Abstract
Background
Multidisciplinary interventions may be useful for children and adolescents with diabetes mellitus (DM), especially in areas where new blood glucose monitoring and control technologies are difficult to access.
Methods
PAANDA, a care program for adolescents and children with diabetes, was implemented in patients aged 0 to 18 years and 11 months. The effect of the intervention was determined by self‐blood glucose monitoring (SBGM) and glycosylated hemoglobin (HbA1C) levels at start and after 6 months.
Results
A total of 121 patients with DM were evaluated, mean age of 14.27 years (SD: 4.60 years). Blood glucose measurements in range (70‐120 mg/dL pre‐prandial or 70‐180 mg/dL post‐prandial) increased by 20.67% before breakfast, 8.14% after breakfast (both P‐value <.001), 5.02% before lunch (P‐value = .02), 8.66% after lunch (P‐value <.001), 11.50% before dinner (P‐value <.001), 11.87% after dinner (P‐value <.001), and 8.00% at dawn (P‐value = .001). This change was accompanied by fewer values in the hyperglycemic category (−19.49% before breakfast, −7.73% after breakfast, both P‐value <.001) and hypoglycemia (−1.18%). HbA1C levels decreased significantly 1.8% (P‐value = .018). Multivariate logistic regression analysis showed an increase in glycemic control associated with each month after the intervention time in the PAANDA program (P‐value <.001 for all the time points evaluated) and a significant decrease in glycemic variability.
Conclusions
The multidisciplinary PAANDA intervention had a beneficial effect on glycemic control, with an improved time in range in a population of children and adolescents with DM.
Keywords: childhood and adolescent diabetes mellitus, diabetes education program, intervention, multidisciplinary, PAANDA, SBGM, self‐monitoring, time in range
1. INTRODUCTION
Diabetes mellitus is the most common metabolic disorder of childhood and its treatment requires patients to perform multiple actions throughout the day, including insulin dosing, blood glucose testing, and regular physical activity. Despite multiple advances in the treatment of type 1 diabetes mellitus (T1DM) (eg, use of blood glucose meters and insulin pumps, among others), more than 70% of T1DM patients maintain glycosylated hemoglobin (HbA1C) levels above 7%.1 In addition, non‐compliance rates in patients with diabetes often exceed 50%, highlighting the need for interventions focused on behavior change.2
Studies of multidisciplinary interventions in T1DM have shown that they generate additional benefits compared to traditional diabetes education programs.3 Some interventions in particular, such as psycho‐educational interventions, have been shown to be effective in improving glycemic control4 and other outcomes in T1DM (diabetes stress, depressive symptoms, resilience, etc.).5 Other interventions, such as the “Herdecker Kids with Diabetes” program, which is based on an anthropological and philosophical understanding of the human being, offer individualized, patient‐oriented approaches to developing comprehensive human and diabetes‐related skills.6 However, despite the benefit of these multidisciplinary programs based on education and self‐management, life expectancy in these patients is still much lower than that observed in non‐diabetic persons.7
Self‐blood glucose monitoring (SBGM)8, 9 remains the predominant modality of glucose monitoring in diabetes compared to continuous glucose monitoring (CGM).10 Although very useful, CGM is expensive, invasive, requires high management skills to be effective, and is difficult to implement in low‐income countries.11 In the Diabetes Control and Complications Trial (DCCT), the time in range has been associated, using 7‐point monitoring, with lower risk of developing chronic complications in patients with T1DM. This strategy offers the possibility of improving success from any intervention, in situations where CGM is not feasible (eg, due to cost of CGM in developing countries).8, 9 Multidisciplinary interventions, particularly those based on SBGM, may therefore be effective in helping people with T1DM achieve their therapeutic goals in developing countries.
To date, in developing countries such as Mexico, there is no information available on the effect of multidisciplinary interventions that use glycemic self‐management as a fundamental tool on the management of children and adolescents with DM. The purpose of this study was therefore to evaluate an intervention (PAANDA: program of care for adolescents and children with diabetes mellitus) that includes multidisciplinary care at the first level of care. The 6‐month study focused on improving disease control through self‐management of blood glucose by SBGM at seven‐time points of the day, as well as HbA1C levels.
2. METHODS
2.1. Population
Patients admitted to the program were referred by local health centers, general hospitals, pediatric hospitals, National Institutes of Health, childhood obesity clinics, and private hospitals of any level of care. The patients included in this study were all those referred by local health centers. Inclusion criteria were age (0‐18 years, 11 months) and absence of acute complications at baseline. Patients were selected from January 2014 to September 2018. All patients had received some form of treatment at the time of inclusion. This program began as a private initiative in 2014 and is currently a primary care program for the very low‐income population of Mexico City, Mexico. This project was approved by the Institutional Review Board of the National Cancer Institute—Mexico (INCAN/CEI/699/19).
2.2. Registration of clinical characteristics
Data were recorded in predefined formats and given to the patient during medical consultations; rigorous training was provided to the patient and the responsible family member. Trained staff then scanned each form into Excel spreadsheets to create standardized for analysis.
2.3. Intervention
The PAANDA program includes the processes necessary to empower children and adolescents living with diabetes in a logical and systematic way. PAANDA consists of a series of multidisciplinary interventions—supported by up‐to‐date documentation—that form an individualized approach for child or adolescent with diabetes. This intervention, designed primarily as an educational model, has been formalized over time with the primary goal of allowing the patient to correct blood glucose levels through dietary measures or administration of additional insulin, trying to maintain glucose levels within the normal range, and thus prevent acute and chronic complications. Patients are encouraged to monitor glucose levels before and after breakfast, before and after lunch, before and after dinner, and in the early morning. The interdisciplinary care plan includes three integrative areas: social work, pediatric nursing and endocrinology; and several complementary services: ophthalmology, orthopedics, sports medicine, diabetes education, nutrition, psychology, podiatry, gastronomy, dentistry, and physical activity. Patients were treated with insulin and metformin, depending on the specific needs of each case. Although the study lasted 6 months, children were asked to continue with clinical and metabolic profiling after the 6‐month period, as part of their standard care follow‐up.
2.4. Statistical analysis
A descriptive analysis of the available clinical variables and the various multidisciplinary evaluations were carried out. Glucose levels and HbA1C levels were determined during the first month of the intervention and compared to the measurement of the same variables obtained during the sixth month of the intervention. Statistically significant differences were also determined between the frequencies of patients in the American Diabetes Association categories (before meals: hypoglycemia, ADA 1, <70 mg/dL; normal range, ADA 2, 70‐120 mg/dL; hyperglycemia, ADA 3, >120 mg/dL; after meals: ADA 1, <70 mg/dL; ADA 2, 70‐180 mg/dL; and ADA 3, >180 mg/dL). Finally, using a multivariate‐adjusted logistic regression model, we determined the effect of time spent in the program on achieving adequate blood glucose levels (ADA 2). It was adjusted by age at the start of the program, type of diabetes (T1DM vs T2DM), sex, and diabetes duration at the start of the program. We included data on all patients, regardless of type of diabetes. P‐values <.05 were considered statistically significant. The analyses were carried out using R software (R Project for Statistical Computing, CRAN, The Comprehensive R Archive Network, Vienna).
3. RESULTS
3.1. Characteristics of the population studied and the intervention
A total of 121 patients were included in the study, with a mean age of 14.27 years (SD 4.46 years); 59.50% were female. Mean diabetes duration at start of the program was 3.3 years (SD: 3.96 years). Patients had an average of 1.2 hospitalizations before entering the program. The majority was classified as T1DM (74.38%), the rest as T2DM, according to clinical criteria (ADA or ISPAD: International Society for Pediatric and Adolescent Diabetes). Details of population characteristics are shown in Table 1. All patients received multidisciplinary care.
Table 1.
Socio‐demographic and clinical characteristics of patients seen within the first 6 months of the program of care for adolescents and children with diabetes mellitus (PAANDA, N = 121)
| Variable | Mean | SD |
|---|---|---|
| Age at time of entry | 14.27 | 4.46 |
| Age at time of diagnosis, years | 11.06 | 3.81 |
| Years elapsed from diagnosis to start of program | 3.33 | 3.96 |
| Number of hospitalizations | 1.2 | 1.62 |
| Number of glucose testsa | 163.9 | 70.55 |
| Variable | n | % |
|---|---|---|
| Gender | ||
| Women | 72 | 59.50 |
| Men | 49 | 40.50 |
| Type of diabetes mellitus | ||
| Type 1 | 90 | 74.38 |
| Type 2 | 31 | 25.62 |
| Interventionsb | ||
|---|---|---|
| Variable | Meanc | SD |
| Nutrition | 3.11 | 2.73 |
| Educational Sessions | 4.72 | 3.31 |
| Physical activity (sport) | 1.72 | 2.03 |
| Gastronomy | 1.23 | 1.37 |
| Orthopedics | 1.7 | 0.89 |
| Foot care | 2.1 | 1.42 |
| Psychology | 2.48 | 2.29 |
| Social work | 1.64 | 1.36 |
| Ophthalmology | 1.91 | 1.08 |
| Dentist | 2.08 | 2.43 |
Glucose tests per patient.
Some services (gastronomy, physical activation, social work and nursing) are not shown in the table.
Sessions per patient.
3.2. Effect of intervention on glycemic control
It was found that glucose levels, at all points of testing showed statistically significant differences in the sixth month, compared to the levels recorded in the first month (P‐value <.001). Before breakfast, there was a reduction in the rates of hypoglycemia (−1.18%), as well as hyperglycemia (−19.50%), with a 20.67% increase in the frequency of levels in the normal ADA range. After breakfast, similarly, there were lower rates of hypoglycemia (−0.41%) and hyperglycemia (−7.73%) with an 8.14% increase in frequency normal values. The same trend was observed in almost all other time points, with similar intensities of change: ADA 1: mean, −0.55% (SD 1.72%); ADA 2: mean 10.55% (SD 5.03%); ADA 3: mean − 10% (SD 4.75%), which were statistically significant (P‐value <.05, Table 2).
Table 2.
Effect of the intervention on the percentage of patients with low, normal, or elevated glucose levels according to the American Diabetes Association (ADA) in patients in the program of care for adolescents and children with diabetes mellitus (PAANDA) at baseline (first month, n = 121) and 6 months after the intervention (n = 54)
| First month | Sixth month | ||||
|---|---|---|---|---|---|
| na | % | na | % | P‐value | |
| Glucose levels before breakfast | |||||
| Hypoglycemia (ADA 1), <70 mg/dL | 29 | 5.21 | 44 | 4.03 | <.001 |
| Normoglycemia (ADA 2), 70‐120 mg/dL | 171 | 30.70 | 561 | 51.37 | |
| Hyperglycemia (ADA 3), >120 mg/dL | 357 | 64.09 | 487 | 44.60 | |
| Glucose levels after breakfast | |||||
| ADA 1, <70 mg/dL | 82 | 4.83 | 35 | 4.42 | <.001 |
| ADA 2, 70‐180 mg/dL | 1071 | 63.07 | 564 | 71.21 | |
| ADA 3, >180 mg/dL | 545 | 32.10 | 193 | 24.37 | |
| Pre‐meal glucose levels | |||||
| ADA 1, <70 mg/dL | 126 | 7.32 | 67 | 8.22 | .02 |
| ADA 2, 70–120 mg/dL | 621 | 36.08 | 335 | 41.10 | |
| ADA 3, >120 mg/dL | 974 | 56.60 | 413 | 50.67 | |
| Post‐meal glucose levels | |||||
| ADA 1,<70 mg/dL | 133 | 8.15 | 55 | 7.05 | <.001 |
| ADA 2, 70–180 mg/dL | 1045 | 64.03 | 567 | 72.69 | |
| ADA 3, >180 mg/dL | 454 | 27.82 | 158 | 20.26 | |
| Pre‐dinner glucose levels | |||||
| ADA 1, <70 mg/dL | 62 | 3.75 | 43 | 5.40 | <.001 |
| ADA 2, 70–120 mg/dL | 554 | 33.54 | 359 | 45.04 | |
| ADA 3, >120 mg/dL | 1036 | 62.71 | 395 | 49.56 | |
| After‐dinner glucose levels | |||||
| ADA 1, <70 mg/dL | 95 | 6.65 | 21 | 2.96 | <.001 |
| ADA 2, 70–180 mg/dL | 918 | 64.29 | 540 | 76.16 | |
| ADA 3, >120 mg/dL | 415 | 29.06 | 148 | 20.87 | |
| Early morning glucose levels | |||||
| ADA 1, <70 mg/dL | 71 | 4.51 | 24 | 4.49 | .001 |
| ADA 2, 70–180 mg/dL | 1067 | 67.70 | 405 | 75.70 | |
| ADA 3, >180 mg/dL | 438 | 27.79 | 106 | 19.81 | |
Note: P‐values in bold: Statistically significant (P = 0.001), corresponds to the three categories.
Number of glycometrics by month in the total population evaluated.
3.3. Effect of intervention on HbA1C levels
Statistically significant reduction in HbA1C levels was seen when all age groups were included: average reduction of 1.8% (P‐value = .018). This reduction was −1.5% in the <8 years age group, −2.3% in the 8 to 13 years age group, and − 1.4% for the >13 age group; however, the reductions by age group did not reach statistical significance (P‐values: .157 (<8 years), 0.087 (8‐13 years), and .135 (>13 years)(Table 3), perhaps because of a reduction in statistical power due to smaller numbers in each category.
Table 3.
Effect of intervention on HbA1C levels in adolescent and child care program with diabetes mellitus (PAANDA) patients at baseline and 4 to 6 months after intervention
| First month | Fourth to sixth month | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | P‐value | |
| All participants | 10.01 | 2.47 | 8.18 | 2.26 | 0.018 |
| <8 years old | 9.66 | 2.32 | 8.18 | 2.26 | 0.157 |
| 8‐13 years old | 9.57 | 2.19 | 7.32 | 2.60 | 0.087 |
| >13 years old | 10.21 | 2.58 | 8.79 | 1.96 | 0.135 |
Note: P‐values in bold: Statistically significant.
3.4. Effect of time in the program on glycemic control
To determine, as a complementary outcome, the effect of the duration of intervention on glycemic control, a multivariate linear regression analysis was performed, including age at program entry, type of diabetes, gender, hospitalization, and diabetes duration as covariates. It was found that for each 30‐day treatment period, there was an average 13% increase in blood glucose levels in the normal range, which was statistically significant for all time points (Table 4). In addition to the general downward trend in blood glucose levels, there was a decrease in measurement variability, both for pre‐meal (Figure 1) and post‐meal (Figure 2) quantifications. Notably, no increase in the frequency of hypoglycemia was observed. Finally, a Kernel diagram clearly showed a decrease in measurements above 200 mg/dL and an increase in peak measurements close to normal levels (Figure 3).
Table 4.
Multivariable logistic regression model for the association between time in the program (every 30 days) and glycemic control in patients of the care program for adolescents and children with diabetes mellitus (PAANDA)
| Normal glucose | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| levels (ADA) | HR | 95% CI | P‐value | HR | 95% CI | P‐value |
| Before breakfast | 1.131 | (1.094, 1.162) | <.001 | 1.136 | (1.094, 1.162) | <.001 |
| After breakfast | 1.125 | (1.094, 1.162) | <.001 | 1.131 | (1.094, 1.162) | <.001 |
| Before lunch | 1.099 | (1.062, 1.127) | <.001 | 1.117 | (1.094, 1.162) | <.001 |
| After lunch | 1.159 | (1.127, 1.197) | <.001 | 1.172 | (1.127, 1.197) | <.001 |
| Before dinner | 1.127 | (1.094, 1.162) | <.001 | 1.144 | (1.127, 1.161) | <.001 |
| After dinner | 1.157 | (1.127, 1.197) | <.001 | 1.160 | (1.127, 1.197) | <.001 |
Note: Adjusted by age at start of program, type of diabetes, gender, hospitalization, and time with illness. Before meals: 70‐120 mg/dL; after meals: 70‐180 mg/dL.
Abbreviations: ADA, American Diabetes Association; HR, hazards ratio; 95% CI, 95% confidence Interval.
Figure 1.
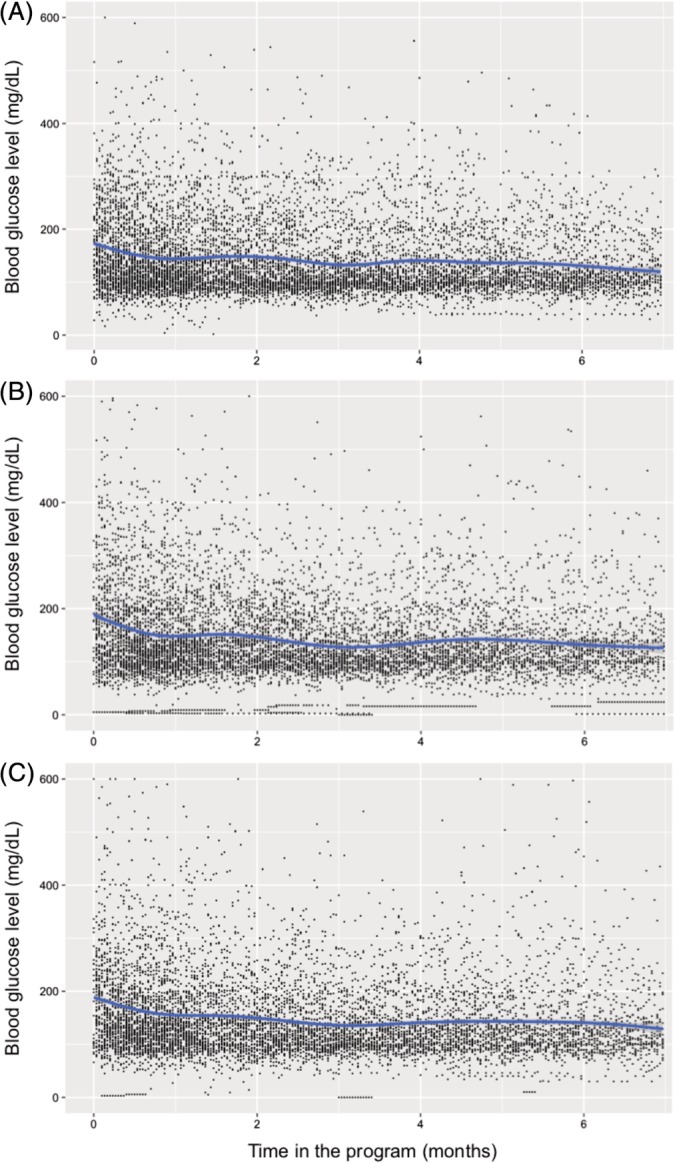
Conditional mean smoothed for the effect of time in the program on glucose levels before: A, breakfast, B, lunch, and C, dinner, in children and adolescents with diabetes mellitus treated within the PAANDA (Care Program for Adolescents and Children with Diabetes Mellitus) program
Figure 2.

Conditional mean smoothed for the effect of time in the program on glucose levels, after: A, breakfast, B, lunch, and C, dinner, in children and adolescents with diabetes mellitus treated within the Care Program for Adolescents and Children with Diabetes Mellitus (PAANDA) program
Figure 3.
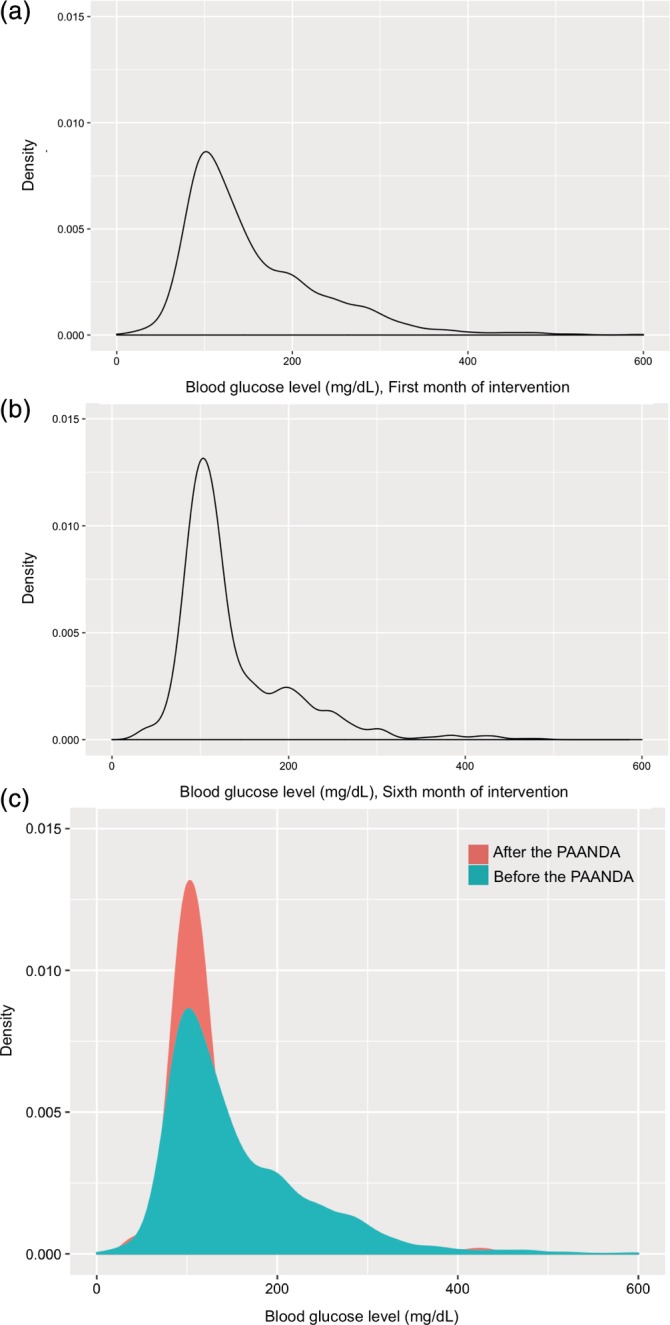
Kernel curve for the effect of the Care Program for Adolescents and Children with Diabetes Mellitus (PAANDA) program in children and adolescents with diabetes mellitus on glucose levels. A, first month of treatment, B, sixth month of treatment, and C, first and sixth month combined
4. DISCUSSION
This study showed that an interdisciplinary intervention that included medical, psychological, nutritional, social, diabetes education, and other interventions improve not only glycemic levels, but also glycemic variability, and HbA1C levels within 6 months. Despite advances in insulin preparations, insulin delivery devices, and new blood glucose monitoring technologies, glycemic control in most patients with T1DM remains sub‐optimal. In this context, multidisciplinary interventions represent a good opportunity to improve glycemic control, especially where new technologies are not available for any reason—high costs, logistics, etc. One of the authors of this program (F.R.) has suggested that they be included in more recent national recommendations for the treatment of DM in young people.12 Although empowering patients through multidisciplinary interventions will allow them to optimize resources to improve diabetes control, and avoid acute and chronic complications, appreciation of the costs of the multidisciplinary interventions themselves, for example, the cost of training and making available the manpower involved, in relation to new technologies, which requires further research.
It is important to reduce glycemic variability because it is well known that it is an important predictor of hypoglycemia13, 14 and correlates with poor glycemic control.14 Glycemic variability has a strong association with the thickening of the carotid intima‐media, with the risk of micro‐vascular complications,15 and is an independent predictor of patient satisfaction with an intensive insulin regimen, independent of other glycemic control measures.16 In addition to the decrease in blood glucose levels, it was clearly observed in this study that there was also a significant decrease in its variability over the period evaluated. This suggests that patients in the PAANDA program may benefit from additional changes that contribute to better long‐term management.
Data from the T1D Exchange Clinical Registry (T1D Ex), which maintains records of 26 000 T1DM patients in the United States, indicate that the average HbA1C in patients under 25 years of age ranges from 8.3% to 8.7%.17 This study showed an average of 8.18% during the sixth month, with the intervention of the PAANDA program having significantly reduced HbA1C by an average of 2.6%. The improved blood glucose monitoring and lower HbA1C show better glycemic control after the intervention, even though the mean HbA1C of 8.18% is higher than the recommended levels (<7%).
The T1D Ex has identified that, in patients with T1DM, the use of CGM and frequent SBGM, as well as the use of insulin pumps, white race, family income, higher patient or parental education, and private insurance, are factors associated with better glycemic control.17 The population assessed in this study tends to be homogeneous from the socio‐demographic point of view. However, there were internal factors, such as shortage of insulin supplies, whose impact on glycemic control in these patients should be examined, and which will be evaluated in future studies. In this study, we did not calculate any changes in insulin requirements, either in terms of their effect on clinical outcomes or the cost of treatment; these aspects will be published in subsequent analyses.
SBGM, which began with the introduction of glucose meters during the 1980s, had a major effect on the control of T1DM. Multiple studies have shown a good correlation between the frequency of self‐monitoring and glycemic control.18 In this study, the main tool for glycemic control tool was self‐monitoring, which showed a significant improvement in almost all parameters evaluated. It is striking to note that the number of blood glucose tests performed is more than the number of suggested tests in countries with higher income levels (four times a day in Sweden).19 PAANDA, with its strong educational component, could contribute to better control, by improving HbA1C and time in range, by optimizing resources such as limited test strips. However, the ability to do self‐monitoring is limited by the availability of test strips, their cost, and, the lack of coverage by some insurers.20 Further studies are needed to determine the relevance of these factors within this type of multidisciplinary programs.
We also observed significant changes in the number of absolute blood glucose measurements after the sixth month of intervention, for example, the absolute number of glucose measurements before breakfast increased from 557 at baseline to 1092 at 6 months, although in other time slabs, it decreased substantially. These changes in the number of glucose measurements may reflect lower compliance status in our patients, due to families' economic constraints, in spite of the medical advice given in the program. The influence of socio‐economic factors will be assessed in future studies with this group of patients.
This study presents notable limitations. For example, there was a significant loss during follow‐up, which could suggest a potential bias in the conclusions. The study evaluates a program implemented for the first level of public attention that collected real‐world data. Differences in the number of measurements and the number of patients at the beginning and end are also evident. The study showed more patient measurements in the first month. In addition, the cross‐sectional analysis considered only those patients who reached the sixth month of the intervention, while many were at that time in month two, three, four, and five. Another key parameter to confirm better control is the presence of hypoglycemic or ketoacidosis events. Because of the before vs after design, our study was unable to determine whether the intervention improved these outcomes, although all the parameters suggest a metabolic improvement.
Other limitations of the study are: (a) Patients usually lack sufficient economic resources, which prevent the availability of adequate test strips for regular testing (ie, families had to pay for the test strips out of their pocket). Other patient factors which interfere with regular monitoring include denial of the disease and refusal of the patient to be monitored; (b) Diagnostic inputs: the availability of laboratory inputs is variable, which affects the frequency of HbA1C measurements; (c) Commercial aspects: patients use multi‐brand meters, which they choose and pay for themselves, so the variability of the results obtained increases. However, despite all the above limitations, this is one of the first studies to collect and analyze data under real‐world conditions, based on which clinical decisions can be made, and which go beyond the perspective of a clinical trial, where many of the factors described are controlled. These factors, particularly poverty, lack of availability of adequate test strips, denial of the disease, and refusal of the patient to be monitored, etc. have to be tackled by all health care teams to varying degrees. The potential influence of those factors needs further research.
Most children and adolescents with DM have suboptimal glycemic control. This can be attributed to the demanding nature of the disease, the presence of long‐term complications, self‐care capacity and associated costs, but there are also factors that can be improved, such as knowledge of the disease, physical activity, diet, and self‐care. The present study has shown that multidisciplinary intervention involving medical, nutritional, social, educational, and other interventions can improve glycemic levels, glycemic variability, and HbA1C levels. Finally, it is necessary to mention that further studies are needed on the effect of interventions such as PAANDA on other equally relevant areas such as quality of life and the cost‐benefit ratios.
CONFLICT OF INTEREST
J.G.H., E.G., M.C., M.V.C, D.C., A.V., Z.C., M.S., M.R., M.D., A.M.M., J.G.G., D.C.L., and D.P. declare that they have no conflicts of interest. F.R.M. has been a speaker for Merck, Medtronic and ROCHE, as well as principal investigator for Boeringher Ingelhaim.
AUTHOR CONTRIBUTIONS
F.R.M. designed the study. J.G.H., E.G., M.C., M.V.C., D.C., A.V., Z.C., M.S., M.R., M.D., A.M.M., and F.R.M contributed with the interventions from the PAANDA program. J.G.G., D.C.L., D.P., and F.R.M clean the datasets, run the statistical analysis, and wrote the manuscript.
ACKNOWLEDGEMENTS
The authors thank Novo Nordisk México S.A. de C.V., for the financial support necessary for the development of this study and declare that: (a) they have no conflict of interest other than having received the sponsorship of Novo Nordisk México S.A. de C.V., and (b) the design of the study, the collection and analysis of data, as well as the drafting of this document and the decision to submit it for publication, were carried out under absolute autonomy.
Ramírez‐Mendoza F, González JE, Gasca E, et al. Time in range and HbA1C after 6 months with a multidisciplinary program for children and adolescents with diabetes mellitus, real world data from Mexico City. Pediatr Diabetes. 2020;21:61–68. 10.1111/pedi.12921
Peer Review The peer review history for this article is available at https://publons.com/publon/10.1111/pedi.12921.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Gomes MB, Coral M, Cobas RA, et al. Prevalence of adults with type 1 diabetes who meet the goals of care in daily clinical practice: a nationwide multicenter study in Brazil. Diabetes Res Clin Pract. 2012;97(1):63‐70. [DOI] [PubMed] [Google Scholar]
- 2. Dean AJ, Walters J, Hall A. A systematic review of interventions to enhance medication adherence in children and adolescents with chronic illness. Arch Dis Child. 2010;95(9):717‐723. [DOI] [PubMed] [Google Scholar]
- 3. Magalhães TPC, Fóscolo RB, Soares AN, Reis JS. Type 1 diabetes mellitus: can coaching improve health outcomes? Arch Endocrinol Metab. 2018;62(4):485‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Zoysa N, Rogers H, Stadler M, et al. A psychoeducational program to restore hypoglycemia awareness: the DAFNE‐HART pilot study. Diabetes Care. 2014;37(3):863‐866. [DOI] [PubMed] [Google Scholar]
- 5. Hood KK, Iturralde E, Rausch J, Weissberg‐Benchell J. Preventing diabetes distress in adolescents with type 1 diabetes: results 1 year after participation in the STePS program. Diabetes Care. 2018;41(8):1623‐1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berger B, Sethe D, Hilgard D, Martin D, Heusser P. Design of a Self‐Management Program for children aged 6‐12 years with type 1 diabetes mellitus at the community hospital Herdecke, Germany. Complement Med Res. 2017;24(4):255‐263. [DOI] [PubMed] [Google Scholar]
- 7. Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008‐2010. JAMA. 2015;313(1):37‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beck RW, Bergenstal RM, Riddlesworth TD, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsch IB, Sherr JL, Hood KK. Connecting the dots: validation of time in range metrics with microvascular outcomes. Diabetes Care. 2019;42(3):345‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. J Diabetes Sci Technol. 2013;7(2):562‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vigersky RA. Escaping the Hemoglobin A1c‐centric world in evaluating diabetes mellitus interventions. J Diabetes Sci Technol. 2015;9(5):1148‐1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Monnier L, Colette C, Leiter L, et al. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2007;30(1):185‐186. author reply 187–188. [DOI] [PubMed] [Google Scholar]
- 13. Qu Y, Jacober SJ, Zhang Q, Wolka LL, DeVries JH. Rate of hypoglycemia in insulin‐treated patients with type 2 diabetes can be predicted from glycemic variability data. Diabetes Technol Ther. 2012;14(11):1008‐1012. [DOI] [PubMed] [Google Scholar]
- 14. Rodbard D, Bailey T, Jovanovic L, Zisser H, Kaplan R, Garg SK. Improved quality of glycemic control and reduced glycemic variability with use of continuous glucose monitoring. Diabetes Technol Ther. 2009;11(11):717‐723. [DOI] [PubMed] [Google Scholar]
- 15. Esposito K, Ciotola M, Carleo D, et al. Post‐meal glucose peaks at home associate with carotid intima‐media thickness in type 2 diabetes. J Clin Endocrinol Metab. 2008;93(4):1345‐1350. [DOI] [PubMed] [Google Scholar]
- 16. Testa MA, Gill J, Su M, Turner RR, Blonde L, Simonson DC. Comparative effectiveness of basal‐bolus versus premix analog insulin on glycemic variability and patient‐centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J Clin Endocrinol Metab. 2012;97(10):3504‐3514. [DOI] [PubMed] [Google Scholar]
- 17. Beck RW, Tamborlane WV, Bergenstal RM, et al. The T1D exchange clinic registry. J Clin Endocrinol Metab. 2012;97(12):4383‐4389. [DOI] [PubMed] [Google Scholar]
- 18. Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self‐monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009‐2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moström P, Ahlén E, Imberg H, Hansson P‐O, Lind M. Adherence of self‐monitoring of blood glucose in persons with type 1 diabetes in Sweden. BMJ Open Diabetes Res Care. 2017;5(1):e000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pollack A. A panel decides Washington State's health care costs. The New York Times 2011; Accessed November 23, 2018. https://www.nytimes.com/2011/03/22/business/22care.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


