ABSTRACT
RpoS is a key regulator of general stress responses in Escherichia coli. Its expression is post-transcriptionally up-regulated by the small RNAs (sRNAs), ArcZ, DsrA and RprA, through sRNA-rpoS mRNA interactions. Although overexpression of the sRNA, CyaR, was reported to down-regulate rpoS expression, how CyaR regulates rpoS has not been determined. Here, we report that CyaR represses rpoS expression by base-pairing with a region next to the ArcZ binding site in the 5ʹ UTR of rpoS mRNA and that CyaR expression itself is down-regulated by ArcZ through sRNA-sRNA interaction. The short form of ArcZ, but not the full-length form, can base-pair with CyaR. This ArcZ-CyaR interaction triggers degradation of CyaR by RNase E, alleviating the CyaR-mediated rpoS repression. These results suggest that ArcZ not only participates in rpoS translation as an activator, but also acts as a regulator of the reciprocally acting CyaR, maximizing its rpoS-activating effect.
KEYWORDS: Small RNAs, ArcZ, CyaR, rpoS, post-transcriptional regulation
Introduction
Bacteria are constantly exposed to a variety of stressful environments, experiencing specific stresses, such as temperature variation and acidic shock, and general stresses, such as entry into stationary phase and nutrient depletion [1]. Numerous targeted response mechanisms to specific stresses have been identified, but response mechanisms that function in general stresses also exist. In Escherichia coli, RpoS (sigma factor S) is a key regulator of general stress responses that controls approximately 500 genes [2].
RpoS expression is regulated at the levels of transcription, translation, and protein stability. The small sRNAs (sRNAs), ArcZ, DsrA and RprA, activate rpoS at the post-transcriptional level by directly base-pairing with rpoS mRNA [3,4]. These three rpoS-activating sRNAs stimulate rpoS translation by unfolding the rpoS mRNA 5ʹ UTR, exposing the translation start site of rpoS that is blocked by a folded stem-loop structure in the 5ʹ UTR [5]. ArcZ is highly expressed under aerobic conditions, but upon anaerobic stress, ArcA suppresses ArcZ, leading to down-regulation of rpoS expression [4]. ArcZ is processed into a short, 56-nt (nucleotide) form from the 3ʹ end of the 120-nt primary transcript [4,6], and the short form of ArcZ binds to the 5ʹ leader of rpoS mRNA [5]. RprA is activated by the Rcs phosphorelay, which is necessary for expression of genes needed for colonic capsule synthesis [7,8]. Activation of rpoS translation by RprA helps ensure properly timed expression of RpoS during biofilm maturation [9]. DsrA biosynthesis is activated in low-temperature environments [10]. OxyS is only one sRNA that has been shown to down-regulate rpoS, although whether it directly interacts with rpoS mRNA remains unclear [11]. Considering that RpoS is regulated in a variety of ways at the post-transcriptional level [1,12–16], the prediction is that additional rpoS-repressing sRNAs would be needed to cope with stresses imposed on bacteria.
Previous studies have reported that, upon overexpression, multiple sRNAs, including CyaR, down-regulate a rpoS-lacZ translational fusion [4]. CyaR is a cyclic AMP-activated RNA; thus, its expression is regulated by cAMP-CRP and the CpxA/R two-component system [17,18]. CyaR is sigma factor E-dependent and represses a variety of targets, including ompX, yqaE, nadE, luxS, yobF and hdeD mRNA [17–21]. Recently, Eric Masse’s group analysed the targetomes of CyaR using MS2-affinity purification coupled with RNA sequencing (MAPS) technology, revealing that additional direct base-pairing target mRNAs (including rpoS mRNA) for CyaR may exist[21].
RNA sequencing-based experiments employing RIL-seq (RNA interaction by ligation and sequencing) or MAPS [21,22] have also identified sRNA-sRNA interaction fragments, implying that sRNA-sRNA interactions are integrated in sRNA-mediated regulatory mechanisms [21,22]. An example of regulation by sRNA-sRNA interaction is RNA ‘sponges’. RNA sponges were first named for eukaryotic RNAs that contain sites capable of base-pairing with target microRNAs and minimizing microRNA-mediated mRNA repression[23]. Circular RNAs and long noncoding RNAs can also act as RNA sponges [24,25]. In bacteria, SroC sRNA, which is produced by mRNA decay from the gltIJKL locus encoding an amino acid ABC transporter, acts as an RNA sponge for GcvB sRNA. Because gltIJKL mRNA itself is a target of GcvB, the GcvB/SroC sponge system forms a feed-forward loop that regulates amino acid ABC transporters[26]. RIL-seq data[22] revealed the presence of ArcZ-CyaR interactions. However, the physiological consequences that might result from ArcZ-CyaR interactions in cells are not yet known.
In this study, we determined (i) whether CyaR-mediated rpoS repression occurs through interaction of CyaR with rpoS mRNA, and (ii) how CyaR-ArcZ interactions contribute to rpoS regulation. We found that CyaR represses rpoS expression by binding a region immediately upstream of the ArcZ binding site in the 5ʹ UTR of rpoS mRNA. CyaR also base-pairs with the short form of ArcZ. This CyaR-ArcZ interaction triggers degradation of CyaR by RNase E, alleviating the CyaR-mediated rpoS repression.
Results
Repression of rpoS expression by CyaR
A previous study reported that several sRNAs down-regulate LacZ expression in a rpoS-lacZ translational fusion when overexpressed[27]. However, whether repression by each sRNA occurs through direct targeting of rpoS mRNA has remained unclear. Of these putative rpoS-repressing sRNAs, we focused on CyaR, first examining the rpoS-repressive effect of overexpressed CyaR. For this purpose, we used PM1409 strain carrying a translational rpoS-lacZ fusion to determine whether the rpoS translational repression effect occurred upon overexpression of CyaR. We found that CyaR overexpression caused a significant reduction in LacZ activity (to ~60% of controls) in rpoS-lacZ fusion cells (Fig 1A,B). This suggests that overexpressed CyaR represses expression of rpoS mRNA.
Figure 1.
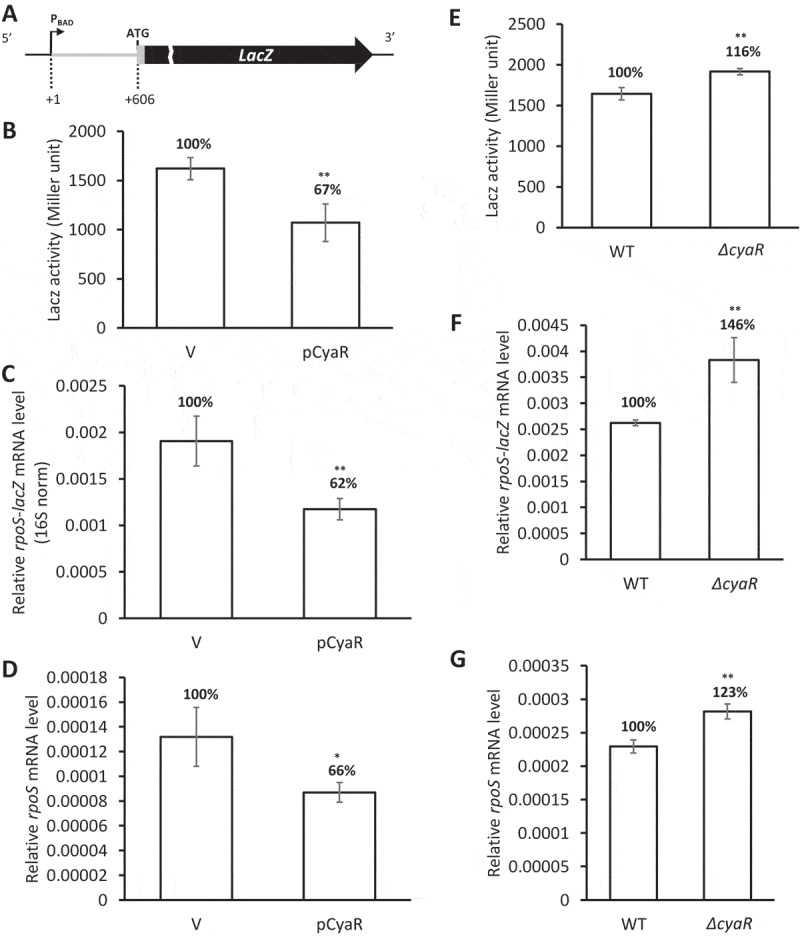
Repression of rpoS expression by CyaR. (A) Schematic presenting the rpoS-lacZ translational fusion. PBAD, the arabinose-inducible pBAD promoter; +1, the rpoS transcription start site; ATG, the rpoS translation start codon. The sequence encoding the 5ʹ-upstream 606 nt of the rpoS mRNA was fused in frame to lacZ. (B-D) Repression of rpoS by overexpression of CyaR in PM1409 (WT) cells carrying the rpoS-lacZ translational fusion. (B) LacZ activity, (C) rpoS mRNA level, and (D) rpoS-lacZ mRNA level were measured. mRNA levels were analysed by qRT-PCR and were normalized to rrsA expression. (E-G) rpoS activation in the absence of CyaR. (E) LacZ activity, (F) rpoS-lacZ mRNA level, and (G) rpoS mRNA level for WT and ΔcyaR cells were measured. The ratios of LacZ activity, and rpoS-lacZ and rpoS mRNA levels to those of WT are shown. Values are means ± SD; n = 3; **P < 0.01, *P < 0.05; ns, not significant (Student’s t-test, equal variance with the control vector across expression values). pCyaR, a plasmid overexpressing CyaR; V, vector control; ΔcyaR, PM1409ΔcyaR.
Next, we quantified levels of rpoS-lacZ transcripts and rpoS mRNA using qRT-PCR (Fig 1C,D). The levels of both rpoS-lacZ transcripts and rpoS mRNA were reduced to about 50–70% of controls by CyaR overexpression, a result consistent with the reduced level of LacZ activity (Fig 1B).
While the magnitude of CyaR repression on rpoS is as low as 30% ~ 40% when it is overexpressed, rpoS-activating sRNAs ArcZ, RprA, and DsrA increase rpoS expression by about 20%, 70%, and 80%, respectively, under the same condition[28]. The magnitude of CyaR repression on other known targets varies from about 50% to 70% (Fig. S1). Although the CyaR repression level on rpoS is lower than those on other known repressible targets, it is marginally comparable to their lowest level.
Rifampicin chase experiments showed that overexpression of CyaR decreased the half-life of rpoS mRNA (Fig. S2). This result indicates that the repressive effect of CyaR on rpoS is mainly caused by degradation of rpoS mRNA. We examined whether RNase E, a key regulator of RNA metabolism [29–32], is involved in CyaR-mediated rpoS mRNA degradation using RNase E temperature-sensitive cells (rnets). To this end, we analysed rpoS mRNA levels following overexpression of CyaR in rnets cells (Fig. S3). We found that CyaR was not able to degrade rpoS mRNA at nonpermissive temperatures, suggesting that RNase E is crucial for CyaR-mediated rpoS degradation.
We also examined rpoS expression levels in a mutant strain lacking CyaR. In these experiments, we measured LacZ activity and rpoS-lacZ transcript levels generated by the rpoS-lacZ fusion, as well as endogenous rpoS mRNA levels. Compared with the WT strain, all three values were significantly increased in ΔcyaR cells (Fig 1E–G). This suggests that chromosomally expressed CyaR, like overexpressed CyaR, also participates in rpoS repression.
Structural mapping of the CyaR-rpoS mRNA interaction
To identify CyaR binding sites on the rpoS sequence, we performed RNA footprinting using lead acetate. For this purpose, we used the 284-nt rpoS301 leader sequence[33]. In the presence of CyaR, we observed protection of rpoS mRNA from nucleotides 434 to 449 relative to the transcription start site. The protected site, which matches the pairing site for CyaR-rpoS mRNA interaction predicted by IntaRNA software[34], is located immediately upstream of the binding region for rpoS-activating sRNAs (Fig 2). Although another protected region from nucleotides 342 to 349 was observed, it probably results from the change of the secondary structure of rpoS mRNA by binding to CyaR because potential binding sites do not exist in this region. In addition, we also found that the region of nucleotides 449 to 460 became more vulnerable to PbAc cleavage, suggesting that this enhanced cleavage is due to the structural change of rpoS mRNA (Fig 2A).
Figure 2.
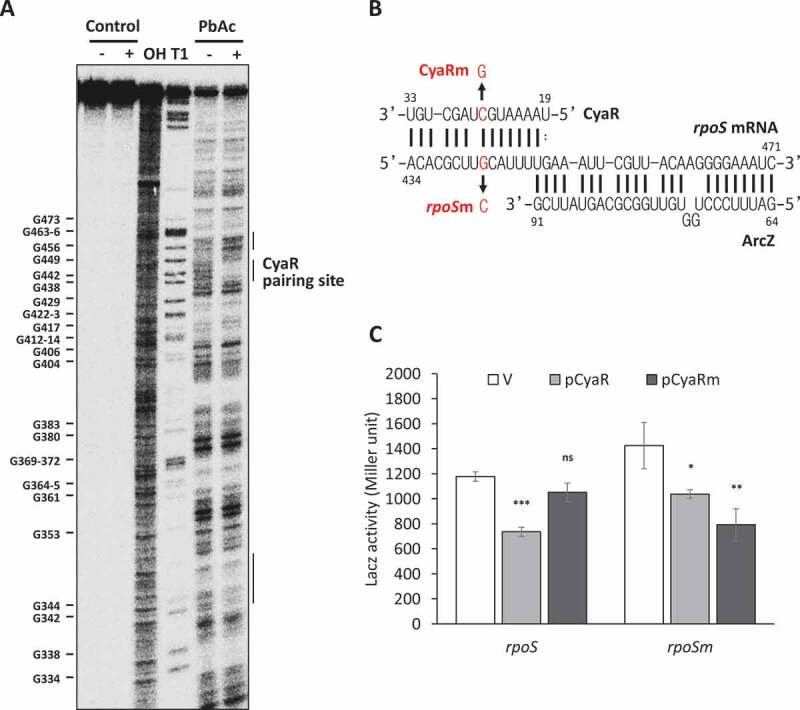
Probing of rpoS mRNA-CyaR interactions. (A) Probing of 5ʹ end-radiolabeled rpoS301 by lead acetate (PbAc) in the presence or absence of CyaR. OH, alkaline ladders; T1, RNase T1 ladders. The numbers to the left indicate G-nucleotide positions with respect to the +1 position relative to the rpoS transcription start site. Regions on rpoS mRNA that revealed significant increases or decreases in PbAc cleavage are indicated by bars on the right of the figure. (B) The base-pairing region between CyaR and rpoS mRNA. The C-to-G mutation at position 26 of CyaR is indicated by CyaRm, and the compensatory mutation at position +442 relative to the rpoS transcription start site in PM1409 is indicated by rpoSm. (C) Plasmids pCyaR and pCyaRm expressing CyaR and CyaRm, respectively, were introduced into strains PM1409 and PM1409m containing the compensatory mutation in rpoS. LacZ activity of each strain was measured and fold changes compared to the vector control (pHMB1) are shown. In (C), values are means ± SD; n = 3; ***P < 0.001, *P < 0.05 by Student’s t-test. rpoS, PM1409; rpoSm, PM1409m.
To validate the CyaR-rpoS mRNA interaction in vivo, we mutated a C nucleotide at nucleotide 26 in the potential binding site of CyaR (Fig 2B). This C-to-G point mutation in CyaR suppressed CyaR-mediated rpoS repression (Fig 2C). A Northern blot analysis showed that the mutated CyaR was expressed at almost the same level as wild-type CyaR (Fig. S4), suggesting that the suppression of rpoS repression was not attributable to a decrease in the level of CyaR, but instead was caused by a loss of the interaction between CyaR and rpoS mRNA. To further confirm the CyaR-rpoS mRNA interaction, we introduced a G-to-C compensatory mutation at nucleotide 442 of rpoS mRNA in the rpoS-lacZ fusion. This G-to-C mutation also mildly suppressed rpoS repression by the wild type CyaR, but slightly restored the ability of the mutant CyaR to repress rpoS expression (Fig 2C). The G-to-C mutation at nucleotide 442 of rpoS mRNA disrupts a base-pair in a structural model of the full-length rpoS leader, which could affect the secondary structure by disrupting the 411-417/570-576 stem. We observed that LacZ activity from the rpoSm-lacZ fusion was increased by 20%, suggesting that the disruption of this stem causes rpoS activation. However, the mutant rpoS-lacZ fusion was activated by rpoS-activating sRNA ArcZ, DsrA or RprA and the increase of LacZ activity by rpoS-activating sRNAs in mutant cells was similar with that in wild type cells, suggesting that the rpoS mutation affects binding to CyaR, but binding to ArcZ, DsrA and RprA (Fig. S1A). On the other hand, the C-to-G mutation at nucleotide 26 of CyaR did not significantly affect a secondary model structure of CyaR. Furthermore, the mutant CyaR was effectively able to repress other known CyaR target genes (Fig. S1B). It seems likely, therefore, that the weak restoration effects of the mutations both in rpoS mRNA and CyaR is due to the rpoS mutation that could affect the secondary structure of the rpoS mRNA. Altogether, these results suggest that base-pairing between CyaR and rpoS is responsible for rpoS repression by CyaR.
Hfq dependence of CyaR-mediated rpoS repression
Since CyaR is an Hfq-dependent sRNA, we examined whether CyaR-dependent rpoS repression is Hfq dependent by measuring rpoS mRNA levels by qRT-PCR in Δhfq cells overexpressing CyaR and in ΔcyaRΔhfq double mutant cells. Interestingly, both WT and Δhfq cells showed similar reductions in the levels of rpoS mRNA upon CyaR overexpression, even though CyaR expression was decreased in the absence of Hfq (Fig 3A,C). In contrast, there was no further activation of rpoS by ΔcyaR mutation in Δhfq cells (Fig 3B), suggesting that endogenous CyaR cannot repress rpoS in Δhfq mutant cells. The failure of the rpoS repression in Δhfq mutant cells seems to be due to extremely low levels of CyaR (Fig 3C) in the absence of Hfq rather than the Hfq requirement for the CyaR-mediated rpoS repression, which is reminiscent of Hfq effects of DsrA on rpoS activation[28]. Therefore, it is likely that CyaR-mediated rpoS repression occurs in an Hfq-independent manner, despite the fact that the stability of CyaR is Hfq-dependent.
Figure 3.
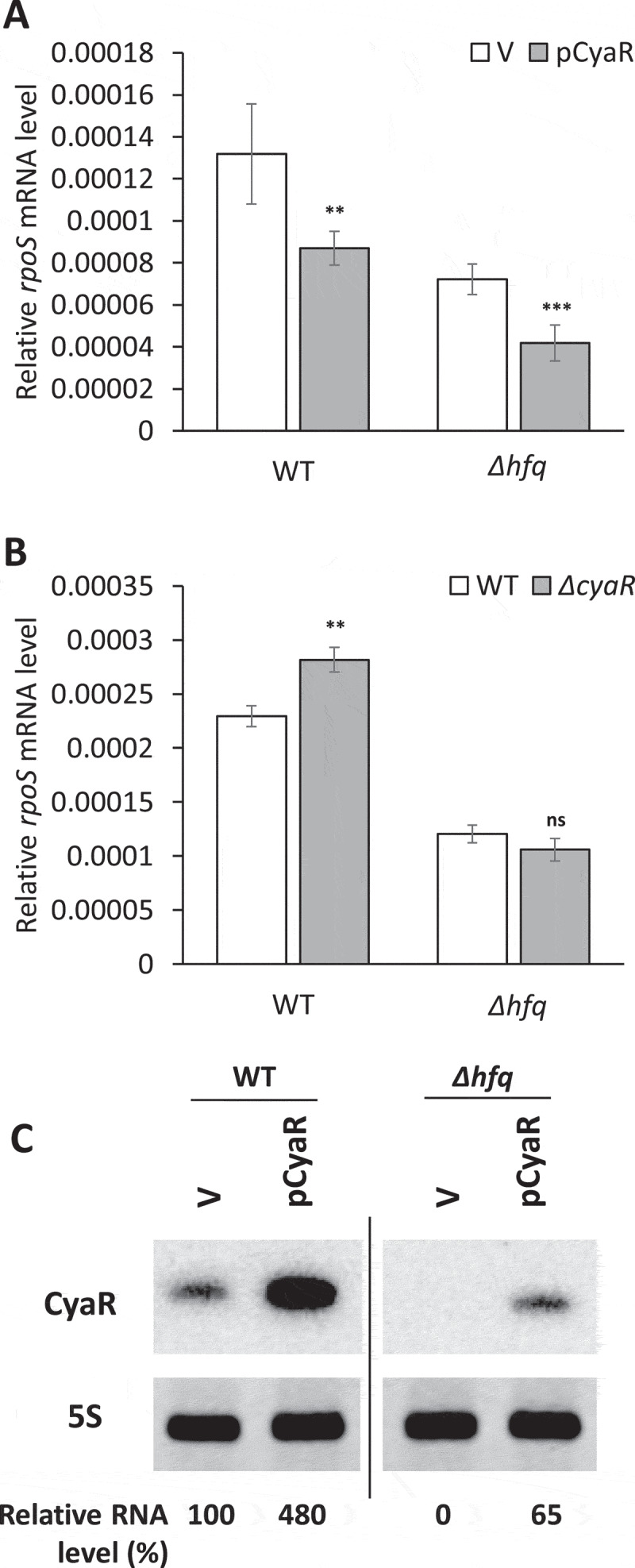
Hfq-independent rpoS repression by CyaR. The rpoS mRNA levels upon CyaR overexpression (A) and the ΔcyaR mutation (B) in Δhfq cells. The rpoS mRNA levels were measured by qRT-PCR and normalized to rrsA expression. Values are means ± SD; n = 3; ***P < 0.001, **P < 0.01 by Student’s t-test. (C) CyaR levels were measured by Northern blot analysis. CyaR was probed with an anti-CyaR oligonucleotide; 5S rRNA is shown as a loading control. CyaR RNA quantities are expressed relative to vector controls for each strain. The spliced image from the same Northern blot membrane is shown with a dividing line inserted between spliced lanes. WT, PM1409; Δhfq, PM1409Δhfq; ΔcyaRΔhfq, PM1409ΔcΔh; V, vector control.
Post-transcriptional down-regulation of CyaR by ArcZ
Results of RIL-seq using Hfq[22] suggest that ArcZ and CyaR interact. We hypothesized that ArcZ and CyaR regulate each other’s expression and that this regulation also contributes to rpoS regulation.
To test this, we examined CyaR in arcZ-mutant cells and ArcZ in cyaR-mutant cells, analysing their levels in cells at different growth time points. CyaR levels were increased at all time points examined in the absence of ArcZ (Fig 4A), whereas ArcZ levels were not significantly changed in the absence of CyaR (Fig 4B). The fold increase in CyaR levels in arcZ-mutant cells was largest at the time point (6 h) at which ArcZ was normally highly expressed. These results suggest that ArcZ down-regulates CyaR expression, but CyaR does not affect ArcZ expression.
Figure 4.
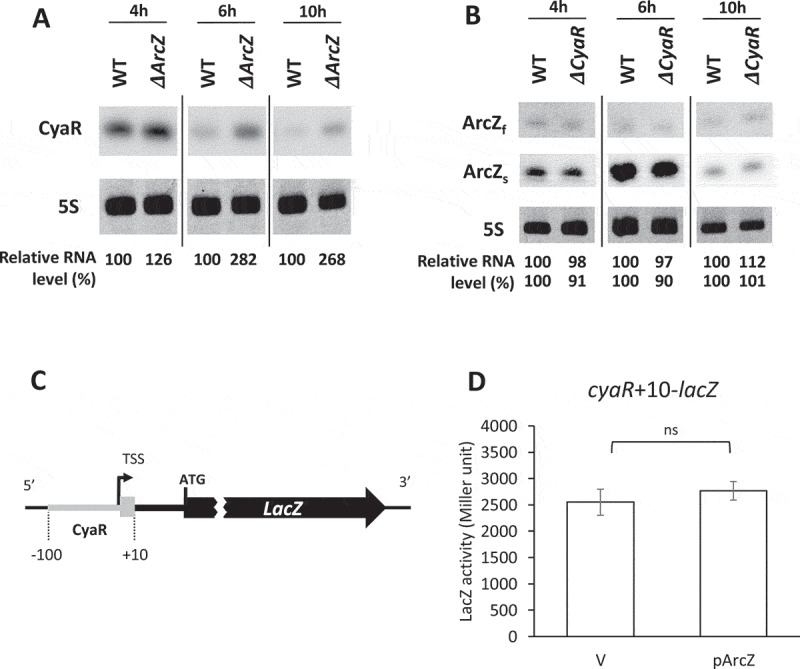
CyaR down-regulation by ArcZ. Overnight cultures of MG1655 (WT), ΔarcZ, and ΔcyaR cells were diluted 1:100 in LB medium and grown at 37°C. Aliquots of cells were sampled from the cultures at specific time intervals, and total cellular RNA was isolated. Cellular levels of CyaR and ArcZ were analysed by Northern blotting. (A) Cellular levels of CyaR were measured in WT and ΔarcZ cells during growth. CyaR was probed with an anti-CyaR oligonucleotide; 5S rRNA was detected as a loading control. (B) Cellular levels of ArcZ were measured in WT and ΔcyaR cells during growth. ArcZ was probed with an anti-ArcZ oligonucleotide; 5S rRNA was detected as a loading control. ArcZf, full-length form of ArcZ; ArcZs, short form of ArcZ. In (A) and (B), the spliced image from the same Northern blot membrane is shown with a dividing line inserted between spliced lanes. (C) Schematic representation of the cyaR-lacZ transcriptional fusion (cyaR+10-lacZ). TSS, transcription start site of CyaR. A sequence from −100 to +10 with respect to the transcription start site of CyaR was fused to lacZ mRNA. (D) Cells carrying the cyaR-lacZ transcriptional fusion were transformed with the plasmid, pArcZ, and LacZ activity was measured. Values are means ± SD; n = 3; ns, non-significant by Student’s t-test; V, vector control.
We further investigated how ArcZ affects CyaR expression. To this end, we constructed a transcriptional cyaR-lacZ fusion strain (cyaR+10-lacZ) and examined whether ArcZ overexpression affects LacZ activity (Fig 4C). LacZ activity was not changed upon ArcZ overexpression (Fig 4D), suggesting that ArcZ does not affect CyaR expression at the transcriptional level, but possibly does at the post-transcriptional level.
Degradation of CyaR through interaction with ArcZ
Since ArcZ is present as the full-length sRNA (ArcZf) and the short form (ArcZs), we examined which form of ArcZ interacts with CyaR. For this purpose, we performed RNA-RNA electrophoretic mobility shift assays (EMSAs). We found that ArcZs bound to CyaR to form an ArcZs-CyaR complex with Kd of ~20 nM, whereas ArcZf did not (Fig 5A–C). However, Hfq did not facilitate ArcZ-CyaR complex formation, suggesting that Hfq had little effect on ArcZ-CyaR interaction (Fig 5A,C).
Figure 5.
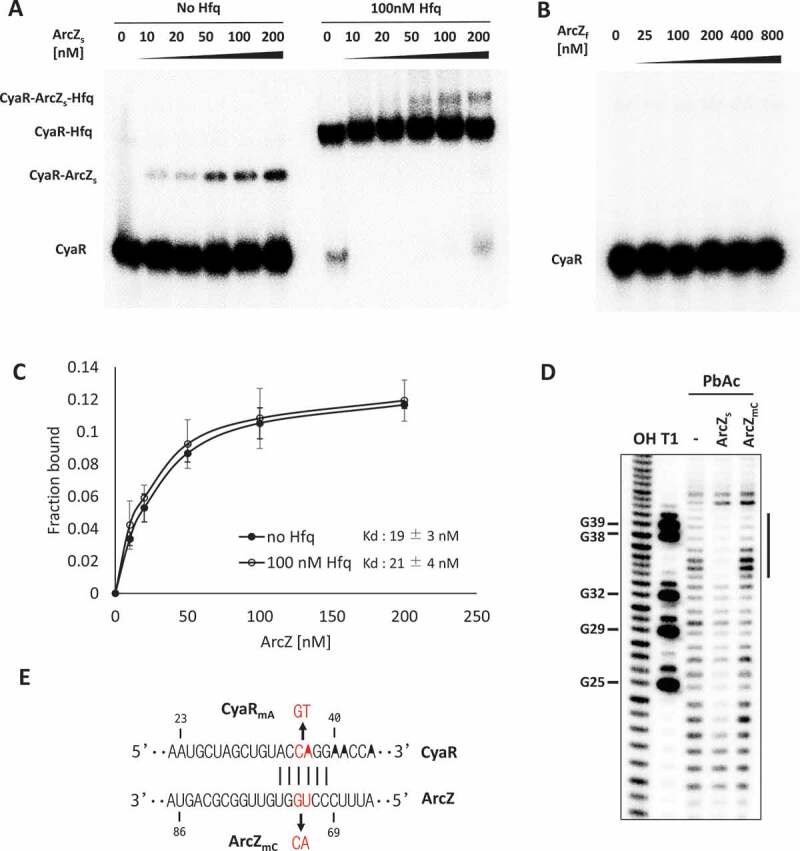
Interaction of CyaR with the short form of ArcZ. (A and B) EMSAs of in vitro-synthesized ArcZ and CyaR. 32P-labelled RNA (2 nM) was incubated with the indicated concentrations of (A) ArcZs with or without 100 nM Hfq and (B) ArcZf without Hfq. (C) Fractions of CyaR bound to ArcZ are shown with increasing concentration of ArcZ with Hfq (open circle) or without Hfq (closed circle). Values are means ± SD; n = 3. (D) Probing of 5ʹ 32P-labelled CyaR with lead acetate (PbAc) in the presence or absence of ArcZs or ArcZmC. OH, alkaline ladders; T1, RNase T1 ladders. The numbers to the left indicate sequence positions with respect to the +1 position of CyaR. A significantly protected region indicated by a bar on the right of the figure. (E) The base-pairing region between CyaR and ArcZ and the UG-to-AC mutation at positions 72 and 73 of ArcZ is indicated by ArcZmC and the compensatory mutation at positions 36 and 37 of CyaR is indicated by CyaRmA.
To identify base-pairing regions between ArcZs and CyaR, we probed the structures of CyaR with lead acetate in the presence of ArcZs in vitro. We observed protection of CyaR nucleotides 34 to 39 in the presence of ArcZs. However, a CyaR mutant with the CA-to-GT mutation at nucleotides 36 and 37 of CyaR failed to bind to ArcZs (Fig 5D). This protected region is in accord with the pairing site predicted by IntaRNA software (Fig 5E)[35].
To address the involvement of the ArcZ-CyaR interaction in CyaR degradation in vivo, we designed a two-plasmid system in which plasmids pACyaR (for CyaR expression) and pArcZ (for ArcZ expression) were introduced into the cells. Introduction of a UG-to-AC mutation at nucleotides 72 and 73 of ArcZ in pArcZ, which would disrupt interaction with CyaR, substantially increased the level of CyaR (Fig 6). This increase in CyaR levels was reduced by a compensatory CA-to-GU mutation at nucleotides 36 and 37 of CyaR in pACyaR, showing that the compensatory mutation restored the ability of the ArcZ mutant to reduce the level of CyaR (Fig 6).
Figure 6.
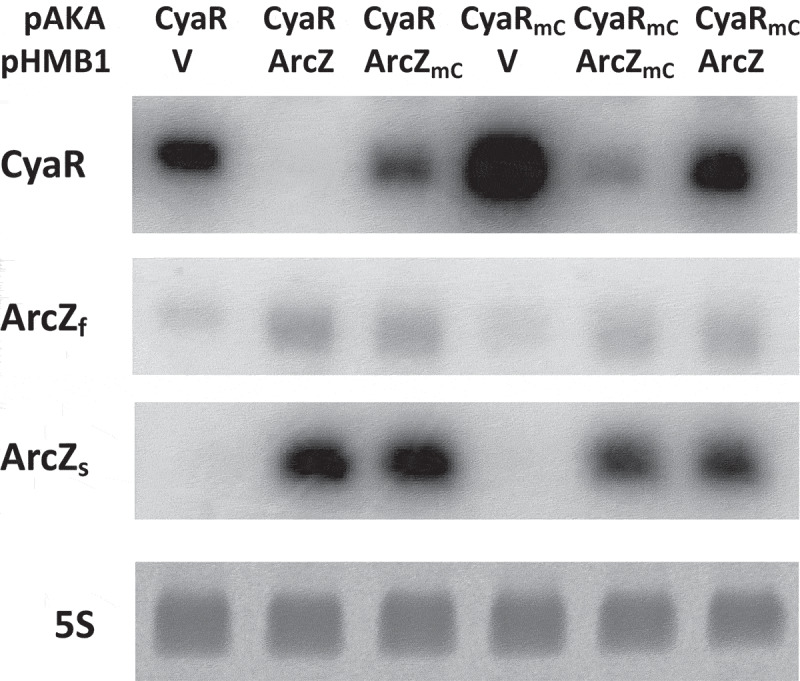
Degradation of CyaR by ArcZ. MG1655 cells were co-transformed with pACyaR or pACyaRmA and pArcZ or pArcZmC. IPTG was added into the culture 2 h after a 1/100 dilution of the culture (grown at 37°C) and the culture was further incubated for 2 h. Cellular levels of CyaR and ArcZ were measured by Northern blot analysis; 5S rRNA was detected as a loading control. V, vector control; CyaR, pACyaR (a derivative of pAKA); CyaRmA, pACyaRmA (a derivative of pAKA); ArcZ, pArcZ (a derivative of pHMB1); ArcZmC, pArcZmC (a derivative of pHMB1).
We also performed RNA-RNA EMSA using the ArcZ mutant and the compensatory CyaR mutant. The CyaR mutant bound to the ArcZ mutant with Kd of ~200 nM, but not to the wild-type ArcZ, whereas the wild type CyaR did not bind to the ArcZ mutant (Fig. S5A-D). When probed with lead acetate, nucleotides 34 to 39 of the CyaR mutant were protected by the ArcZ mutant, but not the wild-type ArcZ (Fig. S5E).
Altogether, these results suggest that CyaR degradation by ArcZ is attributable to base-pairing between CyaR and ArcZ.
RNase E-dependent degradation of CyaR by ArcZ
Since the endoribonuclease RNase III cleaves double-stranded RNA regions in E. coli [32,36–38], we examined how CyaR expression is changed by ArcZ overexpression, and vice versa, in rnc− cells lacking RNase III. Expression of ArcZ and CyaR was not significantly changed by overexpression of CyaR and ArcZ, respectively (Fig. S6), suggesting that RNase III is not involved in ArcZ-mediated CyaR degradation.
Finally, we examined whether RNase E is involved in this process using RNase E temperature-sensitive cells (rnets). To this end, rne+ and rnets cells were transformed with pArcZ or control vector.We analysed CyaR levels following overexpression of ArcZ and measured ArcZ levels following overexpression of CyaR (Fig 7). We observed that CyaR levels were higher with vector controls in both rne+ and rnets cells at 42°C than at 30°C, suggesting CyaR expression is highly dependent on growth condition. Importantly, CyaR accumulated at 42°C upon ArcZ overexpression, whereas the level of ArcZ was not changed by CyaR overexpression. The accumulation of CyaR upon ArcZ overexpression at 42°C declined compared with vector controls. This decline in CyaR levels could be explained by the difference of the growth condition. During 15 min-IPTG induction at 30°C before shift to 42°C, CyaR in rnets cells could be degraded by overexpressed ArcZ, resulting in a decline in CyaR levels. Altogether, the results suggest that RNase E is required for ArcZ-mediated degradation of CyaR.
Figure 7.
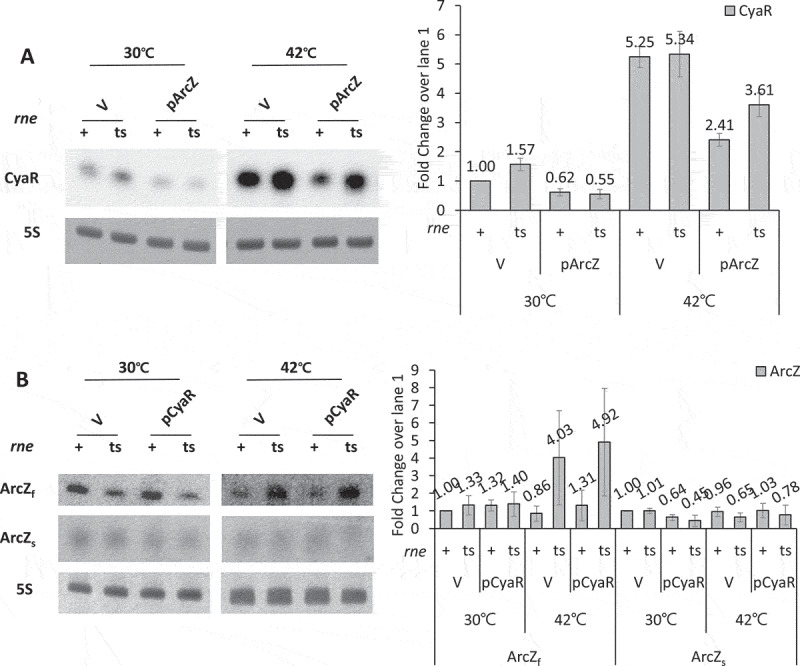
RNase E-dependent degradation of CyaR by ArcZ. (A) MCE+ (rne+) and MCE- (rnets) cells were transformed with pArcZ. IPTG was added 4 h after a 1/100 dilution of culture (grown at 30°C), and the culture was further incubated for 15 min. Cultures were heat-shocked for 15 min at 42°C or incubated for 15 min at 30°C. Total cellular RNA was prepared from the cultures, and cellular levels of CyaR were measured by Northern blot analysis using an anti-CyaR oligonucleotide probe; 5S rRNA was detected as a loading control. (B) The rne+ and rnets cells were transformed with pCyaR. ArcZ levels were analysed as described in (A). Bar graphs show fold changes of ArcZ transcripts (ArcZf and ArcZs) compared with vector controls at 30°C.
Translational regulation of rpoS by ArcZ-CyaR interaction
We examined whether ArcZ-CyaR interaction actually affects rpoS regulation in vivo. To analyse CyaR-mediated rpoS repression when ArcZ is lacking, we compared the increase of LacZ activity of the rpoS-lacZ fusion by the cyaR deletion in the ΔarcZ cells with that in the WT cells. We found that the cyaR deletion increased the LacZ activity more in ΔarcZ cells than in WT cells (Fig 8A) because ΔarcZ cells would lose not only rpoS activation but also cyaR repression.
Figure 8.
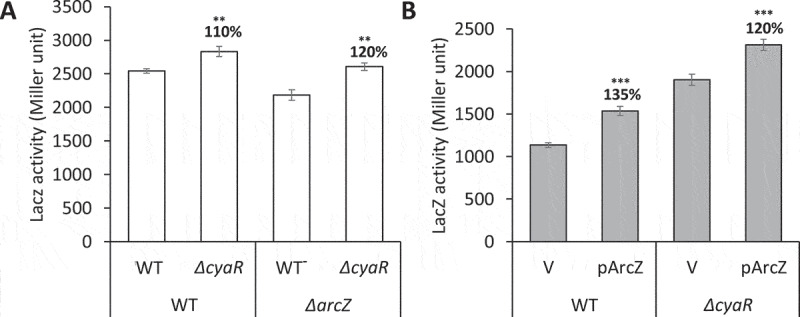
Translational regulation of rpoS by CyaR-ArcZ interaction in vivo. (A) Overnight cultures of PM1409 (WT), ΔcyaR, ΔarcZ, or ΔcyaRΔarcZ were diluted 1:100 in LB medium. Cells were grown at 37°C for 4 h and induced with 0.002% arabinose. The culture was incubated further for 2 h and LacZ activity was measured. (B) WT or ΔcyaR were transformed with pArcZ. The overnight cultures were diluted 1:100 and grown at 37°C. Arabinose of 0.002% and 0.1 mM IPTG were added at 2 h and 3.5 h after 1/100 dilution, respectively, and the culture was incubated further for 0.5 h. LacZ activity was measured. Values are means ± SD; n = 3; ***P < 0.001, **P < 0.01, by student’s t-test. ΔcyaR, PM1409ΔcyaR; ΔarcZ, PM1409ΔarcZ; ΔcyaRΔarcZ, PM1409ΔcΔa.
We also compared rpoS activation upon ArcZ overexpression in WT and ΔcyaRcells. We found that when ArcZ is overexpressed, ΔcyaR cells caused less ArcZ-mediated rpoS activation than WT cells (Fig 8B). The less ArcZ-mediated activation would be due to the absence of ArcZ-mediated cyaR repression. Altogether, the results suggest that CyaR-mediated rpoS repression is affected by ArcZ in vivo.
Discussion
In this study, we demonstrate that CyaR degrades rpoS mRNA by base-pairing with a region next to the ArcZ binding site in the 5ʹ UTR of rpoS mRNA, suggesting that CyaR is a rpoS-repressing sRNA. CyaR also interacts with the rpoS-activating sRNA, ArcZ. The short form of ArcZ, but not the full-length form, base-pairs with CyaR, and this ArcZ-CyaR interaction leads to degradation of CyaR and relieves rpoS repression by CyaR (Fig 9A).
Figure 9.
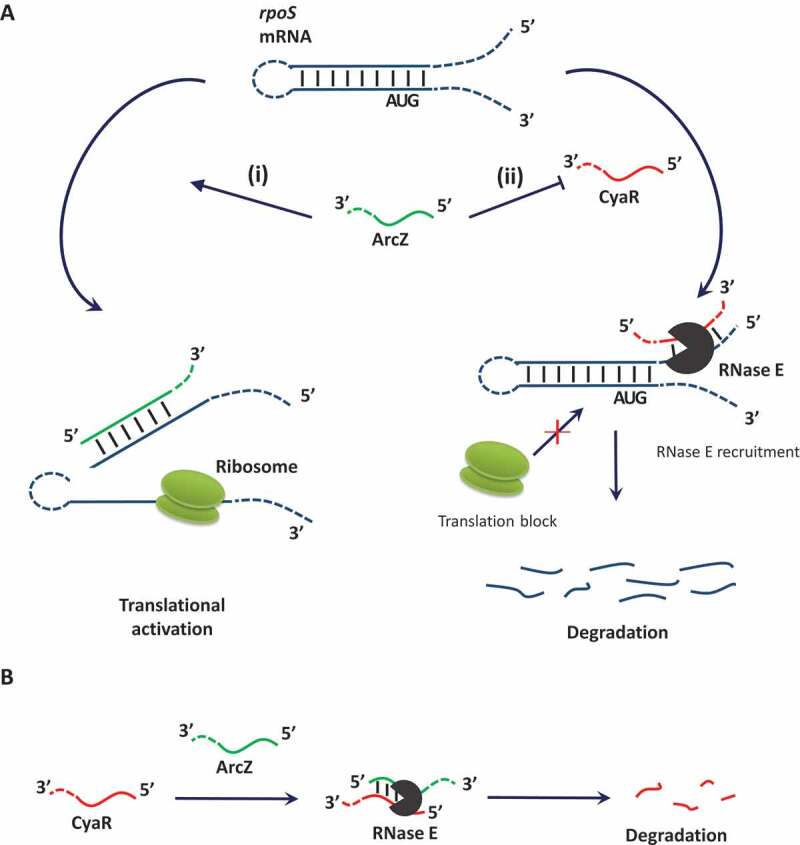
A model for coordinate regulation of rpoS by ArcZ-CyaR interactions. (A) ArcZ up-regulates rpoS expression by different mechanisms: (i) base-pairing with the 5ʹ UTR of rpoS to open up an inhibitory hairpin; and (ii) repression of CyaR-dependent rpoS degradation. (B) Mechanism of CyaR regulation by ArcZ. ArcZ base-pairs with CyaR and degrades CyaR through RNase E.
CyaR is down-regulated by CpxR, which binds the promoter region of CyaR[39]. The CpxA/R two-component system is a complex control system that has been shown to be activated by alkaline pH shock, metal, surface adhesion, and high salt concentration[40]. In addition, genes closely involved in pH homoeostasis, such as grcZ, focA and mgrB, were predicted as targets of CyaR[21]. Here, we showed that CyaR acts as an antisense sRNA to down-regulate rpoS expression, which increases not only under general stress conditions, but also under acidic stress[1]. Therefore, CyaR appears to be involved in cellular regulation of more diverse stress responses.
We found that CyaR-mediated rpoS repression occurs in an Hfq-independent manner in the context of CysR overexpression. However, CyaR levels are very low in the absence of Hfq. Therefore, it is likely that CyaR itself can repress rpoS expression without Hfq, although Hfq is required for CyaR stabilization, as exemplified by rpoS activation by DsrA[28].
Both ArcZ and CyaR target rpoS mRNA while simultaneously interacting with each other. This interaction induces CyaR decay via an RNase E-dependent route (Fig 9B) and would maximize rpoS activation by ArcZ. Degradation of CyaR by ArcZ manifests in the form of ArcZ acting as a sponge for CyaR. Since SroC sRNA is known to function as a sponge for GcvB sRNA[26], and additional putative sRNA-sRNA interactions are predicted by RIL-seq data[22], it is likely that there are many other sRNA-sRNA interactions that modulate cellular metabolism in E. coli. Since the ArcZ-CyaR–interacting region corresponds to the rpoS binding sites of the respective sRNAs[4], there may be competition between sRNA-target mRNA interaction and sRNA-sRNA interaction, which could also contribute to fine-tuning of rpoS expression.
It was previously reported that CyaR and RprA, another rpoS-activating sRNA [7,8], down-regulate the same target gene, hdeD, through different mechanisms: CyaR induces degradation of hdeD mRNA, whereas two molecules of RprA block hdeD translation initiation[21]. Since our study showed that CyaR acts as an rpoS-repressing sRNA, a pair of CyaR and RprA sRNAs could also participate in fine-tuning rpoS expression.
ArcZ expression is suppressed by ArcA under anaerobic conditions[4], where ATP is synthesized by anaerobic respiration. Anaerobic respiration can oxidize NADH to NAD+ in the absence of oxygen, but the efficiency of this pathway is less than that of aerobic respiration. Therefore, cells may need less NAD+ under anaerobic conditions. NAD synthetase, encoded by the CyaR target gene nadE, is an essential enzyme involved in both de novo biosynthesis and salvage of NAD+ [41–43]. Because CyaR is the only sRNA that represses nadE mRNA expression at the post-transcriptional level, anaerobic down-regulation of ArcZ could increase CyaR-mediated nadE repression by increasing CyaR levels. CyaR regulation via ArcZ-CyaR interactions may be involved in modulating the amount of NAD synthetase during the transition from aerobic to anaerobic metabolism.
In summary, this study shows that (i) CyaR represses rpoS expression by directly interacting with rpoS mRNA, and (ii) the rpoS-activating sRNA ArcZ base-pairs with CyaR and degrades it in an RNase E-dependent manner. Our results suggest that ArcZ not only participates in rpoS translation as an activator, but also acts as a regulator of CyaR, maximizing its rpoS-activating effect. This coordinate regulation of rpoS by sRNA-sRNA interactions could contribute to the fine-tuning of rpoS expression to enable cells to more effectively cope with stresses.
Material and methods
Bacterial strains and plasmids
Bacterial strains and plasmids used in this study are listed in Table S1. E. coli strain PM1409 carrying a rpoS-lacZ translational fusion was a gift from Dr. S. Gottesman. PM1409ΔcyaR, PM1409ΔarcZ, PM1409ΔcyaRΔhfq (a cyaR− and hfq− double mutant), and PM1409ΔcyaRΔarcZ (a cyaR− and arcZ− double mutant) were obtained by P1 transduction [44–46] using the relevant mutant strains [47,48]. PM1409ΔcyaRΔhfq and PM1409ΔcyaRΔarcZ were constructed by removing the FRT-flanked kanamycin cassette in PM1409ΔcyaR using the Flp recombinase from pCP20 plasmid[49] and introducing the second ΔarcZ mutation and Δhfq mutation by P1 transduction, respectively[50]. The G-to-C mutation at position +442 relative to the rpoS transcription start in the rpoS-lacZ translational fusion was generated using scarless mutagenesis, as described previously[51]. A strain carrying the cyaR-lacZ transcriptional fusion (cyaR+10-lacZ) was also constructed as described previously[50]. Briefly, a cyaR promoter-containing fragment (−100 to +10 relative to the cyaR transcription start) was cloned into the EcoRI and BamHI sites of the pRS1553 vector, and the resulting recombinant plasmid was introduced into E. coli strain DH408. A lysogen strain carrying the rpoS-lacZ transcriptional fusion was constructed using λRS468. RNA expression vectors pHMB1 and pAKA, derived from pBR322 and p15A, respectively, were used to generate CyaR- or ArcZ-expressing plasmids, as described previously[52]. The oligonucleotides employed are listed in Table S2.
LacZ activity assay
Three colonies for each strain were cultured overnight in LB medium, then overnight cultures were diluted 1:100 and grown in fresh medium. Where necessary, ampicillin (100 μg/ml) or both ampicillin (100 μg/ml) and tetracycline (10 μg/ml) were added to the medium to maintain plasmid-bearing cells. For cyaR-lacZ transcriptional fusion-containing cells, 0.1 mM IPTG (isopropyl β-D-1-thiogalactopyranoside) was added 3.5 h after diluting, and the culture was further incubated for 0.5 h. For rpoS-lacZ translational fusion-containing cells, 0.002% arabinose and IPTG were added at 2 h and 3.5 h, respectively, and the culture was incubated for an additional 0.5 h. LacZ activity was assayed as described previously[53]. At least three independent measurements were made for each strain.
Northern blot analysis
Cells were grown as described in the LacZ activity assay section, above. Total cellular RNA was extracted from the culture using acidic hot phenol, as described previously[54]. Total RNA (10 μg) was fractionated on a 7 M urea, 5% polyacrylamide gel, and electrotransferred onto a Hybond-XL membrane (Amersham Biosciences), as previously described[55]. The membrane was hybridized with 32P-labelled DNA probes in Rapid-Hyb buffer (Amersham Biosciences) according to the manufacturer’s instructions. Hybridization signals were analysed using an FLA7000 Image Analyser (Fuji). The utilized probes are listed in Table S2.
RNA stability assay
Three colonies for each strain were cultured in LB medium containing ampicillin (100 μg/ml) at 37°C, and the overnight culture was diluted to 1:100 and cultured with the fresh medium. Arabinose (0.002%) and 0.1 mM IPTG were added at 2 h and 3 h 50 min, respectively, and the culture was incubated further for 10 min. Transcription were halted by the addition of rifampicin (a final concentration of 500 μg/ml) and aliquots of the culture were sampled at intervals. Total cellular RNAs were prepared and subjected to Northern blot analysis or qRT-PCR.
In vitro transcription
CyaR, rpoS301 carrying the 284-nt rpoS leader sequence[33], the full-length form of ArcZ, and the short form of ArcZ were prepared by in vitro transcription. DNA templates were obtained by polymerase chain reaction (PCR) using appropriate primer pairs (Table S2), and in vitro transcription was carried out using T7 RNA polymerase (Promega). RNA transcripts were gel-purified, as described[53].
Quantitative reverse transcription-PCR (qRT-PCR)
Total cellular RNA was treated to remove any DNA contaminants using a TURBO DNA-free Kit (Ambion). cDNA was synthesized from DNase-treated RNA using a SuPrimeScript RT-PCR premix (Genet Bio) and amplified with SuPrimeScript qRT-PCR Premix (Genet Bio) on a Bioneer Exicycle 96 Real-Time Quantitative Thermal Block (Bioneer). Primer pairs specific to the lacZ ORF, rpoS ORF, or rrsA mRNA were used for qRT-PCR (Table S2).
Electrophoretic mobility shift assay (EMSA)
The Hfq protein was purified as described previously[56]. All purified RNAs were renatured by heating for 1 min at 95°C and slowly cooling to 25°C. CyaR (2 nM), labelled at the 5ʹ end with 32P, was incubated with increasing amounts of ArcZ transcripts for 20 min at 25°C in 20 μl TMN buffer [100 mM Tris–acetate pH 7.6, 500 mM NaOAc, 25 mM Mg(OAc)2][57] in the presence or absence of 0.1 μM Hfq. The reaction was stopped by adding 1/6th volume of non-denaturing loading buffer (0.025% xylene cyanol, 2% glycerol, 1X TBE), after which reaction mixtures were analysed on 5% non-denaturing polyacrylamide gels at 4°C.
RNA footprinting
All purified RNAs were renatured as described in the EMSA section, above. 32P-labelled rpoS301 and CyaR (20 nM each) were incubated with 500 nM CyaR in 10 μl annealing buffer (250 mM Tris-HCl pH7.5, 1.25 M NaCl, 1.25 M KCl)[58] and 500 nM ArcZ in 10 μl TMN buffer, respectively, for 20 min at 25°C. Then, 20 mM lead acetate was added, followed by incubation at 25°C for 15 min in 20 μl of 1x structure buffer (Ambion). Reactions were stopped by adding 1 μl of 0.5 M EDTA and 20 μl of 2x RNA dye. The same 32P-labelled rpoS301 and CyaR were separately incubated for 5 min at 95°C in alkaline buffer or 15 min at 25°C with ribonuclease T1 (0.1 U; Ambion) to generate alkaline (OH) and T1 ladders, respectively. Cleavage products were heated at 95°C for 3 min and analysed on a 4% (for rpoS301) or 7% (for CyaR) polyacrylamide-9 M urea sequencing gel.
Funding Statement
This work was supported by the KAIST; National Research Foundation of Korea (NRF) Grants [2019R1H1A2039730].
Acknowledgments
This study was supported by National Research Foundation of Korea (NRF) Grants from the Korean government (MSIT) (2019R1H1A2039730) and by KAIST. The authors would like to thank Dr. S. Gottesman for providing strain PM1409 and Prof. S. A. Woodson for providing a vector expressing Hfq.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary data
Supplemental Data for this article can be accessed here.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- [1].Battesti A, Majdalani N, Gottesman S.. The RpoS-mediated general stress response in escherichia coli. Annu Rev Microbiol. 2010;65:189–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hirsch M, Elliott T. Stationary-phase regulation of RpoS translation in Escherichia coli. J Bacteriol. 2005;187(21):7204–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].McCullen CA, Benhammou JN, Majdalani N, et al. Mechanism of positive regulation by DsrA and RprA small noncoding RNAs: pairing increases translation and protects rpoS mRNA from degradation. J Bacteriol. [Internet] 2010;192:5559–5571. [cited 2014 February27]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2953674&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mandin P, Gottesman S. Integrating anaerobic/aerobic sensing and the general stress response through the ArcZ small RNA. Embo J. [Internet] 2010; 29:3094–3107. [cited 2014 February26]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2944060&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Soper T, Mandin P, Majdalani N, et al. Positive regulation by small RNAs and the role of Hfq. Proc Natl Acad Sci U S A. [Internet] 2010; 107:9602–9607. [cited 2014 February12]. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2906882&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Papenfort K, Said N, Welsink T, et al. Specific and pleiotropic patterns of mRNA regulation by ArcZ, a conserved, Hfq-aependent small RNA. Mol Microbiol. 2009;74:139–158. [DOI] [PubMed] [Google Scholar]
- [7].Majdalani N, Hernandez D, Gottesman S. Regulation and mode of action of the second small RNA activator of RpoS translation, RprA. Mol Microbiol. 2002;46(3):813–826. [DOI] [PubMed] [Google Scholar]
- [8].Mika F, Busse S, Possling A, et al. Targeting of csgD by the small regulatory RNA RprA links stationary phase, biofilm formation and cell envelope stress in Escherichia coli. Mol Microbiol. 2012;84(1):51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ferrières L, Thompson A, Clarke DJ. Elevated levels of σS inhibit biofilm formation in Escherichia coli: A role for the Rcs phosphorelay. Microbiology. 2009;155(11):3544–3553. [DOI] [PubMed] [Google Scholar]
- [10].Sledjeski DD, Gupta A, Gottesman S. The small RNA, DsrA, is essential for the low temperature expression of RpoS during exponential growth in Escherichia coli. Embo J. [Internet] 1996; 15:3993–4000. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC452119/ [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang A, Altuvia S, Tiwari A, et al. The OxyS regulatory RNA represses rpoS translation and binds the Hfq (HF-I) protein. Embo J. 1998;17(20):6061–6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Loewen PC, Hu B, Strutinsky J, et al. Regulation in the rpoS regulon of Escherichia coli. Can J Microbiol. 2011;44(8):707–717. [DOI] [PubMed] [Google Scholar]
- [13].Gottesman S. The small RNA regulators of Escherichia coli: roles and mechanisms*. Annu Rev Microbiol. 2004;58:303–328. [DOI] [PubMed] [Google Scholar]
- [14].Dong T, Schellhorn HE. Control of RpoS in global gene expression of Escherichia coli in minimal media. Mol Genet Genomics. 2009;281(1):19–33. [DOI] [PubMed] [Google Scholar]
- [15].Patten CL, Kirchhof MG, Schertzberg MR, et al. Microarray analysis of RpoS-mediated gene expression in Escherichia coli K-12. Mol Genet Genomics. 2004;272(5):580–591. [DOI] [PubMed] [Google Scholar]
- [16].Sedlyarova N, Shamovsky I, Bharati BK, et al. sRNA-mediated control of transcription termination in E. Coli Cell. 2016;167:111–121.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].De Lay N, Gottesman S. The crp-activated small noncoding regulatory RNA CyaR (RyeE) links nutritional status to group behavior. J Bacteriol. 2009;191:461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Johansen J, Eriksen M, Kallipolitis B, et al. Down-regulation of outer membrane proteins by noncoding RNAs: unraveling the cAMP-CRP- and σE-dependent CyaR-ompX regulatory case. J Mol Biol. 2008;383:1–9. [DOI] [PubMed] [Google Scholar]
- [19].Papenfort K, Pfeiffer V, Lucchini S, et al. Systematic deletion of Salmonella small RNA genes identifies CyaR, a conserved CRP-dependent riboregulator of OmpX synthesis. Mol Microbiol. 2008;68:890–906. [DOI] [PubMed] [Google Scholar]
- [20].Wright PR, Richter AS, Papenfort K, et al. Comparative genomics boosts target prediction for bacterial small RNAs. Proc Natl Acad Sci. 2013;110(37):E3487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lalaouna D, Prévost K, Laliberté G, et al. Contrasting silencing mechanisms of the same target mRNA by two regulatory RNAs in Escherichia coli. Nucleic Acids Res. 2018;46:2600–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Melamed S, Peer A, Faigenbaum-Romm R, et al. Global mapping of small RNA-target interactions in bacteria. Mol Cell. 2016;63:884–897. Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods. 2007;4(9):721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Salzman J. Circular RNA expression: its potential regulation and function. Trends Genet. 2016;32(5):309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Du Z, Sun T, Hacisuleyman E, Fei T, Wang X, Brown M, Rinn JL, Lee MG-S, Chen Y, Kantoff PW, et al. Integrative analyses reveal a long noncoding RNA-mediated sponge regulatory network in prostate cancer. Nature Communications [Internet] 2016;7:10982. Available from: https://doi.org/10.1038/ncomms10982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miyakoshi M, Chao Y, Vogel J. Cross talk between ABC transporter mRNAs via a target mRNA-derived sponge of the GcvB small RNA. Embo J. 2015;34:1478–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moon K, Gottesman S. Competition among Hfq-binding small RNAs in Escherichia coli. Mol Microbiol. 2011;82:1545–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim W, Choi JS, Kim D, et al. Mechanisms for Hfq-independent activation of rpoS by DsrA, a small RNA, in Escherichia coli. Mol Cells. 2019;42:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mackie GA, RNase E. At the interface of bacterial RNA processing and decay. Nat Rev Microbiol. 2013;11:45–57. [DOI] [PubMed] [Google Scholar]
- [30].Carpousis AJ. The RNA degradosome of escherichia coli : an mRNA-degrading machine assembled on RNase E. Annu Rev Microbiol. 2007;61:71–87. [DOI] [PubMed] [Google Scholar]
- [31].Morita T, Maki K, Aiba H. RNase E-based ribonucleoprotein complexes: mechanical basis of mRNA destabilization mediated by bacterial noncoding RNAs. Genes Dev. 2005;19:2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Massé E, Escorcia FE, Gottesman S. Coupled degradation of a small regulatory RNA and its mRNA targets in Escherichia coli. Genes Dev. 2003;17:2374–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peng Y, Soper TJ, Woodson SA. Positional effects of AAN Motifs in rpoS regulation by sRNAs and Hfq. J Mol Biol. [Internet] 2013; 426:275–285. [cited 2014 March6]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24051417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Sorescu DA, Mann M, Kleinkauf R, et al. CopraRNA and IntaRNA: predicting small RNA targets, networks and interaction domains. Nucleic Acids Res. 2014;42:W119–23. Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sorescu DA, Mann M, Kleinkauf R, et al. CopraRNA and IntaRNA: predicting small RNA targets, networks and interaction domains. Nucleic Acids Res. 2014;42:W119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lybecker M, Zimmermann B, Bilusic I, et al. The double-stranded transcriptome of Escherichia coli. Proc Natl Acad Sci. 2014;111(8):3134–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Court DL, Gan J, Liang Y-H, et al. RNase II: genetics and function; structure and mechanism. Annu Rev Genet. 2013;47:405–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nicholson AW. Ribonuclease III mechanisms of double-stranded RNA cleavage. Wiley Interdiscip Rev RNA. 2014;5(1):31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Vogt SL, Evans AD, Guest RL, et al. The Cpx envelope stress response regulates and is regulated by small noncoding RNAs. J Bacteriol. 2014;196:4229–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hunke S, Keller R, Müller VS. Signal integration by the Cpx-envelope stress system. FEMS Microbiol Lett. 2012;326(1):12–22. [DOI] [PubMed] [Google Scholar]
- [41].Hughes KT, Olivera BM, Roth JR. Structural gene for NAD synthetase in Salmonella typhimurium. J Bacteriol. 1988;170:2113–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schneider BL, Reitzer LJ. Salmonella typhimurium nit is nadE: defective nitrogen utilization and ammonia-dependent NAD synthetase. J Bacteriol. 1998;180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Willison JC, Tissot G. The Escherichia coli efg gene and the Rhodobacter capsulatus adgA gene code for NH3-dependent NAD synthetase. J Bacteriol. 1994;176:3400–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Macdonald DW, Buesching CD, Stopka P, et al. Encounters between two sympatric carnivores: red foxes (Vulpes vulpes) and European badgers (Meles meles). J Zool. 2004;263:385–392. [Google Scholar]
- [45].Moore SD. Assembling new escherichia coli strains by transduction using phage P1. Methods Mol Biol. 2011;765:155–169. [DOI] [PubMed] [Google Scholar]
- [46].Thomason LC, Costantino N, Court DL. E. coli genome manipulation by P1 transduction [Internet]. Curr Protoc Mol Biol. 2007;79(1):1–7. [DOI] [PubMed] [Google Scholar]
- [47].Bak G, Lee J, Suk S, et al. Identification of novel sRNAs involved in biofilm formation, motility, and fimbriae formation in Escherichia coli. Sci Rep. 2015;5:15287. Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Bak G, Han K, Kim D, et al. Roles of rpoS- activating small RNAs in pathways leading to acid resistance of Escherichia coli. Microbiologyopen. 2014;3:15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: tcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. [Internet] 1995; 158:9–14. Available from: http://www.sciencedirect.com/science/article/pii/037811199500193A [DOI] [PubMed] [Google Scholar]
- [50].Müller-Hill B. Experiments with gene fusions. Trends Genet. 1985;1:61. [Google Scholar]
- [51].Blank K, Hensel M, Gerlach RG. Rapid and highly efficient method for scarless mutagenesis within the salmonella enterica chromosome. PLoS One. 2011. cited 2019 April3;6:e15763. Internet [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Han K, Kim KS, Bak G, et al. Recognition and discrimination of target mRNAs by Sib RNAs, a cis-encoded sRNA family. Nucleic Acids Res. 2010;38:5851–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Xiangyang ZHB. Control of the Escherichia coli rrnB P1 promoter strength by ppGpp. J Biol Chem. 1995;270:11181–11189. [DOI] [PubMed] [Google Scholar]
- [54].Kim S, Kim H, Park I, et al. Mutational analysis of RNA structures and sequences postulated to affect 3ʹ processing of M1 RNA, the RNA component of Escherichia coli RNase P. J Biol Chem. 1996;271:19330–19337. [DOI] [PubMed] [Google Scholar]
- [55].Park H, Bak G, Kim SC, et al. Exploring sRNA-mediated gene silencing mechanisms using artificial small RNAs derived from a natural RNA scaffold in Escherichia coli. Nucleic Acids Res. [Internet] 2013; 41:3787–3804. [cited 2014 February20]. Available from: http://nar.oxfordjournals.org/cgi/content/long/41/6/3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Santiago-Frangos A, Kavita K, Schu DJ, et al. C-terminal domain of the RNA chaperone Hfq drives sRNA competition and release of target RNA. Proc Natl Acad Sci. [Internet] 2016; 113:E6089–96. Available from: http://www.pnas.org/lookup/doi/10.1073/pnas.1613053113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Han K, Kim K, Bak G, et al. Recognition and discrimination of target mRNAs by Sib RNAs, a cis -encoded sRNA family. Nucleic Acids Res. 2010;38:5851–5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Soper TJ, Woodson SA. The rpoS mRNA leader recruits Hfq to facilitate annealing with DsrA sRNA. RNA. [Internet] 2008; 14:1907–1917. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2525945/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


