Abstract
Background/Aims
Although acid suppressants are widely used for the prevention or treatment of drug-induced upper gastrointestinal bleeding (GIB), evidence regarding the prevention of anticoagulant-related GIB is scarce. The aim of this study was to evaluate the protective effect of acid suppressants against anticoagulant-related GIB.
Methods
A systematic review was conducted of studies that evaluated the protective effect of acid suppressants against anticoagulant-related GIB found in PubMed, the Cochrane library, Embase, and KoreaMed from the date of database inception to April 2018. Random effect model meta-analyses with sensitivity analyses were conducted. The methodological quality of each included publication was evaluated using the Risk of Bias Assessment Tool for Nonrandomized Studies. Publication bias was assessed.
Results
In total, six nested case-control or cohort studies were identified and analyzed. Proton-pump inhibitors (PPI) had a protective effect against upper GIB in patients on dicumarinics (risk ratio [RR], 0.56; 95% confidence interval [CI], 0.38 to 0.83; I2, 0%); however, the histamine-2 receptor antagonist did not have the same effect (RR, 0.97; 95% CI, 0.52 to 1.81; I2, 0%). Acid suppressants did not have a protective effect against GIB in patients on dabigatran (hazard ratio, 0.78; 95% CI, 0.44 to 1.37; I2, 81.8%).
Conclusions
The protective effect of PPIs against dicumarinics-related upper GIB was clear, while there was no evidence supporting the protective effect of acid suppressants against dabigatran-related GIB. However, in the absence of randomized trials demonstrating a lack of bias, solid conclusions cannot be drawn.
Keywords: Anticoagulants, Acid suppressants, Gastrointestinal hemorrhage
INTRODUCTION
Anticoagulants, including dicumarinics (vitamin K antagonists; warfarin) and direct-acting oral anticoagulants (DOACs), are used to prevent or treat venous thromboembolism and to prevent arterial thromboembolism in patients with high-risk conditions, such as atrial fibrillation and valvular heart disease.1 With the aging process and increasing prevalence of cerebrocardiovascular disease in the general population, the use of anticoagulants has been increasing.2 Use of these drugs has been associated with increased risk of gastrointestinal bleeding (GIB), carrying substantial morbidity and mortality (1% to 13%).3–5 Although DOACs have the advantage of stable bioavailability and lack of required routine serum concentration monitoring, meta-analysis showed no significant difference in the rate of GIB compared to warfarin.4
Acid suppressants, such as proton-pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs), have been used to treat or to prevent drug-induced GIB, especially by nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, in the upper gastrointestinal tract.6 These agents have been recommended in patients with risk factors for gastrointestinal injury, including past history of peptic ulcer or peptic ulcer complications, old age, other comorbidities, high dose-, long-term use of NSAIDs, and co-administration of corticosteroids, antiplatelet agents or anticoagulants.6,7 Although the use of anticoagulants is well known to increase the risk of GIB, data on which risk factors increase GIB and what protective measures are available are lacking.8 The aim of this study was to evaluate the protective effect of acid suppressants on anticoagulant-related GIB.
MATERIALS AND METHODS
This systematic review and meta-analysis fully adhered to the principles of the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) checklist (Supplementary Material 1).9
1. Literature searching strategy
PubMed, the Cochrane library, Embase, and KoreaMed were searched using common keywords associated with acid suppressants and anticoagulant-related GIB (from inception to April 2018) by two independent evaluators (C.S.B. and M.K.J.). Medical Subject Heading or Emtree keywords were selected for searching electronic databases. The abstracts of all identified studies were reviewed to exclude irrelevant publications. Full-text reviews were performed to determine whether the inclusion criteria were satisfied in the remaining studies, and the bibliographies of relevant articles were rigorously reviewed to identify additional studies. Disagreements between the evaluators were resolved by discussion or consultation with a third evaluator (B.W.K.). The detailed searching strategy is described in Supplementary Material 2.
2. Selection criteria
We included studies that met the following criteria: (1) patients: patients on dicumarinics or DOACs or who were newly prescribed these agents; (2) intervention: acid suppressants administration irrespective of primary or secondary prophylaxis goal; (3) comparison: no administration of acid suppressants; (4) outcome: the incidence of GIB; (5) study design: all types including randomized, prospective or retrospective studies; (6) studies of human subjects; (7) publications in English; and (8) full-text publications. Studies that met all of the inclusion criteria were sought and selected. The exclusion criteria were as follows: (1) review articles; (2) guidelines, consensus documents or expert position papers; (3) comments, letters, brief reports, proceedings, or protocol studies; (4) case reports; (5) publications with incomplete data; and (6) meta-analysis articles. Studies meeting at least 1 of the exclusion criteria were excluded from this analysis.
3. Methodological quality
The methodological quality of the included publications was assessed using the Risk of Bias Assessment tool for Non-randomized Studies (RoBANS).10 The RoBANS tool contains six domains, including the selection of participants, confounding variables, measurement of intervention (exposure), blinding of outcome assessment, incomplete outcome data, and selective outcome reporting.10 RoBANS is a validated tool that is reliable and feasible for the assessment of methodological quality of non-randomized studies. Review Manager version 5.3.3 (RevMan for Windows 7; the Nordic Cochrane Centre, Copenhagen, Denmark) was used to generate the summary of RoBANS results. Two of the evaluators (C.S.B. and M.K.J.) independently assessed the methodological qualities of all the included studies, and any disagreements between the evaluators were resolved by discussion or consultation with a third evaluator (B.W.K.).
4. Primary and modifier-based analyses
Two evaluators (C.S.B. and M.K.J.) independently used the same data fill-in form to collect the primary summary outcome and modifiers in each study. The outcome was the effect of acid suppressants on anticoagulant-related GIB. The common effect size extracted from each study was either risk ratios (RRs) or hazard ratios (HRs). We also performed sensitivity analyses to identify the source of heterogeneity based on the modifiers identified during the systematic review and to confirm the robustness of the main result.
5. Handling dependence from multiple outcomes
The independent study was the primary unit of analysis in this meta-analysis. Therefore, for the studies that reported multiple outcomes, an approach of selecting a representative single outcome to include based on the focus of the meta-analysis was used for resolving dependence.11,12
6. Statistics
Comprehensive Meta-Analysis Software version 3 (Biostat; Borenstein M, Hedges L, Higgins J and Rothstein H., Englewood, NJ, USA) was used for this meta-analysis. We extracted the adjusted RRs or HRs with 95% confidence intervals (CIs) from the original articles to evaluate the effect of acid suppressants on anticoagulant-related GIB whenever possible. Heterogeneity was determined using the I2 test developed by Higgins, which measures the percentage of total variation across studies.13 I2 was calculated as follows: I2 (%)=100×(Q−df)/Q, where Q is Cochrane’s heterogeneity statistic, and df signifies the degrees of freedom. Negative values for I2 were set to zero, and an I2 value over 50% was considered to be of substantial heterogeneity (range, 0% to 100%).14 Pooled-effect sizes with 95% CIs were calculated using a random effects model and the method of DerSimonian and Laird.15 Significance was set at p=0.05. Publication bias was evaluated using Egger’s test of the intercept and Begg and Mazumdar’s rank correlation test.16,17
RESULTS
1. Identification of relevant studies
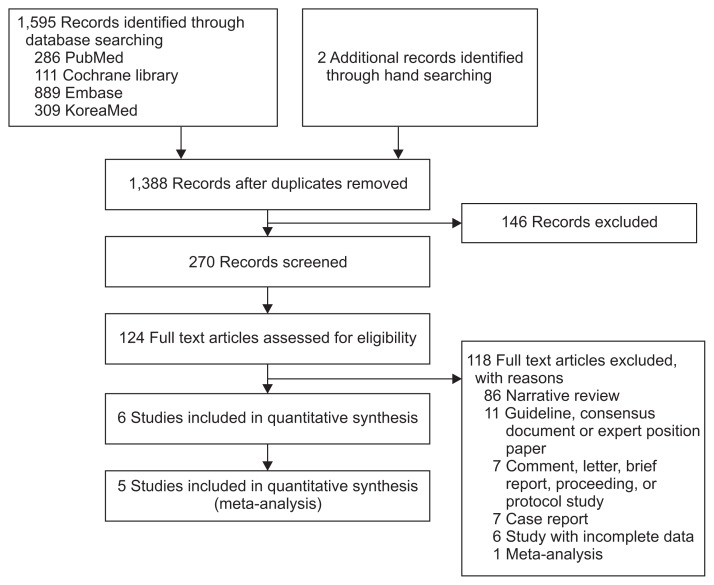
Fig. 1 shows how relevant studies were identified. In total, 1,597 articles were identified by the search of four databases and additional hand searching. In all, 209 were duplicate studies, and an additional 1,264 studies were excluded during the initial screening via a review of titles and abstracts. The full texts of the remaining 124 studies were then thoroughly reviewed. Among these studies, 118 articles were excluded from the final analysis. The reasons for study exclusion during the final review were as follows: narrative review article (n=86), guideline, consensus document or expert position paper (n=11), comment, letter, brief report, proceeding or protocol study (n=7), case report (n=7), incomplete data (n=6), and meta-analysis (n=1). The remaining 6 studies5,18–22 were included in the qualitative synthesis.
Fig. 1.
Flow diagram of the identification of relevant studies.
2. Characteristics of included studies
In the six nested case-control or cohort studies, we identified a total of 31,645 patients (4,893 upper GIB cases vs 26,752 controls) from three nested case-control studies18–20 and 123,504 patients newly prescribed anticoagulants from three retrospective cohort studies.5,21,22 Five studies5,19–22 performed analyses based on cohorts from a database; however, a study by Lanas et al.18 enrolled consecutive patients with upper GIB and controls in multicenter hospitals. The included studies were published between 2007 and 2016. Only one study was conducted in Asia,21 whereas the remaining studies were conducted in Europe18–20 or in the United States.5,22 The age of the enrolled population ranged from 40 to 84 years. The anticoagulants used in each study were dicumarinics in four studies18–20,22 and dabigatran in two studies5,21 (Table 1).
Table 1.
Clinical Data from the Included Studies Regarding the Association between Anticoagulant-Related GIB and Acid Suppressants
| Study (year) | Outcome | Acid suppressants | Anticoagulants | Outcome measure | Study format | Patients and controls | Age, mean±SD, yr | Nationality |
|---|---|---|---|---|---|---|---|---|
| Lanas et al. (2007)18 | UGIB | PPI | Dicumarinics | RR 0.67 (95% CI, 0.37–1.21) | Case-control, multicenter (prospective case ascertainment and retrospective data collection from 2001 to 2004) (adjusted for age, sex, ulcer history, nitrate, gastroprotective drug, and aspirin use) | 2,777 Consecutive patients with UGIB (confirmed by endoscopy) with 5,532 controls | Patients: 61.4±16.1, controls: 61.2±16.2 | Spain |
| UGIB | H2RA | Dicumarinics | RR 0.88 (95% CI, 0.32–2.45) | |||||
| Massó González and García Rodríguez (2008)19 | Recurrent UGIB | PPI | Dicumarinics | RR 0.51 (95% CI, 0.26–0.99) | Prospective cohort study with nested case-control analysis using The Health Improvement Network (THIN) database in the UK, mean follow up 3 years (adjusted for age, gender, length of follow-up and calendar year) | 1,287 Patients (67 with recurrent UGIB) including 215 PPI non-users (assigned to the control group in this meta-analysis) | Unknown | The authors were Spanish, but the data from UK were analyzed |
| H2RA | Dicumarinics | RR 1.68 (95% CI, 0.51–5.50) | ||||||
| Lin et al. (2011)20 | UGIB | PPI for more than 1 month | Dicumarinics | RR 0.48 (95% CI, 0.22–1.04) | Population-based, nested, case-control study using primary care database in the UK (THIN) from 2000 to 2007 (adjusted for age, sex, calendar year, primary care practitioners visits, prior hospitalizations, prior history of peptic ulcer disease, smoking status, alcohol consumption, use of steroids, selective serotonin reuptake inhibitors, oral anticoagulants, NSAIDs, low-dose aspirin, nitrates, H2RAs, and PPIs) | 2,049 UGIB, 20,000 controls | Range: 40–84 | The authors were Spanish, but the data from UK were analyzed |
| UGIB | H2RA for more than 1 month | Warfarin | RR 0.69 (95% CI, 0.24–2.02) | |||||
| Chan et al. (2015)21 | GIB | Overall acid suppressant including PPI and H2RA | Dabigatran | HR 0.57 (95% CI, 0.38–0.85) | Retrospective cohort study using population-wide database managed by the Hong Kong Hospital Authority from 2010 to 2013 (adjusted for patient characteristics, comorbidities, and concurrent medications) | 5,041 Patients who newly prescribed dabigatran including 1,640 acid suppressants non-users (assigned to the control group in this meta-analysis) | 72.0±10.9 | Hong Kong |
| GIB | PPI | Dabigatran | HR 0.57 (95% CI, 0.34–0.97) | |||||
| GIB | H2RA | Dabigatran | HR 0.66 (95% CI, 0.43–1.01) | |||||
| GIB | Both PPI and H2RA | Dabigatran | HR 0.19 (95% CI, 0.07–0.49) | |||||
| Lauffenburger et al. (2015)5 | GIB | Gastrointestinal protective agents | Dabigatran | HR 1.02 (95% CI, 0.78–1.35) | Retrospective cohort study using nationwide United States commercial insurance database from 2010 to 2012 (adjusted for age, sex, region, comorbidities, current medications) | 21,033 Patients with nonvalvular atrial fibrillation who initiated dabigatran including 18,773 acid suppressants non-users (assigned to the control group in this meta-analysis) | 67.5±12.4 | US |
| Ray et al. (2016)22 | UGIB | PPI | Dicumarinics | HR 0.76 (95% CI, 0.63–0.91) | Retrospective cohort study using Tennessee Medicaid and the 5% National Medicare Sample (adjusted for the study population (Medicaid or Medicare), demographic characteristics, warfarin indication and treatment duration, gastrointestinal disease, risk factors for warfarin-related bleeding, medications thought to affect bleeding risk, cardiovascular comorbidity, alcohol abuse, liver disease, and recent medical care utilization) | 97,430 Newly prescribed warfarin treatment with 75,720 person-years of active treatment (52,442 from Tennessee Medicaid & 23,278 from the Medicare sample) including 52,407 person-years of acid suppressants non-users (assigned to the control group in this meta-analysis) | PPI therapy: mean 68.2, no PPI therapy: mean 67.7 | US |
GIB, gastrointestinal bleeding; UGIB, upper GIB; PPI, proton-pump inhibitor; RR, risk ratio; CI, confidence interval; H2RA, histamine-2 receptor antagonist; NSAID, nonsteroidal anti-inflammatory; HR, hazard ratio.
Although six studies were finally sought, the effect size presented by each study was different. Three nested case-control studies18–20 presented outcomes using adjusted RRs, and three retrospective cohort studies5,21,22 presented outcomes using adjusted HRs. The site and type of primary outcome of each study was also categorized by an upper GIB in three studies,18,20,22 overall GIB in two studies,5,21 and recurrent upper GIB in one study.19 The type of intervention (acid suppressants) was also categorized based on the type of acid suppressants (either PPI or H2RA) described in each study; however, the study by Lauffenburger et al.5 presented outcome values by gastrointestinal protective agents that could not be classified by subgroups (PPI or H2RA) used in the remaining studies.
Therefore, the main analysis could be categorized based on the effect size (either using HRs or RRs). The site of primary outcome of studies presenting adjusted RRs was upper GIB in two studies18,20 and recurrent upper GIB in one study19 and the type of anticoagulants of studies presenting adjusted RRs was dicumarinics in all throughout the studies.18–20 Consequently, as the first-step of analysis, meta-analysis of three nested case-control studies using adjusted RRs reflecting the effect of PPI or H2RA on dicumarinics-related upper GIB was performed. Thereafter, sensitivity meta-analysis excluding study focused on the recurrent upper GIB19 was performed to confirm the robustness of the main analysis. Another meta-analysis of three retrospective cohort studies using adjusted HRs reflecting the effect of acid suppressants on dabigatran-related GIB was performed. Study by Ray et al.22 was included in the systematic review, however, due to the difference in effect size compared to the remaining studies (dicumarinics-associated upper GIB was the primary outcome, however, HR was the effect size, not RR), this study could not be included in the meta-analysis (Fig. 1). The clinical characteristics of the included studies are shown in Table 1.
3. PPI or H2RA on dicumarinics-related upper GIB
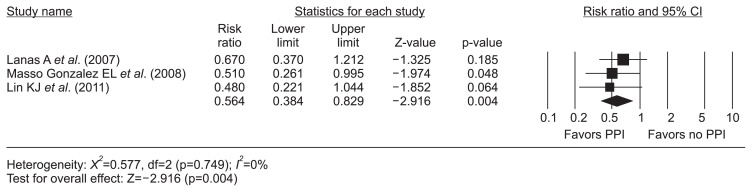
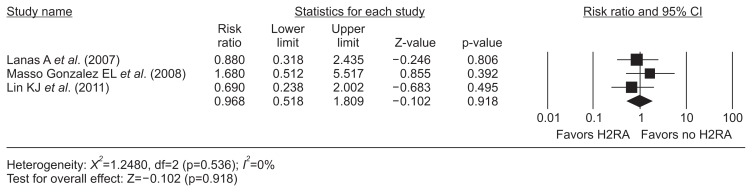
The meta-analysis of three nested case-control studies18–20 exhibited a protective effect of PPIs (RR, 0.56; 95% CI, 0.38 to 0.83; I2, 0%) on dicumarinics-related upper GIB (Fig. 2). However, the H2RA did not show this efficacy (RR, 0.97; 95% CI, 0.52 to 1.81; I2, 0%) (Fig. 3). There was no evidence of methodological heterogeneity.
Fig. 2.
PPI and dicumarinics-related upper gastrointestinal bleeding. The size of each square is proportional to the study’s weight. The diamond indicates the summary estimate from the pooled studies (random effect model).
PPI, proton-pump inhibitor; CI, confidence interval.
Fig. 3.
H2RA and dicumarinics-related upper gastrointestinal bleeding. The size of each square is proportional to the study’s weight. The diamond indicates the summary estimate from the pooled studies (random effect model).
H2RA, histamin-2 receptor antagonist; CI, confidence interval.
4. Sensitivity analysis according to the modifier
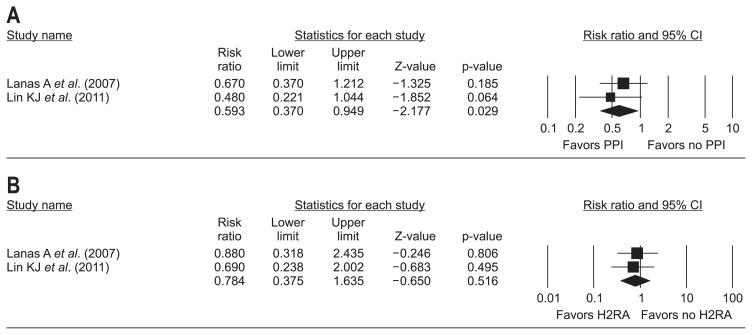
Modifier found during systematic review was difference in type of primary outcome of each study. Only study by Massó González and García Rodríguez (2008)19 focused on the recurrent upper GIB, whereas remaining two studies in main analysis18,20 focused on the upper GIB. Sensitivity meta-analysis of two nested case control studies18,20 excluding study by Massó González and García Rodríguez19 exhibited a consistent protective effect of PPIs (RR, 0.59; 95% CI, 0.37 to 0.95) (Fig. 4A), and a consistent non-significant protective effect of H2RA (RR, 0.78; 95% CI, 0.38 to 1.64) for the dicumarinics-related upper GIB (Fig. 4B).
Fig. 4.
Sensitivity analysis according to the modifiers. (A) PPI and dicumarinics-related upper GIB, excluding the study by Massó González EL et al. (B) H2RA and dicumarinics-related upper GIB, excluding the study by Massó González EL et al. The size of each square is proportional to the study’s weight. The diamond indicates the summary estimate from the pooled studies (random effect model).
PPI, proton-pump inhibitor; GIB, gastrointestinal bleeding; H2RA, histamin-2 receptor antagonist; CI, confidence interval.
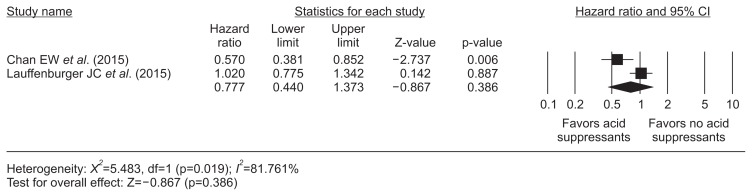
5. Acid suppressants on dabigatran-related GIB
Two retrospective cohort studies5,21 using adjusted HRs reflecting the effect of acid suppressants on dabigatran-related GIB was performed and overall acid suppressants did not show a protective effect (HR, 0.78; 95% CI, 0.44 to 1.37; I2, 81.8%) (Fig. 5).
Fig. 5.
Acid suppressants and dabigatran-related gastrointestinal bleeding. The size of each square is proportional to the study’s weight. The diamond indicates the summary estimate from the pooled studies (random effect model).
CI, confidence interval.
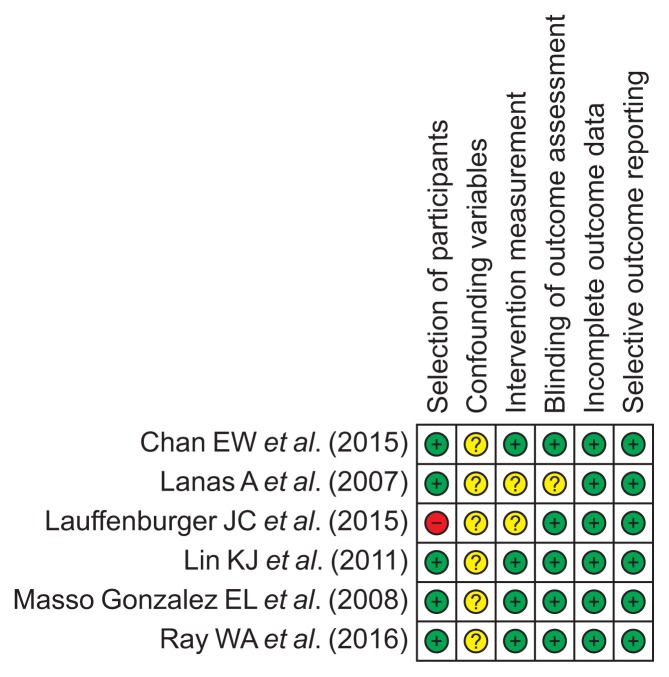
6. Methodological quality
The methodological qualities of the included studies were similar, although the study by Lauffenburger et al.5 included a population with a specific disease condition of nonvalvular atrial fibrillation compared to the remaining studies in the general population. However, subgroup analysis of methodological quality was not performed because only a small number of studies were included in this meta-analysis. The pitfalls inherent in retrospective studies make it difficult to exclude the use of over-the-counter NSAIDs, aspirin, or acid suppressants in all of the included studies. Therefore, rating for confounding variables was ranked as “unclear” risk of bias in all studies. A detailed summary of the methodological qualities of the enrolled studies is described in Fig. 6.
Fig. 6.
RoBANS for the assessment of the methodological quality of each enrolled study (+) denotes low risk of bias, (?) denotes unclear risk of bias, (–) denotes high risk of bias.
RoBANS, Risk of Bias Assessment Tool for Nonrandomized Studies.
7. Analysis of publication bias
Three studies were included in the analysis of PPI or H2RA on dicumarinics-related upper GIB (Figs 2, 3)18–20 and only two studies were included in the analysis of acid suppressants on dabigatran-related GIB (Fig. 5).5,21 Funnel plot or trim and fill method for the detection of publication bias was not used because of too small number of included studies. Although all the methods for the detection of publication bias were underpowered with this small number of publications, thorough statistical analyses were done in two meta-analyses (Figs 2, 3).
Egger’s regression test in the analysis of PPI on dicumarinics-related upper GIB revealed that the intercept was –3.64 (95% CI, –25.40 to 18.12), t-value 2.13, p=0.14 (1-tailed) and p=0.28 (2-tailed). The rank correlation test showed that Kendall’s tau was –0.67 with a continuity correction (p=0.15 [1-tailed] and p=0.30 [2-tailed]). Overall, there was no evidence of publication bias in this meta-analysis.
Egger’s regression test in the analysis of H2RA on dicumarinics-related upper GIB revealed that the intercept was 8.37 (95% CI, –62.44 to 79.18), t-value 1.50, p=0.19 (1-tailed) and p=0.37 (2-tailed). The rank correlation test showed that Kendall’s tau was 0.00 with a continuity correction (p=0.50 [1-tailed] and p>0.99 [2-tailed]) Overall, there was no evidence of publication bias in this meta-analysis.
Because only two studies were included in the analysis of acid suppressants on dabigatran-related GIB, analyses for the detection of publication bias were impossible.
DISCUSSION
GIB is a major adverse event associated with the use of anticoagulants and is a frequent cause of cessation of these drugs, potentially leading to thromboembolic events. Although findings in these meta-analyses suggested the protective effect of PPIs for the development of dicumarinics-related upper GIB, this effect was attenuated by or limited in the high baseline risk of gastrointestinal injury found consistently in the enrolled studies.18,20–22 Moreover, less potent inhibition of gastric acid by H2RA showed a non-significant protective effect, suggesting that baseline ulcerogenic properties (pre-existent erosion/ulcers on the upper gastrointestinal tract, Helicobacter pylori infection, and unrecognized use of NSAIDs or aspirin) of enrolled patients determines the magnitude of the protective effect of acid suppressants on anticoagulant-related upper GIB. Recent large-scale retrospective cohort study showed consistent results with our findings. Although primary end point was the hospitalization for upper GIB, which is different from our study and published after April 2018, which could not be included in our searching strategy, PPI coadministration was associated with lower hospitalization rate for upper GIB irrelevant to the type of anticoagulants (incidence rate ratio for overall anticoagulants: 0.66, for warfarin: 0.65) (vs no PPI coadministration).23
In contrast to NSAIDs or aspirin, anticoagulants are not ulcerogenic drugs. Pathophysiologic evidence for anticoagulants in the development of GIB is scarce. One of the suspected mechanisms is the potential for topical mucosal injury by incomplete absorption of DOACs, while warfarin is more than 95% absorbed in the gastrointestinal tract.24 However, the increased rate of major GIB in patients taking warfarin reflects that the systemic effect of this drug is also important for the development of adverse events.24 Therefore, the exact mechanism of the protective effect of PPIs on anticoagulant-related upper GIB has not been established. Chan et al.21 presented the hypothesis that acid suppression interferes with the absorption of dabigatran (the tartaric acid core of dabigatran needs a low pH for absorption) and results in lower rates of GIB, implying a protective effect of acid suppressants on dabigatran-related GIB. However, the enrolled studies in our meta-analysis used dicumarinics and showed a protective effect of PPIs on dicumarinics-related upper GIB. Only the study by Chan et al.21 and Lauffenburger et al.5 enrolled patients on dabigatran and the main outcome was GIB, not upper GIB in our meta-analysis. Therefore, the hypothesis by Chan et al. should be evaluated with further studies. Consequently, anticoagulants appear to potentiate GIB, especially in the upper gastrointestinal tract with pre-existent erosion/ulcers, H. pylori infection, and unrecognized use of NSAIDs or aspirin.
Although the results of this study revealed that co-administration of PPIs might reduce gastrointestinal bleeding, the routine use of PPIs in patients on anticoagulants should be cautious. There have been two different concerns about the overuse of PPIs in patients without risk factors and the underuse of PPIs in patients with risk factors for upper gastrointestinal injury on anticoagulant treatment.25,26 Considering that PPIs share a common metabolic enzyme (CYP3A4), implying that their use might increase the serum concentration of warfarin, some PPIs might accelerate the absorption of warfarin, and the combination of some PPIs and the CYP2C19 intermediate metabolizer could increase bleeding events.26–29 An approach that balances the risk-benefit of co-administration of PPIs and anticoagulants with estimating the individualized risk factors is needed.30 In cases with PPI and warfarin co-administration, close monitoring of prothrombin time with dose adjustment is needed. The interaction of the co-administration of PPIs and oral factor Xa inhibitors (xabans) does not appear to be of significant concern based on previous studies.27,31,32 However, the interaction of PPIs and the direct thrombin inhibitor dabigatran was reported (low on-treatment level of dabigatran) from recently published studies,33–35 and potential adverse events related to the long-term use of PPIs is another concern. Therefore, balancing the risk-benefit approach is still necessary before the co-prescription of anticoagulants and acid suppressants.
Risk factors for the development of GIB in patients taking warfarin include past history of GIB, old age, comorbidities, H. pylori infection, and co-administration of antiplatelet agents.7,8 However, little is known about risk factors for the development of GIB in patients taking DOACs. A systematic review revealed that similar risk factors consistently affected the adverse events related to dabigatran treatment,5 and these factors should be investigated using an individualized approach for the prevention of anticoagulant-related GIB.
The strength of this study was the enrollment of a large population from a balanced database reflecting real clinical practice, as it did not merely contain a group at high risk for GIB. Sensitivity analysis based on the modifier was performed, and publication bias was thoroughly investigated. Despite the strengths, several limitations were detected. First, a relatively small number of studies were enrolled in the meta-analysis, which makes it difficult to draw a solid conclusion. Second, authors could not recommend the duration or dose of PPI treatment with anticoagulants. All of the enrolled studies were nested case-control or cohort studies mostly from database information. Therefore, the duration, dose, and adherence to PPI could not be assessed. Third, confounding variables, including over-the-counter NSAIDs, aspirin, and antisecretory agents use, might have influenced the main results in all of the included studies. Fourth, there was no study on lower GIB, and only one study evaluated the secondary protective effect of PPIs on warfarin-related upper GIB.19 Therefore, we could not perform subgroup analysis divided by primary or secondary protective effects of acid suppressants.
In conclusion, the protective effect of PPIs on dicumarinics-related upper GIB was valid and there was no evidence supporting the protective effect of acid suppressants on dabigatran-related GIB. However, without randomized controlled trials, solid conclusions cannot be made.
Supplementary Information
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conception and design of the study: C.S.B., B.W.K. Generation, collection, assembly, analysis and/or interpretation of data: M.K.J., B.W.K., J.S.K., C.H.P., J.Y.A., J.H.L., B.E.L., H.J.Y., Y.K.C., J.M.P., B.J.K., H.K.J. Drafting or revision of the manuscript: C.S.B. Approval of the final version of the manuscript: B.W.K.
REFERENCES
- 1.Weitz JI, Eikelboom JW, Samama MM. New antithrombotic drugs: antithrombotic therapy and prevention of thrombosis, 9th ed. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e120S–e151S. doi: 10.1378/chest.11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes GD, Lucas E, Alexander GC, Goldberger ZD. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300–1305. doi: 10.1016/j.amjmed.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman CI, Sobieraj DM, Winkler S, et al. Effect of pharmacological therapies for stroke prevention on major gastrointestinal bleeding in patients with atrial fibrillation. Int J Clin Pract. 2012;66:53–63. doi: 10.1111/j.1742-1241.2011.02809.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller CS, Dorreen A, Martel M, Huynh T, Barkun AN. Risk of gastrointestinal bleeding in patients taking non-vitamin K antagonist oral anticoagulants: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15:1674–1683. doi: 10.1016/j.cgh.2017.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Lauffenburger JC, Rhoney DH, Farley JF, Gehi AK, Fang G. Predictors of gastrointestinal bleeding among patients with atrial fibrillation after initiating dabigatran therapy. Pharmacotherapy. 2015;35:560–568. doi: 10.1002/phar.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol. 2010;105:2533–2549. doi: 10.1038/ajg.2010.445. [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Díaz S, Rodríguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation: an overview of epidemiologic studies published in the 1990s. Arch Intern Med. 2000;160:2093–2099. doi: 10.1001/archinte.160.14.2093. [DOI] [PubMed] [Google Scholar]
- 8.Barada K, Abdul-Baki H, El Hajj II, Hashash JG, Green PH. Gastrointestinal bleeding in the setting of anticoagulation and antiplatelet therapy. J Clin Gastroenterol. 2009;43:5–12. doi: 10.1097/MCG.0b013e31811edd13. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66:408–414. doi: 10.1016/j.jclinepi.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Card N. Applied meta-analysis for social science research. New York: The Guilford Press; 2012. [Google Scholar]
- 12.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. London: The Cochrane Collaboration; 2011. [Google Scholar]
- 13.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanas A, García-Rodríguez LA, Arroyo MT, et al. Effect of antisecretory drugs and nitrates on the risk of ulcer bleeding associated with nonsteroidal anti-inflammatory drugs, antiplatelet agents, and anticoagulants. Am J Gastroenterol. 2007;102:507–515. doi: 10.1111/j.1572-0241.2006.01062.x. [DOI] [PubMed] [Google Scholar]
- 19.Massó González EL, García Rodríguez LA. Proton pump inhibitors reduce the long-term risk of recurrent upper gastrointestinal bleeding: an observational study. Aliment Pharmacol Ther. 2008;28:629–637. doi: 10.1111/j.1365-2036.2008.03780.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin KJ, Hernández-Díaz S, García Rodríguez LA. Acid suppressants reduce risk of gastrointestinal bleeding in patients on antithrombotic or anti-inflammatory therapy. Gastroenterology. 2011;141:71–79. doi: 10.1053/j.gastro.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 21.Chan EW, Lau WC, Leung WK, et al. Prevention of dabigatran-related gastrointestinal bleeding with gastroprotective agents: a population-based study. Gastroenterology. 2015;149:586–595. doi: 10.1053/j.gastro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Ray WA, Chung CP, Murray KT, et al. Association of proton pump inhibitors with reduced risk of warfarin-related serious upper gastrointestinal bleeding. Gastroenterology. 2016;151:1105–1112. doi: 10.1053/j.gastro.2016.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. 2018;320:2221–2230. doi: 10.1001/jama.2018.17242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Minno A, Spadarella G, Prisco D, Scalera A, Ricciardi E, Di Minno G. Antithrombotic drugs, patient characteristics, and gastrointestinal bleeding: clinical translation and areas of research. Blood Rev. 2015;29:335–343. doi: 10.1016/j.blre.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Savarino V, Dulbecco P, de Bortoli N, Ottonello A, Savarino E. The appropriate use of proton pump inhibitors (PPIs): need for a reappraisal. Eur J Intern Med. 2017;37:19–24. doi: 10.1016/j.ejim.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 26.Sugano K. How do we manage serious gastrointestinal adverse events associated with anti-thrombotic therapy? Expert Rev Gastroenterol Hepatol. 2015;9:5–8. doi: 10.1586/17474124.2014.945913. [DOI] [PubMed] [Google Scholar]
- 27.Agewall S, Cattaneo M, Collet JP, et al. Expert position paper on the use of proton pump inhibitors in patients with cardiovascular disease and antithrombotic therapy. Eur Heart J. 2013;34:1708–1713. doi: 10.1093/eurheartj/eht042. [DOI] [PubMed] [Google Scholar]
- 28.Teichert M, van Noord C, Uitterlinden AG, et al. Proton pump inhibitors and the risk of overanticoagulation during acenocoumarol maintenance treatment. Br J Haematol. 2011;153:379–385. doi: 10.1111/j.1365-2141.2011.08633.x. [DOI] [PubMed] [Google Scholar]
- 29.Hata M, Shiono M, Akiyama K, et al. Incidence of drug interaction when using proton pump inhibitor and warfarin according to cytochrome P450 2C19 (CYP2C19) genotype in Japanese. Thorac Cardiovasc Surg. 2015;63:45–50. doi: 10.1055/s-0034-1383814. [DOI] [PubMed] [Google Scholar]
- 30.Ko D, Hylek EM. Anticoagulation in the older adult: optimizing benefit and reducing risk. Semin Thromb Hemost. 2014;40:688–694. doi: 10.1055/s-0034-1389083. [DOI] [PubMed] [Google Scholar]
- 31.EINSTEIN Investigators. Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 32.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 33.Kuwayama T, Osanai H, Ajioka M, et al. Influence of proton pump inhibitors on blood dabigatran concentrations in Japanese patients with non-valvular atrial fibrillation. J Arrhythm. 2017;33:619–623. doi: 10.1016/j.joa.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bolek T, Samoš M, Stančiaková L, et al. The impact of proton pump inhibition on dabigatran levels in patients with atrial fibrillation. Am J Ther. 2019;26:e308–e313. doi: 10.1097/MJT.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 35.Bolek T, Samoš M, Škorňová I, et al. Proton pump inhibition in patients treated with novel antithrombotic drugs: should we worry about thrombosis? J Cardiovasc Pharmacol. 2018;72:71–76. doi: 10.1097/FJC.0000000000000593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.