Abstract
Objective
To investigate whether midlife atherosclerosis is associated with different dementia subtypes and related underlying pathologies.
Methods
Participants comprised the cardiovascular cohort of the Swedish prospective population‐based Malmö Diet and Cancer Study (N = 6,103). Carotid plaques and intima media thickness (IMT) were measured at baseline (1991–1994). Dementia incidence until 2014 was obtained from national registers. Diagnoses were reviewed and validated in medical records. In a cognitively unimpaired subcohort (n = 330), β‐amyloid42 and tau were quantified in cerebrospinal fluid (CSF), and white matter hyperintensity volume, lacunar infarcts, and cerebral microbleeds were estimated on magnetic resonance imaging (2009–2015).
Results
During 20 years of follow‐up, 462 individuals developed dementia (mean age at baseline = 57.5 ± 5.9 years, 58% women). Higher IMT in midlife was associated with an increased hazard ratio (HR) of all‐cause dementia (adjusted HR = 1.14 [95% confidence interval (CI) = 1.03–1.26]) and vascular dementia (adjusted HR = 1.32 [95% CI = 1.10–1.57]) but not Alzheimer disease (AD) dementia (adjusted HR = 0.95 [95% CI = 0.77–1.17]). Carotid plaques were associated with vascular dementia when assessed as a 3‐graded score (adjusted HR = 1.90 [95% CI = 1.07–3.38]). In the cognitively unimpaired subcohort (53.8 ± 4.6 years at baseline, 60% women), higher IMT in midlife was associated with development of small vessel disease (adjusted odds ratio [OR] = 1.47 [95% CI = 1.05–2.06]) but not significantly with abnormal CSF AD biomarkers (adjusted OR = 1.28 [95% CI = 0.87–1.90] for Aβ42 and 1.35 [95% CI = 0.86–2.13] for Aβ42/p‐tau). Carotid plaques revealed no significant association with any of the underlying brain pathologies.
Interpretation
Our findings support an association between midlife atherosclerosis and development of vascular dementia and cerebral small vessel disease but not between atherosclerosis and subsequent AD dementia or AD pathology. ANN NEUROL 2020;87:52–62
Dementia is a major global health issue affecting an increasing number of individuals worldwide.1 Vascular pathology may be significant in development of Alzheimer disease (AD)2, 3 and not only vascular dementia (VaD). It is not yet clear if this relationship is mediated through a direct effect of vascular factors on key AD pathology like β‐amyloid (Aβ) and tau accumulation or through aggravated cognitive symptoms by concomitant cerebrovascular pathology. Recent studies indicate that risk factors for vascular disease may be associated with cerebral Aβ accumulation, measured by positron emission tomography or in cerebrospinal fluid (CSF).4, 5, 6 However, vascular risk factors like dyslipidemia might increase the risk of both atherosclerosis and Aβ accumulation via separate and independent pathways.5, 7, 8, 9
Carotid intima media thickness (IMT) and carotid plaques are markers of atherosclerosis measured by ultrasound.10 The potential association between IMT and dementia has not yet been clarified. Previous longitudinal, population‐based studies show diverging results,11, 12, 13, 14 and the relationship between IMT measured in midlife and subsequent dementia has not yet been studied. Moreover, associations between IMT and Aβ accumulation have only been studied cross‐sectionally in a small cohort (n = 34), where no significant association was found.15
We aimed to investigate if these markers of atherosclerosis, measured in midlife, were associated with development of AD dementia and VaD in a population‐based cohort (N = 6,103) during 20 years of follow‐up. We further studied if the same markers were associated with abnormal accumulation of Aβ and tau or small vessel disease 20 years later in a subcohort (n = 330) with no signs of cognitive impairment at follow‐up.
Subjects and Methods
Malmö Diet and Cancer Study
Participants comprised the cardiovascular cohort (N = 6,103) of the Malmö Diet and Cancer study (MDCS‐CV). The MDCS is a Swedish population‐based study,16 and the cardiovascular cohort was initiated to study the epidemiology of carotid artery disease. MDCS‐CV constituted a random sample of participants entering the MDCS in 1991–1994 (Fig).17 Recruitment and attrition have been described previously.16, 17, 18 All participants provided written informed consent, and the Ethics Committee of Lund University approved the study.
At baseline (1991–1994), participants completed a questionnaire on health status and lifestyle factors and underwent clinical investigation, including carotid ultrasound, blood sampling, and body measurements. All participants were followed in the Swedish National Patient Register (NPR) throughout 2014, when all registered dementia diagnoses were obtained (see Figure). The NPR covers both the Swedish Inpatient Register and the hospital‐based outpatient care register. It started in the 1960s, and since 1987 it has provided information on all inpatient care in Sweden with a coverage of 99%. Since 2001, the NPR has also included hospital outpatient visits with almost full coverage from public caregivers.19 At discharge or at the outpatient visit, primary and secondary diagnoses are routinely registered by the treating physician according to the International Classification of Diseases. Dementia diagnoses included in the present register outtake are AD dementia (F00, G30, 331A/331.0), VaD (F01, 290E/290.4), Parkinson disease dementia (F023), Lewy body dementia (F028, G318A), frontotemporal dementia (F020, G310, 331B/331.1), or unspecified dementia (F03, 290, 294B/294.1, 331C/331.2).
Figure 1.
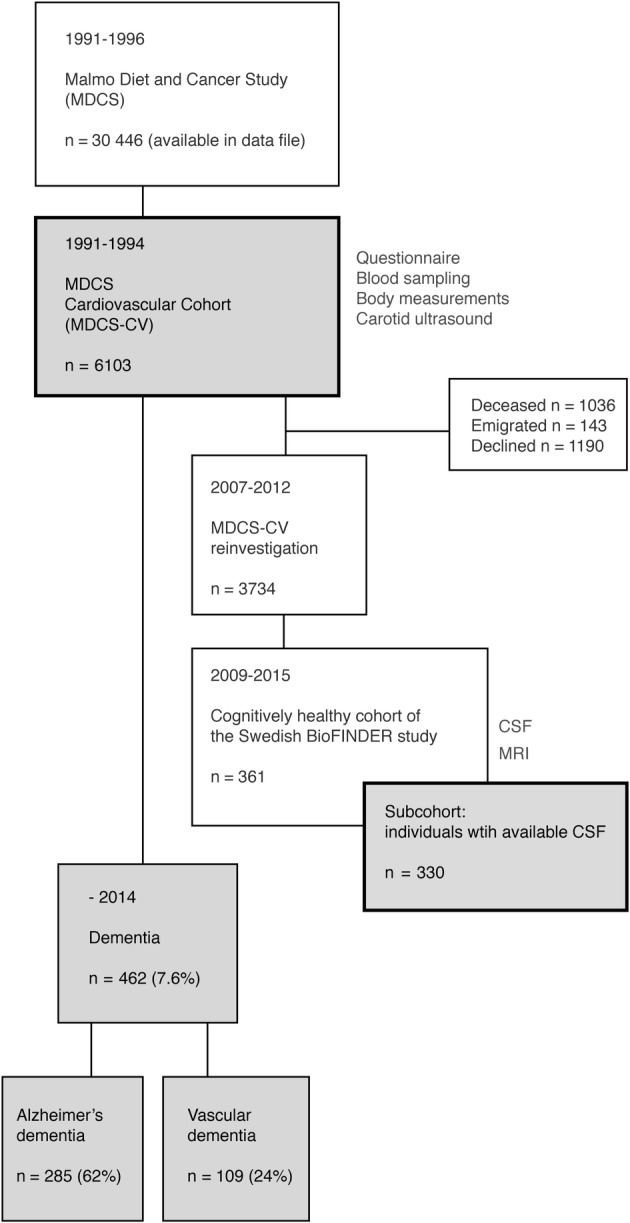
Flow diagram describing the study cohort. CSF = cerebrospinal fluid; MRI = magnetic resonance imaging.
After the register outtake, all diagnoses were thoroughly reviewed in medical records (electronic charts) by medical doctors at the Memory Clinic at Skåne University Hospital. The register diagnoses were assessed based on symptom presentation, cognitive test results, brain imaging (computed tomography or magnetic resonance imaging [MRI]), and CSF analyses (when available) in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐V).20 There were 480 dementia diagnoses first identified in the register, and 378 (78.8%) of these individuals had been assessed at a tertiary unit (Memory Clinic). In 95.4%, neuroimaging was performed in connection to diagnosis. Information from neuroimaging was used both to validate a diagnosis of VaD and to differentiate between pure AD and AD with concomitant cerebrovascular disease. Presence of significant cerebrovascular disease led to a mixed AD diagnosis if AD was considered the primary cause. Based on the diagnostic review process, 18 of 480 individuals (3.8%) did not meet criteria for dementia and were instead regarded as nondemented participants (eg, mild cognitive impairment, reversible delirium, or depressive disorder). The validation process of the remaining 462 individuals with all‐cause dementia resulted in the following incidence numbers (with proportion of cases within the dementia group in parentheses); 109 (23.6%) VaD, 138 (29.9%) Alzheimer dementia without mixed pathologies, 147 (31.8%) Alzheimer dementia with concomitant cerebrovascular pathology, 14 (3.0%) dementia with Lewy bodies, 12 (2.6%) Parkinson disease dementia, 8 (1.7%) frontotemporal dementia, 4 (0.9%) normal pressure hydrocephalus, 4 (0.9%) alcohol‐induced dementia, 1 (0.2%) progressive supranuclear palsy, 1 (0.2%) corticobasal degeneration, 2 (0.4%) multiple sclerosis with vascular comorbidity, and 22 (4.8%) unspecified dementia where medical records did not provide enough information to further characterize the probable/possible origin. The original register diagnosis was refined in 160 of 462 (34.6%) cases with dementia, mainly from unspecified dementia to AD with concomitant vascular pathology. In the analyses, we used AD dementia (both pure and with concomitant cerebrovascular disease), VaD, and all‐cause dementia as event variables.
Cognitively Healthy Swedish BioFINDER Subcohort
This subcohort originates from the MDCS‐CV and is part of the cognitively healthy cohort of the Swedish BioFINDER study (http://www.biofinder.se), approved by the Ethics Committee of Lund University. All participants provided written informed consent. Recruitment was performed during the MDCS‐CV reinvestigation in 2007–201221 (see Figure), and participants were eligible if they were aged >60 years, scored ≥28 points on the Mini‐Mental State Examination (MMSE) at the screening visit, and did not experience any subjective cognitive impairments (Clinical Dementia Rating score = 0). The recruitment took part during random periods of the MDCS‐CV reinvestigation, and it terminated when the predefined cohort size was arrived at. In all, 437 potential participants underwent clinical examinations at the Memory Clinic, and 76 individuals were excluded based on exclusion criteria (stroke, severe neurologic or psychiatric disease, dementia, or mild cognitive impairment). This resulted in 361 participants in the cognitively healthy cohort of the Swedish BioFINDER study; included here are the 330 participants (91%) with available CSF data.
Ultrasound Markers of Atherosclerosis
At baseline (1991–1994), all participants in MDCS‐CV underwent ultrasound examination of the right carotid artery by specially trained and certified sonographers, previously described in more detail, including intra‐ and interobserver variations.17 In short, carotid IMT was measured in the far wall of the common carotid artery according to the leading‐edge principle using a specially designed computer‐assisted imaging system. Plaques were measured in a prespecified area of the bifurcation comprising 3cm of the right common carotid artery, the bifurcation, and 1cm of both the internal and external carotid artery. Plaques were measured using 2 different plaque scores. In the first 1,600 individuals (until May 31, 1992) a 3‐graded scale was used, with the following categorization: 0 = no plaque, 1 = 1 plaque, and 2 = 1 circumferential plaque or 2 or more plaques. In the following 4,249 individuals, a 6‐graded scale was used, with the following categorization: 0 = no plaque and no wall thickening, 1a = 1 plaque <10mm2 or wall thickening (>1.2mm), 1b = 2 or more 1a, 2a = 1 plaque >10mm2, 2b = 1 plaque >10mm2 and 1 or more 1a, and 3 = 1 circumferential plaque and/or 1 large plaque (>50% stenosis) and/or 2 plaques, regardless of presence of 1a. Presence of any carotid plaque was defined as a focal IMT of >1.2mm, equal to ≥1 in both the 3‐ and 6‐graded scale (binary definition). In an attempt to model plaques categorically instead of dichotomously, the 6‐graded scale was converted to the 3‐graded scale as follows; 0 = 0; 1 = 1a, 1b, and 2a; and 2 = 2b and 3. In total, 6,057 participants (99.2%) provided data on IMT, and 5,849 participants (95.8%) provided data on plaques.
CSF and MRI
Within the Swedish BioFINDER study, participants in the subcohort underwent lumbar puncture (n = 330) and MRI (n = 320). CSF was collected between 2010 and 2015 and analyzed simultaneously according to a standardized protocol.22 ELISA (INNOTEST; Fujirebio Europe, Ghent, Belgium) was used for quantification of Aβ42 and tau phosphorylated at Thr181 (p‐tau). The estimated cutoff for abnormal Aβ42 was <500pg/ml and for abnormal Aβ42/p‐tau ratio was <7.7, based on mixture modeling.23
MRI at 3T was performed between 2009 and 2015 and comprised axial T2 fluid‐attenuated inversion recovery (FLAIR), coronal magnetization‐prepared rapid gradient echo (MPRAGE) sequence, and coronal gradient‐echo T2*‐weighted images (GRE) or susceptibility weighted images (SWI). The Lesion Segmentation Tool (version 1.2.3, as implemented in SPM8) was used to segment total white matter lesion volume (ml) from the MPRAGE and FLAIR images.24 Presence of lacunar infarcts was assessed visually on FLAIR and MPRAGE according to Wardlaw et al.25 Cerebral microbleeds were rated on GRE (n = 256) and SWI (n = 63) according to the Microbleed Anatomical Rating Scale and dichotomized as present or absent. Cerebral small vessel disease was defined as either white matter hyperintensity (WMH) volume > median, presence of lacunar infarcts, and/or presence of cerebral microbleeds.
Covariates
Covariates were derived from the MDCS‐CV baseline visit (1991–1994) to adjust for comorbidities and lifestyle factors at the time of the ultrasound measure. Smoking status (categorized as ever or never), education (categorized as noted in Table 1), and medication use were self‐reported and derived from the baseline questionnaire. Blood pressure–lowering medication was defined as any drug with blood pressure–lowering effect, regardless of indication, and consisted of diuretics (Anatomical Therapeutic Chemical [ATC] group C03), beta‐blocking agents (ATC group C07), calcium channel blockers (ATC group C08), or agents acting on the renin‐angiotensin system (ATC group C09). Lipid‐lowering medication was defined as any drug with serum lipid‐reducing effect (ATC group C10). Systolic blood pressure was measured in a supine position after 10 minutes of rest. Body mass index (calculated as kg/m2) was based on weight and height measures at the baseline visit. APOE was analyzed from frozen blood samples collected at the baseline examination (n = 5,706), and participants with at least one ε4 allele were categorized as APOE ε4 carriers. Prevalence of diabetes mellitus (type I or II) at baseline originated from multiple sources, mainly from the HbA1c register at the Clinical Chemistry at the Skåne University Hospital, the NPR, from screening in the Malmö Preventive Project, and from screening at the MDCS baseline. Stroke prevalence was derived from the NPR and the Stroke Register of Malmö.
Table 1.
Baseline Characteristics of the Total Study Population and the Subcohort
| Characteristics, 1991–1994 | MDCS‐CV Cohort, N = 6,103, Mean ± SD or n (%) | BioFINDER Subcohort, n = 330, Mean ± SD or n (%) |
|---|---|---|
| Age, yr | 57.5 ± 5.9 | 53.8 ± 4.6 |
| Women | 3,531 (57.9) | 198 (60.0) |
| APOE ε4 carrier | 1,704 (29.9) | 92 (28.1) |
| Primary/elementary school, ≤8 years | 2,676 (46.3) | 79 (24.2) |
| Secondary school/high school, 9–12 years | 1,982 (34.3) | 143 (43.9) |
| Higher education/university, ≥13 years | 1,123 (19.4) | 104 (31.9) |
| Systolic blood pressure, mmHg | 141 ± 19.1 | 133 ± 16 |
| Body mass index, kg/m2 | 25.8 ± 4.0 | 24.8 ± 3.5 |
| Ever smoker | 3,535 (61.1) | 194 (59.2) |
| Diabetes | 290 (4.8) | 7 (2.1) |
| Stroke | 45 (0.7) | 0 (0) |
| Blood pressure–lowering medication | 1,010 (16.5) | 31 (9.4) |
| Lipid‐lowering medication | 141 (2.3) | 3 (0.9) |
| IMT, mm | 0.77 ± 0.16 | 0.73 ± 0.13 |
| Carotid plaque, any | 3,577 (61.2) | 144 (44.7) |
| Carotid plaque score | ||
| 0 | 2,272 (38.8) | 178 (55.3) |
| 1 | 2,350 (40.2) | 117 (36.3) |
| 2 | 1,227 (21.0) | 27 (8.4) |
Stroke and diabetes data were obtained from hospital registers. Education level, smoking habits, and medication use were self‐reported and were obtained from the baseline questionnaire. The BioFINDER cohort was derived from the total cohort.
IMT = intima media thickness; MDCS‐CV = Malmö Diet and Cancer Study–cardiovascular cohort; SD = standard deviation.
Statistical Analyses
Statistical analyses were performed using SPSS version 25 for Mac (SPSS, Chicago, IL) and R statistical software. IMT was converted to z score, using the raw measure (x), mean (μ), and standard deviation (SD, σ) according to the formula z = (x − μ)/σ to present results per SD increase. To explore nonlinear effects, we categorized IMT based on quartiles (25th, 50th, and 75th percentiles) and compared participants in the second, third, and fourth quartiles, to the lowest quartile (reference category). Quartiles were categorized based on the distribution in the studied cohort, rendering different values in the total cohort as compared to the subcohort (values are presented in Tables 2 and 3).
Table 2.
Associations between IMT in Midlife and Clinical Dementia Diagnoses during 20 Years of Follow‐up, in a Population‐Based Setting (N = 6,103)
| IMT | Alzheimer Dementia, n = 138, HR (95% CI) | Mixed Dementia, n = 147, HR (95% CI) | Vascular Dementia, n = 109, HR (95% CI) | Any Dementia, n = 462, HR (95% CI) |
|---|---|---|---|---|
| 1 SD increase | ||||
| Model 1 | 0.95 (0.79–1.15) | 1.19 (1.02–1.39)a | 1.36 (1.16–1.59)a | 1.16 (1.06–1.27)a |
| Model 2 | 0.93 (0.76–1.14) | 1.18 (0.99–1.40) | 1.40 (1.19–1.66)a | 1.17 (1.06–1.29)a |
| Model 3 | 0.95 (0.77–1.17) | 1.14 (0.95–1.36) | 1.32 (1.10–1.57)a | 1.14 (1.03–1.26)a |
| Quartiles, mm | ||||
| Model 1 | ||||
| Q1, 0.36–0.66 | 1 | 1 | 1 | 1 |
| Q2, 0.66–0.74 | 0.88 (0.53–1.44) | 1.24 (0.71–2.16) | 1.03 (0.53–2.02) | 1.03 (0.76–1.40) |
| Q3, 0.74–0.84 | 0.93 (0.57–1.51) | 1.10 (0.63–1.92) | 1.25 (0.66–2.36) | 1.11 (0.83–1.49) |
| Q4, 0.84–2.66 | 0.77 (0.47–1.28) | 1.75 (1.04–2.95)a | 1.92 (1.06–3.50)a | 1.45 (1.09–1.92)a |
| Model 2 | ||||
| Q1, 0.36–0.66 | 1 | 1 | 1 | 1 |
| Q2, 0.66–0.74 | 0.76 (0.45–1.26) | 1.24 (0.70–2.20) | 1.10 (0.51–2.37) | 0.97 (0.70–1.34) |
| Q3, 0.74–0.84 | 0.89 (0.55–1.45) | 0.96 (0.54–1.72) | 1.37 (0.66–2.82) | 1.07 (0.78–1.45) |
| Q4, 0.84–2.66 | 0.69 (0.41–1.17) | 1.56 (0.91–2.67) | 2.27 (1.15–4.48)a | 1.40 (1.04–1.88)a |
| Model 3 | ||||
| Q1, 0.36–0.66 | 1 | 1 | 1 | 1 |
| Q2, 0.66–0.74 | 0.77 (0.46–1.29) | 1.20 (0.68–2.13) | 1.03 (0.48–2.33) | 0.94 (0.68–1.30) |
| Q3, 0.74–0.84 | 0.91 (0.56–1.49) | 0.95 (0.53–1.72) | 1.26 (0.70–2.61) | 1.05 (0.77–1.43) |
| Q4, 0.84–2.66 | 0.74 (0.43–1.26) | 1.42 (0.81–2.48) | 1.88 (0.94–3.77) | 1.31 (0.96–1.78) |
Adjusted cause‐specific hazard models for dementia. Results are presented both continuously (per SD increase in IMT) and categorically (by IMT quartiles). Mixed dementia denotes Alzheimer disease with cerebrovascular pathology. Number of events (cases) and total number of individuals per model are presented in Supplementary Table 1.
Model 1: Age. Model 2: Age, sex, APOE ε4, and education. Model 3: Age, sex, APOE ε4, education, systolic blood pressure, body mass index, smoking, diabetes mellitus, blood pressure–lowering medication, lipid‐lowering medication, and stroke.
Significant, as can be noted by the CI.
CI = confidence interval; HR = hazard ratio; IMT = intima media thickness; SD = standard deviation.
Table 3.
Associations between IMT in Midlife and AD Pathology and Cerebral Small Vessel Disease at 20‐Year Follow‐up, in a Subgroup of Cognitively Unimpaired Elderly (n = 330)
| IMT | Abnormal Aβ42, OR (95% CI) | Abnormal Aβ42/p‐tau, OR (95% CI) | Small Vessel Disease, OR (95% CI) |
|---|---|---|---|
| 1 SD increase | |||
| Model 1 | 1.41 (1.02–1.95)a | 1.45 (1.00–2.09)a | 1.50 (1.10–2.03)a |
| Model 2 | 1.28 (0.89–1.84) | 1.27 (0.85–1.89) | 1.52 (1.11–2.08)a |
| Model 3 | 1.28 (0.87–1.90) | 1.35 (0.86–2.13) | 1.47 (1.05–2.06)a |
| Quartiles, mm | |||
| Model 1 | |||
| Q1, 0.46–0.63 | 1 | 1 | 1 |
| Q2, 0.63–0.71 | 1.72 (0.80–3.72) | 1.19 (0.46–3.04) | 1.03 (0.55–1.93) |
| Q3, 0.71–0.81 | 1.42 (0.64–3.17) | 1.69 (0.69–4.15) | 1.32 (0.68–2.56) |
| Q4, 0.81–1.18 | 2.26 (1.03–4.95)a | 1.90 (0.77–4.69) | 2.27 (1.14–4.53)a |
| Model 2 | |||
| Q1, 0.46–0.63 | 1 | 1 | 1 |
| Q2, 0.63–0.71 | 1.82 (0.79–4.22) | 1.19 (0.44–3.24) | 1.11 (0.59–2.10) |
| Q3, 0.71–0.81 | 1.20 (0.51–2.85) | 1.52 (0.59–3.92) | 1.36 (0.70–2.65) |
| Q4, 0.81–1.18 | 1.94 (0.82–4.59) | 1.48 (0.56–3.92) | 2.34 (1.15–4.74)a |
| Model 3 | |||
| Q1, 0.46–0.63 | 1 | 1 | 1 |
| Q2, 0.63–0.71 | 1.70 (0.73–3.99) | 1.02 (0.36–2.86) | 0.99 (0.51–1.89) |
| Q3, 0.71–0.81 | 1.25 (0.52–3.01) | 1.59 (0.61–4.19) | 1.29 (0.65–2.58) |
| Q4, 0.81–1.18 | 1.96 (0.78–4.91) | 1.51(0.53–4.32) | 2.07 (0.98–4.40) |
Adjusted ORs for abnormal AD biomarkers (CSF‐Aβ42 < 500 or CSF‐Aβ42/p‐tau <7.7) or cerebral small vessel disease. Results are presented both continuously (per SD increase in IMT) and categorically (by IMT quartiles). Small vessel disease was defined as white matter hyperintensity volume > median, presence of lacunar infarcts, and/or cerebral microbleeds. Number of positive events in the dependent variable and total number of individuals per model are presented in Supplementary Table 3.
Model 1: age. Model 2: age, sex, APOE ε4, and education. Model 3: age, sex, APOE ε4, education, systolic blood pressure, body mass index, smoking, diabetes mellitus, blood pressure‐lowering medication, and lipid lowering medication. No individuals in the subcohort had stroke at baseline.
Significance, as can be noted by the CI.
Aβ = amyloid β; AD = Alzheimer disease; CI = confidence interval; CSF = cerebrospinal fluid; IMT = intima media thickness; OR = odds ratio; p‐tau = phosphorylated tau; SD = standard deviation.
We performed Cox regression models to estimate proportional hazards with 95% confidence interval (CI) for developing dementia per SD increase in IMT (continuous variable), by IMT quartiles (categorical variable), by presence of any versus no carotid plaque (binary variable), and by the 3‐graded plaque score (categorical variable). The event was either all‐cause dementia, pure AD, mixed dementia (AD with cerebrovascular pathology), or VaD. Censoring occurred at time of register outtake (December 31, 2014) or at death (available from Statistics Sweden and the Causes of Death Register). Time was defined as years between baseline and event or censoring. The proportionality assumption was confirmed using Schoenfeld residuals. Because our study objective concerned disease etiology, competing risks were assessed by cause‐specific hazard models rather than Fine and Gray subdistribution hazard models.26, 27 Thereby, death was treated as a competing risk event by censoring individuals at time of death (as stated previously). The hazard ratio (HR) of dementia is thus estimated in subjects who are alive and event free (nondemented).
Further, other dementia diagnoses were also treated as competing risk events when subtypes of dementia were the event of interest (ie, when AD, mixed dementia, or VaD were used as event variable, censoring also occurred when subjects received another dementia diagnosis than the one specifically studied). The HRs of AD, mixed dementia, and VaD were thus estimated in subjects who were alive and free of any dementia event (including dementia diagnoses other than the one specifically studied).
In the subcohort, we used logistic regression models to estimate odds ratios (ORs) for cerebral pathology per SD increase in IMT (continuous variable), by IMT quartiles (categorical variable), by presence of any versus no carotid plaque (binary variable), and by the 3‐graded plaque score (categorical variable). To explore associations to AD pathology, Aβ42 and Aβ42/p‐tau ratio were dichotomized and used as dependent variables. The estimated cutoffs were abnormal Aβ42 < 500pg/ml and abnormal Aβ42/p‐tau ratio < 7.7. These cutoffs were calculated by mixture modeling, using an assumption that the data were from a mixed sample of 2 different normal distributions. The model thus revealed a cutoff point using this bimodal distribution.23 Small vessel disease was used as dependent variable to explore associations to cerebrovascular pathology and defined as present in individuals with either WMH volume > median, lacunar infarcts, and/or cerebral microbleeds.
We constructed 3 models and added covariates stepwise to control for known potential confounders. Age was included in all models because of its strong association with both the predictors of interest (IMT and carotid plaques) and the outcomes (dementia and brain pathology). Sex, APOE ε4, and education were included in the second model, and vascular factors were added in the third model, controlling for systolic blood pressure, smoking, diabetes, body mass index, blood pressure–lowering medication, lipid‐lowering medication, and stroke at baseline.
The same covariates were used in both Cox and logistic regression models, but no individuals in the subcohort had stroke at baseline because this was an exclusion criterion. We performed complete case analyses, only including individuals with observed data on all entered variables in the models (exact numbers are specified in Supplementary Tables 1–4).
Sensitivity Analyses
In sensitivity analyses, we excluded individuals with either prevalent or incident stroke. Further, for AD, mixed dementia, and VaD we ran all analyses without treating other dementia diagnoses as competing risks (ie, we did not censor individuals with other dementia diagnoses). We also performed all analyses restricted to individuals with complete data in the full model, thus excluding individuals with missing data on any of the covariates used in model 3 and including fewer individuals in model 1 and 2 (n = 5,360 for IMT analyses and n = 5,188 for plaque analyses).
Interaction statistics for APOE ε4 were applied by simultaneously entering IMT and APOE ε4 together with a variable consisting of their product (IMT*APOE ε4) in a Cox regression with AD as event variable. The same procedure was done for plaques.
Data Availability
MDCS‐CV data can be requested through an application to the MDCS steering committee. Anonymized data from the Swedish BioFINDER study can be made available upon request to the corresponding author as long as data transfer agrees with European Union legislation on the general data protection regulation. Data will only be shared by requests from qualified investigators for the sole purpose of replicating results.
Results
Descriptive statistics are presented in Table 1. In the MDCS‐CV (N = 6,103), mean follow‐up time (baseline to end of study or death) was 20 ± 5.0 years (mean ± SD), with a median of 22 years (range, 0–23 years). During this period, 462 individuals (7.6%) were diagnosed with dementia. Of these, 285 (63%) were classified as AD, out of which 138 were classified as pure AD and 147 as AD with concomitant cerebrovascular pathology (mixed dementia); 109 (24%) were classified as VaD. Mean age at dementia diagnosis was 77.7 ± 5.8 years, and mean age for separate diagnoses was 77.9 ± 5.7 years for AD and 77.9 ± 5.8 years for VaD. By the end of follow‐up, 1,805 (30%) were deceased, accounting for 28% of participants without a dementia diagnosis and 51% of those diagnosed with dementia.
In the cognitively healthy Swedish BioFINDER subcohort (n = 330), mean time between baseline and subsequent lumbar puncture was 20 ± 1.6 years. CSF revealed abnormal Aβ42 in 75 participants (23%) and abnormal Aβ42/p‐tau ratio in 52 participants (16%). Mean time between baseline and MRI was 19 ± 1.6 years. Cerebral small vessel disease was present in 170 (53%) participants, either as WMH volume > median, lacunar infarcts (present in 12 participants), or cerebral microbleeds (present in 27 participants).
Atherosclerosis and Dementia
Neither midlife IMT nor carotid plaques were associated with AD during 20 years of follow‐up (see Tables 2 and 4). There was a trend toward an association between IMT and mixed dementia, defined as AD with concomitant cerebrovascular pathology.
Table 4.
Associations between Carotid Plaques in Midlife and Clinical Dementia Diagnoses during 20 Years of Follow‐up, in a Population‐Based Setting (N = 6,103)
| Alzheimer Dementia, n = 138, HR (95% CI) | Mixed Dementia, n = 147, HR (95% CI) | Vascular Dementia, n = 109, HR (95% CI) | Any Dementia, n = 462, HR (95% CI) | |
|---|---|---|---|---|
| Any carotid plaque | ||||
| Model 1 | 0.98 (0.68–1.42) | 1.20 (0.82–1.75) | 1.82 (1.13–2.94)a | 1.23 (1.00–1.52) |
| Model 2 | 0.98 (0.67–1.43) | 1.25 (0.84–1.87) | 1.60 (0.97–2.66) | 1.20 (0.96–1.50) |
| Model 3 | 1.03 (0.70–1.51) | 1.18 (0.78–1.78) | 1.46 (0.87–2.43) | 1.15 (0.92–1.44) |
| Carotid plaque score | ||||
| Model 1 | ||||
| 0 | 1 | 1 | 1 | 1 |
| 1 | 1.04 (0.70–1.54) | 1.11 (0.74–1.67) | 1.42 (0.84–2.40) | 1.12 (0.89–1.41) |
| 2 | 0.87 (0.53–1.40) | 1.36 (0.87–2.13) | 2.57 (1.51–4.35)a | 1.44 (1.12–1.84)a |
| Model 2 | ||||
| 0 | 1 | 1 | 1 | 1 |
| 1 | 1.09 (0.73–1.63) | 1.14 (0.74–1.76) | 1.31 (0.76–2.28) | 1.11 (0.87–1.42) |
| 2 | 0.75 (0.45–1.26) | 1.46 (0.91–2.34) | 2.12 (1.21–3.71)a | 1.36 (1.04–1.77)a |
| Model 3 | ||||
| 0 | 1 | 1 | 1 | 1 |
| 1 | 1.12 (0.74–1.67) | 1.11 (0.71–1.72) | 1.23 (0.70–2.14) | 1.08 (0.85–1.38) |
| 2 | 0.82 (0.48–1.40) | 1.32 (0.81–2.16) | 1.90 (1.07–3.38)a | 1.29 (0.98–1.70) |
Adjusted cause‐specific hazard models for dementia. Results are presented by presence of any versus no carotid plaque (dichotomous variable) and by carotid plaque score (categorical variable). Mixed dementia denotes Alzheimer disease with cerebrovascular pathology. Number of events (cases) and total number of individuals per model are presented in Supplementary Table 2.
Model 1: Age. Model 2: Age, sex, APOE ε4, and education. Model 3: Age, sex, APOE ε4, education, systolic blood pressure, body mass index, smoking, diabetes mellitus, blood pressure–lowering medication, lipid‐lowering medication, and stroke.
Significant, as can be noted by the CI.
CI = confidence interval; HR = hazard ratio.
Higher IMT in midlife was associated with VaD and all‐cause dementia (see Table 2). When IMT was modeled linearly per SD increase, the association was significant after adjustments for other cardiovascular risk factors. When IMT was modeled categorically, the association was not robust to full adjustments.
Presence of carotid plaques (any vs none) was not significantly associated with all‐cause dementia nor VaD, except in the age‐adjusted model (see Table 4). When plaques were modeled as a 3‐graded score, higher plaque score (2 vs 0) was significantly associated with VaD, but there was no association with AD and only partly significant associations with all‐cause dementia.
Atherosclerosis and Brain Pathologies
Increased IMT in midlife was weakly associated with abnormal CSF Aβ42 and Aβ42/p‐tau ratio 20 years later in age‐adjusted regression models in the Swedish BioFINDER subcohort. No significant associations were found in further adjusted models (see Table 3). Higher IMT was associated with small vessel disease, independent of other vascular risk factors when IMT was modeled linearly. This association was not robust to full adjustments when IMT was modeled categorically.
Carotid plaques in midlife were not associated with any of the measured brain pathologies 20 years later (Table 5).
Table 5.
Associations between Carotid Plaques in Midlife and AD Pathology and Cerebral Small Vessel Disease at 20‐Year Follow‐up, in a Subgroup of Cognitively Unimpaired Elderly (n = 330)
| Abnormal Aβ42, OR (95% CI) | Abnormal Aβ42/p‐tau, OR (95% CI) | Small Vessel Disease, OR (95% CI) | |
|---|---|---|---|
| Any carotid plaque | |||
| Model 1 | 1.20 (0.69–2.06) | 1.06 (0.56–2.00) | 1.00 (0.62–1.63) |
| Model 2 | 1.03 (0.57–1.85) | 0.91 (0.47–1.78) | 0.97 (0.59–1.58) |
| Model 3 | 1.05 (0.57–1.94) | 0.94 (0.47–1.90) | 0.99 (0.59–1.65) |
| Carotid plaque score | |||
| Model 1 | |||
| 0 | 1 | 1 | 1 |
| 1 | 1.24 (0.70–2.19) | 0.98 (0.50–1.92) | 1.02 (0.61–1.70) |
| 2 | 1.02 (0.38–2.75) | 1.44 (0.52–4.02) | 0.95 (0.39–2.29) |
| Model 2 | |||
| 0 | 1 | 1 | 1 |
| 1 | 1.10 (0.59–2.04) | 0.86 (0.42–1.76) | 0.99 (0.59–1.67) |
| 2 | 0.76 (0.26–2.23) | 1.12 (0.37–3.38) | 0.86 (0.35–2.10) |
| Model 3 | |||
| 0 | 1 | 1 | 1 |
| 1 | 1.11 (0.58–2.10) | 0.83 (0.39–1.76) | 1.01 (0.59–1.72) |
| 2 | 0.83 (0.27–2.56) | 1.56 (0.49–4.94) | 0.89 (0.34–2.29) |
Adjusted ORs for abnormal AD biomarkers (CSF‐Aβ42 < 500 or CSF‐Aβ42/p‐tau <7.7) or cerebral small vessel disease. Results are presented by presence of any versus no carotid plaque (dichotomous variable) and by carotid plaque score (categorical variable). Small vessel disease was defined as white matter hyperintensity volume > median, presence of lacunar infarcts, and/or cerebral microbleeds. Number of positive events in the dependent variable and total number of individuals per model are presented in Supplementary Table 4.
Model 1: Age. Model 2: Age, sex, APOE ε4, and education. Model 3: Age, sex, APOE ε4, education, systolic blood pressure, body mass index, smoking, diabetes mellitus, blood pressure–lowering medication, and lipid‐lowering medication. No individuals in the subcohort had stroke at baseline.
Aβ = amyloid β; AD = Alzheimer disease; CI = confidence interval; CSF = cerebrospinal fluid; OR = odds ratio; p‐tau = phosphorylated tau.
Sensitivity Analyses
When all individuals with either prevalent (n = 45/6,103) or incident stroke (n = 607/6,103) were excluded from the analyses, the association between IMT and VaD (n = 55) remained significant (fully adjusted HR per SD increase = 1.45, 95% CI = 1.15–1.82) but not between plaque score and VaD (fully adjusted HR for grade 2 vs 0 = 1.40, 95% CI = 0.70–2.78). The association between IMT and all‐cause dementia (n = 339) remained significant (fully adjusted HR per SD increase = 1.19, 95% CI = 1.06–1.33) when individuals with stroke were excluded, and overall it did not result in any nonsignificant results turning significant (eg, associations between IMT/plaque and AD, data not shown).
All results from the Cox regression models remained unchanged when individuals with dementia diagnoses other than the one specifically studied were treated as nondemented (ie, were not censored at time of diagnosis) in analyses where AD, mixed dementia, and VaD were used as event variables (data not shown). Similarly, results were not altered when analyses were restricted to individuals with complete data across all models, thus excluding individuals with any missing covariates in model 3 from models 1 and 2 (data not shown).
We found no statistical interactions between IMT and APOE ε4 (interaction term 0.94, p = 0.71) or between plaque and APOE ε4 (interaction term 1.16, p = 0.70) in Cox models with AD as event variable.
Discussion
In this longitudinal study with 20 years of follow‐up, we found that higher IMT in midlife was associated with subsequent all‐cause dementia and VaD, as well as with development of cerebral small vessel disease. However, our results do not support an association between atherosclerosis in midlife and AD.
Our findings are in line with most previous studies reporting increased HRs of all‐cause dementia with higher IMT measured in late life.12, 13, 14 On the contrary, in accordance with the Three‐City Study,11 we did not find IMT to be associated with AD, as opposed to other previous studies.12, 13, 14 In these studies, the hazard of AD was higher for individuals in the highest IMT category compared to the lowest category (based on quartiles or quintiles), but results for continuous IMT were either not reported12, 13 or nonsignificant.14 Further, we found an association between higher IMT and an increased HR of VaD, which was not reported by either of the previous cohorts that included VaD as an event variable.11, 12 Presence of carotid plaques was not associated with dementia in our study population. However, when we used a 3‐graded scale, we found higher plaque score to be significantly associated with VaD, in line with results from the Three‐City Study11 but not the Rotterdam study.12 Because the plaque score was not measured uniformly in our cohort, these results need to be interpreted with some caution.
Discrepancies with previous studies may be due to the aforementioned difference in design, where we measured IMT in midlife compared to late life (age > 65 years). Two of the previous studies did not include VaD as an event variable,13, 14 and because relatively few cases are classified as VaD in the studies that do (83 and 78 cases),11, 12 stronger associations are needed to detect significant effects. Further, diagnostic procedures vary between studies, and clinically derived diagnoses encompass uncertainty.28, 29 In the Three‐City Study, cases with mixed AD and vascular pathology were included in the VaD group,11 whereas we assessed AD in combination with cerebrovascular disease as a separate group (mixed dementia). Indeed, neuropathological studies conclude that joint pathology is common in community‐based samples and accounts for approximately half of clinically diagnosed AD, with one‐third being mainly attributed to vascular comorbidities.28, 29 This emphasizes the importance of studying underlying pathologies directly and not relying only on clinical diagnoses.
In our study, neither IMT nor carotid plaques were significantly associated with AD pathology measured as abnormal CSF Aβ42 and CSF Aβ42/p‐tau ratio in a cognitively unimpaired subcohort (n = 330). This finding is in line with a previous cross‐sectional study on IMT and Aβ PET15 and a small study of participants with unilaterally occluded carotid arteries, where no association between hypoperfusion and Aβ or tau accumulation was found.30 Our results may be interpreted as being suggestive of an association between higher IMT and abnormal AD biomarkers (OR = 1.28 and 1.35; see Table 3) although encompassing statistical uncertainty because the CIs overlap 1, which may be due to the relatively small sample size yielding large CIs. Indeed, some neuropathological31 and experimental32 studies suggest a link between intracerebral atherosclerosis and Aβ pathology, possibly mediated by hypoxia leading to increased Aβ cleavage and accumulation via enzymatic upregulation as well as by vascular narrowing leading to reduced Aβ clearance.33 Aβ may in turn promote atherogenesis through endothelial dysfunction and inflammation.33 If Aβ and atherosclerosis are inextricably linked, it highlights the need of longitudinal assessments and may in part explain why cross‐sectional studies demonstrate significant associations between intracerebral atherosclerosis and Aβ.31, 32 In the Rotterdam study, the association between IMT and AD was attenuated with longer follow‐up.12 Further, another neuropathological assessment of a longitudinal cohort did not reveal any associations between intracranial or carotid atherosclerosis and AD pathology but instead found atherosclerosis to be significantly associated with clinical dementia and cerebral infarcts.34 In line with this, we found significant associations between higher midlife IMT and all‐cause dementia and VaD. We also found higher midlife IMT to be significantly associated with late‐life small vessel disease, as previously suggested in other cohorts.35, 36 In a review on vascular risk factors and AD, the authors concluded that prospective studies with autopsy (highest evidence level) did not support an association between vascular risk factors and AD pathology.37
Epidemiological studies that do report associations between vascular risk factors and clinical AD generally show that the relative risks of mixed dementia or VaD are higher.37 However, recent research found that multiple vascular risk factors were associated with Aβ pathology.4, 15 When separate factors are considered, mainly dyslipidemia seems to be significantly associated with Aβ pathology.5, 6 Dyslipidemia may exert its effect through lipid metabolism8 rather than via vascular pathology such as atherosclerosis. Indeed, in our previous work, we found higher triglycerides to be significantly associated with Aβ pathology, regardless of IMT.5 In summary, the existing literature is diverse, and the role of vascular risk factors and atherosclerosis in AD development has yet to be resolved.
The use of register‐based diagnoses is a study limitation. Because no structured dementia assessment was performed on all participants, individuals with dementia most likely were included as nondemented participants to some extent. Further, diagnoses derived from clinical routine may be less well characterized than diagnoses derived from a research protocol. Because we aimed to study differences between dementia subtypes, a correct classification is very important. Therefore, we reevaluated available medical history in accordance with DSM‐V20 to optimize diagnostic accuracy. When reviewing medical records, we concluded that almost 80% of participants were diagnosed in tertiary care (specialized Memory Clinic), which suggests good diagnostic accuracy. Further, 95% underwent neuroimaging, indicating good conditions to reveal cerebrovascular pathology of significance. Regardless, misclassification needs to be considered in interpretation of results because concordance between clinical and neuropathological diagnoses may vary.28, 29 Other limitations include the use of complete case analyses, which can induce bias because data may not be missing completely at random, as well as health selection bias. Participants in the MDCS are generally healthier than nonparticipants,18 and participants in the Swedish BioFINDER subcohort were per definition cognitively unimpaired at follow‐up. As anticipated, they were even healthier than the recruitment cohort.5 Because individuals with clinically overt cognitive disease were excluded from the biomarker analyses by study design, it is possible that an existing association between midlife atherosclerosis and AD pathology may have been overlooked. The direction of the association between midlife IMT and AD biomarkers in this study may suggest a possible relation, although not statistically significant. This highlights the need for further research, preferably in a larger sample size covering both cognitively unimpaired participants and individuals with biomarker‐verified AD.
Strengths of the study include the longitudinal design and the direct measures of cerebral pathology. By using register‐based diagnoses, we did not lose participants to follow‐up, and we were able to perform analyses in a large sample. The well‐characterized study population allowed for multiple adjustments for known potential confounders and sensitivity analyses to further address possible bias. In conclusion, our study does not support a strong involvement of atherosclerosis in AD development but does support that atherosclerosis is involved in development of VaD. Together with previous literature, this suggests that the role of atherosclerosis in AD is not yet clarified and warrants continued research, preferably in large settings with AD biomarker data.
Author Contributions
A.‐M.G., K.N., and O.H. contributed to the conception and design of the study; A.‐M.G., D.V.W., E.S., E.G., K.N., and O.H. contributed to the acquisition and analysis of data; and A.‐M.G., K.N., and O.H. contributed to drafting the text and preparing the figure and tables.
Potential Conflicts of Interest
Nothing to report.
Supporting information
Supplementary table 1 Associations between intima media thickness in midlife and clinical dementia diagnoses during 20 years of follow up in a population‐based setting, including number of events and total number of individuals per model.
Supplementary table 2. Associations between carotid plaques in midlife and clinical dementia diagnoses during 20 years of follow up in a population‐based setting, including number of events and total number of individuals per model.
Supplementary table 3. Associations between intima media thickness in midlife and AD pathology and cerebral small vessel disease at 20 year follow up in a subgroup of cognitively unimpaired elderly, including number of events and total number of individuals per model.
Supplementary table 4. Associations between carotid plaques in midlife and AD pathology and cerebral small vessel disease at 20 year follow up in a subgroup of cognitively unimpaired elderly, including number of events and total number of individuals per model.
Acknowledgment
This study was supported by the European Research Council, Swedish Research Council, Knut and Alice Wallenberg Foundation, Marianne and Marcus Wallenberg Foundation, Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson's Disease) at Lund University, Swedish Alzheimer Foundation, Swedish Brain Foundation, Parkinson Foundation of Sweden, Parkinson Research Foundation, and Swedish federal government under the ALF agreement.
We thank all our collaborators, including O. Melander, P. M. Nilsson, and all research nurses involved in the Malmö Diet and Cancer study; everyone involved in collecting data for the Swedish BioFINDER study; and S. Ullén for statistical consultation. Foremost, we thank all study participants.
References
- 1. Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kivipelto M, Helkala EL, Laakso MP, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late‐life Alzheimer disease. Ann Intern Med 2002;137:149–155. [DOI] [PubMed] [Google Scholar]
- 3. Qiu C, Xu W, Fratiglioni L. Vascular and psychosocial factors in Alzheimer's disease: epidemiological evidence toward intervention. J Alzheimers Dis 2010;20:689–697. [DOI] [PubMed] [Google Scholar]
- 4. Gottesman RF, Schneider AL, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nagga K, Gustavsson AM, Stomrud E, et al. Increased midlife triglycerides predict brain beta‐amyloid and tau pathology 20 years later. Neurology 2018;90:e73–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vemuri P, Knopman DS, Lesnick TG, et al. Evaluation of amyloid protective factors and Alzheimer disease neurodegeneration protective factors in elderly individuals. JAMA Neurol 2017;74:718–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guerreiro R, Hardy J. Genetics of Alzheimer's disease. Neurotherapeutics 2014;11:732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lim WLF, Martins IJ, Martins RN. The involvement of lipids in Alzheimer's disease. J Genet Genom 2014;41:261–274. [DOI] [PubMed] [Google Scholar]
- 9. Lusis AJ. Atherosclerosis. Nature 2000;407:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lorenz MW, Markus HS, Bots ML, et al. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation 2007;115:459–467. [DOI] [PubMed] [Google Scholar]
- 11. Carcaillon L, Plichart M, Zureik M, et al. Carotid plaque as a predictor of dementia in older adults: the Three‐City Study. Alzheimers Dement 2015;11:239–248. [DOI] [PubMed] [Google Scholar]
- 12. van Oijen M, de Jong FJ, Witteman JC, et al. Atherosclerosis and risk for dementia. Ann Neurol 2007;61:403–410. [DOI] [PubMed] [Google Scholar]
- 13. Newman AB, Fitzpatrick AL, Lopez O, et al. Dementia and Alzheimer's disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J Am Geriatr Soc 2005;53:1101–1107. [DOI] [PubMed] [Google Scholar]
- 14. Wendell CR, Waldstein SR, Ferrucci L, et al. Carotid atherosclerosis and prospective risk of dementia. Stroke 2012;43:3319–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reed BR, Marchant NL, Jagust WJ, et al. Coronary risk correlates with cerebral amyloid deposition. Neurobiol Aging 2012;33:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berglund G, Elmstahl S, Janzon L, Larsson SA. The Malmo Diet and Cancer Study. Design and feasibility. J Intern Med 1993;233:45–51. [DOI] [PubMed] [Google Scholar]
- 17. Rosvall M, Ostergren PO, Hedblad B, et al. Occupational status, educational level, and the prevalence of carotid atherosclerosis in a general population sample of middle‐aged Swedish men and women: results from the Malmo Diet and Cancer Study. Am J Epidemiol 2000;152:334–346. [DOI] [PubMed] [Google Scholar]
- 18. Manjer J, Carlsson S, Elmstahl S, et al. The Malmo Diet and Cancer Study: representativity, cancer incidence and mortality in participants and non‐participants. Eur J Cancer Prev 2001;10:489–499. [DOI] [PubMed] [Google Scholar]
- 19. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
- 21. Rosvall M, Persson M, Ostling G, et al. Risk factors for the progression of carotid intima‐media thickness over a 16‐year follow‐up period: the Malmo Diet and Cancer Study. Atherosclerosis 2015;239:615–621. [DOI] [PubMed] [Google Scholar]
- 22. Palmqvist S, Zetterberg H, Blennow K, et al. Accuracy of brain amyloid detection in clinical practice using cerebrospinal fluid beta‐amyloid 42: a cross‐validation study against amyloid positron emission tomography. JAMA Neurol 2014;71:1282–1289. [DOI] [PubMed] [Google Scholar]
- 23. Benaglia T, Chauveau D, Hunter DR, Young DS. mixtools: an R package for analyzing finite mixture models. J Stat Softw 2009;32:1–29. [Google Scholar]
- 24. Schmidt P, Gaser C, Arsic M, et al. An automated tool for detection of FLAIR‐hyperintense white‐matter lesions in multiple sclerosis. Neuroimage 2012;59:3774–3783. [DOI] [PubMed] [Google Scholar]
- 25. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly—an update. J Alzheimers Dis 2006;9(suppl):61–70. [DOI] [PubMed] [Google Scholar]
- 29. Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol 2009;66:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hansson O, Palmqvist S, Ljung H, et al. Cerebral hypoperfusion is not associated with an increase in amyloid beta pathology in middle‐aged or elderly people. Alzheimers Dement 2018;14:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Honig LS, Kukull W, Mayeux R. Atherosclerosis and AD: analysis of data from the US National Alzheimer's Coordinating Center. Neurology 2005;64:494–500. [DOI] [PubMed] [Google Scholar]
- 32. Li L, Cao D, Garber DW, et al. Association of aortic atherosclerosis with cerebral beta‐amyloidosis and learning deficits in a mouse model of Alzheimer's disease. Am J Pathol 2003;163:2155–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gupta A, Iadecola C. Impaired Aβ clearance: a potential link between atherosclerosis and Alzheimer's disease. Front Aging Neurosci 2015;7:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dolan H, Crain B, Troncoso J, et al. Atherosclerosis, dementia, and Alzheimer disease in the Baltimore Longitudinal Study of Aging cohort. Ann Neurol 2010;68:231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Della‐Morte D, Dong C, Markert MS, et al. Carotid intima‐media thickness is associated with white matter hyperintensities: the Northern Manhattan Study. Stroke 2018;49:304–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke 2009;40:1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chui HC, Zheng L, Reed BR, et al. Vascular risk factors and Alzheimer's disease: are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence‐based review. Alzheimers Res Ther 2012;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table 1 Associations between intima media thickness in midlife and clinical dementia diagnoses during 20 years of follow up in a population‐based setting, including number of events and total number of individuals per model.
Supplementary table 2. Associations between carotid plaques in midlife and clinical dementia diagnoses during 20 years of follow up in a population‐based setting, including number of events and total number of individuals per model.
Supplementary table 3. Associations between intima media thickness in midlife and AD pathology and cerebral small vessel disease at 20 year follow up in a subgroup of cognitively unimpaired elderly, including number of events and total number of individuals per model.
Supplementary table 4. Associations between carotid plaques in midlife and AD pathology and cerebral small vessel disease at 20 year follow up in a subgroup of cognitively unimpaired elderly, including number of events and total number of individuals per model.
Data Availability Statement
MDCS‐CV data can be requested through an application to the MDCS steering committee. Anonymized data from the Swedish BioFINDER study can be made available upon request to the corresponding author as long as data transfer agrees with European Union legislation on the general data protection regulation. Data will only be shared by requests from qualified investigators for the sole purpose of replicating results.


