Abstract
Background
Few studies have analyzed differences in radiographic parameters and patient-reported outcomes (PROs) between expandable and static interbody devices in patients undergoing minimally invasive transforaminal lumbar interbody fusion (MIS TLIF).
Questions/Purposes
To evaluate differences in radiographic parameters and PROs following MIS TLIF between static and expandable interbody devices.
Methods
Patients undergoing primary, single-level MIS TLIF between 2014 and 2017 were retrospectively identified. Radiographic measurements including lumbar lordosis (LL), segmental lordosis (SL), disc height (DH), and foraminal height (FH) were performed on lateral radiographs before and after MIS TLIF with a static or expandable articulating interbody device. Radiographic outcomes and PROs were compared using paired and unpaired Student’s t test.
Results
Thirty patients received expandable interbody devices and 30 patients received static interbody devices. The expandable device cohort exhibited significantly greater improvement in DH and FH at final follow-up compared with those receiving a static device. Both device cohorts experienced significant improvements in PROs at 6 months post-operatively.
Conclusion
MIS TLIF with an expandable interbody device led to a greater increase of DH and FH than with a static interbody device. Patients undergoing MIS TLIF can expect similar improvements in PROs whether receiving a static or an expandable interbody device. Further studies are required to better understand improvements in clinical outcomes afforded by expandable interbody devices.
Electronic supplementary material
The online version of this article (10.1007/s11420-019-09677-z) contains supplementary material, which is available to authorized users.
Keywords: minimally invasive transforaminal interbody fusion, interbody device, expandable, static, patient-reported outcomes, radiographic outcomes, lordosis, disc height, foraminal height
Introduction
Transforaminal lumbar interbody fusion (TLIF) is an effective option in the treatment of degenerative lumbar spine pathology. Interbody devices have been developed for maximizing mechanical stability, promoting arthrodesis, and allowing for sufficient deformity correction and restoration of disc height.
Minimally invasive (MIS) techniques for interbody fusion procedures, such as MIS TLIF, have provided several benefits over extensile approaches. These benefits include decreased intra-operative blood loss, shorter length of inpatient stay, reduced post-operative opioid use, and greater cost efficacy [1, 9, 19, 20, 22, 23]. The smaller access corridor in MIS TLIF, however, may introduce a size constraint for the interbody device and limit the degree of distraction that can be obtained. In an attempt to overcome this limitation, expandable interbody devices have been developed [4]. Expandable interbody devices feature a collapsed design that permits passage through the narrow corridor of an MIS approach, then expands in situ to larger final dimensions. This unique attribute is thought to facilitate fusion and enhance the restoration of disc height and sagittal balance.
Previous studies have evaluated the biomechanical and radiographic outcomes with expandable devices. While some reports indicate improvement in sagittal balance with expandable cages, others have identified similar radiographic outcomes to static cages [26, 27]. However, few have investigated differences in patient-reported outcomes (PROs) between expandable and static devices for MIS TLIF.
The purpose of this study was to compare radiographic parameters and PROs among MIS TLIF patients treated with either an expandable or static articulating interbody device. The specific aims were to compare (1) the change of measured radiographic parameters—disc height (DH), foraminal height (FH), lumbar lordosis (LL), and segmental lordosis (SL)—from the pre-operative period to 6-month follow-up in patients undergoing MIS TLIF using either a static or an expandable device and (2) to compare the magnitude of improvement in PROs—Oswestry Disability Index (ODI), visual analog scale (VAS) back pain, and VAS leg pain—as a function of implanted device 6 months after the MIS TLIF procedure.
Methods
Institutional review board approval was obtained for this retrospective comparative study (ORA #14051301). Patients who underwent a primary, single-level MIS TLIF for degenerative pathology by a single surgeon from 2014 to 2017 were retrospectively identified. A static articulating interbody device was used in MIS TLIF procedures from 2014 to 2015 and an expandable articulating interbody device from 2016 to 2017. A standard MIS TLIF procedure was performed in all patients through a paramedian approach [18]. The static interbody devices used (T-PAL™, DePuy Synthes, West Chester, PA, USA) were composed of polyether ether ketone (PEEK) and featured a 5° sagittal profile, a 12 × 32-mm footprint, and a 9-to-13-mm height range. The expandable interbody devices used (ALTERA™, Globus Medical, Inc., Audubon, PA, USA) were composed of titanium-coated PEEK and featured an 8° sagittal profile. The expandable device featured the following dimension ranges: width, 8 to 14 mm; length, 26 to 33 mm; and a 4-mm height-based expansion range (8 to 12 mm, 9 to 13 mm, 10 to 14 mm, 12 to 16 mm). Patients with less than 6 months of post-operative follow-up or those who underwent MIS TLIF for nondegenerative pathology, such as trauma or infection, were excluded.
A total of 60 patients who underwent a primary, single-level MIS TLIF were retrospectively identified, including 30 consecutive patients for whom a static articulating interbody device was implanted from 2014 to 2015 and 30 consecutive patients for whom an expandable articulating interbody device was implanted from 2016 to 2017. The average age of the patient sample was 52.8 years and 42 (70%) were male. A majority of patients in each cohort underwent MIS TLIF at the L4 to L5 level (static, N = 19; expandable, N = 19; p = 0.333). There were no differences recorded in body mass index (BMI), smoking status, insurance status, or comorbidity burden between cohorts. Peri-operative variables including operative level, operative time, estimated blood loss, and length of stay were similar between groups (Table 1).
Table 1.
Baseline characteristics by device type
| Static device (N = 30) | Expandable device (N = 30) | p value* | |
|---|---|---|---|
| Age (mean ± SD, years) | 53.5 ± 11.7 | 52.2 ± 12.1 | 0.683 |
| Sex (n) | 0.260 | ||
| Female | 36.7% (11) | 23.3% (7) | |
| Male | 63.3% (19) | 76.7% (23) | |
| Body mass index | 0.606 | ||
| Non-obese (< 30 kg/m2) | 46.7% (14) | 53.3% (16) | |
| Obese (≥ 30 kg/m2) | 53.3% (16) | 46.7% (14) | |
| Smoking status (n) | 0.718 | ||
| Non-smoker | 86.7% (26) | 83.3% (25) | |
| Smoker | 10.0% (4) | 16.7% (5) | |
| Insurance status (n) | |||
| Non-WC | 70.0% (21) | 73.3% (22) | 0.774 |
| WC | 30.0% (9) | 26.7% (8) | |
| CCI (mean ± SD) | 2.0 ± 1.6 | 2.2 ± 1.8 | 0.597 |
| Operative level (n) | 0.333 | ||
| L3L4 | 0.0% (0) | 3.3% (2) | |
| L4L5 | 63.3% (19) | 63.3% (19) | |
| L5S1 | 36.7% (11) | 30.0% (9) | |
| Operative time (mean ± SD, minutes) | 112.4 ± 31.1 | 114.7 ± 28.0 | 0.764 |
| Estimated blood loss (mean ± SD, mL) | 53.2 ± 29.6 | 48.5 ± 28.3 | 0.535 |
| Length of stay (mean ± SD, hours) | 38.2 ± 17.1 | 32.1 ± 15.7 | 0.156 |
SD, standard deviation; WC, worker’s compensation; CCI, Charlson Comorbidity Index
*p values calculated using χ2 analysis (categorical) and Student’s t test and p < 0.001 (continuous)
Patients were stratified by the interbody device used for the fusion procedure (static or expandable). Demographic and peri-operative characteristics were recorded for all patients. Demographic variables included age, sex, BMI, smoking status, insurance status, and Charlson Comorbidity Index (CCI). Peri-operative variables included operative level, operative time, estimated intra-operative blood loss, and length of post-operative stay. PROs were recorded at pre-operative and 6-week, 12-week, and 6-month post-operative visits. PROs included Oswestry Disability Index (ODI), VAS back pain, and VAS leg pain. Post-operative achievement of minimum clinically important difference (MCID) for ODI, VAS back pain, and VAS leg pain scores was determined using cutoffs of 12.8, 1.2, and 1.6, respectively [6]. Arthrodesis was defined as the presence of bony bridging on three sequential coronal and sagittal sections on computed tomographic scan at 1 year post-operatively.
Radiographic measurements were completed using the Opal-RAD Digital Radiology suite® (Konica Minolta Medical Imaging, Garner, NC, USA). Two investigators independently performed the radiographic measurements. Significant disagreements between measured values (> 5°or 3 mm) were re-measured by both reviewers independently. Reviewer measurements were averaged for use in data analyses.
Radiographic measurements were performed on pre-operative and 6-month lateral lumbar spine plain radiographs. Parameters measured included SL, intervertebral DH, and FH at the operative level, as well as global LL. LL was defined as the sagittal Cobb angle between the superior endplate of L1 and the sacral endplate. SL was defined as the sagittal Cobb angle created by the superior endplate of the cephalad vertebrae and the inferior endplate of the caudal vertebrae at the operative level. DH was determined by averaging the distance between the inferior endplate of the cephalad vertebrae and superior endplate of the caudal vertebrae at the anterior border, middle, and posterior border of the vertebral bodies. FH was measured as the distance between the inferior border of the superior pedicle and the superior border of the inferior pedicle.
Statistical Analysis
Statistical analysis was performed using Stata/MP® 13.1 for Mac (StataCorp LP, College Station, TX, USA). Pre-operative demographics and peri-operative characteristics were compared between static and expandable groups using χ2 analysis and Student’s t test for categorical and continuous variables, respectively. Changes in post-operative radiographic measurements and PROs from pre-operative values were determined using paired t tests. Post-operative changes in radiographic parameters and PROs were compared between static and expandable cohorts using Student’s t tests. Achievement of MCID for PROs was compared between cohorts using χ2 analysis. Inter-observer reliability was assessed for radiographic measurements using Pearson correlation coefficients. Strength of association was interpreted using guidelines described by Cohen, with 0.1 ≤ r < 0.3, 0.3 ≤ r < 0.5, and r ≥ 0.5, indicating low, moderate, and strong correlations, respectively [5]. Statistical significance was set at p < 0.05. A post-hoc analysis for determining the minimum number of subjects needed for a power of 90% was calculated to be 21 patients for each cohort, with the goal of detecting a 25% increase in disc height at the 6-month time point.
Results
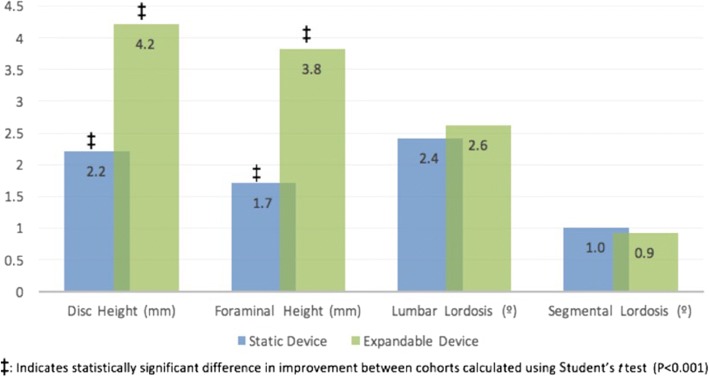
Radiographic parameters were improved in both cohorts following surgery. Both static and expandable device cohorts were identified to have significant improvements in DH, FH, LL, and SL at 6 months post-operatively (p < 0.001 for all), with the exception of SL improvement in the static cohort (p = 0.054) (Table 2). When comparing the degree of post-operative improvement of radiographic parameters between cohorts, expandable devices afforded a greater increase in DH (static + 2.2 mm, expandable + 4.2 mm; p < 0.001) and FH (static + 1.7 mm, expandable + 3.8 mm; p < 0.001; Fig. 1). No differences in post-operative improvements in LL and SL were determined between cohorts (p > 0.05 for each). Strong inter-observer reliability was exhibited for each radiographic measure (r range 0.7798–0.9367, p < 0.05).
Table 2.
Comparison of pre-operative and post-operative radiographic parameters
| Pre-operative | Post-operative (≥ 6 months) | Percent increase | p value* | |
|---|---|---|---|---|
| Static device (mean ± SD) | ||||
| Disc height (mm) | 9.0 ± 1.9 | 11.2 ± 1.8 | 24.4% | < 0.001 |
| Foraminal height (mm) | 20.0 ± 3.1 | 21.6 ± 3.0 | 8.0% | < 0.001 |
| Lumbar lordosis (°) | 58.0 ± 11.5 | 60.4 ± 12.8 | 4.1% | 0.021 |
| Segmental lordosis (°) | 18.1 ± 5.4 | 19.1 ± 5.5 | 5.5% | 0.054 |
| Expandable device (mean ± SD) | ||||
| Disc height (mm) | 9.5 ± 2.1 | 13.7 ± 2.3 | 44.2% | < 0.001 |
| Foraminal height (mm) | 19.8 ± 2.6 | 23.6 ± 2.8 | 19.2% | < 0.001 |
| Lumbar lordosis (°) | 52.4 ± 10.5 | 54.9 ± 10.5 | 4.8% | 0.008 |
| Segmental lordosis (°) | 19.0 ± 6.0 | 20.0 ± 5.7 | 5.3% | 0.048 |
SD, standard deviation
Italic indicates statistical significance
*p values calculated using paired Student’s t tests
Fig. 1.
Post-operative comparison in improvements in radiographic measurements, static and expandable interbody devices.
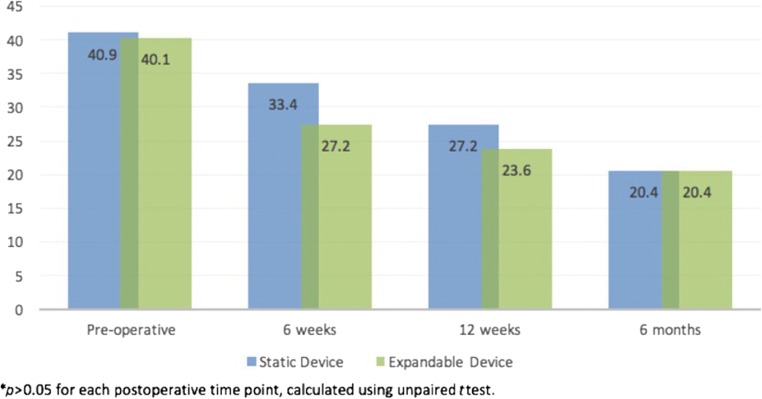
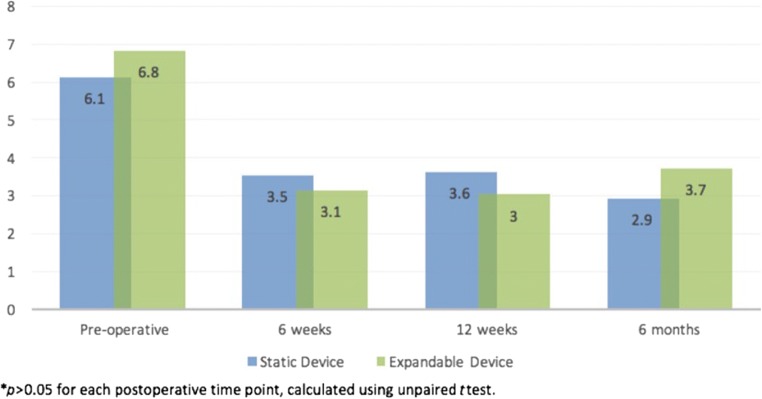
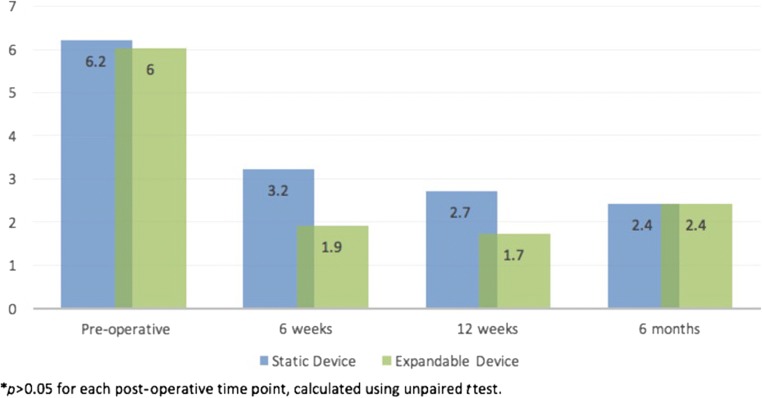
Patients in both cohorts demonstrated significant improvements in ODI, VAS back pain, and VAS leg pain at 6 months post-operatively (p < 0.001 for each; Table 3). When comparing post-operative improvements in PROs between static and expandable cohorts, no differences were identified at the 6-week, 12-week, and 6-month post-operative time points (p > 0.05 for each; Figs. 2, 3, 4). Additionally, similar rates of MCID achievement were identified for ODI and VAS back pain and VAS leg pain among device cohorts (Table 4).
Table 3.
Comparison of pre-operative and post-operative patient-reported outcomes by device type
| Pre-operative (N = 30) | 6-month post-operative (N = 30) | †p value* | |
|---|---|---|---|
| Static device (mean ± SD) | |||
| ODI | 40.9 ± 15.2 | 20.4 ± 15.7 | < 0.001 |
| VAS back pain | 6.1 ± 2.6 | 2.8 ± 2.5 | < 0.001 |
| VAS leg pain | 6.2 ± 2.4 | 2.4 ± 2.7 | < 0.001 |
| Expandable device (mean ± SD) | |||
| ODI | 40.1 ± 16.2 | 20.4 ± 16.8 | < 0.001 |
| VAS back pain | 6.8 ± 2.5 | 3.7 ± 3.0 | < 0.001 |
| VAS leg pain | 6.0 ± 2.7 | 2.9 ± 2.8 | < 0.001 |
ODI, Oswestry Disability Index; VAS, visual analog scale
Italics indicates statistical significance
*p values calculated using paired Student’s t tests
Fig. 2.
Post-operative comparison in improvements in Oswestry Disability Index scores, static and expandable interbody devices.
Fig. 3.
Post-operative comparison in improvements in visual analog scale back pain scores, static and expandable interbody devices.
Fig. 4.
Post-operative comparison in improvement in visual analog scale leg pain scores, static and expandable interbody devices.
Table 4.
Percent of patients who achieved minimum clinically important difference
| Static device (N = 30) | Expandable device (N = 30) | p value* | |
|---|---|---|---|
| ODI (N) | 63.3% (19) | 60.0% (18) | 0.791 |
| VAS back pain (N) | 73.3% (22) | 66.7% (20) | 0.573 |
| VAS leg pain (N) | 70.0% (21) | 70.0% (21) | 1.000 |
VAS, visual analog scale; ODI, Oswestry Disability Index
*p values calculated using χ2 analysis
Discussion
As popularity in MIS approaches to lumbar fusion continues to rise, so has interest in their effectiveness in the correction of spinal radiographic parameters [2, 4, 10, 12, 16, 20, 27]. Few studies exist comparing outcomes between patients undergoing MIS spinal fusion with static vs. expandable interbody devices. This study aimed to identify differences in radiographic and clinical outcomes in patients undergoing MIS TLIF with a static or an expandable interbody device.
Our study is only the second investigation of differences in radiographic and clinical outcomes between static and expandable interbody devices in MIS TLIF. Our study addresses some important limitations in the existing literature, demonstrated by the homogeneity of our cohorts and 100% survey compliance up to the 6-month post-operative time point. However, this study is not without limitations. First, due to its retrospective nature, selection bias may have been present. Second, the change in sagittal parameters that was observed may be directly related to technical aspects of decompression, including facet osteotomy and disruption of spinal ligaments. However, the variation of outcomes was limited by our single-surgeon and single-approach study, with the surgeon placing all cages in the anterior one-third of the disc space. Third, we were unable to comment on long-term fusion rates. However, previous studies have demonstrated fusion rates following TLIF to be greater than 90% [3, 7, 8, 11, 14, 15, 21, 25]. Finally, poor compliance with PRO survey completion at 1- and 2-year follow-up prevented long-term analysis of PROs. Data was assessed up to 6 months post-operatively, when survey completion was highest. Long-term, prospective studies with large patient samples are necessary to make definitive conclusions.
In our study, patients undergoing MIS TLIF with an expandable device experienced significantly greater improvement in DH and FH than patients undergoing MIS TLIF with a static device. Similar to our results, Hawasli et al. observed that patients receiving an expandable device demonstrated a significantly greater improvement in DH (+ 0.84 cm vs. + 0.40 cm; p = 0.02) compared with static devices [10]. In contrast to our study, however, no differences were noted in FH (p > 0.05). Taken together, these findings suggest expandable interbody devices afford patients greater improvement in DH than static devices but may not consistently lead to greater improvements in FH (Table 5).
Table 5.
Comparison of pre-operative and post-operative FH and DH for static vs. expandable devices
| Cage details (mm) | Pre-operative FH (mm) | Post-operative FH (mm) | Pre-operative DH (mm) | Post-operative DH (mm) | |
|---|---|---|---|---|---|
| Static device | |||||
| 1 | 12 × 32 × 9 | 16.8 | 19.0 | 5.9 | 10.5 |
| 2 | 12 × 32 × 8 | 9.7 | 12.2 | 7.7 | 8.2 |
| 3 | 12 × 32 × 10 | 22.8 | 23.8 | 7.1 | 8.4 |
| 4 | 12 × 32 × 9 | 17.5 | 18.7 | 8.1 | 11.8 |
| 5 | 12 × 32 × 12 | 21.7 | 23.0 | 10.4 | 11.2 |
| 6 | 12 × 32 × 10 | 20.7 | 21.8 | 10.0 | 13.3 |
| 7 | 12 × 32 × 9 | 15.5 | 17.9 | 7.8 | 10.0 |
| 8 | 12 × 32 × 11 | 18.7 | 19.8 | 9.8 | 11.7 |
| 9 | 12 × 32 × 13 | 21.2 | 22.3 | 11.6 | 14.1 |
| 10 | 12 × 32 × 12 | 20.0 | 21.7 | 10.8 | 13.6 |
| 11 | 12 × 32 × 12 | 23.2 | 23.8 | 12.1 | 14.2 |
| 12 | 12 × 32 × 11 | 21.6 | 23.0 | 10.7 | 11.6 |
| 13 | 12 × 32 × 11 | 24.7 | 25.8 | 12.2 | 13.3 |
| 14 | 12 × 32 × 9 | 18.3 | 21.2 | 8.1 | 9.9 |
| 15 | 12 × 32 × 11 | 19.5 | 23.3 | 8.9 | 12.4 |
| 16 | 12 × 32 × 12 | 21.6 | 24.3 | 9.7 | 13.3 |
| 17 | 12 × 32 × 13 | 21.2 | 22.5 | 11.5 | 11.3 |
| 18 | 12 × 32 × 11 | 19.1 | 22.0 | 7.3 | 10.4 |
| 19 | 12 × 32 × 12 | 24.1 | 26.7 | 8.2 | 11.3 |
| 20 | 12 × 32 × 12 | 25.0 | 26.3 | 10.2 | 13.5 |
| 21 | 12 × 32 × 8 | 22.0 | 22.6 | 9.5 | 10.7 |
| 22 | 12 × 32 × 9 | 20.2 | 22.6 | 6.3 | 7.6 |
| 23 | 12 × 32 × 10 | 22.3 | 21.9 | 10.7 | 11.0 |
| 24 | 12 × 32 × 10 | 22.3 | 25.0 | 8.5 | 9.5 |
| 25 | 12 × 32 × 10 | 18.9 | 20.2 | 9.7 | 11.2 |
| 26 | 12 × 32 × 10 | 18.2 | 19.3 | 8.9 | 9.7 |
| 27 | 12 × 32 × 9 | 17.8 | 18.5 | 9.0 | 12.4 |
| 28 | 12 × 32 × 9 | 17.4 | 18.3 | 6.3 | 9.5 |
| 29 | 12 × 32 × 9 | 18.7 | 20.0 | 5.3 | 10.2 |
| 30 | 12 × 32 × 10 | 19.0 | 21.9 | 6.9 | 9.4 |
| Expandable device | |||||
| 31 | 14 × 33 | 18.9 | 22.1 | 13.1 | 14.8 |
| 32 | 13 × 32 | 21.0 | 23.6 | 11.3 | 16.3 |
| 33 | 10 × 26 | 17.6 | 23.5 | 6.8 | 13.4 |
| 34 | 10 × 31 | 16.0 | 18.7 | 9.5 | 13.6 |
| 35 | 9 × 31 | 22.1 | 26.8 | 9.3 | 15.5 |
| 36 | 8 × 28 | 24.9 | 29.9 | 9.0 | 13.7 |
| 37 | 11 × 31 | 22.8 | 28.2 | 11.9 | 15.9 |
| 38 | 9 × 31 | 17.8 | 24.4 | 9.8 | 12.8 |
| 39 | 9 × 28 | 20.3 | 24.2 | 11.4 | 13.8 |
| 40 | 10 × 32 | 19.0 | 24.3 | 13.4 | 20.3 |
| 41 | 10 × 31 | 22.0 | 25.9 | 9.8 | 16.9 |
| 42 | 10 × 31 | 17.3 | 18.6 | 8.1 | 13.1 |
| 43 | 10 × 31 | 18.9 | 22.5 | 10.4 | 12.2 |
| 44 | 9 × 31 | 14.2 | 17.5 | 8.4 | 16.1 |
| 45 | 10 × 31 | 19.2 | 24.6 | 9.0 | 12.0 |
| 46 | 10 × 31 | 19.0 | 24.1 | 7.4 | 11.0 |
| 47 | 8 × 26 | 18.9 | 25.0 | 7.2 | 12.5 |
| 48 | 10 × 31 | 18.2 | 22.2 | 4.2 | 9.4 |
| 49 | 10 × 31 | 25.1 | 25.6 | 12.5 | 15.6 |
| 50 | 9 × 28 | 20.0 | 22.7 | 6.5 | 10.5 |
| 51 | 10 × 31 | 21.5 | 25.2 | 9.6 | 14.9 |
| 52 | 9 × 26 | 19.9 | 26.5 | 10.4 | 14.9 |
| 53 | 10 × 31 | 19.6 | 22.3 | 9.8 | 13.7 |
| 54 | 9 × 31 | 16.8 | 20.2 | 5.7 | 9.9 |
| 55 | 10 × 31 | 19.8 | 23.4 | 10.3 | 14.5 |
| 56 | 10 × 26 | 20.0 | 21.8 | 8.7 | 11.5 |
| 57 | 8 × 31 | 17.7 | 21.9 | 8.6 | 12.1 |
| 58 | 10 × 28 | 18.9 | 20.9 | 10.1 | 12.8 |
| 59 | 9 × 26 | 24.9 | 26.8 | 10.6 | 13.2 |
| 60 | 12 × 28 | 23.0 | 24.5 | 11.4 | 14.8 |
FH, foraminal height; DH, disc height
Unlike DH and FH, we found no significant differences in LL and SL between cohorts. This finding is also in keeping with the existing literature [13, 17, 24, 27]. Yee et al. assessed changes in SL and LL following MIS TLIF in a retrospective review of 89 patients [27]. No significant differences were recorded in the improvement of SL (static + 1.0°, expandable + 3.0°; p = 0.41) or LL (static 2°, expandable 5°; p = 0.15). Hawasli et al. did note greater increases in SL in the expandable cohort (5.13° vs. 1.84° for static) but this difference did not reach statistical significance (p = 0.10) [10]. As with Yee et al., there were no significant differences in LL (static + 4.4°, expandable + 4.7°; p = 0.09). These findings are not surprising, however, as the static and expandable devices used in our study both have lordotic sagittal profiles. These findings suggest that improvements in lordosis may be more related to the sagittal profile of the device and less to the intervertebral height expansion provided by this type of expandable interbody device.
In our study, both cohorts exhibited significant improvements in PRO measures, and a majority of patients reached MCID at 6 months post-operatively in each PRO measure. Furthermore, no differences were identified in PRO improvements between interbody device cohorts, despite the expandable device cohort demonstrating significantly greater improvement in DH and FH. These findings contrast with those of Hawasli et al., who reported patients undergoing MIS TLIF with an expandable device experienced a significantly greater improvement in ODI score at final follow-up as compared with patients undergoing MIS TLIF with a static device (expandable − 22.3, static − 13.6; p = 0.02) [10]. The findings of Hawasli et al., however, are limited by inconsistent follow-up times between cohorts. The static device cohort reported a 14.6-month follow-up compared with a 7.1-month follow-up for the expandable cage group (p < 0.01). Our findings suggest that despite greater improvements in radiographic parameters (DH, FH), the use of expandable cages does not lead to greater improvements in clinical outcomes. While there is excellent evidence to suggest that interbody devices can help indirectly decompress the foramen [2, 4, 10, 12, 16, 20, 27], the minimum amount of distraction required to reduce symptoms is unknown. It is possible that both static and expandable cages provide sufficient foraminal distraction to alleviate patient symptoms.
While our study does suggest that there are limited differences in clinical improvement with the use of expandable cages, it is important to note that they offer several technical advantages over static cages, most notably easier sizing and placement with expansion once in the disc space. These advantages are especially important to MIS spinal fusion when surgeons are working through a small access corridor. Additional prospective, long-term studies are required to gain a better understanding of the improvements in clinical outcomes afforded by expandable interbody devices and whether increased segmental height has a protective effect on the development of adjacent-segment degeneration and the need for additional interventions.
In conclusion, MIS TLIF with an expandable interbody device led to a greater increase of DH and FH than with a static interbody device. Patients reported significant improvements in PROs following MIS TLIF in both expandable and static interbody device cohorts; however, no differences in improvement were demonstrated between surgical cohorts. Although the use of expandable interbody devices led to greater increases in DH and FH, it did not translate to superior clinical outcomes. Therefore, patients undergoing MIS TLIF can expect similar improvements in PROs, whether receiving a static or expandable interbody device. Further studies are required to better understand the improvements in clinical outcomes afforded by expandable interbody devices.
Electronic supplementary material
(PDF 1225 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Compliance with Ethical Standards
Conflict of Interest
Benjamin Khechen, BA, Brittany E. Haws, MD, Dil V. Patel, BS, Joon S. Yoo, BA, Jordan A. Guntin, BS, Kaitlyn L. Cardinal, BS, and Sravisht Iyer, MD, declare that they have no conflicts of interest. Kern Singh, MD, reports royalties from Zimmer, Stryker, Pioneer, Lippincott Williams & Wilkins, Thieme, Jaypee Publishing, and Slack Publishing; stock ownership in Avaz Surgical, LLC, and Vital 5, LLC; board membership and a grant from Cervical Spine Research Society; board membership at the International Society for the Advancement of Spine Surgery, American Academy of Orthopaedic Surgeons, Scoliosis Research Society, and Vertebral Column–ISASS, all outside the submitted work.
Human/Animal Rights
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
Informed Consent
Informed consent was waived from all patients for being included in this study.
Required Author Forms:
Disclosure forms provided by the authors are available with the online version of this article.
Footnotes
Level of Evidence: Level III: Therapeutic Study.
References
- 1.Adogwa O, Parker SL, Bydon A, Cheng J, McGirt MJ. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech. 2011;24:479–484. doi: 10.1097/BSD.0b013e3182055cac. [DOI] [PubMed] [Google Scholar]
- 2.Alimi M, Shin B, Macielak M, et al. Expandable polyaryl-ether-ether-ketone spacers for interbody distraction in the lumbar spine. Global Spine J. 2015;5:169–178. doi: 10.1055/s-0035-1552988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine (Phila Pa 1976) 2002;27:S26–31. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 4.Cannestra AF, Peterson MD, Parker SR, Roush TF, Bundy JV. Turner AW. MIS expandable interbody spacers: a literature review and biomechanical comparison of an expandable MIS TLIF with conventional TLIF and ALIF. Spine (Phila Pa 1976) 2016;41(Suppl 8):S44–49. doi: 10.1097/BRS.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 5.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 6.Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J. 2008;8:968–974. doi: 10.1016/j.spinee.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 7.DeBowes RM, Grant BD, Bagby GW, Gallina AM, Sande RD, Ratzlaff MH. Cervical vertebral interbody fusion in the horse: a comparative study of bovine xenografts and autografts supported by stainless steel baskets. Am J Vet Res. 1984;45:191–199. [PubMed] [Google Scholar]
- 8.Glassman SD, Dimar JR, Carreon LY, Campbell MJ, Puno RM, Johnson JR. Initial fusion rates with recombinant human bone morphogenetic protein-2/compression resistant matrix and a hydroxyapatite and tricalcium phosphate/collagen carrier in posterolateral spinal fusion. Spine (Phila Pa 1976) 2005;30:1694–1698. doi: 10.1097/01.brs.0000172157.39513.80. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein CL, Macwan K, Sundararajan K, Rampersaud YR. Perioperative outcomes and adverse events of minimally invasive versus open posterior lumbar fusion: meta-analysis and systematic review. J Neurosurg Spine. 2016;24:416–427. doi: 10.3171/2015.2.SPINE14973. [DOI] [PubMed] [Google Scholar]
- 10.Hawasli AH, Khalifeh JM, Chatrath A, Yarbrough CK, Ray WZ. Minimally invasive transforaminal lumbar interbody fusion with expandable versus static interbody devices: radiographic assessment of sagittal segmental and pelvic parameters. Neurosurg Focus. 2017;43:E10. doi: 10.3171/2017.5.FOCUS17197. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh PC, Koski TR, O’Shaughnessy BA, et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine. 2007;7:379–386. doi: 10.3171/SPI-07/10/379. [DOI] [PubMed] [Google Scholar]
- 12.Kim CW, Doerr TM, Luna IY, et al. Minimally invasive transforaminal lumbar interbody fusion using expandable technology: a clinical and radiographic analysis of 50 patients. World Neurosurg. 2016;90:228–235. doi: 10.1016/j.wneu.2016.02.075. [DOI] [PubMed] [Google Scholar]
- 13.Lee DY, Jung TG, Lee SH. Single-level instrumented mini-open transforaminal lumbar interbody fusion in elderly patients. J Neurosurg Spine. 2008;9:137–144. doi: 10.3171/SPI/2008/9/8/137. [DOI] [PubMed] [Google Scholar]
- 14.Lind M, Bunger C. Factors stimulating bone formation. Eur Spine J. 2001;10(Suppl 2):S102–109. doi: 10.1007/s005860100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig SC, Kowalski JM, Boden SD. Osteoinductive bone graft substitutes. Eur Spine J. 2000;9(Suppl 1):S119–S125. doi: 10.1007/PL00008317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Massie LW, Zakaria HM, Schultz LR, Basheer A, Buraimoh MA, Chang V. Assessment of radiographic and clinical outcomes of an articulating expandable interbody cage in minimally invasive transforaminal lumbar interbody fusion for spondylolisthesis. Neurosurg Focus. 2018;44:E8. doi: 10.3171/2017.10.FOCUS17562. [DOI] [PubMed] [Google Scholar]
- 17.Min SH, Yoo JS. The clinical and radiological outcomes of multilevel minimally invasive transforaminal lumbar interbody fusion. Eur Spine J. 2013;22:1164–1172. doi: 10.1007/s00586-012-2619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mobbs RJ, Phan K, Malham G, Seex K, Rao PJ. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg. 2015;1:2–18. doi: 10.3978/j.issn.2414-469X.2015.10.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parker SL, Adogwa O, Bydon A, Cheng J, McGirt MJ. Cost-effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion for degenerative spondylolisthesis associated low-back and leg pain over two years. World Neurosurg. 2012;78(1–2):178–184. doi: 10.1016/j.wneu.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Peng CW, Yue WM, Poh SY, Yeo W, Tan SB. Clinical and radiological outcomes of minimally invasive versus open transforaminal lumbar interbody fusion. Spine (Phila Pa 1976) 2009;34:1385–1389. doi: 10.1097/BRS.0b013e3181a4e3be. [DOI] [PubMed] [Google Scholar]
- 21.Ray CD. Threaded titanium cages for lumbar interbody fusions. Spine (Phila Pa 1976) 1997;22:667–679. doi: 10.1097/00007632-199703150-00019. [DOI] [PubMed] [Google Scholar]
- 22.Seng C, Siddiqui MA, Wong KP, et al. Five-year outcomes of minimally invasive versus open transforaminal lumbar interbody fusion: a matched-pair comparison study. Spine (Phila Pa 1976) 2013;38:2049–2055. doi: 10.1097/BRS.0b013e3182a8212d. [DOI] [PubMed] [Google Scholar]
- 23.Singh K, Nandyala SV, Marquez-Lara A, et al. A perioperative cost analysis comparing single-level minimally invasive and open transforaminal lumbar interbody fusion. Spine J. 2013;14(8):1694–1701. doi: 10.1016/j.spinee.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 24.Uribe JS, Myhre SL, Youssef JA. Preservation or restoration of segmental and regional spinal lordosis using minimally invasive interbody fusion techniques in degenerative lumbar conditions: a literature review. Spine (Phila Pa 1976) 2016;41(Suppl 8):S50–58. doi: 10.1097/BRS.0000000000001470. [DOI] [PubMed] [Google Scholar]
- 25.Vaccaro AR, Sharan AD, Tuan RS, et al. The use of biologic materials in spinal fusion. Orthopedics. 2001;24:191–197. doi: 10.3928/0147-7447-20010701-29. [DOI] [PubMed] [Google Scholar]
- 26.Wang MY. Improvement of sagittal balance and lumbar lordosis following less invasive adult spinal deformity surgery with expandable cages and percutaneous instrumentation. J Neurosurg Spine. 2013;18:4–12. doi: 10.3171/2012.9.SPINE111081. [DOI] [PubMed] [Google Scholar]
- 27.Yee TJ, Joseph JR, Terman SW, Park P. Expandable vs static cages in transforaminal lumbar interbody fusion: radiographic comparison of segmental and lumbar sagittal angles. Neurosurgery. 2017;81:69–74. doi: 10.1093/neuros/nyw177. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1225 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)