Abstract
A 4-year-old castrated male dog was presented because of acute, severe neurologic signs 4 months after a 10-meter fall. Liver enzyme activity was high. Imaging and surgery revealed diaphragmatic hernia, liver entrapment, and multiple acquired portosystemic shunts. Initial recovery indicated improved liver enzyme activity and neurologic status although pancreatitis ultimately ensued.
Résumé
Shunts porto-systémiques multiples acquis à la suite d’une hernie diaphragmatique traumatique chez un chien. Un chien mâle castré âgé de 4 ans fut présenté à cause de signes neurologiques aigus sévères 4 mois après une chute de 10 mètres. L’activité des enzymes hépatiques était élevée. L’imagerie et la chirurgie ont révélé une hernie diaphragmatique, un coincement du foie et de multiples shunts porto-systémiques acquis. Le rétablissement initial montrait une amélioration de l’activité des enzymes hépatiques et du statut neurologique bien qu’une pancréatite ait finalement suivi.
(Traduit par Dr Serge Messier)
Abnormal vessels that allow the diversion of portal blood from circulating through the hepatic sinusoids and liver and into the systemic circulation are termed portosystemic shunts (PSS) (1). Acquired portosystemic shunts are extrahepatic, and are multiple, tortuous vessels that develop from the opening of embryonic blood vessels between the portal system and the vena cava due to increased portal pressures; they function to relieve portal hypertension (1,2). Both fibrosis and constriction of the liver can lead to portal hypertension (3). Fibrosis can cause intrahepatic portal hypertension by causing mechanical obstruction and contributing to increased pressure in the sinusoids (3). A high-pressure differential between the portal system and the vena cava can then lead to the formation of multiple vessels and cause functional shunting to develop over time (2,3). In this report, we postulate that in our patient, a diaphragmatic hernia caused from a fall resulted in trauma to the liver and subsequently lead to formation of multiple acquired portosystemic shunts. As far as the authors are aware, this is the first report in a dog of acquired PSS associated with trauma.
Case description
A 4-year-old, 27.6-kg castrated male Labrador retriever dog was presented to the Western College of Veterinary Medicine (WCVM) because of a 24-hour history of head pressing, ataxia, and vomiting. Four months before presentation, the dog had leaped over a rail to jump off a bridge, landing on rocks 10 m below. At the time of the accident, the dog was taken to a veterinarian who performed radiographs of the whole body and found the only injury to be possible pulmonary contusions. No corresponding clinical signs were in evidence, and therefore, no treatment was initiated. Two months later, the dog was taken to the referring veterinarian because he had decreased appetite and less overall willingness to engage in energetic play during the preceding weeks, progressing in severity since the accident. The dog had lost weight (~7 kg). An in-house serum biochemistry showed severely elevated alanine transaminase [ALT; 1699 U/L, reference range (RR): 20 to 150 U/L] and alkaline phosphatase (ALP; 1694 U/L, RR: 10 to 118 U/L) activities. The dog was prescribed S-adenosylmethionine containing Silybin complexed with phosphatidylcholine (Zentonil Advanced 400, Vétoquinol, Lavaltrie, Quebec) 400 mg, PO, q24h, and a hepatic diet.
On presentation to the WCVM, the dog had a dull demeanor. His heart rate was elevated at 108 beats/min, he was panting, and his temperature was 38.8°C. He was hypersalivating, head pressing, and ataxic in his pelvic limbs. Other abnormalities included muscle atrophy over the pelvic limbs and of the temporalis muscles. He was estimated to be approximately 7% dehydrated. Serum was collected and noted to be icteric. An electrocardiogram showed no abnormalities. Blood pressure was within normal limits.
Emergency treatment was initiated on presentation. Mannitol (Pharmaceutical Partners of Canada, Mississauga, Ontario), 0.5 g/kg body weight (BW), IV, and lactulose (Pharmascience, Montreal, Quebec), 241 mg/kg BW, PO, q8h, were administered as treatment for the dog’s neurologic signs. Differential diagnoses for his neurologic signs included metabolic disorders [e.g., hyper- or hypo-natremia and hepatic encephalopathy (HE)], lead poisoning, head trauma leading to intracranial disease, immune-mediated/inflammatory disease (e.g., meningoencephalitis of unknown etiology, steroid-responsive immunemediated meningitis) and infectious processes (viral, bacterial or protozoal). However, with his history of trauma and elevated liver enzyme activity, HE and intracranial disease were considered to be the most likely of the differential diagnoses. The dog was administered Normosol R supplemented with 30 mEq/L potassium chloride (Hospira, Saint-Laurent, Quebec), IV, for 4 h at 8.2 mL/kg BW per hour and then at 6.1 mL/kg BW per hour.
Results of a complete blood (cell) count (CBC) were within laboratory reference ranges. Serum biochemistry revealed severely elevated bilirubin (total 10.5 μmol/L, RR: 1 to 4 μmol/L; direct 3.0, RR: 0 to 2 μmol/L; and indirect 7.5, RR: 0 to 2.5 μmol/L); ALP 978 U/L, RR: 9 to 90 U/L; gamma-glutamyl transpeptidase (GGT) 24 U/L, RR: 0 to 8 U/L; ALT 553 U/L, RR: 19 to 59 U/L; and glutamate dehydrogenase (GLDH) 93 U/L, RR: 0 to 7 U/L. There was a decrease in concentrations of urea at 2 mmol/L (RR: 3.5 to 11.4 mmol/L) and cholesterol at 2.13 mmol/L (RR: 2.7 to 5.94 mmol/L). Albumin was at the low end of normal at 32 g/L (RR: 32 to 42 g/L). Urine analysis revealed urine specific gravity of 1.015, with 2+ bilirubin and 1+ blood on the reagent strip. Resting ammonia level was 99 μmol/L (RR: 0 to 98 μmol/L). Prothrombin and partial thromboplastin (PT/PTT) times were 14 s (RR: 11 to 17 s) and 97 s (RR: 72 to 102 s), respectively.
Thoracic radiographs showed a large, gas-filled tubular structure in the right caudal ventral aspect of the thoracic cavity. This was suspected to be the stomach as it was not seen in the cranial abdomen in of any the projections. A right-sided diaphragmatic hernia was suspected. Abdominal ultrasound performed by a Diplomate of the American College of Veterinary Radiology showed a large portion of the stomach, proximal duodenum, and liver extending cranial to the diaphragm in the mid-ventral thorax. There were multiple tortuous vessels caudal to the kidneys and ventral to the aorta. The liver appeared to be small and smooth with a coarse hyperechoic texture consistent with fibrosis. Other findings included mineral debris in the urinary bladder, bilateral enlargement of the adrenal glands with irregular margination, and dilation of both the cystic and common bile duct. The presumptive diagnosis at this time was a chronic traumatic diaphragmatic hernia with acquired extra hepatic portosystemic shunts resulting in HE.
The patient was prescribed lactulose (Pharmascience), 1 mg/kg BW, PO, q8h, and continued on a liver diet (Hills I/D Liver Health, Mississauga, Ontario), and surgery was scheduled for 1 wk later. Three days before the surgery, the dog was started on levetiracetam (Keppra; Apotex, Toronto, Ontario), 20 mg/kg BW, PO, q8h, to potentially decrease the risk of seizures (4).
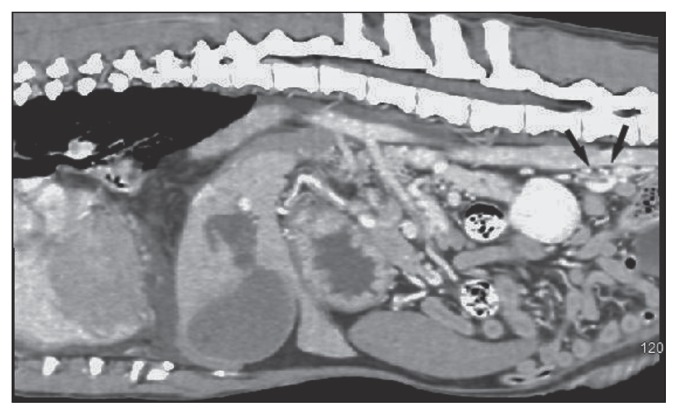
On the day of surgery, computed tomography (CT) revealed a large right-sided diaphragmatic hernia with the pylorus, body of the pancreas, proximal duodenum, and mesentery within the right hemithorax. There was moderate pleural effusion bilaterally with severe reduction of aerated lung. Multifocal groups of tortuous, small mesenteric vessels were located caudal to the left kidney and in the mid-abdomen (Figure 1).
Figure 1.
Sagittal computed tomography (CT) image of the abdomen. Black arrows indicate position of multiple tortuous portosystemic shunt vessels.
The dog was taken to surgery for repair of the right-sided diaphragmatic hernia. Fentanyl (Sandoz, Boucherville, Quebec), 3 μg/kg BW, IV, was used as premedication. Prior to induction of anesthesia, the dog was preoxygenated for 5 min. Propofol (Fresenius Kabi Canada, Mississauga, Ontario), 3 mg/kg BW, IV, was used for induction and anesthesia was maintained with isoflurane (Fresenius Kabi Canada) in 100% oxygen. A standard ventral midline celiotomy was performed. An approximately 10 cm defect was located in the dorsal right crus of the diaphragm. The right medial and lateral lobes of the liver, antrum of the pylorus, and approximately 10 cm of the duodenum extended through the defect, with the margins of the defect tightly encircling the organs. The gall bladder was pulled along with these organs in the direction of the diaphragmatic defect without being herniated, stretching and twisting the cystic and common bile ducts. The defect was extended with an incision in order to facilitate reduction of the herniated organs.
There was slight congestion in the stomach but it quickly became pink with a normal appearance and texture once it was reduced. The duodenum and pancreas were normal in appearance. Although the herniated liver lobes also appeared congested initially, there was no grossly detected vascular compromise of the right medial and lateral lobes, and they appeared viable. The edges of the defect in the diaphragm were debrided and closed using 2-0 polydioxanone (PDS II; Ethicon, Johnson and Johnson, Markham, Ontario) in a simple continuous pattern. A 20 Fr thoracic catheter (Mila International, Florence, Kentucky, USA) was placed through the incision in the diaphragm and exited through the right abdominal wall and secured to the skin using a finger trap pattern with 2-0 nylon (Ethilon; Ethicon). There was a prominent mass of small tortuous vessels noted in the area adjacent to the renal vessels bilaterally and extending cranially towards the hilus of the liver. The linea alba was closed using 0 polydioxanone (PDS II; Ethicon) in a simple continuous pattern, the subcutaneous tissue was closed using 3-0 poliglecaprone 25 (Monocryl; Ethicon) in a simple continuous pattern and the skin was closed using 3-0 poliglecaprone 25 (Monocryl; Ethicon) in an intradermal pattern.
During surgery, the dog did not have any appreciable major vessel compromise but there was severe generalized hemorrhage from the subcutaneous tissues and serosal surfaces. Blood loss was estimated at 2.6 L. The dog was administered approximately 1400 mL of packed red blood cells during surgery and 450 mL of whole blood immediately after surgery followed by 520 mL of fresh frozen plasma. An arterial line was placed for continuous blood pressure measurement. A urinary catheter was placed for measurement of urine output and urine specific gravity. The dog was administered lidocaine (Pfizer Animal Health, Kirkland, Quebec), 50 μg/kg BW per minute, IV, and remifentanyl (Sandoz), 3 μg/kg BW per hour, IV. Oxygen supplementation was provided through nasal lines at 3.5 L/h.
Both PT/PTT times were elevated 1 h after surgery (26 and 126 s, respectively) compared to before surgery, suggestive of comsumptive or dilutional coagulopathy, or disseminated intravascular coagulation. The dog was administered vitamin K (Vétoquinol), 2.5 mg/kg BW, SC. After surgery, other medications included in the dog’s regimen were cefazolin (Pharmascience), 22 mg/kg BW, IV, q6h, metaclopramide (Sandoz), 1 mg/kg BW, q24h, IV, lactulose (Pharmascience), 12 mg/kg BW, PO, q8h, and dexamethasone (Pfizer Animal Health), 0.2 mg/kg BW, IV, once. Normosol R supplemented with 20 mEq/L of potassium chloride (Hospira) was administered IV at a rate of 10 mL/kg BW per hour. Instructions for the thoracic catheter were for aspiration to be performed if an increase in respiratory effort was noted.
The dog was nauseous after recovering from anesthesia and had vomited twice in the 4 h after general anesthesia. Following these episodes, he started to regurgitate frequently. Ultrasonographic examination revealed that the stomach was dilated with fluid, and this was suspected to be the cause of the nausea, regurgitation, and vomiting. A nasogastric tube was placed and suctioned every 2 h.
One day after surgery, both lidocaine and remifentanyl were discontinued and the dog was transitioned to tramadol (compounded in the WCVM pharmacy), 1.78 mg/kg BW, per rectum, q8h. The PT/PTT times (16 and 112 s, respectively) on this day were slightly decreased from the previous day. The dog was given a second transfusion of 460 mL of fresh frozen plasma. Maropitant (Cerenia; Pfizer Animal Health), 1 mg/kg BW, IV, q24h, was added to his medications as he continued to appear nauseous. Intravenous fluid rate was decreased to 5 mL/kg BW per hour. Lactulose was discontinued as the dog’s demeanor had improved, he was responsive to his surroundings, and he had no further neurologic abnormalities.
On the second day after surgery, the thoracic catheter, nasogastric tube, arterial line, and urinary catheter were removed. There was a decreasing amount of fluid that was aspirated from the nasogastric tube and the last aspiration was 5 mL. The dog remained nauseous and had a few episodes of regurgitation; therefore, ondansetron (Zofran; Sandoz, 0.1 mg/kg BW, IV, q8h), was administered. His IV fluid rate was decreased to 3.5 mL/kg BW per hour. The same regimen was continued for the third day after surgery and a serum biochemistry results showed an improvement in liver enzyme activity with ALP at 520 U/L (RR: 9 to 90 U/L), ALT at 481 U/L (RR: 19 to 59 U/L), and GLDH at 58 U/L (RR: 0 to 7 U/L). There was increased bilirubin compared to the previous serum biochemistry (total 42.7 μmol/L, RR: 1 to 4 μmol/L; direct 24.8 μmol/L, RR: 0 to 2 μmol/L; and indirect 17.9 μmol/L, RR: 0 to 2.5 μmol/L).
Immediately after surgery, the dog remained dull and depressed, but there was no ataxia or head pressing. At 3 d after surgery, the dog’s general demeanor was much improved. Although he remained quiet, he was responsive and interactive. The dog appeared to be recovering from surgery and his nausea was improving with medications, although he remained reluctant to eat. Discharge was being tentatively planned when his condition unexpectedly and dramatically declined.
On the fourth day after surgery, the dog developed a dark, mucoid diarrhea. Pantoprazole (Fresenius Kabi Canada), 1 mg/kg BW, IV, q24h, and sucralfate (Aptalis Pharma Canada, Mont-Saint-Hilaire, Quebec), 1 g, PO, q8h, were initiated. A nasoesophageal tube was replaced to start introducing his gastrointestinal tract to nutrition. On the fifth day after surgery, the dog was very depressed and continued to produce dark mucoid diarrhea. A moderate volume of fluid was detected by ultrasonography in the thorax and the abdomen. Cytology of the thoracic fluid showed an exudate suggestive of a mixed inflammation with no evidence of infection. Culture of the thoracic fluid was negative while the culture of the abdominal fluid showed an Enterococcus species. Ampicillin (Pfizer Animal Health), 22 mg/kg BW, IV, q8h, lactulose (Pharmascience) 247 mg/kg BW, per rectum, q8h, and famotidine (Omega, Montreal, Quebec), 1 mg/kg BW, IV, q12h, were added to the dog’s medications. On the sixth day after surgery, an ultrasonographic examination revealed severe pancreatitis. At this point, the owner elected euthanasia and consented to have a necropsy performed.
Gross necropsy findings showed severe acute pancreatitis, severe chronic cholangiohepatitis, severe diffuse hemorrhagic pulmonary congestion and edema, multiple acquired portosystemic vascular shunts extending within the mesentery between the caudal vena cava and the renal veins, and extensive multifocal cutaneous, serosal, and mucosal petechiae and ecchymoses. The pancreas appeared swollen, nodular, and edematous with small areas of hemorrhage. Results of histopathological analysis were consistent with the gross diagnosis of acute pancreatic necrosis. The interlobular septae of the pancreas were widened with edema, hemorrhage, basophilic material, and a small population of inflammatory cells. The liver showed increased fibrosis around the portal triads with biliary hyperplasia and arteriolar proliferation. The fibrous tissue extended into the parenchyma, isolating small groups of hepatocytes and connecting with other portal areas, which obscured normal liver architecture
Discussion
Diaphragmatic hernias are thought to occur as a consequence of increased intraabdominal pressure with an open glottis (5). Diaphragmatic hernia was not diagnosed from thoracic radiographs taken shortly after this dog jumped from a great height. When these radiographs were read by a Diplomate of the American College of Veterinary Radiology for the purposes of this report, a diaphragmatic hernia was not in evidence, and the liver was noted to be of normal size.
The presence of liver fibrosis indicates extensive damage to the liver that may have been initiated at the trauma. Exacerbation of initial injury continued as the liver lobe was compressed and constricted by contraction of the diaphragmatic defect. Portosystemic encephalopathy (PSE) occurs in the presence of both severely decreased liver function and portosystemic shunting (2). The neurologic signs displayed by the dog after the incident were consistent with PSE, and this diagnosis was supported with imaging (abdominal ultrasonographic examination and CT). At surgery, it was observed that due to its intimate anatomic association with the right liver, the gall bladder and its outflow tract were pulled and twisted in the direction of the herniated organs. We hypothesize that this disruption may have caused obstruction to bile flow, leading to accumulation in the liver and eventually fibrosis. Biliary hyperplasia and arteriolar proliferation were reported on postmortem examination. The ensuing increase in portal pressure would lead to development of multiple acquired PSS. Alternatively, the disrupted anatomy may have caused an obstruction of the portal vein primarily, with concomitant decreased delivery of blood to the liver, and leading to formation of multiple acquired PSS.
Although it was difficult to predict the long-term outcome of surgery, it was hoped that reduction of the liver and re- establishment of the normal arrangements of the hepatobiliary system would restore normal hepatic circulation, relieve portal hypertension, and decrease flow through the multiple acquired PSS. The dog’s postoperative improvement in demeanor and neurologic signs may support a theory that outcome may have been good. Unfortunately, due to the complication of acute necrotizing pancreatitis, the long-term outcome resulting from reduction of the liver is unknown. However, with the decrease in ALP, ALT, and GLDH activity and the improving demeanor of the dog with immediate resolution of his neurologic deficits, we postulate that reducing the liver initiated a process of reestablishment of normal hepatic circulation and was already showing a positive effect.
Since the gross appearance of the pancreas was normal during surgery and preoperative imaging, we believe pancreatitis was not caused by the trauma or the chronic diaphragmatic hernia. Further supportive evidence includes the absence of clinical signs of pancreatitis prior to surgery. Ultrasonographic examination and CT before surgery showed no indication of inflammation or edema of the pancreas. Possible etiologies for the pancreatitis were a hypotensive episode during general anesthesia or ischemia and/or inflammation due to intraoperative handling of the pancreas (6).
There is 1 reported case of multiple acquired portosystemic shunts secondary to chronic diaphragmatic hernia in a rescue cat with an unknown history (7). Neurologic signs developed 5 mo after adoption. The cat did not have elevated liver enzyme activity but did have a moderately increased blood ammonia level. Exploratory laparotomy revealed that the jejunum, ileum, ascending and transverse colon, and left medial and left lateral liver lobes were herniated into the thoracic cavity. Multiple acquired shunts were noted between the portal vein and the caudal vena cava at the level of the right limb of the pancreas. At 9 mo after surgery, the cat was clinically normal and weaned off all medications. We postulate that our patient may have recovered similarly had he not developed pancreatitis, as there were indicators in blood analysis and behavior consistent with improvement in liver condition.
We cannot exclude the possibility that either or both the diaphragmatic hernia and portosystemic shunts existed prior to the trauma. The dog had no diagnostic tests performed before the trauma because he had never had any clinical signs requiring investigation. The dog may have had subclinical liver disease which manifested shortly after but unrelated to the trauma. If the remainder of the liver were healthy, loss of function of the right medial and lateral liver lobes alone would not be expected to cause the diffuse liver changes or formation of multiple acquired PSS. Therefore, it must remain a possible etiology that the trauma was only coincident with liver disease.
Since the dog had been clinically normal until after the trauma, we consider it more likely that the trauma and the clinical condition at presentation were connected. There is no means by which to ascertain the status of the dog’s liver before the accident. The immediate decline of liver enzyme activity and the resolution of neurologic abnormalities after surgery lead us to conclude that the underlying cause had been addressed. Radiographs taken immediately after the event do not show evidence of herniated organs, and therefore the organs would have moved through a presumed defect in the intervening time before presentation with neurologic signs 4 mo after the event. We propose that the compromise to the hepatobiliary system as a result of the anatomic disruption caused by the herniation may have led to increasing portal pressure and the formation of multiple acquired portosystemic shunts. To the authors’ knowledge, this is the first reported case of acquired portosystemic shunts associated with trauma in a dog.
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Berent AC, Tobias KM. Portosystemic vascular anomalies. Vet Clin Small Anim. 2009;39:513–541. doi: 10.1016/j.cvsm.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Rothuizen J. Important clinical syndromes associated with liver disease. Vet Clin Small Anim Pract. 2009;39:419–437. doi: 10.1016/j.cvsm.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Buob S, Johnston AN, Webster CRL. Portal hypertension: Pathophysiology, diagnosis, and treatment. J Vet Intern Med. 2011;25:169–186. doi: 10.1111/j.1939-1676.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- 4.Fryer KJ, Levine JM, Peycke LE, Thompson JA, Cohen ND. Incidence of postoperative seizures with and without levetiracetam pretreatment in dogs undergoing postosystemic shunt attenuation. J Vet Intern Med. 2011;25:1379–1384. doi: 10.1111/j.1939-1676.2011.00819.x. [DOI] [PubMed] [Google Scholar]
- 5.Hunt GB, Johnson KA. Diaphragmatic hernia. In: Tobias KM, Johnson KA, editors. Veterinary Surgery Small Animal. 1st ed. St. Louis, Missouri: Elsevier Saunders; 2012. pp. 1380–1390. [Google Scholar]
- 6.Cornell K. Pancreas. In: Tobias KM, Johnson KA, editors. Veterinary Surgery Small Animal. 1st ed. St. Louis, Missouri: Elsevier Saunders; 2012. pp. 1659–1673. [Google Scholar]
- 7.Barfield DM, Gibson AD, Lipscomb VJ. Multiple acquired portosystemic shunts in a cat secondary to chronic diaphragmatic rupture. J Feline Med Surg Open Reports. 2015;1:1. doi: 10.1177/2055116915585020. [DOI] [PMC free article] [PubMed] [Google Scholar]