Abstract
Background
Athletes with chronic lower leg pain present a diagnostic challenge for clinicians due to the differential diagnoses that must be considered.
Purpose/Questions
We aimed to review the literature for studies on the diagnosis and management of chronic lower leg pain in athletes.
Methods
A literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). The PubMed, Scopus, and Cochrane library databases were searched, and articles that examined chronic lower leg pain in athletes were considered for review. Two independent reviewers conducted the search utilizing pertinent Boolean operations.
Results
Following two independent database searches, 275 articles were considered for initial review. After the inclusion and exclusion criteria were applied, 88 were included in the final review. These studies show that the most common causes of lower leg pain in athletes include medial tibial stress syndrome, chronic exertional compartment syndrome, tibial stress fractures, nerve entrapments, lower leg tendinopathies, and popliteal artery entrapment syndrome. Less frequently encountered causes include saphenous nerve entrapment and tendinopathy of the popliteus. Conservative management is the mainstay of care for the majority of cases of chronic lower leg pain; however, surgical intervention may be necessary.
Conclusions
Multiple conditions may result in lower leg pain in athletes. A focused clinical history and physical examination supplemented with appropriate imaging studies can guide clinicians in diagnosis and management. We provide a table to aid in the differential diagnosis of chronic leg pain in the athlete.
Electronic supplementary material
The online version of this article (10.1007/s11420-019-09669-z) contains supplementary material, which is available to authorized users.
Keywords: athlete, chronic pain, lower leg, tendinopathy, stress fracture, compartment syndrome, entrapment
Introduction
Chronic lower leg pain often affects amateur and professional athletes [69]. There are numerous causes of pain in this demographic, and symptoms are often ambiguous. It is important for the physician to have a thorough knowledge of the anatomy and biomechanics of the lower leg in order to effectively diagnose and treat these patients.
The lower leg is divided into four compartments: anterior, lateral, superficial posterior, and deep posterior. The anterior compartment contains the tibialis anterior, extensor hallucis longus (EHL), extensor digitorum longus (EDL), peroneus tertius, deep peroneal nerve, anterior tibial artery, and anterior tibial vein (Fig. 1). The lateral compartment contains peroneus longus, peroneus brevis, and the superficial peroneal nerve. The superficial posterior compartment contains the gastrocnemius, the soleus, the plantaris, and the sural nerve [30]. The deep posterior compartment contains the popliteus, flexor hallucis longus, flexor digitorum longus, tibialis posterior, tibial nerve, posterior tibial artery and vein, and peroneal artery and vein [30]. The lower leg provides critical support for stable movement. Performance tests of lower leg function have been shown to accurately predict disability across diverse populations [36].
Fig. 1.
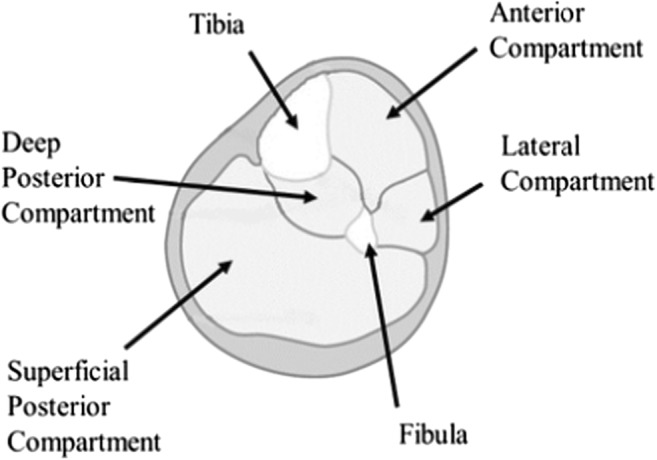
Compartments of the lower leg.
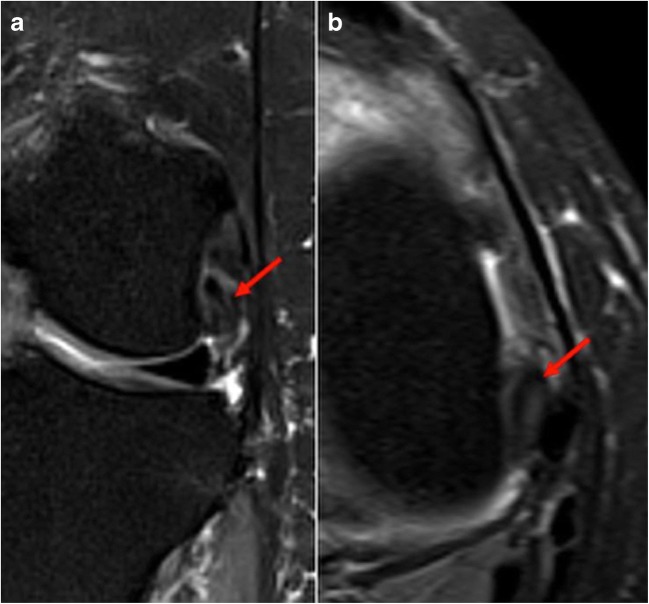
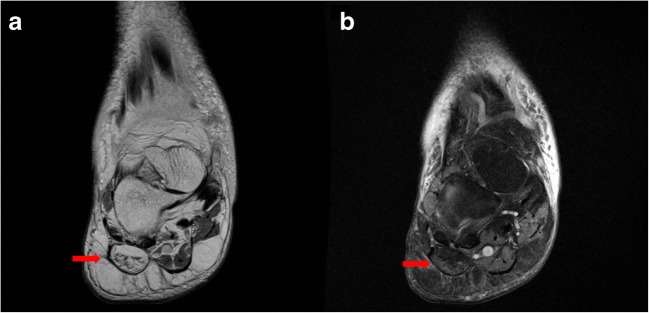
There is a wide range of diagnoses for chronic exercise-induced lower leg pain in athletes. Common etiologies include medial tibial stress syndrome (MTSS), chronic exertional compartment syndrome (CECS), tibial stress fractures, and popliteal artery entrapment syndrome (PAES) [13, 22]. A recent review of athletes presenting with exercise-induced leg pain found that 33% were diagnosed with CECS, 25% with stress fractures, 13% with MTSS, and 10% with nerve entrapment syndromes [13]. Other less frequently considered etiologies including saphenous nerve entrapment and tendinopathy of the popliteus should also be considered (Fig. 2a, b).
Fig. 2.
Coronal (a) and axial (b) T2 fat-suppressed images demonstrate thickening and increased T2 signal of the popliteus near its femoral insertion, indicative of tendinopathy.
The purpose of this literature review was to ask the following questions on chronic lower leg pain in athletes: (1) What are the appropriate imaging modalities to discern the specific etiology of bone, soft tissue, and neurovascular structures in athletes with chronic lower leg pain? (2) What are the optimal symptomatic therapies for alleviating chronic lower leg pain in athletes? (3) When is surgical treatment warranted in athletes with chronic lower leg pain?
Methods
A literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines using a PRISMA checklist [49]. The goal was to identify all studies related to chronic lower extremity pain in athletes. Two reviewers independently conducted the search in October 2018 using the PubMed (MEDLINE), Scopus, and Cochrane library databases. Each search included the following terms and Boolean operations: chronic AND (“lower extremity” OR leg) AND pain AND athlete AND (diagnosis OR treatment). Both reviewers independently filtered returned articles based on title and abstract, and any discrepancies were co-reviewed. The main criteria for selection were articles focused on the etiologies or treatment of chronic lower leg pain in athletes. Articles focusing on the lower back, hip joint, thigh compartment, and knee joint were excluded. Additionally, articles written in a language other than English were excluded. Our search strategy is highlighted in Fig. 3.
Fig. 3.
Flowchart of search methodology.
Results
Following the two independent authors’ search criteria of the databases, 275 articles were considered for initial review. Duplicate articles were excluded. The authors reviewed the titles and abstracts of the potential articles using the inclusion and exclusion criteria to identify a total of 128 for full-text evaluation review. Of these, 88 were found to meet the inclusion/exclusion criteria. All references in each identified article were cross-referenced for inclusion to ensure all available articles were evaluated.
Based on the results of our search, we divided the etiologies of chronic lower leg pain in athletes into bony, soft tissue, and neurovascular categories. We summarize our findings and recommendations in Table 1. This table can be used as a quick aid in the differential diagnosis of chronic lower leg pain in the athlete. We present each etiology in depth below.
Table 1.
A summary of the different etiologies of chronic lower leg pain in the athlete, as well as anatomic location, clinical presentation, diagnostic requirements, imaging, conservative treatment options, and surgical treatment options FHL flexor hallucis longus, NSAIDs non-steroidal anti-inflammatory drugs, MRI magnetic resonance imaging
| Category | Topic | Anatomiclocation | Clinical presentation | Diagnosis | Imaging | Conservative treatment | Surgical management |
|---|---|---|---|---|---|---|---|
| Bony etiologies | Medial tibial stress syndrome | Deep posterior compartment | Diffuse pain along the posteromedial border of the tibia that occurs during exercise | Clinical diagnosis based on history and physical exam; imaging can aide if uncertainty exists | Radiographs used to rule out other etiologies; MRI T2 reveals cortical thickening with periosteal reaction; bone scans reveals diffuse longitudinal uptake | Rest and ice followed by modification of training—reduced intensity, frequency, and/or duration | Fasciotomy of deep posterior compartment with release of the appropriate periosteum |
| Stress fractures of the tibia | Generally posteriomedial cortex or anterior tibial cortex | Localized pain, relieved by rest, in the posteriomedial cortex (running) or anterior tibial cortex (jumping); reproducible and worse with weight-bearing activities | Clinical diagnosis based on history and physical exam and confirmed on plain radiographs. | Radiographs review cortical lucency of the mid-anterior cortex of the tibia; MRI T2 reviews marrow edema with periosteal reaction | Rest and ice along with reduce weight-bearing for 4–6 weeks, and low-impact therapy | Intramedullary nailing and tension band plating for improved osseus union | |
| Achilles tendinopathy | Distal superficial posterior compartment | Posterior ankle pain and occasional swelling between the mid-portion and insertion of the Achilles tendon | Posterior ankle pain worse with initial loading that subsides with continued activity | Transverse and longitudinal ultrasound 2–7 cm from the calcaneal insertion. MRI can provide addition information if necessary | NSAIDs, glucocortical steroids, and/or extracorporeal shockwave therapy | Minimally invasive stripping, percutaneous longitudinal tenotomies, and open tendon augmentation | |
| Peroneal tendinopathy | Lateral compartment | Pain and swelling posterior to the fibula along the lateral wall of the calcaneus. Tenderness on palpation | Clinical diagnosis based on history and physical exam; imaging can aide if uncertainty exists | Ultrasound can determine presence of subluxation and tears. MRI details hypoechoic synovial fluid complex around the os peroneum | Rest, ice, NSAIDs, glucocortical steroids, physical therapy | Synovectomy and debridement | |
| Soft tissue etiologies | FHL tendinopathy | Deep posterior compartment | Pain in the posteromedial aspect of the ankle with dorsiand/ or plantarflexion | Clinical suspicion with MRI visualization of an abrupt cessation of fluid around the FHL tendon at the level of the posterior talus | MRI reveals: posterior capsular thickening and enhancement of muscle belly; fluid around the tendon. MRI + IV gadolinium shows synovial and soft tissue enhancement | Rest, ice, NSAIDs, steroid injection, and/or steroid injection | Surgical tenolysis |
| Tibialis anterior tendinopathy | Anterior compartment | Tenderness along the length of the Tibialis anterior tendon, swelling, and decreased strength during ankle dorsiflexion | Clinical diagnosis aided by ultrasound imaging | Ultrasound demonstrates hypoechoic, thickened, edematous tendon. MRI reveals fluid enhancement around the tendon | Rest, NSAIDs, physical therapy, extracorporeal shockwave therapy, and radiofrequency ablation | Surgical tenosynovectomy, debridement, and/or repair | |
| Tibialis posterior tendon dysfunction | Deep posterior compartment | Pain and swelling along the Tibialis posterior tendon and plantar medial aspect of the foot and ankle | Clinical diagnosis aided by ultrasound imaging | Ultrasound reveals fluid around the tendon, longitudinal hypoechoic rim, target sign on transverse view. MRI reveals increased signal intensity | Rest, NSAIDs, modification to footwear | Tenosynovectomy, debridement, and/or repair | |
| Chronic exertional compartment syndrome | Generally anterior or lateral compartments | Pain on exertion; pain limited to the anterior compartment; tenderness in affected compartment | Compartment pressures greater than 30 mmHg at 1 min or greater than 20 mmHg at 5 min after exertion is diagnostic | Post-exercise T2-weighted MRI reveals increased signal intensity. Infrared spectroscopy measures tissue oxygen saturation to determine levels of ischemia | Rest, ice, NSAIDs, massage, stretching, modifying workout | Surgical release of the fascia of the affected compartment | |
| Popliteal artery entrapment syndrome | Popliteal artery | Leg cramping and pain with strenuous exercise; patient lacks risk factors of peripheral vascular disease | Clinical suspicion confirmed with imaging | MR angiography reveals structural anomalies that compress the popliteal artery | Rest, modify workout regiment; consult vascular surgeon | Myotomy of head of the gastrocnemius; bypass grafting or stent | |
| Neurovascular etiologies | Common peroneal nerve entrapment | Lateral compartment | Foot drop, toe dragging, and frequent tripping. Paresthesia in the distal lateral leg and dorsum of foot | Clinical suspicion confirmed with nerve conduction and/or electromyography | MRI allows for detailed evaluation of the course and morphology of the nerve and surrounding structures | Activity modification, physical therapy, NSAIDs | Surgical decompression of the nerve |
| Saphenous nerve entrapment | Generally within the adductor canal | Knee pain. Worse with knee flexion; pain and numbness along the medial aspect of the leg and foot | Clinical suspicion confirmed with nerve conduction and/or electromyography | MRI allows for detailed evaluation of the course and morphology of the nerve and surrounding structures | Activity modification, physical therapy, NSAIDs | Surgical decompression of the nerve | |
| Lateral plantar nerve entrapment | Generally within the Tarsal tunnel | Neuropathic pain after prolonged activity and tendness along the medial border of the plantar heel | Clinical suspicion confirmed with nerve conduction and/or electromyography | MRI allows for detailed evaluation of the course and morphology of the nerve and surrounding structures | Activity modification, physical therapy, NSAIDs | Surgical decompression of the nerve |
Bony Etiologies
MTSS presents as pain along the posteromedial border of the tibia associated with activity [10, 13, 21, 22, 69]. Studies show that MTSS accounts for 6 to 16% of all running injuries and can represent up to 50% of lower leg injuries in selected populations such as military personnel [46, 92]. While the etiology of MTSS remains unknown, several theories about its origin include underlying periostitis of the tibia; tendinopathy of the tibialis posterior, tibialis anterior, and soleus muscles; periosteal remodeling; stress reactions; and decreased bone density [5]. Intrinsic risk factors that can predispose to MTSS include hyper-pronation, increased body mass index, female sex, hip internal/external rotation, and hyper-plantarflexion [9, 40, 65, 92]. MTSS pain can present as diffuse pain along the posteromedial border of the tibia, whereas pain associated with stress fractures and tendinopathies are usually more focal. MTSS may persist for hours to days, while CECS subsides within minutes of stopping activity [67].
While history and physical examination are usually sufficient for diagnosis, imaging may be useful in cases of unclear and vague presentations of MTSS. Magnetic resonance imaging (MRI) demonstrates superior sensitivity and specificity in early tibial stress injuries [2, 31]. T2-weighted images reveal cortical thickening with periosteal reaction and reactive marrow edema at the site of maximal pain (Fig. 4a, b). Bone scans show a distinct appearance with uptake along the posteromedial tibia on delayed-phase images. Uptake is longitudinally oriented with greater than one third tibial involvement, as opposed to localized uptake in stress fractures [5].
Fig. 4.
Axial (a) and coronal (b) T2-weighted fat-suppressed magnetic resonance imaging scans demonstrate medial tibial stress syndrome (shin splints). Cortical thickening with periosteal reaction and reactive marrow edema along the anterior medial aspect of the proximal to mid-tibial diaphysis, at the site of maximum perceived patient pain is observed (arrows). No acute stress fracture is noted.
During the acute phase of MTSS, rest and ice are recommended, followed by modification of training to reduce intensity, frequency, and duration [45]. Athletes’ return to activity should be gradual and slowed or halted if symptoms return. Surgery is recommended for recurrent cases of MTSS or failure of conservative management and involves a fasciotomy of the deep posterior compartment with release of the appropriate periosteum [45]. While surgery provides excellent symptomatic outcomes in MTSS by reducing pain levels by 72%, a return to previous high-level activity by athletes is not always achieved [37].
Stress fractures occur from repeated tensile and compressive stresses caused by increased load or increased repetition, resulting in a non-displaced fracture [10, 21, 22, 69]. While less than 1% of the general population is affected, studies have reported that the incidence is 1 to 8% in collegiate athletes, 1 to 31% in military recruits, and 13 to 52% among runners [3, 4, 46, 79, 83, 88]. Rapid changes in duration, intensity, or frequency of activity without proper rest increases bone resorption leading to microfractures. Stress fractures commonly occur in three areas of the tibia: the posteromedial cortex (most common), the anterior cortex (high risk of nonunion and fracture propagation due to low healing potential), and the tibial plateau [25]. Runners experience a propensity of stress fractures in the distal one third of the tibia, while jumping and leaping activities common in gymnasts and volleyball and basketball players often cause fractures of the anterior tibial cortex [22]. The most common symptoms are pain on ambulation (81%), focal tenderness (65.9 to 100%), and edema (18 to 44%) at the site of the injury [29, 56].
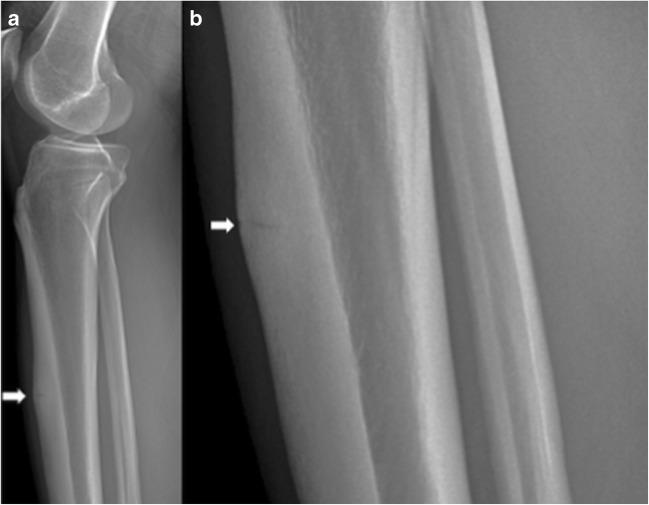
Imaging, though not always necessary, can help confirm the diagnosis. A classic finding on radiographs is the “dreaded black line,” described as a cortical lucency of the mid-anterior cortex of the tibia (Fig. 5a, b) [31]. However, plain radiographs have a low sensitivity (10 to 50%) for detecting stress fractures in the early clinical course. MRI is the imaging modality of choice for diagnosing stress fractures not visible on radiographs, with a sensitivity and specificity of 88 and 99%, respectively [31]. Proton-density and T2-weighted images demonstrate increased signal in the endosteum, reactive soft tissue, and marrow edema with periosteal reaction (Fig. 6) [4, 22, 31, 57].
Fig. 5.
Lateral radiograph of the leg (a) and zoomed area of the lateral radiograph (b) demonstrate horizontal stress fracture within the anterior margin of the tibial diaphysis, with surrounding cortical thickening (arrow) indicative of chronic stress fracture of the anterior margin of the proximal tibial diaphysis.
Fig. 6.
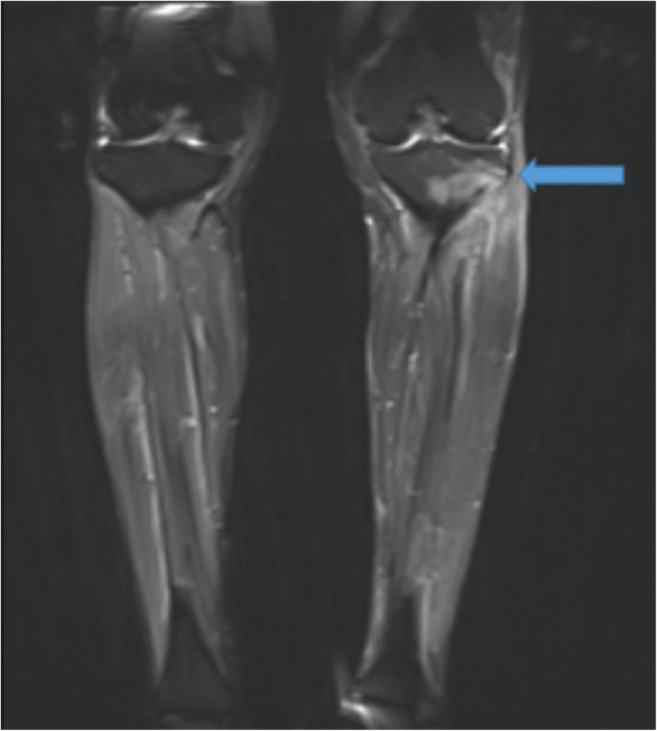
Coronal fluid-suppressed T2-weighted magnetic resonance image demonstrates bone marrow edema pattern in the left lateral tibial plateau with linear hypointensity (arrow) indicative of a stress fracture.
The goal of treatment is to prevent further bone damage, while promoting bone healing. Bone healing requires an increase of osteoblastic relative to osteoclastic activity. Practitioners must consider patient risk factors, location of injury, risk of delayed union or nonunion, and necessity to return to competition. Most tibial stress fractures are located in the posteromedial cortex and heal well with 4 to 8 weeks of conservative management, including rest and low-impact training [22]. Stress fractures of the anterior tibia require longer rest periods and carry a greater risk of conservative treatment failure and need for surgical intervention due to decreased vascularity and increased tensile forces. Regardless of location, surgery should be considered for individuals who fail conservative management resulting in delayed union or nonunion and in athletes who require rapid return to competition. Surgery, including intramedullary nailing and tension band plating for improved osseous union, is associated with high rates of symptom resolution and return to sports in athletes [22, 68, 88]. For instance, in patients with anterior tibial stress fractures, nearly 89% achieve symptom resolution and 95% return to athletic activities following surgical treatment [12].
Soft Tissue Etiologies
Tendinopathy is a broad term used to describe a tendon pathology, often resulting from tendinosis or tendon degeneration [43]. Common sites of tendon pain in athletes include the Achilles and posterior tibialis tendons; uncommon presentation involves the popliteus tendon. In acute tendinopathy, lower leg pain may result from exercise. As the condition progresses, pain may persist with daily activities, occurring at the insertion site or throughout the entire tendon.
Common imaging modalities employed for diagnosis include ultrasound and MRI, with reported sensitivities of 93 and 96%, respectively, in detecting Achilles tendinopathy [44, 73]. Although MRI produces images with greater soft tissue detail and quality, ultrasound is accessible, cost-effective, and better tolerated by patients [11].
Conservative management, including rest, reduced activity, physical therapy, and non-steroidal anti-inflammatory drugs (NSAIDs), is the mainstay of treatment for tendinopathy. Surgical intervention is considered after 3 to 6 months of conservative treatment has failed. Surgery involves excision of fibrotic adhesions and nodules with decompression of the tendon by longitudinal tenotomies [53].
Achilles tendinopathy has an estimated annual incidence of 9% in frequent runners, and it is attributable to numerous extrinsic and intrinsic factors including alterations in training patterns, poor footwear, running on slanted surfaces, and fluoroquinolone/corticosteroid use [16, 52]. Through overuse and repetitive strain, the Achilles tendon degenerates with histopathological changes demonstrating a disorganized collagen structure within the tendon [53]. Tendinopathy of the mid-portion of the Achilles contributes to 55 to 65% of injuries, while 20 to 25% of cases are due to insertional Achilles tendinopathy [52]. Patients present with posterior ankle pain as the cardinal symptom, particularly worse during initial loading and subsiding with continued activity [53]. With tendinopathy progression, swelling of the posterior ankle may often persist, leading to functional impairment [42, 53].
MRI provides extensive information on tendon morphology and stages of degeneration, but ultrasound is the imaging modality of choice based on accessibility, cost, time, ability for contralateral comparison, and patient tolerance [42, 52]. Ultrasound should be performed via a standardized protocol of transverse and longitudinal images along the entire Achilles tendon, with particular attention to the mid-portion, 2 to 7 cm from calcaneal insertion, due to the tenuous blood supply in this region [16]. Imaging findings suggestive of Achilles tendinopathy are characterized by thickening, hypoechogenicity, and neovascularization of the tendon (Fig. 7a, b) [16]. Standard ultrasound criteria include the presence of hypoechogenic focus within the tendon, loss of organized, ribbon-like intratendinous echo structure, or an increase of the anterior diameter of the tendon greater than 50% compared to the contralateral tendon [42].
Fig. 7.
Ultrasound of the right Achilles tendon demonstrates thickening and rounding of the affected portion of the right Achilles tendon to greater than 1 cm, indicative of Achilles tendinopathy. The contralateral normal Achilles tendon is shown for comparison (a), and right Achilles tendon is shown alone (b).
Non-surgical treatment of Achilles tendinopathy includes NSAIDs, steroid injections, and extracorporeal shockwave therapy, often beneficial during early phases of progression [53, 90]. Additionally, eccentric exercises demonstrate beneficial outcomes in patients with mid-portion Achilles tendinopathy [90]. If clinical conditions do not improve after 6 months of conservative modalities, surgery is recommended. Surgical options include endoscopic minimally invasive stripping, percutaneous longitudinal tenotomies, and open procedures involving tendon augmentation or transfer, with technique chosen in part based on degree of tendon degeneration [52]. Surgery provides a valid alterative when conservative management has failed, with an excellent global assessment response in 85% of patients [54]. In long-term studies, 96% of patients achieve complete symptom resolution 7 years after minimally invasive debridement and longitudinal tenotomies [54].
Peroneal tendinopathy, although relatively rare, should be included in the differential diagnosis of runners complaining of lateral ankle pain and instability [34]. The peroneus longus and peroneus brevis tendons play a pivotal role in ankle stability, controlling eversion and plantarflexion of the ankle [61]. While acute tears and dislocation of the peroneus tendons are often observed in younger athletes, tendinopathy is secondary to repetitive mechanical stress [61]. This occurs through direct contact between the peroneus brevis tendon and the lateral malleolus, coupled with compression from the peroneus longus tendon during active contraction [61, 93]. Peroneal tendinopathy often presents with swelling posterior to the fibula, along the lateral wall of the calcaneus [18]. Additionally, athletes complain of tenderness to palpation of the tendon, pain with resisted eversion, passive inversion stretch, and resisted plantarflexion [18].
While ultrasound offers advantages in identifying subluxation and complete peroneal tendon tears, MRI is the most reliable to determine the extent of degenerative and inflammatory changes [18, 77]. Mild thickening of the peroneal tendon with hypoechoic synovial fluid complex and increased vascularity around the os peroneum suggest early progression of the tendinopathy (Fig. 8) [81]. Findings consistent with severe tendinopathy illustrate a boomerang-shaped, irregular, and heterogeneous peroneal tendon on MRI. Circumferential fluid within the common peroneal tendon sheath wider than 3 mm is specific for peroneal tenosynovitis [18]. Tenosynovitis is best visualized via fluid-sensitive MRI sequences, including proton-density and T2-weighted images, which will demonstrate fluid surrounding the tendon [18].
Fig. 8.

Axial proton-density fat-saturation image demonstrates tendinosis and tenosynovitis of the peroneus longus and peroneus brevis, with longitudinal split tear of the peroneus brevis, demonstrating the “Mickey Mouse ears” appearance (arrows) of the peroneus longus, with the split brevis representing the ears.
Treatment of peroneal tendinopathy begins with non-operative management, including NSAIDs, ice, physical therapy, periods of immobilization, and steroid injections into the tendon sheath [75]. Operative treatment, reserved for patients who fail conservative management, often involves synovectomy and debridement via open or endoscopic procedures [75]. Surgery provides significant improvements in functional outcomes, with an average return to sports in 9 months and high levels of patient satisfaction [34].
Flexor hallucis longus (FHL) tendinopathy commonly presents in ballet dancers due to the muscular stress of extreme plantar flexion and metatarsophalangeal (MTP) flexion and extension [71, 76]. The FHL stabilizes the foot, significantly contributing to balance during a heel rise [76]. Since the FHL crosses the ankle and MTP joint, this repetitive action leads to increased muscular demand and subsequent tendinopathy [76]. Patients present with pain in the posteromedial aspect of the ankle during ankle dorsiflexion and plantarflexion [76]. Additionally, the condition may cause a “trigger toe,” resulting in snapping of the toe during transition from plantarflexion to neutral [76].
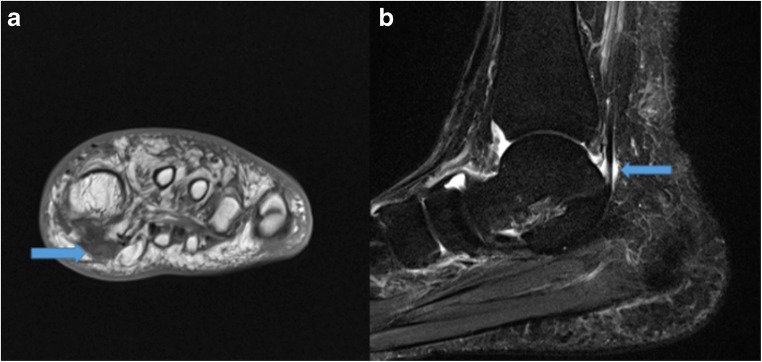
MRI provides superior soft tissue resolution and multiplanar capability to illustrate FHL tendinopathy and commonly associated posterior ankle impingement syndrome (PAIS). PAIS causes limited range of motion, specifically limited ankle plantarflexion as a result of soft tissue or bone impediment [62, 70]. MRI can delineate the posterior capsular thickening and enhancement within the musculotendinous junction of the FHL muscle belly [62]. Additionally, FHL tendinopathy can be diagnosed by visualization of an abrupt cessation of fluid around the FHL tendon at the level of the posterior talus (Fig. 9a, b) [62].
Fig. 9.
Coronal T1-weighted (a) image demonstrates increased signal within an enlarged flexor hallucis longus (FHL) tendon near its insertion (arrow), indicative of FHL tendinopathy. Sagittal T2 short-tau inversion recovery (STIR) (b) image demonstrates irregular, lobulated fluid in the proximal FHL tendon sheath (arrow) indicative of mild tenosynovitis.
Conservative treatment focuses on rest, ice, NSAIDs, physical therapy to mobilize the FHL tendon, and steroid injections. Surgical tenolysis, pursued after 6 months of failed conservative management, provides satisfactory outcomes in 89% of cases and an average period of return in dancers at 16 weeks [70, 71, 76].
While tibialis posterior tendon dysfunction is not common in athletes but rather typically occurs in obese, middle-aged women, it can be a source of disability in runners [8, 15]. The tibialis posterior muscle provides dynamic stabilization of the medial arch and allows for plantarflexion and inversion of the foot [8, 23]. Dysfunction of the tibialis posterior tendon occurs primarily through degenerative changes leading to potential rupture, deformity, and secondary ankle arthritis [1, 47, 84]. Additionally, tibialis posterior tendon dysfunction is the most common etiology of adult-onset pes planus, due to collapse of the medial longitudinal arch leading to a locked, rigid midfoot and hindfoot [8, 23]. Patients present with pain and swelling along the tibialis posterior tendon and plantar medial aspect of the foot and ankle [84]. Disease progression results in decreased ability to perform heel raises or participate in athletics [84].
Although MRI and ultrasonography correlate closely in evaluating structural abnormalities of the tibialis posterior tendon, the latter is preferred as the initial imaging modality based on cost-effectiveness and accessibility [66, 84]. Classic signs of tendinopathy are fluid circumferentially around the tendon, hypoechoic rim on longitudinal sonogram, and a target sign on transverse sonogram [84]. Additionally, the tibialis posterior tendon can demonstrate an irregular contour, longitudinal splits, heterogeneous echogenicity, and empty tibial groove at the medial malleolus in ruptures [84]. While not always necessary, MRI may provide further characterization of the pathology with fluid-sensitive sequences demonstrating increased signal in the tibialis posterior tendon, increased anteroposterior dimension, and increased soft tissue signal intensity surrounding the tendon (Fig. 10a, b) [66].
Fig. 10.
Axial proton-density (a) and proton-density fat-saturation (b) images demonstrate thickening of the posterior tibial tendon with abnormal high-intermediate signal within (arrows), demonstrating posterior tibial tendinosis and reactive tenosynovitis. Additionally, there is intense bone marrow edema pattern within the posteromedial margin of the distal tibia, involving the medial malleolus, reflecting reactive osteitis from the adjacent posterior tibial tendinopathy (red curved arrow).
Patients with mild tendon dysfunction may respond to conservative measures including immobilization, NSAIDs, and footwear modifications [84]. When symptoms persist after 6 months of conservative management, tenosynovectomy, debridement, and repair of tendon tears are indicated and can provide significant improvements in function and pain levels in high-functioning patients under the age of 50 [1, 82, 84].
Tibialis anterior tendinopathy, a rare phenomenon compared to other chronic lower leg pathologies, is typically seen in obese, middle-aged women, but it can also be seen in athletes after alterations in mode, intensity, and duration of activity [39]. The tibialis anterior muscle allows for dorsiflexion and inversion of the foot and controlled plantar flexion following the heel-strike phase of gait [39]. Through recurrent mechanical force, the tibialis anterior tendon degenerates, leading to collagen breakdown and a disorganized matrix of neovascularization and hypercellularity [89]. Patients present with tenderness along the length of the tibialis anterior tendon, local swelling, and decreased strength during ankle dorsiflexion [89]. Passive tension of the tendon via plantar flexion, eversion, abduction, and pronation elicits pain over the medial midfoot [86].
While MRI fluid-sensitive sequences provide excellent visualization of fluid enhancement surrounding the tendon, ultrasound is performed in the majority of cases due to cost-effectiveness and availability (Fig. 11) [86]. Ultrasound will show a hypoechoic, thickened, edematous tendon and may show increased fluid within the tendon sheath [86]. Further progression reveals increased swelling with associated hypervascular change and may progress to longitudinal splits [86]. As bursitis of the distal tibialis anterior tendon can present with tendinopathy, color Doppler ultrasound may reveal hyperemia in the region of the distal bursa [86].
Fig. 11.
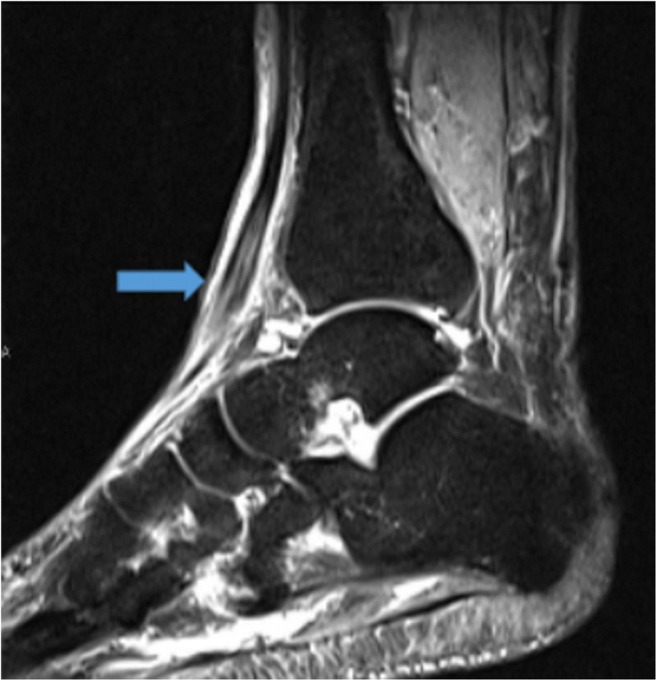
Sagittal T1-weighted turbo inversion recovery magnitude image demonstrates a hyperintense, thickened tibialis anterior tendon (arrow), indicative of tendinopathy.
Conservative management focuses on pain relief through activity limitation, NSAIDs, physical therapy with eccentric strengthening programs, and novel methods including extracorporeal shock wave therapy and radiofrequency ablation [39]. Surgical tenosynovectomy, debridement, and repair are indicated after failure of conservative methods and are effective in restoring function, with an overall satisfaction rate of 92% among patients [19, 89].
Neurovascular Etiologies
CECS is a reversible, exercise-induced compression within the inelastic fascial compartments causing reduction in tissue perfusion leading to functional loss, ischemic pain, and/or neurological symptoms [10, 21, 22, 50, 69]. While the pathophysiology has not been fully elucidated, it is thought that exercise may increase blood flow to contracting muscles, causing expansion and subsequent constriction to surrounding fascia and an overall increase in compartmental pressures [60]. Factors that contribute to CECS are fixed muscular compartments, abnormal muscle swelling with exercise, abnormal fascia, normal muscle hypertrophy in response to resistance training, or dynamic contraction patterns during gait [7]. Military personnel and athletes are most commonly affected and exhibit symptoms of intense pain, burning sensations, or numbness in the lower leg, with resolution of the pain after activity cessation [78]. Clinical suspicion for CECS is indicated if a patient presents with pain induced only by athletic activity, pain limited to the anterior compartment, pain that causes the individual to stop running, and/or tenderness only in the affected compartment [80].
The gold standard for diagnosis of CECS is measurement of pre-exertional and post-exertional intracompartmental pressures with the following criteria: (1) pre-exercise resting pressure of 15 mmHg or greater, (2) 1-min post-exercise pressure greater than 30 mmHg, or (3) 5-min post-exercise pressure of 20 mmHg or greater [63]. Less invasive diagnostic techniques such as MRI and near-infrared spectroscopy (NIRS) have been reported with similar sensitivities to invasive modalities [85]. Fluid-sensitive MRI sequences reveal increased signal intensity after exercise, representing diffuse soft tissue edema consistent with CECS [87]. NIRS allows for measurement of hemoglobin saturation via tissue oxygen saturation (StO2), which reflects the level of local ischemia caused by CECS [85].
Conservative management for CECS includes anti-inflammatory measures such as ice or NSAIDs, as well as stretching, shoe modifications, and gait modifications. Additionally, injections of botulinum toxin have been shown to reduce anterior and lateral intracompartmental pressures by 50% following exercise in a 9-month period [10]. While activity modification has demonstrated the greatest efficacy in treating this condition, many patients remain unwilling to modify their activity levels, leading to high failure rates with conservative management [10]. CECS patients who fail conservative therapy, specifically rest and activity modification, require surgical treatment via open or endoscopic corrective fasciotomies or fasciectomies, typically involving the anterior and lateral compartments [6, 28]. While surgical outcomes are dependent on the degree of compartmental involvement, they remain a valid alternative in providing pain relief and improved function. For instance, following anterolateral fasciotomies, roughly 90% of patients return to equal or higher levels of sports with correlating improvements in satisfaction [32].
Peripheral nerve entrapments of the lower leg are a heterogeneous group of nerve disorders with a variety of etiologies and clinical presentations. Treatment is dependent upon proper identification of the involved nerve and determination of the anatomic location of entrapment [27]. The peroneal nerve fibers derive from the L4 to S1 nerve roots and travel along the lumbosacral plexus. As the common peroneal nerve traverses the superficial head of the peroneus longus muscle, it divides into the deep and superficial peroneal nerves. Common peroneal neuropathy can occur in proximal fibula fractures or in repetitive trauma in runners and cyclists, systemic diseases (diabetes and amyloidosis), or rapid weight loss following bariatric surgery [27]. Patients present with foot drop, toe dragging, foot slapping, and frequent tripping. Paresthesias are noted in the distal lateral leg and dorsal aspect of the foot [27].
Electrodiagnostic studies, specifically nerve conduction velocity (NCV) and electromyography (EMG), localize the site of neuropathy, helping clinicians diagnose entrapment of the common peroneal nerve [55]. Additionally, these studies can be repeated every 3 months to monitor for improvement or deterioration. However, electrodiagnostic modalities are limited in demonstrating the extent of nerve abnormality. MRI allows for detailed evaluation of the course and morphology of the nerve, as well as accurate delineation of surrounding soft tissue and osseous structures that may contribute to nerve entrapment [55]. For instance, in the setting of common peroneal nerve entrapment, short TI inversion recovery MRI sequences demonstrate increased signal intensity and enlargement of the common peroneal nerve at the fibular neck with edematous muscle changes reflecting denervation injury [55].
Conservative management is the mainstay of treatment for peripheral nerve entrapment, consisting of activity modification, biomechanical correction, physical therapy, and NSAIDs. Surgical decompression should be considered 3 to 7 months after injury if conservative management fails [59]. Approximately 80% of patients have improvements of pre-operative motor weakness and pain following decompression of the common peroneal nerve [41].
Popliteal artery entrapment syndrome (PAES) is the compression of the popliteal artery with activity due to repetitive trauma, a congenital deformity of the tendons, or abnormal arterial course. Although rare, with an estimated incidence in the general population between 0.17 and 3.5%, PAES is an important cause of intermittent claudication in young adults [33]. Approximately 85% of patients diagnosed with PAES are male, with 60% of cases in male athletes younger than age 30 [33]. Patients present with calf or foot claudication, with symptoms correlating with intensity of physical activity [48]. MRI and MR angiography provocation and static studies are the gold standard for diagnosis [14, 35, 38, 74]. Axial sequences often reveal structural anomalies causing compression of the popliteal artery. Surgery is recommended in occluded vessels with significant, repetitive symptoms [48, 64]. In diseased arteries, myotomy and arterial reconstruction are performed via bypass grafting, with primary graft patency rates at 1 and 5 years approximating 96 and 92%, respectively [48].
Saphenous nerve entrapment is a rare cause of lower leg pain in athletes. The saphenous nerve, derived from L2 to L4 nerve roots, is the longest cutaneous branch of the femoral nerve, providing sensory innervation to the medial aspect of the leg and foot [58]. As the nerve travels distally in the thigh, entrapment can occur at the adductor canal with referred knee pain via the infrapatellar branch of the saphenous nerve. Saphenous nerve entrapment commonly presents as chronic knee pain in athletes, worsened with knee flexion [22, 58]. Entrapment distal to the infrapatellar branch leads to pain and numbness along the medial aspect of the leg and foot.
Although imaging of the saphenous nerve is challenging due to its small size, identifying the sartorius muscle and femoral artery as anatomical landmarks can aid in localization [17]. Entrapment can result in nerve swelling, which can be seen as increased hypoechogenicity on ultrasound and increased signal intensity on MRI proton-density and T1-weighted sequences [17].
Treatment for saphenous nerve entrapment begins with conservative management, including physical therapy, activity modification, NSAIDs, topical analgesics, and steroid injections. Surgical alternatives, after failure of conservative therapy, include decompression and neurolysis, though studies discussing surgical efficacy and outcomes are limited [91].
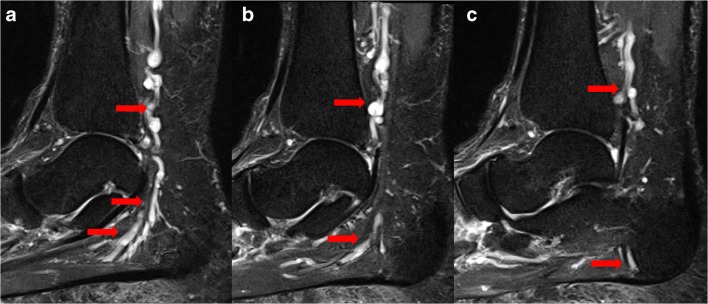
Plantar heel pain is a common presenting complaint to orthopedic surgery practices. While differential diagnoses for neuropathic pain include plantar fasciitis, fat pad inflammation, stress fractures, and inflammatory arthropathy, the entrapment of the first branch of the lateral plantar nerve (inferior calcaneal nerve, also known as Baxter’s nerve) is often missed as an additional cause of pain in this region (Fig. 12) [72]. Also referred to as distal tarsal tunnel syndrome or Baxter’s neuropathy, this condition often presents in runners, ballet dancers, and gymnasts and must be considered in athletes [26]. In these patients, the first branch of the lateral plantar nerve is compressed between the fascia of the abductor hallucis muscle and the medial side of the quadratus plantae muscle within the tarsal tunnel as it travels to innervate the abductor digiti quinti muscle [20, 24]. The isolated nerve entrapment presents as neuropathic pain after prolonged activity, likely due to ischemia of the nerve from engorgement of the accompanying branches of the lateral plantar artery [51]. Patients present with maximal tenderness at the location of nerve entrapment, along the medial border of the plantar heel [51].
Fig. 12.
Sagittal short-tau inversion recovery images (a, b, c) demonstrate varicosities (arrows) in the tarsal tunnel compressing Baxter’s nerve (inferior calcaneal nerve—the first branch of the lateral plantar nerve), resulting in fatty replacement of the abductor digiti quinti.
MRI is useful in identifying lateral plantar nerve entrapment through detection of muscle alterations associated with denervation [72]. In acute and subacute muscle denervation, fluid-sensitive MRI sequences, specifically proton-density and T2-weighted sequences, demonstrate hyperintense signal within the abductor digiti quinti muscle, corresponding to neurogenic muscle edema (Fig. 13a, b) [72]. Chronic denervation leads to atrophy and irreversible fat infiltration that are well depicted on T1-weighted images, demonstrating significant volumetric reduction of the abductor digiti quinti muscle with occupied fatty tissue. Through clear depiction of osseous and soft tissue structures in the heel region, MRI provides highly accurate diagnostic methods to evaluate for lateral plantar nerve entrapment [24].
Fig. 13.
Coronal proton-density (a) and proton-density fat-saturation (b) images demonstrate fatty infiltration of the abductor digiti minimi muscle belly (arrows), indicative of lateral plantar nerve entrapment (Baxter’s neuropathy).
As with management of plantar fasciitis, that of lateral plantar nerve entrapment focuses on conservative methods including rest, activity modification, NSAIDs, stretching exercises, and local steroid injections [51]. Although the literature on long-term outcomes is limited, surgical release of the fascia overlying the abductor hallucis via open or endoscopic decompression is warranted after conservative treatment fails [51].
In summary, lower leg pain in athletes can be caused by multiple conditions, with common etiologies including MTSS, CECS, tibial stress fractures, nerve entrapments, and tendinopathies. Clinicians should also consider rare etiologies including PAES, saphenous nerve entrapment, and tendinopathy of the popliteus. Primary bone tumors should be included as part of the differential diagnosis in athletes experiencing chronic lower leg pain. A focused clinical history and physical examination, with appropriate imaging studies, can help guide clinicians in management.
Electronic supplementary material
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
Compliance with Ethical Standards
Conflict of Interest
Neil Mohile, BS, Jose Perez, MD, Michael Rizzo, MD, Christopher P. Emerson, MS, Greg Foremny, MD, Paul Allegra, MD, Harry Greditzer IV, MD, and Jean Jose, DO, declare that they have no conflicts of interest.
Human/Animal Rights
N/A
Informed Consent
N/A
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Abousayed MM, Tartaglione JP, Rosenbaum AJ, Dipreta JA. Classifications in Brief: Johnson and Strom classification of adult-acquired flatfoot deformity. Clin Orthop Relat Res. 2016;474(2):588–593. doi: 10.1007/s11999-015-4581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aoki Y, Yasuda K, Tohyama H, Ito H, Minami A. Magnetic resonance imaging in stress fractures and shin splints. Clin Orthop Relat Res. 2004;421:260–267. doi: 10.1097/01.blo.0000126333.13806.87. [DOI] [PubMed] [Google Scholar]
- 3.Beck BR. Tibial stress injuries. An aetiological review for the purposes of guiding management. Sports Med. 1998;26(4):265–279. doi: 10.2165/00007256-199826040-00005. [DOI] [PubMed] [Google Scholar]
- 4.Boden BP, Osbahr DC, Jimenez C. Low-risk stress fractures. Am J Sports Med. 2001;29(1):100–111. doi: 10.1177/03635465010290010201. [DOI] [PubMed] [Google Scholar]
- 5.Bonasia DE, Rosso F, Cottino U, Rossi R. Exercise-induced leg pain. Asia Pac J Sports Med Arthrosc Rehabil Technol. 2015;2(3):73–84. doi: 10.1016/j.asmart.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braver RT. Chronic exertional compartment syndrome. Clin Podiatr Med Surg. 2016;33(2):219–233. doi: 10.1016/j.cpm.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Brennan FH, Jr, Kane SF. Diagnosis, treatment options, and rehabilitation of chronic lower leg exertional compartment syndrome. Curr Sports Med Rep. 2003;2(5):247–250. doi: 10.1249/00149619-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Bubra PS, Keighley G, Rateesh S, Carmody D. Posterior tibial tendon dysfunction: an overlooked cause of foot deformity. J Family Med Prim Care. 2015;4(1):26–29. doi: 10.4103/2249-4863.152245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burne SG, Khan KM, Boudville PB, Mallet RJ, Newman PM, Steinman LJ, et al. Risk factors associated with exertional medial tibial pain: a 12 month prospective clinical study. Br J Sports Med. 2004;38(4):441–445. doi: 10.1136/bjsm.2002.004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrus MT, Werner BC, Starman JS, Gwathmey FW, Carson EW, Wilder RP, et al. Chronic leg pain in athletes. Am J Sports Med. 2015;43(6):1538–1547. doi: 10.1177/0363546514545859. [DOI] [PubMed] [Google Scholar]
- 11.Campbell RS, Grainger AJ. Current concepts in imaging of tendinopathy. Clin Radiol. 2001;56(4):253–267. doi: 10.1053/crad.2000.0653. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhry ZS, Raikin SM, Harwood MI, Bishop ME, Ciccotti MG, Hammoud S. Outcomes of surgical treatment for anterior tibial stress fractures in athletes: a systematic review. Am J Sports Med. 2017;1:363546517741137. doi: 10.1177/0363546517741137. [DOI] [PubMed] [Google Scholar]
- 13.Clanton TO. Solcher BW. Chronic leg pain in the athlete. Clin Sports Med. 1994;13(4):743–759. [PubMed] [Google Scholar]
- 14.Collins PS, McDonald PT, Lim RC. Popliteal artery entrapment: an evolving syndrome. J Vasc Surg. 1989;10(5):484–489. doi: 10.1067/mva.1989.14964. [DOI] [PubMed] [Google Scholar]
- 15.Conti SF. Posterior tibial tendon problems in athletes. Orthop Clin North Am. 1994;25(1):109–121. [PubMed] [Google Scholar]
- 16.Counsel P, Comin J, Davenport M, Connell D. Pattern of Fascicular involvement in midportion achilles tendinopathy at ultrasound. Sports Health. 2015;7(5):424–428. doi: 10.1177/1941738115595226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damarey B, Demondion X, Wavreille G, Pansini V, Balbi V, Cotten A. Imaging of the nerves of the knee region. Eur J Radiol. 2013;82(1):27–37. doi: 10.1016/j.ejrad.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 18.Davda K, Malhotra K, O'Donnell P, Singh D, Cullen N. Peroneal tendon disorders. EFORT Open Rev. 2017;2(6):281–292. doi: 10.1302/2058-5241.2.160047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Carvalho Junior AE, Bittar CK, Salomao O, Miranda JB, Ninomiya A, Silva DB. Tendinopathy of the anterior compartment of the ankle. Rev Bras Ortop. 2010;45(2):141–147. doi: 10.1016/S2255-4971(15)30283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Maeseneer M, Madani H, Lenchik L, et al. Normal anatomy and compression areas of nerves of the foot and ankle: us and mr imaging with anatomic correlation. Radiographics. 2015;35(5):1469–1482. doi: 10.1148/rg.2015150028. [DOI] [PubMed] [Google Scholar]
- 21.Detmer DE. Chronic leg pain. Am J Sports Med. 1980;8(2):141–144. doi: 10.1177/036354658000800221. [DOI] [PubMed] [Google Scholar]
- 22.Edwards PH, Jr, Wright ML, Hartman JF. A practical approach for the differential diagnosis of chronic leg pain in the athlete. Am J Sports Med. 2005;33(8):1241–1249. doi: 10.1177/0363546505278305. [DOI] [PubMed] [Google Scholar]
- 23.Erol K, Karahan AY, Kerimoğlu Ü, et al. An important cause of pes planus: the posterior tibial tendon dysfunction. Clin Pract. 2015;5(1):699. doi: 10.4081/cp.2015.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farooki S, Theodorou DJ, Sokoloff RM, Theodorou SJ, Trudell DJ, Resnick D. MRI of the medial and lateral plantar nerves. J Comput Assist Tomogr. 2001;25(3):412–416. doi: 10.1097/00004728-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Feldman JJ, Bowman EN, Phillips BB, Weinlein JC. Tibial stress fractures in athletes. Orthop Clin North Am. 2016;47(4):733–741. doi: 10.1016/j.ocl.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 26.Ferkel E, Davis WH, Ellington JK. Entrapment neuropathies of the foot and ankle. Clin Sports Med. 2015;34(4):791–801. doi: 10.1016/j.csm.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Flanigan RM, DiGiovanni BF. Peripheral nerve entrapments of the lower leg, ankle, and foot. Foot Ankle Clin. 2011;16(2):255–274. doi: 10.1016/j.fcl.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Fraipont MJ, Adamson GJ. Chronic exertional compartment syndrome. J Am Acad Orthop Surg. 2003;11(4):268–276. doi: 10.5435/00124635-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Fredericson M, Jennings F, Beaulieu C, Matheson GO. Stress fractures in athletes. Top Magn Reson Imaging. 2006;17(5):309–325. doi: 10.1097/RMR.0b013e3180421c8c. [DOI] [PubMed] [Google Scholar]
- 30.Frink M, Hildebrand F, Krettek C, Brand J, Hankemeier S. Compartment syndrome of the lower leg and foot. Clin Orthop Relat Res. 2010;468(4):940–950. doi: 10.1007/s11999-009-0891-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaeta M, Minutoli F, Scribano E, et al. CT and MR imaging findings in athletes with early tibial stress injuries: comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235(2):553–561. doi: 10.1148/radiol.2352040406. [DOI] [PubMed] [Google Scholar]
- 32.Gatenby G, Haysom S, Twaddle B, Walsh S. Functional outcomes after the surgical management of isolated anterolateral leg chronic exertional compartment syndrome. Orthop J Sports Med. 2017;5(11):2325967117737020. doi: 10.1177/2325967117737020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gourgiotis S, Aggelakas J, Salemis N, Elias C, Georgiou C. Diagnosis and surgical approach of popliteal artery entrapment syndrome: a retrospective study. Vasc Health Risk Manag. 2008;4(1):83–88. doi: 10.2147/vhrm.2008.04.01.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grasset W, Mercier N, Chaussard C, Carpentier E, Aldridge S, Saragaglia D. The surgical treatment of peroneal tendinopathy (excluding subluxations): a series of 17 patients. J Foot Ankle Surg. 2012;51(1):13–19. doi: 10.1053/j.jfas.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Greenwood LH, Yrizarry JM, Hallett JW., Jr Popliteal artery entrapment: importance of the stress runoff for diagnosis. Cardiovasc Intervent Radiol. 1986;9(2):93–99. doi: 10.1007/BF02577908. [DOI] [PubMed] [Google Scholar]
- 36.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–231. doi: 10.1093/gerona/55.4.m221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haentjens P, Autier P, Collins J, Velkeniers B, Vanderschueren D, Boonen S. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women. A meta-analysis. J Bone Joint Surg Am. 2003;85-A(10):1936–1943. doi: 10.2106/00004623-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Hai Z, Guangrui S, Yuan Z, Zhuodong X, Cheng L, Jingmin L, et al. CT angiography and MRI in patients with popliteal artery entrapment syndrome. AJR Am J Roentgenol. 2008;191(6):1760–1766. doi: 10.2214/AJR.07.4012. [DOI] [PubMed] [Google Scholar]
- 39.Harkin E, Pinzur M, Schiff A. Treatment of acute and chronic tibialis anterior tendon rupture and tendinopathy. Foot Ankle Clin. 2017;22(4):819–831. doi: 10.1016/j.fcl.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Hubbard TJ, Carpenter EM, Cordova ML. Contributing factors to medial tibial stress syndrome: a prospective investigation. Med Sci Sports Exerc. 2009;41(3):490–496. doi: 10.1249/MSS.0b013e31818b98e6. [DOI] [PubMed] [Google Scholar]
- 41.Humphreys DB, Novak CB, Mackinnon SE. Patient outcome after common peroneal nerve decompression. J Neurosurg. 2007;107(2):314–318. doi: 10.3171/JNS-07/08/0314. [DOI] [PubMed] [Google Scholar]
- 42.Hutchison AM, Evans R, Bodger O, et al. What is the best clinical test for Achilles tendinopathy? Foot Ankle Surg. 2013;19(2):112–117. doi: 10.1016/j.fas.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Khan KM, Cook JL, Taunton JE, Bonar F. Overuse tendinosis, not tendinitis. Part 1: a new paradigm for a difficult clinical problem. Phys Sportsmed. 2000;28(5):38–48. doi: 10.3810/psm.2000.05.890. [DOI] [PubMed] [Google Scholar]
- 44.Khoury NJ, el-Khoury GY, Saltzman CL, Kathol MH. Peroneus longus and brevis tendon tears: MR imaging evaluation. Radiology. 1996;200(3):833–841. doi: 10.1148/radiology.200.3.8756941. [DOI] [PubMed] [Google Scholar]
- 45.Korkola M, Amendola A. Exercise-induced leg pain: sifting through a broad differential. Phys Sportsmed. 2001;29(6):35–50. doi: 10.3810/psm.2001.06.825. [DOI] [PubMed] [Google Scholar]
- 46.Kortebein PM, Kaufman KR, Basford JR, Stuart MJ. Medial tibial stress syndrome. Med Sci Sports Exerc. 2000;32(3 Suppl):S27–33. doi: 10.1097/00005768-200003001-00005. [DOI] [PubMed] [Google Scholar]
- 47.Kulig K, Reischl SF, Pomrantz AB, Burnfield JM, Mais-Requejo S, Thordarson DB, et al. Nonsurgical management of posterior tibial tendon dysfunction with orthoses and resistive exercise: a randomized controlled trial. Phys Ther. 2009;89(1):26–37. doi: 10.2522/ptj.20070242. [DOI] [PubMed] [Google Scholar]
- 48.Levien LJ, Veller MG. Popliteal artery entrapment syndrome: more common than previously recognized. J Vasc Surg. 1999;30(4):587–598. doi: 10.1016/s0741-5214(99)70098-4. [DOI] [PubMed] [Google Scholar]
- 49.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu B, Barrazueta G, Ruchelsman DE. Chronic exertional compartment syndrome in athletes. J Hand Surg Am. 2017;42(11):917–923. doi: 10.1016/j.jhsa.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 51.Lui TH. Endoscopic decompression of the first branch of the lateral plantar nerve and release of the plantar aponeurosis for chronic heel pain. Arthrosc Tech. 2016;5(3):e589–594. doi: 10.1016/j.eats.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maffulli N, Via AG, Oliva F. Chronic Achilles tendon disorders: tendinopathy and chronic rupture. Clin Sports Med. 2015;34(4):607–624. doi: 10.1016/j.csm.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Magnan B, Bondi M, Pierantoni S, Samaila E. The pathogenesis of Achilles tendinopathy: a systematic review. Foot Ankle Surg. 2014;20(3):154–159. doi: 10.1016/j.fas.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Maquirriain J. Surgical treatment of chronic achilles tendinopathy: long-term results of the endoscopic technique. J Foot Ankle Surg. 2013;52(4):451–455. doi: 10.1053/j.jfas.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 55.Masakado Y, Kawakami M, Suzuki K, Abe L, Ota T, Kimura A. Clinical neurophysiology in the diagnosis of peroneal nerve palsy. Keio J Med. 2008;57(2):84–89. doi: 10.2302/kjm.57.84. [DOI] [PubMed] [Google Scholar]
- 56.Matheson GO, Clement DB, McKenzie DC, Taunton JE, Lloyd-Smith DR, MacIntyre JG. Stress fractures in athletes. A study of 320 cases. Am J Sports Med. 1987;15(1):46–58. doi: 10.1177/036354658701500107. [DOI] [PubMed] [Google Scholar]
- 57.Meyer SA, Saltzman CL, Albright JP. Stress fractures of the foot and leg. Clin Sports Med. 1993;12(2):395–413. [PubMed] [Google Scholar]
- 58.Morganti CM, McFarland EG, Cosgarea AJ. Saphenous neuritis: a poorly understood cause of medial knee pain. J Am Acad Orthop Surg. 2002;10(2):130–137. doi: 10.5435/00124635-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 59.Morimoto D, Isu T, Kim K, Sugawara A, Yamazaki K, Chiba Y, et al. Microsurgical decompression for peroneal nerve entrapment neuropathy. Neurol Med Chir (Tokyo) 2015;55(8):669–673. doi: 10.2176/nmc.oa.2014-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mubarak SJ, Pedowitz RA, Hargens AR. Compartment syndromes. Curr Orthop. 1989;3:36–40. doi: 10.1016/0268-0890(89)90069-8. [DOI] [PubMed] [Google Scholar]
- 61.Park HJ, Cha SD, Kim HS, et al. Reliability of MRI findings of peroneal tendinopathy in patients with lateral chronic ankle instability. Clin Orthop Surg. 2010;2(4):237–243. doi: 10.4055/cios.2010.2.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peace KA, Hillier JC, Hulme A, Healy JC. MRI features of posterior ankle impingement syndrome in ballet dancers: a review of 25 cases. Clin Radiol. 2004;59(11):1025–1033. doi: 10.1016/j.crad.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 63.Pedowitz RA, Hargens AR, Mubarak SJ, Gershuni DH. Modified criteria for the objective diagnosis of chronic compartment syndrome of the leg. Am J Sports Med. 1990;18(1):35–40. doi: 10.1177/036354659001800106. [DOI] [PubMed] [Google Scholar]
- 64.Pillai J, Levien LJ, Haagensen M, Candy G, Cluver MD, Veller MG. Assessment of the medial head of the gastrocnemius muscle in functional compression of the popliteal artery. J Vasc Surg. 2008;48(5):1189–1196. doi: 10.1016/j.jvs.2008.06.057. [DOI] [PubMed] [Google Scholar]
- 65.Plisky MS, Rauh MJ, Heiderscheit B, Underwood FB, Tank RT. Medial tibial stress syndrome in high school cross-country runners: incidence and risk factors. J Orthop Sports Phys Ther. 2007;37(2):40–47. doi: 10.2519/jospt.2007.2343. [DOI] [PubMed] [Google Scholar]
- 66.Premkumar A, Perry MB, Dwyer AJ, et al. Sonography and MR imaging of posterior tibial tendinopathy. AJR Am J Roentgenol. 2002;178(1):223–232. doi: 10.2214/ajr.178.1.1780223. [DOI] [PubMed] [Google Scholar]
- 67.Reinking MF, Austin TM, Richter RR, Krieger MM. Medial Tibial stress syndrome in active individuals: a systematic review and meta-analysis of risk factors. Sports Health. 2017;9(3):252–261. doi: 10.1177/1941738116673299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rettig AC, Shelbourne KD, McCarroll JR, Bisesi M, Watts J. The natural history and treatment of delayed union stress fractures of the anterior cortex of the tibia. Am J Sports Med. 1988;16(3):250–255. doi: 10.1177/036354658801600309. [DOI] [PubMed] [Google Scholar]
- 69.RFt P, Khanuja HS, Cooley GR. Leg pain in the running athlete. J Am Acad Orthop Surg. 2004;12(6):396–404. doi: 10.5435/00124635-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 70.Rietveld ABMB, Hagemans FMT. Operative treatment of posterior ankle impingement syndrome and flexor hallucis longus tendinopathy in dancers open versus endoscopic approach. J Dance Med Sci. 2018;22(1):11–18. doi: 10.12678/1089-313X.22.1.11. [DOI] [PubMed] [Google Scholar]
- 71.Rietveld ABMB, Hagemans FMT, Haitjema S, Vissers T, Nelissen RGHH. Results of treatment of posterior ankle impingement syndrome and flexor hallucis longus tendinopathy in dancers: a systematic review. J Dance Med Sci. 2018;22(1):19–32. doi: 10.12678/1089-313X.22.1.19. [DOI] [PubMed] [Google Scholar]
- 72.Rodrigues RN, Lopes AA, Torres JM, Mundim MF, Silva LL, Silva BR. Compressive neuropathy of the first branch of the lateral plantar nerve: a study by magnetic resonance imaging. Radiol Bras. 2015;48(6):368–372. doi: 10.1590/0100-3984.2013.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenberg ZS, Cheung Y, Jahss MH, Noto AM, Norman A, Leeds NE. Rupture of posterior tibial tendon: CT and MR imaging with surgical correlation. Radiology. 1988;169(1):229–235. doi: 10.1148/radiology.169.1.3420263. [DOI] [PubMed] [Google Scholar]
- 74.Rosset E, Hartung O, Brunet C, et al. Popliteal artery entrapment syndrome. Anatomic and embryologic bases, diagnostic and therapeutic considerations following a series of 15 cases with a review of the literature. Surg Radiol Anat. 1995;17(2):161–169. doi: 10.1007/BF01627578. [DOI] [PubMed] [Google Scholar]
- 75.Roster B, Michelier P, Giza E. Peroneal tendon disorders. Clin Sports Med. 2015;34(4):625–641. doi: 10.1016/j.csm.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 76.Rowley KM, Jarvis DN, Kurihara T, Chang YJ, Fietzer AL, Kulig K. Toe Flexor strength, flexibility and function and flexor hallucis longus tendon morphology in dancers and non-dancers. Med Probl Perform Art. 2015;30(3):152–156. doi: 10.21091/mppa.2015.3029. [DOI] [PubMed] [Google Scholar]
- 77.Schubert R. MRI of peroneal tendinopathies resulting from trauma or overuse. Br J Radiol. 2013;86(1021):20110750. doi: 10.1259/bjr.20110750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shah SN, Miller BS, Kuhn JE. Chronic exertional compartment syndrome. Am J Orthop (Belle Mead NJ) 2004;33(7):335–341. [PubMed] [Google Scholar]
- 79.Sterling JC, Edelstein DW, Calvo RD, Webb R., 2nd Stress fractures in the athlete. Diagnosis and management. Sports Med. 1992;14(5):336–346. doi: 10.2165/00007256-199214050-00005. [DOI] [PubMed] [Google Scholar]
- 80.Styf JR, Korner LM. Diagnosis of chronic anterior compartment syndrome in the lower leg. Acta Orthop Scand. 1987;58(2):139–144. doi: 10.3109/17453678709146460. [DOI] [PubMed] [Google Scholar]
- 81.Taljanovic MS, Alcala JN, Gimber LH, Rieke JD, Chilvers MM, Latt LD. High-resolution US and MR imaging of peroneal tendon injuries. Radiographics. 2015;35(1):179–199. doi: 10.1148/rg.351130062. [DOI] [PubMed] [Google Scholar]
- 82.Tellisi N, Lobo M, O'Malley M, Kennedy JG, Elliott AJ, Deland JT. Functional outcome after surgical reconstruction of posterior tibial tendon insufficiency in patients under 50 years. Foot Ankle Int. 2008;29(12):1179–1183. doi: 10.3113/FAI.2008.1179. [DOI] [PubMed] [Google Scholar]
- 83.Touliopolous S, Hershman EB. Lower leg pain. Diagnosis and treatment of compartment syndromes and other pain syndromes of the leg. Sports Med. 1999;27(3):193–204. doi: 10.2165/00007256-199927030-00005. [DOI] [PubMed] [Google Scholar]
- 84.Trnka HJ. Dysfunction of the tendon of tibialis posterior. J Bone Joint Surg Br. 2004;86(7):939–946. doi: 10.1302/0301-620x.86b7.15084. [DOI] [PubMed] [Google Scholar]
- 85.van den Brand JG, Nelson T, Verleisdonk EJ, van der Werken C. The diagnostic value of intracompartmental pressure measurement, magnetic resonance imaging, and near-infrared spectroscopy in chronic exertional compartment syndrome: a prospective study in 50 patients. Am J Sports Med. 2005;33(5):699–704. doi: 10.1177/0363546504270565. [DOI] [PubMed] [Google Scholar]
- 86.Varghese A, Bianchi S. Ultrasound of tibialis anterior muscle and tendon: anatomy, technique of examination, normal and pathologic appearance. J Ultrasound. 2014;17(2):113–123. doi: 10.1007/s40477-013-0060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Verleisdonk EJ, van Gils A, van der Werken C. The diagnostic value of MRI scans for the diagnosis of chronic exertional compartment syndrome of the lower leg. Skeletal Radiol. 2001;30(6):321–325. doi: 10.1007/s002560100361. [DOI] [PubMed] [Google Scholar]
- 88.Verma RB, Sherman O. Athletic stress fractures: part I. History, epidemiology, physiology, risk factors, radiography, diagnosis, and treatment. Am J Orthop (Belle Mead NJ) 2001;30(11):798–806. [PubMed] [Google Scholar]
- 89.Waizy H, Goede F, Plaass C, Stukenborg-Colsman C. Tendinopathy of the tibialis anterior tendon: surgical management. Orthopade. 2011;40(7):630–632. doi: 10.1007/s00132-010-1703-4. [DOI] [PubMed] [Google Scholar]
- 90.Wiegerinck JI, Kerkhoffs GM, van Sterkenburg MN, Sierevelt IN, van Dijk CN. Treatment for insertional Achilles tendinopathy: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2013;21(6):1345–1355. doi: 10.1007/s00167-012-2219-8. [DOI] [PubMed] [Google Scholar]
- 91.Worth RM, Kettelkamp DB, Defalque RJ, Duane KU. Saphenous nerve entrapment. A cause of medial knee pain. Am J Sports Med. 1984;12(1):80–81. doi: 10.1177/036354658401200114. [DOI] [PubMed] [Google Scholar]
- 92.Yates B, White S. The incidence and risk factors in the development of medial tibial stress syndrome among naval recruits. Am J Sports Med. 2004;32(3):772–780. doi: 10.1177/0095399703258776. [DOI] [PubMed] [Google Scholar]
- 93.Ziai P, Benca E, Wenzel F, et al. Peroneal tendinosis as a predisposing factor for the acute lateral ankle sprain in runners. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1175–1179. doi: 10.1007/s00167-015-3562-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)
(PDF 1224 kb)