Version Changes
Revised. Amendments from Version 1
Minor changes were made to the manuscript between versions 1 and 2, in line with reviewer comments (see responses to reviewers for consideration of each specific change). The most substantial changes were (1) amending "palliative and end-of-life care" to "palliative care" throughout, starting with the title, as a reviewer found the original confusing; (2) a more detailed justification for exclusion of decedent cohort study designs in Methods>Eligibility criteria>Types of studies and reports.
Abstract
Introduction: Improving palliative care is a priority worldwide as this population experiences poor outcomes and accounts disproportionately for costs. In clinical practice, physician judgement is the core method of identifying palliative care needs but has important limitations. Machine learning (ML) is a subset of artificial intelligence advancing capacity to identify patterns and make predictions using large datasets. ML has the potential to improve clinical decision-making and policy design, but there has been no systematic assembly of current evidence.
Methods: We conducted a rapid review, searching systematically seven databases from inception to December 31st, 2018: EMBASE, MEDLINE, Cochrane Library, PsycINFO, WOS, SCOPUS and ECONLIT. We included peer-reviewed studies that used ML approaches on routine data to improve palliative care for adults. Our specified outcomes were survival, quality of life (QoL), place of death, costs, and receipt of high-intensity treatment near end of life. We did not search grey literature.
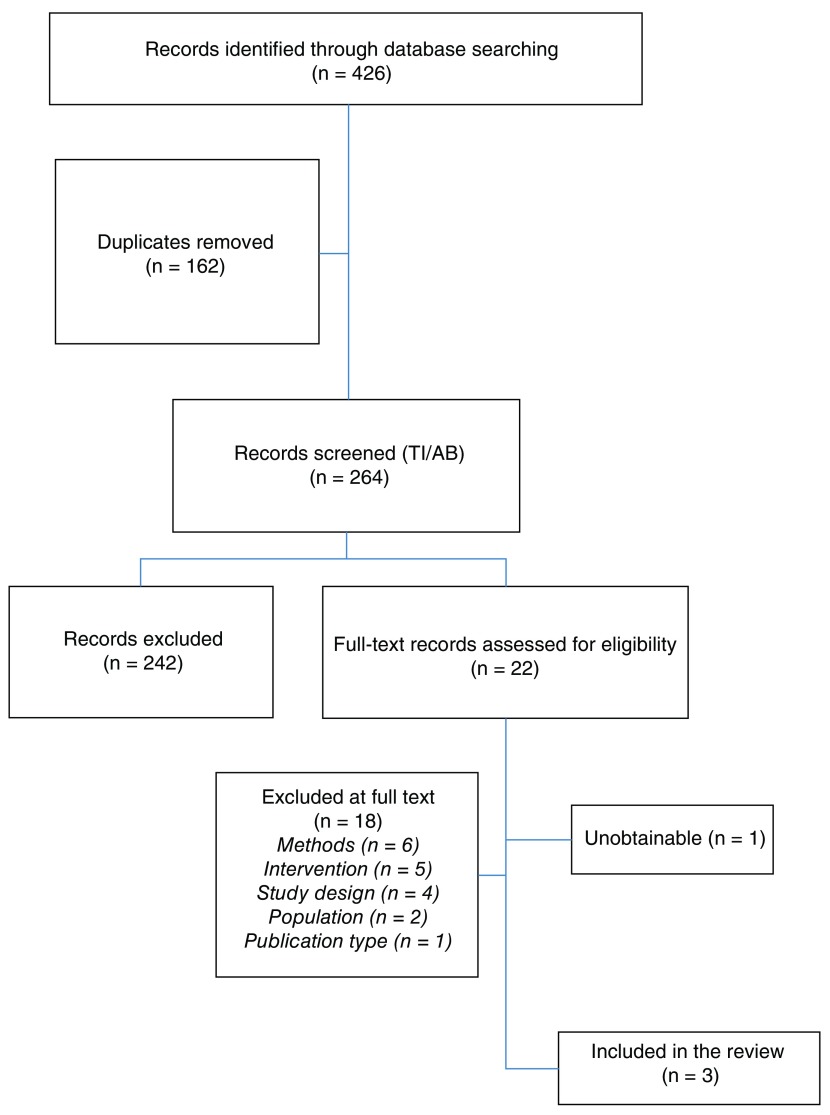
Results: The database search identified 426 citations. We discarded 162 duplicates and screened 264 unique title/abstracts, of which 22 were forwarded for full text review. Three papers were included, 18 papers were excluded and one full text was sought but unobtainable. One paper predicted six-month mortality, one paper predicted 12-month mortality and one paper cross-referenced predicted 12-month mortality with healthcare spending. ML-informed models outperformed logistic regression in predicting mortality where data inputs were relatively strong, but those using only basic administrative data had limited benefit from ML. Identifying poor prognosis does not appear effective in tackling high costs associated with serious illness.
Conclusion: While ML can in principle help to identify those at risk of adverse outcomes and inappropriate treatment, applications to policy and practice are formative. Future research must not only expand scope to other outcomes and longer timeframes, but also engage with individual preferences and ethical challenges.
Keywords: Machine learning, artificial intelligence, palliative care, terminal care, multimorbidity, quality of life, costs, decision-making
Introduction
Background
Improving care for people with serious and complex medical illness is a health system priority worldwide. Between 2016 and 2060 there will be an estimated 87% increase globally in the number of deaths that occur following serious health-related suffering, with low-income countries experiencing the largest proportional increases 1. Health systems originally configured to provide acute, episodic treatment often provide poor-value care to complex multimorbid populations 2.
Palliative care is an approach “that improves the quality of life (QOL) of patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual” 3. Studies suggest that palliative care improves outcomes and reduces health care costs for people with serious medical illness, although significant gaps in the evidence base persist 4. Insufficient palliative care capacity is reported even among those nations whose services perform strongly on international rankings 5, 6, and need will only grow given demographic ageing 7.
In clinical practice, physician judgement remains the de facto method of identifying palliative care needs and predicting adverse outcomes including mortality 8. However, studies have repeatedly shown that clinicians tend to make imprecise and overly optimistic predictions of survival in metastatic cancer, where prognosis is most accurate of major terminal illnesses 9– 11. No subgroup of clinicians are proven to be more accurate than others in late-stage predictions 8.
For research and policy, primary research studies are rare due to ethical and practical issues, increasing reliance on the use of routine data 4, 12. Evaluations of programmes and interventions employing traditional analytic approaches encounter challenges in missing data and unobserved confounding 13.
Health care is entering an era of ‘big data’ in which researchers and providers will have access to unprecedented levels of information on patients 14. Machine learning (ML) is a subset of artificial intelligence that is rapidly advancing capacity to identify patterns and make predictions using large datasets 15. In contrast to traditional analytic methods, where the analyst specifies data inputs according to hypotheses and/or conceptual models, ML approaches leverage computing power to identify patterns in available data and can make inferences without explicit user instruction 16. These have a well-documented potential to improve clinical decision-making by analysing electronic health records and other routinely collected health data that are commonly large and “dirty”, i.e. erroneous and incomplete 17, although significant data missingness may be still be a source of bias 18.
Rationale and aim
ML approaches to improving decision-making may be strongly appropriate for palliative care. First, patient care is complex with high illness burden and management challenges including polypharmacy 19. ML tools may be effective at identifying patterns in data on complex patients, for example denoting risk of functional decline or mortality, that are not obvious to healthcare professionals with limited time and analytic expertise to interpret available data. Second, the volume of projected palliative care need means that a minority of patients will receive care from palliative care specialists 7, 20. Tools that can aid non-specialists to identify need and provide appropriate care are essential to avoiding population health crises in an era of demographic ageing 21. While multiple face-to-face clinical assessment tools exist for this purpose, meeting challenges of scale requires improved use of routinely collected data. Third, in the context of reliance on routine data 4, 12, 13. Since ‘big’ data are largely routine data, optimising analytic approaches to these may be particularly impactful in palliative care compared to fields where randomised trials and large primary data collection are more feasible and commonplace.
Multiple recent editorials in topic journals reflect the growing interest in principle in using big data and ML to improve palliative care 22– 25. Less has been written on the empirical evidence of these applications. In this context we conducted a rapid review of research studies using ML to improve palliative care. By identifying and organising this evidence for the first time, we anticipated that our findings could inform ongoing and future efforts in this field.
Methods
Eligibility criteria
We included studies meeting the following PICOS (Population, Intervention, Comparison, Outcomes and Study design) criteria.
Types of participants. Studies that reported on adults (≥ 18 years). We specified this criterion as children are a distinct and functionally different population. Studies that reported adults and children separately were eligible, but only results for adults would be considered; studies that pooled adults and children in one sample were excluded 26. We sought studies seeking to improve palliative care: care that “improves the quality of life of patients and their families facing the problems associated with a life-threatening illness” 3. Studies not addressing palliative care, or evaluating a specific discrete treatment (e.g. stent, chemotherapy) were excluded.
Types of outcomes. We specified three domains of interest: patient outcomes (survival; QoL); caregiver outcomes (survival; QoL); and economic outcomes (costs; receipt of cost-(in)effective treatment, e.g. high-intensity treatment at end of life). We specified these as established measures for quality in palliative and EOL care, and so of particular interest to practitioners and policymakers 27.
Types of studies and reports. We included the following types of studies: those that used routinely collected data with ML approaches to improve palliative care, connecting our outcomes of interest with patients’ characteristics before or at diagnosis. Any prospective study meeting the other criteria was therefore eligible; retrospective studies were eligible provided that the analysis was conducted counting forwards, i.e. defining samples and treatments at baseline. Studies that counted backwards, examining samples defined by characteristics at death or according to outcome, were excluded for three reasons. First, participant selection at death biases substantially derived results by distorting timeframe of analysis 28. Second, mortality is an outcome and many characteristics at death are outcomes; they are not independent of treatment choices but instead endogenous and therefore inappropriate as an eligibility criteria for evaluating the treatment 29. Third, treatment effect estimates from decedent cohort studies are in practice evidence that “treatment t should be provided to population p”, where p is partly defined by imminent death 28, 29. They are therefore only useful under scenarios of very good prognostic accuracy, and this is seldom the case in the real world 8– 11.
This was a rapid review of published literature. Rapid reviews are a well-established methodology for gathering evidence in a limited timeframe, provided that search methods are transparent and reproducible, clear inclusion criteria are applied, and a rigorous if time-limited appraisal is performed 30, 31. Only peer-reviewed papers in academic journals were considered eligible. Selection of relevant papers was restricted to English-language publications only. We excluded conference abstracts and book chapters returned by the database search, and we did not search grey literature.
Information sources
Database search. The following electronic databases were searched:
- EMBASE
- MEDLINE
- PsycINFO
- SCOPUS
- ECONLIT
Searches were conducted on February 14 th, 2019. All databases were searched from their inception dates to December 31 st, 2018.
A full search strategy is provided in Table 1. Search terms for all seven databases are provided as extended data 32.
Table 1. Example search strategy, EMBASE.
| # | Limiters |
|---|---|
| 1 | 'palliative therapy'/exp OR 'terminal care'/exp OR 'terminally ill patient'/exp OR 'hospice'/exp |
| 2 | Palliat*:ti,ab |
| 3 | (‘terminal illness’ OR ‘end of life’ OR ‘end-of-life’ OR ‘end-stage disease’ OR ‘last year of life’):ab,ti |
| 4 | #1 OR #2 OR #3 |
| 5 | ‘machine learning’/exp |
| 6 | (‘data mining’ OR ‘artificial intelligence’ OR ‘machine learning’ OR ‘deep learning’ OR ‘neural networks’):ti,ab |
| 7 | #5 OR #6 |
| 8 | #4 AND #7 |
| 9 | Limiters on #8: To end of 2018; articles and reviews and articles in press only. NOT conference proceedings or a book chapters. |
Study selection
Screening of titles and abstracts. Two reviewers (any two authors) independently screened each title and abstract of retrieved citations based on the eligibility criteria. Subsequently, conflicts were resolved on the basis of consensus. The online reviewer tool Covidence was used to manage the screening and selection process.
Screening of full-text reports. The second phase of screening involved downloading and assessing the full-text papers for all of the citations retained from the first phase of screening and applying the eligibility criteria. Two independent reviewers (any two of VS, AOH, SA, RA) screened each full-text paper.
Hand searching. One author (PM) searched the ferences of all included papers by hand, applying the same criteria.
Data extraction. We extracted study design features to a standardised template: setting (country, year, health care environment), aim, principle methods, data sources and sample size. These are presented in Table 2.
Table 2. Key characteristics of included studies.
| Lead author
(year) |
Setting | Aim | Principle methods | Data and sample | Key Results |
|---|---|---|---|---|---|
| Einav (2018) 33 | US: random sample
of Medicare fee-for- service beneficiaries in 2008 |
To analyse healthcare
spending by predicted 12-mortality, i.e. can high end-of-life care costs be identified ex ante |
Ensemble of RF, gradient
boosting and LASSO |
Administrative data: demographics,
ICD codes, chronic conditions, prior utilization for a baseline sample of 5,631,168 Trajectories of health care use and diagnosis in the prior 12-month period were included |
ML model attributed a higher risk
score to those who died within one year than those who did not in 87% of cases End-of-life spending is high but deaths do not account heavily for high spending in Medicare overall Focusing on end-of-life spending is not a useful way to identify inappropriate treatment choices |
| Makar (2015) 34 | US: Medicare fee-for-
service beneficiaries in 2010 |
To quantify six-month
mortality risk in four disease cohorts: cancer, COPD, CHF, dementia. |
Six ML approaches and
logistic regression were used in each cohort, of which RF models performed best in primary analysis. |
Administrative data: demographics,
ICD codes, chronic conditions, functional status, durable medical equipment, prior utilization for 20,000 randomly selected subjects in each disease cohort Traditional baseline characteristics were augmented with values in prior 12-month period, thus capturing disease progression, functional decline, etc. |
ML model attributed a higher risk
score to those who died within six months than those who did not in 82% of cases Augmented variables key to predictive power; models using only traditional variables were less accurate |
| Sahni (2018) 35 | Minnesota, US:
Six-hospital network (one large tertiary care centre; five community hospitals), 2012–2016 |
To quantify 1-year
mortality risk in a cohort of clinically diverse hospitalized patients. |
RF models and logistic
regression were applied separately and performance compared. |
Electronic medical record data,
including vital signs, blood count, metabolic panel, demographics and ICD codes for 59,848 patients |
ML model attributed a higher risk
score to those who died within one year than those who did not in 86% of cases RF model outperforms logistic regression Demographic and lab data key to predictive power; models using ICD codes alone are less reliable |
US: United States; RF: random forest; ML: machine learning; COPD: chronic obstructive pulmonary disease; CHF: congestive heart failure; ICD: international classification of disease; SEER: Surveillance, Epidemiology and End Results.
Data synthesis. Narrative synthesis was employed to review the included studies and combine their key findings. Narrative synthesis involves appraisal of all relevant material, grouping the findings into a coherent thematic narrative. We chose this approach ex ante in the context of our broad aims: to understand how ML has been used to improve palliative care to date, and to consider the implications for future research and practice. At no point did we anticipate quantitative results for combination in meta-analysis, since both available data and ML methods would not be standardised in a way that permitted meaningful pooling.
Results
Database search
The database search identified 426 citations for consideration against the eligibility criteria for this review. Following removal of duplicates (n=162 ), 264 unique citations were forwarded for title and abstract screening. Of these, 242 citations were excluded based on title and abstract, as they clearly did not meet the review’s pre-specified eligibility criteria. A full-text review of the remaining 22 citations was performed, following which a further 18 citations were excluded and one 36 was unobtainable. We corresponded via email with the author of the unobtainable text, who confirmed she did not have access to a copy of the manuscript and was not able to source one.
Full details for each of the 18 exclusions is provided as extended data 32. Reasons for exclusion at full text review were wrong methods used, e.g. not using artificial intelligence/computer learning in the study design (n=6); wrong intervention, e.g. a named drug, a stent, a surgery or chemotherapy (n=5); wrong study design, i.e. defining the sample by outcome (n=4); wrong population, i.e. under 18 (n=2); and not a peer-reviewed article (n=1). Three studies were therefore judged as eligible and included in narrative synthesis 33– 35; a hand-search of all references in these three papers identified no further studies of interest.
The review process is displayed in a PRISMA flow chart ( Figure 1).
Figure 1. PRISMA flow diagram.
Included studies
Three studies meeting the criteria are presented in Table 1. All came from the United States, two 33, 34 using data from the national Medicare programme for those aged 65 and over, and one 35 data on adults admitted to a network of six hospitals in a single urban area. Sampling strategy and sample sizes varied: 59,848 35 hospitalised adults (18+); 80,000 34 Medicare beneficiaries divided equally into four disease cohorts (cancer, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), dementia); and a representative sample of 5,631,168 33 Medicare beneficiaries.
ML methods were similar: one 35 used random forest (RF) models only, one 34 used six approaches in which RF models performed best, and one 33 used an ensemble method combing RF, LASSO and gradient boosting approaches. All three studies used area-under-the-curve (AUC) as a measure of predictive accuracy. In this context AUC represents the probability that a randomly selected patient who died within the time horizon had a higher risk-of-mortality score than one who did not. Two 34, 35 studies compared the performance of their ML models to traditional logistic regression (LR) in predicting mortality.
Main findings
In predicting 12-month mortality amongst a cohort of hospitalised adults, Sahni et al. report an AUC of 0.86 with ML methods versus 0.82 with logistic regression 35.
In predicting six-month mortality amongst disease cohorts of Medicare beneficiaries, Makar et al. report AUC scores of 0.83 (cancer), 0.81 (COPD), 0.76 (CHF) and 0.72 (dementia) 34. This performance is superior to LR using the authors’ own dataset. Sensitivity analyses suggest that unmeasured disease severity accounts for the difference in model performance across disease cohorts.
In analysing association between predicted 12-month mortality and health care expenditures in a representative sample of Medicare beneficiaries, Einav et al. report a weak relationship 33. Their AUC for 12-month mortality is 0.87 but for a population with low mortality rates (about 5% of Medicare beneficiaries died in 2008 and this proportion has since fallen), the 1% of beneficiaries with the highest mortality risk (46%<) account for less than 5% of programme expenditures. Nearly half of this “high-risk” group did not die in the 12-month time horizon. High spending is not concentrated among decedents and ex ante identification of mortality does not appear a useful way to identify poor value care.
Variable selection
All papers additionally evaluate variable selection in their models.
Sahni et al. use a model in primary analysis that includes as predictors both routinely collected administrative data (demographics; International Classification of Disease (ICD) codes identifying presence of specific conditions) and clinical data (physiologic variables such as blood pressure, respiratory rate, body mass index; and biochemical variables such as potassium, creatinine, albumin, white blood count, platelet count).
Age, blood urea nitrogen, platelet count, haemoglobin and creatinine were the most important predictors of 12-month mortality. Comorbidities were the least important domain for additional variables. When the model was restricted to comorbidity variables only, predictive power of the model dropped significantly (AUC=0.64) 35.
Makar et al. use as predictors routinely collected administrative data: demographics, ICD codes, chronic conditions, functional status, durable medical equipment and prior healthcare utilization. In their primary analysis, they not only employ these variables cross-sectionally at baseline but derive ‘augmented’ versions that capture trajectories, e.g. when diagnoses were made (and so how long the person is living with the condition at baseline), when functional limitations set in, when prior ED visits occurred, etc..
Augmented variables are important for model accuracy. All six models employing ML approaches lose predictive power when relying on traditional baseline variables only, e.g. the RF AUC for cancer drops to 0.77 34.
Einav et al. employ a similar approach to Makar et al., taking Medicare database variables at baseline and incorporating some prior trajectories. Additionally, they perform a sensitivity analysis with an additional variable of observed outcome, weighted to 10%. While this endogenous predictor improves the AUC to 0.96, it does not improve the model’s predictive powers for high Medicare spending 33.
Model choice in the context of available variables
Sahni et al. and Makar et al. both report superior performance from RF models to traditional LR using their full datasets.
However, as noted above, RF model performance is contingent on available predictors. It is notable that when LR and RF models are compared with fewer available predictors, the superiority of RF approaches is less clear.
For Sahni et al., LR outperforms RF (AUC 0.71 and 0.64 respectively) when the only available clinical data are routinely collected ICD codes 35. For Makar et al., LR performs better with only traditional variables (AUC=0.73) than with augmented (AUC=0.56). It also performs much more favourably compared to six ML approaches when only traditional variables are available.
Discussion
Key results
Palliative care is a field of health that may be improved through ML techniques that identify patterns and make predictions using large and complex data. A systematic search of peer-reviewed papers on this topic identifies three relevant studies with important insights.
First, ML approaches are powerful in predicting mortality in older and/or hospitalised adults. All three studies report AUC statistics that compare favourably with prior mortality prediction attempts.
Second, in reported studies these ML methods are superior to traditional LR, but only provided sufficient data are available. Particularly notable were the results of Sahni et al., for whom RF model performance relied on physiologic and biochemical data. When these data were excluded, LR performance was superior to RF and this performance was modest. Many studies have used comorbidity counts derived from ICD codes as predictors of important outcomes for people with serious chronic illness including short-term mortality 37– 39, but the papers in this review suggest these indices may not be as powerful predictors as previously understood.
Makar et al. similarly observe much improved RF model performance with more considered inputs, though it is notable that these were not additional clinical data but simply an inventive approach to handling routine data. The predictive power of their four models varied across disease, reflecting unmeasured underlying severity, further emphasising that input data quality decides model performance.
Third, strong predictive power does not correspond to policy implications. Einav et al. was the only included paper seeking to apply explicitly its mortality predictions to a policy problem (high costs near end of life), but found a strong mortality prediction model was not useful for their specific purpose.
Corresponding implications for researchers and practitioners seeking to follow these examples are clear. Certainly, ML approaches have significant potential to improve identification of trajectories in seriously-ill populations. However, be mindful of the truism that a model is only as good as its inputs. Where possible, collect granular, individual-level clinical data. Optimise available routine data with innovative approaches where possible. Do not assume that ML approaches are de facto superior to traditional LR, and compare performance of different models. Perhaps most important, recognise that improving clinical decision-making will require more than simply improving the predictive power of mortality models. Expand analyses to other outcomes of interest and to the processes underpinning those outcomes.
Limitations
This review has a number of important limitations. We conducted a rapid review of published peer review literature and not a full systematic review incorporating grey literature. Rapid reviews are a well-established methodology for gathering evidence in a limited timeframe 30, and we considered this appropriate for our aims of characterising the landscape of a fast-emerging field 31. Our results can inform ongoing attempts by researchers and practitioners to harness the power of ML methods in improving palliative care, a policy priority worldwide. A particular strength of our methods was that we retained two independent reviewers at each stage, per systematic review methodology, an approach many rapid reviews eschew 40. Nevertheless this is a rapidly evolving field 16, 41, and there are likely conference abstracts and other works in progress that would have featured in a systematic review but are excluded here.
Another limitation of the rapid review timeframe is that we did not formally assess quality or risk of bias in included papers. Instead we incorporated considerations of data quality and study usefulness in reporting, e.g. in interpreting differences in available data across studies in the context of their results. While we specified at the outset that we would not conduct a meta-analysis, we do not consider this a serious limitation in the context of our findings: specific data inputs vary across included papers yet are central to the performance of ML methods; pooling results from these papers is therefore of limited relevance.
Our eligibility criteria led to some exclusions of papers applying ML methods to improve palliative care (see extended data 32 for 18 papers excluded at full text review). In particular, four papers that were otherwise-eligible and examined patient outcomes, e.g. QoL, a comfortable death and related factors, were excluded for defining their sample by vital status (a sub-sample of decedents were extracted ex post) 42– 45. While decedent cohort studies have well established value in some research contexts 12, we established this criterion ex ante given endogeneity and bias concerns 28, 29. This decision was validated by the demonstration of one included study that found analyses of the ex post dead are not particularly useful in analysing ex ante the sick 33. Additionally we excluded one otherwise-eligible paper for pooling children and adults in the sample 46, but this decision was based in standard research practice in this field 26 and this was reflected in 19 of 21 (90%) full texts using adulthood as an eligibility criterion. Finally, we did not include studies where the endpoints of interest were process measures, e.g. patient-physician interaction, advance care planning and expression of patient/family preferences 47. While it is rational to assume that improved processes using ML will lead to improved outcomes in the long run 16, 41, we required that this effect on outcomes be evaluated to be eligible for our review.
One paper was excluded as unobtainable within the timeframe of the analysis, although we did subsequently obtain a copy. We did not consider the paper met our eligibility criteria. While we decided at the outset to exclude papers not in the English language, no paper was ruled ineligible on that basis.
Future research
At the outset of this review we identified outcomes of interest in three domains: patient outcomes (survival; QoL); caregiver outcomes (survival; QoL); and economic outcomes (costs, receipt of cost-(in)effective treatment, high-intensity treatment at end of life). We specified these as established measures for quality in palliative care, and so of direct relevance to practitioners and policymakers 27. Most of these domains were unaddressed and so stand as priority areas for future work.
Our review included three studies with a predominant interest in patient mortality. No included study examined patient outcomes such as QoL. Evaluations of QoL are not straightforward because the outcome of interest is an individual and subjective concept where mortality is an observable binary state. Nevertheless, the reality is that living and dying with serious illness, and caring for those populations, is messy and complex. Studies characterising palliative care need beyond mortality, for example those at risk of pain or unmet need or death anxiety 42– 45, and accurately predicting risk would have the capacity to improve clinical decision-making and treatment pathways.
No included study, or any study rejected at full text, incorporated caregiver perspective. This is perhaps not surprising as the patient is naturally the primary focus of clinical practice and associated research. Nevertheless the role of unpaid carers is well recognised in this field, and evaluations of dyad and family outcomes is increasingly common 48. Identifying caregiver needs in advance would also have vast potential benefit.
One study examined an economic dimension – the long-established association between end-of-life phase and high costs. The authors conclude that this is not a useful lens by which to improve resource allocation in the care of the seriously ill. Rather, those dying with terminal illness and multimorbidity are a subset of all people living with high illness burden and high associated health care use. Identification of appropriate care and supports for this group using ML must be cognisant of recent work identifying substantive treatment effect heterogeneity by disease profile and burden in palliative care 49. In turn, this review has important insights for treatment effect heterogeneity work that has been based on routinely collected clinical data such as ICD codes: these data, it appears, are relatively weak predictors of relevant outcomes 50.
An additional concern for future studies across all outcomes of interest is timeframe. The three included studies here used six- and 12-month survival as their outcomes of interest, but there is increasing recognition that palliative care has benefits across the trajectory of life-limiting illness 3. For example, American Society of Clinical Oncology guidelines recommend that palliative care is provided across the disease trajectory 51, and the last 12 months of life accounts for less than a third of US cancer care costs 52. Treatment choices from diagnosis have the greatest scope to impact outcomes and costs 53, 54, and so studies that can inform these choices are the most useful.
Ethical issues receive scant attention in the studies read for this review. Questions arise with respect to how systems will use the data collected. For example, demographics may be important drivers of outcomes and treatment effects in the context of different experiences and preferences for care across social groups 55, 56. But offering or providing treatments to some groups and not others based on sociodemographic characteristics is, to say the least, ethically problematic. Also important are the data not collected. Fulfilling the goal of a “good death” involved fixed and modifiable dimensions of patient personality, experience and preferences. Personal resources associated with improved QoL near end of life may include religiosity and beliefs, ‘‘acceptance of reality’’, ‘‘life meaning and purpose’’, ‘‘self-worth’’, ‘‘hope’,’ and ‘‘caregivers’ support and acceptance’’ 57.
These limitations may be best addressed in the context of broad conceptual considerations for the optimal applications of big data 58. In all fields it is critical to ground big data collection and measurement in conceptual frameworks to guard against results that are specious, not generalisable or not actionable 59. This is never more true than in palliative care, where many areas of data collection and conceptual understanding are formative. No matter how powerful the artificial intelligence driving models to improve decision-making, it remains paramount that research, policy and practice protects space for the human wisdom of patients, their families and health care professionals in optimising experience of a unique life event.
Conclusion
Improving palliative care is a policy priority worldwide. ML has the potential to support clinicians in improved decision-making by identifying those at heightened risk of inappropriate care, poor outcomes and mortality. To date studies have demonstrated capacity to improve mortality prediction. Other outcomes have not received equivalent attention. Applications of ML approaches to policy and practice remains formative. Derived results depend on available data and must be interpreted in this context. Future research must not only expand scope to consider other outcomes and longer timeframes, but also address individual needs and preferences in the context of prognosis, and engage with the profound ethical challenges of this emerging field.
Data availability
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Open Science Framework: Appendix to: [Improving palliative care with machine learning and routine data: a rapid review]. https://doi.org/10.17605/OSF.IO/AC6TR
This project contains the following extended data:
20190601 Appendix.doc (Summary of search strategies; details of studies rejected at full text review) 32
Reporting guidelines
Open Science Framework: PRISMA checklist and diagram for ‘Improving palliative care with machine learning and routine data: a rapid review’, https://doi.org/10.17605/OSF.IO/AC6TR 32
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Funding Statement
Health Research Board Ireland [ARPP-A-2018-005 to PM]
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 3 approved]
References
- 1. Knaul FM, Bhadelia A, Rodriguez NM, et al. : The Lancet Commission on Palliative Care and Pain Relief: findings, recommendations, and future directions. Lancet Glob Health. 2018;6(S1):S5–S6. 10.1016/S2214-109X(18)30082-2 [DOI] [Google Scholar]
- 2. Lehnert T, Heider D, Leicht H, et al. : Review: health care utilization and costs of elderly persons with multiple chronic conditions. Med Care Res Rev. 2011;68(4):387–420. 10.1177/1077558711399580 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization: WHO Definition of Palliative Care. Published 2017. Accessed August 15th, 2017. Reference Source [Google Scholar]
- 4. Higginson IJ, Evans CJ, Grande G, et al. : Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med. 2013;11:111 10.1186/1741-7015-11-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Economist Intelligence Unit: The Quality of Death Index: Ranking palliative care across the world.2015. Reference Source [Google Scholar]
- 6. Arias-Casais N, Garralda E, Rhee J, et al. : EAPC Atlas of Palliative Care in Europe 2019. Vilvoorde.2019. Reference Source [Google Scholar]
- 7. Etkind SN, Bone AE, Gomes B, et al. : How many people will need palliative care in 2040? Past trends, future projections and implications for services. BMC Med. 2017;15(1):102. 10.1186/s12916-017-0860-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. White N, Reid F, Harris A, et al. : A Systematic Review of Predictions of Survival in Palliative Care: How Accurate Are Clinicians and Who Are the Experts? PLoS One. 2016;11(8):e0161407. 10.1371/journal.pone.0161407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glare P, Virik K, Jones M, et al. : A systematic review of physicians' survival predictions in terminally ill cancer patients. BMJ. 2003;327(7408):195–198. 10.1136/bmj.327.7408.195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clément-Duchêne C, Carnin C, Guillemin F, et al. : How accurate are physicians in the prediction of patient survival in advanced lung cancer? Oncologist. 2010;15(7):782–789. 10.1634/theoncologist.2009-0149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gripp S, Moeller S, Bölke E, et al. : Survival prediction in terminally ill cancer patients by clinical estimates, laboratory tests, and self-rated anxiety and depression. J Clin Oncol. 2007;25(22):3313–3320. 10.1200/JCO.2006.10.5411 [DOI] [PubMed] [Google Scholar]
- 12. Earle CC, Ayanian JZ: Looking back from death: the value of retrospective studies of end-of-life care. J Clin Oncol. 2006;24(6):838–840. 10.1200/JCO.2005.03.9388 [DOI] [PubMed] [Google Scholar]
- 13. May P, Garrido MM, Del Fabbro E, et al. : Evaluating Hospital Readmissions for Persons With Serious and Complex Illness: A Competing Risks Approach. Med Care Res Rev. 2019; 1077558718823919. 10.1177/1077558718823919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Raghupathi W, Raghupathi V: Big data analytics in healthcare: promise and potential. Health Inf Sci Syst. 2014;2(1):3. 10.1186/2047-2501-2-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Arel I, Rose D, Karnowski T: Deep Machine Learning - A New Frontier in Artificial Intelligence Research [Research Frontier].2010;5(4):13–18. 10.1109/MCI.2010.938364 [DOI] [Google Scholar]
- 16. Chan A, Chien I, Moseley E, et al. : Deep learning algorithms to identify documentation of serious illness conversations during intensive care unit admissions. Palliat Med. 2019;33(2):187–196. 10.1177/0269216318810421 [DOI] [PubMed] [Google Scholar]
- 17. Miotto R, Wang F, Wang S, et al. : Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform. 2018;19(6):1236–1246. 10.1093/bib/bbx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gianfrancesco MA, Tamang S, Yazdany J, et al. : Potential Biases in Machine Learning Algorithms Using Electronic Health Record Data. JAMA Intern Med. 2018;178(11):1544–1547. 10.1001/jamainternmed.2018.3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Institute of Medicine: Dying in America: improving quality and honoring individual preferences near the end of life. Washington, DC: The National Academies Press;2014. Reference Source [PubMed] [Google Scholar]
- 20. Brereton L, Clark J, Ingleton C, et al. : What do we know about different models of providing palliative care? Findings from a systematic review of reviews. Palliat Med. 2017;31(9):781–797. 10.1177/0269216317701890 [DOI] [PubMed] [Google Scholar]
- 21. Maas EA, Murray SA, Engels Y, et al. : What tools are available to identify patients with palliative care needs in primary care: a systematic literature review and survey of European practice. BMJ Support Palliat Care. 2013;3(4):444–451. 10.1136/bmjspcare-2013-000527 [DOI] [PubMed] [Google Scholar]
- 22. Tanuseputro P: Delivering care to those in need: Improving palliative care using linked data. Palliat Med. 2017;31(6):489–491. 10.1177/0269216317704629 [DOI] [PubMed] [Google Scholar]
- 23. Nwosu AC, Collins B, Mason S: Big Data analysis to improve care for people living with serious illness: The potential to use new emerging technology in palliative care. Palliat Med. 2018;32(1):164–166. 10.1177/0269216317726250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies JM, Gao W, Sleeman KE, et al. : Using routine data to improve palliative and end of life care. BMJ Support Palliat Care. 2016;6(3):257–62. 10.1136/bmjspcare-2015-000994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Gunten CF, Teno JM, Sean Morrison R: Big Data and End-of-Life Care: Promise and Peril. J Palliat Med. 2016;19(12):1240. 10.1089/jpm.2016.0374 [DOI] [PubMed] [Google Scholar]
- 26. Groh G, Feddersen B, Führer M, et al. : Specialized home palliative care for adults and children: differences and similarities. J Palliat Med. 2014;17(7):803–810. 10.1089/jpm.2013.0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Earle CC, Park ER, Lai B, et al. : Identifying potential indicators of the quality of end-of-life cancer care from administrative data. J Clin Oncol. 2003;21(6):1133–1138. 10.1200/JCO.2003.03.059 [DOI] [PubMed] [Google Scholar]
- 28. Bach PB, Schrag D, Begg CB: Resurrecting treatment histories of dead patients: a study design that should be laid to rest. JAMA. 2004;292(22):2765–2770. 10.1001/jama.292.22.2765 [DOI] [PubMed] [Google Scholar]
- 29. May P, Garrido MM, Cassel JB, et al. : Using Length of Stay to Control for Unobserved Heterogeneity When Estimating Treatment Effect on Hospital Costs with Observational Data: Issues of Reliability, Robustness, and Usefulness. Health Serv Res. 2016;51(5):2020–2043. 10.1111/1475-6773.12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ganann R, Ciliska D, Thomas H: Expediting systematic reviews: methods and implications of rapid reviews. Implement Sci. 2010;5(1):56. 10.1186/1748-5908-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khangura S, Konnyu K, Cushman R, et al. : Evidence summaries: the evolution of a rapid review approach. Syst Rev. 2012;1(1):10. 10.1186/2046-4053-1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. May P: Appendix to: [Improving Palliative and End-of-Life Care with Machine Learning and Routine Data: A Rapid Review]. OSF.Web.2019. 10.17605/OSF.IO/AC6TR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Einav L, Finkelstein A, Mullainathan S, et al. : Predictive modeling of U.S. health care spending in late life. Science. 2018;360(6396):1462–1465. 10.1126/science.aar5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Makar M, Ghassemi M, Cutler DM, et al. : Short-term Mortality Prediction for Elderly Patients Using Medicare Claims Data. Int J Mach Learn Comput. 2015;5(3):192–197. 10.7763/IJMLC.2015.V5.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sahni N, Simon G, Arora R: Development and Validation of Machine Learning Models for Prediction of 1-Year Mortality Utilizing Electronic Medical Record Data Available at the End of Hospitalization in Multicondition Patients: a Proof-of-Concept Study. J Gen Intern Med. 2018;33(6):921–928. 10.1007/s11606-018-4316-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamang S, Kopec D, Shagas G, et al. : Improving palliative care: An information systems approach. Journal on Information Technology in Healthcare. 2005;3(3):159–171. Reference Source [PubMed] [Google Scholar]
- 37. Yurkovich M, Avina-Zubieta JA, Thomas J, et al. : A systematic review identifies valid comorbidity indices derived from administrative health data. J Clin Epidemiol. 2015;68(1):3–14. 10.1016/j.jclinepi.2014.09.010 [DOI] [PubMed] [Google Scholar]
- 38. Diederichs C, Berger K, Bartels DB: The measurement of multiple chronic diseases--a systematic review on existing multimorbidity indices. J Gerontol A Biol Sci Med Sci. 2010;66(3):301–311. 10.1093/gerona/glq208 [DOI] [PubMed] [Google Scholar]
- 39. Huntley AL, Johnson R, Purdy S, et al. : Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10(2):134–141. 10.1370/afm.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tricco AC, Antony J, Zarin W, et al. : A scoping review of rapid review methods. BMC Med. 2015;13(1):224. 10.1186/s12916-015-0465-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lindvall C, Lilley EJ, Zupanc SN, et al. : Natural Language Processing to Assess End-of-Life Quality Indicators in Cancer Patients Receiving Palliative Surgery. J Palliat Med. 2019;22(2):183–187. 10.1089/jpm.2018.0326 [DOI] [PubMed] [Google Scholar]
- 42. Almasalha F, Xu D, Keenan GM, et al. : Data mining nursing care plans of end-of-life patients: a study to improve healthcare decision making. Int J Nurs Knowl. 2013;24(1):15–24. 10.1111/j.2047-3095.2012.01217.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lodhi MK, Cheema UI, Stifter J, et al. : Death anxiety in hospitalized end-of-life patients as captured from a structured electronic health record: differences by patient and nurse characteristics. Res Gerontol Nurs. 2014;7(5):224–234. 10.3928/19404921-20140818-01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lodhi MK, Ansari R, Yao Y, et al. : Predictive Modeling for Comfortable Death Outcome Using Electronic Health Records.In: Barbara C, Khan L, eds. Proc IEEE Int Congr Big Data. 2015;2015:409–415. 10.1109/BigDataCongress.2015.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lodhi MK, Stifter J, Yao Y, et al. : Predictive Modeling for End-of-Life Pain Outcome using Electronic Health Records.In: Perner P, ed. Adv Data Min. 2015;9165:56–68. 10.1007/978-3-319-20910-4_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Avati A, Jung K, Harman S, et al. : Improving palliative care with deep learning. BMC Med Inform Decis Mak. 2018;18(Suppl 4):122. 10.1186/s12911-018-0677-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Udelsman B, Chien I, Ouchi K, et al. : Needle in a Haystack: Natural Language Processing to Identify Serious Illness. J Palliat Med. 2019;22(2):179–182. 10.1089/jpm.2018.0294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ornstein KA, Kelley AS, Bollens-Lund E, et al. : A National Profile Of End-Of-Life Caregiving In The United States. Health Aff (Millwood). 2017;36(7):1184–1192. 10.1377/hlthaff.2017.0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. May P, Normand C, Cassel JB, et al. : Economics of Palliative Care for Hospitalized Adults With Serious Illness: A Meta-analysis. JAMA Intern Med. 2018;178(6):820–829. 10.1001/jamainternmed.2018.0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yourman LC, Lee SJ, Schonberg MA, et al. : Prognostic indices for older adults: a systematic review. JAMA. 2012;307(2):182–192. 10.1001/jama.2011.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ferrell BR, Temel JS, Temin S, et al. : Integration of Palliative Care Into Standard Oncology Care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2017;35(1):96–112. 10.1200/JCO.2016.70.1474 [DOI] [PubMed] [Google Scholar]
- 52. National Cancer Institute: Graph by Cancer Site & Phase of Care.National Institutes of Health.2018; Accessed December 4th, 2018. Reference Source [Google Scholar]
- 53. Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363(8):733–742. 10.1056/NEJMoa1000678 [DOI] [PubMed] [Google Scholar]
- 54. May P, Normand C: Analyzing the Impact of Palliative Care Interventions on Cost of Hospitalization: Practical Guidance for Choice of Dependent Variable. J Pain Symptom Manage. 2016;52(1):100–106. 10.1016/j.jpainsymman.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 55. Frost DW, Cook DJ, Heyland DK, et al. : Patient and healthcare professional factors influencing end-of-life decision-making during critical illness: a systematic review. Crit Care Med. 2011;39(5):1174–1189. 10.1097/CCM.0b013e31820eacf2 [DOI] [PubMed] [Google Scholar]
- 56. Orlovic M, Smith K, Mossialos E: Racial and ethnic differences in end-of-life care in the United States: Evidence from the Health and Retirement Study (HRS). SSM Popul Health. 2018;7: 100331. 10.1016/j.ssmph.2018.100331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chan WCH: Can personal resources make a difference? an exploratory study of chinese cancer patients in palliative care. J Soc Work End Life Palliat Care. 2009;5(3–4):186–200. 10.1080/15524250903555585 [DOI] [Google Scholar]
- 58. Scott IA: Hope, hype and harms of Big Data. Intern Med J. 2019;49(1):126–129. 10.1111/imj.14172 [DOI] [PubMed] [Google Scholar]
- 59. Coveney PV, Dougherty ER, Highfield RR: Big data need big theory too. Philos Trans A Math Phys Eng Sci. 2016;374(2080): pii: 20160153. 10.1098/rsta.2016.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]