Abstract
Background
Red tattoos are prone to allergic reactions. The identity of the allergen(s) is mostly unknown.
Objectives
Chemical analysis of human skin biopsies from chronic allergic reactions in red tattoos to identify culprit pigment(s) and metals.
Material and methods
One hundred four dermatome biopsies were analyzed by matrix‐assisted laser desorption/ionization tandem mass spectrometry (MALDI‐MS/MS) for identification of commonly used organic pigments. Metal concentrations were assessed by inductively coupled plasma (ICP)‐MS and x‐ray fluorescence (XRF). Fourteen patients had cross‐reactions in other red tattoos.
Results
In total, the identified pigments were mainly azo Pigment Red (P.R.) 22 (35%), P.R. 210 (24%), P.R. 170 (12%), P.R. 5 (0.9%), P.R. 112 (0.9%), and Pigment Orange (P.O.) 13 (11%). P.R. 122 (0.9%) and Pigment Violet (P.V.) 23 (8%) were also common. P.R. 22, P.R. 170, and P.R. 210 also dominated in patients with cross‐reactions. In 22% of the biopsies, no red pigment was detected. Element analysis indicated the presence of the sensitizers nickel and chromium.
Conclusions
P.R. 22, P.R. 170, and P.R. 210 were identified as the prevailing pigments behind chronic allergic reactions in red tattoos. The epitope causing the reaction might be a pigment‐degradation product. Metal contamination may derive from different sources, and its role in red tattoo allergy cannot be ascertained.
Keywords: allergy, nickel, pigments, Pigment Red 170/210, Pigment Red 22, tattoo reaction
First study on chemical analysis of human skin biopsies from chronic allergic reactions in red tattoos to identify culprit pigment(s) and metals.
Pigment Red (P.R.) 22, P.R.170, and P.R.210 were the prevailing pigments behind chronic allergic reactions in red tattoos. The epitope causing the reaction might be a pigment‐degradation product.
Metal contamination is very common in any tattoo ink stock product, and its role in red tattoo allergy appears dubious.
1. INTRODUCTION
Allergy in tattoos is seen mainly in red colors and in shades of red.1, 2, 3, 4 In a review of 405 patients with 493 tattoo reactions treated at the Bispebjerg University Hospital, Department of Dermatology, the “Tattoo Clinic” in Copenhagen, chronic allergic reactions were predominant and found in 37% of all reactions.2 This was confirmed in a study of 101 patients reported by the “Tattoo Clinic” in Amsterdam using the same diagnostic classification system.4 Dermatitis is a manifestation of a delayed‐type allergic response. Patients experience the reaction as severe itching and discomfort, reducing their quality of life on a level similar to that of pruritic dermatologic diseases involving larger skin areas.5 Tattoo allergies often develop with a latency of months or years, but then their occurrence is mostly abrupt. Tattoo allergy also may occur in purple, violet, green, blue, and yellow tattoos but seemingly not in black tattoos.2 Black pigment is composed of amorphous carbon (carbon black) or, exceptionally, of black iron oxides.6 Soluble potential sensitizers such as preservatives and chemical impurities will be removed quickly from the site of the tattoo and are thus not likely to cause the aforementioned delayed local reactions.
In the last decades, mineral pigments have been widely replaced by highly colored, brilliant organic pigments.7, 8 Their main chemical classes are azo pigment, quinacridones, and phthalocyanines.6 Case reports indicate that the two former classes may be sensitizers and the main reason for allergy in red nuanced tattoos.9 However, most studies fail to prove a causative relationship of allergic reactions of tattoos and organic pigments. This is because reports often identify pigments through the list of ingredients on the labels on ink bottles, and do not verify pigments by chemical analysis in ink or in the patients' skin. Approximately one‐third of ink labels provide false information concerning the pigments used.10 So far, the only study providing evidence of sensitization and on presence of the same organic pigment contained in the ink was related to the thioindigo derivative Pigment Red (P.R.) 181.11 However, this report included only four patients, who were tattooed with cosmetic tattoo inks originating from the same manufacturer.
Patch testing, with the putative inks suspected of causing the allergic tattoo reaction, fails to induce a positive outcome of the test.9, 12, 13 Therefore, it was speculated whether the allergen may be a hapten formed in the skin over time, possibly with some pigment‐derived decomposition product making up the epitope.12 Photochemical breakdown of pigments by UV or laser irradiation is suggested to contribute to tattoo allergy induction.12, 14, 15 In a cohort of 101 patients, 32% reported worsening of allergy symptoms after sun exposure, suggesting that sunlight might play a role in the development of allergic reactions.4 However, intermittent sun‐induced complaints are common in tattoos and occur at a similarly high rate.16 These complaints might be induced partly by titanium dioxide, a white pigment used for color blending in tattoo inks. Titanium dioxide is used in the crystal structures rutile and anatase, of which the latter is known to cause formation of reactive oxygen species upon UV irradiation and that also occurs in tattoo and permanent makeup inks.17
Until now, no large‐scale clinical study has been carried out aiming to identify specific organic pigments that are causing tattoo allergies. Particularly chemical analysis of the organic tattoo pigments present in the reactive skin is still missing. The aim of the current study was to identify organic pigments and metals in the skin of 104 patients with tattoo allergy. Because the preferred treatment of the “Tattoo Clinic” in Copenhagen is dermatome shaving, it was possible to harvest and freeze tissue samples for chemical analysis.18 Hence, dermatome‐shaving biopsies of the epidermis and outer dermis were obtained as a by‐product of surgery. Matrix‐assisted laser desorption/ionization tandem mass spectrometry (MALDI‐MS/MS) was used for identification of organic pigments and inductively coupled plasma (ICP)‐MS for the quantification of elements present in the skin biopsies.
2. MATERIALS AND METHODS
2.1. Patients and biopsies
In total, 104 shave‐biopsy samples were obtained from 104 patients who underwent surgery from 2015 to 2017 in the “Tattoo Clinic” of Bispebjerg University Hospital in Copenhagen. Samples were taken in accordance with the current Helsinki Declaration; patients accepted that the biologic waste material from dermatome shaving performed as a routine treatment of their tattoo allergy was donated for research and education. Patients are referred primarily from greater Copenhagen, but patients from other parts of Denmark with more serious complications are treated as well. Seventy‐one women and thirty‐three men participated with a mean age of 36 years (range: 18‐65). Sixty‐eight patients (65%) had tattoos localized on sun‐exposed areas: for example, neck (1 patient), forearm (29), wrist (8 patients), lower leg (20 patients), and ankle/foot (10 patients). Forty‐eight of 104 patients (46%) stated that they had no known allergies before, and 28 (27%) stated that they had metal allergy. Only patients with objective plaque elevation or excessive hyperkeratosis in a red tattoo or in tattoos of red nuances (light red, bordeaux, violet) were included (Figure 1).19 Fourteen patients had very strong allergic symptoms manifested as cross‐reactions, that is, when a recently tattooed skin area started to trigger a simultaneous reaction in one or more hitherto well‐tolerated tattoos of the same color at distant sites. The shave samples were stored immediately after surgery in a freezer at −18°C until analysis was performed. The shave biopsies were made by thin‐cut horizontal slicing performed from the skin surface down to a level in the dermis devoid of visible pigments. Samples were blinded and dispatched on dry ice to German Federal Institute for Risk Assessment (BfR) for chemical analysis. Wherever possible, patients were asked to collect inks from their tattooist for analysis.
Figure 1.
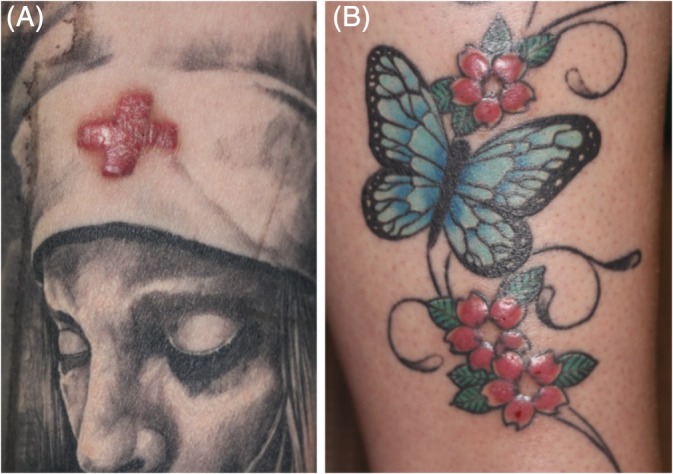
Clinical examples of allergic reactions in red tattoos, type plaque elevation (A) and type excessive hyperkeratosis (B) according to clinical classification used by the “Tattoo Clinics” in Copenhagen and Amsterdam2, 19
2.2. Identification of organic pigments by MALDI‐MS/MS
The 104 biopsies and 12 inks were analyzed by means of MALDI‐MS/MS to identify the organic pigments present in the samples. Skin specimens were digested with collagenase followed by mechanical disruption as described previously.20 A library of 40 known pigments comprising 19 red and violet pigments was used for identification (Table S1). P.R. 210 actually represents a mixture including P.R. 170 but was subsequently referred to only as P.R. 210. In case of equivocal results, lithium cation attachment was applied to verify the pigment's identity, as recently described by Schreiver et al.20 Identification of pigments can be carried out only by the targeted approach. Thus, pigments not present in the in‐house library could not be identified.
2.3. Quantification of metals by ICP‐MS
Elemental compositions in a total of 104 skin biopsies were analyzed using microwave digest for sample preparation and ICP‐MS as described elsewhere.21 In brief, 50 to 200 mg tissue or ink sample were digested in 1.5 mL ultrapure water, 2.5 mL nitric acid, and 1 mL hydrogen peroxide in Teflon vessels for microwave digestion (Ultraclave, MLS, Leutkirch, Germany). Elemental concentrations given in ppm are calculated in relation to the weight of digested sample. Copper and nickel standards for ICP were purchased from Sigma Aldrich (Munich, Germany). For chromium, iron, manganese, titanium and cadmium 1000 mg/L standard solutions in diluted nitric acid were obtained from VWR (Darmstadt, Germany). XSeries II ICP‐MS (Thermo Fischer Scientific, Bremen, Germany) together with an ESI SC2 autosampler (Elemental Service & Instruments, Mainz, Germany) was used for sample analysis. The collision cell was operated in −3.0 V mode. Data were processed with PlasmaLab 2.5.11.321 (Thermo Fischer Scientific).
2.4. XRF imaging and titanium speciation
X‐ray fluorescence (XRF) imaging was carried out on 35 biopsy lysates to screen for the presence of iron particles at beamline ID21. A rhodium‐coated mirror was used and the energy was tuned to 8.4 keV. Titanium speciation analysis by means of x‐ray absorption near edge structure (XANES) was carried out at beamline ID21 at the European Synchrotron (ESRF) in Grenoble as described elsewhere with the following amendments: Lysates prepared for MALDI‐MS/MS (see above) were placed on ultralene foils for analysis.21 XANES spectroscopy was carried out for 44 biopsy lysates. The samples size was restricted due to the limited amount of allocated beamtime at ESRF.
3. RESULTS
3.1. Identification of organic pigments
In total, 104 dermatome shaving biopsies from patients with a clinical diagnosis of allergic reaction in a red tattoo or in tattoos of red nuances (light red, bordeaux, violet) were included in the study. Typical clinical reactions are shown in Figure 1. Samples were analyzed with MALDI‐MS/MS to identify known organic pigments. Since the allergic reactions of interest occurred in the red part of the tattoo, identified pigments other than nuances of red were considered deviant. This is justified by the fact that dermatome shaving biopsies can include adjacent parts of a multi‐coloured tattoo and therefore may include pigments surrounding the red tattoo reaction. Samples from patients with strong allergy manifested as secondary allergic cross‐reactions in old and hitherto well‐tolerated tattoos were obtained from the most recent tattoo only; the triggering tattoo.
The shave biopsies contained the naphthol AS pigments P.R. 22 (35%), P.R. 210 (24%), P.R. 170 (36%), P.R. 5 (0.9%) and P.R. 112 (0.9%) in over 55% of all samples, see Table 1, Figure S1. Some biopsies contained more than one red pigment. Pigments of different chemical structures present in the biopsies were the diazo Pigment Orange (P.O.) 13 (12%), the dioxazine Pigment Violet (P.V.) 23 (8%) and the quinacridone P.R. 122, see Table 1, Figure 2. In 37 biopsies (36%), more than one type of pigment was detected. Fourteen (13%) patients presented clinical cross‐reactions in older tattoos. P.R. 210 was found in 43% of these samples compared to 21% in samples without cross‐reactions. P.R. 170 alone was similar for the two groups of allergies, 14% versus 11%. This contrasts analysis of pigment P.R. 22 which was found in 21% of the samples displaying cross‐reactions versus 37% in samples without cross‐reactions, Table 1.
Table 1.
Identified organic pigments in dermatome shave biopsies obtained from 104 patients with allergic reactions in red tattoos
| Identified pigments | C.I. number | Frequency in all biopsies N = 104 | Frequency in biopsies from patients with cross‐reaction (s) N = 14 | Frequency in biopsies from patients without cross‐reaction (s) N = 90 | Pigment class | |||
|---|---|---|---|---|---|---|---|---|
| P.R. 22 | 12 315 | 36 | 35% | 3 | 21% | 33 | 37% | Azo (Naphthol AS) |
| P.R. 210a | 12 477 | 25 | 24% | 6 | 43% | 19 | 21% | Azo (Naphthol AS) |
| P.R. 170 | 12 475 | 12 | 12% | 2 | 14% | 10 | 11% | Azo (Naphthol AS) |
| P.R. 122 | 73 915 | 1 | 0.9% | 1 | 7% | 0 | 0% | Quinacridone |
| P.R. 112 | 12 370 | 1 | 0.9% | 0 | 0% | 1 | 1% | Azo (Naphthol AS) |
| P.R. 5 | 12 490 | 1 | 0.9% | 0 | 0% | 1 | 1% | Azo (Naphthol AS) |
| P.V. 23 | 51 319 | 8 | 8% | 3 | 21% | 5 | 6% | Dioxazine |
| P.V. 19 | 73 900 | 1 | 0.9% | 0 | 0% | 1 | 1% | Quinacridone |
| P.O. 13 | 21 110 | 12 | 12% | 3 | 21% | 9 | 10% | Diazo |
| P.O. 16 | 21 160 | 2 | 2% | 0 | 0% | 2 | 2% | Diazo |
| P.Y. 74 | 11 741 | 5 | 5% | 0 | 0% | 5 | 6% | Azo |
| P.Y. 151 | 13 980 | 1 | 0.9% | 1 | 7% | 1 | 1% | Azo |
| P.Y. 138 | 56 300 | 1 | 0.9% | 0 | 0% | 1 | 1% | Quinaphthalone |
| P.Y. 1 | 11 680 | 1 | 0.9% | 0 | 0% | 1 | 1% | Azo |
| P.B. 15 | 74 160 | 17 | 16% | 2 | 14% | 15 | 17% | Phthalocyanine |
| P.G. 7 | 74 260 | 10 | 10% | 1 | 7% | 9 | 10% | Phthalocyanine |
| Not identified | ‐ | 19 | 18% | 0 | 0% | 19 | 21% | ‐ |
Abbreviations: C.I., color index; P.B., Pigment Blue; P.G., Pigment Green; P.O., Pigment Orange; P.R., Pigment Red; P.V., Pigment Violet; P.Y., Pigment Yellow.
P.R. 210 is a mixture containing also P.R. 170.
Figure 2.
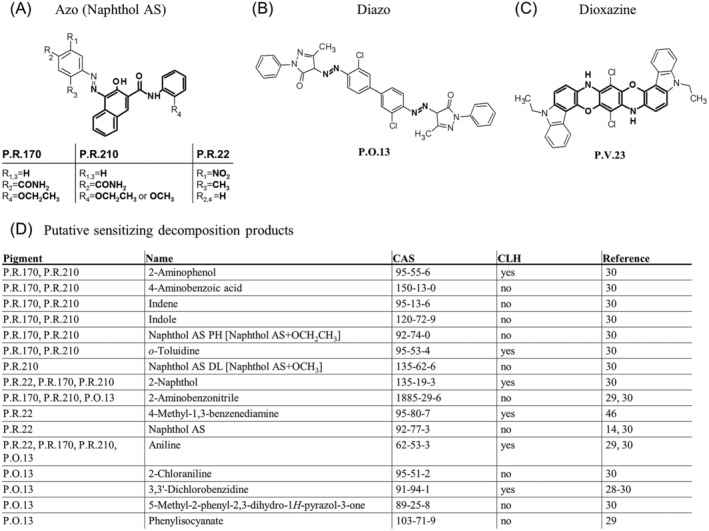
Main organic pigments in red tattoo allergy biopsies and their sensitizing decomposition products. (A) In the majority of biopsies the red pigments found are the naphthol AS azo pigments. The diazo P.O. 13 (B) and dioxazine P.V. 23 (C) pigments were found in 8% and 11% of all biopsies, respectively. (A‐C) Structural features determining the chemical group are marked in bold. (D) Known decomposition products of these pigments that are classified as sensitizers by manufacturers or the CLH system are listed with literature references and CAS number. CAS, chemical registry number; CLH, harmonized classification and labeling; P.O., Pigment Orange; P.R., Pigment Red; P.V., Pigment Violet
In 23 biopsies (22%), no red to violet organic pigment or iron particles could be identified (Table S2). In six biopsies with red to violet organic pigments, iron particles were found by means of XRF analysis indicating the use of inorganic red iron oxide pigment, Figure 3. The lack of pigment identification may either be due to low and non‐detectable pigment concentrations in these samples, or due to the presence of an unknown pigment. It has to be noted that many of the biopsy lysates did not appear red but rather dark when black ink particles dominated the biopsy, or unstained indicating that hardly any pigments were present.
Figure 3.
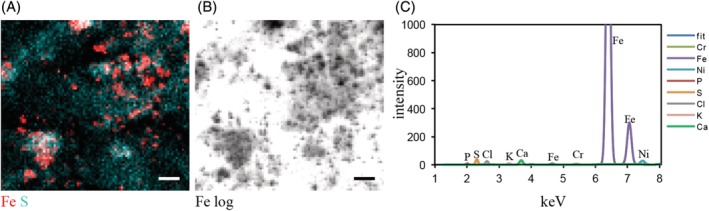
Shave biopsy from a patient analyzed by synchrotron x‐ray fluorescence (XRF) imaging. (A) Synchrotron‐XRF imaging with 1 × 1 μm resolution shows co‐localization of Fe with S from skin proteins. (B) Logarithmic display of Fe shows particle structure of Fe in the sample. (C) XRF spectrum averaged over the total area displayed in (A, B) with elements used for curve fitting. The spectrum shows high count‐rates at the iron K lines and less intensity for Ni and Cr K lines. In total, 35 biopsy lysates were analyzed with 14 showing presence of Fe particles. Scale bar = 10 μm. Ca, calcium; Cl, chlorine; Cr, chromium; Fe, iron; K, potassium; Ni, nickel; P, phosphorus; S, sulphur
In addition to the skin biopsies, 12 inks deriving from nine patients were analyzed. For six inks, pigment declaration from the label was available. Only three inks had correct labels displaying all pigments that have also been found in the inks by chemical analysis. In four cases, at least one pigment found in the ink bottle was also detectable in the skin biopsy of the corresponding patient. In two biopsies, a different red pigment was found. For three biopsies, the identification of the red pigments was unsuccessful again either due to low pigment concentrations or an unknown pigment not being declared on the list of ingredients on the ink bottle.
3.2. Quantification of metals in skin biopsies
Metals related to tattoos were also quantified in the skin samples. The presence of iron, titanium, and copper can be indicators of the use of iron oxide, titanium dioxide, and copper phthalocyanines as tattoo pigments, respectively (Table 2, Figure 4). Iron and copper are physiologically present in the human skin and therefore reach levels above the limit of quantification in the samples (Table 2, Table S2). Concentrations in the tattoo allergy biopsies were compared to reference data from the literature. Because these are based on postmortem data, environmental or occupational exposure is unknown. The maximum control values of nontattooed skin were compared to our samples to indicate which concentrations exceed the worst‐case background. In addition, literature values of ink contamination are displayed (Table 2). When estimating that a mean of 2.4% of the ink will still be present in the tattooed skin after years, only mean ink contamination with copper, chromium, and manganese (and likely titanium) would lead to a detectable increase above worst‐case background levels in control skin. The analysis of elements in pig skin when prepared with the knife used for dermatome shaving did not show increased metal concentrations compared to ceramic knifes (data not shown). Metals in samples from patients with and without cross‐reactions were on par. In addition, no associations between nickel or chromium concentrations and cross‐reactive patients were identified. Iron concentrations were increased in some samples, partly originating from iron‐containing tattoo pigments visible as iron particles in XRF analysis in 14 of 35 analyzed biopsies (Figure 3). Blood residues containing iron‐heme complexes might also play a role. The sensitizing elements chromium and nickel were found in many samples.
Table 2.
Metal concentrations (ppm) detected in 104 dermatome shave biopsies
| Metals in biopsies in the total materiala N = 104 | Mean concentration in biopsies (range) | Metals in biopsies from patients with cross‐sensitivity reaction N = 14 | Control values from human skin | Biopsies above max. control values | Mean concentrations in inks (range)b | ||||
|---|---|---|---|---|---|---|---|---|---|
| Fe | 104 | 100% | 42.93 (5.07‐216.05) | 14 | 100% | 9.0‐59c | 22 | 21% | 1608.7 (0.7‐88 443) |
| Cu | 102 | 98% | 3.48 (0.25‐35.01) | 13 | 93% | 0.35‐2.48c | 60 | 58% | 2317.8 (0.1‐31 310) |
| Cr | 96 | 92% | 2.17 (0.1‐24.57) | 13 | 93% | 0.16‐0.6c | 70 | 67% | 3.7 (0‐147.2) |
| Ti | 94 | 90% | 78.23 (0.02‐426.83) | 11 | 79% | 1.06‐27.7c | 49 | 47% | |
| Mn | 87 | 84% | 0.77 (0.05‐4.28) | 13 | 93% | 0.01‐6.1 ppbc | 87 | 84% | 2.4 (0.1‐98.8) |
| Ni | 70 | 67% | 1.05 (0.02‐7.75) | 11 | 79% | 0.08‐0.15c | 57 | 54% | 0.7 (0‐9.6) |
| Cd | 55 | 53% | 0.32 (0.05‐1.24) | 8 | 57% | 0.02‐0.25c | 30 | 29% | 0.6 (0‐4.7) |
Abbreviations: Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Mn, manganese; Ni, nickel; ppm, parts per million; Ti, titanium.
Samples under the limit of quantification of the analytical method were not included in the results shown in the table.
Concentrations of elements in tattoo inks.22
Figure 4.
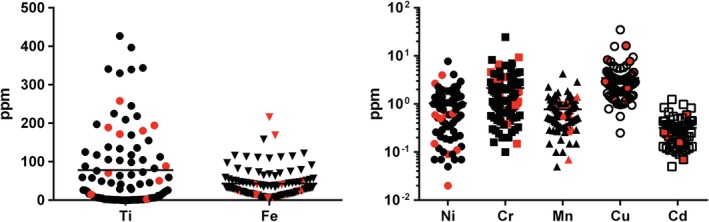
Plot of metal concentrations and mean of 104 biopsies with data of cross‐reactive patients marked in red. Cd, cadmium; Cr, chromium; Cu, copper; Fe, iron; Mn, manganese; Ni, nickel; ppm, parts per million; Ti, titanium
Titanium was found in the majority of samples, probably because the white pigment titanium dioxide is used for the blending of inks into different color shades. Speciation of titanium was carried out by XANES analysis for 44 samples with high titanium concentrations. The titanium dioxide pigment in these biopsies consisted mostly of rutile (38/44 [86%]). Anatase was found in only 2 of 44 samples (5%) and a mix of rutile and anatase in 4 of 44 of the analyzed biopsies (9%).
3.3. Reports of pigment frequencies extracted from literature
Frequencies of pigments found in the human shave biopsies were compared to pigments in tattoo inks purchased in Denmark, market‐monitoring data from Switzerland, as well as an Internet search study in the United States (Table 3). The Danish study lists pigments taken from the labels of 36 tattoo inks of different colors purchased over the Internet, guided by tattooist reports of their popularity. The inks investigated in the two market surveys in Switzerland were taken directly from the tattoo studios and compiled analytical data from 190 and 229 tattoo inks in 2011 and 2014, respectively. The study from the United States reports on pigments that have been listed on the safety data sheets of more than 1400 inks. All four studies generally show the same frequencies for most of the pigments (Table 3). Although P.R. 22 was uncommon in all four studies it was revealed with high percentage in the shave biopsies.
Table 3.
Pigments in dermatome shave biopsies of 104 allergic tattoo reactions compared to pigments in tattoo ink stock products according to market surveys; red tattoo reactions and red tattoo inks
| Pigment | C.I. number | Present study of biopsies N = 104 | Danish screening of inks by product label25 N = 36 | 2011 Swiss study of inks by chemical analysis9 N = 190 | 2014 Swiss study of inks by chemical analysis26 N = 229 | Internet survey of inks by SDS27 N = 1416 |
|---|---|---|---|---|---|---|
| P.R. 22 | 12 315 | 35% | 0% | 0% | 3.5% | 0.1% |
| P.R. 210a, P.R. 170 | 12 475, 12 477 | 24% | 25% | 11% | 11% | 16% |
| P.R. 122b | 73 915 | 0.9% | 5.5% | 7% | 6% | 2.0% |
| P.R. 112b | 12 370 | 0.9% | 0% | 2.1% | 0.4% | 0% |
| P.R. 5b | 12 490 | 0.9% | 5.5% | 3% | 0.9% | 0% |
| P.R. 202 | 73 907 | 0% | 0% | 1.1% | 3.1% | 0% |
| P.R. 254 | 56 110 | 0% | 0% | 5% | 10% | 0.4% |
| P.V. 23b | 51 319 | 8% | 0% | 7% | 5% | 2.3% |
| P.V. 19b | 73 900 | 0.9% | 2.7% | 0.5% | 3.1% | 0.4% |
| P.O. 13 | 21 110 | 11% | 14% | 4% | 6% | 13% |
| P.O. 16 | 21 160 | 2% | 5.5% | 0% | 2.2% | 1.5% |
| P.Y. 74, P.Y. 65† | 11 740, 11 741 | 5% | 22% | 9.1% | 11% | 16% |
| P.Y. 151 | 13 980 | 0.9% | 5.5% | 1.6% | 2.2% | 0.6% |
| P.Y. 138 | 56 300 | 0.9% | 0% | 3% | 8% | 0.8% |
| P.Y. 1b | 11 680 | 0.9% | 0% | 3% | 0.9% | 0% |
| P.B. 15b | 74 160 | 16% | 22% | 18% | 18% | 21% |
| P.G. 7b | 74 260 | 10% | 19% | 8% | 7% | 4.6% |
Abbreviations: C.I., color index; P.B., Pigment Blue; P.G., Pigment Green; P.O., Pigment Orange; P.R., Pigment Red; P.V., Pigment Violet; P.Y., Pigment Yellow; SDS, safety data sheet.
P.R. 210 is a mixture that contains P.R. 170; P.Y. 74, and P.Y. 65 are positional isomers and are therefore not distinguishable with the methods applied.
Ban recommended by the Council of Europe, ResAP(2008)1.
3.4. Sensitizing pigment decomposition products extracted from the literature
The pigments found in this study are known to be cleaved upon sunlight exposure or laser irradiation for tattoo removal. Metabolic breakdown in the skin has been studied only rarely and is thus largely unknown. From the literature, 16 substances that descend from the most frequently found pigments—P.R. 22, P.R. 210, P.R. 170, P.O. 13, and P.V. 23 are—classified as sensitizers either by manufacturers or by the European Chemical Agency (Figure 2D). Among these sensitizers, known carcinogens such as aniline and 3,3'‐dichlorobenzidine14, 25, 28, 29, 30 were also found. Aniline presents the simplest primary aromatic amine and is cleaved off by a multitude of pigments. The Naphthol AS pigments cleave into their corresponding Naphthol AS derivatives, thereby releasing 2‐naphthol.
4. DISCUSSION
The study identified Naphthol AS pigments in more than 55% of the shave biopsies, with allergic reactions in the 104 patients tattooed with red or red nuances. Therefore this structural element is to be considered as a contributing factor in the development of tattoo‐related allergic responses. However, in 23 biopsies (22%), no organic pigment or iron particles indicating the use of iron oxide pigments could be detected. The main reasons for incomplete identification will be insufficient pigment concentrations in the biopsy lysates or pigments not yet included in the pigment library.20, 31 This is, because the detection limit of pigments with MALDI is dependent on other components in the mixture and on the pigment itself and can range from 0.1% in our own experiments and up to 20% w/w in extreme cases.31 In addition, pigments not yet known to occur in tattoo inks may be contained. In some samples, iron oxide pigments may have been used to create the red color but were not analyzed via XRF imaging due to limited synchrotron beamtime. Of the pigments identified, P.R. 22, P.R. 170, and P.R. 210 were most frequent. Regarding cross‐reactions at distant sites, which have been tattooed well back in the past, particularly P.R. 210 appeared associated with this special kind of allergic reaction. When compared to its putative occurrence on the market, the increase in the frequency of adverse skin reactions was especially obvious for P.R. 22. However, it is a limitation that product content labels were used for pigment identification in two of four reference studies from the literature, given that the declaration of content on the label can deviate substantially from the actual content, as proven by chemical analysis.25, 31, 32
Other human data from pigment analysis of nonreacting red tattoos exist only for nine cases in forensic material.33 P.R. 22 was detected in two biopsies (22%) and P.R. 112 in three biopsies (33%), whereas none of these pigments could be detected in four biopsies (44%). The same study revealed several impurities such as methyl‐naphthol‐AS present in commercial pigment preparations. The identified pigments may have been more commonly used in the past.
Based on the present data, we still cannot conclude on the precise azo pigment‐related allergenic fragment that serves as hapten causing the allergy, even though P.R. 22, P.R. 170 and P.R. 210 seem to be capable of sensitization. In clinical studies, allergic reactions in tattoos may start after a few weeks or even after months or years.2, 12 An early debut points to an allergen already present in the tattoo ink. Conversely, late debut rather indicates the formation of an allergen over time due to local metabolic breakdown or photodegradation. These breakdown products are thought to be components of haptens, which include tissue proteins.12 Sensitization to such hapten‐protein complexes and consecutive allergy development may occur at any time during the individual's lifetime; tattoos can be tolerated for years before adverse reactions suddenly emerge a long time after the original tattoo was acquired. Thus, even with one isolated pigment such as P.R. 22, more than one allergenic hapten might be formed (Figure 2D). The degradation hypothesis leading to hapten formation is strengthened by the observation that pigment concentrations in skin decrease over time. Concentrations of P.R. 22 and P.R. 112 in skin biopsies were, respectively, 87% to 99% lower than previously found in fresh tattoos performed on mice and humans, thus indicating that elimination takes place during healing, with washout or breakdown of pigments weeks, months, or years after tattooing.33, 34 Authors also found that up to 60% of P.R. 22 disappeared within 32 days when animals were exposed to solar radiation. Likewise, the azo breakdown products may also be found as impurities in tattoo ink preparations. Because neither of the decomposition products of the pigments that can be detected in biopsies was tested in standard patch test series, no evidence on the supposable associated sensitization rate and the putative hapten in the investigated patients can be provided yet.
Metals were very common in all biopsies investigated in this study. The results are in accordance with metal contaminants commonly found in tattoo ink stock products on the market. According to the literature, a broad range of metals are usually present in tattoo inks.22, 35 However, when estimating that a mean of 2.4% of the ink is staying in the skin,33 only mean concentrations of copper, chromium, and manganese impurities in inks would result in levels higher than the background control values in human skin. Even if highly contaminated inks are used, cadmium might not rise above the background level in skin, in contrast to the other elements. The control values of skin include unknown environmental factors that might have caused these concentrations in the cited studies and our tattooed skin biopsies. Hence, the metals found in the current study originate from pigment impurities, unknown environmental factors, or tattoo needle wear (composed of iron, nickel, and chromium) deposited in the skin, as described recently.36 Mean concentrations of chromium and nickel deriving from tattoo needle wear can raise the metal concentration in tattooed skin above the background level if titanium dioxide was in the ink used for tattooing.36 The mean values of nickel and cadmium in the tattooed biopsies are rather high and might derive from a combination of environmental factors, ink impurities, and metal wear of tattoo needles.
With respect to tattoo safety and risk of allergic sensitization, the metals nickel and chromium are of primary interest. A previous study of allergy patch testing in patients with chronic tattoo reactions, including allergy in red tattoos and cases of cross‐reaction, showed positive reaction to nickel sulfate in 21% of the cases.12 This is close to the known level of 18% in the background population of dermatitis patients documented in large European studies.12, 37 The same patch test study could not verify that chromium plays any role in tattoo allergy.
The chemical analysis used in this investigation quantifies total metal contents and cannot distinguish metallic forms and soluble ions. Soluble metal ions may induce allergy (eg, nickel ions) and are considered to be constantly cleared from tissues with a short half‐life in the body, for example, 17–39 hours for clearance of nickel ions measured in the urine after single dose intake.38, 39 Metal allergy due to metal joint replacement is considered rare despite the huge amount of nickel and chromium present in these prostheses.40, 41 Still, the rate of patients sensitized to metals is higher among those with implant failure.42 Thus metal deposition in the body does not necessarily lead to adverse effects, but may still play a role during local inflammatory responses due to their high local concentrations in tissue surrounding joints with implants.42 We therefore think that metals do not play a major role in chronic tattoo allergy reactions in red tattoos observed as typical reactions in the clinic of today.
Titanium is used widely in metal implants and accepted to be rather nonallergenic. However, a recent case report suggested that titanium release from an implant as a contributing factor in tattoo‐related allergic reactions.43 The problem arose in black tattoos, in which the histology showed that granulomatous inflammation and patch tests with titanium were inconclusive, although the patient reacted to the processed implant material. Titanium is therefore unlikely to have caused the tattoo reaction. The case fulfilled the criteria of papulonodular reaction with autoimmune rush phenomenon, a known condition associated with sarcoidosis.19
Titanium dioxide found in the shave biopsies was present mainly as rutile, with only 14% containing the anatase crystal structure. It is known that titanium dioxide has photocatalytic properties toward the degradation of organic compounds in aqueous solutions, even at low concentrations—especially in the anatase crystal structure.44 It is therefore possible that the increased release of putative sensitizers originating from azo‐based pigments might be supported by anatase, which thus may have an indirect, but nevertheless active adjuvant role, in clinically manifested tattoo allergy. Hohl and Hauri recently showed that titanium dioxide exerts a strong and rapid photodegradation on diazo pigments when mixed with rutile and exposed to daylight in a collagen solution.45 However, the translation from in vitro to in vivo is controversial and no data exists on titanium dioxide triggering degradation of azo pigments in tattooed skin.
P.R. 22, P.R. 170, and P.R. 210 are accepted for tattoo ink manufacturing according to the nonbinding European Council resolution on requirements and criteria for the safety of tattoos and permanent makeup (ResAP(2008)1). Seven member states transferred this nonbinding guidance into national law. The upcoming EU restriction of tattoo and permanent makeup inks under the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation would limit the use of P.R. 22, P.R. 112, P.R. 210, P.Y. 74, P.Y. 1, P.O. 16, and P.O. 13 to 0.1% w/w according to the current draft. P.R. 122 and P.V. 19 are banned due to their listing in Annex IV of Regulation (EC) 1223/2009 as rinse‐off product. P.R. 5 is banned because it appears in Annex II of Regulation (EC) 1223/2009. Hence of the pigments found in this study, P.R. 170 found in 36% of all biopsies may be the only red organic pigment left for unlimited use in tattoo ink production on the market in the future. Unfortunately, a multitude of other pigments not mentioned in the negative lists of the REACH restriction might be used as substitutes and serve as a new generation of organic pigments used in tattoo inks with unpredictable risks. The REACH regulation of tattoo inks primarily addresses potential carcinogenicity and reprotoxicity as safety concerns. In contrast, allergic sensitizers in tattoo ink manufacturing, distribution, and use will be only insufficiently addressed. The outcome of the present study indicates that future research aimed at production and distribution of allergy‐safe tattoo inks should primarily address the group of azo pigments with P.R. 22, P.R. 170, and P.R. 210 as lead suspects.
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report.
Supporting information
Table S1. List of 40 pigments included in the pigment library used for MALDI‐MS/MS analysis.
Figure S1. Example of pigment identification with MALDI‐MS/MS. (A) Spectra of a biopsy containing P.R. 170 (m/z 318, m/z 477), P.R. 210 (m/z 318, m/z 463), and P.O. 13 (m/z 623) with the corresponding masses specific for each pigment indicated. (B) Ink containing P.R. 170, P.R. 210, and P.O. 13. (C) Reference spectra of P.R. 210. Because P.R. 210 is a mixture containing P.R. 170 also m/z 477 was detected. (D) Reference spectra of P.O. 13. (A‐D) MS/MS spectra of pigment‐specific masses in biopsy; ink and reference spectra are displayed. These are used for pigment identification. MS, mass spectrometry; m/z, mass to charge ratio; P.O., Pigment Orange; P.R., Pigment Red.
Table S2. Experimental data linked to individual donors.
ACKNOWLEDGEMENTS
Nadine Dreiack (BfR) and Dr. Hiram Castillo‐Michel (ESRF) are acknowledged for their technical help with sample preparation, ICP‐MS and XRF analyses, respectively. This work was supported by the intramural research project (SFP #1322‐604) at the German Federal Institute for Risk Assessment (BfR).
Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73–82. 10.1111/cod.13423
Funding information German Federal Institute for Risk Assessment (BfR), Grant/Award Number: Intramural research project (SFP #1322‐604)
REFERENCES
- 1. Wenzel SM, Rittmann I, Landthaler M, Bäumler W. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138‐147. [DOI] [PubMed] [Google Scholar]
- 2. Serup J, Sepehri M, Hutton Carlsen K. Classification of tattoo complications in a hospital material of 493 adverse events. Dermatology. 2016;232:668‐678. [DOI] [PubMed] [Google Scholar]
- 3. Kluger N. Cutaneous complications related to tattoos: 31 cases from Finland. Dermatology. 2017;233:100‐109. [DOI] [PubMed] [Google Scholar]
- 4. van der Bent SAS, de Winter RW, Wolkerstorfer A, Rustemeyer T. Red tattoo reactions, a prospective cohort on clinical aspects. J Eur Acad Dermatol Venereol. 2019;33:e384‐e386. [DOI] [PubMed] [Google Scholar]
- 5. Hutton Carlsen K, Serup J. Patients with tattoo reactions have reduced quality of life and suffer from itch: Dermatology Life Quality Index and Itch Severity Score measurements. Skin Res Technol. 2015;21:101‐107. [DOI] [PubMed] [Google Scholar]
- 6. Dirks M. Making innovative tattoo ink products with improved safety: possible and impossible ingredients in practical usage. Curr Probl Dermatol. 2015;48:118‐127. [DOI] [PubMed] [Google Scholar]
- 7. Prior G, ed. Tattoo Inks: Analysis, Pigments, Legislation. Berlin, Germany: epubli GmbH; 2014. [Google Scholar]
- 8. Serup J, Kluger N, Bäumler W, eds. Tattooed Skin and Health. Basel, Switzerland: Karger; 2015. [Google Scholar]
- 9. Gaudron S, Ferrier‐Le Bouedec MC, Franck F, D'Incan M. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97‐105. [DOI] [PubMed] [Google Scholar]
- 10. Hauri U. Inks for tattoos and PMU (permanent make‐up)/Organic pigments, preservatives and impurities such as primary aromatic amines and nitrosamines. Report of the State Laboratory of the Canton Basel City; 2011.
- 11. Wenzel SM, Welzel J, Hafner C, Landthaler M, Bäumler W. Permanent make‐up colorants may cause severe skin reactions. Contact Dermatitis. 2010;63:223‐227. [DOI] [PubMed] [Google Scholar]
- 12. Serup J, Hutton Carlsen K. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255‐263. [DOI] [PubMed] [Google Scholar]
- 13. Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265‐266. [DOI] [PubMed] [Google Scholar]
- 14. Engel E, Spannberger A, Vasold R, König B, Landthaler M, Bäumler W. Photochemical cleavage of a tattoo pigment by UVB radiation or natural sunlight. J Dtsch Dermatol Ges. 2007;5:583‐589. [DOI] [PubMed] [Google Scholar]
- 15. Vasold R, Naarmann N, Ulrich H, et al. Tattoo pigments are cleaved by laser light—the chemical analysis in vitro provide evidence for hazardous compounds. Photochem Photobiol. 2004;80:185‐190. [DOI] [PubMed] [Google Scholar]
- 16. Hutton Carlsen K, Serup J. Photosensitivity and photodynamic events in black, red and blue tattoos are common: a 'Beach Study'. J Eur Acad Dermatol Venereol. 2013;28:231‐237. [DOI] [PubMed] [Google Scholar]
- 17. Wamer WG, Yin JJ. Photocytotoxicity in human dermal fibroblasts elicited by permanent makeup inks containing titanium dioxide. J Cosmet Sci. 2010;62:535‐547. [PubMed] [Google Scholar]
- 18. Sepehri M, Jørgensen B, Serup J. Introduction of dermatome shaving as first line treatment of chronic tattoo reactions. J Dermatolog Treat. 2015;26:451‐455. [DOI] [PubMed] [Google Scholar]
- 19. Serup J. How to diagnose and classify tattoo complications in the clinic: a system of distinctive patterns. Curr Probl Dermatol. 2017;52:58‐73. [DOI] [PubMed] [Google Scholar]
- 20. Schreiver I, Eschner LM, Luch A. Matrix‐assisted laser desorption/ionization tandem mass spectrometry for identification of organic tattoo pigments in inks and tissue samples. Analyst. 2018;143:3941‐3950. [DOI] [PubMed] [Google Scholar]
- 21. Schreiver I, Hesse B, Seim C, et al. Synchrotron‐based nano‐XRF mapping and micro‐FTIR microscopy enable to look into the fate and effects of tattoo pigments in human skin. Sci Rep. 2017;7:11395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Forte G, Petrucci F, Cristaudo A, Bocca B. Market survey on toxic metals contained in tattoo inks. Sci Total Environ. 2009;407:5997‐6002. [DOI] [PubMed] [Google Scholar]
- 23. Saltzman BE, Gross SB, Yeager DW, Meiners BG, Gartside PS. Total body burdens and tissue concentrations of lead, cadmium, copper, zinc, and ash in 55 human cadavers. Environ Res. 1990;52:126‐145. [DOI] [PubMed] [Google Scholar]
- 24. Goldblum RW, Derby S, Bunsen Lerner A. The metal content of skin, nails and hair. J Invest Dermatol. 1953;20:13‐18. [DOI] [PubMed] [Google Scholar]
- 25. Jacobsen E, Toenning K, Pedersen E, et al. Chemical substances in tattoo ink. Survey of chemical substances in consumer products. Report of the Danish Environmental Protection Agency no. 116; 2012. ISBN 978‐87‐92779‐87‐8.
- 26. Hauri U. Inks for tattoos and permanent make‐up/pigments, preservatives, aromatic amines, polyaromatic hydrocarbons and nitrosamines. Report of the Department of Health, Kanton Basel‐Stadt; 2014.
- 27. Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379‐385. [DOI] [PubMed] [Google Scholar]
- 28. Hauri U, Hohl C. Photostability and breakdown products of pigments currently used in tattoo inks. Curr Probl Dermatol. 2015;48:164‐169. [DOI] [PubMed] [Google Scholar]
- 29. Hering H, Sung AY, Roder N, et al. Laser irradiation of organic tattoo pigments releases carcinogens with 3,3'‐dichlorobenzidine inducing DNA strand breaks in human skin cells. J Invest Dermatol. 2018;138:2687‐2690. [DOI] [PubMed] [Google Scholar]
- 30. Schreiver I, Hutzler C, Andree S, Laux P, Luch A. Identification and hazard prediction of tattoo pigments by means of pyrolysis—gas chromatography/mass spectrometry. Arch Toxicol. 2016;90:1639‐1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niederer M, Hauri U, Kroll L, Hohl C. Identification of organic pigments in tattoo inks and permanent make‐up using laser desorption ionisation mass spectrometry. F1000Res. 2017;6:2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Høgsberg T, Saunte DM, Frimodt‐Møller N, Serup J. Microbial status and product labelling of 58 original tattoo inks. J Eur Acad Dermatol Venereol. 2013;27:73‐80. [DOI] [PubMed] [Google Scholar]
- 33. Lehner K, Santarelli F, Penning R, et al. The decrease of pigment concentration in red tattooed skin years after tattooing. J Eur Acad Dermatol Venereol. 2011;25:1340‐1345. [DOI] [PubMed] [Google Scholar]
- 34. Engel E, Vasold R, Santarelli F, et al. Tattooing of skin results in transportation and light‐induced decomposition of tattoo pigments ‐ a first quantification in vivo using a mouse model. Exp Dermatol. 2010;19:54‐60. [DOI] [PubMed] [Google Scholar]
- 35. Timko AL, Miller CH, Johnson FB, Ross V. In vitro quantitative chemical analysis of tattoo pigments. Arch Dermatol. 2004;137:143‐147. [PubMed] [Google Scholar]
- 36. Schreiver I, Hesse B, Seim C, et al. Distribution of nickel and chromium containing particles from tattoo needle wear in humans and its possible impact on allergic reactions. Part Fibre Toxicol. 2019;16:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Uter W, Hegewald J, Aberer W, et al. The European standard series in 9 European countries, 2002/2003‐ first results of the European Surveillance system on Contact Allergies. Contact Dermatitis. 2005;53:136‐145. [DOI] [PubMed] [Google Scholar]
- 38. Tossavainen A, Numinen M, Tola S. Application of mathematical modelling for assessing the biological half‐times of chromium and nickel in field studies. Br J Ind Med. 1980;37:285‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Christinsen OB, Lagesson V. Nickel concentration of blood and urine after oral administration. Ann Clin Lab Sci. 1981;11:119‐125. [PubMed] [Google Scholar]
- 40. Teo WZW, Schalock PC. Metal hypersensitivity reactions to orthopedic implants. Dermatol Ther (Heidelb). 2017;7:53‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saccomanno MF, Sircana G, Masci G, et al. Allergy in total knee replacement surgery: is it a real problem? World J Orthop. 2019;10:63‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hallib NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67:182‐188. [PubMed] [Google Scholar]
- 43. de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo‐related hypersensitivity reactions? A case report. Contact Dermatitis. 2017;77:397‐405. [DOI] [PubMed] [Google Scholar]
- 44. Wold A. Photocatalytic properties of titanium dioxide (TiO2). Chem Mater. 1993;5:280‐283. [Google Scholar]
- 45. Hohl C. Diarylide pigments under sunlight – what do in vitro tests tell us? http://ectp2017.org/fileadmin/user_upload/ECTP/Abstracts/O12._DIARYLIDE_PIGMENTS_UNDER_SUNLIGHT_-_WHAT_DO_IN_VITRO_TESTS_TELL_US.pdf. Accessed June 6, 2019.
- 46. NICNAS. Australien Government, Department of Health . Characterisation of tattoo inks used in Australia, report; 2016. https://www.nicnas.gov.au/__data/assets/pdf_file/0006/42297/NICNAS-Tattoo-ink-report-for-publication-May-2018-updates.pdf. Assessed July 23, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of 40 pigments included in the pigment library used for MALDI‐MS/MS analysis.
Figure S1. Example of pigment identification with MALDI‐MS/MS. (A) Spectra of a biopsy containing P.R. 170 (m/z 318, m/z 477), P.R. 210 (m/z 318, m/z 463), and P.O. 13 (m/z 623) with the corresponding masses specific for each pigment indicated. (B) Ink containing P.R. 170, P.R. 210, and P.O. 13. (C) Reference spectra of P.R. 210. Because P.R. 210 is a mixture containing P.R. 170 also m/z 477 was detected. (D) Reference spectra of P.O. 13. (A‐D) MS/MS spectra of pigment‐specific masses in biopsy; ink and reference spectra are displayed. These are used for pigment identification. MS, mass spectrometry; m/z, mass to charge ratio; P.O., Pigment Orange; P.R., Pigment Red.
Table S2. Experimental data linked to individual donors.


