Abstract
Malaria is one of the life-threatening diseases in the world. The spread of resistance to antimalarial drugs is a major challenge, and resistance to artemisinin has been reported in the Southeast Asian region. In the previous study, the active compound of Streptomyces hygroscopicus subsp. Hygroscopicus (S. hygroscopicus), eponemycin, has been shown to have antimalarial effects. To further analyze the effects of other active compounds on the Plasmodium parasite, identifying and analyzing the effectiveness of compounds contained in S. hygroscopicus through instrumentation of liquid chromatography/mass spectrometry (LC/MS) and in silico studies were very useful. This study aimed at identifying other derivative compounds from S. hygroscopicus and screening the antimalarial activity of the compound by assessing the binding affinity, pharmacokinetic profile, and bond interaction. The derivative compounds were identified using LC/MS. Protein targets for derivative compounds were found through literature studies, and the results of identification of compounds and protein targets were reconstructed into three-dimensional models. Prediction of pharmacokinetic profiles was carried out using Swiss ADME. Screening of protein targets for the derivative compound was carried out using the reverse molecular docking method. Analyzing bond interaction was done by LigPlot. One compound from S. hygroscopicus, i.e., 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione, was successfully identified using LC/MS. This compound was an isoquinoline derivative compound. Through literature studies with inclusion criteria, thirteen protein targets were obtained for reverse molecular docking. This isoquinoline derivative had the potential to bind to each protein target. The pharmacokinetic profile showed that this compound had the drug-likeness criteria. Conclusion. 6,7-Dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione has antimalarial activity as shown by reverse molecular docking studies and pharmacokinetic profiles. The best inhibitory ability of compounds based on bond affinity is with adenylosuccinate synthetase.
1. Introduction
Malaria is one of the top ten deadly diseases in the world and has been a global health threat for centuries [1]. At present, malaria is estimated to be endemic in 91 countries and one of them is Indonesia [2]. The incidence of malaria cases or Annual Parasite Incidence (API) in Indonesia in 2011 to 2015 continued to decline from 1.75% to 0.85% per 1000 population. The API number reaches zero or malaria-free in several regions, such as DKI Jakarta, West Java, East Java, Banten, and Bali. However, most parts of Eastern Indonesia still have high API numbers, one of which reaches 31.93% in the Papua region [3].
Facing malaria is a big challenge because of the presence of antimalarial drug resistance [4]. The endemic countries have changed therapeutic policies, from monotherapy to combination therapy, artemisinin-based combination therapies (ACT), but artemisinin resistance in P. falciparum in Southeast Asia has been reported [5]. In addition, resistance to antimalarial drugs such as chloroquine and sulfadoxine-pyrimethamine has also been noted, especially in malaria-endemic areas. Other antimalarial drugs such as mefloquine, halofantrine, atovaquone, proguanil, artemether, and lumefantrine have good efficacy, but there are limitations such as cost [6].
The emergence of resistance to antimalarial drugs has become an urgent need to develop effective new antimalarial compounds. The process of finding a drug starts from the identification of unmet medical needs, it is the condition in which there is no satisfaction with the method of diagnosis, therapy, and prevention, and then proceeds with the identification of biological targets for drug-able targets. Protein targets that are compatible with the drug or compound will improve the symptoms of the disease or have a relation to the causative process of the disease [6]. In this revolution era of molecular biology, there are a lot of protein targets that had been successfully identified in Plasmodium, such as plasmepsins, falcipains, proteases, peptidases, reductoisomerases, purine nucleoside phosphorylase, thymidylate synthase, pyruvate kinase, and dihydrofolate reductase [6]. The known protein targets lead to clear understanding about the mechanism of action of the antimalarial target towards each target. The prior known antimalarial also has different pathways towards Plasmodium; quinoline derivatives act by accumulation of food vacuoles in parasites, inhibition of heme detoxication, and inhibition of respiration reaction of parasites in cytochrome BC1 complex. Antifolate derivatives have another pathway; they inhibit dihydropteroate synthetase (PfDHPS), biosynthesis of folic acid, and dihydrofolate reductase (PfDHFR). Antibiotics such as tetracycline, doxycycline, and clindamycin have antimalarial effect by inhibiting protein synthetase pathway and preventing the binding of aminoacyl-tRNA to mRNA ribosome complex. However, there are several antimalarial drugs that have unclear mechanisms such as artemisinin derivatives and primaquine as quinoline derivative [7].
The use of natural products as a therapy for the disease has been carried out since several years ago because it can produce certain biological activities and has properties such as a drug [8]. One proof of the successful development of drugs from natural products is the discovery of artemisinin and quinine. These drugs have been used extensively as antimalarial therapy [9]. One study conducted that utilized natural product is Streptomyces hygroscopicus subsp. hygroscopicus (S. hygroscopicus), by extracting the active fraction of bacterial secondary metabolites. The active fraction of this bacterium has an antimalarial activity with the protein target the ubiquitin-proteasome system (UPS) [10]. The active fraction of the S. hygroscopicus, eponemycin, is a proteasome inhibitor that can inhibit the function of the UPS in eukaryotic cells, including Plasmodium. Analysis of eponemycin analogues from the metabolite extract of S. hygroscopicus has also been carried out by another research by Fitri et al. [11] who used the thin-layer chromatography (TLC) and high-performance liquid chromatography (HPLC) methods and stated that the active fraction of S. hygroscopicus has the potential to be a candidate for new antimalarial drugs and can cause stress on Plasmodium berghei [11].
The process of further analysis and identification of other derivative compounds of S. hygroscopicus is very necessary to find out more details about the profile and potential of the derivative compound from S. hygroscopicus as an antimalarial agent. In silico study as a visual screening method is one of the choices in the drug discovery process. In silico method aims at predicting the orientation of molecular bonds (ligands) with other molecules (protein targets) to form a stable complex [12]. In silico approach is often used in the process of discovering new drugs because it is considered to have many advantages in terms of cost, time efficiency, and work effectiveness [13]. The main focus of the in silico is docking, which is the program used to predict the accuracy of the bonding mode of the protein-ligand complex through the conformation and scoring stages. Docking is usually used to predict various types of ligands for one specific protein target. There is another method known as reverse docking. In contrast to docking, reverse docking is used to predict the bonding of one ligand with various protein targets. Reverse docking is usually used to find out new targets of a drug whose mechanism of action is known or natural products have unknown therapeutic effects [14]. Reverse docking in this study can be used to determine and assess the potential of the active compound of S. hygroscopicus as an antimalarial agent through its association with protein targets in Plasmodium parasite.
Based on the above, we report the newest antimalarial agent, the isoquinoline derivative from S. hygroscopicus through in silico approach.
2. Materials and Methods
2.1. Design and Settings
The design of the study was explorative method using LC/MS and continued to reveal the antimalarial activities of the compound in silico through protein-ligand interactions in reverse molecular docking. This research was conducted from September to November 2018. S. hygroscopicus culture was handled in the Laboratory of Microbiology, Faculty of Medicine, Universitas Brawijaya. S. hygroscopicus extraction was carried out at Laboratory of Pharmacy, Faculty of Medicine, Universitas Brawijaya. LC/MS analysis activities were carried out at PT. Angler Biochemlab, Surabaya, and the in silico study was carried out together with Inbio Indonesia.
2.2. Culture of Streptomyces hygroscopicus subsp. hygroscopicus
Bacteria S. hygroscopicus was obtained from LIPI Microbial Collection, Cibinong, Indonesia. The colony S. hygroscopicus then confirmed its characterization based on the macroscopic colony, morphology, and microscopic staining of Gram. It is Gram-positive bacteria, has endospore, and produces hyphae.
2.3. Secondary Metabolic Fermentation of Streptomyces hygroscopicus subsp. hygroscopicus
Fermentation using ISP4 liquid was in the amount of 50 ml under aseptic conditions in 250 ml Erlenmeyer flask. Bacterial colonies were scraped from ISP4 culture medium and homogenized in ISP4 broth media. It was incubated at 28°C on a 150 rpm shaking incubator for 5 days. After that, the inoculum for the fermentation process was ready to use. 100 ml of 7.0–7.4 adjusted pH ISP4 broth was autoclaved. Next, 25.8 × 106 bacteria from inoculum were added to the broth and fermented at 28°C with 150 rpm of shaking incubator for 5 days [10, 11].
2.4. Extraction Procedure for Metabolites of Streptomyces hygroscopicus subsp. hygroscopicus
After fermentation, the media were harvested and centrifuged to remove cells and debris. The filtrate was collected in a separating funnel. The fermented broth was filtered. The filtrate was mixed with an ethyl acetate ratio of 1 : 1 (v/v) and hand-shaken for 1 hour in a separating funnel. The solvent phase containing metabolic compounds was separated from the aqueous phase. The solvent phase was evaporated to dryness in a water bath.
2.5. Liquid Chromatography/Mass Spectrometry (LC/MS) Analysis
Crude extract samples from S. hygroscopicus were injected and analyzed by the UPLC and ultra high-resolution time-of-flight mass spectrometry (TOF-MS) detector in cooperation with PT. Angler Indonesia, Surabaya.
2.6. Pharmacokinetic Profile
The derivative compound of S. hygroscopicus was analyzed by pharmacokinetic profile using Swiss ADME by entering the SMILES formula.
2.7. Construction of Protein Target Database
In this study, we obtained protein target database through the literature studies and Therapeutic Target Database (TTD) [6, 15, 16]. The protein target screening processes were done by searching using the keywords “isoquinoline,” “malaria,” and “protein target.” We selected and downloaded the protein targets that fulfil the criteria in Protein Data Bank via the http://www.rscb.organd can be downloaded as an extension file .pdb. The protein targets had to fulfil the criteria: (1) they have significant roles in Plasmodium survival and their inhibition may cause parasite death, (2) the protein targets have their own native ligands, (3) the native ligands could not be peptide or protein, and (4) it has been docking to other isoquinoline compounds related to malaria disease (Table 1). Then, we did the residual separation and optimization of protein target. Optimization involved removing the remaining water, ligands from the crystallization process, and cofactors with the PyMol 2.0 program. The residue that is still in the protein will affect the bond between the protein and the ligand, so it needs to be separated first so that the docking results can be optimal. The results of this process were saved in the form of .pdb.
Table 1.
The results of protein target screening.
| No. | Protein target | PDB ID | Res (Å) | Organism | Native ligand | Percent identity to human protein (accession no.) [17, 18]∗ | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| 1. | PfdUTPDXRase | Deoxyuridine 5′-triphosphate nucleotidohydrolase | 1VYQ | 2.40 | P. falciparum | 2,3-Deoxy-3-fluoro-5-O-trityluridine (DUX) | 36.59% | 1Q5H_A | [16, 19] |
| 2. | PfDHODH | Dihydroorotate dehydrogenase | 3O8A | 2.30 | P. falciparum | N-Cyclopropyl-5-[2-methyl-5-(trifluoromethoxyl)-1H-benzimidazol-1-yl]thiophene-2-carboxamide (O8A) | 38.52% | 5K9C_A | [20] |
| 3. | PvDHFR | Dihydrofolate reductase-thymidylate synthase | 2BLA | 2.50 | P. vivax | Pyrimethamine (CP6) | 28.71% | 3GHV_A | [21–23] |
| 4. | PfPMT | Phosphoethanolamine N-methyltransferase | 4FGZ | 1.994 | P. falciparum | Amodiaquine, flavoquine (CQA) | 45.83%† | 3O0Z_A | [16, 24, 25] |
| 5. | PfAdSS | Adenylosuccinate synthetase | 1P9B | 2.00 | P. falciparum | Hadacidin (HDA) | 46.03% | 2V40_A | [16, 26, 27] |
| 6. | PfGK | Glycerol kinase | 2W41 | 2.41 | P. falciparum | Adenine diphosphate (ADP) | 41.67%† | 2GY5_A | [16, 28] |
| 7. | GMP synthetase | Guanosine monophosphate synthetase | 3UOW | 2.72 | P. falciparum | Xanthosine monophosphate (XMP) | 30.77% | 2VXO_A | [16, 29] |
| 8. | PfENR | Enoyl-acyl carrier protein reductase | 3LT0 | 1.96 | P. falciparum | 4-(2,4-Dichlorophenoxy)-3-hydroxybenzaldehyde (FT1); nicotinamide-adenine-dinucleotide (NAD) | 30.00%‡ | 6JLQ_A | [16,30–32] |
| 9. | PfG6PI | Glucose-6-phosphate isomerase | 3PR3 | 2.45 | P. falciparum | Fructose-6-phosphate (F6P) | 38.89% | 1JLH_A | [16, 33, 34] |
| 10. | Pf-cTrpRNA | Tryptophanyl tRNA synthetase | 4J75 | 2.40 | P. falciparum | Tryptophanyl-5′amp (TYM) | 50.25% | 1ULH_A | [16, 35, 36] |
| 11. | DXR | 1-Deoxy-D-xylulose 5-phosphate reductoisomerase | 4Y67 | 1.60 | P. falciparum | [(2R)-2-{2-[Hydroxy(methyl)amino]-2-oxoethyl}-5-phenylpentyl]phosphonic acid (RC5) | 28.57% | 3FJC_A | [16, 37, 38] |
| 12. | DXR | 1-Deoxy-D-xylulose 5-phosphate reductoisomerase | 3AU9 | 1.90 | P. falciparum | 3-[Formyl(hydroxyl)amino]propylphosphonic (fosmidomycin/FOM) | 28.57% | 3FJC_A | [39] |
| 13. | FP2 | Falcipain 2 | 3BPF | 2.90 | P. falciparum | N-[N-[1-Hydroxycarboxyethyl-carbonyl]leucylamino-butyl]-guanindine (E64) | 38.50% | 1FH0_A | [6,40–42] |
| 14. | FP3 | Falcipain 3 | 3BWK | 2.42 | P. falciparum | N∼2∼-(Morpholin-4-ylcarbonyl)-N-[(3S)-1-phenyl-5-phenylsulfonyl)pentan-3-yl)]-L-leucinamide (C1P) | 78.00% | 4AHH_A | [6, 40, 43] |
∗Determined using blast.ncbi.nlm.nih.gov (see supplementary files and ). Percent identity was noted by comparing the sequence of protein targets with the most similar protein sequence existed on humans (accession no. of protein). The Expect value (E value) used was default in the BLAST system (<10), except percent with marks (†10–100, ‡100–1000).
2.8. Preparation of Ligands and Target Structures
The ligand structure was prepared. The ligand was obtained from the identification of the derivative compound of S. hygroscopicus via LC/MS. The derivative compound had been identified and its chemical structure was manually drawn with MarvinSketch and downloaded in the form of .sdf or .pdb. The information of native ligands for their protein target was found in RSCB Protein Data Bank, and the 3D structure was downloaded from PubChem as .sdf file, and another native ligand was taken from cryocrystallized ligand in each protein target and saved as .pdb file.
2.9. Reverse Molecular Docking Studies
Reverse molecular docking was done using PyRx 0.9.5, i.e., a visual screening software for computational drug discovery that could be used in various chemical compounds against potential drug targets [44], with specific dimension of grid box based on native ligands' protein targets (Table 2). We had docked each protein target with its native ligand and the derivative compound of S. hygroscopicus three to five times for accuracy. The results were binding affinity scores between the ligands and the protein targets. After that, the binding affinities were compared between the ligand from S. hygroscopicus and protein targets' native ligands to each protein target. Docking results were visualized using Chimera 1.13.1 program.
Table 2.
Grid box scale of reverse molecular docking.
| No. | Protein targets (PDB ID) | Control ligand | Grid box scale | |||||
|---|---|---|---|---|---|---|---|---|
| Center X | Center Y | Center Z | Size X | Size Y | Size Z | |||
| 1 | 1VYQ | DUX | 39.642 | −−11.423 | −9.416 | 20.929 | 18.116 | 18.059 |
| 2 | 3O8A | O8A | 27.327 | −11.500 | 17.077 | 19.029 | 14.315 | 14.932 |
| 3 | 2BLA | CP6 | 28.120 | 3.267 | 11.793 | 11.999 | 13.358 | 17.359 |
| 4 | 4FGZ | CQA | 19.138 | 26.353 | 14.428 | 31.372 | 25.182 | 30.762 |
| 5 | 1P9B | HDA | 26.315 | 86.090 | 28.993 | 13.599 | 14.757 | 14.304 |
| 6 | 2W41 | ADP | 7.699 | −11.466 | 110.859 | 13.817 | 19.345 | 20.104 |
| 7 | 3UOW | XMP | −10.921 | 30.878 | 43.840 | 22.647 | 19.535 | 21.819 |
| 8 | 3LT0 | FT1 | 49.285 | 86.071 | 37.026 | 37.026 | 29.896 | 18.603 |
| NAD | ||||||||
| 9 | 3PR3 | F6P | 15.645 | 12.408 | 12.578 | 20.050 | 20.150 | 19.285 |
| 10 | 4J75 | TYM | 21.709 | 12.607 | −0.525 | 21.240 | 29.604 | 28.658 |
| 11 | 4Y67 | RC5 | 2.187 | 12.936 | 19.733 | 21.369 | 18.817 | 19.252 |
| 12 | 3BPF | E64 | −59.109 | −3.131 | −18.321 | 25.043 | 20.228 | 22.512 |
| 13 | 3BWK | C1P | 5.661 | −23.586 | 50.308 | 24.306 | 22.568 | 19.700 |
| 14 | 3AU9 | FOM | 11.486 | −15.360 | 16.082 | 20.921 | 16.018 | 18.591 |
2.10. Visualization of Interaction
The stronger binding affinities between the derivative compound and protein targets compared to the native ligands were visualized using LigPlot 1.4.5. The visualization is used to assess whether the residues that formed are consistent with the score of binding affinities [14]. The derivative compound is predicted to have strong bond to the targets if it is capable of binding strongly through hydrogen bonds with the same amino acid residue compared to the control ligand [45].
3. Results and Discussion
3.1. LC/MS Analysis
The purpose of identifying the compound of S. hygroscopicus using the LC/MS analysis method was to find out the type of the compound in S. hygroscopicus. Analysis was done by looking at mass-to-charge ratio (m/z) with the percentage of abundance (% abundance) or intensity. In addition, in the total ion chromatogram (TIC), derivative compounds could be observed based on molecular weight and certain retention time (RT) peaks. The molecular weight of time would be compared with the LC/MS database so that the name of the active compound can be known (Table 3).
Table 3.
The results of derivative compound identification using LC/MS.
| No. | RT | Precursor ion | Possibility name | Product ion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ion formula | m/z | Err (mDa) | M sigma | Ion formula | m/z | Err (mDa) | m sigma | |||
| 1 | 2.52 | C7H13N2O1- | 141.1022 | 0.1 | 6.7 | — | C6H11N21+ | 111.0917 | 0.6 | 8.5 |
| 2 | 3.77 | C14H7N6O61+ | 355.0422 | 1.6 | 6.9 | 6,7-Dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione | C6HN6O21+ | 189.0155 | 0.9 | 5.2 |
| C8H7O41+ | 167.0339 | 0.2 | 2.9 | |||||||
| 3 | 4.08 | C16H19N4O51+ | 347.1350 | −1.7 | −4.9 | — | — | — | — | — |
| 4 | 8.15 | C25H40N5O81+ | 538.2871 | −0.5 | 6.10 | — | C16H22NO31+ | 276.1594 | −0.3 | 17.9 |
| 5 | 9.22 | C22H30NO61+ | 404.2100 | −1.40 | 5.80 | — | C22H27NO61+ | 387.1802 | −1.4 | 8.8 |
| C14H19O51+ | 267.1227 | 0.6 | 13.1 | |||||||
| C16H24NO21+ | 262.1802 | 1.7 | 2.1 | |||||||
| C14H26NO21+ | 240.1958 | −0.9 | 11.4 | |||||||
| C8H9O1+ | 121.0648 | 0.1 | 12.1 | |||||||
| C8H91+ | 105.0699 | −0.1 | 17.6 | |||||||
The results of the LC/MS analysis showed several peaks of the compounds. Each peak was identified based on the molecular weight and fragments, and the results of the identification of the five highest peaks were obtained. Of the five highest peaks analyzed by RT 0–15 minutes, there was one identifiable compound which is 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione with a molecular weight of 355.0422 kDa, while four other compounds have not been identified. The ligand was prepared for its structure. It was obtained from the depiction in MarvinSketch (Figure 1). SMILES formula of ligand, c1cc2c(=O)n(c(=O)c3c2c(c1[N+](=O)[O-])c(cc3)[N+](=O)[O-])n1cnnc1, was done by using Open Babel. The ligand structure was then converted to the .pdb format.
Figure 1.

Chemical structure of 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione.
6,7-Dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione has the chemical formula C14H7N6O6+1 at 3.77 minutes, and it produces two ion fragments, namely, C6HN6O2+1 and C8H7O4+1. 6,7-Dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione is one of the derivatives of isoquinoline. Isoquinoline is a heterocyclic organic aromatic compound, isomer with quinoline. The term isoquinoline is used to make references to isoquinoline derivatives. For example, benzylisoquinoline is a compound with a backbone structure naturally similar to isoquinoline and also papaverine and morphine. Cellular location of isoquinoline is in the cytoplasm [46]. Isoquinoline and its derivatives have been widely used as therapeutic agents for atherosclerosis therapy [47] and antibacterial [48], antituberculosis [49], anticancer [50], and antimalarial [51, 52] agents, and others.
In the previous study, the active compound of the secondary metabolite of S. hygroscopicus, eponemycin, is known to have antimalarial effects through inhibition of parasitic UPS [10, 11]. Another compound that was identified in this study was 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione; this compound is a derivative of isoquinoline. Previous studies have suggested that isoquinoline and its derivatives have antimalarial effects, for example, isoquinoline alkaloids and 10-demethylxylopinine, which originate from Actinodaphne macrophylla [51], protopine and coreximine from Corydalis crispa, and crude alkaloid extracts from Brazilian plant species [52].
3.2. Prediction of Pharmacokinetic Profile
The pharmacokinetic profile of a compound greatly influences the effectiveness of drugs in the body. Pharmacokinetic describes how the body treats drugs or compounds by looking at the processes of absorption, distribution, metabolism, and excretion (ADME) [53]. To find out the effectiveness of compound drugs that are good in the oral route administration, there are several criteria that must be met. One method of assessment is the Rule of Five from Lipinski et al. [54], which includes the following: (1) the molecular weight of the compound is less than 500 mg/dL; (2) a lipophilicity (clogP) is less than 5; (3) hydrogen bond donor is less than 5; and (4) hydrogen bond acceptor is less than 10.
Based on Table 4, this isoquinoline derivative is complied with Lipinski's criteria, molecular weight 354.23 g/mol, 8 H-bond acceptor, 0 H-bond donor, and clogP of 0.08. These results indicated that isoquinoline derivative compounds can be administered via the oral route. cLogP or lipophilicity of isoquinoline derivative compounds is 0.08 which means of having a good lipophilicity (< 5). Good lipophilicity will facilitate compounds in the absorption process, not released by albumin easily, so that the toxicity effects can be reduced and they can be rapidly metabolized and slightly excreted in the kidneys [55].
Table 4.
Prediction of pharmacokinetic profile.
| Molecular weight | 354.23 g/mol |
|---|---|
| Hydrogen bond donor | 0 |
| Hydrogen bond acceptor | 8 |
| cLogP | 0.08 |
3.3. Reverse Molecular Docking Results
The reverse molecular docking results in Figure 2 showed that 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione can bind to protein targets with various binding affinities compared to each native ligand of protein targets. The binding affinity is the ability of ligands to bind to the protein target; the more negative the binding affinity between the ligand and the protein, the better the binding so that the compound can have a biological activity on the protein target [56]. The results of reverse molecular docking between the isoquinoline derivatives with fourteen protein targets showed that the isoquinoline derivative has different binding affinities with each of the protein targets compared to the native ligands. There are eight protein targets (1P9B, 3BPF, 3PR3, 2W41, 2BLA, 3BWK, 3UOW, and 4Y67) that have more negative values than the native ligands, four protein targets (1VYQ, 4FGZ, 3O8A, and 4J75) that have more positive value than the native ligands, one protein target (3AU9) that has same binding affinity, and one protein target (3LT0) that has more positive and negative values compared to two different native ligands.
Figure 2.
The result of reverse molecular docking. Binding affinities of control ligands toward each protein target (green bars) are compared with binding affinities of isoquinoline (ISO) toward same protein targets. Red bars show binding affinity differences between the two ligands. Asterisk indicates the highest and most negative binding affinity differences.
In order to identify specific protein target for which the isoquinoline derivative compound can be therapeutic, based on the most negative delta in Figure 2, there are six protein targets that potentially have stronger interaction than the native ligands based on the binding affinities.
The strongest binding affinity of this compound to the protein target was a bond with the adenylosuccinate synthetase (PDB ID: 1P9B) compared to its native ligand, hadacidin, as shown in Figure 2. The binding affinity between the isoquinoline derivative and adenylosuccinate synthetase is more negative, which is −9.4 kcal/mol, while the binding affinity between the control ligand (hadacidin) and a adenylosuccinate synthetase is −5.1 kcal/mol. The difference in the binding affinity of isoquinoline derivatives with hadacidin is −4.3 kcal/mol; this value is the highest difference between the isoquinoline derivative compound ligand and other native ligands of protein target.
Adenylosuccinate synthetase plays an important role in purine biosynthesis in both de novo pathways and salvage pathways [57]. This enzyme catalyzes the reaction of adenylosuccinate formation from IMP and aspartate in the salvage pathway from hypoxanthine [57, 58]. Inhibition of adenylosuccinate synthetase will affect the balance of the AMP/GMP ratio in cells and lead to death of the parasites [57, 58]. Hadacidin as a native ligand compared to the compound 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione inhibits the adenylosuccinate synthetase through competition with aspartate. Hadacidin is an analogous metabolite of L-aspartate and becomes a potent inhibitor of adenylosuccinate synthetase, thus inhibiting the synthesis of AMP [59–61].
Adenylosuccinate synthetase in Plasmodium falciparum (PfAdSS) has kinetic resemblance to acidic isozyme from mice whose main function is to carry out purine biosynthesis, whereas in other species such as E. coli and humans, they show several characteristic differences [61]: (1) In P. falciparum, the content of A/T (adenine/thymine) is higher (>70%) than in E. coli and mice, so it takes 3-4 times more adenine (AMP precursors) than the number of guanine (GMP precursors). Adenylosuccinate synthetase regulation in maintaining the AMP/GMP ratio in Plasmodium cells has a very important role because of this difference in A/T levels; (2) the absence of the de novo pathway in Plasmodium makes adenylosuccinate synthetase the only source of AMP biosynthesis; (3) the bond sequence between adenylosuccinate synthetase and the other three substrates (IMP, guanosine triphosphate/GTP, and aspartate) to form adenylosuccinate and GDP (guanosine diphosphate) is different from other species. On PfAdSS, the sequence is important, starting with the binding of IMP to adenylosuccinate synthetase, followed by GTP, followed by bonding with aspartate, whereas in other species the sequence of bonds tends to be random; (4) transfer of phosphoryl groups from GTP to IMP in E. coli enzymes and mice can occur in the absence of aspartate, but in P. falciparum, the transfer requires aspartate assistance; and (5) fructose-1,6-biphosphate (F16B) is a potent inhibitor of the adenylosuccinate synthetase in humans, whereas in Plasmodium this compound is an activator of the adenylosuccinate synthetase enzyme, especially in situations where GTP is limited.
The results of virtual screening using LigPlot showed the interaction of the compound 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione with the adenylosuccinate synthetase. This compound has 6 hydrogen bonds and 10 hydrophobic interactions while the native ligand (hadacidin) has 7 hydrogen bonds and 3 hydrophobic interactions, as shown in Table 5 and Figures 3(b) and 3(c). There are 9 same bonds between the compound–1P9B interaction and the control ligand–1P9B interaction. The hydrogen bonds that formed in compound–1P9B interaction shared the same amino acid residues compared to the control ligand, these were Arg311(A), Arg313(A), Thr307(A), and Gly53(A). Besides, the compound-1P9B's hydrophobic interactions shared several same residues as well compared to the control, these were Thr141(A), Val281(A), and Gly306(A). For the same amino acid residues involved, some had different types of bonds, these were Asp26(A) and Thr308(A). In 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione hydrophobic interactions are formed, while in the hadacidin, hydrogen bonds are formed. Raman et al. [57] stated that hadacidin binds to AdSS on Thr307 and the bond formed is a hydrogen bond. Isoquinoline derivative is also able to bind to the Thr307 amino acid from adenylosuccinate synthetase. Based on these data, the bonding interactions between the compound and protein target indicate that the compounds are able to work on the same side of the protein target and are able to interact better than the control assessed based on the number of bonds formed and the number of bond similarities compared to its native ligand.
Table 5.
Interaction between the isoquinoline derivative and the protein target compared to its native ligand.
| No. | Protein targets (PDB ID) | Ligand | Interactions |
|---|---|---|---|
| 1 | 1P9B | ISO | Hydrogen bond: Val246(A), Arg311(A), Arg313(A), Thr307(A), Gly53(A), Asn232(A) |
| Hydrophobic interaction: Thr247(A), Leu236(A), Thr 141(A), Val281(A), Gly306(A), Thr308(A), Asp26(A), His54(A), Asn51(A), Ala52(A) | |||
| HDA | Hydrogen bond: Thr307(A), Gly53(A), Arg313(A), Thr309(A), Thr 308(A), Arg311(A), Asp26(A). | ||
| Hydrophobic interaction: Gly306(A), Val281(A), Thr141(A) | |||
|
| |||
| 2 | 3BPF | ISO | Hydrogen bond: Asn77(A), Ala110(A), Gln208(C) |
| Hydrophobic interaction: Ser74(A), Gly79(A), Phe75(A), Tyr78(A), Asn81(A), Cys80(A), Asp109(A), Asn112(A), Pro111(A), Asp72(A), Val171(A) | |||
| E64 | Hydrogen bond: Lys76(A), Asn77(A), Asn87(A), Gly83(A), | ||
| Hydrophobic interaction: Leu84(A), Asp234(A), Ile85(A), Tyr78(A), Ser149(A), Gly82(A), Asn173(A), Ala175(A), His174(A), Cys42(A) | |||
|
| |||
| 3 | 3PR3 | ISO | Hydrogen bond: Lys540(A), Arg91(A), Gly293(A), Lys232(A), Gln533(A) |
| Hydrophobic interaction: Thr236(A), Ile156(A), Thr233(A), Gly292(A), Gln376(A), Arg294(A), Glu380(A) | |||
| F6P | Hydrogen bond: Glu380(A), Arg294(A), Thr233(A), Ser231(A), Thr236(A), Ser159(A), Lys232(A) | ||
| Hydrophobic interaction: Gln533(A), Gly293(A), Gln376(A) | |||
|
| |||
| 4 | 2W41 | ISO | Hydrogen bond: Ser317(A), Thr268(A), Ser332(A) |
| Hydrophobic interaction: Val316(A), Gly313(A), Pro328(A), Ser329(A), Gly414(A), Asn418(A), Lys417(A), Met415(A) | |||
| ADP | Hydrogen bond: Asn418(A), Ser332(A), Pro328(A) | ||
| Hydrophobic interaction: Thr268(A), Gly313(A), Gly267(A), Val316(A), Gly414(A), Lys417(A), Ala331(A), Met415(A) | |||
|
| |||
| 5 | 3LT0 | ISO | Hydrogen bond: Gly104(A), Thr266(A), Ser215(A), Ala312(A), Ser317(A) |
| Hydrophobic interaction: Leu216(A), Ala319(A), Ala320(A), Pro314(A), Gly313(A), Tyr267(A), Tyr111(A), Leu265(A) | |||
| FT1 | Hydrogen bond: Thr266(A) | ||
| Hydrophobic interaction: Tyr111(A), Ser215(A), Pro314(A), Gly313(A), Ala312(A), Tyr267(A), Tyr277(A), Leu265(A) | |||
|
| |||
| 6 | 2BLA | ISO | Hydrogen bond: Tyr179(A), Ile13(A) |
| Hydrophobic interaction: Ile173(A), Cys14(A), Phe57(A), Met54(A), Leu128(A), Tyr125(A), Ile121(A) | |||
| CP6 | Hydrogen bond: Ile13(A), Asp53(A), Ile173(A) | ||
| Hydrophobic interaction: Ala15(A), Cys14(A), Phe57(A), Ile121(A), Asn117(A) | |||
Protein targets: adenylosuccinate synthetase (1P9B), falcipain 2 (3BPF), glucose-6-phosphate isomerase (3PR3), glycerol kinase (2W41), enoyl-acyl carrier protein reductase (3LT0), and dihydrofolate reductase-thymidylate synthase (2BLA). Ligands: 6,7-dinitro-2-[1, 2, 4]triazol-4-yl-benzo[de]isoquinoline-1,3-dione (ISO), hadacidin (HDA), hydroxycarboxyethyl-carbonyl [leucylamino-butyl]-guanindine (E64), fructose-6-phosphate (F6P), adenine diphosphate (ADP), 4-(2,4-dichlorophenoxy)-3-hydroxybenzaldehyde (FT1), and pyrimethamine (CP6).
Figure 3.
Binding complex and interaction visualization between adenylosuccinate synthetase (1P9B, blue), hadacidin (HDA, green), and isoquinoline derivative (ISO, yellow). In the ISO-HDA-1P9B binding complex (a), HDA and ISO share the same pocket. Interaction visualization of HDA-1P9B (b) shows it shares some similar residue interactions with ISO-1P9B (c).
The next most potential protein target was falcipain 2 (PDB ID: 3BPF, shown in Figure 4). The binding affinity between the isoquinoline derivative compound is −8.1 kcal/mol. It was stronger than the native ligand which is −5.7 kcal/mol. Falcipain 2 is known as the cysteine protease enzyme of Plasmodium falciparum. The role of falcipain 2 is to degrade haemoglobin in the trophozoite phase and to cleave the ankyrin and protein 4.1, i.e., an element of cytoskeleton for stabilizing red blood cell membrane in schizont phase [40, 62]. There were two same interaction bonds between isoquinoline derivative compound compared to the native ligand of falcipain 2, Asn77(A) as hydrogen bond and Tyr78(A) as hydrophobic interaction, as shown in Table 5 and Figure 4.
Figure 4.
Binding complex and interaction visualization between falcipain 2 (3BPF, light purple), hydroxycarboxyethyl-carbonyl [leucylamino-butyl]-guanindine (E64, green), and isoquinoline derivative (ISO, yellow). In the ISO-E64-3BPF binding complex (a), E64 and ISO share the different binding pockets. Interaction visualization of E64-3BPF (b) shows mostly different residue interactions with ISO-3BPF (c).
Glucose-6-phosphate isomerase (PDB ID: 3PR3), as shown in Figure 5, was the third in the list of potential protein targets for the isoquinoline derivative compound (−8.8 kcal/mol) compared to its native ligand (−6.6 kcal/mol). The native ligand and the isoquinoline derivative compound shared several same-interacted residues, these were Lys232(A) as hydrogen bond and Gln376(A) as hydrophobic interaction. The remaining same interacted residues, i.e., Gln533(A), Thr236(A), Thr233(A), Gly293(A), Arg294(A), and Glu380(A), had different types of bonds, as shown in Table 5 and Figures 5(b) and 5(c). Glucose-6-phosphate isomerase is a key enzyme in glucose metabolism, i.e., glycolysis and gluconeogenesis. It catalyzes the second step of glycolysis, glucose-6-phosphate to fructose-6-phosphate [63–65]. This enzyme not only is a housekeeping enzyme of the sugar metabolism inside the cell, but also plays a role in various properties of cytokines and several other protein targets outside the cell [64]. Since Plasmodium falciparum use glucose as the major substrate of energy, this enzyme can be the potential target for antimalarial drugs [65].
Figure 5.
Binding complex and interaction visualization between glucose-6-phosphate isomerase (3PR3, grey), fructose-6-phosphate (F6P, green), and isoquinoline derivative (ISO, yellow). In the ISO-F6P-3PR3 binding complex (a), F6P and ISO share the same binding pocket. Interaction visualization of F6P-3PR3 (b) shows it shares some similar residue interactions with ISO-3PR3 (c).
Glycerol kinase (PDB ID: 2W41), as shown in Figure 6, interacts with the isoquinoline derivative compound at −8.4 kcal/mol and its native ligand at −6.3 kcal/mol. This protein target plays a role in the catalyzation of utilization of glycerol in plants and animals. It is very important for the carbohydrate and lipid metabolisms [28]. Glycerol kinase is the second most highly upregulated gene in Plasmodium falciparum gametocytes, with its expression not detectable in the asexual blood stage. Deletion of this gene had no effect on asexual and sexual (exflagellation) parasite growth. In kinetic studies, Plasmodium falciparum glycerol kinase is not regulated by fructose-1,6-biphosphate [28]. The interaction with this protein occurred with hydrogen bonds at Ser332(A) and hydrophobic interactions at Val316(A), Gly313(A), Gly414(A), Met415(A), and Lys417(A). The different types of interactions occur in the hydrogen bond Thr268(A) of the isoquinoline derivative compound; meanwhile, this bond in the native ligand's protein target is the hydrophobic interaction. Respectively, in the isoquinoline derivative compound, Pro328(A) and Asn418(A) formed hydrophobic interactions, but they formed hydrogen bonds in the native ligand, as shown in Table 5 and Figures 6(b) and 6(c).
Figure 6.
Binding complex and interaction visualization between glycerol kinase (2W41, black), adenine diphosphate (ADP, green), and isoquinoline derivative (ISO, yellow). In the ISO-ADP-2W41 binding complex (a), ADP and ISO share the same binding pocket. Interaction visualization of ADP-2W41 (b) shows it shares some similar residue interactions with ISO-2W41 (c).
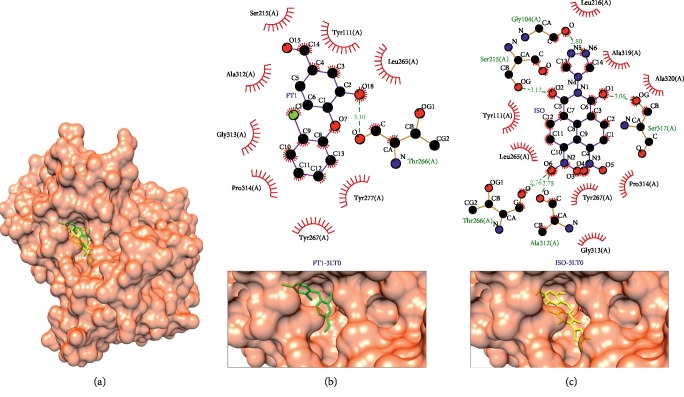
Enoyl-acyl carrier protein reductase (PDB ID: 3LT0) and dihydrofolate reductase-thymidylate synthase (PDB ID: 2BLA), as shown in Figures 7 and 8, respectively, have the same difference as a the result of reverse docking, which is −1.8 kcal/mol compared to each native ligand of protein targets. Enoyl-acyl carrier protein reductase plays role in catalyzation of the final reaction in the type II fatty acid synthesize (FAS) system; this protein mediates the reduction of NADH-dependent trans-2-enoyl-ACP (acyl carrier protein) to acyl-ACP [66]. In contrast in humans, this enzyme utilizes type I FAS [67]. The same interaction between this complex protein target-ligand to its native ligand is at Thr266(A) as hydrogen bond and Pro314(A), Gly313(A), Tyr267(A), Tyr111(A), and Leu265(A) as hydrophobic interaction. Ser215(A) and Ala312(A) formed hydrogen bonds in the isoquinoline derivative compound, yet they formed hydrophobic interactions in the native ligand, as shown in Table 5 and Figures 7(b) and 7(c). On the other hand, dihydrofolate reductase-thymidylate synthase has five same interactions: Ile173(A), Cys14(A), Ile121(A), and Phe57(A) formed hydrophobic interactions and Ile13(A) formed hydrogen bonds in the isoquinoline derivative compound. However, both Ile13(A) and Ile173(A) formed the hydrophobic interaction in the native ligand and the rest formed the hydrophobic interaction, as shown in Table 5 and Figures 8(b) and 8(c). This protein target can be inhibited by antifolate agents [23, 64]. In Plasmodium, dihydrofolate reductase-thymidylate synthase (DHFR) and thymidylate synthase (TS) have a single bifunctional protein that codes for both DHFR and TS activities. Meanwhile, in mammalian cells, these two proteins have separate genes that code for small monofunctional DHFR and TS. However, it has not been fully understood if this bifunctional status of DHFR-TS in Plasmodium can play a role in selective chemotherapy [68].
Figure 7.
Binding complex and interaction visualization between enoyl-acyl carrier protein reductase (3LT0, orange), 4-(2,4-dichlorophenoxy)-3-hydroxybenzaldehyde (FT1, green), and isoquinoline derivative (ISO, yellow). In the ISO-FT1-3LT0 binding complex (a), FT1 and ISO share the same binding pocket. Interaction visualization of FT1-3LT0 (b) shows it shares some similar residue interactions with ISO—3LT0 (c).
Figure 8.
Binding complex and interaction visualization between dihydrofolate reductase, thymidylate synthase (2BLA, dark purple), pyrimethamine (CP6, green), and isoquinoline derivative (ISO, yellow). In the ISO-CP6-2BLA binding complex (a), CP6 and ISO share the same binding pocket. Interaction visualization of CP6-2BLA (b) shows a few similar hydrophobic interactions with ISO-2BLA (c).
4. Conclusions
On the basis of the results of this research and discussion, it can be concluded that the compound of Streptomyces hygroscopicus subsp. hygroscopicus can be identified using LC/MS as 6,7-dinitro-2-[1, 2, 4]triazole-4-yl-benzo[de]isoquinoline-1,3-dione, which is one of the derivatives of isoquinoline. Reverse molecular studies of this compound revealed that this compound has drug-likeness criteria based on Lipinski's rule of five, and thus, it can be administered by the oral route and has antimalarial activity assessed by the strength of binding affinities in each protein target compared to each native ligand using the reverse molecular docking approach. Based on the results, this novel compound can be formulated as an antimalarial drug candidate. The most possible mechanism of action is through the interaction of compound with several key proteins in accordance with their binding affinities; they are as follows: adenylosuccinate synthetase, falcipain 2, glucose-6-phosphate isomerase, glycerol kinase, enoyl-acyl carrier protein reductase, and dihydrofolate reductase-thymidylate synthase. For further study, the compound 3D model should be geometrically optimized, and it should be purified and analyzed further by in vitro and in vivo analysis.
Acknowledgments
This study was funded by Directorate for Research and Community Service, Directorate General of Research Strengthening and Development, Ministry of Research, Technology and Higher Education Republic of Indonesia. The authors would like to thank Mrs. Agustina Tri Endharti, SSi, PhD, for her kind review, and Faculty of Medicine, Universitas Brawijaya, for providing the research facility.
Data Availability
The protein structures can be accessed with the PDB codes 1VYQ, 3O8A, 2BLA, 4FGZ, 1P9B, 2W41, 3UOW, 3LT0, 3PR3, 4J75, 4Y67, 3BPF, and 3BWK, and the native ligands can be accessed in PubChem.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Supplementary Materials
Supplementary 1 (S1): BLAST results of protein targets used (determined using blast.ncbi.nlm.nih.gov). Percent of identity was noted by comparing the sequence of protein targets with the most similar protein sequence existed on human (accession no. of protein). The Expect value (E value) used was default in the BLAST system. Supplementary 2 (S2): distance tree results of the protein targets acquired from BLAST analysis (determined using blast.ncbi.nlm.nih.gov).
References
- 1.Melmane P., Shetty S., Gulati D. A study of drug resistance in malaria. JIACM. 2014;15(1):9–12. [Google Scholar]
- 2.World Health Organization (WHO) Geneva, Switzerland: WHO; 2016. World malaria report summary. [Google Scholar]
- 3.Depkes R. I. Infodatin pusat data dan informasi kesehatan RI malaria. 2016. http://www.depkes.go.id/download.php?file=download/pusdatin/infodatin/InfoDatin-Malaria-2016.pdf.
- 4.World Health Organization (WHO) Drug Resistance in Malaria. Geneva, Switzerland: WHO; 2001. http://www.who.int/csr/resources/publications/drugresist/malaria.pdf. [Google Scholar]
- 5.World Health Organization (WHO) Artemisinin Resistance and Artemisinin-Based Combination Therapy Efficacy. Geneva, Switzerland: WHO; 2018. http://apps.who.int/iris/bitstream/handle/10665/274362/WHO-CDS-GMP-2018.18-eng.pdf. [Google Scholar]
- 6.Oladele T. O., Bewaji C. O., Sadiku J. S. Drug target selection for malaria : molecular basis for the drug discovery process. Centrepoint Journal (Science Edition) 2012;18(2):111–124. [Google Scholar]
- 7.Antony H. A., Parija S. C. Antimalarial drug resistance: an overview. Tropical Parasitology. 2016;6(1):30–41. doi: 10.4103/2229-5070.175081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan H., Ma Q., Ye L., Piao G. The traditional medicine and modern medicine from natural products. Molecules. 2016;21(5):p. 559. doi: 10.3390/molecules21050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muschietti L., Vila R., Filho V. C., Setzer W. Tropical protozoan diseases: natural product drug discovery and development. Evidence-Based Complementary and Alternative Medicine. 2013;2013:2. doi: 10.1155/2013/404250.404250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivo Y. B., Alkarimah A., Ramadhani N. N., et al. Metabolite extract of Streptomyces hygroscopicus inhibit the growth of Plasmodium berghei through inhibition of ubiquitin—proteasome system. Tropical Biomedicine. 2013;30(2):291–300. [PubMed] [Google Scholar]
- 11.Fitri L. E., Cahyono A. W., Nugraha R. Y. B., et al. Analysis of eponemycin (α’β’ epoxyketone) analog compound from Streptomyces hygroscopicus subsp hygroscopicus extracts and its antiplasmodial activity during plasmodium berghei infection. Biomedical Research. 2017;28(1) [Google Scholar]
- 12.Gohlke H., Klebe G. Approaches to the description and prediction of the binding affinity of small-molecule ligands to macromolecular receptors. Angewandte Chemie International Edition. 2002;41(15):2644–2676. doi: 10.1002/1521-3773(20020802)41:15<2644::aid-anie2644>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Meng X.-Y., Zhang H.-X., Mezei M., Cui M. Molecular docking: a powerful approach for structure-based drug discovery. Current Computer Aided-Drug Design. 2011;7(2):146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park K., Cho A. E. Using reverse docking to identify potential targets for ginsenosides. Journal of Ginseng Research. 2017;41(4):534–539. doi: 10.1016/j.jgr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y. H., Yu C. Y., Li X. X., et al. Therapeutic target database update 2018: enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Research. 2018;46(D1):D1121–D1127. doi: 10.1093/nar/gkx1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negi A., Bhandari N., Shyamlal B. R. K., Chaudhary S. Inverse docking based screening and identification of protein targets for Cassiarin alkaloids against Plasmodium falciparum. Saudi Pharmaceutical Journal. 2018;26(4):546–567. doi: 10.1016/j.jsps.2018.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altschul S., Madden T. L., Schaffer A. A., et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altschul S. F., Wootton J. C., Gertz E. M., et al. Protein database searches using compositionally adjusted substitution matrices. FEBS Journal. 2005;272(20):5101–5109. doi: 10.1111/j.1742-4658.2005.04945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whittingham J. L., Leal I., Nguyen C., et al. dUTPase as a platform for antimalarial drug design: structural basis for the selectivity of a class of nucleoside inhibitors. Structure. 2005;13(2):329–338. doi: 10.1016/j.str.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 20.Booker M. L., Bastos C. M., Kramer M. L., et al. Novel inhibitors of Plasmodium falciparum Dihydroorotate dehydrogenase with anti-malarial activity in the mouse model. Journal of Biological Chemistry. 2010;285(43):33054–33064. doi: 10.1074/jbc.m110.162081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bilsland E., Van Vliet L., Williams K., et al. Plasmodium dihydrofolate reductase is a second enzyme target for the antimalarial action of triclosan. Scientific Reports. 2018;8(1):1–8. doi: 10.1038/s41598-018-19549-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuthavong Y., Tarnchompoo B., Vilaivan T., et al. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proceedings of the National Academy of Sciences. 2012;109(42):16823–16828. doi: 10.1073/pnas.1204556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kongsaeree P., Khongsuk P., Leartsakulpanich U., et al. Crystal structure of dihydrofolate reductase from Plasmodium vivax: pyrimethamine displacement linked with mutation-induced resistance. Proceedings of the National Academy of Sciences. 2005;102(37):13046–13051. doi: 10.1073/pnas.0501747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause R. G. E., Goldring J. P. D. Phosphoethanolamine-N-methyltransferase is a potential biomarker for the diagnosis of P. knowlesi and P. falciparum malaria. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193833.e0193833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S. G., Alpert T. D., Jez J. M. Crystal structure of phosphoethanolamine methyltransferase from Plasmodium falciparum in complex with amodiaquine. Bioorganic & Medicinal Chemistry Letters. 2012;22(15):4990–4993. doi: 10.1016/j.bmcl.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crowther G. J., Napuli A. J., Gilligan J. H., et al. Identification of inhibitors for putative malaria drug targets among novel antimalarial compounds. Molecular and Biochemical Parasitology. 2011;175(1):21–29. doi: 10.1016/j.molbiopara.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaazhisai K., Jayalakshmi R., Gayathri P., et al. Crystal structure of fully ligated adenylosuccinate synthetase from plasmodium falciparum. Journal of Molecular Biology. 2004;335(5):1251–1264. doi: 10.1016/j.jmb.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 28.Schnick C., Polley S. D., Fivelman Q. L., et al. Structure and non-essential function of glycerol kinase in Plasmodium falciparum blood stages. Molecular Microbiology. 2009;71(2):533–545. doi: 10.1111/j.1365-2958.2008.06544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wernimont A., Dong A., Hills T., et al. Crystal Structure of PF10_0123, a GMP Synthetase from Plasmodium Falciparum. Protein Data Bank; 2011. https://www.rcsb.org/structure/3UOW. [Google Scholar]
- 30.Maity K., Bhargav S. P., Sankaran B., Surolia N., Surolia A., Suguna K. X-ray crystallographic analysis of the complexes of enoyl acyl carrier protein reductase of Plasmodium falciparum with triclosan variants to elucidate the importance of different functional groups in enzyme inhibition. IUBMB Life. 2010;62(6):467–476. doi: 10.1002/iub.327. [DOI] [PubMed] [Google Scholar]
- 31.Nicola G., Smith C. A., Lucumi E., et al. Discovery of novel inhibitors targeting enoyl-acyl carrier protein reductase in Plasmodium falciparum by structure-based virtual screening. Biochemical and Biophysical Research Communications. 2007;358(3):686–691. doi: 10.1016/j.bbrc.2007.04.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tallorin L., Durrant J. D., Nguyen Q. G., McCammon J. A., Burkart M. D. Celastrol inhibits Plasmodium falciparum enoyl-acyl carrier protein reductase. Bioorganic & Medicinal Chemistry. 2014;22(21):6053–6061. doi: 10.1016/j.bmc.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gileadi T., Wernimont A., Hutchinson A., et al. Crystal Structure of Plasmodium Falciparum Glucose-6-Phosphate Isomerase (PF14_0341) in Complex with Fructose-6-Phosphate. Protein Data Bank; 2011. https://www.rcsb.org/structure/3pr3. [Google Scholar]
- 34.Alam A., Neyaz M. K., Hasan S. I. Exploiting unique structural and functional properties of malarial glycolytic enzymes for antimalarial drug development. Malaria Research and Treatment. 2014;2014:13. doi: 10.1155/2014/451065.451065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koh C. Y., Kim J. E., Napoli A. J., et al. Crystal structures of Plasmodium falciparum cytosolic tryptophanyl-tRNA synthetase and its potential as a target for structure-guided drug design. Molecular and Biochemical Parasitology. 2013;189(1-2):26–32. doi: 10.1016/j.molbiopara.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pasaje C. F. A., Cheung V., Kennedy K., et al. Selective inhibition of apicoplast tryptophanyl-tRNA synthetase causes delayed death in Plasmodium falciparum. Scientific Reports. 2016;6:1–13. doi: 10.1038/srep27531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chofor R., Sooriyaarachchi S., Risseeuw M. D. P., et al. Synthesis and bioactivity of β-substituted fosmidomycin analogues targeting 1-deoxy-d-xylulose-5-phosphate reductoisomerase. Journal of Medicinal Chemistry. 2015;58(7):2988–3001. doi: 10.1021/jm5014264. [DOI] [PubMed] [Google Scholar]
- 38.Yajima S., Hara K., Iino D., et al. Structure of 1-deoxy-D-xylulose 5-phosphate reductoisomerase in a quaternary complex with a magnesium ion, NADPH and the antimalarial drug fosmidomycin. Acta Crystallographica Section F Structural Biology and Crystallization Communications. 2007;63(6):466–470. doi: 10.1107/s1744309107024475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Umeda T., Tanaka N., Kusakabe Y., Nakanishi M., Kitade Y., Nakamura K. T. Molecular basis of fosmidomycin’s action on the human malaria parasite Plasmodium falciparum. Scientific Reports. 2011;14:1–9. doi: 10.1038/srep00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marco M., Miguel Coteron J. Falcipain inhibition as a promising antimalarial target. Current Topics in Medicinal Chemistry. 2012;12(5):408–444. doi: 10.2174/156802612799362913. [DOI] [PubMed] [Google Scholar]
- 41.Kerr I. D., Lee J. H., Pandey K. C., et al. Structures of falcipain-2 and falcipain-3 bound to small molecule inhibitors: implications for substrate specificity. Journal of Medicinal Chemistry. 2009;52(3):852–857. doi: 10.1021/jm8013663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chakka S. K., Kalamuddin M., Sundararaman S., et al. Identification of novel class of falcipain-2 inhibitors as potential antimalarial agents. Bioorganic & Medicinal Chemistry. 2015;23(9):2221–2240. doi: 10.1016/j.bmc.2015.02.062. [DOI] [PubMed] [Google Scholar]
- 43.Kerr I. D., Lee J. H., Farady C. J., et al. Vinyl sulfones as antiparasitic agents and a structural basis for drug design. Journal of Biological Chemistry. 2009;284(38):25697–25703. doi: 10.1074/jbc.m109.014340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dallaaykan S. PyRx—Virtual Screening Tool. La Jolla, CA, USA: TSRI; 2012. http://mgltools.scripps.edu/documentation/links/pyrx-virtual-screening-tool. [Google Scholar]
- 45.Damayanti D. S., Utomo D. H., Kusuma C. Revealing the potency of Annona muricata leaves extract as FOXO1 inhibitor for diabetes mellitus treatment through computational study. In Silico Pharmacology. 2016;5(1):p. 3. doi: 10.1007/s40203-017-0023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.NCBI. Pubchem Compound Database; CID-8405. Bethesda, MD, USA: NCBI; 2018. https://pubchem.ncbi.nlm.nih.gob/compound/8405. [Google Scholar]
- 47.Zhang Y., Li M., Li X., Zhang T., Qin M., Ren L. Isoquinoline alkaloids and indole alkaloids attenuate aortic atherosclerosis in apolipoprotein E deficient mice: a systematic review and meta-analysis. Frontiers in Pharmacology. 2018;9:p. 602. doi: 10.3389/fphar.2018.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galán A., Moreno L., Párraga J., et al. Novel isoquinoline derivatives as antimicrobial agents. Bioorganic & Medicinal Chemistry. 2013;21(11):3221–3230. doi: 10.1016/j.bmc.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 49.Rotthier G., Cappoen D., Nguyen Q. T., et al. Synthesis and anti-tubercular activity of N2-arylbenzo[g]isoquinoline-5,10-dione-3-iminium bromides. Organic & Biomolecular Chemistry. 2016;14(6):2041–2051. doi: 10.1039/c5ob02138c. [DOI] [PubMed] [Google Scholar]
- 50.Yang X., Yang S., Chai H., et al. A novel isoquinoline derivative anticancer agent and its targeted delivery to tumor cells using transferring-conjugated liposomes. PLoS One. 2015;10(8) doi: 10.1371/journal.pone.0136649.e0136649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fadaeinasab M., Taha H., Fauzi P. N. M., Ali H. M., Widyawaruyanti A. Anti-malarial activity of isoquinoline alkaloids from the stem bark of Actinodaphne macrophylla. Natural Product Communications. 2015;10(9):1541–1542. doi: 10.1177/1934578x1501000913. [DOI] [PubMed] [Google Scholar]
- 52.Amoa Onguéné P., Ntie-Kang F., Lifongo L., Ndom J., Sippl W., Mbaze L. The potential of anti-malarial compounds derived from African medicinal plants, part I: a pharmacological evaluation of alkaloids and terpenoids. Malaria Journal. 2013;12(1):p. 449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Scientific Reports. 2017;7(1):p. 42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced Drug Delivery Reviews. 2001;46(1–3):3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 55.Waring M. J. Lipophilicity in drug discovery. Expert Opinion on Drug Discovery. 2010;5(3):235–248. doi: 10.1517/17460441003605098. [DOI] [PubMed] [Google Scholar]
- 56.Ramirez D., Caballero J. Is it reliable to use common molecular docking methods for comparing the binding affinities of enantiomer pairs for their protein target? International Journal of Molecular Sciences. 2016;17(4):p. 525. doi: 10.3390/ijms17040525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raman J., Mehrotra S., Anand R. P., Balaram H. Unique kinetic mechanism of Plasmodium falciparum adenylosuccinate synthetase. Molecular and Biochemical Parasitology. 2004;138(1):1–8. doi: 10.1016/j.molbiopara.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Jayalakshmi R., Sumathy K., Balaram H. Purification and characterization of recombinant Plasmodium falciparum adenylosuccinate synthetase expressed in Escherichia coli. Protein Expression and Purification. 2002;25(1):65–72. doi: 10.1006/prep.2001.1610. [DOI] [PubMed] [Google Scholar]
- 59.De Bruyn C. H. M. M., Simmonds H. A., Muller M. M. Purine Metabolism in Man-IV Part A: Clinical and Therapeutic Aspect; Regulatory Mechanism. New York, NY, USA: Plenum Press; 1984. [Google Scholar]
- 60.Hatch M. D. Inhibition of plant adenylosuccinate synthetase by hadacidin and the mode of action of hadacidin and structurally related compounds on plant growth. Phytochemistry. 1967;6(1):115–119. doi: 10.1016/0031-9422(67)85015-5. [DOI] [Google Scholar]
- 61.Shigeura H. T., Gordon C. N. The mechanism of action of hadacidine. The Journal of Biological Chemistry. 1962;237(6):1937–1941. [PubMed] [Google Scholar]
- 62.Ettari R., Bova F., Zappalà M., Grasso S., Micale N. Falcipain-2 inhibitors. Medicinal Research Reviews. 2010;30(1):136–167. doi: 10.1002/med.20163. [DOI] [PubMed] [Google Scholar]
- 63.Seo M., Crochet R. B., Lee Y.-H. Neidle SBT-CDD and D. Second. San Diego, CA, USA: Academic Press; 2014. Chapter 14—targeting altered metabolism—emerging cancer therapeutic strategies; pp. 427–448. [Google Scholar]
- 64.Mharakurwa S., Kumwenda T., Mkulama M. A. P., et al. Malaria antifolate resistance with contrasting Plasmodium falciparum dihydrofolate reductase (DHFR) polymorphisms in humans and Anopheles mosquitoes. Proceedings of the National Academy of Sciences. 2011;108(46):18796–18801. doi: 10.1073/pnas.1116162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaslow D. C., Hill S. Cloning metabolic pathway genes by complementation in Escherichia coli. Isolation and expression of Plasmodium falciparum glucose phosphate isomerase. The Journal of Biological Chemistry. 1990;265(21):12337–12341. [PubMed] [Google Scholar]
- 66.Lindert S., McCammon J. A. Dynamics of Plasmodium falciparumenoyl-ACP reductase and implications on drug discovery. Protein Science. 2012;21(11):1734–1745. doi: 10.1002/pro.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kapoor M., Gopalakrishnapai J., Surolia N., Surolia A. Mutational analysis of the triclosan-binding region of enoyl-ACP (acyl-carrier protein) reductase from Plasmodium falciparum. Biochemical Journal. 2004;381(3):735–741. doi: 10.1042/bj20040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shallom S., Zhang K., Jiang L., Rathod P. K. Essential protein-protein interactions betwee Plasmodium falciparum Thymidylate synthase and dihydrofolate reductase domains. Journal of Biological Chemistry. 1999;274(53):37781–37786. doi: 10.1074/jbc.274.53.37781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary 1 (S1): BLAST results of protein targets used (determined using blast.ncbi.nlm.nih.gov). Percent of identity was noted by comparing the sequence of protein targets with the most similar protein sequence existed on human (accession no. of protein). The Expect value (E value) used was default in the BLAST system. Supplementary 2 (S2): distance tree results of the protein targets acquired from BLAST analysis (determined using blast.ncbi.nlm.nih.gov).
Data Availability Statement
The protein structures can be accessed with the PDB codes 1VYQ, 3O8A, 2BLA, 4FGZ, 1P9B, 2W41, 3UOW, 3LT0, 3PR3, 4J75, 4Y67, 3BPF, and 3BWK, and the native ligands can be accessed in PubChem.