Abstract
Cell death is now reclassified into several types based on the mechanisms and morphologic phenotype. Understanding of such classifications offers insights into the pathogenesis of liver disease, as well as diagnostic or therapeutic implications. Apoptosis is recognized relatively easily due to its unique morphology, but lytic cell death may occur in the form of accidental necrosis, mitochondria permeability transition-driven necrosis, necroptosis, pyroptosis, ferroptosis, and parthanatos. The cell may be engulfed by neighboring cells due to a loss of integrin signaling or cancer cell competition by entosis, a type of cell death. The classification also includes mechanistically termed cell death such as autophagy-dependent cell death and lysosome-dependent cell death. These different types of cell death may occur uniquely in certain liver diseases but may coexist in the evolution of the disease. They occur in parenchymal and non-parenchymal liver cells, as well as inflammatory cells, causing distinct pathologic consequences. This review briefly covers the recently revised classifications of cell death and discusses their relevance to liver diseases of different etiologies.
Keywords: Pyroptosis, Necroptosis, Apoptosis, Ferroptosis, Mitochondria permeability transition-driven necrosis
INTRODUCTION
Cell death represents the most critical pathologic entity in liver disease. Although it fundamentally takes place as an adaptive and homeostatic response to internal or external perturbations to achieve targeted elimination of damaged or harmful cells, it also occurs due to a failure to cope with excessive insults or stress and more importantly dictates pathologic consequences such as inflammation, fibrosis, and even transformation. Many different types of cell death have been described and most, except accidental cell death (e.g., death due to physical or chemical injury), are mediated by built-in mechanisms and thus termed regulated cell death. Based on the type of insult, initiation mechanisms and phenotype, cell death is classified into different types: apoptosis, mitochondrial permeability transition (MPT)-driven necrosis, necroptosis, pyroptosis, ferroptosis, parthanatos, and entosis as summarized in Table 1.1 These different types of death may be manifested by hepatocytes, biliary epithelial cells, or non-parenchymal liver cells in a manner dependent on etiology, the nature and extent of co-morbidities, and disease stage.2,3
Table 1.
Summary of Different Cell Death Types
| Cell death type | Regulated | Insults | Effectors | Morphologic features | Liver disease |
|---|---|---|---|---|---|
| Apoptosis | Yes | Intrinsic: loss of growth signals; organelle stress, DNA damage, ROS, mitotic defects Extrinsic: FAS/TNFR1 activation |
BAX/BAK>APAF1/CASP9>CASP3/CASP7 CASP8/10>CASP3 |
PM integrity, PS exposure DNA fragmentation/nuclear condensation |
Cholestatic, autoimmune, ALD, NASH viral, HCC |
| MPT-necrosis | Yes | ROS, cytosolic Ca2+ overload | BAX/BAK CYPD |
Initial apoptotic features followed by mitochondria abnormality and lytic death | NAFLD, I/R, ALD* |
| Necroptosis | Yes | FAS/TNFR1 activation TLR3/4 receptor activation |
RIPK3>MLKL | Lytic death due to PM permeability | NASH, ALD, drug autoimmune |
| Ferroptosis | No/yes | Iron catalyzed ROS and lipid peroxidation | Independent of BAX/BAK, CYPD, CASPs, RIPK3 | Necrotic morphology Mitochondria shrinkage, cristae loss, ruptured outer mitochondria membrane |
NASH* ALD* |
| Pyroptosis | Yes | Intracellular LPS or bacteria | CASP1 CASP11/4>GSDMD |
Lytic death due to PM pore formation | NASH, ALD |
| Parthanatos | Yes | Alkylating DNA damage, ROS, RNS, hypoxia | PARP1 | DNA fragmentation/nuclear condensation | Drug |
| Entosis | Yes | Loss of integrin signaling; cancer cell competition | Myosin/RhoA/ROCK | Invasion of entotic cells into engulfing cells | HCC |
| Autophagy-dependent | Yes | Toxicity, hypoxia, ischemia | Autophagy machinery | Autophagosome | Drug* |
| Lysosome-dependent | Yes | TRAIL activation | BAX | LMP/lysosomal abnormality followed by mitochondria dysfunction | Lipotoxicity (NASH), ALD |
ROS, reactive oxygen species; TNFR1, tumor necrosis factor 1; BAX, BCL-2 associated X protein; BAK, BCL-2 homologous antagonist killer protein; APAF1, apoptotic protease activating factor 1; CASP, caspase; PM, plasma membrane; PS, phosphatidylserine; ALD, alcoholic liver disease; NASH, nonalcoholic steatohepatitis; HCC, hepatocellular carcinoma; MPT-necrosis, mitochondrial permeability transition necrosis; CYPD, cyclophilin D; NAFLD, nonalcoholic fatty liver disease; I/R, ischemia-reperfusion; TLR3/4, Toll-like receptor 3/4; RIPK3, receptor interacting protein kinase 3; MLKL, mixed lineage kinase domain like pseudokinase; LPS, lipopolysaccharide; GSDMD, gasdermin-D; RNS, reactive nitrogen species; PARP1, poly [ADP-ribose] polymerase 1; ROCK, rho-associated kinase; TRAIL, tumor necrosis factor related apoptosis-inducing ligand; LMP, latent membrane protein.
These relationships have not yet been fully established.
APOPTOSIS
Apoptosis is described as programmed cell death and characterized by nuclear fragmentation, chromatin condensation, and cellular shrinkage. Apoptosis occurs normally during development and aging and as a homeostatic mechanism to maintain cell populations in tissues. Apoptosis also occurs as a defense mechanism such as in immune reactions or when cells are damaged by disease or noxious agents.1 Apoptotic cells generate apoptotic bodies, which are phagocytosed (also termed efferocytosed) by immune cells without eliciting inflammation as well as by adjacent parenchymal or non-parenchymal cells. Although apoptosis has a less pro-inflammatory consequence than necrotic cell death, phagocytosis of apoptotic bodies by Kupffer cells upregulates death ligand and cytokines in cholestatic liver injury in mice.4 In the same model, the Fas-mediated apoptosis of hepatocytes is associated with activation of hepatic stellate cells (HSCs) and liver fibrosis, linking apoptosis to liver fibrosis.5 Thus, apoptosis may not be as innocuous as originally presumed. Apoptosis primarily is dependent on activation of caspases, but caspase-independent apoptosis also occurs. In general, apoptosis can either be triggered via intrinsic mitochondrial or extrinsic death receptor-mediated pathways.1
Intrinsic apoptosis occurs due to a variety of abnormal conditions such as growth factor withdrawal, DNA damage, endoplasmic reticulum stress, reactive oxygen species (ROS) overload, replication stress, microtubular alterations or mitotic defects.6 These cellular stresses cause BAX/BAK-induced mitochondrial outer membrane pore formation and mitochondrial release of apoptotic factors such as cytochrome c and SMAC/DIABLO, which bind to APAF1 and pro-caspase 9 (CASP9) to form apoptosome and to activate CASP9. CASP9 activates the apoptosis executioner caspases, CASP3 and CASP7, which mediate DNA fragmentation and phosphatidylserine externalization of the plasma membrane.1,6,7
The extrinsic death receptor pathway is initiated by the binding of the ligands to the death receptors such as FAS and TNFR1. This initiates activation of CASP8 via FADD and TRADD, respectively and subsequent activation of CASP3 and CASP7 and apoptosis.1
Both intrinsic and extrinsic apoptosis are involved in cholestatic liver injury, alcoholic and nonalcoholic steatohepatitis (ASH and NASH), and milder hepatotoxic injury.2,3,8 The family of dependence receptors encompasses around 20 members which include netrin1 receptors (DCC and UNC5A-D), neurotrophin receptor (NTRK3), and the sonic hedgehog receptor patched. Although ligand-mediated activation of these dependence receptors promotes cell proliferation, differentiation, and survival, the ligand depletion triggers death pathways via CASP9-CASP3 activation or a p53-dependent mechanism.1 UNC5A in hepatocytes counteracts hepatitis C virus (HCV) persistence but HCV infection downregulates its expression.9 UNC5A is depleted in HCV cirrhosis and hepatocellular carcinoma (HCC), and its role in regulation of liver tumorigenesis is suggested.9 HSCs express p75 neurotrophin receptor and its loss causes stellate cell apoptosis and impairs liver regeneration.10,11
Hepatitis B virus (HBV) infection is known to regulate apoptosis of hepatocytes. HBV HBx protein sensitizes cells to apoptosis triggered by various insults such as tumor necrosis factor (TNF)-α,12 anti-Fas antibody, growth factor deprivation, and oxidative stress13 in culture and spontaneously increases apoptosis of hepatocytes when expressed in vivo.13 This pro-apoptotic effect is p53-independent13 and may be mediated by loss of mitochondrial membrane potential14 or HBx interaction with cellular FLICE inhibitory protein (c-FLIP), a key regulator of the death-inducing signaling complex, inhibiting the c-FLIP’s anti-apoptotic function.15 Conversely, HBx protein abrogates p53-induced apoptosis by sequestering this pro-apoptotic protein in the cytoplasm16 and inhibits Fas-mediated apoptosis in a p53-independent manner involving the MEKK1-JNK intrinsic apoptotic pathway.17 HBx also promotes autophagic and lysosomal degradation of the TNFSF10/TRAIL (tumor necrosis factor related apoptosis-inducing ligand) death receptors, supporting survival of HBV-infected cells and evading antiviral immunity.18 In fact, HBx stimulates Parkin-mediated mitophagy and suppresses mitochondrial apoptosis, a mechanism which may contribute to HBV-induced hepatocarcionogenesis.19
MPT-DRIVEN NECROSIS
Necrosis is characterized by cell swelling, membrane rupture, and release of cell contents that leads to a subsequent inflammatory response. However, a current consensus is that necrosis is mostly mediated by MPT, which characterizes the formation of permeability transition pore at inner and outer mitochondria membranes, causing rapid dissipation of the membrane potential gradient, loss of ATP synthesis, osmotic breakdown of both membranes, and cell death.1 Peptidylprolyl isomerase F, also known as cyclophilin D (CYPD), is shown to participate in this pore formation. Pharmacologic inhibitors of CYPD such as Cyclosporin A, ameliorate MPT and cell death in diseases where oxidative stress and cytosolic Ca2+ overload play major pathogenetic roles.1 In animal model of acetaminophen hepatotoxicity,20 CYPD deficiency prevents hepatotoxicity although a conflicting result is also published.21 CYPD deficiency, either global or liver-specific, prevents high fat diet-induced fatty liver disease.22,23 GSK3β translocates to mitochondria and phosphorylates CYPD, promoting its association with the adenine nucleotide translocator and Ca2+-mediated MPT in ischemia-reperfusion (I/R) liver injury. GSK3β inhibition with indirubin prevents these events.24 Similarly, NAD-stimulated SIRT3 activity deacetylates and inactivates CYPD and prevents MPT in fatty livers after warm I/R.24 Acute alcohol dosing causes transient dose-dependent mitochondrial depolarization in hepatocytes in a manner independent of MPT but in close association with lipid accumulation.25 Further, CYPD global deficiency failed to prevent alcoholic fatty liver.26 Thus, the role of CYPD in MPT-mediated hepatocellular necrosis in alcoholic liver disease (ALD) is yet to be clarified.
NECROPTOSIS
Another necrotic type of regulated cell death is necroptosis, which is caused by extracellular or intracellular perturbations and prevalent in most chronic liver diseases including viral hepatitis, autoimmune hepatitis, NASH, and ALD.8,27 Extracellular signals may be mediated via the death receptors (FAS and TNFR1) and Toll-like receptors (TLRs; TLR3 and TLR4). This pathway is caspase-independent and triggered by sequential activation of receptor interacting protein kinase 3 (RIPK3) and mixed lineage kinase domain like pseudokinase (MLKL). Upon TNFR1 ligation, for example, the formation of “necrosome” takes place where RIPK1 and RIPK3 undergo trans- and auto-phosphorylation. Active RIPK3 phosphorylates MLKL, resulting in MLKL oligomerization and translocation to the plasma membrane where they increase the permeability via activation of ADAM proteases, Ca2+ influx by targeting the cation channel (TRPM7), and phosphatidylserine externalization.1
There are considerable crosstalk regulations between apoptosis versus necroptosis pathways.1,8 Active CASP8 cleaves and inactivates RIPK3, suppressing necroptosis. Necroptosis is also inhibited by c-IAPs, the inhibitors of apoptosis due to their ability to ubiquitinate RIPK1, which functions immediate downstream of TNFR1 along with TRADD for the survival pathway but also forms a complex with FADD and RIPK3 for the necroptosis pathway. CYPD deubiquitinates RIPK1 but can be targeted by CASP8. IKK, which activates NF-kB, phosphorylates and inhibits RIPK1 while transcriptionally upregulating anti-apoptotic molecules such as c-IAPs and c-FLIP. High levels of damage associated molecular patterns (DAMPs) are released by lytic necroptotic cell death as compared to apoptosis, activating inflammasome and TLRs and stimulating inflammation. CASP8 also inhibits NLRP inflammasome while RIPK3 activates it, serving as another mechanism for differential effects on inflammation by apoptosis versus necroptosis. The balance between these two cell death types not only determines the extent of inflammation but also appears to dictate the type of liver cancer developed. In a recent study,28 a necroptotic microenvironment with inflammatory cytokine expression promoted intrahepatic cholangiocarcinoma development caused by genetic oncogenic activation while an apoptotic environment favored the development of HCC. Active MLKL is also shown to be capable of activating the NLRP3 inflammasome and pro-interleukin (IL)-1β just preceding the necroptotic cell death.29 It is noteworthy that RIPK3 deficient mice fed high fat diet, which failed to phosphor-activate MLKL, were shown to exhibit exacerbated liver inflammation, apoptosis, and fibrosis.30 This finding contrasts to the protective effect observed in an ALD model27 but also suggests that the role of necroptosis is context-dependent and a shift from necroptosis to apoptosis may be more inflammatory and fibrogenic in nonalcoholic fatty liver disease (NAFLD).
FERROPTOSIS
Ferroptosis is a form of regulated cell death, which is dependent on intracellular iron catalyzing the generation of ROS and consequent oxidative damage. Such condition is experimentally induced by erastin and sulfasalazine, which inhibit cystine-glutamate antiporter system xCT, resulting in deprivation of cysteine and suppressed glutathione synthesis.31 In ferroptosis, mitochondria are reduced in size and condensed with loss of crista and rupture of the outer membrane.31 Accordingly, this process is characterized by the accumulation of lipid peroxidation products and can be pharmacologically ameliorated with iron chelators such as deferoxamine and lipid peroxidation inhibitors like ferrostatin.31 Besides hemochromatosis, which is obviously based on iron-catalyzed oxidative injury,32 acetaminophen hepatotoxicity may also involve ferroptosis.33 As iron accumulation and iron-catalyzed lipid peroxidation are common complications of ALD and NASH, this cell death pathway may be expected to co-exist with apoptotic, MPT-necrotic, or necroptotic pathways. As recently shown in neurodegenerative diseases, ferritin degradation in lysosomes and subsequent release of catalytically active iron may underlie ferroptosis in the pathogenesis of these diseases.34 This raises a question as to if enhanced autophagy of ferritin, the major intracellular protein complex for iron storage, may risk iron-loaded cells with compromised antioxidant defense for ferroptosis rather than offering protection. A recent study addressed this question in HSCs. In this study, the mRNA-binding protein ELAV1 (HuR) which is upregulated in activated HSCs and contributes to liver fibrosis,35 was shown to enhance autophagy via Beclin1 mRNA stabilization and to promote ferritinophagy and ferroptosis in HSCs.36 Further, the treatment with sorafenib inhibited this pathway and ameliorated liver fibrosis in mice.36 Although the latter data need to be scrutinized due to multiple effects of sorafenib besides autophagy, regulation of HSCs via ferroptosis is an interesting notion which needs to be further investigated.
PYROPTOSIS
Pyroptosis is a type of regulated cell death, which mainly occurs in response to intracellular pathogens or pathogen-associated molecular patterns (PAMPs), most notably LPS. Pyroptosis was originally described as a type of cell death associated with cell swelling and rapid plasma membrane lysis due to the membrane pore formation in a CASP1-dependent manner.37 However, caspase-1 independent pathway triggered by activation of CASP11 (CASP4/5 in man), has recently been described.38 Unlike canonical inflammasome CASP1, which requires a complex with a Nod-like receptor sensor protein and the ACS adopter protein for its activation, CASP11 undergo oligomerization upon binding of the LPS lipid A moiety to CASP11 N-termini. Similar to apoptosis, cells undergoing pyroptosis have extensive nuclear DNA fragmentation but DNA fragmentation in pyroptosis does not require caspase-activated DNAse. Recently, the executioner molecule responsible for pore formation was identified to be gasdermin-D (GSDMD), which is activated by CASP11-mediated proteolytic activation of pro-GSDMD, releasing a 30 to 31 kD N-terminal fragment.39,40 This fragment is recruited to the plasma membrane via binding to phosphatidylinositol phosphates, phosphatidylserine, and cardiolipin and forms an oligomerized ring structure to create a pore.41,42 The importance of the CASP11-GSDMD pathway in endotoxemia-induced lethality was highlighted by remarkable protection of Casp11−/− or Gasmd−/− mice from death caused by an excessive LPS challenge which killed almost all wild type mice.39
Alcoholic hepatitis (AH) is one of the most severe form of ALD and carries a very high 3-month mortality rate of 30% to 50%.43 Its common clinical symptoms are hepatomegaly, jaundice, nausea, vomiting and abdominal pain, and ascites.44,45 Histologically, the patient liver shows ballooned hepatocytes often containing Mallory-Denk bodies accompanied by neutrophilic infiltration.45,46 Patients with severe AH develop sepsis, liver failure, and multiorgan dysfunction, raising the risk of death. The treatment option for AH is very limited. Although most guidelines recommend corticosteroid use, 30% to 40% of AH patients fail to respond to this treatment. Liver transplantation has a favorable outcome for AH patients, however, most transplant centers require 6-month abstinence from alcohol. Since the short-term mortality of severe AH is very high, the 6-month abstinence requirement is often waived for such patients who show no improvement after the standard treatment.47,48
Pursuing for the development of a new therapeutic approach for AH, gut dysbiosis, leaky gut and bacterial translocation, neutrophilic inflammation, and sepsis are important considerations. Through transcriptomic analysis of the mouse model, which undergoes mild chronic steatohepatitis and is mainly characterized by steatohepatitis with mononuclear cell inflammation and chicken-wire fibrosis versus AH with intense neutrophilic infiltration, we looked for a driver(s) which cause(s) a transition from the former mild form to the latter life-threatening form.49 Pathway analysis for differentially regulated transcripts for this comparison, revealed the genes associated with infection were most abundantly and significantly regulated in the AH model liver. One such gene was CASP11. Our subsequent analysis revealed the pro-CASP11 was not only induced but also activated in AH, concomitant with the appearance of the 30 kD fragment of GSDMD, demonstrating activation of CASP11-GSDMD pathway.49 This biochemical evidence was associated with increased bacterial load in the liver, hepatocellular necrosis, and neutrophilic infiltration, all of which were ameliorated in CASP11 deficient-mice. Conversely, the deficiency of IL-18, a key anti-microbial cytokine, worsened CASP11-GSDMD activation, lytic hepatocellular death, liver bacterial load, and neutrophilic inflammation. Further, hepatocyte-specific overexpression of active GSDMD reproduced the similar phenotype. Finally, activation of CASP11-GSDMD was also evident in livers from AH patients but not normal subjects.49 These results support a notion that the CASP11-GSDMD pathway is activated in bacteria- or LPS-loaded hepatocytes and causes pyroptosis of such cells, aggravating neutrophilic infiltration via release of DAMP, bacteria, PAMPs, IL-1, and IL-18 from pyroptotic cells in AH. If this process is severe enough, it may lead to septicemia, one of the most common complications of AH in patients.
As gut dysbiosis is also commonly evident in NAFLD and NASH, it is not surprising that NASH is associated with activation of the pyroptotic GSDMD pathway.50 Human hepatoma cell lines infected with HCV also undergo pyroptosis besides apoptosis,51 although its relevance to HCV-infected patient livers is yet to be determined. However, this raises an outstanding question as to what PAMPs or microorganisms are capable of eliciting the pyroptotic pathway besides gram-negative bacteria and LPS. Another crucial question is what cells are targeted by the pyroptotic pathways in liver diseases. Hepatic macrophages, which serve as the first line of defense against invading microorganisms, are a natural target. Indeed, in the mouse AH model, isolated hepatic macrophages show increased levels of activated GSDMD.49 In the AH patient livers, functional macrophages are often depleted and pyroptosis may be involved in such condition which further aggravate neutrophilic infiltration to fight against bacteria. Eosinophils52 and invariant natural killer T cells53 are also shown to undergo pyroptosis in response to liver injury. Future studies have to address how pyroptosis of parenchymal and non-parenchymal liver cells determines the pathologic outcome in the disease-specific setting.
PARTHANATOS
Parthanatos is a form of regulated cell death caused by excessive DNA damage response primarily mediated by poly(ADP-ribose) polymerase 1 (PARP1). Parthanatos occurs after severe and prolonged alkylating DNA damage, oxidative stress, hypoxia, hypoglycemia, or inflammation.1 Reactive nitrogen species, such as NO, are a major trigger of PARP1 activation which causes NAD+ and ATP depletion, accumulation of poly(ADP-ribose) polymers and poly(ADP-ribosyl)ated proteins in mitochondria, culminating to the loss of membrane potential. Poly(ADP-ribose) polymers also binds apoptosis-inducing factor (AIF) and promotes AIF nuclear translocation, leading to DNA fragmentation and nuclear condensation. Macrophage migration inhibitory factor, which is implicated in various liver diseases including ALD,54 is recently shown to be an AIF binding partner and catalyze DNA cleavage.55 In fact, there appears to be a cross-relationship between necroptosis and parthanatos as activated RIPK1 and RIPK3 may stimulate the enzymatic activity of PARP1 and promote ATP depletion and AIF release.56,57 Although parthanatos is primarily described in the context of cardiovascular and renal diseases, diabetes, and neurodegeneration, its role in liver diseases is suspected based on the known involvement of PARP1 in liver cell death.
ENTOSIS
Entosis is a form of cell cannibalism that takes place in either healthy or abnormal tissues via engulfment of viable cells by non-phagocytic cells of homotypic or heterotypic variety.1 Entosis of epithelial cells commonly occurs when the cells lose integrin signaling by detachment from the matrix. The process of entosis is mediated by cellular invasion, which appears to depend on E-cadherin, α-catenin, RhoA, and rho-associated kinase (ROCK). Entosis takes place in cancer cell competition. Activation of KRAS and RAC1 facilitates myosin downregulation in engulfing cells to allow invasion of target cells.58 The cells with AMPK activation due to nutrient deprivation appear to succumb to entosis, suggesting a competition based on nutrient recovery.59 Emperipolesis represents entosis of hematopoietic cells by host cells, and emperipolesis of activated T cells by hepatocytes occurs which may render immune tolerance. In chronic liver disease such as chronic HBV and autoimmune hepatitis, emperipolesis is increased, suggesting its involvement in liver injury or defective T clearance.60 Recently, HSCs are shown to engage in emperipolesis of anti-fibrotic natural killer cells in HBV patients with liver cirrhosis in a transforming growth factor-β dependent manner as a potential novel mechanism of fibrosis amplification.61
OTHER CELL DEATH TYPES CLASSIFIED BY THE PRIMARY MECHANISMS
Autophagy-dependent cell death is a type of regulated cell death dictated by the autophagic machinery primarily for an adaptive cytoprotective purpose. Examples of experimental evidence for autophagy-dependent cell death include neuronal cell death caused by hypoxia-ischemia in neonatal mice, which is prevented by neuron-specific deletion of Atg7.1 Cocaine-induced neurotoxicity is also prevented by pharmacologic or genetic abrogation of autophagic process. The lncRNA autophagy-promoting factor which promotes ATG7 expression is implicated in myocardial infarction.1 In a certain case of hepatocellular toxicity, autophagy-dependent apoptosis may be induced in hepatocytes via a lysosomal-mitochondrial axis.62
Lysosome-dependent cell death is a regulated cell death type induced by the permeabilization of lysosomal membranes (LMP), the condition relevant to inflammation, tissue involution, aging, neurodegeneration, and intracellular pathogen response.1 LMP may occur following mitochondrial membrane permeabilization as a consequence of apoptotic and necroptotic processes. But the lysosomes can be permeabilized before mitochondria via BAX recruitment to the lysosomal membrane. Oxidative stress and lipid peroxidation of lysosomal membrane may also contribute to LMP. Primary LMP can also occur via TRAIL signaling or viral infection. In fact, c-FLIP prevents TRAIL-induced apoptosis in liver cancer cells by inhibiting LMP.63 Treatment of hepatocytes with free saturated fatty acids causes LMP prior to mitochondrial dysfunction and pharmacologic or genetic inhibition of cathepsin B prevents mitochondrial dysfunction of hepatocellular lipotoxicity,64 suggesting the relevance of lysosomal-dependent cell death to NASH. ALD is also associated with impaired lysosomal functions,65 and a recent study demonstrates restoration of lysosome biogenesis and autophagy prevents alcohol-induced liver injury,66 suggesting the role of lysosomal effects in the pathogenesis. In the mammary epithelial cell involution model, STAT3 is shown to be important in lysosome-dependent cell death via its ability to upregulate cathepsin B and L and inhibit their inhibitors Spi2A.67 Cathepsins and STAT3 are commonly overexpressed and activated in many malignancies including liver cancer, yet these tumors evade lysosome-dependent cell death for reasons yet to be elucidated.
CROSS-REGULATION AMONG DIFFERENT CELL DEATH PATHWAYS AND WITH INFLAMMATION
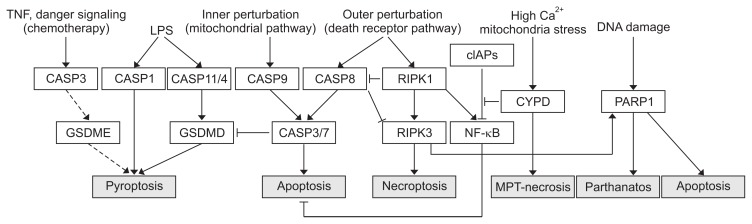
The death pathways discussed above cross-regulate to determine a type of cell death which dominates in the end or co-exists to culminate to cell death of mixed phenotypes. Thus, unless the liver is acutely injured, the liver cells must undergo different phases of cell death pathway activation and adaptations in the evolution of chronic liver disease, with a relatively more dominant cell death type in each phage rendering different pathologic consequences such as inflammation, fibrosis, and transformation. Most notable cross-regulation is CASP8-mediated inhibition of necroptosis by RIPK3 degradation, favoring apoptosis. RIPK1 can be directly inhibited via ubiquitination by anti-apoptotic cIAPs and this prevents TNF-mediated apoptosis or necroptosis.68 In contrast, CYPD required for MPT-driven necrosis deubiquitinates RIPK1 to facilitate necrosome formation to enforce the cell death pathway.69 Active RIPK3 and MLKL activates NLRP3 inflammasome,29 serving as a direct link between necroptosis and inflammation besides DAMP-mediated inflammasome activation. However, the extrinsic apoptosis effector CASP8 also forms a NLRP3 inflammasome like CASP1 to process pro-IL-1β to incite inflammation,70 underscoring the complexity of the cross-regulation. Partial examples of such cross-regulations among the different death pathways are summarized in Table 256,57,69,71–77 and Fig. 1.
Table 2.
Cross-Regulations of Different Cell Death Pathways
| Cell death pathway | Effector | Action | Cell death pathway regulated |
|---|---|---|---|
| Apoptosis | CASP8 | Inactivates RIPK3 and necroptosis71 | Necroptosis |
| Inactivates CYPD and inhibits MPT-necrosis72 | MPT-necrosis | ||
| Activated by NLRC4 inflammasome and induces pyroptosis73 | Pyroptosis | ||
| CASP3 | Activates GSDME-mediated pyroptosis in chemotherapy74 | Pyroptosis | |
| CASP3/7 | Inactivates GSDMD and pyroptosis in monocytes75 | Pyroptosis | |
| MPT-necrosis | CYPD | Rescues cIAP-induced ubiquitination of RIPK1 and promotes necroptosis69 | Necroptosis |
| Necroptosis | RIPK1 | Inhibits CASP8 dependent apoptosis76 | Apoptosis |
| Stimulates anti-apoptotic NF-kB activation77 | Apoptosis | ||
| RIPK1/RIPK3 | Activates PARP1 and stimulates parthanatos56,57 | Parthanatos |
CASP, caspase; RIPK1 and 3, receptor interacting protein kinase 1 and 3; CYPD, cyclophilin D; MPT-necrosis, mitochondrial permeability transition necrosis; NLRC4, NLR family CARD domain-containing protein 4; GSDME, gasdermin-E; GSDMD, gasdermin-D; cIAP, cellular inhibitor of apoptosis protein; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; PARP1, poly (ADP-ribose) polymerase 1.
Fig. 1.
A schematic diagram depicting the cross-regulations of different cell death pathways. Solid arrows represent activating interactions, while T-shaped lines represent inhibitory interactions. Hypothesized interactions are represented by dashed line.
TNF, tumor necrosis factor; CASP, caspase; GSDME, gasdermin-E; LPS, lipopolysaccharide; RIPK, receptor interacting protein kinase; cIAPs, cellular inhibitor of apoptosis proteins; NF, nuclear factor; CYPD, cyclophilin D; MPT-necrosis, mitochondrial permeability transition necrosis; PARP1, poly (ADP-ribose) polymerase 1.
CONCLUSIONS
A major difficulty in studying cell death in vivo is the absence of specific markers that can be used for detection of different types of cell death in situ. TUNEL staining which was originally considered to be specific for apoptosis is now known to be detected in several other cell death types. CASP3 activation is generally considered as a good marker for apoptosis as it serves as a final step toward this type of cell death. However, CASP3 can also be activated for non-apoptotic functions such as regulation of cell proliferation and differentiation.78 Although recognition of apoptotic cells is relatively easy due to their distinct morphology, distinction among other necrotic, necroptotic, and pyroptotic cell death currently has to rely on assessment of the effector activation status via biochemical analysis and their in situ validation is very difficult if not possible. Development of sensitive in situ markers of these different cell types will advance our ability to enhance the pathogenetic insights into liver diseases.
As cell death is a critical pathogenetic event in acute and chronic liver diseases, the effectors of cell death pathways obviously are potential therapeutic targets. Many of such pharmacologic compounds are under clinical trials. Just to list a few, Emricasan (IDN-6556) is a pan-caspase inhibitor which is under randomized clinical trials for NASH patients with stage 1–3 fibrosis, decompensated cirrhosis, or severe portal hypertension. Apoptosis signal-regulating kinase (ASK1) is a member of the MAP3K family which activates JNK and p38MAPK to promote apoptosis, inflammation, and fibrosis. Selonsertib (GS-4997), an ASK1 inhibitor, is under phase 3 trial for NASH, and its anti-fibrotic effect may benefit NASH patients advancing to cirrhosis. RIPK1 inhibitors have been developed for chronic inflammatory diseases, and phase 2 studies are underway for GSK2982772, a selective RIPK1 inhibitor, for psoriasis and rheumatoid arthritis. The same drug and another RIPK1 inhibitor, GSK2983559, are currently under clinical trials for ulcerative colitis and inflammatory bowel diseases (https://clinicaltrials.gov/ct2/results?term=RIP1+inhibitor). These RIPK1 and RIPK3 inhibitors will likely be tested for chronic liver diseases very soon.
ACKNOWLEDGEMENTS
This study was supported by the NIH grants (P50AA011999, U01AA018663, R24AA012885) and by Medical Research Service of Department of Veterans Affairs (5I01BX001991 and IK6BX004205). S.A.’s research scholarship in the senior author’s laboratory was supported in part by Japan Student Services Association.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang S, Pacher P, De Lisle RC, Huang H, Ding WX. A mechanistic review of cell death in alcohol-induced liver injury. Alcohol Clin Exp Res. 2016;40:1215–1223. doi: 10.1111/acer.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765–783. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canbay A, Feldstein AE, Higuchi H, et al. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 5.Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323–1330. doi: 10.1053/gast.2002.35953. [DOI] [PubMed] [Google Scholar]
- 6.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwabe RF, Luedde T. Apoptosis and necroptosis in the liver: a matter of life and death. Nat Rev Gastroenterol Hepatol. 2018;15:738–752. doi: 10.1038/s41575-018-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plissonnier ML, Lahlali T, Raab M, et al. Reciprocal antagonism between the netrin-1 receptor uncoordinated-phenotype-5A (UNC5A) and the hepatitis C virus. Oncogene. 2017;36:6712–6724. doi: 10.1038/onc.2017.271. [DOI] [PubMed] [Google Scholar]
- 10.Kendall TJ, Hennedige S, Aucott RL, et al. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49:901–910. doi: 10.1002/hep.22701. [DOI] [PubMed] [Google Scholar]
- 11.Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853–1856. doi: 10.1126/science.1137603. [DOI] [PubMed] [Google Scholar]
- 12.Su F, Schneider RJ. Hepatitis B virus HBx protein sensitizes cells to apoptotic killing by tumor necrosis factor alpha. Proc Natl Acad Sci U S A. 1997;94:8744–8749. doi: 10.1073/pnas.94.16.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terradillos O, Pollicino T, Lecoeur H, et al. p53-independent apoptotic effects of the hepatitis B virus HBx protein in vivo and in vitro. Oncogene. 1998;17:2115–2123. doi: 10.1038/sj.onc.1202432. [DOI] [PubMed] [Google Scholar]
- 14.Shirakata Y, Koike K. Hepatitis B virus X protein induces cell death by causing loss of mitochondrial membrane potential. J Biol Chem. 2003;278:22071–22078. doi: 10.1074/jbc.M301606200. [DOI] [PubMed] [Google Scholar]
- 15.Kim KH, Seong BL. Pro-apoptotic function of HBV X protein is mediated by interaction with c-FLIP and enhancement of death-inducing signal. EMBO J. 2003;22:2104–2116. doi: 10.1093/emboj/cdg210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elmore LW, Hancock AR, Chang SF, et al. Hepatitis B virus X protein and p53 tumor suppressor interactions in the modulation of apoptosis. Proc Natl Acad Sci U S A. 1997;94:14707–14712. doi: 10.1073/pnas.94.26.14707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diao J, Khine AA, Sarangi F, et al. X protein of hepatitis B virus inhibits Fas-mediated apoptosis and is associated with up-regulation of the SAPK/JNK pathway. J Biol Chem. 2001;276:8328–8340. doi: 10.1074/jbc.M006026200. [DOI] [PubMed] [Google Scholar]
- 18.Shin GC, Kang HS, Lee AR, Kim KH. Hepatitis B virus-triggered autophagy targets TNFRSF10B/death receptor 5 for degradation to limit TNFSF10/TRAIL response. Autophagy. 2016;12:2451–2466. doi: 10.1080/15548627.2016.1239002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang XY, Li D, Chen ZX, et al. Hepatitis B Virus X protein elevates Parkin-mediated mitophagy through Lon Peptidase in starvation. Exp Cell Res. 2018;368:75–83. doi: 10.1016/j.yexcr.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LoGuidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology. 2011;54:969–978. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Du H, Shao S, et al. Cyclophilin D deficiency attenuates mitochondrial perturbation and ameliorates hepatic steatosis. Hepatology. 2018;68:62–77. doi: 10.1002/hep.29788. [DOI] [PubMed] [Google Scholar]
- 23.Laker RC, Taddeo EP, Akhtar YN, Zhang M, Hoehn KL, Yan Z. The mitochondrial permeability transition pore regulator cyclophilin D exhibits tissue-specific control of metabolic homeostasis. PLoS One. 2016;11:e0167910. doi: 10.1371/journal.pone.0167910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teodoro JS, Varela AT, Duarte FV, Gomes AP, Palmeira CM, Rolo AP. Indirubin and NAD(+) prevent mitochondrial ischaemia/reperfusion damage in fatty livers. Eur J Clin Invest. 2018;48:e12932. doi: 10.1111/eci.12932. [DOI] [PubMed] [Google Scholar]
- 25.Zhong Z, Ramshesh VK, Rehman H, et al. Acute ethanol causes hepatic mitochondrial depolarization in mice: role of ethanol metabolism. PLoS One. 2014;9:e91308. doi: 10.1371/journal.pone.0091308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King AL, Swain TM, Mao Z, et al. Involvement of the mitochondrial permeability transition pore in chronic ethanol-mediated liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2014;306:G265–G277. doi: 10.1152/ajpgi.00278.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seehawer M, Heinzmann F, D’Artista L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562:69–75. doi: 10.1038/s41586-018-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conos SA, Chen KW, De Nardo D, et al. Active MLKL triggers the NLRP3 inflammasome in a cell-intrinsic manner. Proc Natl Acad Sci U S A. 2017;114:E961–E969. doi: 10.1073/pnas.1613305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roychowdhury S, McCullough RL, Sanz-Garcia C, et al. Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury. Hepatology. 2016;64:1518–1533. doi: 10.1002/hep.28676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, An P, Xie E, et al. Characterization of ferroptosis in murine models of hemochromatosis. Hepatology. 2017;66:449–465. doi: 10.1002/hep.29117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lőrincz T, Jemnitz K, Kardon T, Mandl J, Szarka A. Ferroptosis is involved in acetaminophen induced cell death. Pathol Oncol Res. 2015;21:1115–1121. doi: 10.1007/s12253-015-9946-3. [DOI] [PubMed] [Google Scholar]
- 34.Biasiotto G, Di Lorenzo D, Archetti S, Zanella I. Iron and neurodegeneration: is ferritinophagy the link? Mol Neurobiol. 2016;53:5542–5574. doi: 10.1007/s12035-015-9473-y. [DOI] [PubMed] [Google Scholar]
- 35.Woodhoo A, Iruarrizaga-Lejarreta M, Beraza N, et al. Human antigen R contributes to hepatic stellate cell activation and liver fibrosis. Hepatology. 2012;56:1870–1882. doi: 10.1002/hep.25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Z, Yao Z, Wang L, et al. Activation of ferritinophagy is required for the RNA-binding protein ELAVL1/HuR to regulate ferroptosis in hepatic stellate cells. Autophagy. 2018;14:2083–2103. doi: 10.1080/15548627.2018.1503146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–114. doi: 10.1016/S0966-842X(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 38.Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 39.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 40.Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mulvihill E, Sborgi L, Mari SA, Pfreundschuh M, Hiller S, Müller DJ. Mechanism of membrane pore formation by human gasdermin-D. EMBO J. 2018;37:e98321. doi: 10.15252/embj.201798321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathurin P, Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62(1 Suppl):S38–S46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209–219. [PMC free article] [PubMed] [Google Scholar]
- 45.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 46.Basra S, Anand BS. Definition, epidemiology and magnitude of alcoholic hepatitis. World J Hepatol. 2011;3:108–113. doi: 10.4254/wjh.v3.i5.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marroni CA, Fleck AM, Jr, Fernandes SA, et al. Liver transplantation and alcoholic liver disease: history, controversies, and considerations. World J Gastroenterol. 2018;24:2785–2805. doi: 10.3748/wjg.v24.i26.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–1800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 49.Khanova E, Wu R, Wang W, et al. Pyroptosis by caspase11/4-gasdermin-D pathway in alcoholic hepatitis in mice and patients. Hepatology. 2018;67:1737–1753. doi: 10.1002/hep.29645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu B, Jiang M, Chu Y, et al. Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 2018;8:773–782. doi: 10.1016/j.jhep.2017.11.040. [DOI] [PubMed] [Google Scholar]
- 51.Kofahi HM, Taylor NG, Hirasawa K, Grant MD, Russell RS. Hepatitis C virus infection of cultured human hepatoma cells causes apoptosis and pyroptosis in both infected and bystander cells. Sci Rep. 2016;6:37433. doi: 10.1038/srep37433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palacios-Macapagal D, Connor J, Mustelin T, et al. Cutting edge: eosinophils undergo caspase-1-mediated pyroptosis in response to necrotic liver cells. J Immunol. 2017;199:847–853. doi: 10.4049/jimmunol.1601162. [DOI] [PubMed] [Google Scholar]
- 53.Lan P, Fan Y, Zhao Y, et al. TNF superfamily receptor OX40 triggers invariant NKT cell pyroptosis and liver injury. J Clin Invest. 2017;127:2222–2234. doi: 10.1172/JCI91075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marin V, Poulsen K, Odena G, et al. Hepatocyte-derived macrophage migration inhibitory factor mediates alcohol-induced liver injury in mice and patients. J Hepatol. 2017;67:1018–1025. doi: 10.1016/j.jhep.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, An R, Umanah GK, et al. A nuclease that mediates cell death induced by DNA damage and poly(ADP-ribose) polymerase-1. Science. 2016;354 doi: 10.1126/science.aad6872. aad6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park EJ, Min KJ, Lee TJ, Yoo YH, Kim YS, Kwon TK. β-Lapachone induces programmed necrosis through the RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma SK-Hep1 cells. Cell Death Dis. 2014;5:e1230. doi: 10.1038/cddis.2014.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jouan-Lanhouet S, Arshad MI, Piquet-Pellorce C, et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun Q, Luo T, Ren Y, et al. Competition between human cells by entosis. Cell Res. 2014;24:1299–1310. doi: 10.1038/cr.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hamann JC, Surcel A, Chen R, et al. Entosis is induced by glucose starvation. Cell Rep. 2017;20:201–210. doi: 10.1016/j.celrep.2017.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sierro F, Tay SS, Warren A, et al. Suicidal emperipolesis: a process leading to cell-in-cell structures, T cell clearance and immune homeostasis. Curr Mol Med. 2015;15:819–827. doi: 10.2174/1566524015666151026102143. [DOI] [PubMed] [Google Scholar]
- 61.Shi J, Zhao J, Zhang X, et al. Activated hepatic stellate cells impair NK cell anti-fibrosis capacity through a TGF-beta-dependent emperipolesis in HBV cirrhotic patients. Sci Rep. 2017;7:44544. doi: 10.1038/srep44544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang Y, Liu Y, Liu X, et al. Citreoviridin induces autophagy-dependent apoptosis through lysosomal-mitochondrial axis in human liver HepG2 cells. Toxins (Basel) 2015;7:3030–3044. doi: 10.3390/toxins7083030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guicciardi ME, Bronk SF, Werneburg NW, Gores GJ. cFLIPL prevents TRAIL-induced apoptosis of hepatocellular carcinoma cells by inhibiting the lysosomal pathway of apoptosis. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1337–G1346. doi: 10.1152/ajpgi.00497.2006. [DOI] [PubMed] [Google Scholar]
- 64.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–1503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kharbanda KK, McVicker DL, Zetterman RK, Donohue TM., Jr Ethanol consumption reduces the proteolytic capacity and protease activities of hepatic lysosomes. Biochim Biophys Acta. 1995;1245:421–429. doi: 10.1016/0304-4165(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 66.Chao X, Wang S, Zhao K, et al. Impaired TFEB-mediated lysosome biogenesis and autophagy promote chronic ethanol-induced liver injury and steatosis in mice. Gastroenterology. 2018;155:865–879. doi: 10.1053/j.gastro.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kreuzaler PA, Staniszewska AD, Li W, et al. Stat3 controls lysosomal-mediated cell death in vivo. Nat Cell Biol. 2011;13:303–309. doi: 10.1038/ncb2171. [DOI] [PubMed] [Google Scholar]
- 68.Annibaldi A, Wicky John S, Vanden Berghe T, et al. Ubiquitin-mediated regulation of RIPK1 kinase activity independent of IKK and MK2. Mol Cell. 2018;69:566–580. doi: 10.1016/j.molcel.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hitomi J, Christofferson DE, Ng A, et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chung H, Vilaysane A, Lau A, et al. NLRP3 regulates a non-canonical platform for caspase-8 activation during epithelial cell apoptosis. Cell Death Differ. 2016;23:1331–1346. doi: 10.1038/cdd.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alvarez-Diaz S, Dillon CP, Lalaoui N, et al. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Donnell MA, Perez-Jimenez E, Oberst A, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mascarenhas DP, Cerqueira DM, Pereira MS, et al. Inhibition of caspase-1 or gasdermin-D enable caspase-8 activation in the Naip5/NLRC4/ASC inflammasome. PLoS Pathog. 2017;13:e1006502. doi: 10.1371/journal.ppat.1006502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Gao W, Shi X, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 75.Taabazuing CY, Okondo MC, Bachovchin DA. Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem Biol. 2017;24:507–514. doi: 10.1016/j.chembiol.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orozco S, Yatim N, Werner MR, et al. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death Differ. 2014;21:1511–1521. doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ting AT, Pimentel-Muiños FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NF-kappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. doi: 10.1002/j.1460-2075.1996.tb01007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Basu S, Rajakaruna S, Menko AS. Insulin-like growth factor receptor-1 and nuclear factor kappaB are crucial survival signals that regulate caspase-3-mediated lens epithelial cell differentiation initiation. J Biol Chem. 2012;287:8384–8397. doi: 10.1074/jbc.M112.341586. [DOI] [PMC free article] [PubMed] [Google Scholar]