Summary
Plants exist in an environment of changing abiotic and biotic stresses. They have developed a complex set of strategies to respond to these stresses and over recent years it has become clear that sphingolipids are a key player in these responses. Sphingolipids are not universally present in all three domains of life. Many bacteria and archaea do not produce sphingolipids but they are ubiquitous in eukaryotes and have been intensively studied in yeast and mammals. During the last decade there has been a steadily increasing interest in plant sphingolipids. Plant sphingolipids exhibit structural differences when compared with their mammalian counterparts and it is now clear that they perform some unique functions. Sphingolipids are recognised as critical components of the plant plasma membrane and endomembrane system. Besides being important structural elements of plant membranes, their particular structure contributes to the fluidity and biophysical order. Sphingolipids are also involved in multiple cellular and regulatory processes including vesicle trafficking, plant development and defence. This review will focus on our current knowledge as to the function of sphingolipids during plant stress responses, not only as structural components of biological membranes, but also as signalling mediators.
Keywords: abiotic stress, biotic stress, pathogens, plant defence, programmed cell death, sphingolipid
Introduction
The strategies that plants employ to endure stressful conditions are varied and involve a multitude of molecular, metabolic and physiological adaptations. There is now a significant body of work to indicate that sphingolipids are an important part of the arsenal of tools the plant has at its disposal to respond to stress. Sphingolipids are an incredibly diverse group of compounds (Pata et al., 2010) with a vast array of physical properties that facilitate their function in a variety of cellular processes. Sphingolipids form a significant proportion of the lipids present in higher plants. Studies suggest sphingolipids constitute up to 40% of lipids in the plasma membrane of plant cells (Cacas et al., 2016) and are enriched in the endosomes and tonoplasts (Moreau et al., 1998). More comprehensive extraction techniques have been developed over recent years that, when coupled with technological advances in mass spectrometry and chromatography, have allowed improved sphingolipid identification and the discovery of novel structures from smaller quantities of material (Cacas et al., 2016). This situation has enabled researchers to determine the contribution that sphingolipid metabolites make in different cellular processes.
An overview of the sphingolipid biosynthetic pathway is presented in Fig. 1. The term sphingolipid covers a class of lipids whose defining component is a long‐chain or sphingoid base (LCB; for ease of reference, Supporting Information Table S1 lists the abbreviations used in this review). The LCB is a carbon amino‐alcohol backbone most commonly of 18 carbons that is synthesised by the condensation of serine and palmitoyl‐CoA catalysed by serine palmitoyl transferase (SPT) in the endoplasmic reticulum (ER) (Chen et al., 2006). The product of this reaction, 3‐ketosphinganine, is then reduced by the action of the 3‐ketosphinganine reductase to sphinganine (d18:0) (Beeler et al., 1998). The LCB is considered the simplest functional sphingolipid and can have a range of modifications including phosphorylation, desaturation and hydroxylation. It is sometimes referred to as the free LCB. The LCB may be linked to a very‐long‐chain fatty acid via an amide bond to form a ceramide. The fatty acyl component is usually 16–26 carbons. This reaction is catalysed by ceramide synthase. In Arabidopsis thaliana (hereafter Arabidopsis) three ceramide synthases have been identified, LOH1–3. Ceramidases catalyse the reverse reaction and are a component in regulating the ceramide pool and sphingolipid homeostasis (Pata et al., 2008). Ceramides can be phosphorylated in the endoplasmic reticulum (ER) by ceramide kinases (CerK) or ACD5 (accelerated cell death 5) or further modified to form the complex sphingolipids glycosylceramides (GlcCers) in the ER and glycosyl inositol phosphorylceramides (GIPCs) by the addition of simple or multiple sugars on ceramide at the C1 position in the Golgi. These reactions are catalysed by glucosylceramide synthase (GCS) and at least three functional IPC‐synthases and several glycosyl or glucuronyl transferases (Wang et al., 2008; Mina et al., 2010; Rennie et al., 2014; Msanne et al., 2015). The complex sphingolipids can exhibit very high levels of sugar decoration. One study of 23 plant species identified at least 21 different patterns showing variation in number, type and order of glycan substitutions (Cacas et al., 2013). The biosynthesis of complex sphingolipids is tightly controlled and the GIPC pool is regulated by the hydrolysis of GIPC to phytoceramide‐1 phosphate by the action of a phospholipase D (PLD) (Tanaka et al., 2013). Functional characterisations of enzymes of the sphingolipid biosynthetic pathway have also pointed to the controls on the pathway and the specific pool sizes and structures that are generated. This flexibility enables sphingolipids to constitute both a structural membrane component and a signalling molecule from the same basic lipid backbones. For more details about sphingolipid biosynthesis, see the recent reviews by Luttgeharm et al., 2016; Michaelson et al., 2016 and Mamode Cassim et al., 2019.
Figure 1.
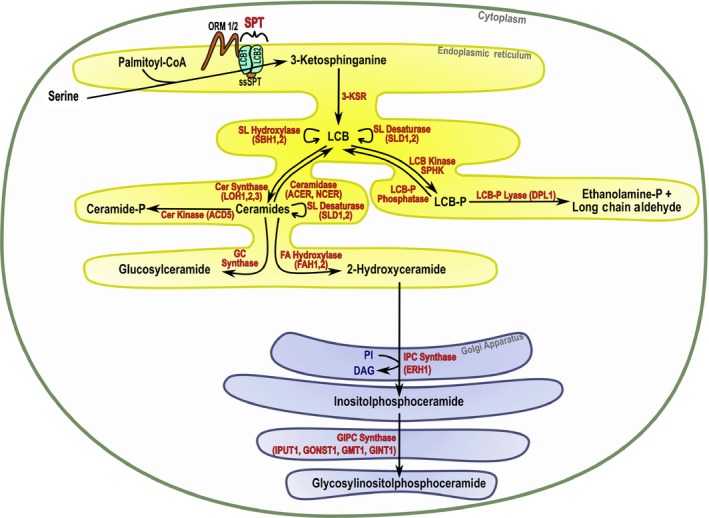
Schematic representation of the sphingolipid biosynthetic pathway in plants. 3‐KSR, 3‐ketosphinganine reductase; ACD5, accelerated cell death 5; ACER, alkaline ceramidase; Cer, ceramide; ceramide‐P, ceramide‐phosphate; CoA, coenzyme A; DAG, diacylglycerol; DPL1, dihydrosphingosine phosphate lyase; ERH1, enhancing RPW8‐mediated HR‐like cell death; FA, fatty acid; FAH, fatty acid hydroxylase; GC, glucosylceramide; GINT1, glucosamine inositol phosphorylceramide transferase 1; GIPC, glycosyl inositol phosphoceramide; GMT1, GIPC mannosyl‐transferase 1; GONST1, Golgi localized nucleotide sugar transporter 1; IPC, inositol phosphorylceramide; IPUT, inositol phosphorylceramide glucuronosyltransferase 1; LCB1,2, subunit of serine palmitoyltransferase 1 and 2; LCB, long‐chain base; LCB‐P, long‐chain base phosphate; LOH, LAG1 homolog; NCER, neutral ceramidase; ORM, orosomucoid‐like protein; PI, phosphoinositol; SBH, sphingoid base hydroxylase; SL, sphingolipid; SLD, sphingolipid Δ8 long‐chain base desaturase; SPHK, sphingosine kinase; ssSPT, small subunit of serine palmitoyl transferase; SPT, serine palmitoyl transferase.
In plants, the size of the different sphingolipid pools tends to vary in a species‐specific and tissue‐dependent manner. For example, the occurrence of the LCB d18:2 containing GlcCer in Arabidopsis is mainly confined to floral and pollen tissue (Michaelson et al., 2009) and sphingolipid distribution changes during fruit development and ripening (Ines et al., 2018). However outside the Brassicaceae family d18:2 production occurs throughout the plant and, in species such as tomato and soybean, it is the most abundant GlcCer (Markham et al., 2006). Wheat was found to contain much higher levels of d18:1 in its LCBs when compared with rice (Goto et al., 2012). In addition, the different tissues in rice have been found to contain a similar quantity of sphingolipids, but distribution across the lipid classes was altered. A survey of 21 different plant species from different phylogenetic groups found d18:1Δ4 to be present in nonseed land plants and monocots but absent from Arabidopsis and soybean (Islam et al., 2012).
The functional significance of variations in sphingolipid chemical diversity and abundance is still in the early stages of investigation. The different classes and modifications offer a variety of differing solubility, charge, shape and size. It is this array of properties that confers the potential of sphingolipids to function both as bio‐active components of cells involved in regulating cellular processes and as integral components involved in the structural integrity of the membranes. Regulation of sphingolipid metabolism enables plants to facilitate cell growth and to appropriately respond to stress, both biotic and abiotic, using different metabolites to modulate its response.
Here, we summarise our current knowledge on the role of sphingolipids in plants in response to environmental cues and stress.
Signals in programmed cell death
Recent work utilising genetically altered plants and plants exposed to sphingolipid biosynthesis inhibitors have revealed that sphingolipids are regulators of programmed cell death (PCD) occurring either during plant development or immunity. Perception of a stress often occurs at the plasma membrane level. Therefore its integrity is essential for cell signalling and survival. Sphingolipids are major structural constituents of plant plasma membrane microdomains and their relationship with other components of the plasma membrane is crucial. Changes in sphingolipid biosynthesis therefore affect the microdomain composition and this could affect protein content and distribution due to altered interactions between plasma membrane components. For example, Bax‐inhibitor‐1 (AtBI‐1, an inhibitor of Bax‐induced cell death) interacts with both FAH1 and FAH2 (fatty acid 2‐hydroxylase). Plants overexpressing AtBI‐1 therefore displayed enrichment in 2‐hydroxy fatty acid‐containing GlcCer in microdomains as well as a loss of two proteins that are usually specifically localised to microdomains (Ishikawa et al., 2015). These two proteins feature in plant defence, both being involved in cell death triggered by salicylic acid (SA) or oxidative stress. This reduction in protein content led to an enhanced tolerance to SA or oxidative stress in AtBI‐1‐overexpressing plants (Ishikawa et al., 2015). These data suggest that the integrity of microdomains is critical to cell death and sphingolipids are central to these structures.
Sphingolipids are involved in the control of PCD, either as structural components of membranes but also as initiators in the cell death regulatory pathway. The existence of a rheostat between ceramides/LCBs and their phosphorylated counterparts, already described in animal cells, is thought to exist in plants and similarly to control cell fate. According to this model, ceramides and LCBs are able to trigger cell death, whereas ceramide phosphates and LCB‐Ps promote cell survival (Shi et al., 2007; Alden et al., 2011) (Fig. 2). The induction of PCD by LCB was based on the activation of protein kinases, MPK6 (Saucedo‐Garcia et al., 2011) or 14‐3‐3‐regulated CPK3 (Lachaud et al., 2013). The spontaneous PCD observed in the acd5 mutant, defective in ceramide kinase and with enhanced levels of ceramides, was due to a strong accumulation of mitochondrial reactive oxygen species (ROS) (Bi et al., 2014). This finding suggests that ROS are a component of sphingolipid‐induced PCD. The mycotoxin fumonisin B1 (FB1) has been widely used to study both sphingolipid biosynthesis and PCD. Indeed, FB1 is a strong inhibitor of ceramide synthase and has been shown to induce PCD. When applied to plants, FB1 also triggered the accumulation of LCBs and LCB‐Ps (Shi et al., 2007; Tsegaye et al., 2007; Saucedo‐Garcia et al., 2011; Yanagawa et al., 2017). Overexpression of AtLCBK1 (Arabidopsis sphingoid LCB kinase) in a recent study in plants induced resistance to FB1 treatment and, conversely, AtLCBK1 knockdown plants exhibited a sensitivity to such a treatment (Yanagawa et al., 2017). Moreover, the authors demonstrated that transgenic alteration of proteins involved in LCB/LCB‐P homeostasis (AtLCBK1, AtSPP1 and AtDPL1) resulted in a positive correlation between the levels of free LCBs and the degree of FB1‐induced cell death (Yanagawa et al., 2017).
Figure 2.
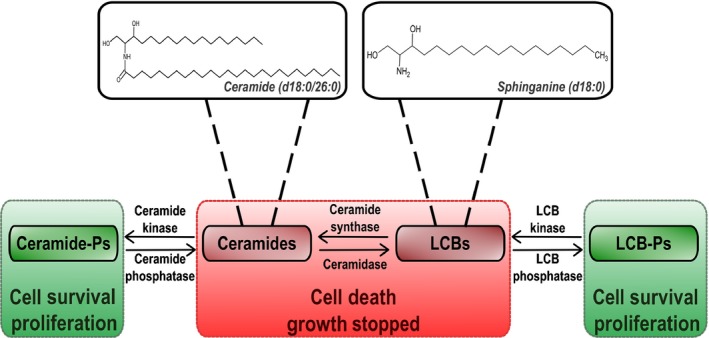
Sphingolipid rheostat. The equilibrium between ceramides/long‐chain bases (LCBs) and ceramide phosphates (ceramide‐Ps)/LCB‐Ps defines cell fate.
Increase in SPT activity, by overexpression of AtssSPTa (small subunit of SPT), resulted in an accumulation of LCBs and reduced tolerance to FB1, whereas AtssSPTa suppression lines displayed lower levels of LCBs but enhanced tolerance to FB1 (Kimberlin et al., 2013). It was recently demonstrated by two independent studies that orosomucoid‐like proteins AtORM1 and AtORM2 physically interact with the core SPT complex and function as a repressor of SPT activity (Kimberlin et al., 2016; Li et al., 2016). ORM proteins therefore regulate sphingolipid homeostasis by differently modulating functionally different ceramide synthase activities (Kimberlin et al., 2016). AtORM1 and AtORM2 overexpressing plants were more tolerant to FB1 treatment when compared with wild‐type (WT) plants. This tolerance is accompanied by a lower accumulation of C16 ceramides, LCBs and their phosphorylated counterparts. Conversely, AtORM RNAi lines were more sensitive to such treatment, and displayed higher content of C16 ceramides, LCBs and LCB‐Ps (Kimberlin et al., 2016). Similarly, the ceramide synthase LOH2 overexpressing lines resulted in the accumulation of ceramides containing C16 fatty acids and dihydroxy LCBs and had reduced accumulation of free LCBs and LCB‐Ps in response to FB1. This overexpression also resulted in constitutive induction of PCD and increased resistance to FB1 (Luttgeharm et al., 2015). These findings suggested that FB1‐induced PCD is primarily due to the accumulation of free LCBs rather than the accumulation of ceramides containing C16 fatty acids/dihydroxy LCBs. Curiously, growth and increased cell division were promoted in LOH1 and LOH3 overexpressing plants, which displayed enhanced production of ceramides with very‐long‐chain fatty acids (VLCFAs) and trihydroxy LCBs (Luttgeharm et al., 2015). These unexpected outcomes for growth and development could be due to a ceramide synthesis with a certain chain length fatty acid and quantity and in response to the correct stimuli. It is also known that VLCFA ceramides are important for Golgi trafficking and cell plate or phragmoplast formation during cell division in Arabidopsis (Molino et al., 2014). It is therefore possible that increased cell expansion could be due to sphingolipid targeting to plant membranes that contributes directly to cell expansion. In addition, the fatty acid hydroxylase double mutant fah1/fah2 fails to form spontaneous lesions under standard culture conditions, despite an accumulation in free trihydroxy LCBs, C16 ceramides and VLCFA ceramides and SA (König et al., 2012). Moreover, the gonst1 (Golgi localised nucleotide sugar transporter1, involved in glycosylation of GIPCs) mutant displayed spontaneous hypersensitive reaction (HR)‐like lesions but did not accumulate ceramides or LCBs (Mortimer et al., 2013). One potential explanation for these observed differences is that several different mechanisms could be responsible for inducing cell death.
Sphingolipids as structural components in response to abiotic stress
Several studies have recently reported a role for sphingolipids in response to temperature stress. Acclimation capacity was correlated with changes in the content of TAGs (triacylglycerols), MGDG (monogalactosyldiacylglycerol), DGDG (digalactosyldiacylglycerol) and a GlcCer (Degenkolbe et al., 2012). Analysis of oat, rye and Arabidopsis lipid profiles during cold acclimation demonstrated that GlcCer contents decreased in the plasma membrane, whereas they were unchanged in microdomains (Minami et al., 2009; Takahashi et al., 2016). These changes could contribute to a greater hydration of the plasma membrane that could, in turn, increase membrane stability during cold stress. In a study focusing on grapevine leaves, it was found that high levels of t18:1 (8Z) in complex sphingolipids were correlated with freezing tolerance (Kawaguchi et al., 2000). The sphingolipid Δ8 long‐chain base desaturases (SLD), which desaturate the LCB at the Δ8 position in both cis and trans orientations, appear to play a role in cold tolerance in Arabidopsis (Chen et al., 2012) and tomato (Zhou et al., 2016). In Arabidopsis, the sld1sld2 double mutant is sensitive to cold stress (Chen et al., 2012). Similarly, SlSLD knockdown tomato plants displayed greater membrane damage and physiological indicators of chilling damage after stress than WT plants. Chloroplasts are the main organelle affected by cold and many studies have reported that chloroplast morphology is affected by changes in lipid unsaturation. Chloroplasts in SlSLD knockdown were more severely damaged than in WT plants and the surviving organelles were not surrounded by an extra membrane (Zhou et al., 2016). GlcCers, believed to stabilise membranes, were detected in the envelope membrane of chloroplasts (Spassieva & Hille, 2003), suggesting that sphingolipids are structurally important for chloroplast membrane for cold tolerance. This finding illustrated that disrupting SlSLD transcript accumulation reduced chilling tolerance of tomato. Lipid desaturation is a way for plants to mitigate the effects of chilling or freezing temperatures. SlSLD knockdown plant sensitivity to chilling could therefore be related to membrane properties such as fluidity, which is diminished due to depletion of sphingolipids with unsaturated LCBs. Another explanation for the decrease in cold tolerance could be a change in the formation and content of microdomains in the membrane. It is conceivable that activity of some microdomain‐localised proteins important for cold tolerance could be modified in perturbed microdomains (Chen et al., 2012). There has been no characterised function for sphingolipids in tolerance of high temperature by contrast with the high concentration of trienoic fatty acids in the thylakoid membranes that have been shown to be involved in both chilling and high temperature tolerance (Murakami et al., 2000; Routaboul et al., 2012; Tovuu et al., 2016).
Sphingolipids as structural components in response to biotic stress
The rice Osfah1/2 plants displayed similar SA levels to WT and a decreased tolerance to the hemibiotrophic fungus Magnaporthe oryzae. Nagano and colleagues demonstrated that products of these enzymes, 2‐hydroxy‐sphingolipids, were critical in the formation of microdomains and disruption of OsFah1/2 activity disturbed organisation of defence proteins localized in these microdomains, such as the NADPH oxidase RbohB, required for ROS production involved in rice immunity (Nagano et al., 2016).
Recent work has identified three genes involved in GIPC glycosylation: GONST1, IPUT1 (inositol phosphorylceramide glucuronosyltransferase1) and GMT1 (GIPC mannosyl‐transferase1) (Mortimer et al., 2013; Fang et al., 2016; Tartaglio et al., 2017). These three mutants displayed high SA and ROS levels coupled to a constitutive HR and defence‐gene induction, suggesting a constitutive biotic stress response. Interestingly, gmt1 also had a decrease in cellulose accompanied by an increase in lignin content, a well known process in disease resistance.
Eudicot plant‐specific GIPCs appeared to act as NLP (necrosis and ethylene‐inducing peptide 1‐like protein) cytolysin receptors (Lenarcic et al., 2017). NLP are produced by bacterial, fungal and oomycete plant pathogens. Monocots did not develop necrotic lesions upon challenge with NLP. The difference between the two clades resides in the length of terminal hexose residues in GIPCs (two for eudicots and three for monocots). The GIPC sugar moiety is exposed at the surface of the plasma membrane and is therefore accessible to NLP binding. The presence of a third hexose unit in monocots impeded NLP insertion into the plasma membrane. The structural and molecular consequences for the plasma membrane that could occur downstream of this recognition requires further study. These studies demonstrate that GIPC glycosylation and the identity of the glycan headgroup are important for the plant immune response.
Sphingolipids as signalling messengers in abiotic stress
The sessile nature of plants has driven them to develop a myriad of strategies to resist cell damage. Abiotic stress affects plant growth and development, resulting in loss of vigour and ultimately death. The altered physical and chemical composition of cell membranes under temperature, salt stress or hypoxia is a problem the plant must manage. As a major component of plasma membranes, sphingolipids are significant in mitigating abiotic stress, both in plasma membrane remodelling, and as signal transduction molecules (Ali et al., 2018). A summary of the available data on the enzymes and genes of the sphingolipid pathway involved in response to both abiotic and biotic stress is presented in Table 1.
Table 1.
Enzymes and genes of sphingolipid metabolism involved in response to (a)biotic stress.
| Enzyme | Name | Mutant/transgenic plants | Phenotype under (a)biotic stress | References |
|---|---|---|---|---|
| Sphingolipid ∆8 long‐chain base desaturases | SLD | sld1sld2 (Arabidopsis) | Sensitive to cold | Chen et al. (2012) |
| SlSLD‐KD (tomato) | Sensitive to chilling | Zhou et al. (2016) | ||
| Long‐chain base kinase | LCBK1 | lcbk1 (Arabidopsis) | Freezing tolerant | Huang et al. (2017) |
| lcbk1‐KD (Arabidopsis) | Sensitive to FB1 treatment | Yanagawa et al. (2017) | ||
| OsLCBK1‐OE (rice) | Tolerance to oxidative stress | Zhang et al. (2013) | ||
| AtLCBK1‐OE (Arabidopsis) | Tolerance to FB1 treatment | Yanagawa et al. (2017) | ||
| Long‐chain base kinase | LCBK2 | lcbk2 (Arabidopsis) | Tolerance to intermediate cold (12°C) | Dutilleul et al. (2012) |
| Long‐chain base kinase | SPHK1 | SPHK1‐OE (Arabidopsis) | Sensitive to ABA treatment | Worrall et al. (2008) |
| Ceramide kinase | ACD5 | acd5 (Arabidopsis) | Seed germination sensitive to cold | Dutilleul et al. (2015) |
| Tolerance to powdery mildew | Wang et al. (2008) | |||
| Susceptibility to B. cinerea | Bi et al. (2014) | |||
| Ceramide synthase | LOH1LOH2LOH3 | loh1, loh2, loh3 (Arabidopsis) | Sensitivity to dark submergence | Xie et al. (2015a) |
| loh1‐1 loh3‐1 (Arabidopsis) | Sensitivity to dark and light submergence | Xie et al. (2015a) | ||
| LOH2‐OE (Arabidopsis) | Tolerance to FB1 treatment | Luttgeharm et al. (2015) | ||
| Neutral ceramidase | nCER1 | ncer1 (Arabidopsis) | Sensitivity to oxidative stress | Li et al. (2015) |
| nCer1‐OE (Arabidopsis) | Tolerance to oxidative stress | Li et al. (2015) | ||
| Alkaline ceramidase | AtACER | Atacer (Arabidopsis) | Sensitivity to oxidative stress | Zheng et al. (2018) |
| Susceptibility to P. syringae strain DG3 | Wu et al. (2015a) | |||
| Atacer, AtACER RNAi (Arabidopsis) | Sensitivity to salinity | Wu et al. (2015a) | ||
| AtACER‐OE (Arabidopsis) | Tolerance to salinity | Wu et al. (2015a) | ||
| Sphingosine‐1 phosphate lyase | OsSPL1 | OsSPL1‐OE (rice) | Sensitivity to salinity | Zhang et al. (2012) |
| Susceptibility to P. syringae pv. tabaci | Zhang et al. (2014) | |||
| Sphingoid phosphate phosphatase1 | AtSPP1 | Atssp1 (Arabidopsis) | Sensitive to ABA treatment | Nakagawa et al. (2012) |
| Dihydrosphingosine‐1‐phosphate lyase1 | AtDPL1 | Atdpl1 (Arabidopsis) | Susceptibility to P. syringae pv. tomato and tolerant to B. cinerea | Magnin‐Robert et al. (2015) |
| Fatty acid alpha‐hydroxylase | FAH1FAH2 | fah1/fah2 (Arabidopsis) | Tolerance to powdery mildew | König et al. (2012) |
| OsFah1/OsFah2 (rice) | Susceptibility to Magnaporthe oryzae | Nagano et al. (2016) | ||
| Enhancing RPW8‐mediated HR‐like cell death | ERH1 | erh1 (Arabidopsis) | Tolerance to powdery mildew | Wang et al. (2008) |
| Glucosamine inositol phosphorylceramide transferase1 | AtGINT1 | Atgint1 (Arabidopsis) | Tolerance to moderate salinity | Ishikawa et al. (2018) |
| Serine palmitoyltransferase | SPT | SPT‐silenced (tobacco) | Susceptibility to Alternaria alternata f. sp. lycopersici | Rivas‐San Vicente et al. (2013) |
| Small subunit of serine palmitoyltransferase | ssSPTa | AtssSPTa‐OE (Arabidopsis) | Sensitivity to FB1 treatment | Kimberlin et al. (2013) |
| AtssSPTa RNAi (Arabidopsis) | Tolerance to FB1 treatment | Kimberlin et al. (2013) | ||
| Subunit of serine palmitoyltransferase | LCB2a1 | OsLCB2a‐OE (rice) | Tolerance to Myzus persicae infestation | Begum et al. (2016) |
| Orosomucoid‐like proteins | ORM1ORM2 | orm1 amiR‐ORM2 (Arabidopsis) | Tolerance to P. syringae strain DG3 | Li et al. (2016) |
| Tolerance to oxidative stress | Li et al. (2016) | |||
| AtORM1‐OE, AtORM2‐OE (Arabidopsis) | Tolerance to FB1 treatment | Kimberlin et al. (2016) | ||
| AtORM1 RNAi, AtORM2 RNAi(Arabidopsis) | Sensitivity to FB1 treatment | Kimberlin et al. (2016) |
KD, knocked‐down; OE, overexpressing line.
Temperature stress
Sphingolipids are involved in cold acclimation as structural components of membranes and also as signalling molecules. In Arabidopsis WT plants, low temperatures trigger an accumulation of total sphingolipids, whereas the ratio of unsaturated LCBs is not increased by low temperatures (Nagano et al., 2014). This situation suggests that sphingolipids containing unsaturated LCBs are potential candidates for natural resistance to low temperatures but not for induced tolerance to cold. The cell death suppressor AtBI‐1 is involved in sphingolipid synthesis in response to cold by interacting with AtSLD1, AtFAH1, AtSBH2 (a LCB C‐4 hydroxylase) and AtADS2 (acyl lipid desaturase 2) through Arabidopsis cytochrome b5 (Nagano et al., 2014). Moreover, chilling induced a decrease in LCB production (especially t18:1) (Guillas et al., 2013). An Arabidopsis mutant exhibiting low levels of nitric oxide (NO) displayed an accumulation of t18:1. A rapid and transient production of t18:0‐P and ceramide phosphates is induced by cold. This accumulation was negatively regulated by NO (Cantrel et al., 2011) and was specifically impaired in lcbk2 (but not in lcbk1) or acd5 mutants, respectively (Dutilleul et al., 2012, 2015). Whether NO is able to directly regulate enzymes involved in LCB/LCB‐P and Cer/Cer‐P rheostat or their substrate availability is still unknown. lcbk2 displayed a constitutive activation of a cold‐responsive MAPK, AtMPK6, at 22°C. AtMPK6 activation was also stimulated by t18:0‐P treatment (Dutilleul et al., 2012). The expression of some cold‐responsive genes and phenotypical cold responses were impaired in the lcbk2 mutant but not in acd5. In addition, acd5 seed germination was hypersensitive to cold and abscisic acid (ABA), however gibberellic acid (GA) treatment reverted the acd5 germination phenotype at 4°C. Germination is regulated by ABA and GA, two hormones that function antagonistically. This finding suggests that defects in the ABA/GA balance and CerK activity could be responsible for acd5 seed hypersensitivity (Dutilleul et al., 2015). Therefore, some responses are regulated by phosphorylated sphingolipids, ABA and NO signalling during cold stress. Recent data have described a role for LCBK1 in Arabidopsis freezing tolerance (Huang et al., 2017). Typical responses including osmolyte accumulation, induction of cold‐ and membrane lipid‐related genes occurring during this abiotic stress are all impaired in the lcbk1 mutant. This situation suggested a fine‐tuned regulation in which LCBK1 acts as a signal in response to freezing temperatures and LCBK2 in response to chilling temperatures.
There are only a small number of studies indicating that sphingolipid metabolism is also involved in heat stress. It was shown that exogenous LCB‐phosphate contributed to heat stress tolerance in Arabidopsis cell culture (Alden et al., 2011). Moreover, a recent transcriptome analysis showed that AtSLD1 expression is significantly decreased in response to a combination of heat wave and drought at ambient and elevated CO2, mimicking global changes in climate (Zinta et al., 2018).
Hypoxia and oxidative stress
Hypoxia leads to an increase in ceramides, hydroxyceramides, GlcCers and GIPCs (Xie et al., 2015a,b). In hypoxic conditions, GIPCs are elevated in Arabidopsis and increased further in Atacbp3 (acyl‐CoA binding protein 3), whereas AtACBP3‐overexpressors were hypersensitive to submergence (Xie et al., 2015b; Lung & Chye, 2019). Similarly, a reduction of unsaturated VLC‐ceramides in loh1, loh2 and loh3 mutants due to the disruption of ceramide synthase is accompanied by an enhanced sensitivity to dark submergence. The loh1‐1 loh3‐1 double mutant displayed a reduction in unsaturated very‐long‐chain (VLC)‐ceramides and impaired tolerance to dark and light submergence. Unsaturated VLC ceramides are therefore seen as defence molecules for plant tolerance to hypoxia (Xie et al., 2015a). The mechanism underlying this tolerance involves the modulation of ethylene signalling. These molecules were shown to interact with constitutive triple response1 (CTR1; a negative regulator in ethylene signalling) and to inhibit its kinase activity (Xie et al., 2015a) and subsequent ethylene signalling. Furthermore, the hypersensitivity of loh mutants to dark submergence was rescued by introduction of the crt1‐1 mutation that constitutively induces the ethylene response. Overexpression of long‐chain base kinase (OsLCBK1) in tobacco led to an increased tolerance to oxidative stress provoked by treatment with either methyl viologen or H2O2, accompanied with an induction of oxidative stress‐related gene expression (Zhang et al., 2013). orm1 amiR‐ORM2 plants exhibited an early senescence phenotype accompanied by ROS production and they displayed higher survival rates to oxidative stress (Li et al., 2016). Measurement of sphingolipids showed an increase in LCBs and ceramides and an active vesicular transport that could contribute to the onset of the senescence phenotype and the resistance to oxidative stress. A homolog of human ceramidase, the neutral ceramidase nCer1, was recently characterised. ncer1 Arabidopsis plants accumulated hydroxyceramides and were more sensitive to oxidative stress. Conversely, nCer1 overexpressing plants were more tolerant to oxidative stress (Li et al., 2015). Loss of AtACER, encoding an alkaline ceramidase, inhibited autophagy and its overexpression stimulated autophagy under oxidative stress (Zheng et al., 2018). The Atacer mutant is highly sensitive to oxidative stress, whereas the complementation line showed a similar tolerance to this stress as the WT plant (Zheng et al., 2018). This result suggests that AtACER improves adaptation to oxidative stress by regulating autophagy.
Salt stress
During the early stage of salt stress in Carex rigescens, an iTRAQ‐based proteome study showed a reduction of the enzyme that catalyses the second step of the biosynthesis of phytosphingosine, 3‐ketosphingosine reductase (KDSR) (Li et al., 2017). Based on work performed in yeast where 3‐ketosphinganine reductase suppressed Ca2+ sensitivity (Beeler et al., 1998), the authors hypothesised that KDSR acts as a suppressor of the calcium signal during salt stress. Seeds of Atgint1 (glucosamine inositol phosphorylceramide transferase1, responsible for the glycosylation of some GIPCs) mutants displayed a higher germination rate than WT in response to salt stress, although this difference disappeared at higher salt concentrations (Ishikawa et al., 2018). The Atacer mutant and AtACER RNAi lines displayed high ceramide levels but reduced LCBs due to a disruption of an alkaline ceramidase gene (Wu et al., 2015a). Whereas these plants showed increased sensitivity to salinity, AtACER overexpression led to an increased tolerance to such a stress, highlighting the involvement of ceramides in response to salt stress. More precisely, it has recently been shown that AtACER regulates autophagy induced by high salt stress (Zheng et al., 2018). Overexpression of a rice S1P (sphingosine‐1‐phosphate) lyase gene in tobacco led to a decrease in tolerance to salt and changes in salt stress related genes (Zhang et al., 2012). By contrast, overexpression of OsLCBK1 in tobacco plants triggered no alteration in expression of salt stress‐related genes or tolerance/sensitivity phenotype compared with control plants in response to salt stress (Zhang et al., 2013), suggesting that this enzyme is not involved in salt stress responses in rice. Bioinformatic analysis supported the hypothesis that there are at least two OsLCBKs (Zhang et al., 2013). No sphingolipidomic analysis has been performed to reveal how the LCB content could vary between these two overexpressing plants. Previously published papers suggested that the sphingolipid metabolism could be adjusted, so that length chain, concentration and threshold are important for sphingolipid function.
Interplay with ABA signalling pathway
ABA has a key function in cold/drought stress responses. Pioneering work on sphingolipids showed that d18:1‐P and t18:0‐P were rapidly induced by drought and were involved in ABA signalling pathway to control guard‐cell turgor and therefore stomatal aperture (Ng et al., 2001; Coursol et al., 2003, 2005). This sphingolipid signalling pathway involved Ca2+ mobilisation, modification of ion channel activity, and heterotrimeric G‐protein. Consistent with this, AtLCBK1 was reported to be induced by low‐humidity or ABA treatments (Imai & Nishiura, 2005). Moreover, ABA also induces the accumulation of several LCB‐Ps (Guo et al., 2012). SPHK1 is an enzyme that phosphorylates d18:1 and t18:0. Stomata of SPHK1‐OE and of Atspp1 mutant (which accumulates d18:1‐P) displayed a higher sensitivity than WT to ABA (Worrall et al., 2008; Nakagawa et al., 2012). Therefore, LCB‐P content regulated by LCB kinases and phosphatases plays a key role in the ABA signalling pathway.
Interplay with phospholipid metabolism
Similar to sphingolipids, phosphatidic acid (PA) is considered as a lipid messenger involved in plant response to both biotic and abiotic stress. Like sphingolipids, PA interacts with MPK6 during salt stress response in Arabidopsis (Yu et al., 2010) and NADPH oxidase to regulate ROS production during ABA‐regulated stomatal closure (Zhang et al., 2009). The PA biosynthetic pathway responds to temperature and salt stress and interacts with sphingosine kinases (Guo et al., 2011). Moreover, addition of exogenous PA induced LCB‐P production and LCB‐P levels are diminished in pldα1 in response to ABA (Guo et al., 2012). Overexpression of sphingosine kinase increased PA accumulation. Altogether, the crosstalk between PA and sphingolipids should be a critical point to coordinate a stress response that needs to be elucidated (Fig. 3) (Guo & Wang, 2012; Ng & Coursol, 2012). DAG is a by‐product of the IPC synthase and is known to promote stomatal opening (Lee & Assmann, 1991; Peters et al., 2010). Although there is no direct evidence for a relationship between sphingolipids and DAG (Fig. 3), lipidome remodelling under stress could yet prove a link.
Figure 3.
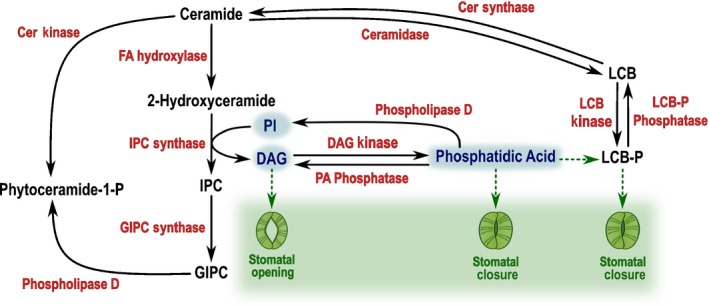
Interplay between sphingolipid and phospholipid metabolisms and their involvement in stomatal aperture. Phospholipid compounds are highlighted in blue. Solid arrows represent enzymatic reactions and dashed arrows indicate a stimulation reaction. Cer, ceramide; DAG, diacylglycerol; FA, fatty acid; GIPC, glycosyl inositol phosphoceramide; IPC, inositol phosphorylceramide; LCB, long‐chain base; LCB‐P, long‐chain base phosphate; PA, phosphatidic acid; PI, phosphoinositol.
Signalling messengers in biotic stress
Biotic stress caused by plant pathogens and insects is a major threat to both plant survival and productivity. Plants have developed a complex set of defences when challenged by pathogens. A successful innate immune response depends on the capability of the plant to recognise its invader and then to translate the different stimuli to an adaptive response. As structural plasma membrane components, sphingolipids are important molecules on the front line of pathogen recognition. Sphingolipid disruption also has an impact on PCD and accumulation of several well known defence molecules (such as ROS, MAPK, and hormones) and sphingolipids therefore act as mediators in the defence signalling cascade.
Very recently, metabolomic profiling identified changes in the sphingolipid pool after exposure to biotic stress. Xanthomonas campestris pv. campestris infection on Brassica oleracea triggered dynamic changes in sphingolipid metabolism including a reduction in the levels of ceramide N‐palmitoylsphinganine (Tortosa et al., 2018). Treatment of tomato fruit with the β‐aminobutyric acid elicitor increased the detected levels of ceramide phosphatidylinositol (Wilkinson et al., 2017). These metabolomic studies suggested that biotic stresses could impact sphingolipid metabolism.
Interplay with SA signalling pathway
Genetic and biochemical data suggest that sphingolipids are involved in the regulation of SA levels. Several mutants with altered sphingolipid metabolism displayed higher SA content and activation of SA‐dependent responses. Conversely, both SA and its analogue benzothiadiazole affected sphingolipid metabolism (Shi et al., 2015). The Arabidopsis fah1/2 mutant displayed SA accumulation in addition to an increase in ceramides but moderate changes in LCB accumulation (König et al., 2012). This suggests that elevated ceramide levels lead to an increase in salicylate levels. By contrast, the Arabidopsis loh1 mutant displayed an accumulation of C16‐ceramides but no changes in SA levels (Ternes et al., 2011). This discrepancy suggests the sphingolipid trigger for SA accumulation may be more complicated than initially expected. It is noteworthy that these mutants displayed other changes in sphingolipid homeostasis (e.g. fah1/2 also shows a decrease in glucosylceramides) that maybe have previously been overlooked. The induction of SA could therefore be due to alterations in sphingolipid classes other than LCBs or ceramides. The link between sphingolipid metabolism and SA may rely on MPK6, ROS/NO and/or calcium accumulation but this is still unclear (Sanchez‐Rangel et al., 2015). For example, overexpression of LCBK1 in tobacco cell culture triggered the accumulation of ROS in response to cryptogein. Loss of LCBK activity by using inhibitors resulted in a decrease in ROS production in elicited tobacco cells (Coursol et al., 2015).
In conjunction with activation of the SA pathway, several studies revealed that plants disrupted in sphingolipid biosynthesis are also affected in their ability to tolerate biotrophic pathogens. Whereas SA is considered essential for resistance to biotrophic and hemibiotrophic pathogens, it has been demonstrated that jasmonic acid (JA) and ethylene (ET) signalling pathways are important for resistance to necrotrophic pathogens in Arabidopsis (Thomma et al., 2001; Glazebrook, 2005). In Arabidopsis, it is now acknowledged that SA has a reciprocal antagonistic effect on JA signalling (Glazebrook, 2005). Using orm1 amiR‐ORM2 plants, Li et al. (2016) demonstrated that the loss of ORM function triggered a constitutive induction of SA‐dependent gene and a tolerance to Pseudomonas syringae strain DG3 compared with WT plants. acd5, erh1 (enhancing RPW8‐mediated HR‐like cell death) and fah1/2 mutants also exhibited a constitutive activation of SA pathway and enhanced resistance to powdery mildew. However, they had a similar phenotype to WT after challenge with the hemibiotrophic pathogens P. syringae pv. maculicola or Verticillium longisporum (Wang et al., 2008; König et al., 2012). Similarly, overexpression of OsSPL1 in tobacco dramatically reduced SA‐dependent gene expression and increased susceptibility to P. syringae pv. tabaci. Conversely, PDF1.2, a JA‐dependent gene, expression is slightly enhanced (Zhang et al., 2014). SA‐dependent pathogenesis‐related (PR) gene expressions were constitutively lower in Atacer‐1 plants compared with WT plants. This profile was similar, but enhanced, when these plants were infected by the P. syringae strain DG3. As a consequence, Atacer‐1 plants were found to be more susceptible to the biotrophic P. syringae strain DG3 (Wu et al., 2015a). In light of the antagonistic relationship between SA and JA, it would be interesting to analyse SA and JA levels alongside JA‐responsive genes in Atacer‐1 plants.
Few studies have analysed the role of sphingolipids during plant/necrotrophic pathogen interaction. Tobacco plants in which SPT was silenced accumulated SA, constitutively expressed SA‐induced genes and showed an increased susceptibility to the necrotrophic fungus Alternaria alternata f. sp. lycopersici (Rivas‐San Vicente et al., 2013). Similarly, the SA accumulating acd5 showed increased susceptibility to B. cinerea (Bi et al., 2014).
The role of sphingolipid metabolism in response to herbivory has been analysed (Begum et al., 2016). Overexpression of OsLCB2a in Arabidopsis led to the accumulation of LCB and ceramides compared with WT. These transgenic plants also displayed increased callose and wax deposition, an induction of SA‐dependent and camalexin‐dependent genes but a reduction of JA‐related genes, and inhibited aphid infestation (Begum et al., 2016).
Interplay with JA signalling pathway
The Atdpl1 mutant displayed a sensitivity towards the hemibiotrophic bacterium Pseudomonas syringae pv. tomato but a tolerance when infected by the necrotrophic fungus Botrytis cinerea (Magnin‐Robert et al., 2015). However, SA levels were similar or even reduced compared with WT, whereas JA levels and JA‐dependent gene expression were higher in the Atdpl1 infected mutant. This situation suggested a link between the sphingolipid and JA pathway. By using SPHK1 overexpressing plants, SA production was enhanced in response to FB1 treatment. Conversely SPHK1‐KD plants displayed an increase in JA‐related transcripts and metabolites (Qin et al., 2017). Therefore, it was suggested that the balance between LCBs and LCB‐Ps modulated by the activity of SPHK1 acted as a signal upstream of the SA/JA signalling pathways during FB1‐induced cell death (Qin et al., 2017).
Interplay with ethylene signalling pathway
It was recently shown that sphingolipid metabolism has connections with not only SA and JA pathways but also with ethylene signalling. Ethylene or its precursor (1‐aminocyclopropane carboxylic acid) inhibits sphingolipid biosynthesis. Mutants disturbed in ethylene biosynthesis or signalling displayed constitutive modifications in sphingolipid content (Wu et al., 2015b). For example, ctr1‐1 mutants, which have enhanced ethylene signalling, contained lower levels of ceramides and hydroxyceramides compared with WT. Some constitutive ethylene response mutants displayed a higher tolerance to FB1, and mutants deficient in ethylene signalling exhibited more sensitivity to FB1, showing that enhanced ethylene signalling rescues FB1‐induced cell death.
Conclusions and future directions
Over the last few decades we have learned much about the role of sphingolipids during the plant stress response. Functional analyses have demonstrated that sphingolipids are involved in the response to environmental cues. The role of sphingolipids during PCD is well studied. Significant progress has been made but the precise identity of sphingolipids involved in this process is not clearly defined. It is clear that PCD is tightly regulated and further consideration should be given to the different stresses triggering PCD and also the plant species in question. The plasma membrane mediates contact with the environment and is the likely initial source of signal transduction. Recent evidence has shown that GIPC glycosylation involved different regulation processes in the plasma membrane. The composition, distribution and dynamic association of sphingolipids are therefore of high importance for plasma membrane function. It is essential to unravel the dynamic association between sphingolipids, plasma membrane lipids and proteins to better understand the recognition step of the immune response. While a body of evidence has revealed functions for LCBs/LCB‐Ps, ceramides and GIPCs, the roles of GlcCers in plants have yet to be fully investigated, other than the observation that they are essential for normal plant growth and development. The relationship between sphingolipids and SA is long acknowledged and recent studies have shown interconnections with other defence signalling pathways such as JA and ethylene. The regulation of stomatal aperture is of crucial importance during plant defence responses, especially in response to foliar pathogens. ABA‐mediated stomatal closure inhibits pathogen penetration to the apoplast. As the sphingolipid signalling pathway has some interconnections during this process in response to drought stress, the relationship between sphingolipids and ABA in response to foliar pathogens remains to be elucidated.
Despite the range of different structures of sphingolipids and differing physical properties they exhibit, understanding sphingolipid regulation and function is not comprehensive. The interactions with other cellular lipids are also yet to be fully resolved but there are known relationships with several other lipid classes. The wider lipidome is subject to remodelling when the plant is under stress and it is likely that sphingolipids form part of a coordinated response. The mechanisms for action and whether sphingolipids regulate stress responsive gene expression or are themselves regulated by stress responsive transcription factors are not yet fully understood. There is still a gap in understanding the role of sphingolipids in the plant stress response, but the advent of genome editing technology opens the possibility to develop crops with a greater ability to tolerate stress based on the manipulation of their sphingolipid biosynthetic pathway.
Supporting information
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Table S1 Abbreviations used in this review.
Acknowledgements
LVM and JAN are supported by the Biotechnology and Biological Sciences Research Council (BBSRC) Institute Strategic Programme Tailoring Plant Metabolism (BBS/E/C/000I0420). EH and SD‐C are funded by SFR Condorcet (18ARC107). We extend our apologies to researchers whose work could not be cited here due to space constraints.
Contributor Information
Louise V. Michaelson, Email: louise.michaelson@rothamsted.ac.uk.
Sandrine Dhondt‐Cordelier, Email: sandrine.cordelier@univ-reims.fr.
References
- Alden KP, Dhondt‐Cordelier S, McDonald KL, Reape TJ, Ng CK, McCabe PF, Leaver CJ. 2011. Sphingolipid long chain base phosphates can regulate apoptotic‐like programmed cell death in plants. Biochemical and Biophysical Research Communications 410: 574–580. [DOI] [PubMed] [Google Scholar]
- Ali U, Li H, Wang X, Guo L. 2018. Emerging roles of sphingolipid signaling in plant response to biotic and abiotic stresses. Molecular Plant 11: 1328–1343. [DOI] [PubMed] [Google Scholar]
- Beeler T, Bacikova D, Gable K, Hopkins L, Johnson C, Slife H, Dunn T. 1998. The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3‐ketosphinganine reductase is identified in a screen for temperature‐sensitive suppressors of the Ca2+‐sensitive csg2Delta mutant. Journal of Biological Chemistry 273: 30688–30694. [DOI] [PubMed] [Google Scholar]
- Begum MA, Shi XX, Tan Y, Zhou WW, Hannun Y, Obeid L, Mao C, Zhu ZR. 2016. Molecular characterization of rice OsLCB2a1 gene and functional analysis of its role in insect resistance. Frontiers in Plant Science 7: 1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi FC, Liu Z, Wu JX, Liang H, Xi XL, Fang C, Sun TJ, Yin J, Dai GY, Rong C, et al 2014. Loss of ceramide kinase in Arabidopsis impairs defenses and promotes ceramide accumulation and mitochondrial H2O2 bursts. Plant Cell 26: 3449–3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacas JL, Bure C, Furt F, Maalouf JP, Badoc A, Cluzet S, Schmitter JM, Antajan E, Mongrand S. 2013. Biochemical survey of the polar head of plant glycosylinositolphosphoceramides unravels broad diversity. Phytochemistry 96: 191–200. [DOI] [PubMed] [Google Scholar]
- Cacas JL, Bure C, Grosjean K, Gerbeau‐Pissot P, Lherminier J, Rombouts Y, Maes E, Bossard C, Gronnier J, Furt F, et al 2016. Revisiting plant plasma membrane lipids in tobacco: a focus on sphingolipids. Plant Physiology 170: 367–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrel C, Vazquez T, Puyaubert J, Reze N, Lesch M, Kaiser WM, Dutilleul C, Guillas I, Zachowski A, Baudouin E. 2011. Nitric oxide participates in cold‐responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana . New Phytologist 189: 415–427. [DOI] [PubMed] [Google Scholar]
- Chen M, Han G, Dietrich CR, Dunn TM, Cahoon EB. 2006. The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase. Plant Cell 18: 3576–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Markham JE, Cahoon EB. 2012. Sphingolipid Delta8 unsaturation is important for glucosylceramide biosynthesis and low‐temperature performance in Arabidopsis. The Plant Journal 69: 769–781. [DOI] [PubMed] [Google Scholar]
- Coursol S, Fan LM, Le Stunff H, Spiegel S, Gilroy S, Assmann SM. 2003. Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 423: 651–654. [DOI] [PubMed] [Google Scholar]
- Coursol S, Fromentin J, Noirot E, Briere C, Robert F, Morel J, Liang YK, Lherminier J, Simon‐Plas F. 2015. Long‐chain bases and their phosphorylated derivatives differentially regulate cryptogein‐induced production of reactive oxygen species in tobacco (Nicotiana tabacum) BY‐2 cells. New Phytologist 205: 1239–1249. [DOI] [PubMed] [Google Scholar]
- Coursol S, Le Stunff H, Lynch DV, Gilroy S, Assmann SM, Spiegel S. 2005. Arabidopsis sphingosine kinase and the effects of phytosphingosine‐1‐phosphate on stomatal aperture. Plant Physiology 137: 724–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolbe T, Giavalisco P, Zuther E, Seiwert B, Hincha DK, Willmitzer L. 2012. Differential remodeling of the lipidome during cold acclimation in natural accessions of Arabidopsis thaliana . The Plant Journal 72: 972–982. [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Benhassaine‐Kesri G, Demandre C, Reze N, Launay A, Pelletier S, Renou JP, Zachowski A, Baudouin E, Guillas I. 2012. Phytosphingosine‐phosphate is a signal for AtMPK6 activation and Arabidopsis response to chilling. New Phytologist 194: 181–191. [DOI] [PubMed] [Google Scholar]
- Dutilleul C, Chavarria H, Reze N, Sotta B, Baudouin E, Guillas I. 2015. Evidence for ACD5 ceramide kinase activity involvement in Arabidopsis response to cold stress. Plant, Cell & Environment 38: 2688–2697. [DOI] [PubMed] [Google Scholar]
- Fang L, Ishikawa T, Rennie EA, Murawska GM, Lao J, Yan J, Tsai AY, Baidoo EE, Xu J, Keasling JD, et al 2016. Loss of inositol phosphorylceramide sphingolipid mannosylation induces plant immune responses and reduces cellulose content in Arabidopsis. Plant Cell 28: 2991–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43: 205–227. [DOI] [PubMed] [Google Scholar]
- Goto H, Nishikawa K, Shionoya N, Taniguchi M, Igarashi T. 2012. Determination of sphingoid bases from hydrolyzed glucosylceramide in rice and wheat by online post‐column high‐performance liquid chromatography with O‐phthalaldehyde derivatization. Journal of Oleo Science 61: 681–688. [DOI] [PubMed] [Google Scholar]
- Guillas I, Guellim A, Reze N, Baudouin E. 2013. Long chain base changes triggered by a short exposure of Arabidopsis to low temperature are altered by AHb1 non‐symbiotic haemoglobin overexpression. Plant Physiology and Biochemistry 63: 191–195. [DOI] [PubMed] [Google Scholar]
- Guo L, Mishra G, Markham JE, Li M, Tawfall A, Welti R, Wang X. 2012. Connections between sphingosine kinase and phospholipase D in the abscisic acid signaling pathway in Arabidopsis. Journal of Biological Chemistry 287: 8286–8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Mishra G, Taylor K, Wang X. 2011. Phosphatidic acid binds and stimulates Arabidopsis sphingosine kinases. Journal of Biological Chemistry 286: 13336–13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Wang X. 2012. Crosstalk between phospholipase D and sphingosine kinase in plant stress signaling. Frontiers in Plant Science 3: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Zhang Y, Zhang X, Shi Y. 2017. Long‐chain base kinase1 affects freezing tolerance in Arabidopsis thaliana . Plant Science 259: 94–103. [DOI] [PubMed] [Google Scholar]
- Imai H, Nishiura H. 2005. Phosphorylation of sphingoid long‐chain bases in Arabidopsis: functional characterization and expression of the first sphingoid long‐chain base kinase gene in plants. Plant Cell Physiology 46: 375–380. [DOI] [PubMed] [Google Scholar]
- Ines C, Parra‐Lobato MC, Paredes MA, Labrador J, Gallardo M, Saucedo‐Garcia M, Gavilanes‐Ruiz M, Gomez‐Jimenez MC. 2018. Sphingolipid distribution, content and gene expression during olive‐fruit development and ripening. Frontiers in Plant Science 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Aki T, Yanagisawa S, Uchimiya H, Kawai‐Yamada M. 2015. Overexpression of BAX INHIBITOR‐1 links plasma membrane microdomain proteins to stress. Plant Physiology 169: 1333–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Fang L, Rennie EA, Sechet J, Yan J, Jing B, Moore W, Cahoon EB, Scheller HV, Kawai‐Yamada M, et al 2018. Glucosamine inositolphosphorylceramide transferase1 (GINT1) is a GlcNAc‐containing glycosylinositol phosphorylceramide glycosyltransferase. Plant Physiology 177: 938–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MN, Jacquemot MP, Coursol S, Ng CK. 2012. Sphingosine in plants–more riddles from the Sphinx? New Phytologist 193: 51–57. [DOI] [PubMed] [Google Scholar]
- Kawaguchi M, Imai H, Naoe M, Yasui Y, Ohnishi M. 2000. Cerebrosides in grapevine leaves: distinct composition of sphingoid bases among the grapevine species having different tolerances to freezing temperature. Bioscience, Biotechnology, and Biochemistry 64: 1271–1273. [DOI] [PubMed] [Google Scholar]
- Kimberlin AN, Han G, Luttgeharm KD, Chen M, Cahoon RE, Stone JM, Markham JE, Dunn TM, Cahoon EB. 2016. ORM expression alters sphingolipid homeostasis and differentially affects ceramide synthase activity. Plant Physiology 172: 889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin AN, Majumder S, Han G, Chen M, Cahoon RE, Stone JM, Dunn TM, Cahoon EB. 2013. Arabidopsis 56‐amino acid serine palmitoyltransferase‐interacting proteins stimulate sphingolipid synthesis, are essential, and affect mycotoxin sensitivity. Plant Cell 25: 4627–4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König S, Feussner K, Schwarz M, Kaever A, Iven T, Landesfeind M, Ternes P, Karlovsky P, Lipka V, Feussner I. 2012. Arabidopsis mutants of sphingolipid fatty acid alpha‐hydroxylases accumulate ceramides and salicylates. New Phytologist 196: 1086–1097. [DOI] [PubMed] [Google Scholar]
- Lachaud C, Prigent E, Thuleau P, Grat S, Da Silva D, Briere C, Mazars C, Cotelle V. 2013. 14‐3‐3‐regulated Ca2+‐dependent protein kinase CPK3 is required for sphingolipid‐induced cell death in Arabidopsis. Cell Death & Differentiation 20: 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Assmann SM. 1991. Diacylglycerols induce both ion pumping in patch‐clamped guard‐cell protoplasts and opening of intact stomata. Proceedings of the National Academy of Sciences, USA 88: 2127–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarcic T, Albert I, Bohm H, Hodnik V, Pirc K, Zavec AB, Podobnik M, Pahovnik D, Zagar E, Pruitt R, et al 2017. Eudicot plant‐specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 358: 1431–1434. [DOI] [PubMed] [Google Scholar]
- Li J, Bi FC, Yin J, Wu JX, Rong C, Wu JL, Yao N. 2015. An Arabidopsis neutral ceramidase mutant ncer1 accumulates hydroxyceramides and is sensitive to oxidative stress. Frontiers in Plant Science 6: 460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yin J, Rong C, Li KE, Wu JX, Huang LQ, Zeng HY, Sahu SK, Yao N. 2016. Orosomucoid proteins interact with the small subunit of serine palmitoyltransferase and contribute to sphingolipid homeostasis and stress responses in Arabidopsis. Plant Cell 28: 3038–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhang K, Long R, Sun Y, Kang J, Zhang T, Cao S. 2017. iTRAQ‐based comparative proteomic analysis reveals tissue‐specific and novel early‐stage molecular mechanisms of salt stress response in Carex rigescens . Environmental & Experimental Botany 143: 99–114. [Google Scholar]
- Lung SC, Chye ML. 2019. Arabidopsis acyl‐CoA‐binding proteins regulate the synthesis of lipid signals. New Phytologist 223: 113–117. [DOI] [PubMed] [Google Scholar]
- Luttgeharm KD, Chen M, Mehra A, Cahoon RE, Markham JE, Cahoon EB. 2015. Overexpression of Arabidopsis ceramide synthases differentially affects growth, sphingolipid metabolism, programmed cell death, and mycotoxin resistance. Plant Physiology 169: 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttgeharm KD, Kimberlin AN, Cahoon EB. 2016. Plant sphingolipid metabolism and function. Sub‐Cellular Biochemistry 86: 249–286. [DOI] [PubMed] [Google Scholar]
- Magnin‐Robert M, Le Bourse D, Markham J, Dorey S, Clement C, Baillieul F, Dhondt‐Cordelier S. 2015. Modifications of sphingolipid content affect tolerance to hemibiotrophic and necrotrophic pathogens by modulating plant defense responses in Arabidopsis. Plant Physiology 169: 2255–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamode Cassim A, Gouguet P, Gronnier J, Laurent N, Germain V, Grison M, Boutte Y, Gerbeau‐Pissot P, Simon‐Plas F, Mongrand S. 2019. Plant lipids: key players of plasma membrane organization and function. Progress in Lipid Research 73: 1–27. [DOI] [PubMed] [Google Scholar]
- Markham JE, Li J, Cahoon EB, Jaworski JG. 2006. Separation and identification of major plant sphingolipid classes from leaves. Journal of Bioogical Chemistry 281: 22684–22694. [DOI] [PubMed] [Google Scholar]
- Michaelson LV, Napier JA, Molino D, Faure JD. 2016. Plant sphingolipids: their importance in cellular organization and adaption. Biochimica et Biophysica Acta 1861: 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson LV, Zauner S, Markham JE, Haslam RP, Desikan R, Mugford S, Albrecht S, Warnecke D, Sperling P, Heinz E, et al 2009. Functional characterization of a higher plant sphingolipid Delta4‐desaturase: defining the role of sphingosine and sphingosine‐1‐phosphate in Arabidopsis. Plant Physiology 149: 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina JG, Okada Y, Wansadhipathi‐Kannangara NK, Pratt S, Shams‐Eldin H, Schwarz RT, Steel PG, Fawcett T, Denny PW. 2010. Functional analyses of differentially expressed isoforms of the Arabidopsis inositol phosphorylceramide synthase. Plant Molecular Biology 73: 399–407. [DOI] [PubMed] [Google Scholar]
- Minami A, Fujiwara M, Furuto A, Fukao Y, Yamashita T, Kamo M, Kawamura Y, Uemura M. 2009. Alterations in detergent‐resistant plasma membrane microdomains in Arabidopsis thaliana during cold acclimation. Plant and Cell Physiology 50: 341–359. [DOI] [PubMed] [Google Scholar]
- Molino D, Van der Giessen E, Gissot L, Hematy K, Marion J, Barthelemy J, Bellec Y, Vernhettes S, Satiat‐Jeunemaitre B, Galli T, et al 2014. Inhibition of very long acyl chain sphingolipid synthesis modifies membrane dynamics during plant cytokinesis. Biochimica et Biophysica Acta 1842: 1422–1430. [DOI] [PubMed] [Google Scholar]
- Moreau P, Bessoule JJ, Mongrand S, Testet E, Vincent P, Cassagne C. 1998. Lipid trafficking in plant cells. Progress in Lipid Research 37: 371–391. [DOI] [PubMed] [Google Scholar]
- Mortimer JC, Yu X, Albrecht S, Sicilia F, Huichalaf M, Ampuero D, Michaelson LV, Murphy AM, Matsunaga T, Kurz S, et al 2013. Abnormal glycosphingolipid mannosylation triggers salicylic acid‐mediated responses in Arabidopsis. Plant Cell 25: 1881–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msanne J, Chen M, Luttgeharm KD, Bradley AM, Mays ES, Paper JM, Boyle DL, Cahoon RE, Schrick K, Cahoon EB. 2015. Glucosylceramides are critical for cell‐type differentiation and organogenesis, but not for cell viability in Arabidopsis. The Plant Journal 84: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Tsuyama M, Kobayashi Y, Kodama H, Iba K. 2000. Trienoic fatty acids and plant tolerance of high temperature. Science 287: 476–479. [DOI] [PubMed] [Google Scholar]
- Nagano M, Ishikawa T, Fujiwara M, Fukao Y, Kawano Y, Kawai‐Yamada M, Shimamoto K. 2016. Plasma membrane microdomains are essential for Rac1‐RbohB/H‐mediated immunity in rice. Plant Cell 28: 1966–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Ishikawa T, Ogawa Y, Iwabuchi M, Nakasone A, Shimamoto K, Uchimiya H, Kawai‐Yamada M. 2014. Arabidopsis Bax inhibitor‐1 promotes sphingolipid synthesis during cold stress by interacting with ceramide‐modifying enzymes. Planta 240: 77–89. [DOI] [PubMed] [Google Scholar]
- Nakagawa N, Kato M, Takahashi Y, Shimazaki K, Tamura K, Tokuji Y, Kihara A, Imai H. 2012. Degradation of long‐chain base 1‐phosphate (LCBP) in Arabidopsis: functional characterization of LCBP phosphatase involved in the dehydration stress response. Journal of Plant Research 125: 439–449. [DOI] [PubMed] [Google Scholar]
- Ng CK, Carr K, McAinsh MR, Powell B, Hetherington AM. 2001. Drought‐induced guard cell signal transduction involves sphingosine‐1‐phosphate. Nature 410: 596–599. [DOI] [PubMed] [Google Scholar]
- Ng CK, Coursol S. 2012. New insights into phospholipase d and sphingosine kinase activation in Arabidopsis. Frontiers in Physiology 3: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pata MO, Hannun YA, Ng CK. 2010. Plant sphingolipids: decoding the enigma of the Sphinx. New Phytologist 185: 611–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pata MO, Wu BX, Bielawski J, Xiong TC, Hannun YA, Ng CK. 2008. Molecular cloning and characterization of OsCDase, a ceramidase enzyme from rice. The Plant Journal 55: 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Li M, Narasimhan R, Roth M, Welti R, Wang X. 2010. Nonspecific phospholipase C NPC4 promotes responses to abscisic acid and tolerance to hyperosmotic stress in Arabidopsis. Plant Cell 22: 2642–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Zhang RX, Ge S, Zhou T, Liang YK. 2017. Sphingosine kinase AtSPHK1 functions in fumonisin B1‐triggered cell death in Arabidopsis. Plant Physiology and Biochemistry 119: 70–80. [DOI] [PubMed] [Google Scholar]
- Rennie EA, Ebert B, Miles GP, Cahoon RE, Christiansen KM, Stonebloom S, Khatab H, Twell D, Petzold CJ, Adams PD, et al 2014. Identification of a sphingolipid alpha‐glucuronosyltransferase that is essential for pollen function in Arabidopsis. Plant Cell 26: 3314–3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas‐San Vicente M, Larios‐Zarate G, Plasencia J. 2013. Disruption of sphingolipid biosynthesis in Nicotiana benthamiana activates salicylic acid‐dependent responses and compromises resistance to Alternaria alternata f. sp. lycopersici . Planta 237: 121–136. [DOI] [PubMed] [Google Scholar]
- Routaboul JM, Skidmore C, Wallis JG, Browse J. 2012. Arabidopsis mutants reveal that short‐ and long‐term thermotolerance have different requirements for trienoic fatty acids. Journal of Experimental Botany 63: 1435–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez‐Rangel D, Rivas‐San Vicente M, de la Torre‐Hernandez ME, Najera‐Martinez M, Plasencia J. 2015. Deciphering the link between salicylic acid signaling and sphingolipid metabolism. Frontiers in Plant Science 6: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo‐Garcia M, Guevara‐Garcia A, Gonzalez‐Solis A, Cruz‐Garcia F, Vazquez‐Santana S, Markham JE, Lozano‐Rosas MG, Dietrich CR, Ramos‐Vega M, Cahoon EB, et al 2011. MPK6, sphinganine and the LCB2a gene from serine palmitoyltransferase are required in the signaling pathway that mediates cell death induced by long chain bases in Arabidopsis. New Phytologist 191: 943–957. [DOI] [PubMed] [Google Scholar]
- Shi L, Bielawski J, Mu J, Dong H, Teng C, Zhang J, Yang X, Tomishige N, Hanada K, Hannun YA, et al 2007. Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Cell Research 17: 1030–1040. [DOI] [PubMed] [Google Scholar]
- Shi C, Yin J, Liu Z, Wu JX, Zhao Q, Ren J, Yao N. 2015. A systematic simulation of the effect of salicylic acid on sphingolipid metabolism. Frontiers in Plant Science 6: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassieva S, Hille J. 2003. Plant sphingolipids today - are they still enigmatic? Plant Biology 5: 125–136. [Google Scholar]
- Takahashi D, Imai H, Kawamura Y, Uemura M. 2016. Lipid profiles of detergent resistant fractions of the plasma membrane in oat and rye in association with cold acclimation and freezing tolerance. Cryobiology 72: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Kida T, Imai H, Morishige J, Yamashita R, Matsuoka H, Uozumi S, Satouchi K, Nagano M, Tokumura A. 2013. Identification of a sphingolipid‐specific phospholipase D activity associated with the generation of phytoceramide‐1‐phosphate in cabbage leaves. FEBS Journal 280: 3797–3809. [DOI] [PubMed] [Google Scholar]
- Tartaglio V, Rennie EA, Cahoon R, Wang G, Baidoo E, Mortimer JC, Cahoon EB, Scheller HV. 2017. Glycosylation of inositol phosphorylceramide sphingolipids is required for normal growth and reproduction in Arabidopsis. The Plant Journal 89: 278–290. [DOI] [PubMed] [Google Scholar]
- Ternes P, Feussner K, Werner S, Lerche J, Iven T, Heilmann I, Riezman H, Feussner I. 2011. Disruption of the ceramide synthase LOH1 causes spontaneous cell death in Arabidopsis thaliana . New Phytologist 192: 841–854. [DOI] [PubMed] [Google Scholar]
- Thomma BP, Penninckx IA, Broekaert WF, Cammue BP. 2001. The complexity of disease signaling in Arabidopsis. Current Opinion in Immunology 13: 63–68. [DOI] [PubMed] [Google Scholar]
- Tortosa M, Cartea ME, Rodriguez VM, Velasco P. 2018. Unraveling the metabolic response of Brassica oleracea exposed to Xanthomonas campestris pv. campestris . Journal of the Science of Food and Agriculture 98: 3675–3683. [DOI] [PubMed] [Google Scholar]
- Tovuu A, Zulfugarov IS, Wu G, Kang IS, Kim C, Moon BY, An G, Lee CH. 2016. Rice mutants deficient in omega‐3 fatty acid desaturase (FAD8) fail to acclimate to cold temperatures. Plant Physiology and Biochemistry 109: 525–535. [DOI] [PubMed] [Google Scholar]
- Tsegaye Y, Richardson CG, Bravo JE, Mulcahy BJ, Lynch DV, Markham JE, Jaworski JG, Chen M, Cahoon EB, Dunn TM. 2007. Arabidopsis mutants lacking long chain base phosphate lyase are fumonisin‐sensitive and accumulate trihydroxy‐18:1 long chain base phosphate. Journal of Biological Chemistry 282: 28195–28206. [DOI] [PubMed] [Google Scholar]
- Wang W, Yang X, Tangchaiburana S, Ndeh R, Markham JE, Tsegaye Y, Dunn TM, Wang GL, Bellizzi M, Parsons JF, et al 2008. An inositolphosphorylceramide synthase is involved in regulation of plant programmed cell death associated with defense in Arabidopsis. Plant Cell 20: 3163–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson SW, Pastor V, Paplauskas S, Pétriacq P, Luna E. 2017. Long‐lasting β‐aminobutyric acid‐induced resistance protects tomato fruit against Botrytis cinerea . Plant Pathology 67: 30–41. [Google Scholar]
- Worrall D, Liang YK, Alvarez S, Holroyd GH, Spiegel S, Panagopulos M, Gray JE, Hetherington AM. 2008. Involvement of sphingosine kinase in plant cell signalling. The Plant Journal 56: 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JX, Li J, Liu Z, Yin J, Chang ZY, Rong C, Wu JL, Bi FC, Yao N. 2015a. The Arabidopsis ceramidase AtACER functions in disease resistance and salt tolerance. The Plant Journal 81: 767–780. [DOI] [PubMed] [Google Scholar]
- Wu JX, Wu JL, Yin J, Zheng P, Yao N. 2015b. Ethylene modulates sphingolipid synthesis in Arabidopsis. Frontiers in Plant Science 6: 1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LJ, Chen QF, Chen MX, Yu LJ, Huang L, Chen L, Wang FZ, Xia FN, Zhu TR, Wu JX, et al 2015a. Unsaturation of very‐long‐chain ceramides protects plant from hypoxia‐induced damages by modulating ethylene signaling in Arabidopsis. PLoS Genetics 11: e1005143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LJ, Yu LJ, Chen QF, Wang FZ, Huang L, Xia FN, Zhu TR, Wu JX, Yin J, Liao B, et al 2015b. Arabidopsis acyl‐CoA‐binding protein ACBP3 participates in plant response to hypoxia by modulating very‐long‐chain fatty acid metabolism. The Plant Journal 81: 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagawa D, Ishikawa T, Imai H. 2017. Synthesis and degradation of long‐chain base phosphates affect fumonisin B1‐induced cell death in Arabidopsis thaliana . Journal of Plant Research 130: 571–585. [DOI] [PubMed] [Google Scholar]
- Yu L, Nie J, Cao C, Jin Y, Yan M, Wang F, Liu J, Xiao Y, Liang Y, Zhang W. 2010. Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana. New Phytologist 188: 762–773. [DOI] [PubMed] [Google Scholar]
- Zhang H, Huang L, Li X, Ouyang Z, Yu Y, Li D, Song F. 2013. Overexpression of a rice long‐chain base kinase gene OsLCBK1 in tobacco improves oxidative stress tolerance. Plant Biotechnology 30: 9–16. [Google Scholar]
- Zhang H, Jin X, Huang L, Hong Y, Zhang Y, Ouyang Z, Li X, Song F, Li D. 2014. Molecular characterization of rice sphingosine‐1‐phosphate lyase gene OsSPL1 and functional analysis of its role in disease resistance response. Plant Cell Reports 33: 1745–1756. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhai J, Mo J, Li D, Song F. 2012. Overexpression of rice sphingosine‐1‐phoshpate lyase gene OsSPL1 in transgenic tobacco reduces salt and oxidative stress tolerance. Journal of Integrative Plant Biology 54: 652–662. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhu H, Zhang Q, Li M, Yan M, Wang R, Wang L, Welti R, Zhang W, Wang X. 2009. Phospholipase dalpha1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA‐mediated stomatal closure in Arabidopsis. Plant Cell 21: 2357–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Wu JX, Sahu SK, Zeng HY, Huang LQ, Liu Z, Xiao S, Yao N. 2018. Loss of alkaline ceramidase inhibits autophagy in Arabidopsis and plays an important role during environmental stress response. Plant, Cell & Environment 41: 837–849. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zeng L, Fu X, Mei X, Cheng S, Liao Y, Deng R, Xu X, Jiang Y, Duan X, et al 2016. The sphingolipid biosynthetic enzyme Sphingolipid delta8 desaturase is important for chilling resistance of tomato. Scientific Reports 6: 38742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinta G, AbdElgawad H, Peshev D, Weedon JT, Van den Ende W, Nijs I, Janssens IA, Beemster GTS, Han A. 2018. Dynamics of metabolic responses to combined heat and drought spells in Arabidopsis thaliana under ambient and rising atmospheric CO2 . Journal of Experimental Botany 69: 2159–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: Wiley Blackwell are not responsible for the content or functionality of any Supporting Information supplied by the authors. Any queries (other than missing material) should be directed to the New Phytologist Central Office.
Table S1 Abbreviations used in this review.


