been reported that PCCRC can be traced to missed lesions, incomplete resection or rapid new growth of cancer.19, 20 According to the previous study, the incidence rates of new and missed CRC after colonoscopy ranged from 2% to 6%.20 Lieberman et al.3 reported that the cumulative incidence of invasive cancer within 5.5 years was 0.6% (5/875) in subjects with no AN at baseline, and 3.3% (9/273) in patients with AN at baseline. In the present study, the cumulative incidence of PCCRC was 0.14% (4/2768) among the N/D and small adenoma group, whereas it was 1.4% (5/347) for subjects with AN at baseline (Table 3). All of the PCCRC were detected by the second surveillance colonoscopies (Table 4), which suggests that at least two surveillance colonoscopies be recommended in order to ensure detection of PCCRC.
There were several limitations of the present study. First, the current findings were based on a retrospective cohort analysis of endoscopic records without any relevant clinical information, such as family history, smoking status, alcohol intake, medication, and indications for colonoscopy. Also, the quality indicators of colonoscopy were not considered. Second, the number of subjects for the follow ups was small compared to that for the non‐follow ups. In addition, the proportion of excluded subjects with and without neoplasia was not available. Therefore, we may have overestimated or underestimated the risk of AN development. In this way, selection bias may be involved in the present study. Third, several subjects in whom invasive cancers at baseline colonoscopy were detected and who had treatments at other facilities were not included as study participants. It is possible that we estimated their risks as lower than what they actually were. The last limitation is regarding the treatment of diminutive polyps. Because there are no definitive guidelines or unification of treatment regimens for these in Japan, in the present study, they were left untreated. Regardless of these limitations, the present study does indicate that the endoscopic findings for baseline colonoscopy can be used to stratify the risk of AN development and that undergoing two endoscopic interventions can reduce the incidence of AN over a 5‐year period.
In conclusion, this study showed there was a close relationship between the endoscopic findings of baseline colonoscopy and subsequent AN development, thereby suggesting that risk stratification for AN based on the findings of baseline colonoscopy could serve as a useful index for determining the optimal interval and frequency of colonoscopies over a period of 5 years.
Author Contributions
TAKASHI SHONO, SHINICHIRO Oyama: Study concept and design, collection and analysis of data, drafting of the manuscript. (These two authors contributed equally to this article.). Kazunori Yokomine: Collection and analysis of data, drafting of the manuscript. Yasushi Oda: Study concept and design, analysis of data, drafting of the manuscript. Yoshitaka Murakami: Interpretation of data, statistical analysis. Hideaki Miyamoto: Collection and analysis of data. Motohiko Tanaka: Collection and analysis of data. Hideaki Naoe: Collection and analysis of data. Yutaka Sasaki: Study concept and design, revision of the manuscript, approval of the final version of the manuscript.
Conflicts of Interest
Authors declare no conflicts of interest for this article.
Acknowledgments
We appreciated the Kumamoto Colon Cancer Study Group for helpful data collection. The collaborators of the Kumamoto Colon Cancer Study Group were as follows: Dr Yoshimitsu Adachi, Dr Naotsugu Ueno, Dr Hidemitsu Ohkado, Dr Tsuyoshi Ozaki, Dr Shinichi Yoshimatsu, Dr Ryuukichi Akashi, Dr Katsuhiko Mitsuzaki, Dr Takao Kawakami, Dr Ryoichi Nozaki, Dr Hiromitsu Tsuruta, Dr Yuji Toyama, Dr Seiichi Furusho, Dr Norio Mitsunaga, Dr Toshiaki Yamaoka, Dr Akio Yamaguchi, Dr Kenji Yamasaki, Dr Sumio Waki.
Takashi Shono and Shinichiro Oyama contributed equally to this article.
References
- 1. Cancer Statistics in Japan (Japanese literature) (The Editorial Board of the Cancer Statistics in Japan , ed). Tokyo: Foundation for Promotion of Cancer Research (FPCR), 2016. [Cited 1 Mar 2019.] Available from URL: http://www.fpcr.or.jp/ [Google Scholar]
- 2. Winawer SJ, Zauber AG, O'Brien MJ et al Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N. Engl. J. Med. 1993; 328: 901–6. [DOI] [PubMed] [Google Scholar]
- 3. Lieberman DA, Weiss DG, Harford WV et al Five‐year colon surveillance after screening colonoscopy. Gastroenterology 2007; 133: 1077–85. [DOI] [PubMed] [Google Scholar]
- 4. Imperiale TF, Glowinski EA, Lin‐Cooper C et al Five‐year risk of colorectal neoplasia after negative screening colonoscopy. N. Engl. J. Med. 2008; 359: 1218–24. [DOI] [PubMed] [Google Scholar]
- 5. Baxter NN, Goldwasser MA, Paszat LF et al Association of colonoscopy and death from colorectal cancer. Ann. Intern. Med. 2009; 150: 1–8. [DOI] [PubMed] [Google Scholar]
- 6. Boolchand V, Olds G, Singh J et al Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann. Intern. Med. 2006; 145: 654–9. [DOI] [PubMed] [Google Scholar]
- 7. Brenner H, Chang‐Claude J, Seiler CM et al Protection from colorectal cancer after colonoscopy: a population‐based, case‐control study. Ann. Intern. Med. 2011; 154: 22–30. [DOI] [PubMed] [Google Scholar]
- 8. Baxter NN, Warren JL, Barrett MJ et al Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J. Clin. Oncol. 2012; 30: 2664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atkin WS, Valori R, Kuipers EJ et al European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition–Colonoscopic surveillance following adenoma removal. Endoscopy 2012;44 (Suppl 3):SE151–63. [DOI] [PubMed] [Google Scholar]
- 10. Lieberman DA, Rex DK, Winawer SJ et al Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi‐Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143: 844–57. [DOI] [PubMed] [Google Scholar]
- 11. Sano Y, Byeon JS, Li XB et al Colorectal cancer screening of the general population in East Asia. Dig. Endosc. 2016; 28: 243–9. [DOI] [PubMed] [Google Scholar]
- 12. Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest. Endosc. 2009; 69: 620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsuda T, Fujii T, Sano Y et al Five‐year incidence of advanced neoplasia after initial colonoscopy in Japan: a multicenter retrospective cohort study. Jpn. J. Clin. Oncol. 2009; 39: 435–42. [DOI] [PubMed] [Google Scholar]
- 14. Leung WK, Lau JY, Suen BY et al Repeat‐screening colonoscopy 5 years after normal baseline‐screening colonoscopy in average‐risk Chinese: a prospective study. Am. J. Gastroenterol. 2009; 104: 2028–34. [DOI] [PubMed] [Google Scholar]
- 15. Hawkins NJ, Ward RL. Sporadic colorectal cancers with microsatellite instability and their possible origin in hyperplastic polyps and serrated adenomas. J. Natl Cancer Inst. 2001; 93: 1307–13. [DOI] [PubMed] [Google Scholar]
- 16. Rutter MD, Beintaris I, Valori R et al World Endoscopy Organization consensus statements on post‐colonoscopy and post‐imaging colorectal cancer. Gastroenterology 2018; 155: 909–25 e3. [DOI] [PubMed] [Google Scholar]
- 17. Chung SJ, Kim YS, Yang SY et al Five‐year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut 2011; 60: 1537–43. [DOI] [PubMed] [Google Scholar]
- 18. Kawamura T, Oda Y, Murakami Y et al Relationship between frequency of surveillance colonoscopy and colorectal cancer prevention. Dig. Endosc. 2014; 26: 409–16. [DOI] [PubMed] [Google Scholar]
- 19. Laiyemo AO, Doubeni C, Sanderson AK II et al Likelihood of missed and recurrent adenomas in the proximal versus the distal colon. Gastrointest. Endosc. 2011; 74: 253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bressler B, Paszat LF, Chen Z et al Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population‐based analysis. Gastroenterology 2007; 132: 96–102. [DOI] [PubMed] [Google Scholar]


