Abstract
OBJECTIVE
To determine the prevalence and prognostic significance of unrecognized myocardial infarction (MI) by delayed-enhancement MRI (DE-MRI) in asymptomatic patients with diabetes.
RESEARCH DESIGN AND METHODS
In this prospective, two-center study of asymptomatic patients without known cardiac disease (n = 120), two prespecified cohorts underwent a research MRI: 1) a high-risk group with type 1 diabetes and chronic renal insufficiency (n = 50) and 2) an average-risk group with type 2 diabetes (n = 70). The primary end point was a composite of all-cause mortality and clinical MI.
RESULTS
Overall, the prevalence of unrecognized MI was 19% by DE-MRI (28% high-risk group and 13% average-risk group) and 5% by electrocardiography. During up to 5 years of follow-up with a total of 460 patient-years of follow-up, the rate of death/MI was markedly higher in patients with diabetes with (vs. without) unrecognized MI (all 44% vs. 7%, high-risk group 43% vs. 6%, and average-risk group 44% vs. 8%; all P < 0.01). After adjustment for Framingham risk score, left ventricular ejection fraction, and diabetes type, the presence of unrecognized MI by DE-MRI conferred an eightfold increase in risk of death/MI (95% CI 3.0–21.1, P < 0.0001). Addition of unrecognized MI to clinical indices significantly improved model discrimination for adverse events (integrated discrimination improvement = 0.156, P = 0.001).
CONCLUSIONS
Unrecognized MI is prevalent in asymptomatic patients with diabetes without a history of cardiac disease and confers a markedly increased risk of death and clinical MI.
Introduction
Complications of coronary artery disease (CAD) in patients with diabetes, such as myocardial infarction (MI), heart failure, and premature death, represent a major health care issue. Globally, the prevalence of diabetes is expected to exceed 625 million people by 2045 (1). The high cardiovascular event rates inherent in diabetes have led some to advocate the performance of cardiac imaging studies in asymptomatic patients with diabetes to identify a high-risk group that may benefit from aggressive intervention. However, the role of noninvasive imaging in asymptomatic patients with diabetes is poorly defined given the limited prospective data addressing this issue (2). Accordingly, the American College of Cardiology Foundation/American Heart Association (AHA) guideline for assessment of cardiovascular risk in asymptomatic adults does not recommend the routine use of cardiac testing (exercise treadmill testing, stress echocardiography, nuclear perfusion imaging, and coronary computed tomography angiography) in asymptomatic patients with diabetes (3).
Recent advances in cardiovascular imaging allow for the detection of subclinical disease in asymptomatic patients. However, up to 70% of asymptomatic individuals with diabetes have evidence of CAD by coronary computed tomography angiography (4). Hence, given its common presence in diabetes, subclinical CAD may not provide sufficient discrimination of cardiovascular risk. In comparison, imaging techniques that can identify downstream adverse consequences of CAD, such as unrecognized MI, may be more tightly linked to future risk of clinical events. Delayed-enhancement MRI (DE-MRI) is a high spatial resolution technique (5) that can accurately identify MI (6), including those that are not clinically recognized or detected by electrocardiography (7,8).
In symptomatic patients with diabetes, the presence of unrecognized MI by DE-MRI is associated with increased risk of cardiac events (9). However, in asymptomatic patients with diabetes, the prognostic significance of unrecognized MI by DE-MRI is unknown. Hence, the aim of the current study was to assess the prevalence and prognostic impact of unrecognized MI as identified by DE-MRI in asymptomatic patients with diabetes.
Research Design and Methods
Population
Asymptomatic patients with diabetes and without a history of cardiac disease were prospectively enrolled. All participants gave informed consent to the study protocol in accordance with the policies of the institutional review board of both institutions. Patients with angina, prior MI, prior coronary revascularization, congestive heart failure, cardiomyopathy, and severe valvular disease were excluded. To evaluate the prevalence and prognostic impact of unrecognized MI across a wide spectrum of risk, the study population consisted of two prespecified cohorts with diabetes; the two patient cohorts were enrolled between January 2001 and January 2005.
The high-risk group consisted of 50 patients with type 1 diabetes and advanced nephropathy (stage 4 or 5 renal insufficiency). This group was chosen because patients with diabetes with nephropathy have a several-fold higher cardiac mortality rate in comparison with patients with diabetes with normal renal function (10,11). Consecutive patients with type 1 diabetes undergoing an evaluation for simultaneous kidney and pancreas transplantation at Northwestern Memorial Hospital were screened. After excluding candidates with chest discomfort or prior cardiac disease (n = 12), the first 50 patients who agreed to participate and could be scheduled for a cardiac MRI scan were prospectively enrolled. Patients with renal insufficiency were enrolled before the U.S. Food and Drug Administration (FDA) alerts regarding the potential occurrence of nephrogenic systemic fibrosis (NSF) associated with gadolinium administration (12). These patients received gadoteridol, a macrocyclic gadolinium-based contrast agent considered to have a substantially reduced risk of NSF (13). None of the study patients developed NSF during the follow-up period.
The average-risk group consisted of 70 patients with type 2 diabetes without known renal insufficiency. The first 70 asymptomatic patients recruited from an internal medicine clinic at Duke University Medical Center who agreed to participate were enrolled in the study.
A control group of 24 healthy volunteers (mean age 36 years; 14 men and 10 women) was also recruited to reduce possible observer bias in the interpretation of DE-MRI images. Images from both groups with diabetes and normal volunteers were randomized into a single group before blinded interpretation. All volunteers were asymptomatic and had a very low probability of developing coronary disease over the next 10 years (lowest Framingham risk score category: 1% for women and 2% for men).
Initial Assessment
All patients enrolled in the study had a complete medical history and examination, including cardiac risk factors, medication regimen, diabetes duration, blood pressure, and lipid profiles. Framingham risk score was calculated. A standard 12-lead electrocardiogram was performed. The presence or absence of Q waves was assessed by the criteria of Minnesota codes 1-1-1 to 1-2-7 (14). The average-risk group also had blood samples drawn for HbA1c and serum creatinine (mg/dL), and glomerular filtration rates (GFR) were calculated using the MDRD equation.
MRI
Cardiac MRI was performed for research purposes (i.e., not clinically ordered scans), and scan results were not used to guide clinical decision making. MRI was performed on 1.5T scanners (Siemens Sonata or Avanto) using phased-array receiver coils. Cine and delayed-enhancement imaging was performed using standard protocols as described previously (8). In brief, cine images were acquired in multiple short-axis views (every 10 mm throughout the entire left ventricle) and three long-axis views using a steady-state free-precession sequence (slice thickness 6 mm, interslice gap 4 mm, temporal resolution 35–40 ms, flip angle 60°, and in-plane resolution 1.7 × 1.4 mm). Delayed-enhancement images were acquired using a segmented inversion-recovery gradient-echo sequence (slice thickness 6 mm, interslice gap 4 mm, flip angle 25°, and in-plane resolution 1.9 × 1.4 mm) 10–15 min after an intravenous bolus of gadoteridol or gadoversetamide contrast (0.125–0.15 mmol/kg body weight). Image planes were identical to that used for cine imaging, and inversion delay time was set to null signal from normal myocardium (5).
Follow-up
Follow-up was performed prospectively in all patients via 1) telephone interview with the patient or, if deceased, with family members; 2) contact with the patient’s physician; and 3) review of hospital and/or outpatient records. The primary end point of the study was the composite of all-cause mortality and clinical MI. Additionally, a secondary end point was the composite of cardiac mortality and MI. Clinical MI was defined in accordance with the joint European Society of Cardiology/American College of Cardiology consensus document for the definition of MI (15). Cardiac death was defined as all deaths in the setting of coronary ischemic syndromes, congestive heart failure, and sudden death (16). All other deaths were recorded as noncardiac. The results of all coronary angiography procedures that patients underwent within the first 6 months of the cardiac MRI exam at the discretion of their managing physicians were obtained. Significant CAD was defined as ≥70% stenosis of a major epicardial coronary artery. Also, all coronary revascularization procedures (coronary artery bypass grafting [CABG]/percutaneous coronary intervention [PCI]) that occurred during the follow-up period were recorded.
Analysis
MRI examinations from all patients and volunteers were randomized into a single group before analysis to limit observer bias. No patient was excluded on the basis of MRI image quality. Left ventricular ejection fraction (LVEF) was measured from manual tracings of end-diastolic and end-systolic endocardial borders from the entire stack of short-axis cine images. The presence and location of hyperenhanced tissue on DE-MRI was determined by visual inspection using the AHA 17-segment model. Additionally, the pattern of hyperenhancement was assessed and used to classify scarring as consistent with MI or a nonischemic disorder (e.g., myocarditis, infiltrative cardiomyopathy, etc.) as described previously (17).
Scar size was measured by planimetry from the stack of short-axis DE-MRI images. The borders were determined visually in our MRI core laboratory. Interobserver and intraobserver agreement for scar size in our core laboratory services using Bland-Altman analysis demonstrates a bias of 1% and −0.1%, respectively, with an SD of differences of 2.6% and 0.8%, respectively. In patients with coronary angiography during follow-up, the myocardial perfusion territory of obstructive coronary lesions was compared with the location of infarction by DE-MRI as described previously (6).
Statistical Analysis
Normally distributed data are presented as the mean ± SD or, in cases where the distribution is not normal, as median and interquartile range. Comparisons of ontinuous data between groups were made using two-sample Student t tests or Wilcoxon rank sum tests as appropriate. χ2 tests were used to compare discrete data between groups; in those cases where the expected cell count was less than five, Fisher exact test was used. Cumulative event rates were calculated according to the Kaplan-Meier method. Differences in event rates between groups were assessed with the log rank test. In order to identify the baseline characteristics associated with adverse outcome, univariable Cox proportional hazards regression analysis was performed. For patients with two or more events during follow-up (MI event followed by death), only the time to the first event was considered per patient.
Two Cox regression multivariable models were subsequently developed. In the first, candidate variables showing a possible association with prognosis by univariable analysis (P < 0.10) were considered one at a time, starting with the most significant variable. Significant variables were determined by stepwise selection (and backward elimination) at the 0.05 level of significance. Relative risks were expressed as hazard ratios (HRs) with associated 95% CIs. In the second multivariable model, only three prespecified variables known to be associated with adverse outcome were included to avoid the potential for overfitting. These were LVEF, Framingham risk score, and diabetes type. To assess the added prognostic value of unrecognized MI by DE-MRI, the final model was compared with a model in which unrecognized MI was included. The global χ2 statistic was calculated for both models and compared using the likelihood ratio test. Model discrimination was also assessed by calculating the integrated discrimination improvement (IDI), which measures the improvement in sensitivity and specificity of the model with addition of the new predictor (unrecognized MI) (18). All SPSS tests were two tailed, and P < 0.05 was regarded as significant.
Results
Baseline Characteristics
The baseline clinical characteristics for the total population and the two subgroups are presented in Table 1. Overall, the mean age was 52 ± 13 years, and nearly half were women (46%). The Framingham risk score (10.5 ± 9) placed the majority of patients in an intermediate risk category for future cardiovascular events (10–20% 10-year risk). The mean duration of diabetes was 17 ± 11 years, and two-thirds were on insulin. Approximately 45% of all patients were receiving aspirin and/or statin therapy, and nearly two-thirds were on ACE inhibitors or angiotensin receptor blockers. The mean LDL levels were relatively low (112 ± 42 mg/dL) for the study population. Left ventricular systolic function was normal with a mean LVEF of 63 ± 9%.
Table 1.
Patient characteristics
| Parameter | All patients with diabetes (n = 120) | High risk (type 1) (n = 50) | Average risk (type 2) (n = 70) | P |
|---|---|---|---|---|
| Clinical history | ||||
| Age (year) | 52 ± 13 | 40 ± 7 | 60 ± 8 | <0.0001 |
| Male sex | 65 (54) | 29 (58) | 36 (51) | 0.51 |
| Atherosclerosis risk factors | ||||
| Hypertension | 102 (85) | 50 (100) | 52 (74) | <0.0001 |
| Tobacco use | 27 (23) | 19 (38) | 8 (11) | 0.02 |
| Hyperlipidemia | 68 (57) | 22 (44) | 44 (66) | 0.0006 |
| Family history of CAD | 28 (23) | 7 (14) | 21 (30) | 0.04 |
| Framingham risk score* | 10.5 (7, 17) | 8.0 (4, 12) | 13.0 (10, 24) | <0.0001 |
| Duration of diabetes (year) | 17 ± 11 | 26 ± 8 | 11 ± 9 | <0.0001 |
| Medications | ||||
| ACE inhibitor/angiotensin receptor blocker | 77 (64) | 30 (60) | 47 (67) | 0.42 |
| β-Blocker | 30 (25) | 11 (22) | 19 (27) | 0.52 |
| Aspirin | 52 (43) | 11 (22) | 41 (59) | <0.0001 |
| Statin | 57 (48) | 22 (44) | 35 (50) | 0.52 |
| Insulin | 79 (66) | 50 (100) | 29 (41) | <0.0001 |
| Sulfonylureas | — | — | 18 (26) | |
| Metformin | — | — | 35 (50) | |
| Baseline assessments | ||||
| Blood pressure (mmHg) | ||||
| Systolic | 143 ± 22 | 150 ± 24 | 139 ± 20 | 0.007 |
| Diastolic | 80 ± 12 | 84 ± 12 | 76 ± 12 | 0.0009 |
| Lipid panel (mg/dL)** | ||||
| Total cholesterol | 200 ± 50 | 213 ± 56 | 190 ± 45 | 0.02 |
| LDL | 112 ± 42 | 124 ± 45 | 103 ± 38 | 0.02 |
| HDL | 55 ± 19 | 61 ± 25 | 51 ± 13 | 0.02 |
| Electrocardiogram, Q wave† | 6 (5) | 1 (2) | 5 (7) | 0.20 |
| HbA1c (%)‡ | — | — | 7.6 ± 1.5 | |
| BMI (kg/m2) | 28 ± 6 | 24 ± 4 | 31 ± 6 | <0.0001 |
| GFR <60 mL/min | 62 (54) | 50 (100) | 11 (17) | <0.0001 |
| Dialysis | 24 (20) | 24 (48) | 0 (0) | <0.0001 |
| Cine MRI, LVEF (%) | 63 ± 9 | 64 ± 9 | 63 ± 9 | 0.45 |
Data are means ± SD or n (%) unless otherwise indicated.
*Median (interquartile range), calculated in the 106 patients who had all relevant blood tests.
**Obtained in 112 patients.
†Minnesota codes 1-1-1 to 1-2-7.
‡HbA1c levels obtained only in patients with type 2 diabetes.
There were many differences in baseline clinical characteristics between the two subgroups. The average-risk patients with type 2 diabetes were older than the high-risk group with type 1 diabetes (60 vs. 40 years, P < 0.0001) and had a higher Framingham risk score (13 vs. 8, P < 0.0001) and BMI (31 vs. 24 kg/m2, P < 0.0001). The high-risk patients with type 1 diabetes had a longer duration of diabetes (26 vs. 11 years, P < 0.0001), higher prevalence of renal insufficiency (100% vs. 17%, P < 0.0001), and higher baseline blood pressure and cholesterol levels. There was no significant difference in baseline LVEF on MRI between the two groups (P = 0.45).
Prevalence and Predictors of Unrecognized MI
Overall, the prevalence of unrecognized MI by DE-MRI was 19% (n = 23; 95% CI 17–21%) for the total patient population. The prevalence was increased for high-risk compared with the average-risk patients with diabetes (28% vs. 13%, P = 0.038). The prevalence of unrecognized MI by electrocardiography was far lower, at 5% (P < 0.001 vs. DE-MRI). However, of the six patients with unrecognized MI by electrocardiography, only one had unrecognized MI by DE-MRI, suggesting that 12-lead electrocardiography had both limited sensitivity and specificity in this cohort. No evidence of non–CAD-type hyperenhancement was observed in the patients. None of the volunteer controls had unrecognized MI by DE-MRI or electrocardiography.
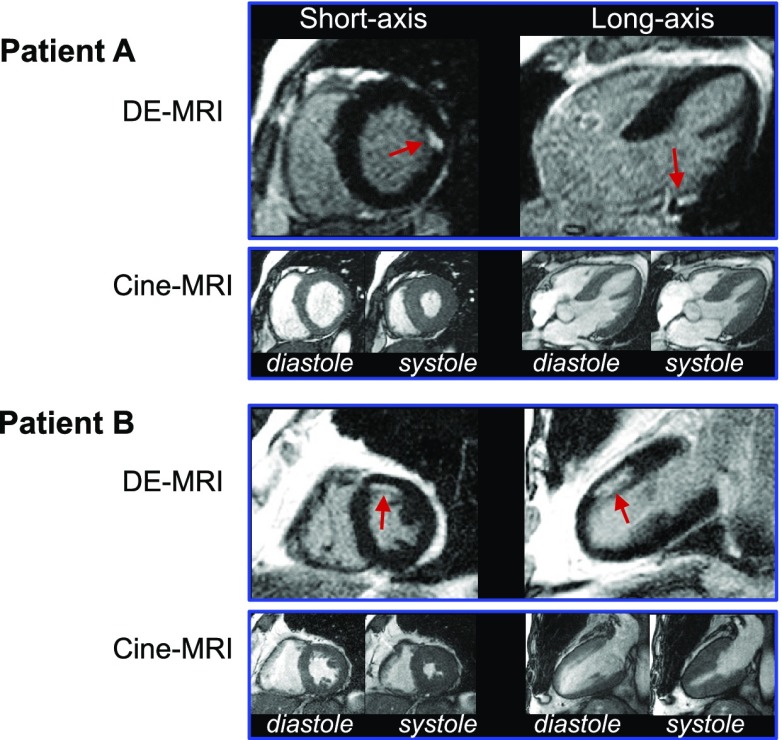
Representative patient examples of unrecognized MI are shown in Fig. 1. Both patient examples demonstrate small subendocardial infarcts with normal wall motion. In all patients with unrecognized MI, infarct size was small, involving only 5.1 ± 4.2% (range 0.8–15.3%) of the left ventricle. Accordingly, LVEF was well preserved in those with unrecognized MI (61 ± 11%) and similar to that in the patients without unrecognized MI (64 ± 8%, P = 0.17).
Figure 1.
Typical DE-MRI and cine-MRI images. Short- and long-axis views in two patients are shown. Patient A demonstrates a small, focal MI (arrows) limited to the basal anterolateral wall. Patient B has a subendocardial infarction (arrows) involving the mid-anterior wall. Both patients had normal LVEF (A, 64%; B, 61%), no regional wall motion abnormalities, and no Q waves on electrocardiography.
To determine any association between baseline clinical variables and the presence of unrecognized MI by DE-MRI, a logistic regression analysis of all patient characteristics in Table 1 was performed. No baseline clinical variable other than diabetes type was determined to be a significant predictor of unrecognized MI.
Coronary Angiography, Location of MI, and Revascularization
Coronary angiography was performed for routine clinical purposes at the managing physician’s discretion within 6 months of MRI in 35 (29%) patients. Given the low threshold for requiring coronary angiography in kidney-pancreas transplant candidates, the high-risk group comprised the majority of patients undergoing angiography (29 of 35, 83%). Significant CAD (≥70% stenosis) was identified in 12 of 29 high-risk and 5 of 6 average-risk patients who underwent angiography.
Among the 23 patients with unrecognized MI by DE-MRI, 12 (56%) underwent coronary angiography and significant CAD was identified in 92% (11/12) of these patients, with multivessel CAD being present in 50% (6/12). The one patient without significant CAD was determined to have diffuse, nonobstructive (<50%) coronary atherosclerosis. Additionally, the location of the infarct by DE-MRI corresponded to the coronary artery perfusion territory of obstructive disease identified by angiography in all 11 patients with significant CAD. Among the 97 patients without unrecognized MI, 23 (24%) underwent coronary angiography and significant CAD was identified in 26% (6 of 23) of these patients, a rate lower than for those with unrecognized MI (92% vs. 26%, P = 0.0003).
A total of 20 patients (17%) underwent coronary revascularization procedures (6 CABG and 14 PCI) during the follow-up period. Revascularization was performed in 43% (n = 10) of the patients with unrecognized MI and in 10% (n = 10) of those without unrecognized MI. No primary end point (death/MI) occurred in the setting of coronary revascularization procedures.
Prognosis
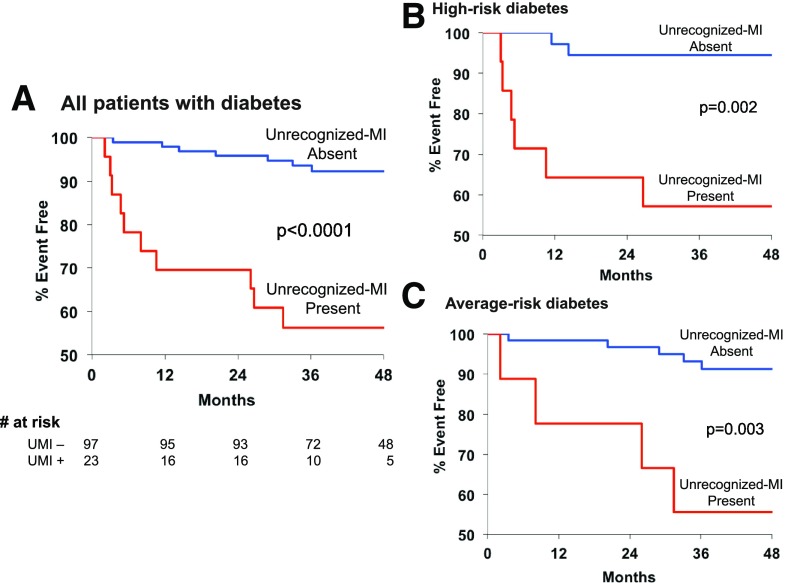
Median follow-up time was 46 months (interquartile range 33, 64), with a total of 460 patient-years of follow-up. Follow-up data were available in all patients. A total of 19 events (12 deaths and 7 MI) occurred in 17 patients, with an event rate of 5.3%/year vs. 2.9%/year for high-risk and average-risk patients, respectively. Figure 2 demonstrates that patients with unrecognized MI had markedly reduced event-free survival compared with those without unrecognized MI (P < 0.0001). Among the 23 with unrecognized MI, 10 (44%) experienced an event. There were six deaths (sudden death in three, heart failure in one, and noncardiac in two) and four nonfatal MIs, representing a total event rate of 16.0%/year and a cardiac event rate of 12.8%/year. Among the 97 without unrecognized MI, 7 (7%) experienced an event. There were six deaths (all noncardiac) and one nonfatal MI, representing a total event rate of 1.8%/year and a cardiac event rate of 0.3%/year.
Figure 2.
Kaplan-Meier estimates of event-free survival (death/MI) for all patients with diabetes (A), high-risk patients with diabetes (B), and average-risk patients with diabetes (C) according to the presence (red line) or absence (blue line) of unrecognized MI. Event-free survival in all patients with diabetes and both subgroups with unrecognized MI was significantly reduced in comparison with those without MI (P < 0.0001, P = 0.002, and P = 0.003, respectively). UMI−, unrecognized MI absent; UMI+, unrecognized MI present.
Figure 2 also demonstrates that the markedly reduced event-free survival among those with unrecognized MI was similar for both cohorts. Over 40% of the high-risk (43%) and average-risk patients (44%) with unrecognized MI experienced an event during the follow-up period. Among high-risk and average-risk patients without unrecognized MI, 6% and 8%, respectively, experienced an event during follow-up (P = 0.002 and P = 0.003 in comparison with those with unrecognized MI by DE-MRI).
Among the baseline characteristics, significant univariable predictors of death and MI were duration of diabetes (HR 1.04 [95% CI 1.00–1.08], P = 0.04), GFR <60 mL/min/1.73 m2 (HR 4.2 [1.2–14.8], P = 0.03), and unrecognized MI by DE-MRI (HR 8.0 [3.0–21.1], P < 0.0001). Stepwise multivariable analysis, however, demonstrated that only the presence of unrecognized MI by DE-MRI was an independent predictor of death and MI, with an eightfold increase in risk compared with those without unrecognized MI (HR 8.0 [3.0–21.1], P < 0.0001). Coronary revascularization (two CABG and four PCI) was performed in six patients who suffered a cardiac event during follow-up. There was no significant difference in the rate of coronary revascularization (prior to death/MI) in patients who experienced clinical events versus those without events.
In the second multivariable modeling approach (using a limited number of prespecified variables), again, only unrecognized MI by DE-MRI was a significant predictor of the primary end point (HR 8.1 [2.9–22.2], P < 0.0001) (see Table 2). The HRs were similar between the high-risk (HR 6.9 [1.3–37.9], P = 0.03) and average-risk patients with diabetes (HR 7.5 [2.0–28.5], P = 0.003).
Table 2.
Prespecified multivariable Cox proportional hazards model predicting adverse events
| Variable | All patients with diabetes |
High-risk diabetes |
Average-risk diabetes |
||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Diabetes type | 0.96 | 0.32–2.86 | 0.94 | — | — | — | — | — | — |
| Framingham risk score | 1.01 | 0.96–1.06 | 0.83 | 1.13 | 1.01–1.26 | 0.03 | 0.98 | 0.93–1.04 | 0.57 |
| LVEF (cine-MRI) | 1.01 | 0.95–1.06 | 0.85 | 0.96 | 0.88–1.05 | 0.35 | 1.04 | 0.96–1.12 | 0.37 |
| Unrecognized MI (DE-MRI) | 8.08 | 2.94–22.22 | <0.0001 | 6.89 | 1.25–37.95 | 0.03 | 7.52 | 1.99–28.51 | 0.003 |
| Model without unrecognized MI | χ2: 1.30; P = 0.73 | χ2: 8.29; P = 0.02 | χ2: 0.87; P = 0.65 | ||||||
| Model with unrecognized MI | χ2: 17.06; P = 0.002 | χ2: 13.81; P = 0.003 | χ2: 8.11; P = 0.04 | ||||||
| Incremental value | χ2: 15.76; P < 0.0001 |
χ2: 5.52; P = 0.02 |
χ2: 7.24; P = 0.007 |
||||||
The addition of unrecognized MI to the model with prespecified clinical variables alone resulted in a significant increase in global χ2 for all patients (from 1.30 to 17.06, P < 0.0001) as well as in both cohorts (Table 2). Moreover, the addition of unrecognized MI resulted in a significant improvement in model discrimination as assessed by the IDI for all patients (0.156, P = 0.001) and both cohorts (high-risk diabetes: IDI = 0.133, P < 0.01; average-risk diabetes: IDI = 0.147, P < 0.05).
Conclusions
We observed that asymptomatic patients with diabetes and without a history of heart disease have a substantial prevalence of unrecognized MI, occurring in 19% of the total study population. Often, these infarcts were small (∼5% of the left ventricle) and not identified by electrocardiography. Despite normal LVEF (61 ± 11%), the presence of a small unrecognized MI conferred an eightfold higher risk for adverse outcome, independent of traditional cardiac risk factors, including age, sex, and Framingham risk score.
In symptomatic patients with CAD, unrecognized MI is identified by DE-MRI at a more than threefold higher rate than by 12-lead electrocardiography (8). Importantly, the presence of unrecognized MI by DE-MRI predicts adverse prognosis, conferring a multifold higher risk for subsequent mortality (8,19). Similarly, in patients with diabetes and symptomatic CAD, unrecognized MI is often detected on DE-MRI and appears to have prognostic significance. Kwong et al. (9) investigated a cohort of symptomatic patients with diabetes undergoing a clinically ordered cardiac MRI. In this referral population, unrecognized MI was identified in 28% of the patients and was the strongest predictor of cardiac outcome in multivariable analysis. Schelbert et al. (19) determined that 21% of the patients with diabetes in the Iceland MI cohort study of older individuals had unrecognized MI on DE-MRI. The patients in this study were elderly (median age 76 years), their symptom status at the time of MRI was unknown, and 36% of the patients with diabetes and unrecognized MI had prior coronary revascularization.
The present investigation differs from the prior studies in three ways: 1) we prospectively enrolled asymptomatic patients with diabetes without known cardiac disease; 2) research MRI scans were performed rather than recruiting patients with clinically ordered scans to reduce referral bias; and 3) we recruited two separate cohorts of patients with diabetes, because it is well recognized that there is heterogeneity of cardiac risk among all patients with diabetes (10,11). Regarding the last point, it is of interest that the prevalence of unrecognized MI differed among our two groups: 28% for the high-risk group with type 1 diabetes and chronic renal insufficiency and 13% for the average-risk group with type 2 diabetes. The greater than twofold difference in rate of unrecognized MI underscores the variance in the prevalence of occult cardiac disease among populations with diabetes and highlights the importance of evaluating separate groups within large and diverse patient populations, such as those with diabetes.
In the current study, it is noteworthy that the presence of unrecognized MI had a similar hazard for adverse outcome independent of group with diabetes (see Table 2). Specifically, the rate of subsequent death or clinical MI during the 4-year follow-up period for patients with unrecognized MI among the two groups with diabetes was nearly identical (high risk 43% and average risk 44%). The implication is not that cardiovascular risk is the same across different groups with diabetes, since we observed that the high-risk group had nearly double the rate of adverse events of the average-risk group. Rather, it is the difference in the prevalence of occult cardiac disease (e.g., unrecognized MI) among groups with diabetes that confers the risk, as there appears to be minimal difference in the hazard associated with unrecognized MI once it occurs.
Furthermore, DE-MRI provided a high level of risk discrimination, as nearly 60% of all patients who experienced an event (10 of 17) and 90% of all who experienced a cardiac event (8 of 9) were those with unrecognized MI on DE-MRI. In comparison, only 38% of patients with diabetes who experienced a cardiac event in the Detection of Ischemia in Asymptomatic Diabetics (DIAD) study had an abnormal nuclear perfusion study (20). Hence, our study data provide compelling evidence that DE-MRI could be a valuable tool in risk stratifying asymptomatic patients with diabetes without known CAD. Additionally, the presence of unrecognized MI on DE-MRI can provide a high-level of cardiac risk stratification for all patients with diabetes regardless of diabetes type and the presence of other cardiac risk factors.
A contrarian opinion to continuing efforts to identify imaging or other biomarkers that refine cardiac risk in asymptomatic patients with diabetes is to adopt an aggressive primary preventive strategy for everyone with diabetes. Ideally, all patients with diabetes should receive guideline-directed medical therapy to lower cardiovascular risk (21). However, prospective randomized clinical trials evaluating the impact of aggressive glycemic and blood pressure control on cardiovascular events have not demonstrated a benefit for patients with type 2 diabetes (22,23). Also, the Stop Atherosclerosis in Native Diabetics Study (SANDS) evaluated the impact of aggressive blood pressure– and cholesterol-lowering therapy on atherosclerosis progression in patients with diabetes (24). Despite the provision of extensive resources, including dedicated study physician and nursing care, less than half of patients in the SANDS trial achieved blood pressure and lipid goals—highlighting the practical limitations of an aggressive preventive therapy strategy for all patients with diabetes. A targeted approach to the selection of intensive medical therapy for cardiovascular risk reduction in patients with diabetes may prove to be more impactful than an unrestricted, universal approach, particularly in asymptomatic patients motivated by the revelation that they have suffered a silent heart attack. In this context, vigorous efforts to target these vulnerable patients with diabetes and unrecognized MI with intensive medical therapy and adjunctive coronary revascularization is a strategy worthy of prospective testing.
Several limitations of our study should be mentioned. Perhaps the most notable limitation is that not all patients with diabetes may be candidates for risk stratification by DE-MRI. Those with advanced nephropathy are unlikely to be candidates given the rare, but serious, occurrence of NSF in association with gadolinium administration (12). In the current study, patients with renal insufficiency were enrolled before the FDA alerts regarding the potential occurrence of NSF associated with gadolinium administration. These patients received gadoteridol, a macrocyclic gadolinium-based contrast agent considered to have a substantially reduced risk of NSF. Indeed, comprehensive reviews and prospective cohort studies of NSF have not demonstrated any unconfounded cases linking gadoteridol exposure with NSF (13). None of the patients in our study developed NSF during the follow-up period. Although cardiac morbidity and mortality rates are extraordinarily elevated for patients with diabetes and renal insufficiency (10), the safety of a risk stratification strategy derived from low-dose (<0.125 mmol/kg) DE-MRI imaging using safer macrocyclic gadolinium contrast agents such as gadoteridol would need to be confirmed by larger investigations in this population before adoption into clinical care. Another limitation is the relatively small size of our study. This limits our ability to determine the prognostic value of DE-MRI with more precision. Although the high HRs for adverse outcome observed for both high- and average-risk groups strongly suggest that DE-MRI could play a valuable role in assessing cardiovascular risk across a wide spectrum of asymptomatic patients with diabetes, the results of this study should be hypothesis generating and need confirmation in larger studies. Other limitations include the fact that not all variables that might influence prognosis were collected (e.g., albuminuria) and the relative low rate of statin usage in our study patients.
In summary, unrecognized MI on DE-MRI imaging is prevalent in asymptomatic patients with diabetes without a history of cardiac disease. Although these infarcts were small, had little impact on systolic function, and were not identified by electrocardiography, their presence conferred a markedly increased risk of death and clinical MI independent of traditional cardiac risk factors.
Article Information
Funding. This research was supported in part by National Institutes of Health grant R01-HL64726 (R.J.K.).
Duality of Interest. R.J. and R.J.K. are inventors on a U.S. patent on DE-MRI, which is owned by Northwestern University. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.D.E., J.F.H., and R.J.K. designed the study, conducted the studies, researched data, and wrote, reviewed, and edited the manuscript. H.K. researched data and reviewed and edited the manuscript. E.W., D.C.L, D.B.K., R.O.B., and R.J. contributed to the discussion and reviewed and edited the manuscript. M.A.P. contributed to the data analysis and interpretation and edited the manuscript. M.D.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is featured in a podcast available at http://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1.International Diabetes Federation IDF Diabetes Atlas. 8th ed Brussels, Belgium, International Diabetes Federation, 2017 [Google Scholar]
- 2.Budoff MJ, Raggi P, Beller GA, et al.; Imaging Council of the American College of Cardiology . Noninvasive cardiovascular risk assessment of the asymptomatic diabetic patient: the Imaging Council of the American College of Cardiology [published correction appears in JACC Cardiovasc Imaging 2016;9:335]. JACC Cardiovasc Imaging 2016;9:176–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenland P, Alpert JS, Beller GA, et al.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines . 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010;122:2748–2764 [DOI] [PubMed] [Google Scholar]
- 4.Muhlestein JB, Lappé DL, Lima JA, et al. Effect of screening for coronary artery disease using CT angiography on mortality and cardiac events in high-risk patients with diabetes: the FACTOR-64 randomized clinical trial. JAMA 2014;312:2234–2243 [DOI] [PubMed] [Google Scholar]
- 5.Simonetti OP, Kim RJ, Fieno DS, et al. An improved MR imaging technique for the visualization of myocardial infarction. Radiology 2001;218:215–223 [DOI] [PubMed] [Google Scholar]
- 6.Kim RJ, Albert TSE, Wible JH, et al.; Gadoversetamide Myocardial Infarction Imaging Investigators . Performance of delayed-enhancement magnetic resonance imaging with gadoversetamide contrast for the detection and assessment of myocardial infarction: an international, multicenter, double-blinded, randomized trial. Circulation 2008;117:629–637 [DOI] [PubMed] [Google Scholar]
- 7.Kwong RY, Chan AK, Brown KA, et al. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation 2006;113:2733–2743 [DOI] [PubMed] [Google Scholar]
- 8.Kim HW, Klem I, Shah DJ, et al. Unrecognized non-Q-wave myocardial infarction: prevalence and prognostic significance in patients with suspected coronary disease. PLoS Med 2009;6:e1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong RY, Sattar H, Wu H, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation 2008;118:1011–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Groop P-H, Thomas MC, Moran JL, et al.; FinnDiane Study Group . The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med 2009;26:142–148 [DOI] [PubMed] [Google Scholar]
- 12.Kanal E, Barkovich AJ, Bell C, et al.; ACR Blue Ribbon Panel on MR Safety . ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol 2007;188:1447–1474 [DOI] [PubMed] [Google Scholar]
- 13.Soulez G, Bloomgarden DC, Rofsky NM, et al. Prospective cohort study of nephrogenic systemic fibrosis in patients with stage 3-5 chronic kidney disease undergoing MRI with injected gadobenate dimeglumine or gadoteridol. AJR Am J Roentgenol 2015;205:469–478 [DOI] [PubMed] [Google Scholar]
- 14.Prineas R, Crow RS. The Minnesota Code Manual of Electrocardiographic Findings. Littleton, MA, John Wright & Sons Ltd, 1982 [Google Scholar]
- 15.Myocardial infarction redefined—a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502–1513 [DOI] [PubMed] [Google Scholar]
- 16.Kadish A, Dyer A, Daubert JP, et al.; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators . Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151–2158 [DOI] [PubMed] [Google Scholar]
- 17.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005;26:1461–1474 [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D’Agostino RB Sr., D’Agostino RB Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 19.Schelbert EB, Cao JJ, Sigurdsson S, et al. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA 2012;308:890–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young LH, Wackers FJT, Chyun DA, et al.; DIAD Investigators . Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA 2009;301:1547–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buse JB, Ginsberg HN, Bakris GL, et al.; American Heart Association; American Diabetes Association . Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2007;115:114–126 [DOI] [PubMed] [Google Scholar]
- 22.Skyler JS, Bergenstal R, Bonow RO, et al.; American Diabetes Association; American College of Cardiology Foundation; American Heart Association . Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association [published correction appears in Circulation 2009;119:e605]. Circulation 2009;119:351–357 [DOI] [PubMed] [Google Scholar]
- 23.Cushman WC, Evans GW, Byington RP, et al.; The Accord Study Group . Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl Med 2010;362:1575–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard BV, Roman MJ, Devereux RB, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial. JAMA 2008;299:1678–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]