Abstract
Introduction
This study compared healing of the scars after cesarean section during the first postpartum year using a single‐ or double‐layer suturing technique. Scarring was assessed by a transvaginal ultrasound. We explored the appearance and localization of uterine scars with regard to the obstetric history. Our aim was to compare the position of the scar or defect, if present, its dimensions, and any residual myometrium with respect to the suturing technique during the cesarean section.
Material and methods
Women with uncomplicated singleton pregnancies indicated for elective or acute cesarean section were randomly allocated to the uterine closure technique group. During the first postpartum year, their lower uterine segment was examined with a transvaginal ultrasound in three consecutive visits at 6 weeks, 6 months and 12 months.
Results
324 women attended the 12‐month visit; of these, 149 underwent single‐layer closure of the uterine incision and 175 double‐layer technique. A higher proportion of the defects is seen in the single‐layer closure technique of suturing. Defects in the single‐layer group were wider (0.002) and the residual myometrial thickness in the single‐layer group were thinner (0.019). Women who underwent cesarean section at the stage of full cervical dilation had scars that were closer to the external cervical os (0.000). The position of the uterus varies greatly between controls (0.000). The combination of uterine position and scar defect presence changed significantly between controls (0.001), and was significantly dependent on the suturing method (0.003). Defects with or without contact with the uterine cavity changed statistically between controls (0.017). Both types of defects were more common in the single‐layer closure technique group.
Conclusions
The findings of this study demonstrate that double‐layer technique with the first continuous nonlocking suture followed by a second continuous nonlocking suture is associated with better suture healing and greater residual myometrial thickness. No difference was observed between single‐ and double‐layer closure for the presence of maternal infectious morbidity, wound infection or blood transfusion.
Keywords: cesarean section, double‐layer technique, suture healing, uterine scar, uterine suture
Abbreviations
- CS
cesarean section
- DLT
double‐layer technique
- RMT
residual myometrial thickness
- SLT
single‐layer technique
Key message.
Deficient uterine scar healing represents a side effect with potential negative long‐term consequences. This study demonstrates that a double‐layer technique with the first continuous nonlocking suture followed by a second continuous nonlocking suture is associated with better uterine suture healing.
1. INTRODUCTION
There are multiple factors driving the increase in cesarean section (CS) rates internationally. Demographic factors in the economically developed world partly explain the rise.1, 2 Deficient uterine scar healing represents a side effect with negative consequences. Serious obstetric complications may occur in the subsequent pregnancy such as uterine scar dehiscence (0.6‐3.8%), uterine scar rupture (0.2‐3.8%) and cesarean scar pregnancy, which may be associated with morbidly adherent placenta.3, 4 In the long‐term, women with a scar defect may also suffer from gynecological problems.5, 6, 7
Transvaginal ultrasound is validated tool to evaluate uterine scar defects commonly referred to as “niche”.8, 9 Healing and scar maturation are influenced by the suture technique, number of previous CS deliveries and patient's medical and obstetric history.10 There are many techniques for the closure of the uterine incision. In the short‐term, no clear benefit of any of the randomized comparisons has been shown. The objective of this study was to compare the effect of single‐ vs double‐layer closure technique on uterine scar morphology in primiparas after CS.
2. MATERIAL AND METHODS
A prospective randomized study was conducted between November 2011 and September 2014 in the Institute for the Care of Mother and Child in Prague (tertiary perinatological center). The study included a cohort of nulliparous women with a singleton pregnancy who underwent first delivery by any type of CS. Women were invited for three consecutive control visits at 6 weeks, 6 months and 12 months postpartum. Women who did not complete all postoperative visits were excluded from the analysis.
The goal of the proposed study is to test the null hypothesis that the mean thickness of the uterine scar in the two groups is equal. Women with a uterine scar defect had thinner full lower uterine segment and thinner myometrial layer. The optimal cut‐off value varied from 2.0 to 3.5 mm for full lower uterine segment thickness and from 1.4 to 2.0 mm for myometrial layer.11 We calculated the sample size to observe a difference of 1.0 mm of myometrial thickness between uterine closures. For the comparison of the influence of both surgical techniques on muscle layer thickness we used a two‐tailed test, which means that an effect in either direction will be interpreted. The significance level for a test of the null hypothesis was set at 0.050 (alpha). With the proposed sample size of 103 each for the two groups, the study would have a power of 90.1% to yield a statistically significant result. This computation assumes that the mean difference for myometrial layer thickness is 1.0 mm and the common within‐group standard deviation is 2.2 mm (on the basis of our feasibility studies). This effect was selected as the smallest effect that would be important to detect, in the sense that any smaller effect would not be of clinical or substantive significance. It was also assumed that this effect size was reasonable, in the sense that an effect of this magnitude could be anticipated in this field of research. Women indicated for CS were randomly allocated after opening a consecutively numbered envelope containing the uterine closure technique group, either single‐layer technique (SLT) or double‐layer technique (DLT) closure of the hysterotomy. This study represents a subanalysis from a large prospective cohort of healthy women with a first singleton pregnancy who delivered at or beyond 37 weeks. The SLT involved a single continuous nonlocking suture including decidua. In DLT the first layer comprised a continuous nonlocking suture including decidua, followed by a second continuous, nonlocking suture (Figure 1). In both techniques, we used 0/0 polyglactin 910 suturing material with a blunt needle (Vicryl, Ethicon®, Diegem, Belgium). Additional hemostasis sutures could be laid regardless of the closure method. All obstetricians who performed the surgery had similar experience, and those with less training were always supervised by a senior attending physician.
Figure 1.
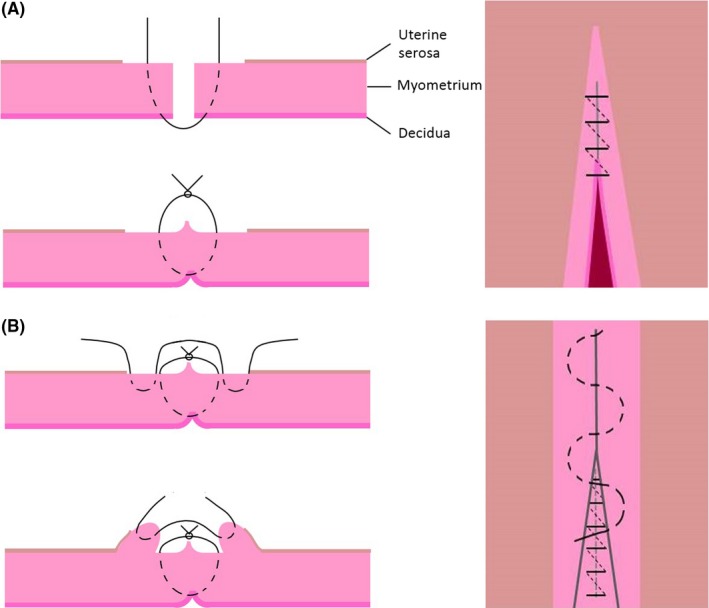
Uterine suture technique. (A) Unlocked single‐layer closure. Decidua was incorporated in the suture. The uterine serosa is not included in the suture. (B) Double‐layer closure. First layer unlocked suture including decidua. Second layer unlocked suture taking superficial part of myometrium. Uterine serosa is not included in the suture [Color figure can be viewed at http://wileyonlinelibrary.com]
During the follow‐up control visits at 6 weeks, 6 and 12 months, all patients underwent a three‐dimensional transvaginal ultrasound of the uterus using a GE Voluson E8 Expert ultrasound system (General Electric, Zipf, Austria) equipped with a 2.8‐10 MHz transvaginal probe in the lithotomy position with an empty urinary bladder. Imaging was performed in the mid‐sagittal plane with the angle of acquisition set at 120°. Two volume datasets in longitudinal and transverse sections were acquired and stored for later analysis using the software 4d view (General Electric Medical Kretz technik, Zipf, Austria). Data analysis were undertaken by two of the authors (J.H. and L.K.), blinded to clinical data. The following sonographic features were assessed: position of the uterus (anteflexion or retroflexion), visibility of the CS scar, presence of a scar defect (yes or no); any visible defect in the scar was classified as a defect. The following scar measurements were taken: total myometrial thickness, residual myometrial thickness (RMT), scar width, the distance between the scar and the external cervical os. We also noted myometrial defects with or without contact with the uterine cavity (scar defect character) (Figure 2).
Figure 2.
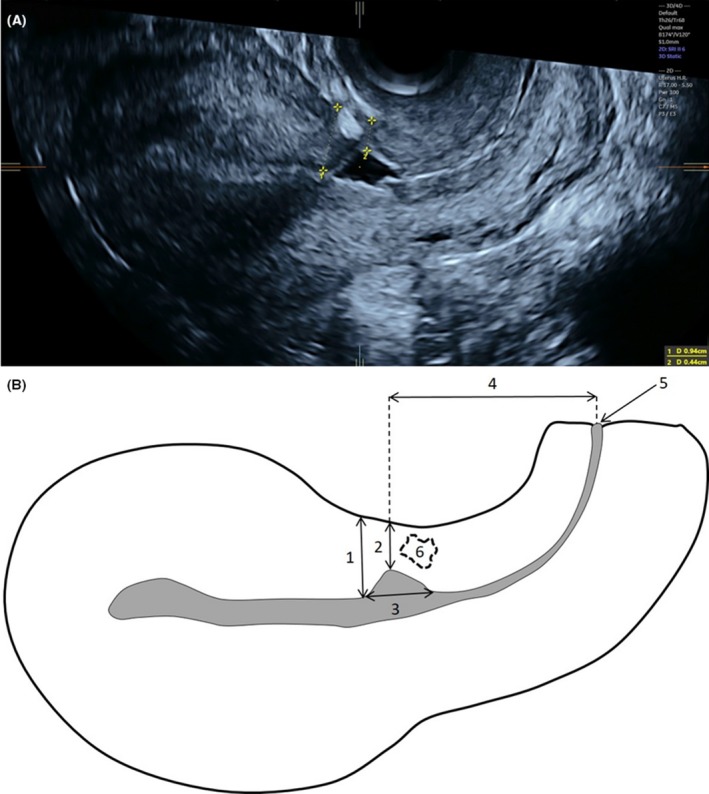
(A) Transvaginal ultrasound demonstrating measurement of total myometrial thickness (1) and residual myometrial thickness (2). (B) Schematic diagram showing CS scar placement and dimensions measurement: total myometrial thickness (1), residual myometrial thickness (2), width of the scar defect (3), distance between the scar and the external cervical ostium (4), external cervical ostium (5), myometrial defects without contact with the uterine cavity (6) [Color figure can be viewed at http://wileyonlinelibrary.com]
Primary outcome was the mean RMT related to the SLT or DLT. Secondary outcomes included: frequency of uterine defects, the position of the uterus, frequency of uterine defects with respect to the position of the uterus, position of the scar and frequency of defects with respect to the stage of labor in which the CS was performed. We recorded the incidence of selected postoperative complications at 6 weeks postpartum.
Statistical analyses were performed using SPSS software version 13.0 (IBM Corp., Armonk, NY, USA). Participant baseline and ultrasound characteristics were presented. The SLT and DLT group were compared. For continuous, normally distributed variables, we used Student's t test. The nonparametric tests (Wilcoxon‐Mann‐Whitney test) were used for continuous, non‐normally distributed variables. To test symmetry in the contingency table with dichotomous variables we used Fisher's exact test. A P value <0.05 was considered to be significant. Apart from simple tests, linear model (two‐way ANOVA) was carried out to examine the effect of vaginal findings during indication for CS on the scar. To test the development of categorized variables (including dichotomous variables) over time and dependence on suturing techniques, the generalized linear mixed model was used.
2.1. Ethical approval
The design of this study was approved by the institutional ethics committee (ethics committee number 3/2010).
3. RESULTS
A total of 540 pregnant women (270 in the SLT, 270 in the DLT) were included in the study. Drop‐out rate was 216 cases (40%) (Figure 3). Table 1 summarizes the basic demographics and obstetric data of the 12‐month visit cohort and those who dropped out. Six weeks postpartum, there were no differences in uterine position, presence or localization of the defect and scar sonomorphology. In all, 324 women attended the 12‐month visit, 149 of whom underwent SLT and 175 DLT. Their demographic and obstetric data did not differ, nor did the incidence of selected complications (Table 2). Repeated observational data on uterine position, presence and type of defects with missing cases are presented in Tables 3, 4, 5, 6. The CS scar measurements at 12 months postpartum are listed in Table 7. Defects in the SLT group were significantly (0.002) wider (4.8 vs 4.0 mm). The RMT in the SLT group were significantly (0.019) thinner (4.6 vs 5.2 mm). At 12 months there were no significant difference in the total myometrial thickness (0.777). One year postpartum, defects in the DLT group were significantly (0.002) closer to the external cervical os (30.0 vs 33.0 mm). Women who underwent CS at the stage of full cervical dilation had scars that were closer to the external cervical os (0.000), RMT was thinner (0.010) and width of the defect smaller (0.001). Other ultrasound scar measurements were not influenced by the vaginal finding at the time of the CS indication.
Figure 3.
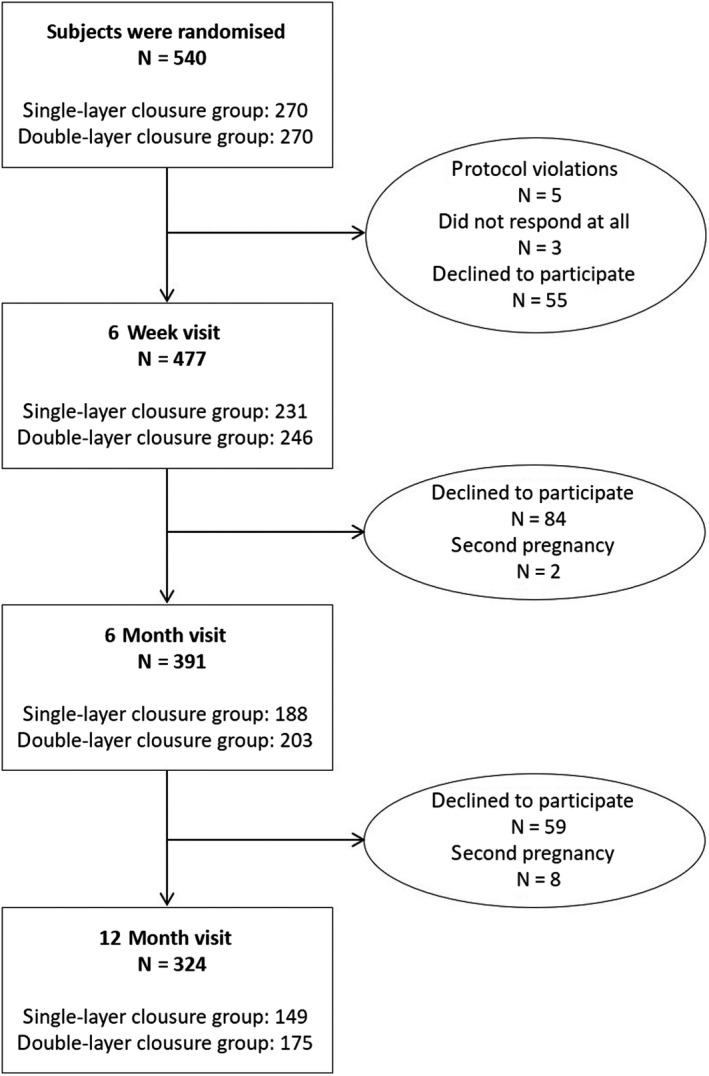
Flow chart summarizing selection of participants who underwent single‐ or double‐layer uterine suture technique. We observed five protocol violations
Table 1.
Demographics and obstetric data comparison of study subjects who attended the 12‐month visit and women who dropped out. Ultrasound outcomes comparison of study subjects attending the 12‐month visit and women who dropped out after the 6‐week check‐up
| Parameter | Follow up | P | |
|---|---|---|---|
| 12‐month visit (n = 324) | Drop out (n = 216) | ||
| Demographic and obstetrics details | |||
| Maternal age (y) | 31.7 ± 3.8 | 31.1 ± 4.2 | 0.139b |
| BMI (kg/m2) | 22.9 (20.2‐24.8) | 23.1 (20.5‐27.8) | 0.925c |
| Gestational age at delivery (gestational weeks) | 40.2 (39‐41) | 40.2 (39‐41) | 0.928c |
| Previous surgery on the uterus | 43 (13.3) | 23 (15.0) | 0.612d |
| Assisted reproduction: IVF/ICSI | 21 (6.5) | 9 (5.9) | 0.489d |
| Gestational diabetes | 12 (3.7) | 7 (4.6) | 0.409d |
| Hypertensive disorders | 11 (3.4) | 4 (2.8) | 0.443d |
| Type of cesarean section | |||
| Acute in pregnancy | 15 (4.6) | 2 (0.9) | 0.062d |
| Acute during labor | 132 (40.7) | 92 (42.6) | |
| Planned in pregnancy | 39 (12.0) | 29 (13.4) | |
| Planned during labor | 138 (42.6) | 93 (43.1) | |
| Hysterotomy closure | |||
| Single‐layer | 149 (46.0) | 100 (46.3) | 0.167d |
| Double‐layer | 175 (54.0) | 116 (53.7) | |
| Cervical dilation | |||
| No dilation | 91 (28.1) | 54 (25.0) | 0.360d |
| Partial dilation | 166 (51.2) | 125 (57.9) | |
| Full dilation | 67 (20.7) | 37 (17.1) | |
| 6‐week control (n = 153)a | |||
| Uterine position | |||
| Anteflexion | 176 (54.3) | 88 (56.1) | 0.570d |
| Retroflexion | 148 (45.7) | 65 (42.5) | |
| CS scar description | |||
| Intact scar | 62 (19.1) | 38 (24.8) | 0.199d |
| Presence of scar defect | 262 (80.9) | 115 (75.2) | |
| Scar measurements and localization | |||
| Total myometrial thickness (mm) | 11.5 (9.7‐13.0) | 10.5 (9.2‐12.1) | 0.115c |
| Residual myometrial thickness (mm) | 5.8 ± 2.2 | 5.6 ± 1.9 | 0.340b |
| Width of the defect (mm) | 4.3 (2.9‐5.9) | 4.2 (2.8‐5.8) | 0.180c |
| Scar‐external cervical os distance (mm) | 31.0 (27.0‐35.0) | 33.0 (28.0‐35.4) | 0.211c |
Abbreviations: CS, cesarean section; IVF/ICSI, in vitro fertilization/intracytoplasmatic sperm injection.
The 63 women who did not attend the 6‐week check‐up were not included. Characteristics are presented as mean ± SD for normally distributed variables, median and interquartile range for non‐normally distributed variables. Categorical variables were presented as total number (percentage in group).
Student′s t test.
Wilcoxon‐Mann‐Whitney test.
Pearson Chi‐square test.
Table 2.
Demographics, obstetric data and selected complications at 6 week postpartum of study subjects who finished the 12‐month follow up. Characteristics are presented as median and interquartile range for non‐normally distributed variables. Categorical variables are presented as total number (percentage in group)
| Parameter | Closure technique | P | |
|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | ||
| Demographic and obstetrics details | |||
| Maternal age (y) | 31.0 (29.0‐34.0) | 32.0 (29.0‐34.0) | 0.392a |
| BMI (kg/m2) | 22.4 (20.4‐25.3) | 22.3 (20.1‐24.2) | 0.602a |
| Gestational age at delivery (gestational weeks) | 40.0 (39.0‐41.0) | 40.0 (40.0‐41.0) | 0.446a |
| Type of cesarean section | |||
| Acute in pregnancy | 8 (5.4) | 8 (4.6) | 0.850b |
| Acute during labor | 62 (41.6) | 70 (40.0) | |
| Planned in pregnancy | 16 (10.7) | 22 (12.6) | |
| Planned during labor | 63 (42.3) | 75 (42.9) | |
| Cervical dilation | |||
| No dilation | 42 (28.2) | 49 (28.0) | 0.245b |
| Partial dilation | 82 (55.0) | 84 (48.0) | |
| Full dilation | 25 (16.8) | 42 (24.0) | |
| 6‐week complications | |||
| None | 135 (90.6) | 159 (90.9) | 0.263b |
| Maternal infectious morbidity | 7 (4.7) | 7 (4.0) | |
| Operative procedures on wound | 4 (2.7) | 1 (0.6) | |
| Other (placental remnants, transfusion) | 3 (2.0) | 8 (4.6) | |
Wilcoxon‐Mann‐Whitney test.
Pearson Chi‐square test.
Table 3.
Repeated observation data on presence of scar defects with missing cases. Categorical variables are presented as total number (percentage in group)
| Follow up | Closure technique | |||
|---|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | |||
| Presence of scar defect | ||||
| Yes | No | Yes | No | |
| 6 weeksa | 187 (81.0) | 44 (19.0) | 191 (77.6) | 55 (22.4) |
| 6 monthsb | 148 (78.7) | 40 (21.3) | 155 (76.4) | 48 (23.6) |
| 12 monthsc | 124 (83.2) | 25 (16.8) | 127 (72.6) | 48 (27.4) |
Total number of women who attended the 6‐week follow up = 477.
Total number of women who attended the 6‐month follow up = 391.
Total number of women who attended the 12‐month follow up = 324.
Table 4.
Repeated observation data on uterine position with missing cases. Categorical variables are presented as total number (percentage in group)
| Follow up | Closure technique | |||
|---|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | |||
| Uterine position | ||||
| Anteflexion | Retroflexion | Anteflexion | Retroflexion | |
| 6 weeksa | 122 (52.8) | 109 (47.2) | 141 (57.3) | 105 (42.7) |
| 6 monthsb | 123 (65.4) | 65 (34.6) | 151 (74.4) | 52 (25.6) |
| 12 monthsc | 90 (60.4) | 59 (39.6) | 125 (71.4) | 50 (28.6) |
Total number of women who attended the 6‐week follow up = 477.
Total number of women who attended the 6‐month follow up = 391.
Total number of women who attended the 12‐month follow up = 324.
Table 5.
Repeated observation data on scar defects in anteflexion/retroflexion (AVF/RVF) uterus with missing cases. Categorical variables are presented as total number (percentage in group)
| Follow up | Closure technique | |||
|---|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | |||
| Scar defect in AVF uterus | ||||
| Yes | No | Yes | No | |
| 6 weeksa | 105 (86.1) | 17 (13.9) | 116 (82.3) | 25 (17.7) |
| 6 monthsb | 102 (82.9) | 21 (17.1) | 117 (77.5) | 34 (22.5) |
| 12 monthsc | 81 (90.0) | 9 (10.0) | 90 (72.0) | 35 (28.2) |
| Follow up | Scar defect in RVF uterus | |||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| 6 weeksa | 82 (75.2) | 27 (24.8) | 75 (71.4) | 30 (28.6) |
| 6 monthsb | 46 (70.8) | 19 (29.2) | 38 (73.1) | 14 (26.9) |
| 12 monthsc | 43 (72.9) | 16 (27.1) | 37 (74.0) | 13 (26.0) |
Total number of women who attended the 6‐week follow up = 477.
Total number of women who attended the 6‐month follow up = 391.
Total number of women who attended the 12‐month follow up = 324.
Table 6.
Repeated observation data on scar defect presence and defect contact with the uterine cavity with missing cases. Categorical variables are presented as total number (percentage in group)
| Follow up | Closure technique | |||||
|---|---|---|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | |||||
| Presence and defect contact with the uterine cavity | ||||||
| Yes | No | Normal scar | Yes | No | Normal scar | |
| 6 weeksa | 111 (48.1) | 76 (32.9) | 44 (19.0) | 118 (48.0) | 73 (29.7) | 55 (22.4) |
| 6 monthsb | 112 (59.6) | 36 (19.1) | 40 (21.3) | 114 (56.2) | 41 (20.2) | 48 (23.6) |
| 12 monthsc | 84 (56.4) | 40 (26.8) | 25 (16.8) | 92 (52.6) | 35 (20.0) | 48 (27.4) |
Yes: scar defect is present and is in contact with the cavity; No: scar defect is present and is not in contact with the cavity, normal scar defect is not present.
Total number of women who attended the 6‐week follow up = 477.
Total number of women who attended the 6‐month follow up = 391.
Total number of women who attended the 12‐month follow up = 324.
Table 7.
Sonographic cesarean section (CS) scar measurement and localization at 12‐month follow up and relation to cervical dilation at time of delivery. Characteristics are presented as mean ± SD for normally distributed variables, median and interquartile range for non‐normally distributed variables
| Parameter | Closure technique | P | |
|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | ||
| CS scar description | |||
| Scar measurements and localization | |||
| Total myometrial thickness (mm) | 10.0 (8.5‐11.5) | 10.0 (8.6‐11.7) | 0.777b |
| Residual myometrial thickness (mm) | 4.6 (±1.9) | 5.2 (±2.2) | 0.019a |
| Width of the defect (mm) | 4.8 (3.2‐6.6) | 4.0 (3.0‐5.4) | 0.002b |
| Scar‐external cervical os distance (mm) | 33.0 (29.0‐37.0) | 30.0 (25.7‐34.7) | 0.002b |
| Residual myometrial thicknes <2.5 mm | 18 (12.2) | 12 (6.8) | 0.019a |
| CS scar description and relation to vaginal finding at CS indication | |||
| Total myometrial thickness | |||
| No cervical dilation | 10.5 (±2.6) | 10.1 (±2.0) | 0.533c |
| Partial cervical dilation | 10.2 (±2.1) | 10.2 (±1.8) | |
| Full cervical dilation | 8.8 (±2.4) | 9.9 (±2.4) | |
| Residual myometrial thickness | |||
| No cervical dilation | 4.8 (±1.7) | 5.7 (±2.1) | 0.010c |
| Partial cervical dilation | 4.7 (±2.0) | 5.0 (±1.9) | |
| Full cervical dilation | 4.3 (±2.2) | 5.0 (±2.5) | |
| Width of the defect | |||
| No cervical dilation | 5.6 (±2.8) | 4.3 (±2.1) | 0.001c |
| Partial cervical dilation | 4.7 (3.2‐6.0) | 4.0 (3.2‐5.5) | |
| Full cervical dilation | 4.0 (2.9‐7.3) | 4.0 (3.1‐5.3) | |
| Scar‐external cervical os distance | |||
| No cervical dilation | 35.5 (29.3‐37.0) | 33.0 (29.0‐39.0) | 0.000c |
| Partial cervical dilation | 33.6 (±6.1) | 30.1 (±5.5) | |
| Full cervical dilation | 28.9 (±9.0) | 27.0 (±5.8) | |
Student′s t test.
Wilcoxon‐Mann‐Whitney test.
Two‐way ANOVA (with interaction).
Longitudinal observational data on uterine position and presence and type of defects in this cohort are presented in Tables 8, 9, 10, 11. The incidence of scar defects was not statistically significant between controls. The difference between groups with different suturing methods was statistically significant (0.036). A higher proportion of the defects were seen in the SLT group.The position of the uterus varied greatly between controls (0.000), especially between 6‐week and 6‐month controls. The difference between the 6‐ and 12‐month controls was already statistically insignificant. The difference between groups with different suturing methods was not statistically significant. The combination of uterine position and scar defect presence changed significantly between controls (0.001) and it varied significantly depending on the suturing method (0.003). A higher incidence of uterine position in retroflexion and defects was seen in the 6‐week control in the SLT group. Defects with or without contact with the uterine cavity changed statistically between controls (0.017). Both types of defects occurred more frequently in the 6‐week follow up than in stage II and III controls. The difference based on suturing method is at the limit of statistical significance (0.065). Both types of defects are more common in the SLT group. For any of the variables uterine position, scar defect, combination of both and scar placement, the difference between groups according to the suturing method did not change statistically significantly over time (interaction of time and suture type is not significant).
Table 8.
Longitudinal observation data on Presence of scar defect in women with all three follow up controls (total number of women who attended the 12‐month follow up, with all three controls = 324). Test of dependence of outcome on time (generalized linear mixed model): test significance of variable Time is 0.247, test significance of variable Closure technique is 0.036 and test significance of interaction Time with Closure technique (change of closure technique outcome in time) is 0.370
| Follow up | Closure technique | |||
|---|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | |||
| Presence of scar defect | ||||
| Yes | No | Yes | No | |
| 6 weeks | 123 (82.6) | 26 (17.4) | 140 (80.0) | 35 (20.0) |
| 6 months | 118 (79.2) | 31 (20.8) | 129 (73.7) | 46 (26.3) |
| 12 months | 124 (83.2) | 25 (16.8) | 127 (72.6) | 48 (27.4) |
Table 9.
Longitudinal observation data on Uterine position in women with all three follow‐up controls (total number of women who attended 12‐month follow up, with all three controls = 324). Test of dependence of outcome on time (generalized linear mixed model): test significance of variable Time is 0.000, test significance of variable Closure technique is 0.132 and test significance of interaction Time with Closure technique (change of closure technique outcome in time) is 0.270
| Follow up | Closure technique | |||
|---|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | |||
| Uterine position | ||||
| Anteflexion | Retroflexion | Anteflexion | Retroflexion | |
| 6 weeks | 78 (52.3) | 71 (47.7) | 97 (55.4) | 78 (46.6) |
| 6 months | 97 (65.1) | 52 (34.9) | 128 (73.1) | 47 (26.9) |
| 12 months | 90 (60.4) | 59 (39.6) | 125 (71.4) | 50 (28.6) |
Table 10.
Longitudinal observation data on Scar defect in anteflexion/retroflexion (AVF/RVF) uterus in women with all three follow‐up controls (total number of women who attended 12‐months follow up, with all three controls = 324). Test of dependence of outcome on time (generalized linear mixed model): test significance of variable Time is 0.001, test significance of variable Closure technique is 0.003 and test significance of interaction Time with Closure technique (change of closure technique outcome in time) is 0.821
| Follow up | Closure technique | |||
|---|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | |||
| Scar defect in AVF uterus | ||||
| Yes | No | Yes | No | |
| 6 weeks | 70 (89.7) | 8 (10.3) | 80 (82.5) | 17 (17.5) |
| 6 months | 81 (83.5) | 16 (16.5) | 94 (73.4) | 34 (26.6) |
| 12 months | 81 (90.0) | 9 (10.0) | 90 (72.0) | 35 (28.2) |
| Follow up | Scar defect in RVF uterus | |||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| 6 weeks | 53 (74.6) | 18 (25.4) | 60 (76.9) | 18 (23.1) |
| 6 months | 37 (71.2) | 15 (28.8) | 35 (74.4) | 12 (25.5) |
| 12 months | 43 (72.9) | 16 (27.1) | 37 (74.0) | 13 (26.0) |
Table 11.
Longitudinal observation data on Scar defect presence and defect contact with the uterine cavity in women with all three follow‐up controls (total number of women who attended 12‐month follow up, with all three controls = 324). Test of dependence of outcome on time (generalized linear mixed model): test significance of variable Time is 0.017, test significance of variable Closure technique is 0.065 and test significance of interaction Time with Closure technique (change of closure technique outcome in time) is 0.493
| Follow up | Closure technique | |||||
|---|---|---|---|---|---|---|
| Single‐layer (n = 149) | Double‐layer (n = 175) | |||||
| Presence and defect contact with the uterine cavity | ||||||
| Yes | No | Normal scar | Yes | No | Normal scar | |
| 6 weeks | 75 (50.3) | 48 (32.2) | 26 (17.4) | 87 (49.7) | 53 (30.3) | 35 (20.0) |
| 6 months | 91 (61.1) | 27 (18.1) | 31 (20.8) | 93 (53.1) | 36 (20.6) | 46 (26.3) |
| 12 months | 84 (56.4) | 40 (26.8) | 25 (16.8) | 92 (52.6) | 35 (20.0) | 48 (27.4) |
4. DISCUSSION
The prevalence of scar defects on transvaginal ultrasound in our study was 83.2% in the SLT and 72.6% in the DLT group at the 12‐month follow up. We observed a higher prevalence of defects compared with other groups after first CS (37‐61%).3, 9, 10, 12 The discrepancy is most likely to be explained not only by the different definitions of defect that have been used in different studies, but also by including defects that were not in contact with the uterine cavity. The 12‐month prevalence of more serious defects (RMT <2.5 mm)9, 13, 14 in contact with the cavity was 12.2% in the SLT and 6.8% in the DLT group.
We show that the presence of a defect and the scar position are relatively stable during the first postpartum year but that their appearance changes. Defects with or without contact with the uterine cavity changed statistically between controls. Both types of defects occurred more frequently in the 6‐week follow up and were more common in the SLT group. The most notable example was the defect without contact with the uterine cavity, which represented 30% of scar defects at 6 weeks and 20% at 6 months. During healing, this structure can disappear and can be changed into defects with contact with the cavity. Healing is a long‐term, ongoing process of tissue remodeling, peaking in intensity within 6 months after the primary insult.15, 16 On the basis our data we assume that CS scar healing should be completed after 6 months. This is consistent with previous studies.14
The most important decision is whether to use an SLT or DLT to improve the scar quality and decrease the risk of uterine rupture and dehiscence in the subsequent pregnancy. Data from Swedish registers demonstrate no significant difference in the rate of uterine rupture.17 An earlier meta‐analysis including retrospective and prospective studies reported that locked SLT, compared with DLT, is associated with a fourfold increase in the risk of uterine rupture.18 We do not have any clinical data from subsequent pregnancies in our patients and are thus unable to confirm this finding.
We have shown that DLT is associated with smaller defects and with thicker RMT. This is in contrast to evidence based on randomized trials which does not support a specific type of uterine closure. SLT and locked first layer are possibly associated with thinner residual RMT.19
Prospective studies using transvaginal ultrasound of the scar, favor an unlocked suture with exclusion of the decidua to optimize the placement of the muscle layers and their regeneration.19, 20 Roberge et al demonstrate in a randomized controlled trial that DLT with a first unlocked layer excluding the decidua, compared with locked SLT including the decidua, is associated with a greater RMT and healing ratio.21 But the lack of statistical power did not allow the authors to draw a definitive conclusion. It is possible that excluding the decidua from the first suture induces a better adaptation of myometrium. We have included decidua in the first suture and thus are unable to confirm this suggestion.
The reason for the more distal scars and defects in women with CS at full dilation is clear. At stage II of labor, the cervix creates a continuous birth canal with the uterine cavity and is pulled up. Therefore, the incision is finally located caudal to the external cervical ostium.
In contrast to other studies, we were not able to prove that scars with larger defects reside more caudally than intact scars or scars with smaller defects.22 But those findings were influenced by cases with more than one CS.
We have shown a change in uterine position between 6 weeks and 1 year from retroflexion to anteflexion, with no relation to closure technique. We do not have data on its position before the pregnancy and thus were unable to demonstrate that the CS and the closure technique affected it. The reason for change in uterine position is unknown: it could be due to tissue healing and scar remodeling, but it may also interfere with healing and tissue extension. Vikhareva Osser et al observed more scar defects in women with a uterus in retroflexion.9 A higher incidence of uterine position in retroflexion and defects was only seen in our study in the 6‐week control in the SLT group.
Our study is not limited only to CS performed in women before or in early labor. Women in advanced labor were also included. The women in both groups were selected from the same caucasian community and the comparability between the two groups was high. The scar was longitudinally evaluated by two independent observers blinded to the treatment allocation in a population having a primary CS. Another strength of our study was the uniform use of a specific suture method for both closures. On the other hand, cervical dilation, duration of labor or oxytocin augmentation are factors that increase the risk of a large scar defect in non‐pregnant women.23
The main limitation of the study is the drop‐out rate. We did not examine further why women discontinued the follow up. Another limitation is that RMT represents an indirect evaluation of scar healing and demonstrates a surrogate outcome for the prediction of negative consequences. On the other hand, there are enough data showing that the presence of scar defects and RMT value are correlated with such adverse events.10, 23 Also, we did not perform an ultrasonographic examination according to menstrual cycle, as recommended by another group.7 Synchronization was not possible because many women were breastfeeding and had secondary amenorrhea. This study was primarily an urogynecological research targeted at the influence of the first pregnancy and delivery on female pelvic floor; therefore this subanalysis, which is focused on scar assessment after CS, was not registered as a randomized control trial.
5. CONCLUSION
Our data demonstrate the benefit of DLT. Defects in SLT group were more common, wider and had thinner RMT. Most changes in the scar area occurred during the first 6 months. Although recent discussion has focused mainly on the number of suture layers, the current knowledge highlights the importance of decidua suture exclusion.
CONFLICT OF INTERESTS
The authors have stated explicitly that there are no conflicts of interest in connection with this article. This study was supported by PROGRES Q 34, Charles University project, Prague, Czech Republic.
Hanacek J, Vojtech J, Urbankova I, et al. Ultrasound cesarean scar assessment one year postpartum in relation to one‐ or two‐layer uterine suture closure. Acta Obstet Gynecol Scand. 2020;99:69–78. 10.1111/aogs.13714
Funding information
This study was supported by PROGRES Q 34, Charles University project, Prague, Czech Republic.
REFERENCES
- 1. Martin JA, Hamilton BE, Osterman MJK, Curtin SC, Mathews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1‐65. [PubMed] [Google Scholar]
- 2. Dietz HP, Campbell S. Toward normal birth—but at what cost? Am J Obstet Gynecol. 2016;215:439‐444. [DOI] [PubMed] [Google Scholar]
- 3. Ofili‐Yebovi D, Ben Nagi J, Sawyer E, et al. Deficient lower segment Caesarean section scars: prevalence and risk factors. Ultrasound Obstet Gynecol. 2008;31:72‐77. [DOI] [PubMed] [Google Scholar]
- 4. Timor‐Tritsch IE, Cali G, Monteagudo A, et al. Foley baloon catheter to prevent or manage bleeding during treatment for cervical and Cesarean scar pregnancy. Ultrasound Obstet Gynecol. 2015;46:118‐123. [DOI] [PubMed] [Google Scholar]
- 5. Wang CB, Chiu WWC, Lee CY, Sun YL, Lin YH. Caesarean scar defect: correlation between Caesarean section number, defect size, clinical symptoms and uterine position. Ultrasound Obstet Gynecol. 2009;34:85‐89. [DOI] [PubMed] [Google Scholar]
- 6. Fabres C, Arriagada P, Fearnandez C, Mackenna A, Zegers F, Fernandez E. Surgical treatment and follow‐up of women with intermenstrual bleeding due to caesarean section scar defect. J Minim Invasive Gynecol. 2005;12:25‐28. [DOI] [PubMed] [Google Scholar]
- 7. Bij de Vaate AJM, Brölmann HAM, Van Der Voet LF, Van Der Slikke JW, Veersema S, Huirne JAF. Ultrasound evaluation of the cesarean scar: relation between a niche and postmenstrual spotting. Ultrasound Obstet Gynecol. 2011;37:93‐99. [DOI] [PubMed] [Google Scholar]
- 8. Baranov A, Gunnarsson G, Salvesen KA, Isberg PE, Vikhareva O. Cesarean hysterotomy scar in non‐pregnant women: reliability of transvaginal sonography with and without contrast enhancement. Ultrasound Obstet Gynecol. 2016;47:499‐505. [DOI] [PubMed] [Google Scholar]
- 9. Vikhareva Osser O, Jokubkiene L, Valentin L. High prevalence of defects in Caesarean section scars at transvaginal ultarsound examination. Ultrasound Obstet Gynecol. 2009;34:90‐97. [DOI] [PubMed] [Google Scholar]
- 10. Roberge S, Boutin A, Chaillet N, et al. Systematic review of cesarean scar assessment in the nonpregnant state: imaging techniques and uterine scar defect. Am J Perinatol. 2012;29:465‐472. [DOI] [PubMed] [Google Scholar]
- 11. Jastrow N, Chaillet N, Roberge S, Morency AM, Lacasse Y, Bujold E. Sonographic lower uterine segment thickness and risk of uterine scar defect: a systematic review. J Obstet Gynaecol Can. 2010;32:321‐327. [DOI] [PubMed] [Google Scholar]
- 12. Armstrong V, Hansen WF, Van Voorhis BJ, Syrop CH. Detection of caesarean scars by transvaginal ultrasound. Obstet Gynecol. 2003;101:61‐65. [DOI] [PubMed] [Google Scholar]
- 13. Vikhareva Osser O, Valentin L. Clinical importance of appearance of caesarean hysterotomy scar at transvaginal ultrasonography in nonpregnant women. Obstet Gynecol. 2011;117:525‐532. [DOI] [PubMed] [Google Scholar]
- 14. Vikhareva Osser O, Jokubkiene L, Valentin L. Caesarean section scar defects: agreement between transvaginal sonographic findings with and without saline contrast enhancement. Ultrasound Obstet Gynecol. 2010;35:75‐83. [DOI] [PubMed] [Google Scholar]
- 15. Whaley K, Burt A. Factors influencing wound healing In: MacSween R, Whaley K, eds. Muir's Textbook of Pathology. London: Edward Arnold;1992:160‐161. [Google Scholar]
- 16. Valenzano Menada M, Lijoi D, Mistrangelo E, Constantini S, Ragni N. Vaginal ultrasonography and hysterosonography evaluation of the low transverse incision after caesarean section: correlation with gynecological symptoms. Gynecol Obstet Invest. 2006;61:216‐222. [DOI] [PubMed] [Google Scholar]
- 17. Hesselman S, Högberg U, Ekhlom‐Selling K, Rassjö EB, Jonsson M. The risk of uterine rupture is not increased with single‐ compared with double‐layer closure: a Swedish cohort study. BJOG. 2015;122:1535‐1541. [DOI] [PubMed] [Google Scholar]
- 18. Roberge S, Berghella V, Chaillet N, Moore L, Bujold E. Impact of single‐ vs double‐layer closure on adverse outcomes and uterine scar defect: a systematic review and metaanalysis. Am J Obstet Gynecol. 2014;211:453‐460. [DOI] [PubMed] [Google Scholar]
- 19. Roberge S, Chaillet N, Boutin A, et al. Single‐ versus double‐layer closure of the hysterotomy incision during caesarean delivery and risk of uterine rupture. Int J Gynaecol Obstet. 2011;115:5‐10. [DOI] [PubMed] [Google Scholar]
- 20. Turan GA, Gur EB, Tatar S, Gokduman A, Guclu S. Uterine closure with unlocked suture in cesarean section: safety and quality. Pak J Med Sci. 2014;30:530‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberge S, Demers S, Girard M, et al. Impact of uterine closure on residual myometrial thickness after cesarean: a randomized controlled trial. Am J Obstet Gynecol. 2016;214:507.e1–507.e6. [DOI] [PubMed] [Google Scholar]
- 22. Vikhareva Osser O, Valentin L. Risk factors for incomplete healing of the uterine incision after caesarea section. BJOG. 2010;117:1119‐1126. [DOI] [PubMed] [Google Scholar]
- 23. van der Voet L, de Vaate B, Veersema S, Brolmann H, Huirne J. Long‐term complications of cesarean section. The niche in the scar: a prospective cohort study on niche prevalence and its relation to abnormal uterine bleeding. BJOG. 2014;121:236‐244. [DOI] [PubMed] [Google Scholar]


