ABSTRACT
Objective
To explore the effect on perinatal outcome of different fetal monitoring strategies for early‐onset fetal growth restriction (FGR).
Methods
This was a cohort analysis of individual participant data from two European multicenter trials of fetal monitoring methods for FGR: the Growth Restriction Intervention Study (GRIT) and the Trial of Umbilical and Fetal Flow in Europe (TRUFFLE). All women from GRIT (n = 238) and TRUFFLE (n = 503) who were randomized between 26 and 32 weeks' gestation were included. The women were grouped according to intervention and monitoring method: immediate delivery (GRIT) or delayed delivery with monitoring by conventional cardiotocography (CTG) (GRIT), computerized CTG (cCTG) only (GRIT and TRUFFLE) or cCTG and ductus venosus (DV) Doppler (TRUFFLE). The primary outcome was survival without neurodevelopmental impairment at 2 years of age.
Results
Gestational age at delivery and birth weight were similar in both studies. Fetal death rate was similar between the GRIT and TRUFFLE groups, but neonatal and late death were more frequent in GRIT (18% vs 6%; P < 0.01). The rate of survival without impairment at 2 years was lowest in pregnancies that underwent immediate delivery (70% (95% CI, 61–78%)) or delayed delivery with monitoring by CTG (69% (95% CI, 57–82%)), increased in those monitored using cCTG only in both GRIT (80% (95% CI, 68–91%)) and TRUFFLE (77% (95% CI, 70–84%)), and was highest in pregnancies monitored using cCTG and DV Doppler (84% (95% CI, 80–89%)) (P < 0.01 for trend).
Conclusions
This analysis supports the hypothesis that the optimal method for fetal monitoring in pregnancies complicated by early‐onset FGR is a combination of cCTG and DV Doppler assessment.
Trial Registration: GRIT ISRCTN41358726 and TRUFFLE ISRCTN56204499. © 2019 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of the International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: cardiotocography, ductus venosus, fetal growth restriction, monitoring, short‐term variation
Short abstract
This article's abstract has been translated into Spanish and Chinese. Follow the links from the http://onlinelibrary.wiley.com/doi/10.1002/uog.20354/abstract to view the translations.
 This article has been selected for Journal Club. Click https://www.isuog.org/resource/uog-journal-club-January-2020.html to view slides and discussion points.
This article has been selected for Journal Club. Click https://www.isuog.org/resource/uog-journal-club-January-2020.html to view slides and discussion points.
RESUMEN
Análisis comparativo de los resultados a los 2 años de edad en los ensayos GRIT y TRUFFLE
Objetivo
Examinar el efecto sobre el resultado perinatal de diferentes estrategias de monitoreo del feto para la restricción del crecimiento fetal (RCF) de inicio precoz.
Métodos
Este estudio realizó un análisis de cohortes de datos de participantes individuales en dos ensayos multicéntricos europeos de métodos de monitoreo fetal para la RCF: el Estudio de Intervención en la Restricción del Crecimiento (GRIT, por sus siglas en inglés) y el Ensayo Europeo de Flujo Umbilical y Fetal (TRUFFLE, por sus siglas en inglés). Se incluyeron todas las mujeres de GRIT (n = 238) y de TRUFFLE (n = 503) que habían sido asignadas al azar entre 26 y 32 semanas de gestación. Las mujeres se agruparon según el método de intervención y monitoreo: parto inmediato (GRIT) o parto diferido con monitoreo mediante cardiotocografía convencional (CTG) (GRIT), solo CTG digital (cCTG, por sus siglas en inglés) (GRIT y TRUFFLE) o cCTG y Doppler del conducto de Arancio (DV) (TRUFFLE). La medida de resultado primaria fue la supervivencia sin deterioro del desarrollo neurológico a los dos años de edad.
Resultados
La edad gestacional al momento del parto y el peso al nacer fueron similares en ambos estudios. La tasa de mortalidad fetal fue similar entre los grupos de GRIT y TRUFFLE, pero la muerte neonatal y tardía fue más frecuente en el grupo de GRIT (18% vs 6%; P < 0,01). La tasa de supervivencia sin deterioro a los dos años fue más baja en los embarazos que se sometieron a un parto inmediato (70% (IC 95%, 61–78%)) o a un parto tardío con monitoreo mediante CTG (69% (IC 95%, 57–82%)), más alta en los monitoreados solo mediante cCTG en GRIT (80% (IC 95%, 68–91%)) y TRUFFLE (77% (IC 95%, 70–84%)), y mayor aun en los embarazos monitoreados mediante cCTG y Doppler DV (84% (IC 95%, 80–89%)) (P < 0,01 para tendencia).
Conclusiones
Este análisis apoya la hipótesis de que el método óptimo para el monitoreo fetal en los embarazos complicados por RCF de inicio precoz es una combinación de cCTG y evaluación Doppler DV.
Inscripción del ensayo
GRIT ISRCTN41358726 y TRUFFLE ISRCTN56204499. © 2019 Los autores. Ultrasonido en Obstetricia y Ginecología publicado por John Wiley & Sons Ltd. en nombre de la Sociedad Internacional de Ultrasonido en Obstetricia y Ginecología.
摘要
GRIT 和 TRUFFLE两年结局的比较分析
目的
探讨早发胎儿生长受限(FGR)不同胎儿监护策略对围产儿结局的影响。
方法
这是对FGR胎儿监测方法的两项欧洲多中心试验的个体参与者数据的队列分析:生长限制干预(GRIT)研究和欧洲脐带血和胎儿血流试验。所有来自GREST(n = 238)和TOULVO(n = 503)的妇女在妊娠26至32周之间随机分组,均纳入研究。根据干预和监测方法对这些妇女进行分组:立即分娩(GRET)或延迟分娩,并通过常规胎儿电子监护(CTG)(GRET)、仅计算机化CTG (cCTG)或cCTG和静脉导管(DV) 多普勒(TRUFFLE)进行监测。主要结局是2岁时无神经发育损害的存活。
结果
两项研究中分娩时的胎龄和出生体重相似。GRITE组和TRUFFLE组的胎儿死亡率相似,但GRITE组新生儿和晚期死亡更频繁(18%对6%;P < 0.01)。在接受CTG监测的立即分娩(70% (95%CI,61‐78%)或延迟分娩(69% (95%CI,57‐82%)的妊娠中,2年无损伤生存率最低,在仅使用cCTG监测的 GRIT (80% (95%CI,68‐91%)和TRUFFLE(77% (95%CI,70‐84%)妊娠中增加,在使用cCTG和 DV多普勒监测的妊娠中最高(84% (95%CI,95%)
结论
这一分析支持这样的假设,即妊娠合并早发性FGR的最佳胎儿监测方法是联合应用cCTG和 DV 多普勒超声评估。
临床试验注册: GRIT ISRCTN41358726和TRUFFLE ISRCTN56204499。 © 2019 作者.John Wiley & Sons Ltd代表International Society of Ultrasound in Obstetrics and Gynecology出版的Ultrasound in Obstetrics & Gynecology。
CONTRIBUTION —
What are the novel findings of this work?
This work provides a comparative analysis of the effect on perinatal outcome of several monitoring techniques for early‐onset fetal growth restriction employed in two randomized trials. The rate of survival without neurodevelopmental impairment at 2 years was highest in pregnancies monitored using computerized cardiotocography and ductus venosus Doppler.
What are the clinical implications of this work?
This analysis supports the hypothesis that the optimal method for fetal monitoring in pregnancies complicated by early‐onset fetal growth restriction is a combination of computerized cardiotocography and ductus venosus Doppler assessment.
INTRODUCTION
Fetal growth restriction (FGR) in the early preterm period before 32 weeks' gestation is a rare but serious complication of pregnancy owing to its association with adverse perinatal outcome. Treatment of the underlying condition is impossible and the challenge therefore lies in optimizing the timing of delivery. The risks associated with prematurity, namely neonatal complications and impaired neurodevelopment, have to be balanced against the risks associated with prolonged fetal exposure to hypoxemia and acidemia, which may result in stillbirth or brain damage. Obstetricians use a range of tests of fetal wellbeing. However, the sequence of events in fetal deterioration is difficult to evaluate in humans because observational case series of growth‐restricted fetuses rarely, if ever, include all tests of wellbeing. More importantly, study outcomes are inevitably ‘censored’ because timing of delivery is subject to the vagaries of parental choice and the decisions of managing clinicians1. The optimal indication for timing of delivery is still open to debate2.
Trials in this patient group are hard to conduct. To date, only two large randomized trials have evaluated timing of delivery in early‐onset preterm FGR: the Growth Restriction Intervention Study (GRIT) and the Trial of Umbilical and Fetal Flow in Europe (TRUFFLE)3, 4. Both have been cited frequently, but their influence on clinical practice is difficult to measure. Although GRIT recruited 548 women (588 fetuses) and TRUFFLE recruited 503 women, and both trials achieved very high follow‐up rates to 2 years, the number of participants at any specific gestational age or with similar clinical risk factors was small. Given the high dependency of clinical decision‐making on gestational age, the available analyses are necessarily underpowered.
In the absence of data from further trials, an interim solution would be to meta‐analyze the existing data. Ideally, this should be an individual patient data meta‐analysis (IPD‐MA).
The aim of the current analysis was to explore the effect on perinatal outcome of different fetal monitoring strategies for early‐onset FGR employed in GRIT and TRUFFLE.
METHODS
This study initially set out to perform a meta‐analysis of trials investigating the impact of fetal monitoring methods for FGR on long‐term infant outcome. A scoping literature search was performed in PubMed, using the following strategy, limited to clinical trials: ‘(fetal compromise[Title/Abstract] or fetal monitoring[Title/Abstract]) AND (growth restriction[Title/Abstract] OR fetal growth[Title/Abstract]) AND (long term[Title/Abstract] OR long‐term[Title/Abstract] OR wellbeing[Title/Abstract] OR neurodevelopment[Title/Abstract] OR Griffith[Title/Abstract] OR Bayley[Title/Abstract])’. This confirmed that GRIT and TRUFFLE are the only monitoring/intervention studies in early‐onset FGR3, 4. Differences in inclusion criteria, study period and study intervention between the two trials precluded an IPD‐MA, so a cohort analysis of individual participant data from the GRIT and TRUFFLE studies was performed. Individual participant data were retrieved from both trial datasets. The methods of both studies have been described previously in full and are summarized below. This study was exempt from review by the institutional ethics review boards.
GRIT
In GRIT3, 5, 548 pregnant women between 24 and 36 completed weeks' gestation were recruited at 69 European hospitals between 1993 and 2001. All fetuses had suspected FGR and the inclusion criterion was clinical uncertainty about whether immediate delivery was indicated. Fetal arterial Doppler and cardiotocography (CTG) were recorded before inclusion, and interpretation of these findings was according to local standards. Ductus venosus (DV) Doppler was not used. The women were allocated randomly to immediate delivery (n = 296 babies) or delayed delivery until the obstetrician was no longer uncertain (n = 292 babies). Mode of delivery and monitoring strategy in the delayed‐delivery group were at the discretion of the attending obstetrician. The main outcome was death or neurodevelopmental impairment at or beyond 2 years of age. Impairment was a composite outcome comprising any of cerebral palsy, little or no vision, requirement for a hearing aid, or Griffith's Mental Development Scales General Quotient ≤ 70, assessed by a Griffith‐trained assessor. For those infants who were not assessed in person at 2 years of age, the former three diagnoses were accepted based on either parental report or the child's family practitioner or pediatrician. The overall rate of death or severe impairment at 2 years was 17.2% in those with known outcome and there was no statistically significant difference between the randomization groups. Neurodevelopmental impairment occurred in 6.5% of assessed children. For the purpose of the current analysis, the original study endpoint definition was used, i.e. survival without neurodevelopmental impairment at or beyond 2 years of age.
TRUFFLE
In TRUFFLE4, 6, 503 pregnant women between 26 and 32 completed weeks' gestation were recruited at 20 European hospitals between 2005 and 2010. All pregnancies had a fetus with FGR, defined based on elevated umbilical artery pulsatility index (PI) and ultrasound biometry. Women were randomized to one of three groups in which delivery was determined either by reduced short‐term variation (STV) in fetal heart rate using computerized CTG (cCTG), or by early (PI > 95th centile) or late (absent or negative A‐wave) abnormalities on Doppler ultrasound assessment of the DV waveforms. The criteria for determining delivery were specified in detail and included specific cut‐offs for STV in all groups, although the intensity of monitoring was not prescribed. All ultrasonographers met predetermined criteria for performing DV Doppler measurements. The main outcome was survival without neurodevelopmental impairment at 2 years of age. Impairment comprised any of cerebral palsy, severe vision or hearing impairment or cognitive composite score on the Bayley Scales of Infant and Toddler Development (3rd edition) of < 85, evaluated by an assessor trained and accredited specifically for the trial. For those infants who were not assessed in person at 2 years of age, the former three diagnoses were accepted based on either parental report or the child's family practitioner or pediatrician. The overall rate of death or severe impairment at 2 years was 18.1% of all infants with known outcome, with no statistical difference between the groups. The overall rate of neurodevelopmental impairment at 2 years in assessed children was 9.7%, and was lower in the groups monitored using DV Doppler than in the group monitored using cCTG.
Data strategy
For this analysis, we used data from the subset of participants in the GRIT study with a singleton pregnancy and gestational age between 26 and 32 completed weeks at study entry. From TRUFFLE, all pregnancies were included, except one in which neonatal data were missing. Baseline variables, process variables and outcomes were compared between groups, with specific focus on their relationship with antenatal death and 2‐year outcome. We combined 2‐year outcome data, defining ‘survival free of impairment’ as survival without cerebral palsy, severe visual impairment or hearing loss requiring a hearing aid, and either Griffith Quotient > 70 (GRIT) or Bayley‐III (or adjusted Bayley‐II) Cognitive Composite Score ≥ 85 (TRUFFLE). The primary outcome was calculated for all infants with known outcome, including those with perinatal or late death, but excluding infants lost to 2‐year follow‐up.
Analysis strategy
Data were analyzed according to intervention and monitoring strategy: immediate delivery when fetal condition was uncertain (GRIT) or delayed delivery with monitoring using conventional CTG (GRIT), cCTG with STV calculation (GRIT and TRUFFLE) or cCTG with STV calculation and DV Doppler (both DV Doppler groups from TRUFFLE were combined, as the results were not statistically significantly different). A secondary analysis was performed to investigate differences in infant outcome over time. The years of the studies were grouped as 1993–1997 and 1998–2001 for GRIT, and 2005–2007 and 2008–2010 for TRUFFLE, i.e. dividing each study into a first and second half.
Parameters analyzed included monitoring strategy, year of randomization, gestational age at randomization, umbilical artery absent or reversed end‐diastolic (ARED) flow and birth‐weight Z‐score. Birth‐weight Z‐scores were calculated using the in‐utero estimated fetal weight model developed by Hadlock et al.7. As estimated fetal weight was not recorded at inclusion in GRIT, we included birth weight in the analysis.
Statistical analysis
Baseline characteristics, process variables and outcomes were compared using two‐tailed tests by ANOVA, the Mann–Whitney U‐test or Pearson's chi‐square test, as appropriate.
The associations of demographic, clinical and diagnostic parameters at study inclusion with the endpoints was first explored using univariable analysis. Those parameters that were significantly different on univariable analysis between infants with normal 2‐year outcome and those with death or neurodevelopmental impairment were entered into multivariable logistic regression analyses to adjust for the association between parameters and to calculate odds ratios (OR) for survival without neurodevelopmental impairment at 2 years7, 8. Regression analysis both with and without gestational age or birth weight was planned in order to determine if the ORs of intervention and monitoring strategy or study period for the primary outcome were affected by these parameters owing to possible collider bias.
Intervention and monitoring strategy or study period was initially entered into the logistic regression analysis and other parameters were added in a stepwise manner, based on the probability of survival without impairment at 2 years. The probability cut‐offs for entry into and removal from the model were set at 0.05 and at 0.10, respectively. Statistical calculations were performed using SPSS version 25 (IBM Corp., Armonk, NY, USA).
RESULTS
We included 238 women from GRIT and 502 women from TRUFFLE. Gestational age at inclusion and at delivery and birth weight were similar between all subgroups (Table 1). Because of the randomization sequence, allocation to different groups was spread evenly across the study years. More pregnancies had ARED flow at study entry in GRIT than in TRUFFLE (70% vs 41%; P < 0.01). However, if later measurements were included, rates of umbilical artery ARED flow were comparable between the groups (64% on average). The interval to delivery was shorter in the GRIT delayed‐delivery groups than in the TRUFFLE groups (median 3 days vs 8 days; P < 0.01). Fetal‐death rate was comparable between GRIT and TRUFFLE, but neonatal or late death was more frequent in GRIT (18% vs 6%; P < 0.01). Fetal‐death rate was similar in women monitored by conventional CTG without STV and in those monitored by cCTG in GRIT (4.8% vs 5.5%), but perinatal mortality was lower in the GRIT cCTG group (13% vs 23%), although the difference did not reach statistical significance. The rate of survival without neurodevelopmental impairment at 2 years was lowest in the immediate‐delivery group and in pregnancies monitored by CTG without STV, with a higher rate in the cCTG groups and the highest rate in the cCTG + DV Doppler group (Pearson chi‐square, P < 0.01). Figure 1 shows a trend of decreasing perinatal‐death rate across these groups at different gestational ages at inclusion, which was most apparent for those included at the lowest gestational age (26–27 weeks). A similar trend was observed when data were grouped by year of randomization (Pearson chi‐square, P < 0.01) (Figure 2). Other parameters that were associated significantly with survival without neurodevelopmental impairment at 2 years were gestational age at randomization, umbilical artery ARED flow, birth weight and birth‐weight Z‐score.
Table 1.
Characteristics and outcome of pregnancies complicated by early‐onset fetal growth restriction in GRIT and TRUFFLE studies, overall and according to intervention and monitoring strategy
| Parameter | GRIT | TRUFFLE | Total (n = 740) | |||
|---|---|---|---|---|---|---|
| Immediate delivery (n = 121) | Delayed delivery with monitoring by: | Delayed delivery with monitoring by: | ||||
| CTG without STV (n = 62) | cCTG with STV (n = 55) | cCTG with STV (n = 165) | cCTG with STV and DV Doppler (n = 337) | |||
| Nulliparous | 74 (61) | 33 (53) | 33 (60) | 100 (61) | 218 (65) | 458 (62) |
| GA at inclusion (weeks) | 29.5 (28.5–31.0) | 29.5 (28.5–31.5) | 29.5 (28.5–30.5) | 29.2 (27.9–30.1) | 29.2 (27.9–30.4) | 29.5 (28.1–30.5) |
| Interval to delivery (days)* | 0 (0–1) | 4 (2–8) | 2 (1–8) | 7 (2–17) | 8 (3–17) | 5 (1–14) |
| Umbilical artery ARED flow | ||||||
| At inclusion* | 85 (70) | 44 (71) | 37 (67) | 61 (37) | 147 (44) | 374 (51) |
| At any time | 85 (70) | 44 (71) | 37 (67) | 94 (57) | 210 (62) | 470 (64) |
| Fetal death | 2 (2) | 3 (5) | 3 (5) | 2 (1) | 10 (3) | 20 (3) |
| Live birth | 119 (98) | 59 (95) | 52 (95) | 163 (99) | 327 (97) | 720 (97) |
| GA at delivery (weeks) | 30.5 (28.6–31.5) | 30.6 (29.0–32.4) | 30.7 (29.6–31.6) | 30.6 (29.0–32.0) | 30.7 (29.3–32.3) | 30.6 (29.1–31.9) |
| Birth weight (g) | 880 (740–1100) | 920 (745–1085) | 928 (733–1068) | 965 (800–1115) | 990 (806–1200) | 953 (780–1160) |
| Birth‐weight Z‐score | –3.3 (–3.9 to –2.4) | –3.3 (–4.3 to –2.7) | –3.5 (–4.1 to –2.8) | –3.3 (–3.8 to –2.7) | –3.3 (–3.8 to –2.8) | –3.3 (–3.8 to –2.8) |
| Neonatal death* | 23 (19) | 11 (18) | 4 (7) | 10 (6) | 17 (5) | 65 (9) |
| Late death† | 3 (2) | 0 (0) | 1 (2) | 1 (1) | 1 (0.3) | 6 (1) |
| Evaluated at 2 years | 91 (75) | 45 (73) | 46 (84) | 131 (79) | 271 (80) | 584 (79) |
| Severe impairment‡, § | 4/91 (4) | 2/45 (4) | 1/46 (2) | 6/131 (5) | 3/271 (1) | 16/584 (3) |
| Abnormal development§ | 8/91 (9) | 4/45 (9) | 3/46 (7) | 20/131 (15) | 19/271 (7) | 54/584 (9) |
| Alive with normal development at 2 years*, ¶ | 83/119 (70 (61–78)) | 41/59 (69 (57–82)) | 43/54 (80 (68–91)) | 111/144 (77 (70–84)) | 252/299 (84 (80–89)) | 530/675 (79 (75–82)) |
Data are given as n (%), median (interquartile range), n/N (%) or n/N (% (95% CI)).
Distribution differed between groups (Kruskal–Wallis test or Pearson chi‐square; P < 0.05).
Defined as death after first discharge and before 2 years of age.
Severe hearing or vision impairment or cerebral palsy.
Percentage of infants evaluated at 2 years.
Percentage of all infants with known outcome.
ARED, absent/reversed end‐diastolic; CTG, cardiotocography; cCTG, computerized CTG; DV, ductus venosus; GA, gestational age; STV, short‐term variation in fetal heart rate.
Figure 1.
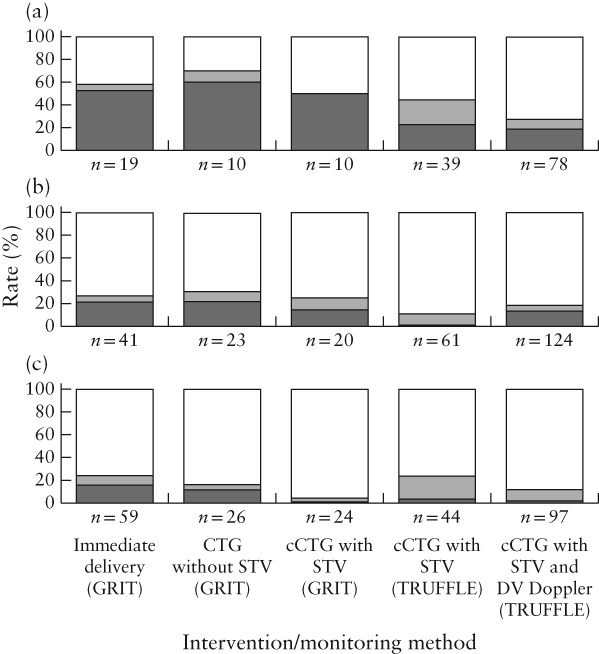
Rates of normal development ( ), neurodevelopmental impairment (
), neurodevelopmental impairment ( ) and perinatal death (
) and perinatal death ( ) by 2 years of age in children from pregnancies included in GRIT and TRUFFLE studies, randomized at 26–27 weeks (a), 28–29 weeks (b) and 30–31 weeks (c), according to intervention/monitoring method. Pearson chi‐square test between subgroups, P < 0.05; N = 675; 65 women lost to follow‐ up were excluded. CTG, cardiotocography; cCTG, computerized CTG; DV, ductus venosus; STV, short‐term variation in fetal heart rate.
) by 2 years of age in children from pregnancies included in GRIT and TRUFFLE studies, randomized at 26–27 weeks (a), 28–29 weeks (b) and 30–31 weeks (c), according to intervention/monitoring method. Pearson chi‐square test between subgroups, P < 0.05; N = 675; 65 women lost to follow‐ up were excluded. CTG, cardiotocography; cCTG, computerized CTG; DV, ductus venosus; STV, short‐term variation in fetal heart rate.
Figure 2.
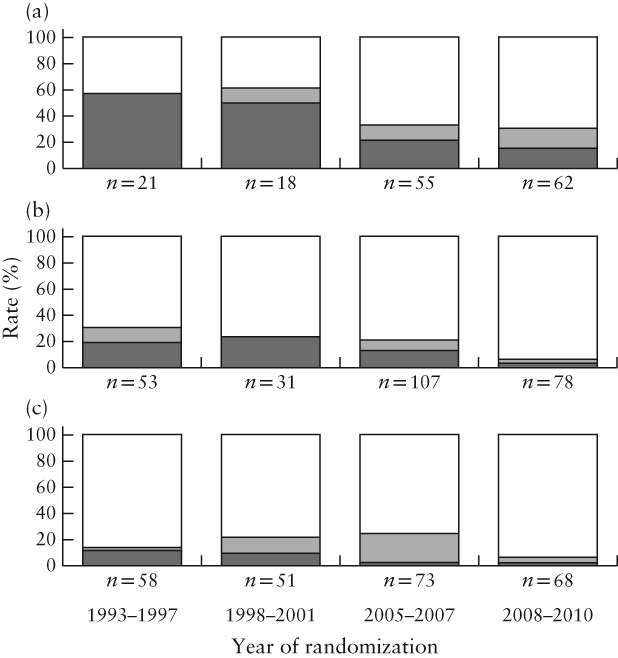
Rates of normal development ( ), neurodevelopmental impairment (
), neurodevelopmental impairment ( ) and perinatal death (
) and perinatal death ( ) by 2 years of age in children from pregnancies included in GRIT and TRUFFLE studies, randomized at 26–27 weeks (a), 28–29 weeks (b) and 30–31 weeks (c), according to year of randomization. Pearson chi‐square test between subgroups, P < 0.05; N = 675; 65 women lost to follow‐up were excluded.
) by 2 years of age in children from pregnancies included in GRIT and TRUFFLE studies, randomized at 26–27 weeks (a), 28–29 weeks (b) and 30–31 weeks (c), according to year of randomization. Pearson chi‐square test between subgroups, P < 0.05; N = 675; 65 women lost to follow‐up were excluded.
Because the study periods of GRIT and TRUFFLE differed (1993–2001 vs 2005–2010) and study period and monitoring strategy were strongly associated (Pearson correlation coefficient, 0.82), it was not appropriate to combine these in one regression analysis. We therefore performed separate regression analyses for monitoring method and for year of inclusion, alongside the other parameters that were associated significantly with survival without impairment at 2 years, except birth weight and birth‐weight Z‐score, which were omitted because they were correlated with both gestational age and umbilical artery ARED flow. The addition of gestational age did not affect the ORs of monitoring strategy and study period for survival without impairment at 2 years, but the area under the receiver–operating characteristics curve (AUC) of the regression model increased from 0.63 to 0.69. The ORs for the model are shown in Figure 3. Gestational age at inclusion and umbilical artery ARED flow, which are both measures of the severity of FGR, had a strong association with survival without impairment at 2 years. Year of inclusion and monitoring strategy had similar, moderate effects.
Figure 3.
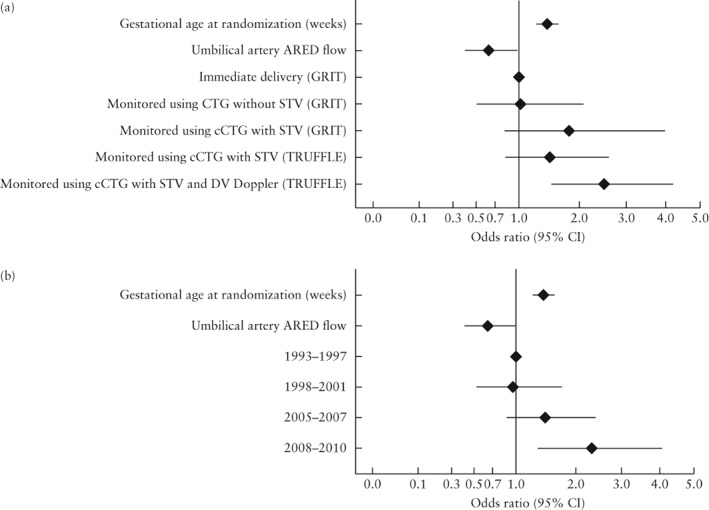
Odds ratios for survival without neurodevelopmental impairment at 2 years in children from pregnancies complicated by early‐onset fetal growth restriction included in GRIT and TRUFFLE studies, calculated using multivariable regression model including intervention/monitoring method (area under receiver–operating characteristics curve (AUC), 0.69 (95% CI, 0.64–0.74)) (a) or year of inclusion (AUC, 0.68 (95% CI, 0.63–0.73)) (b). ARED, absent/reversed end‐diastolic; CTG, cardiotocography; cCTG, computerized CTG; DV, ductus venosus; STV, short‐term variation in fetal heart rate.
DISCUSSION
This study found a trend for improved long‐term infant outcome using more advanced fetal monitoring strategies for early‐onset FGR, with lower rates of neonatal death and neurodevelopmental impairment at 2 years, after adjustment for severity of FGR based on gestational age at delivery and umbilical artery ARED flow.
We had initially intended to perform an IPD‐MA. However, conventional meta‐analysis was not possible owing to differences in inclusion criteria and type of interventions in GRIT and TRUFFLE. Although inclusion criteria differed, the study populations were very similar with regard to gestational age, birth weight and birth‐weight Z‐score. Umbilical artery ARED flow on Doppler examination at inclusion was more frequent in GRIT. GRIT participants were included if there was uncertainty regarding the necessity of delivering the fetus and were probably at a later stage of the pathological process of FGR at inclusion than were TRUFFLE participants. As a result, in TRUFFLE, the interval from inclusion to delivery was longer and, when later Doppler measurements were included, ARED flow became as frequent as in GRIT.
A problem encountered was that the trials were performed during successive time periods, and it was not possible to separate outcome improvement due to advances in obstetric and neonatal care from the effects of differences in monitoring strategy between the trials. On statistical analysis, both study period and intervention/monitoring method were highly correlated. Both seemed to have an effect on survival without neurodevelopmental impairment at 2 years, but the proportional contribution of these parameters could not be determined exactly.
Birth weight and gestational age have a potential for collider stratification bias, as they are not independent parameters in a population of pregnancies with early preterm FGR. They are influenced by severity of FGR, monitoring method and the decision to deliver the fetus at a certain moment. More data on the underlying pathology of FGR would have been useful to improve outcome prediction; unfortunately, except for Doppler variables, there are no appropriate markers for the underlying pathology of FGR. Therefore, gestational age in this study should be regarded as a proxy for the severity of FGR, and not as an independent variable. The study population was fairly uniform in its selection through functional Doppler markers of FGR, and we expect that this resulted in similar underlying pathologies of FGR in the intervention groups. A further argument supporting the notion that collider bias by gestational age is unlikely is that regression analysis with or without the inclusion of gestational age resulted in similar ORs for monitoring strategy and study period, while the AUC for the model increased with the addition of gestational age.
The two studies used two different tests to assess neurodevelopmental outcome; Griffiths General Quotients were used in GRIT and Bayley Cognitive Composite Scores were used in TRUFFLE, with different cut‐offs commensurate with prevailing practice. Using a cut‐off of < 85 in TRUFFLE and a stricter cut‐off of ≤ 70 in GRIT, it would be unlikely that differences in developmental outcome were inflated in favor of TRUFFLE9. This supports our conclusion that outcome was better in pregnancies that underwent monitoring with DV Doppler in TRUFFLE.
GRIT investigated immediate delivery in pregnancies with FGR when the clinician was uncertain as to whether to deliver the fetus. Fetal‐monitoring method and mode of delivery were at the discretion of the attending obstetrician. In contrast, TRUFFLE was designed to compare different monitoring strategies, and delivery criteria were specifically defined for each strategy. Cut‐offs for STV in TRUFFLE were defined according to the criteria of Dawes et al.10, based on associations with fetal acidemia. DV Doppler cut‐offs were substantiated by longitudinal studies on monitoring parameters and adverse perinatal outcomes1, 11. However, the minimum frequency of monitoring was not specified strictly (DV Doppler once per week, cCTG twice per week). Lack of a fixed, frequent schedule for monitoring in TRUFFLE and the complete absence of such data in GRIT preclude a secondary longitudinal analysis of monitoring techniques, which could have provided more information on their relative impact on decision‐making.
In both studies, delivery was indicated by criteria other than fetal distress. In GRIT, the indication for delivery was not included in the database, while in TRUFFLE, it is reported that 30% were delivered outside the prespecified monitoring criteria12. Additionally, there were insufficient data regarding co‐interventions such as administration of corticosteroids, which were probably administered to nearly all patients, and magnesium sulfate, which was probably used in only a few patients for the prevention of eclampsia, as fetal neuroprotection was not used in clinical practice during the study periods. These restrictions hamper the combined analysis of data on the effect of monitoring strategy.
Our attempt to assess the superiority of cCTG over conventional CTG was only partly successful. Only a few studies have compared the predictive value of cCTG with that of CTG using visual inspection, while all previous studies assessed only short‐term outcomes and were underpowered for perinatal outcomes. Turan et al.13 described monitoring parameters in a cohort of 56 growth‐restricted fetuses and showed a slightly better correlation between low STV and low umbilical cord pH, as compared with traditional CTG. Another study showed no significant relationship between both methods and fetal or neonatal survival14. To our knowledge, there are no published data comparing the efficacy of cCTG and standard CTG using visual inspection. Currently, probably owing to the limited association of cCTG with fetal hypoxemia and acidemia15, 16, there is significant variation in the use of cCTG in clinical practice.
We observed an improvement in infant outcome over time and with increasing improvements in fetal monitoring techniques. This correlation is expected, as it is logical that advances in obstetric and neonatal care over the past 20 years will have had an effect on outcome, and that advances in fetal‐monitoring methods are a part of this.
In the group monitored using cCTG in GRIT, infant outcome was better than in the group monitored using CTG with visual assessment, although this difference was not statistically significant and might have been caused by differences between centers that used cCTG and those that did not. The main advantage of cCTG is that it provides a numerical result, which allows for a strict protocol‐based decision on intervention to be made, as applied in TRUFFLE. Its use is therefore essential for future intervention trials. However, it has not been proven to be superior in a head‐to‐head comparison.
In conclusion, this comparative analysis supports the hypothesis that the optimal method of fetal monitoring for early‐onset preterm FGR is a combination of cCTG and DV Doppler assessment.
ACKNOWLEDGMENTS
We gratefully acknowledge the support of Prof. Andy Vail, Centre for Biostatistics, University of Manchester for the provision of the relevant GRIT study data. This analysis was performed without funding. The original TRUFFLE study was partly funded by Grant Number 94506556, ZonMw, POBox 93245, 2509 AE Den Haag, The Netherlands. The GRIT study was funded by the United Kingdom Medical Research Council, a European Union Concerted Action and the Dutch Princess Beatrix Foundation.
Supporting information
Appendix S1 GRIT study group
Appendix S2 TRUFFLE study group
 This article has been selected for Journal Club. Click https://www.isuog.org/resource/uog-journal-club-January-2020.html to view slides and discussion points.
This article has been selected for Journal Club. Click https://www.isuog.org/resource/uog-journal-club-January-2020.html to view slides and discussion points.
Contributor Information
W. Ganzevoort, Email: j.w.ganzevoort@amsterdamumc.nl.
for the GRIT Study Group:
B Van Bulck, G M Kalakoutis, P Sak, K T M Schneider, S E Karpathios, T Major, T Todros, D Arduini, C Flumini, A C Tenore, N Roncaglia, T Frusca, G Ferretti, M J N Weinans, J van Roosmalen, J W van der Slikke, H van Geijn, P J M Pernet, H Wolf, R H Stigter, J Wilczynski, E Vasco, M Abdullah, Z Novak‐Antolic, P Danielian, S D Jenkinson, C R Welch, C Griffin, H Gee, D Tuffnell, J Cresswell, T Tariq, B Sengupta, G Tydeman, D Churchill, S Bewley, L Fusi, S W Lindow, W Johal, F M Fairlie, K Neales, G Mason, I Scudamore, J Konje, S A Walkinshaw, M Griffiths, A Dawson, G Mires, R Johanson, R B Fraser, P Hendy Ibbs, S A Steel, M Ramsay, J B Robins, M J Heard, H M Tonge, I T Manyonda, J Walker, M Maresh, A Yoong, P Soothill, H Cameron, D Byrne, B Beattie, S Bober, M Van Damme, S Kyriakides, P Pokorna, A Zimmerman, H Tsitsikas, E Mani, P Bosisio, E Mastritta, M Nicocia, C Fabris, P Eken, L De Vries, Z Kubicka, R Ramalho, M Rashid, L Cerar‐Kornhauser, S Thomas, A Elliman, T Sim, S Swaminathan, L Walker, D McCormick, Z Sibanda, J Hughes, J Kilding, A O'Hara, V Harpin, N Porter, S Pandey, K Murtagh, K Burton, R MacGregor, B Stewart, H Klenke, P Hallam, N Kai, M Graham, A Harrison, E Saharia, T McGhee, P Rowsell, P Howie, Max Parmar, D Field, A Grant, P Steer, G Breart, M Levene, D Torgeson, S Kitzinger, M Levene, N Marlowe, D Wolke, L De Vries, A Johnson, and G Ferretti
the TRUFFLE Study Group:
Birgit Arabin, Caterina M Bilardo, Christoph Brezinka, Jerome M J Cornette, Jan B Derks, Anke Diemert, Johannes J Duvekot, Enrico Ferrazzi, Nicola Fratelli, Tiziana Frusca, Wessel Ganzevoort, Kurt Hecher, Aleid van Leemhuis, Christoph C Lees, Silvia Lobmaier, Neil Marlow, Pasquale Martinelli, Gianpaolo Maso, Hannah Missfelder‐Lobos, Raffaele Napolitano, Eva Ostermayer, Aris T Papageorghiou, Federico Prefumo, Dietmar Schlembach, K T M Schneider, Baskaran Thilaganathan, Tullia Todros, Adriana Valcamonico, Gerard H A Visser, Hans Wolf, John Kingdom, Karel Marsal, Jim Thornton, and Herbert Valensise
REFERENCES
- 1. Bilardo CM, Wolf H, Stigter RH, Ville Y, Baez E, Visser GH, Hecher K. Relationship between monitoring parameters and perinatal outcome in severe, early intrauterine growth restriction. Ultrasound Obstet Gynecol 2004; 23: 119–125. [DOI] [PubMed] [Google Scholar]
- 2. Brodszki J, Morsing E, Malcus P, Thuring A, Ley D, Marsál K. Early intervention in management of very preterm growth‐restricted fetuses: 2‐year outcome of infants delivered on fetal indication before 30 gestational weeks. Ultrasound Obstet Gynecol 2009; 34: 288–296. [DOI] [PubMed] [Google Scholar]
- 3. GRIT Study Group . A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG 2003; 110: 27–32. [DOI] [PubMed] [Google Scholar]
- 4. Lees CC, Marlow N, van Wassenaer‐Leemhuis A, Arabin B, Bilardo CM, Brezinka C, Calvert S, Derks JB, Diemert A, Duvekot JJ, Ferrazzi E, Frusca T et al; TRUFFLE study group . 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet 2015; 385: 2162–2172. [DOI] [PubMed] [Google Scholar]
- 5. Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M; GRIT study group . Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004; 364: 513–520. [DOI] [PubMed] [Google Scholar]
- 6. Lees C, Marlow N, Arabin B, Bilardo CM, Brezinka C, Derks JB, Duvekot J, Frusca T, Diemert A, Ferrazzi E, Ganzevoort W, Hecher K et al; TRUFFLE Group . Perinatal morbidity and mortality in early‐onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet Gynecol 2013; 42: 400–408. [DOI] [PubMed] [Google Scholar]
- 7. Hadlock FP, Harrist RB, Martinez‐Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology 1991; 181: 129–133. [DOI] [PubMed] [Google Scholar]
- 8. Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput 2004; 36: 717–731. [DOI] [PubMed] [Google Scholar]
- 9. Cirelli I, Bickle Graz M, Tolsa JF. Comparison of Griffiths‐II and Bayley‐II tests for the developmental assessment of high‐risk infants. Infant Behav Dev 2015; 41: 17–25. [DOI] [PubMed] [Google Scholar]
- 10. Dawes GS, Moulden M, Redman CW. Short‐term fetal heart rate variation, decelerations, and umbilical flow velocity waveforms before labor. Obstet Gynecol 1992; 80: 673–678. [PubMed] [Google Scholar]
- 11. Hecher K, Bilardo CM, Stigter RH, Ville Y, Hackeloer BJ, Kok HJ, Senat MV, Visser GH. Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound Obstet Gynecol 2001; 18: 564–570. [DOI] [PubMed] [Google Scholar]
- 12. Visser GHA, Bilardo CM, Derks JB, Ferrazzi E, Fratelli N, Frusca T, Ganzevoort W, Lees CC, Napolitano R, Todros T, Wolf H, Hecher K; TRUFFLE group investigators . Fetal monitoring indications for delivery and 2‐year outcome in 310 infants with fetal growth restriction delivered before 32 weeks' gestation in the TRUFFLE study. Ultrasound Obstet Gynecol 2017; 50: 347–352. [DOI] [PubMed] [Google Scholar]
- 13. Turan S, Turan OM, Berg C, Moyano D, Bhide A, Bower S, Thilaganathan B, Gembruch U, Nicolaides K, Harman C, Baschat AA. Computerized fetal heart rate analysis, Doppler ultrasound and biophysical profile score in the prediction of acid–base status of growth‐restricted fetuses. Ultrasound Obstet Gynecol 2007; 30: 750–756. [DOI] [PubMed] [Google Scholar]
- 14. Madazli R. Prognostic factors for survival of growth‐restricted fetuses with absent end‐diastolic velocity in the umbilical artery. J Perinatol 2002; 22: 286–290. [DOI] [PubMed] [Google Scholar]
- 15. Kapaya H, Jacques R, Rahaim N, Anumba D. “Does short‐term variation in fetal heart rate predict fetal acidaemia?” A systematic review and meta‐analysis. J Matern Fetal Neonatal Med 2016; 29: 4070–4077. [DOI] [PubMed] [Google Scholar]
- 16. Pels A, Mensing van Charante NA, Vollgraff Heidweiller‐Schreurs CA, Limpens J, Wolf H, de Boer MA, Ganzevoort W. The prognostic accuracy of short term variation of fetal heart rate in early‐onset fetal growth restriction: A systematic review. Eur J Obstet Gynecol Reprod Biol 2019; 234: 179–184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 GRIT study group
Appendix S2 TRUFFLE study group


