Abstract
Although oxidative stress has been long associated with the genesis and progression of the atherosclerotic plaque, scanty data on its in situ effects on protein sulfhydryl group modifications are available. Within the arterial wall, protein sulfhydryls and low-molecular-weight (LMW) thiols are involved in the cell regulation of both Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS) levels and are a target for several posttranslational oxidative modifications that take place inside the atherosclerotic plaque, probably contributing to both atherogenesis and atherosclerotic plaque progression towards complicated lesions. Advanced carotid plaques are characterized by very high intraplaque GSH levels, due to cell lysis during apoptotic and/or necrotic events, probably responsible for the altered equilibrium among protein sulfhydryls and LMW thiols. Some lines of evidence show that the prooxidant environment present in atherosclerotic tissue could modify filtered proteins also by protein-SH group oxidation, and demonstrate that particularly albumin, once filtered, represents a harmful source of homocysteine and cysteinylglycine inside the plaque. The oxidative modification of protein sulfhydryls, with particular emphasis to protein thiolation by LMW thiols and its association with atherosclerosis, is the main topic of this review.
1. Introduction
Cardiovascular diseases are the leading cause of death and illness in developed countries, being atherosclerosis a major contributor [1]. Cardiovascular risk factors such as hypertension, diabetes, and hyperlipidaemia play a key role in the onset and progression of atherosclerosis [2]. Atherosclerosis, a chronic inflammatory condition, develops and evolves in a site-specific and patient-specific manner, with a great heterogeneity in growth rate and pathologic features [3]. Atherosclerotic plaques are commonly characterized by accumulation of lipids and fibrous elements in the intimal layer of medium size and large arteries. Erosion of the atherosclerotic plaques could cause plaque ulceration leading to acute thrombosis and artery occlusion, driving major adverse clinical events. Indeed, plaque disruption is a common precipitating factor in the pathogenesis of both acute coronary occlusion and peripheral artery thrombosis. The mechanisms underlying plaque formation and progression towards advanced lesions potentially prone to rupture are not yet completely understood. Despite current systemic application of therapies, such as statins and antiplatelet agents for prevention of both accelerated plaque growth and thrombotic consequences of its rupture, most of major adverse cardiovascular events cannot be averted [3].
Understanding the mechanisms that promote thin fibrous cap formation and stabilization, as opposed to lysis and disruption, would help to effectively counteract the release of prothrombogenic elements and prevent acute thrombotic occlusion. It is generally held that plaque instability is caused by a substantial increase in inflammatory and proteolytic activity [4]. Furthermore, some lines of evidence suggest that unstable plaques are also characterized by pronounced oxidative environment [5]. In situ oxidative events may determine lipid/protein metabolic fate, bioactivity, and antigenic properties.
This review will focus on oxidative modifications of protein sulfhydryl groups, with particular emphasis to protein thiolation by low-molecular-weight thiols (LMW thiols), and their association with atherosclerosis.
2. Oxidative Stress and Atherosclerosis
A great deal of research has shown a contributory role of oxidative modifications of apolipoprotein B-100-containing lipoproteins (LDL and Lp(a)), within the arterial wall, in the early events of atherogenesis [1, 6, 7]. Oxidized LDL is readily internalized by macrophages through the so-called “scavenger receptor” pathway. These early modifications could initiate and/or contribute to atherogenesis, mainly when an imbalance between oxidant and antioxidant agents takes place [7]. Although several studies report that atherosclerotic plaques contain high concentrations of some amino acid oxidation products, caused mainly by carbonylation, ROS and RNS oxidation, or thiolation [8–10], limited information is available regarding the relationship between the accumulation of in situ oxidized proteins and atherosclerosis severity [11]. Furthermore, different oxidation-specific epitopes can be detected in blood and may reflect atherosclerosis manifestations [12]. At present, the mechanisms underlying the formation of these by-products and their relevance for disease progression are not completely understood and deserve further investigation.
ROS and RNS are highly reactive electrophiles that oxidize nucleophilic functional groups of proteins, polysaccharides, and nucleic acids such as -OH, -NH2 and -SH, leading to cell damage and death, if not properly repaired. Due to its chemistry, -SH is more reactive than -OH and -NH2 and, consequently, more prone to oxidation or conjugation [13, 14].
Within the arterial wall, several enzymes as well as nonenzymatic antioxidants take part in counteracting these oxidative modifications [7]. Among them, protein sulfhydryl groups (protein-SH (PSH) groups) and low-molecular-weight thiols (LMW thiols) are involved in the cell regulation of both ROS and RNS levels. Due to its high reducing power and its relatively high concentration, glutathione (GSH) represents the best intracellular reducing agent. In this respect, it has been reported that human atherosclerotic plaques display lack of GSH-peroxidase (Px) activity and a deficient glutathione redox cycle status that may significantly weaken its antioxidant potential [15, 16], so corroborating the hypothesis that the prooxidant environment within the vascular wall might be involved in atherogenesis and complicated lesion formation. Further evidences come from differential proteomic analysis on advanced unstable and stable carotid plaques, where a reduced expression of superoxide dismutase 3 (major defence against the superoxide anion radical in the vascular extracellular matrix) and glutathione S-transferase (vessel protection against reactive species such as α,β-unsaturated carbonyls and 4-hydroxy-2-nonenal), in the former, suggests an even more impaired antioxidant/prooxidant balance in those plaques more prone to rupture [17].
3. Protein Sulfhydryls Oxidation and Atherosclerosis
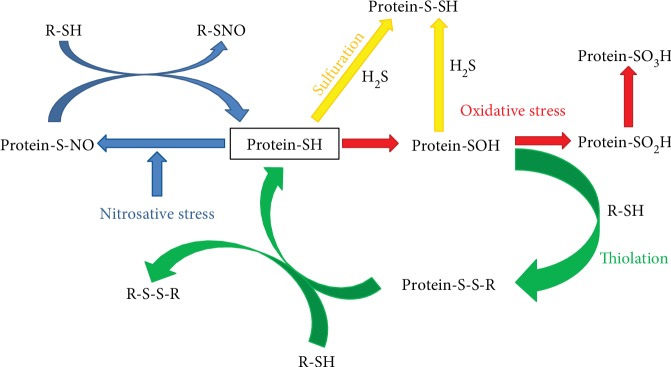
The reactivity of LMW thiols, namely cysteine-glycine (Cys-Gly), homocysteine (HCy), cysteine (Cys), glutathione (GSH), and glutamylcysteine (Glu-Cys), due to their proton lability (pKa), is strictly dependent on their structure, whereas reactivity of protein-SH is affected also by the exposure to the milieu [18]. Some proteins, such as albumin and haemoglobin, have reactive cysteine residues susceptible to some reversible (thiolation, nitrosylation, and sulfenylation) or irreversible (sulfinilation and sulfonation) oxidative modifications (Figure 1). In the last years, it is becoming increasingly clear that also protein S-sulfuration [19] represents an important mechanism of regulation of protein activity mediated by hydrogen sulfide (H2S) (Figure 1). In this respect, it has been reported that these modifications occur on enzymes, receptors, transcription factors, and ion channels and represent key regulatory events in maintaining the physiological function of proteins in the cardiovascular system [20].
Figure 1.
Overview of the wide variety of biochemical modifications that reduced protein sulfhydryl groups may potentially undergo. R-SH or LMW thiols: γGlu-Cys-Gly (γglutamyl-cysteinil-glycine, glutathione), Cys (cysteine), HCy (homocysteine), Cys-Gly (cysteine-glycine), and Glu-Cys (glutamyl-cysteine).
Among the abovementioned reversible reactions, protein S-thiolation by LMW thiols is the most biologically stable and important one [21]. The formation of S-thiolated proteins represents an antioxidant defence mechanism against such reactive molecules, and it has been suggested as a possible redox regulation mechanism of protein function [22, 23] and cell signalling [24, 25]. Indeed, the reversible covalent modification of some protein cysteine residues may be transitory and have critical modulatory effects as suggested for the activation of the latent elastolytic metalloproteinase-2 (pro-MMP-2) by homocysteinylation and S-glutathionylation of the propeptide via the so-called “cysteine switch” mechanism [26–29]. The activation of matrix metalloproteinases (MMPs) by LMW thiol adduction may have a key role in the extracellular matrix degradation and plaque rupture. Furthermore, reversible oxidative modifications, including sulfenylation, S-nitrosylation, and S-thiolation are thought to act as redox sensors in cell signalling pathways [30–32].
S-Glutathionylation is emerging as having a causative role in cardiovascular diseases by regulating numerous physiological processes involved in cardiovascular homeostasis, including myocyte contraction, oxidative phosphorylation, protein synthesis, vasodilation, glycolytic metabolism, and response to insulin [33]. With respect to atherogenesis, there are several in vitro evidences that GSH protects macrophages from OxLDL-induced cell injury [34–37]. Furthermore, Adachi et al. showed that S-glutathionylation of sarco/endoplasmic reticulum calcium (Ca2+) ATPase (SERCA) induces NO-dependent relaxation that is impaired following irreversible oxidation of key thiol(s) during atherosclerosis, so preventing the activation of SERCA [38]. The same team described the mechanisms of redox-dependent p21ras activation induced by oxLDL in endothelial cells [39]. Actually, protein-S-glutathionylation/deglutathionylation in monocytes and macrophages is thought to be an important signalling mechanism that modulates cell response to oxidative stress in key events of plaque initiation (monocyte recruitment and differentiation) and progression (macrophage activation and death) [40]. Increased serum protein glutathionylation has been also correlated with peripheral atherosclerosis [41]. Furthermore, serum levels of glutathione, homocysteine, and cysteine have been independently associated with cardiovascular risk scores at a population level [42].
Elevated plasma levels of homocysteine are a known risk factor for cardiovascular disease and atherothrombosis [43]. Some of its effects include impaired relaxation of blood vessels mediated by the endothelium-derived NO, thiolation of plasma or endothelial proteins, low-density lipoprotein S-homocysteinylation [44, 45], activation of inflammatory pathways and apoptosis, vascular SMC activation and proliferation, and macrophage activation and differentiation [46–50]. Besides GSH, also homocysteine is involved in extracellular matrix remodelling through activation of latent metalloproteinases [26, 28, 29].
Attempting to investigate the relationship between oxidative stress and plaque progression, we studied sulfhydryl group oxidative modifications of extractable proteins from advanced human atherosclerotic plaques, by means of a differential proteomic approach [51]. The study provided evidence that in unstable carotid plaques, and to a lesser extent in stable ones, there is a prooxidant microenvironment conducive to the formation of ROS- and RNS-mediated protein thiol oxidation products. The sulfhydryl group oxidation observed regarded both filtered (e.g., albumin and transferrin) and topically expressed (e.g., α-actin) proteins. Those findings were also corroborated by capillary electrophoresis analysis of LMW thiols bound to extractable proteins that showed higher levels of protein thiolation in unstable plaque extracts. Interestingly, such an increase in protein-bound thiol content was not associated with a concurrent increase in total LMW thiol content. Furthermore, the levels of plasma LMW thiols did not discriminate between patients with stable and unstable plaques, suggesting that the observed differences resulted from oxidative events which take place inside the atherosclerotic plaque [51].
4. Human Serum Albumin as a Carrier of Harmful LMW Thiols inside the Atherosclerotic Plaque
Human serum albumin (HSA) is the most abundant plasma multifunctional protein and the major antioxidant in plasma with a concentration (0.8 mM) higher than the other antioxidants by an exponential factor [52]. Its plasma levels have been strongly inversely correlated with both incident coronary heart disease [53, 54] and some carotid plaque oxidation markers (i.e., TBARS and AOPP) detected in advanced lesions [55]. Recently, its redox state has been associated with some indices of atherosclerosis [56]. Furthermore, a positive correlation between serum total homocysteine and HSA-bound homocysteine in hyperlipidaemic patients has been reported [57]. Interestingly, S-thiolation of HSA occurred not only at Cys34 but also at other cysteine residues, such as Cys90 and Cys101 [57].
Albumin accounts for ROS/RNS scavenging, metal ion binding [58], and transport functions for fatty acids [59, 60], nitric oxide, hemin, and drugs [61–63]. It displays also pseudoenzymatic hydrolytic activity of several endogenous and exogenous compounds [64]. Most of the abovementioned functions are due to its unique redox active free cysteine residue (Cys34) [65]. Thiol concentration in plasma are lower than in the cells, mostly represented by albumin Cys34 residue, which accounts for 80% (500 μmol/L) of total plasma thiols. Due to its reactivity (unexpectedly low Cys34 pKa) and its relatively high concentration, it is the preferential target for oxidants and electrophiles. In fact, in blood as well as in extravascular fluids, albumin is susceptible to different oxidative modifications, especially thiol oxidation and carbonylation [66, 67]. Although albumin circulates primarily in its reduced form, about 30–40% of its reactive Cys34 residues could be variably oxidized, either reversibly as mixed disulphide with low-molecular-weight thiols [68], S-nitroso Cys [69], or sulfenic acid [70] or irreversibly as sulfinic or sulfonic acid [52] (Figure 1). Furthermore, it has been described that albumin, through its nucleophilic Cys34 residue, acts as scavenger for proatherogenic species such as 4-hydroxy-trans-2-nonenal [71].
S-Thiolation of circulating albumin by LMW thiols is the most prevalent Cys34 oxidative modification. Although the proinflammatory mechanisms mediated by LMW thiols are not yet completely understood, it was suggested that albumin could act as homocysteine carrier inside the cells where it could exert its noxious effects by altering the redox potential or modifying intracellular proteins resulting in cellular dysfunction [72].
Starting from this interesting assumption, we have hypothesized that circulating albumin may filter into the atherosclerotic plaque where it may release harmful LMW thiols. Some previous results obtained on advanced human carotid plaques extracts showed that about 70% of extractable proteins were of plasma origin, being albumin the most represented [17], and intraplaque LMW thiol content and distribution deeply differed from plasma [73]. In particular, the high intraplaque glutathione levels, probably consequent to red blood cell lysis, a frequently observed event in atherosclerotic lesions, could contribute to plaque fate by perturbing the physiological LMW thiol redox state.
To assess a possible role for albumin as a carrier of LMW thiols inside the plaque, we set up a very sensitive method for the thiolation analysis of both circulating and intraplaque albumin [74]. The method, which allows for the separation and quantitation of all five LMW thiols starting from 3 μg of albumin, consisted of (1) a preanalytical albumin purification from both plasma and plaque extracts by nonreducing SDS-PAGE, (2) in-gel extraction of LMW thiols, and (3) capillary electrophoresis laser induced fluorescence analysis (CE-LIF).
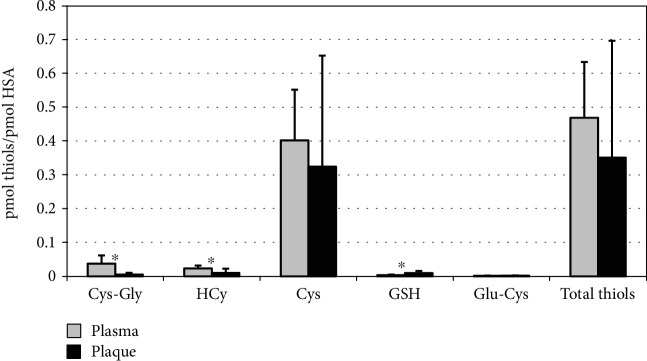
This method was applied to the analysis of HSA Cys34 thiolation/oxidation on twenty-seven atherosclerotic plaque specimens and the corresponding plasma samples collected from patients undergoing carotid endarterectomy [75]. By this way, we evidenced distinct patterns of thiolation for the two HSA forms with a significant reduction of Cys-Gly (∼7-fold) and HCy (∼2-fold), as well as an increase of GSH (∼2.8-fold) in intraplaque HSA (Figure 2). Overall, following infiltration, albumin releases 16.2 ± 11.2 nmol HCy/g proteins and 32.8 ± 23.9 nmol Cys-Gly/g proteins, which represent the bulk of free HCy and Cys-Gly inside the plaque environment [44]. In this regard, carotid plaques are characterized by very high intraplaque GSH levels, with respect to plasma (225 ± 177 nmol/g proteins vs. 59 ± 15 nmol/g proteins, 23.9% vs. 1.72%), probably due to cell lysis during apoptotic and/or necrotic events, responsible for the different equilibrium among protein-bound LMW thiols and the higher Cys34 glutathionylation of the intraplaque form.
Figure 2.
Levels of LMW thiols extracted from both circulating and plaque-filtered HSA, expressed as pmoles/pmoles of albumin, obtained by CE-LIF analysis (from [75]). ∗Significant differences between the two HSA forms (P < 0.001). Cys-Gly: cysteine-glycine; HCy: homocysteine; Cys: cysteine; GSH: glutathione; Glu-Cys: glutamylcysteine.
These in situ evidences have been further supported by recent in vitro data [76] showing equilibria among protein-bound LMW thiols and free LMW thiols released in a thiol-free medium. In particular, the Authors, by using commercial HSA with or without previous incubation with HCy and HCy thiolactone, have evidenced that (1) albumin spontaneously releases HCy in a thiol-free medium (and likely in a thiol-poor medium such as the plaque environment) and such event (2) is positively correlated with the levels of albumin homocysteinylation and (3) could be further increased by the presence in the medium of other LMW thiols, such as Cys and GSH.
5. Conclusions
Understanding the mechanisms that regulate either plaque stabilization or its evolution towards complication and rupture is essential to prevent the clinical events related to acute artery atherothrombosis.
Within the arterial wall, oxidative modifications could initiate and/or contribute to atherogenesis and plaque development. Among them, the formation of mixed disulfides between protein sulfhydryls and LMW thiols may represent an antioxidant defence mechanism, and it has been suggested as a possible redox regulation mechanism of protein function and cell signalling.
Although several in vitro and in vivo evidences show a link between protein thiolation and cardiovascular diseases, only scanty data on these oxidative modifications in situ (inside atherosclerotic plaque) have been reported so far, leaving the issue at a speculative perspective.
In these respect, in the last years, a step forward has been made by reporting the different sulfhydryl oxidation/thiolation of plaque proteins in relation to stable/unstable advanced atherosclerotic lesions and demonstrating that serum albumin, the main plasma protein filtered in plaque, represents a carrier of LMW thiols inside the atherosclerotic lesions, where it releases harmful quantities of homocysteine, probably contributing to plaque progression. However, some aspects, including the equilibria between LMW thiols and protein sulfhydryls, and the effects of specific protein thiolation on their functions remain to be elucidated. They could provide further insight into the relevance of oxidative modifications in atherosclerotic plaque development and progression towards advanced lesions and, surely, deserve further studies.
Acknowledgments
AJ Lepedda thanks Regione Autonoma della Sardegna for its financial support (POR-FSE 2014-2020-Asse Prioritario 3 “Istruzione e Formazione”-Obiettivo Tematico: 10, Priorità d'investimento: 10ii, Obiettivo Specifico: 10.5, Azione dell'Accordo di Partenariato 10.5.12-C.U.P. J86C18000270002). The authors thank the University of Sassari for its financial support (Fondo di Ateneo per la Ricerca 2019).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Libby P., Buring J. E., Badimon L., et al. Atherosclerosis. Nature Reviews Disease Primers. 2019;5(1):p. 56. doi: 10.1038/s41572-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 2.Herrington W., Lacey B., Sherliker P., Armitage J., Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circulation Research. 2016;118(4):535–546. doi: 10.1161/CIRCRESAHA.115.307611. [DOI] [PubMed] [Google Scholar]
- 3.Hansson G. K., Libby P., Tabas I. Inflammation and plaque vulnerability. Journal of Internal Medicine. 2015;278(5):483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geovanini G. R., Libby P. Atherosclerosis and inflammation: overview and updates. Clinical Science. 2018;132(12):1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 5.Madamanchi N. R., Vendrov A., Runge M. S. Oxidative stress and vascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(1):29–38. doi: 10.1161/01.ATV.0000150649.39934.13. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg D., Witztum J. L. Oxidized low-density lipoprotein and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(12):2311–2316. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 7.Stocker R., Keaney J. F., Jr. Role of oxidative modifications in atherosclerosis. Physiological Reviews. 2004;84(4):1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 8.Charles R. L., Eaton P. Redox signalling in cardiovascular disease. Proteomics Clinical Applications. 2008;2(6):823–836. doi: 10.1002/prca.200780104. [DOI] [PubMed] [Google Scholar]
- 9.Gödecke A., Schrader J., Reinartz M. Nitric oxide-mediated protein modification in cardiovascular physiology and pathology. Proteomics Clinical Applications. 2008;2(6):811–822. doi: 10.1002/prca.200780079. [DOI] [PubMed] [Google Scholar]
- 10.Sugamura K., Keaney J. F., Jr. Reactive oxygen species in cardiovascular disease. Free Radical Biology & Medicine. 2011;51(5):978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lepedda A. J. Journal Biomedical and Biopharmaceutical Research. 2017;2(14):p. 296. [Google Scholar]
- 12.Ou H., Huang Z., Mo Z., Xiao J. The characteristics and roles of advanced oxidation protein products in atherosclerosis. Cardiovascular Toxicology. 2017;17(1):1–12. doi: 10.1007/s12012-016-9377-8. [DOI] [PubMed] [Google Scholar]
- 13.Di Simplicio P., Franconi F., Frosalí S., Di Giuseppe D. Thiolation and nitrosation of cysteines in biological fluids and cells. Amino Acids. 2003;25(3-4):323–339. doi: 10.1007/s00726-003-0020-1. [DOI] [PubMed] [Google Scholar]
- 14.Griendling K. K., Touyz R. M., Zweier J. L., et al. Measurement of reactive oxygen species, reactive nitrogen species, and redox-dependent signaling in the cardiovascular system: a scientific statement from the American Heart Association. Circulation Research. 2016;119(5):e39–e75. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lapenna D., de Gioia S., Ciofani G., et al. Glutathione-related antioxidant defenses in human atherosclerotic plaques. Circulation. 1998;97(19):1930–1934. doi: 10.1161/01.CIR.97.19.1930. [DOI] [PubMed] [Google Scholar]
- 16.Lapenna D., Ciofani G., Calafiore A. M., Cipollone F., Porreca E. Impaired glutathione-related antioxidant defenses in the arterial tissue of diabetic patients. Free Radical Biology & Medicine. 2018;124:525–531. doi: 10.1016/j.freeradbiomed.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Lepedda A. J., Cigliano A., Cherchi G. M., et al. A proteomic approach to differentiate histologically classified stable and unstable plaques from human carotid arteries. Atherosclerosis. 2009;203(1):112–118. doi: 10.1016/j.atherosclerosis.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiga O., Summa D., Cirri S., et al. A structurally driven analysis of thiol reactivity in mammalian albumins. Biopolymers. 2011;95(4):278–285. doi: 10.1002/bip.21577. [DOI] [PubMed] [Google Scholar]
- 19.Toohey J. I. The conversion of H2S to sulfane sulfur. Nature Reviews Molecular Cell Biology. 2012;13(12):p. 803. doi: 10.1038/nrm3391-c1. [DOI] [PubMed] [Google Scholar]
- 20.Meng G., Zhao S., Xie L., Han Y., Ji Y. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system. British Journal of Pharmacology. 2018;175(8):1146–1156. doi: 10.1111/bph.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eaton P. Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radical Biology & Medicine. 2006;40(11):1889–1899. doi: 10.1016/j.freeradbiomed.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 22.Hogg P. J. Disulfide bonds as switches for protein function. Trends in Biochemical Sciences. 2003;28(4):210–214. doi: 10.1016/S0968-0004(03)00057-4. [DOI] [PubMed] [Google Scholar]
- 23.Klatt P., Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. European Journal of Biochemistry. 2000;267(16):4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 24.Biswas S., Chida A. S., Rahman I. Redox modifications of protein-thiols: emerging roles in cell signaling. Biochemical Pharmacology. 2006;71(5):551–564. doi: 10.1016/j.bcp.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 25.Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends in Biochemical Sciences. 2009;34(2):85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Bescond A., Augier T., Chareyre C., Garçon D., Hornebeck W., Charpiot P. Influence of homocysteine on matrix metalloproteinase-2: activation and activity. Biochemical and Biophysical Research Communications. 1999;263(2):498–503. doi: 10.1006/bbrc.1999.1391. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto T., Akaike T., Sawa T., Miyamoto Y., van der Vliet A., Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. The Journal of Biological Chemistry. 2001;276(31):29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 28.Tyagi S. C., Smiley L. M., Mujumdar V. S., Clonts B., Parker J. L. Reduction-oxidation (redox) and vascular tissue level of homocyst(e)ine in human coronary atherosclerotic lesions and role in extracellular matrix remodeling and vascular tone. Molecular and Cellular Biochemistry. 1998;181(1-2):107–116. doi: 10.1023/a:1006882014593. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z. S., Jin H., Wang D. M. Influence of hydrogen sulfide on zymogen activation of homocysteine-induced matrix metalloproteinase-2 in H9C2 cardiocytes. Asian Pacific Journal of Tropical Medicine. 2016;9(5):489–493. doi: 10.1016/j.apjtm.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Charles R. L., Schröder E., May G., et al. Protein sulfenation as a redox sensor: proteomics studies using a novel biotinylated dimedone analogue. Molecular & Cellular Proteomics. 2007;6(9):1473–1484. doi: 10.1074/mcp.M700065-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Poole L. B., Nelson K. J. Discovering mechanisms of signaling-mediated cysteine oxidation. Current Opinion in Chemical Biology. 2008;12(1):18–24. doi: 10.1016/j.cbpa.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ying J., Clavreul N., Sethuraman M., Adachi T., Cohen R. A. Thiol oxidation in signaling and response to stress: detection and quantification of physiological and pathophysiological thiol modifications. Free Radical Biology & Medicine. 2007;43(8):1099–1108. doi: 10.1016/j.freeradbiomed.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pastore A., Piemonte F. Protein glutathionylation in cardiovascular diseases. International Journal of Molecular Sciences. 2013;14(10):20845–20876. doi: 10.3390/ijms141020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bea F., Hudson F. N., Chait A., Kavanagh T. J., Rosenfeld M. E. Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circulation Research. 2003;92(4):386–393. doi: 10.1161/01.RES.0000059561.65545.16. [DOI] [PubMed] [Google Scholar]
- 35.Darley-Usmar V. M., Severn A., O'Leary V. J., Rogers M. Treatment of macrophages with oxidized low-density lipoprotein increases their intracellular glutathione content. The Biochemical Journal. 1991;278(2):429–434. doi: 10.1042/bj2780429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gotoh N., Graham A., Nikl E., Darley-Usmar V. M. Inhibition of glutathione synthesis increases the toxicity of oxidized low-density lipoprotein to human monocytes and macrophages. The Biochemical Journal. 1993;296(1):151–154. doi: 10.1042/bj2960151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Qiao M., Mieyal J. J., Asmis L. M., Asmis R. Molecular mechanism of glutathione-mediated protection from oxidized low-density lipoprotein-induced cell injury in human macrophages: role of glutathione reductase and glutaredoxin. Free Radical Biology & Medicine. 2006;41(5):775–785. doi: 10.1016/j.freeradbiomed.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 38.Adachi T., Weisbrod R. M., Pimentel D. R., et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nature Medicine. 2004;10(11):1200–1207. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- 39.Clavreul N., Adachi T., Pimental D. R., Ido Y., Schöneich C., Cohen R. A. S-Glutathiolation by peroxynitrite of p21ras at cysteine-118 mediates its direct activation and downstream signaling in endothelial cells. The FASEB Journal. 2006;20(3):518–520. doi: 10.1096/fj.05-4875fje. [DOI] [PubMed] [Google Scholar]
- 40.Short J. D., Downs K., Tavakoli S., Asmis R. Protein thiol redox signaling in monocytes and macrophages. Antioxidants & Redox Signaling. 2016;25(15):816–835. doi: 10.1089/ars.2016.6697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nonaka K., Kume N., Urata Y., et al. Serum levels of S-glutathionylated proteins as a risk-marker for arteriosclerosis obliterans. Circulation Journal. 2007;71(1):100–105. doi: 10.1253/circj.71.100. [DOI] [PubMed] [Google Scholar]
- 42.Mangoni A. A., Zinellu A., Carru C., Attia J. R., McEvoy M. Serum thiols and cardiovascular risk scores: a combined assessment of transsulfuration pathway components and substrate/product ratios. Journal of Translational Medicine. 2013;11(1):p. 99. doi: 10.1186/1479-5876-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djuric D., Jakovljevic V., Zivkovic V., Srejovic I. Homocysteine and homocysteine-related compounds: an overview of the roles in the pathology of the cardiovascular and nervous systems. Canadian Journal of Physiology and Pharmacology. 2018;96(10):991–1003. doi: 10.1139/cjpp-2018-0112. [DOI] [PubMed] [Google Scholar]
- 44.Zinellu A., Lepedda A., Jr., Sotgia S., et al. Evaluation of low molecular mass thiols content in carotid atherosclerotic plaques. Clinical Biochemistry. 2009;42(9):796–801. doi: 10.1016/j.clinbiochem.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 45.Zinellu A., Sotgia S., Scanu B., et al. Low density lipoprotein S-homocysteinylation is increased in acute myocardial infarction patients. Clinical Biochemistry. 2012;45(4-5):359–362. doi: 10.1016/j.clinbiochem.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 46.Austin R. C., Lentz S. R., Werstuck G. H. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death and Differentiation. 2004;11(Supplement 1):S56–S64. doi: 10.1038/sj.cdd.4401451. [DOI] [PubMed] [Google Scholar]
- 47.Chernyavskiy I., Veeranki S., Sen U., Tyagi S. C. Atherogenesis: hyperhomocysteinemia interactions with LDL, macrophage function, paraoxonase 1, and exercise. Annals of the New York Academy of Sciences. 2016;1363(1):138–154. doi: 10.1111/nyas.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esse R., Barroso M., Tavares de Almeida I., Castro R. The contribution of homocysteine metabolism disruption to endothelial dysfunction: state-of-the-art. International Journal of Molecular Sciences. 2019;20(4):p. 867. doi: 10.3390/ijms20040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lentz S. R. Mechanisms of homocysteine-induced atherothrombosis. Journal of Thrombosis and Haemostasis. 2005;3(8):1646–1654. doi: 10.1111/j.1538-7836.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- 50.Tsai J. C., Perrella M. A., Yoshizumi M., et al. Promotion of vascular smooth muscle cell growth by homocysteine: a link to atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(14):6369–6373. doi: 10.1073/pnas.91.14.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lepedda A. J., Zinellu A., Nieddu G., et al. Protein sulfhydryl group oxidation and mixed-disulfide modifications in stable and unstable human carotid plaques. Oxidative Medicine and Cellular Longevity. 2013;2013:8. doi: 10.1155/2013/403973.403973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruschi M., Candiano G., Santucci L., Ghiggeri G. M. Oxidized albumin. The long way of a protein of uncertain function. Biochimica et Biophysica Acta. 2013;1830(12):5473–5479. doi: 10.1016/j.bbagen.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 53.Nelson J. J., Liao D., Sharrett A. R., et al. Serum albumin level as a predictor of incident coronary heart disease: the atherosclerosis risk in communities (ARIC) study. American Journal of Epidemiology. 2000;151(5):468–477. doi: 10.1093/oxfordjournals.aje.a010232. [DOI] [PubMed] [Google Scholar]
- 54.Pignatelli P., Farcomeni A., Menichelli D., Pastori D., Violi F. Serum albumin and risk of cardiovascular events in primary and secondary prevention: a systematic review of observational studies and Bayesian meta-regression analysis. Internal and Emergency Medicine. 2019:1–9. doi: 10.1007/s11739-019-02204-2. [DOI] [PubMed] [Google Scholar]
- 55.Lapenna D., Ciofani G., Ucchino S., et al. Serum albumin and biomolecular oxidative damage of human atherosclerotic plaques. Clinical Biochemistry. 2010;43(18):1458–1460. doi: 10.1016/j.clinbiochem.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 56.Fujii R., Ueyama J., Aoi A., et al. Oxidized human serum albumin as a possible correlation factor for atherosclerosis in a rural Japanese population: the results of the Yakumo study. Environmental Health and Preventive Medicine. 2018;23(1):p. 1. doi: 10.1186/s12199-017-0690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakashima F., Shibata T., Kamiya K., et al. Structural and functional insights into S-thiolation of human serum albumins. Scientific Reports. 2018;8(1):p. 932. doi: 10.1038/s41598-018-19610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bal W., Sokołowska M., Kurowska E., Faller P. Binding of transition metal ions to albumin: sites, affinities and rates. Biochimica et Biophysica Acta. 2013;1830(12):5444–5455. doi: 10.1016/j.bbagen.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 59.Fujiwara S., Amisaki T. Fatty acid binding to serum albumin: molecular simulation approaches. Biochimica et Biophysica Acta. 2013;1830(12):5427–5434. doi: 10.1016/j.bbagen.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 60.Hamilton J. A. NMR reveals molecular interactions and dynamics of fatty acid binding to albumin. Biochimica et Biophysica Acta. 2013;1830(12):5418–5426. doi: 10.1016/j.bbagen.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Anand U., Mukherjee S. Binding, unfolding and refolding dynamics of serum albumins. Biochimica et Biophysica Acta. 2013;1830(12):5394–5404. doi: 10.1016/j.bbagen.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z. M., Ho J. X., Ruble J. R., et al. Structural studies of several clinically important oncology drugs in complex with human serum albumin. Biochimica et Biophysica Acta. 2013;1830(12):5356–5374. doi: 10.1016/j.bbagen.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 63.Yamasaki K., Chuang V. T., Maruyama T., Otagiri M. Albumin-drug interaction and its clinical implication. Biochimica et Biophysica Acta. 2013;1830(12):5435–5443. doi: 10.1016/j.bbagen.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Kragh-Hansen U. Molecular and practical aspects of the enzymatic properties of human serum albumin and of albumin-ligand complexes. Biochimica et Biophysica Acta. 2013;1830(12):5535–5544. doi: 10.1016/j.bbagen.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 65.Anraku M., Chuang V. T., Maruyama T., Otagiri M. Redox properties of serum albumin. Biochimica et Biophysica Acta. 2013;1830(12):5465–5472. doi: 10.1016/j.bbagen.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 66.Colombo G., Clerici M., Giustarini D., Rossi R., Milzani A., Dalle-Donne I. Redox albuminomics: oxidized albumin in human diseases. Antioxidants & Redox Signaling. 2012;17(11):1515–1527. doi: 10.1089/ars.2012.4702. [DOI] [PubMed] [Google Scholar]
- 67.Turell L., Radi R., Alvarez B. The thiol pool in human plasma: the central contribution of albumin to redox processes. Free Radical Biology & Medicine. 2013;65:244–253. doi: 10.1016/j.freeradbiomed.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ogasawara Y., Mukai Y., Togawa T., Suzuki T., Tanabe S., Ishii K. Determination of plasma thiol bound to albumin using affinity chromatography and high-performance liquid chromatography with fluorescence detection: ratio of cysteinyl albumin as a possible biomarker of oxidative stress. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2007;845(1):157–163. doi: 10.1016/j.jchromb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Stamler J. S., Jaraki O., Osborne J., et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(16):7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bonanata J., Turell L., Antmann L., et al. The thiol of human serum albumin: acidity, microenvironment and mechanistic insights on its oxidation to sulfenic acid. Free Radical Biology & Medicine. 2017;108:952–962. doi: 10.1016/j.freeradbiomed.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 71.Mol M., Regazzoni L., Altomare A., et al. Enzymatic and non-enzymatic detoxification of 4-hydroxynonenal: methodological aspects and biological consequences. Free Radical Biology & Medicine. 2017;111:328–344. doi: 10.1016/j.freeradbiomed.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 72.Sengupta S., Chen H., Togawa T., et al. Albumin thiolate anion is an intermediate in the formation of albumin-S-S-homocysteine. The Journal of Biological Chemistry. 2001;276(32):30111–30117. doi: 10.1074/jbc.M104324200. [DOI] [PubMed] [Google Scholar]
- 73.Zinellu A., Sotgia S., Scanu B., et al. S-homocysteinylated LDL apolipoprotein B adversely affects human endothelial cells in vitro. Atherosclerosis. 2009;206(1):40–46. doi: 10.1016/j.atherosclerosis.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 74.Zinellu A., Lepedda A., Jr., Sotgia S., et al. Albumin-bound low molecular weight thiols analysis in plasma and carotid plaques by CE. Journal of Separation Science. 2010;33(1):126–131. doi: 10.1002/jssc.200900582. [DOI] [PubMed] [Google Scholar]
- 75.Lepedda A. J., Zinellu A., Nieddu G., et al. Human Serum Albumin Cys34 Oxidative Modifications following Infiltration in the Carotid Atherosclerotic Plaque. Oxidative Medicine and Cellular Longevity. 2014;2014:7. doi: 10.1155/2014/690953.690953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zinellu A., Sotgia S., Mangoni A. A., et al. Spontaneous release of human serum albumin S-bound homocysteine in a thiol-free physiological medium. International Journal of Peptide Research and Therapeutics. 2019;25(1):187–194. doi: 10.1007/s10989-017-9663-8. [DOI] [Google Scholar]