Abstract
Cancer is now considered a multifactorial disorder with different aetiologies and outcomes. Yet, all cancers share some common molecular features. Among these, the reprogramming of cellular metabolism has emerged as a key player in tumour initiation and progression. The finding that metabolic enzymes such as fumarate hydratase (FH), succinate dehydrogenase (SDH) and isocitrate dehydrogenase (IDH), when mutated, cause cancer suggested that metabolic dysregulation is not only a consequence of oncogenic transformation, but that it can act as cancer driver. However, the mechanisms underpinning the link between metabolic dysregulation and cancer remain only partially understood. In this review we discuss the role of FH loss in tumorigenesis, focusing on the role of fumarate as a key activator of a variety of oncogenic cascades. We also discuss how these alterations are integrated and converge towards common biological processes. This review highlights the complexity of the signals elicited by FH loss, describes that fumarate can act as a bona fide oncogenic event, and provides a compelling hypothesis of the step wise neoplastic progression after FH loss.
Keywords: FH, mitochondria, fumarate, cancer, metabolism.
1. Introduction
Oncogenesis is a multistep process during which cells acquire molecular features known as “Hallmarks of Cancer”, which pave the way to malignant transformation [1]. The reprogramming of cellular metabolism is now widely considered a pivotal hallmark of cancer that allows cancer cells to survive, proliferate, and metastasize[2]. Although added to the list of the hallmarks only recently [2], a first piece of evidence that cellular metabolism is reprogrammed in cancer was provided already in 1887 by Ernst Freund, a Viennese physicians, who observed high sugar levels in the blood of cancer patients [3]. Based on this observation he proposed that reducing the amount of sugar could impact the tumour growth [3]. In 1911 the German scientist Wassermann postulated that accelerated proliferation of cancer cells was associated with an increased oxygen consumption [4]. To validate this hypothesis, he tried, without success though, to target tumours using inhibitors of respiration such as selenium derivates [4]. Just two years later, in 1913, Eleanor Van Ness Van Alstyne and colleagues showed that increased carbohydrate intake resulted in accelerated rat sarcoma growth [5], which was further confirmed by William Woglom in 1915 [6]. These works supported the notion that tumours use nutrients such as glucose and oxygen in a different way than normal tissue. Few years later, these findings were systematically investigated by Otto Warburg. He demonstrated that cancer cells ferment most of their glucose to lactate even in the presence of normal levels of oxygen, when glucose should be fully oxidised to carbon dioxide through cellular respiration [7]. After the discovery that respiration is carried out by the mitochondria, Warburg concluded that all cancers must originate from a mitochondrial dysfunction [8].
After Warburg’s discoveries, the field of cancer metabolism was neglected until the beginning of the 21st century, when major discoveries and technical advances, including the advent of metabolomics, rekindled the field. Furthermore, thanks to the availability of large collections of gene expression data from cancer patients, the metabolic landscape of cancer could be extensively assessed using gene expression of metabolic enzymes. These bioinformatics analyses showed that both nuclear and mitochondrial DNA-encoded mitochondrial genes are suppressed in cancer [9–11] and this feature is associated with poor clinical outcome and metastasis [9]. Noteworthy, not all tumours exhibit mitochondrial impairment and it should be highlighted that the complete loss of mitochondrial function can be detrimental for cancer cells [12,13]. The role of mitochondrial dysfunction in cancer was further corroborated by recent sequencing efforts that led to the discovery that mitochondrial genes, including fumarate hydratase (FH), succinate dehydrogenase (SDH) and isocitrate dehydrogenase (IDH), when mutated, cause hereditary and sporadic forms of cancer (reviewed in [14]). Although these discoveries were made almost twenty years ago, the mechanisms underpinning transformation in these metabolically impaired tumours are still under intense investigation and could provide unique mechanistic insights into the link between dysregulated mitochondrial function and transformation. In this review we will focus on the role of FH loss in cancerous transformation.
2. Fumarate Hydratase mutations in human diseases
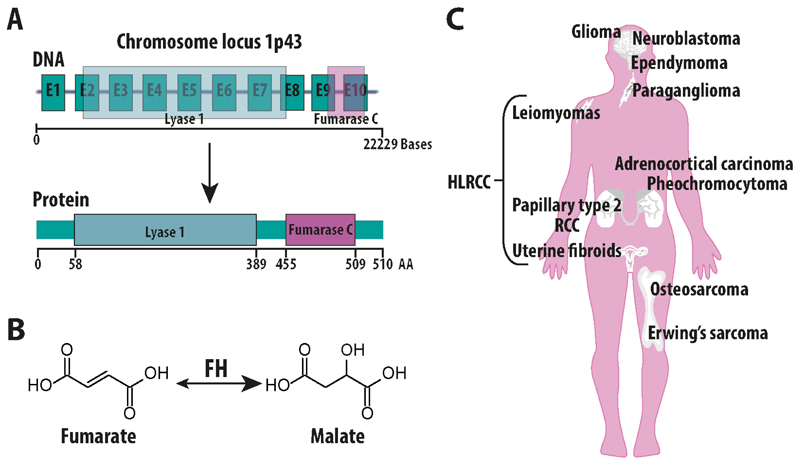
In the human genome the gene encoding FH is located in the chromosome locus 1p43 and encompasses 22229 bases transcribing for 10 exons (NCBI database, NG_012338.1) that give rise to the FH monomer, which exhibits a “tridomain” structure, with a central domain involved in the interactions with the other monomers, an N terminal Lyase 1 domain, and a C-terminal Fumarase C domain (Ensembl database, FH-001 ENST00000366560.3) (Fig. 1A). Interestingly, the FH gene encodes for both the cytosolic and the mitochondrial variant, which differ in the peptide sequence at the N-terminus [15]. Despite the existence of a mitochondrial and a cytosolic isoform of FH, known as echoforms [16], how these two variants are generated was matter of debate. Whilst it was initially proposed that these two isoforms are generated by differential mRNA processing [17], more recent data suggest that they result from an alternative initiation of the transcription [18]. The homotetrameric mitochondrial FH protein, is part of the tricarboxylic acid (TCA) cycle, where it catalyses the reversible hydration of fumarate to malate [19] (Fig. 1B).
Figure 1. Fumarate Hydratase and cancer.
A) schematic representation of Fumarate Hydratase (FH) gene and protein. B) Depiction of the chemical reaction catalysed by FH, which converts fumarate to malate. C) Representation of the various tissue where the sporadic or hereditary loss of FH leads to cancer. HLRCC: hereditary Leiomyomatosis and renal cell carcinoma.
Mutations of FH have been described in the literature and have been implicated in the pathogenesis of various diseases. For instance, the homozygous germline loss of FH is the cause of an autosomal recessive metabolic diseases called fumaric aciduria (OMIM #606812), which was first reported in 1983 by Whelan and colleagues [20]. Patients with fumaric aciduria display a biallelic loss of FH due to missense and frameshift mutations or partial deletions, which results mainly in brain abnormalities, developmental delay, and accumulation of fumarate in the urine [21]. Patients affected by fumaric aciduria rarely survive childhood. Unfortunately, apart from dietary interventions with unclear efficacy, there are no therapies available for this disease [22].
Heterozygous germline mutations of FH predispose to Hereditary Leiomyomatosis and Renal Cell Cancer (HLRCC), a cancer syndrome characterised by cutaneous, uterine leiomyomas, and renal cancer [23]. HLRCC patients harbour one mutant FH allele and the loss of the wild type allele by loss of heterozygosity leads to benign tumours of the skin and uterus, and papillary type II renal cell carcinomas (RCC), one of the most aggressive forms of renal cancer characterised by early metastasis and a poor clinical outcome [24]. So far, no correlation between the site of mutations and clinical outcome in patients has been established, indicating that the loss of FH activity, rather than any neomorphic functions of the mutant protein, is responsible for cellular transformation [25]. Interestingly, the sporadic loss of FH has been reported in other tumour types such as pheochromocytomas, paragangliomas [26,27], adrenocortical carcinoma [28], neuroblastomas [28,29], glioma, ependymoma, osteosarcoma, and Erwing’s sarcoma [28] (Fig. 1C). Consistent with a broader role of FH in tumorigenesis, its transcriptional downregulation was found in sporadic clear cell carcinomas [30] and in colorectal cancer [31], and additional evidence suggests the involvement of FH mutations in breast, bladder, and testicular cancers [32]. These findings hint at a key role of FH loss in human cancers. Yet, how its loss promotes tumorigenesis is still debated. The molecular alterations caused by FH loss that are implicated in tumorigenesis will be discussed in the next paragraphs.
3. FH loss induces a multi-layer cellular reprogramming that leads to transformation
3.1. Metabolic rewiring in FH-deficient cells
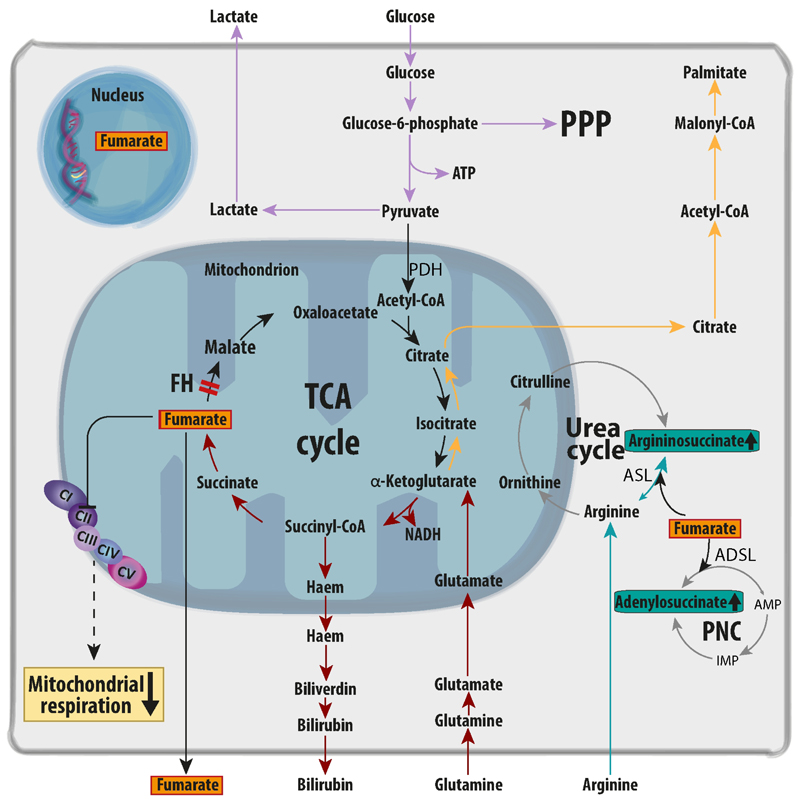
The TCA cycle is a set of metabolic reactions within the mitochondria that represents the final converging route for the oxidation of lipids, carbohydrates, and amino acids [33]. Consequently, TCA cycle enzymes are essential for cell growth and survival, and it came as a surprise that FH loss could not only be tolerated by cells, but that it could also cause cancer. Therefore, it was argued that FH-deficient cells must respond to this mitochondrial impairment by compensatory metabolic changes. We and others have extensively investigated these metabolic changes, which are summarised in Fig. 2. First, as often observed in mitochondrial diseases, FH-deficient cells increase their glycolytic rates and instead of oxidising glucose in the mitochondria they shunt it into lactate production [34] and other glycolytic branches, including the pentose phosphate pathway (PPP) [35]. Interestingly, as will be detailed in section 3.2.2, this glycolytic shift is supported by a transcriptional reprogramming of glycolytic enzymes and the inhibition of pyruvate dehydrogenase (PDH), which in turn blocks the entry of glucose into the mitochondria [36] (Fig. 2 and Fig. 4). To fuel this truncated TCA cycle when glucose entry in the mitochondria is reduced, glucose is replaced by glutamine as the main source of carbons [34] (Fig. 2). Glutamine fuels part of the TCA cycle supplying α-ketoglutarate (αKG) allowing through αKG dehydrogenase the generation of NADH, used by oxidative phosphorylation (OXPHOS) for ATP generation and for the maintenance of mitochondrial membrane potential [34]. The latter is necessary for a variety of mitochondrial processes that needs to be preserved for cell survival, including protein translocation, ion exchange and metabolite transport, and it also regulates mitochondrial quality control, cell death, and mitochondrial retrograde signalling [37]. To maintain this linear set of reactions without reaching saturation due to FH loss, some glutamine-derived carbons are diverted towards the haem biosynthesis and degradation pathway, which is essential for the survival of FH-deficient cells [34] (Fig. 2). Moreover, in human FH-mutant cells, UOK262 cells, glutamine is converted to αKG and eventually to citrate for lipid biosynthesis via the reversal of IDH and aconitase (ACO) in a process called reductive carboxylation [38] (Fig. 2). Of note, this process uses both cytosolic and mitochondrial NADP+-dependent IDH isoforms (IDH1 and 2, respectively) and provides key TCA cycle intermediates normally generated from glucose, such as citrate and isocitrate, and also Acetyl-CoA for lipid biosynthesis [38,39] (Fig. 2). The presence of reductive carboxylation in FH-deficient cells is controversial since it was observed in human FH-deficient cells, but not in mouse Fh1-deficient epithelial cells[34] or fibroblasts [40]. This apparent inconsistency between the mouse and human models could be explained by the fact that the human FH-deficient cells accumulate lower levels of fumarate than the mouse counterparts [41]. As a key component of the set of reactions of reductive carboxylation, the mitochondrial ACO (ACO2), is inactivated by fumarate [40] (more details in section 3.2.1), it is possible that the lower fumarate levels observed in UOK262 could spare ACO2 from fumarate-driven inactivation, preserving the ability to perform reductive carboxylation in these cells. Given that reductive carboxylation offers important bioenergetics and biosynthetic advantages for tumour growth in cells with defective mitochondria [38] it is also possible that FH-deficient clones harbouring lower levels of fumarate and preserved ACO2 function are selected during tumour progression.
Figure 2. Metabolic rewiring in FH-deficient cells.
The biallelic loss of FH leads to the truncation of the TCA cycle and the subsequent accumulation of fumarate (highlighted in orange). The combined disruption of the TCA cycle and the inhibition of Succinate Dehydrogenase (also known as Complex II of the respiratory chain) by fumarate significantly reduce mitochondrial respiration. To compensate for the loss of mitochondrial function, FH-deficient cells engage in a complex biochemical rewiring. First, FH-deficient cells shift towards aerobic glycolysis reducing the oxidation of glucose in the mitochondria (lilac arrows). Part of carbons from glucose are diverted toward the pentose phosphate pathway (PPP) to maintain redox homeostasis (lilac arrow). Furthermore, to maintain the remaining TCA cycle activity and sufficient NADH generation, FH-deficient cells increase glutamine oxidation (red arrows). Glutamine-derived carbons are further metabolized to fumarate and, via the haem pathway, to biliverdin and bilirubin, which is secreted in the medium, or are used to generate lipogenic acetyl-CoA via reductive carboxylation (yellow arrows). FH-deficient cells also activate multiple strategies to buffer the potentially toxic accumulation of fumarate. For instance, fumarate permeates the various intracellular compartments, including the nucleus, and can be released in the extracellular milieu. Fumarate accumulation leads to the aberrant production of argininosuccinate via the reversal of the urea cycle enzyme argininosuccinate lyase (ASL) (turquoise arrows). Of note, FH-deficient cells require constant supply of exogenous arginine to maintain this buffering system active and die when arginine is depleted. Finally, fumarate leads to the accumulation of adenylosuccinate, likely via the reversal of adenylosuccinate lyase (ADSL) within the purine nucleotide cycle (PNC). AMP= adenosine monophosphate; CI-V=Electron transport chain Complex I-V; PDH: pyruvate dehydrogenase; GLUT1=glucose transporter 1; HMOX1=haem oxygenase 1; IMP= inosine monophosphate.
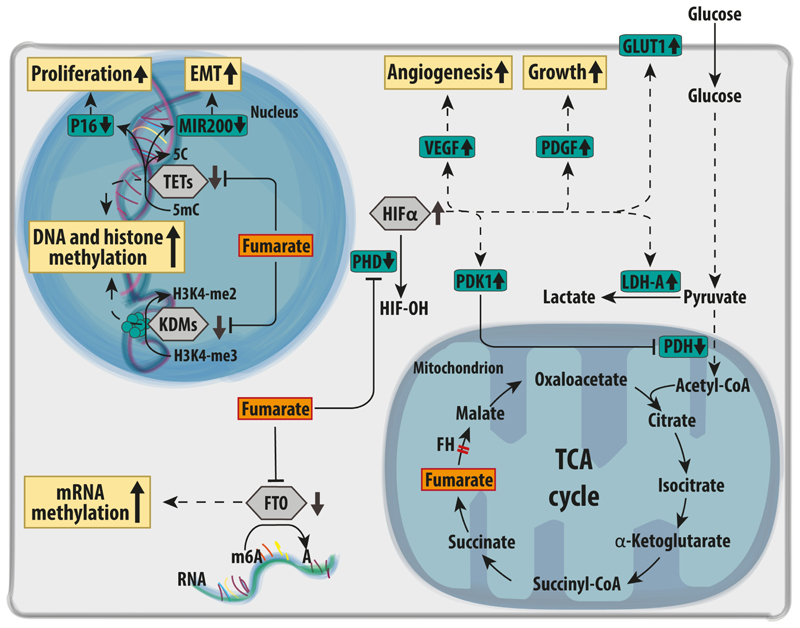
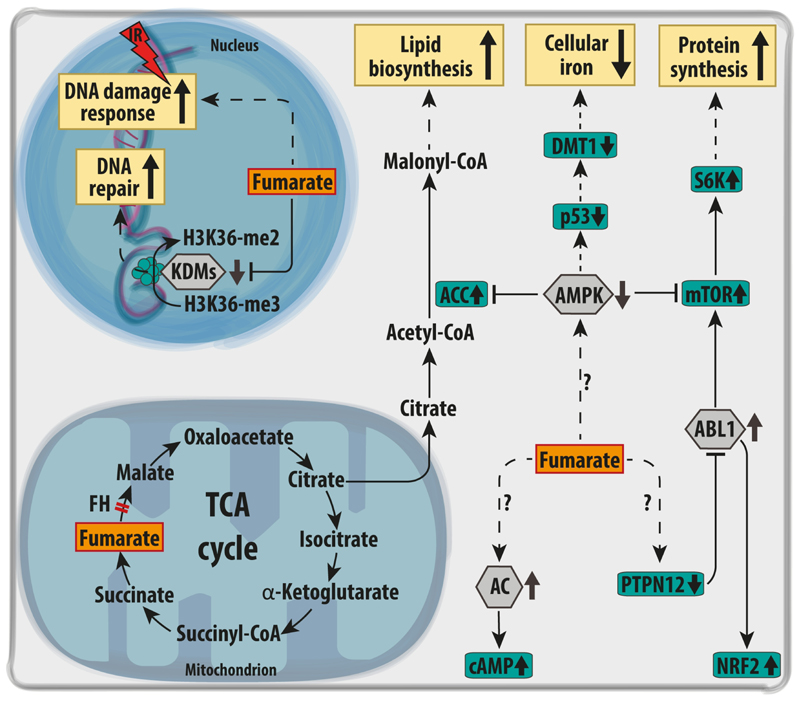
Figure 4. Oncogenic signalling mediated via aKGDDs inhibition in FH-deficient cells.
Upon FH loss, fumarate accumulation inhibits the activity of various aKGDDs (grey hexagons). For instance, fumarate inhibits prolyl hydroxylases (PHDs), causing the stabilisation of the alpha subunit of a family of hypoxia inducible factors (HIFs) even in the presence of normal oxygen levels. The transcriptional response elicited by HIFs promotes angiogenesis, tumour growth, and aerobic glycolysis via increased expression of the glucose transporter GLUT1, and lactate dehydrogenase (LDHA). Furthermore, HIF triggers the expression of pyruvate dehydrogenase kinase 1 (PDK1), which phosphorylates and inhibits pyruvate dehydrogenase complex (PDH), a gatekeeper of glucose-derived pyruvate in the mitochondria. In the nucleus, fumarate accumulation induces a profound epigenetic reprogramming due to the inhibition of both DNA and histone demethylases (TETs and KDMs respectively). In particular, the inhibition of the demethylation of miR200 was shown to trigger an epithelial-to-mesenchymal transition (EMT) in FH-deficient cells. Finally, the inhibition of the RNA demethylase FTO by fumarate accumulation is predicted to increase RNA methylation. A=adenosine; FTO=Fat Mass and Obesity-Associated Protein; H3K4-me2= dimethylated arginine 4 in Histone H3; H3K4-3me= trimethylated H3K4; HIFα= hypoxia inducible factor subunit α; HIF-OH= hydroxylated; KDMs=Lysine Demethylases; miR200-C= unmethylated microRNA 200 gene; miR200-mC= methylated microRNA 200 gene; m6A=N6 methyl-adenosine; PDGF= Platelet Derived Growth Factor; TETs= Ten-Eleven Translocation Gene Proteins; VEGF=Vascular Endothelial Growth Factor.
Fumarate accumulates to millimolar levels in FH-deficient cells[41] and it is the most striking biochemical feature associated to FH loss [34,41]. At this concentration, fumarate could permeate multiple subcellular compartments including mitochondria, cytosol, and nuclei [42–44] as well as the extracellular microenvironment [36]. High fumarate levels could alter the balance of multiple enzymatic reactions in which this metabolite is directly involved as either substrate or product. For instance, it was shown that fumarate accumulation impacts the conversion of succinate to fumarate by SDH in the TCA cycle, reducing SDH-dependent mitochondrial respiration [45] (Fig. 2). Other examples of pathways dysregulated by fumarate accumulation are the urea cycle [43] and the purine nucleotide cycle (PNC) [46] (Fig. 2). Within the urea cycle, argininosuccinate produced from citrulline and aspartate is normally converted to arginine and fumarate by the enzyme argininosuccinate lyase (ASL) [41]. The accumulation of fumarate can reverse this reaction, driving the synthesis of argininosuccinate from exogenous arginine and fumarate [41] (Fig. 2). In turn, FH-deficient cells require arginine to buffer fumarate and its depletion is lethal for FH-deficient cells [41,43]. Another metabolic pathway predicted to be affected by fumarate accumulation is the PNC, whereby the increase in fumarate may cause the reversal of adenylosuccinate lyase (ADSL) to form adenylosuccinate (Fig. 2). However, besides the observation of an accumulation of adenylosuccinate in mouse Fh1-deficient cells [47], there is no formal experimental evidence that ADSL reversal occurs in FH-deficient cells. Overall, these results show that the loss of FH leads to profound metabolic changes that are required to compensate for the truncation of the TCA cycle and for the aberrant accumulation of fumarate. Failure to activate these metabolic scape valves has been shown to be detrimental for FH deficient cells [48], arguing that these changes are a first essential step for tumorigenesis.
3.2. Pro-oncogenic signalling activated by FH loss
3.2.1. Oncogenic signalling via fumarate-dependent succination
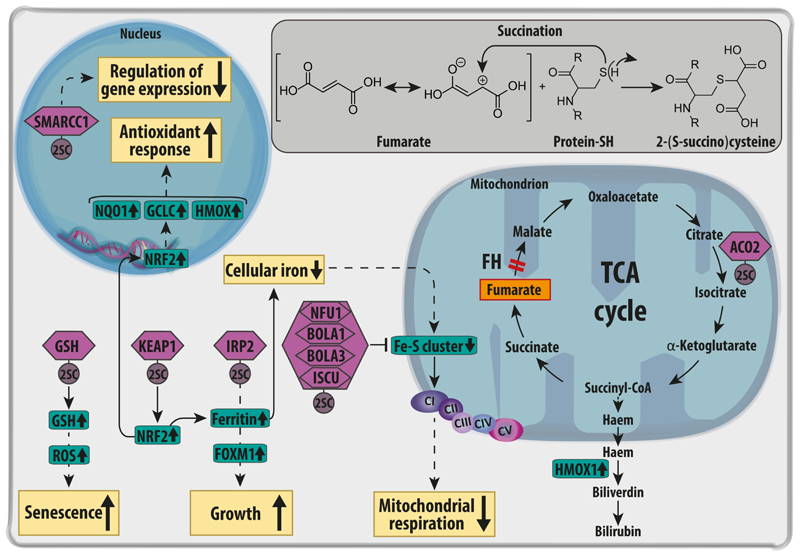
Besides the above-described metabolic reprogramming (section 3.1.), whose contribution to the transformation process is still unclear, FH loss and fumarate accumulation elicit a plethora of pro-oncogenic signals that can directly contribute to transformation. The first type of oncogenic signalling activated by fumarate is related to its chemical structure. Fumarate is a mild electrophilic molecule due to the low electron density in its double bond caused by the conjugation to two carboxylic acid residues [49] (Insert in Fig. 3). At acidic pH conditions typical of cancer cells [50], fumarate can react with nucleophilic residues such as thiol groups from cysteine residues exposed at the surface of proteins, generating a stable thioether known as S-(2-succino) cysteine (2SC) [51]. This post-translational modification, succination [52], is not only irreversible and resistant to acidic hydrolysis, but it manifests only at pathological levels of fumarate, making it an excellent diagnostic marker of FH-deficiency in cancer patients [53]. So far, various proteins have been identified as targets of succination including the Kelch-like ECH-associated protein1 (KEAP1) [54,55], iron regulatory protein 2 (IRP2) [56], the iron-sulfur-cluster (Fe-S cluster) biogenesis family of proteins [45], aconitase (ACO2) [40], glutathione (GSH) [57,58] and SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin Subfamily C Member 1 (SMARCC1)[50] (Fig. 3). We will briefly describe them within this section below.
Figure 3. Targets of succination in FH-deficient cells.
Fumarate, accumulated upon FH loss, leads to a post translational modification of cysteine residues of a variety of proteins (violet hexagons) called succination. The chemical reaction between fumarate and reactive thiol residues of proteins is in depicted in the insert. Succination of KEAP1 causes the stabilisation and activation of the NRF2-mediated anti-oxidant response. One of the targets of NRF2 is Haem Oxygenase 1 (HMOX1), which is required for the haem biosynthesis and degradation pathway, an essential pathway for the survival of FH-deficient cells. Succination of Iron Responsive Element Binding Protein 2 (IRP2) inhibits the repressive function of this protein on the translation of ferritin. The subsequent increase in ferritin causes a drop in free intracellular iron. In parallel, ferritin promotes the expression of Forkhead box protein M1 (FOXM1), a pro-mitotic protein that supports cell growth. Succination of the Fe-S cluster proteins Nfu1, Bola and Iscu impairs the Fe-S clusters assembly required by the electron transport chain complex I, contributing to defects in mitochondrial respiration. The reduction of iron and the succination of key cysteine residues in its catalytic core also inactivates the TCA cycle enzyme Aconitase 2 (ACO2). In the nucleus, the succination of SWI/SNF complex protein SMARCC1 inactivates this complex, affecting gene expression and chromatin remodelling. Finally, GSH succination causes the depletion of glutathione (GSH) stores, increasing oxidative stress, and triggering senescence in primary FH-deficient cells. CI-V=electron transport chain complex I-V; KEAP1=Kelch Like ECH Associated Protein 1; Bola1-3=BolA Family Member 1-3ISCU= Iron-Sulfur Cluster Assembly Enzyme; NFU1=NFU1 Iron-Sulfur Cluster Scaffold; NRF2=Nuclear Factor, Erythroid 2 Like 2; SMARCC1=SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin Subfamily C Member 1. GCLC: glutamate-cysteine ligase; NQO1: NADH quinone oxidase 1.
The succination of KEAP1 was one of the first post-translational modifications triggered by fumarate to be identified [54,55]. Under physiological conditions, KEAP1, a E3 ubiquitin ligase, binds Nuclear Factor, Erythroid 2 Like 2 (NRF2) protein, priming it for proteasomal degradation [59]. NRF2 is a transcription factor that belongs to the family of basic leucine zippers (bZIP) activated in response to oxidative stress [59]. In FH-deficient cells, the succination and subsequent inactivation of KEAP1 prevents the degradation of NRF2, which in turn is allowed to translocate in the nucleus, mounting a potent antioxidant response mediated by genes such as haem oxygenase 1 (HMOX), NAD(P)H dehydrogenase quinone 1 (NQO1), and glutamate-cysteine ligase catalytic subunit (GCLC) [55,60]. The role of NRF2 in tumorigenesis is debated and context-dependent. On one hand, NRF2 activation can prevent cellular transformation triggered by specific carcinogens such as benzo[α]pyrene and aflatoxin B1 by facilitating their detoxification (REF). On the other hand, the antioxidant response activated by NRF2 elicited by oncogenes such as Kras, Braf and Myc can prevent the damages caused by oxidative stress, and therefore favour tumour survival [61]. The contribution of NRF2 to cellular transformation in FH-deficient cells is still unclear but we will discuss its potential role later in the section 4
Another target of succination is the iron regulatory protein 2 (IRP2) [56] (Fig. 3), a protein that normally suppresses the translation of ferritin, a key player in iron homeostasis [62]. When inactivated by succination, IRP2 allows the expression of ferritin, leading to the depletion of freely available iron [56] with important consequences for the Fe-S cluster formation. The depletion of Fe-S clusters may indirectly induce a mitochondrial dysfunction through the impairment of RC and other mitochondrial proteins, potentially leading to transformation. Moreover, the upregulation of ferritin was also shown to activate forkhead box protein M1 (FOXM1), promoting cell growth [56].
Fe-S cluster assembly family of proteins is another target of succination (Fig. 3). These proteins include Fe-S cluster scaffold (NFU1), Fe-S cluster assembly enzyme (ISCU) 1 and 2, and BolA family member (BOLA) 1 and 3 [45]. This family of proteins is required for the correct synthesis and integration of Fe-S cluster in various proteins, including electron transport chain complexes I, II, III, and ACO2 [45]. Through the impairment of the assembly of Fe-S clusters, fumarate indirectly reduces the activity of the RC complex I, which together with the above-described inhibition of complex II (Section 3.1.), reduces the overall activity of the RC [45].
ACO2 was also found to be succinated by fumarate on three different cysteine residues (C385, C448, and C451) in Fh1-/- MEFs [40]. As a consequence of succination, ACO2 activity is impaired and this inactivation may prevent Fh1-deficient MEFs to use glutamine for citrate formation through reductive carboxylation[40].
Succination can also target the tripeptide glutathione (GSH) [57,63]By depleting this important antioxidant molecule, FH-deficient cells experience an increased oxidative stress, which is balanced by an increase in GSH biosynthesis [57].
Finally, very recent work showed that SMARCC1, a member of the SWI–SNF tumour-suppressor complex, is succinated by fumarate on cysteine residue 520 [64]. Of note, this complex functions as a ATP-dependent chromatin remodelling factor and regulates the structure of the nucleosome [64]. Intriguingly, the cysteine sensitive to fumarate is contained within the SWIRM domain of the protein, usually mutated in other cancer types and also responsible for interaction with other downstream proteins such as SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily B, Member 5 (SNF5) [50]. As a consequence of succination, the interaction between SMARCC1 and SNF5 is weakened and HLRCC cells shows similar transcriptional profile compared to SNF5-deficent cell s[50].
Together, these pieces of evidence show that upon FH loss the accumulation of fumarate can trigger a broad range of signalling cascades via succination, and the role of these cascades in tumorigenesis of HLRCC is only now beginning to be understood.
3.2.2. Oncogenic signalling mediated by fumarate via aKGDDs inhibition
Another target of fumarate accumulation is the superfamily of αKG-dependent-dioxygenases (aKGDDs), proteins involved in multiple biological processes, including protein hydroxylation, DNA and histone demethylation, and RNA modifications (Fig. 4). These enzymes use αKG and oxygen as substrates, iron and vitamin C as cofactors, and produce succinate and carbon dioxide [65]. Fumarate acts as a competitive inhibitor of these enzymes, with important biological consequences, which will be described in this section below.
The human genome encodes for three aKGDDs prolyl-hydroxylases (PHD1-3), all of which use molecular oxygen to generate an hydroxyl group on proline residues of proteins [66]. Under normoxic conditions, PHDs hydroxylate the subunit α of Hypoxia Inducible Factors (HIFs) on two proline (Pro) residues, Pro 402 and Pro 564 [66], leading to their proteasomal degradation [67,68]. In the early 2000s it was shown that the competitive inhibition of PHDs by fumarate can lead to the stabilisation of HIF1-α/HIF2-α [69] even at normal oxygen levels, a phenomenon known as pseudohypoxia [32]. FH-deficient cells display the activation of typical HIF targets that are involved in angiogenesis, growth, and metabolism [70,71] (Fig. 4). Among the target genes of HIFs there are several metabolic enzymes including the glucose transporter 1 (GLUT1) [69], which increases glucose uptake, pyruvate dehydrogenase kinases (PDKs) [72,73], which inhibits pyruvate dehydrogenase (PDH), and lactate dehydrogenase A (LDH-A) [74]. Together, these genes switch off mitochondrial oxidative metabolism and redirect glycolytic pyruvate towards lactate production, as described in section 3.1. and in Fig. 2. Furthermore, HIFs activate vascular endothelial growth factor (VEGF), which has important implication for tumour invasiveness and cross activation of other oncogenic signalling mediated by platelet derived growth factor (PDGF) [69,75,76]. Despite these lines of evidence pointing at an important role of HIFs in HLRCC biology, the role of these transcription factors in tumorigenesis is still debated. For instance, it was shown the genetic deletion of both Hif1and Hif2 does not prevent the formation of premalignant lesions in Fh1-deficient animals, suggesting that at least in this model, HIF proteins are dispensable for tumorigenesis [54].
Within the nucleus of a cell, chromatin structure and function are finely regulated by chemical changes of DNA and histones catalysed by aKGDD DNA and histone demethylases. DNA demethylation is catalysed by a family of proteins known as Ten-Eleven Translocation (TETs) proteins [77], whilst histone demethylation is carried out by Lysine demethylases (KDMs) [78]. By altering the activity of these enzymes, fumarate can affect chromatin organisation, eventually perturbing gene expression.
TETs are a family of three proteins that catalyse the demethylation of cytosine residues on DNA [77], a process linked with the activation of gene expression [79]. Therefore, by blocking TET-dependent DNA demethylation, fumarate could suppress the expression of several genes. For instance, FH loss is associated with the hypermethylation and suppression of the tumour suppressor cyclin dependent kinase inhibitor 2A (CDKN2A) [80,81] (Fig. 4), which encodes for p16, an inducer of senescence [82]. Fumarate can also cause the hypermethylation and suppression of a family of antimetastatic miRNAs, MIR200 [83], known inhibitors of the transcription factors Zinc Finger E-Box Binding Homeobox 1/2 (ZEB1 and ZEB2) and Snail homolog 2 (SNAI2), leading to an epithelial-to-mesenchymal transition (EMT), a process known to promote metastatic dissemination [84,85]. Interestingly, the link between the induction of EMT and FH loss was further strengthen by the finding that the chromatin remodelling factor lymphoid-specific helicase (LSH) triggers an EMT by suppressing FH in nasopharyngeal carcinoma [86].
Another important family of aKGDDs involved in epigenetic reprogramming is the Jumonji-containg histone lysine demethylases (JmjC-KDMs) [87] (Fig. 4). These enzymes remove the methyl group from lysine residues of histones, which are known to regulate chromatin accessibility and gene expression [78,87]. Only recently it has been shown that fumarate accumulation, through inhibition of JmjC-KDMs, increases the global levels of methylation of lysine 4, 27, and 79 of histone 3 (H3K4, H3K27, and H3K79, respectively) [88]. These histone marks are associated to activation and repression of gene transcription, respectively. However, the biological consequences of a fumarate-dependent inhibition of histone demethylation are still unclear.
A less characterised aKGDD target of fumarate is the fat mass and obesity associated (FTO) [89] (Fig. 4). FTO was originally characterised as a protein that catalyses the demethylation of 3-methylthymine (3mT) in single stranded DNA of mice [89]. However, only few years ago it was clarified that its main activity is the demethylation of N6-methyladenosine of RNA [90,91]. Even though it was shown that in vitro FTO is sensitive to fumarate levels as the other aKDDG [89], the role of FTO inhibition within FH-dependent tumorigenesis is still largely unexplored and there is no evidence that FTO is inhibited in FH-deficient tumours.
3.3. Other molecular cascades affected by FH loss
Beyond the inhibition of aKGDDs and the targeting of proteins via succination, other molecular pathways that are potentially involved in the tumorigenic process are differentially modulated in FH-deficient cells. For instance, it has been shown that FH loss alters the activation of the AMP-activated protein kinase (AMPK) [92], the mammalian target of rapamycin (mTOR)[92] and Abelson murine leukaemia viral oncogene homolog 1 (ABL-1) [93,94]. Furthermore, FH-deficient cells exhibit alterations of the cyclic AMP (cAMP) [95] signalling and DNA-damage response (DDR) pathway [96,97]. The role of these cascades in FH-deficient cells will be briefly described in this section below (Fig. 5).
Figure 5. Other molecular cascades affected by FH loss.
Upon FH loss, distinct signalling nodes have been found dysregulated (grey hexagons), and to activate key downstream proteins (green rectangles). For instance, the oxidation and inactivation of the protein phosphatase PTPN12 activates the kinase ABL1, which in turn activates mTOR and NRF2. The activation of mTOR is key to increase general protein synthesis via the phosphorylation of S6K. In parallel, AMPK is suppressed in FH-deficient cells, further activating mTOR and Acetyl CoA carboxylase (ACC), thus promoting lipid biosynthesis. The inactivation of AMPK also leads to the p53-dependent suppression of the iron transporter DMT1, decreasing iron uptake and reducing of the free iron pool. FH deficient cells were shown to depend on the activity of a set of Adenylate Cyclases (AC), which increase the total pool of cyclic AMP (cAMP) in the cells. Finally, the accumulation of fumarate increases resistance to DNA damage by ionising radiations (IR) and favours non-homologous end-joining upon DNA damage, via inhibition of KDM6, a key histone demethylase implicated in chromatin unfolding for DNA repair. ABL1= Abelson murine leukaemia viral oncogene homolog 1; DMT1= Divalent metal transporter 1; H3K36-me2= dimethylated arginine 36 on Histone H3; H3K36-3me= trimethylated H3K36; KDMs=Lysine Demethylases; mTOR= mechanistic target of rapamycin; p53= tumour suppressor protein 53; AMPK= AMP-activated protein kinase; PTPN12=Tyrosine-protein phosphatase non-receptor type 12; S6K= S6 ribosomal protein kinase.
AMPK is a heterotrimeric kinase activated by alterations in the energetic balance [98]. The protein complex consists of two catalytic subunits (α1 and α2) and two regulatory subunits (β and γ) [99]. AMPK activation is tightly controlled by both ADP:ATP and AMP:ATP ratios, acting therefore as sensor of the bioenergetic status of the cells [100]. When cells experience energy stress and AMP levels raise, AMPK blocks anabolic processes and activates catabolism, including fatty acid oxidation and autophagy [101]. In HLRCC tumours, AMPK activity and its phosphorylation are reduced compared to normal kidney tissue [92] (Fig. 5). Even though it is still not clear which mechanisms lead to suppression of AMPK in FH-deficient tumours, this cascades increases the activity of acetyl CoA carboxylase (ACC), one of the main regulatory enzymes of the de novo lipid biosynthesis [92], and causes the repression of divalent metal transporter 1 (DMT1), reducing freely available iron [92]. More importantly, it was shown that AMPK inhibition may cross-activate mTOR signalling in FH-deficient tumours [92]. Consistently, S6 ribosomal protein Kinase (S6K), a downstream effector of mTOR, is phosphorylated in FH-deficient cells, and increases the global protein biosynthesis [92]. Additionally, Linehan and co-workers showed that also ABL-1 activation via the oxidation of the protein-tyrosine phosphatase N12 (PTPN12) converge to mTOR signalling in FH-deficient cells [93]. Intriguingly, the connection between FH and mTOR appears bidirectional since the chronic activation of mTOR complex1 (mTORC1) through the deletion of Tuberous Sclerosis complex1/2 (Tsc1/2) represses Fh1 thus contributing to a fumarate-dependent transformation in RCC mouse model [102].
Another important regulator of cell signalling is the cyclic nucleotide cAMP [103] (Fig. 5). cAMP signalling is often increased in cancer through different molecular strategies that depends on the tumour type [103]. cAMP levels are tightly controlled by two different types of enzymes, adenylate cyclases, which are responsible for the generation of cAMP from ATP, and phosphodiesterases, which convert cAMP back to AMP [103]. Both enzymes respond to other signalling cascades including calcium signalling, calmodulin, calcineurin, and receptor tyrosine kinases [103]. A synthetic lethal screenings performed on FH-deficient cells revealed that multiple adenylate cyclases are essential for FH-deficient cells [95]. The higher turnover of cAMP observed upon FH loss suggests that other downstream targets of cAMP, such as cAMP-activated protein kinases (PKA) may play a role in FH-dependent tumorigenesis [95]; however this aspect of FH biology is largely unexplored.
Another important biological process controlled by FH and fumarate levels is DDR (Fig. 5). DDR is a complex and articulated process that follows DNA damage [104]. Its role has been widely studied in cancer research mainly in connection with genomic instability and radiotherapy [104]. Few recent works shed light on a new function of FH and fumarate accumulation in DDR. It was shown that upon DNA damage, FH translocates in the nucleus at the sites of damage, where it produces a local pool of fumarate that causes the inhibition of histone H3K36 demethylation, an important step in DDR, and the binding of pro-non homologous end joining (pro-NEHJ) proteins [105]. Secondly, the accumulation of fumarate was shown to correlate with increased endogenous DNA damage, inhibition of homologous recombination repair, and increased sensitivity towards poly-ADP ribose polymerase inhibitors (PARP inhibitors) [106]. Finally, FH-deficient cells displayed not only a marked resistance to DNA damage caused by ionising radiation but also an early mitotic entry even in a condition of unrepaired damage [96].
4. A possible paradigm of tumorigenesis in HLRCC
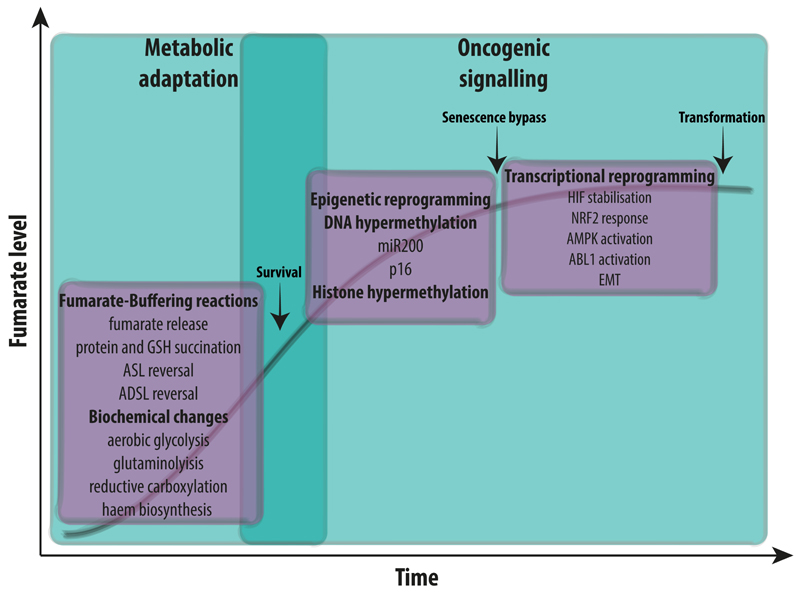
In the previous paragraph of this review we provided compelling evidence that upon FH loss cells orchestrate a multifaceted reprogramming that includes pro-survival metabolic adaptations and the activation of oncogenic cascades. However, the specific contribution of these signalling cascades toward cellular transformation is not fully understood. Based on our current understanding, we postulate that tumorigenesis driven by FH loss occurs via a series of steps over time, largely divided into a “metabolic adaptation” phase, and a subsequent activation of oncogenic signalling cascades mediated by fumarate (Fig. 6). First, upon FH loss, cells must adapt to the profound dysfunction generated by the truncation of the TCA cycle, engaging in a series of compensatory metabolic adaptations, including the switch towards glycolysis, and activation of glutamine oxidation. Under these conditions, fumarate starts to accumulate. In order to tolerate the potentially toxic accumulation of fumarate, cells exploit a series of strategies to buffer this metabolite, including protein succination and the reversal of biochemical pathways that normally produce fumarate. At this stage, FH-deficient cells might experience a profound oxidative stress caused by the disruption of mitochondrial function and depletion of GSH that lead to senescence. The fine tuning of an antioxidant response is likely important for the survival and growth of FH-deficient tumours at this stage. It is tempting to speculate that the activation of antioxidant programmes such as those triggered by NRF2 via succination of KEAP1 are not just important to escape cell death triggered by excessive oxidative stress but could also contribute to avoid senescence, paving the way to transformation in FH-deficient cells. Yet, it should be noted that the activation of NRF2 can be tumour suppressive, by allowing detoxification from excessive fumarate with increased GSH biosynthesis [57] and activating HMOX1 [34]. Additionally, the epigenetic suppression of p16 could be another important step to overcome senescence in FH-deficient cells. In parallel, fumarate accumulation causes the inhibition of multiple aKGDDs. Of note, PHDs/TETs/KDMS inhibition appears to occur via the combination of fumarate accumulation and the parallel decrease in freely available iron, a key cofactor for these enzymes [107]. The inhibition of aKGDDs orchestrates a complex transcriptional and epigenetic rewiring in FH-mutant tumours. Of note, the epigenetic silencing of p16 and activation of EMT can both help to evade senescence and promote cell migration and invasion. Whether these events are involved in the early phases of transformation or if they emerge at a later stage of tumour progression and are implicated in tumour metastasis is still an open question in the field. Understanding this question will be key to elucidate which of the pathways triggered by fumarate should be targeted to prevent tumour formation and/or progression.
Figure 6. Tumorigenesis in FH-deficient cancer.
We hypothesise that tumorigenesis in FH-deficient cells is a multi-step process. First, upon FH loss, cells undergo a series of biochemical adaptations in order to compensate for the loss of FH and for the truncation of the TCA cycle. These compensatory changes support the elevation of intracellular fumarate, which in turn can lead to senescence due, at least in part, to oxidative stress. In parallel, fumarate can induce epigenetic changes, such as hypermethylation of p16, that can enable the bypass of senescence. The activation of additional oncogenic cascades, including those orchestrated by NRF2, ABL1, and HIF contribute to cellular transformation.
Acknowledgments
We thank all the members of the Frezza’s laboratory for insightful discussion.
Authors’ information and funding
CS is a PhD student and MS is a Research Associate in the laboratory of CF. CF is a group leader at the MRC Cancer Unit, University of Cambridge. MS and CF are funded by an MRC Core Funding to the MRC Cancer Unit MRC_MC_UU_12022/6, CS is funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 722605.
Abbreviations
- A
Adenosine
- ABL-1
Abelson murine leukaemia viral oncogene homolog 1
- AC
Adenylate Cyclases
- ACC
Acetyl CoA carboxylase
- ACO
Aconitase
- ADSL
Adenylosuccinate lyase
- AMP
adenosine monophosphate
- AMPK
AMP-activated protein kinase
- ASL
argininosuccinate lyase
- BOLA
BolA family member
- cAMP
cyclic AMP
- CDKN2A
cyclin dependent kinase inhibitor 2A
- CI-V
Electron transport chain Complex I-V
- DMT1
divalent metal transporter 1
- DDR
DNA-damage response
- FOXM1
forkhead box protein M1
- FH
Fumarate hydratase
- FTO
fat mass and obesity associated
- GCLC
glutamate-cysteine ligase catalytic subunit
- GLUT1
glucose transporter 1
- GSH
glutathione
- H3K4-me2
dimethylated arginine 4 in Histone H3
- H3K4-3me
trimethylated arginine 4 in Histone H3
- H3K36-me2
dimethylated arginine 36 on Histone H3
- H3K36-3me
trimethylated arginine 36 on Histone H3
- HIFα
Hypoxia inducible factor subunit α
- HIF-OH
hydroxylated Hypoxia inducible factor
- HIFs
Hypoxia Inducible Factors
- HLRCC
Hereditary Leiomyomatosis and Renal Cell Cancer
- HMOX
haem oxygenase 1
- IDH
isocitrate dehydrogenase
- IMP
inosine monophosphate
- IR
ionising radiations
- IRP2
iron regulatory protein 2
- ISCU
Fe-S cluster assembly enzyme
- JmjC-KDMs
Jumonji-containg histone lysine demethylases
- KDMs
Lysine demethylases
- KEAP1
Kelch-like ECH-associated protein1
- αKG
α-ketoglutarate
- aKGDDs
αKG-dependent-dioxygenases
- LDH-A
lactate dehydrogenase A
- miR200-C
unmethylated microRNA 200 gene
- miR200-mC
methylated microRNA 200 gene
- m6A
N6 methyl-adenosine
- mTORC1
mTOR complex1
- NFU1
Fe-S cluster scaffold
- NQO1
NAD(P)H dehydrogenase quinone 1
- NRF2
Nuclear Factor, Erythroid 2 Like 2
- p53
tumour suppressor protein 53
- PARP
poly-ADP ribose polymerase inhibitors
- PDGF
platelet derived growth factor
- PDH
pyruvate dehydrogenase
- PDKs
pyruvate dehydrogenase kinases
- PHDs
prolyl-hydroxylases
- PNC
purine nucleotide cycle
- PPP
pentose phosphate pathway
- pro-NEHJ
pro-non homologous end joining
- PTPN12
protein-tyrosine phosphatase N12
- RCC
renal cell carcinomas
- SNAI2
Snail homolog 2
- 2SC
S-(2-succino) cysteine
- SDH
succinate dehydrogenase
- S6K
S6 ribosomal protein Kinase
- SMARCC1
SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin Subfamily C Member 1
- SNF5
SWI/SNF Related, Matrix Associated, Actin Dependent Regulator Of Chromatin, Subfamily B, Member 5
- TETs
Ten-Eleven Translocation
- TCA
Tricarboxylic acid
- Tsc1/2
Tuberous Sclerosis complex1/2
- VEGF
vascular endothelial growth factor
- ZEB
Zinc Finger E-Box Binding Homeobox
Footnotes
Competing interests
CF is member of the scientific advisory board of Owlstone Medicals, Cambridge, UK; and a scientific advisor for Istesso Limited, London, UK
Authors’ contribution
CS, MS and CF jointly wrote the manuscript.
References
- [1].Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [2].Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [3].Freund E. Zur Diagnose des Carcinoms. Wiener Medizinische Blätter. 1885;9:268–269. https://www.nytimes.com/1887/12/24/archives/sugar-and-cancer.html. [Google Scholar]
- [4].Wassermann M, Keysser AV, Wassermann F. Beiträge zum problem: Geschwülste von der blutbahn aus therapeutisch zu beeinflussen. Dtsch Medizinische Wochenzeitschrift. 1911;37:2389–2391. [Google Scholar]
- [5].Van Alstyne EV, Beebe SP. Diet Studies in transplantable Tumors : I. The Effect of non-carbohydrate Diet upon the Growth of transplantable Sarcoma in Rats. J Med Res. 1913;29:217–232. [PMC free article] [PubMed] [Google Scholar]
- [6].Woglom WH. DIET AND TUMOR GROWTH. J Exp Med. 1915;22:766–779. doi: 10.1084/jem.22.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Warburg OPK, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochem Zeitschr. 1924;152:309–344. [Google Scholar]
- [8].Warburg O. On the Origin of Cancer Cells. Science (80-. ) 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- [9].Gaude E, Frezza C. Tissue-specific and convergent metabolic transformation of cancer correlates with metastatic potential and patient survival. Nat Commun. 2016;7 doi: 10.1038/ncomms13041. 13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Reznik E, Miller ML, Şenbabaoğlu Y, Riaz N, Sarungbam J, Tickoo SK, Al-Ahmadie HA, Lee W, Seshan VE, Hakimi AA, Sander C. Mitochondrial DNA copy number variation across human cancers. Elife. 2016;5:e10769. doi: 10.7554/eLife.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Reznik E, Wang Q, La K, Schultz N, Sander C. Mitochondrial respiratory gene expression is suppressed in many cancers. Elife. 2017;6:e21592. doi: 10.7554/eLife.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Joshi S, Tolkunov D, Aviv H, Hakimi AA, Yao M, Hsieh JJ, Ganesan S, Chan CS, White E. The Genomic Landscape of Renal Oncocytoma Identifies a Metabolic Barrier to Tumorigenesis. Cell Rep. 2015;13:1895–1908. doi: 10.1016/j.celrep.2015.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bajzikova M, Kovarova J, Coelho AR, Boukalova S, Oh S, Rohlenova K, Svec D, Hubackova S, Endaya B, Judasova K, Bezawork-Geleta A, et al. Reactivation of Dihydroorotate Dehydrogenase-Driven Pyrimidine Biosynthesis Restores Tumor Growth of Respiration-Deficient Cancer Cells. Cell Metab. 2019;29:399–416.e10. doi: 10.1016/j.cmet.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sciacovelli M, Frezza C. Oncometabolites: Unconventional triggers of oncogenic signalling cascades. Free Radic Biol Med. 2016;100:175–181. doi: 10.1016/j.freeradbiomed.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Picaud S, Kavanagh KL, Yue WW, Lee WH, Muller-Knapp S, Gileadi O, Sacchettini J, Oppermann U. Structural basis of fumarate hydratase deficiency. J Inherit Metab Dis. 2011;34:671–676. doi: 10.1007/s10545-011-9294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yogev O, Naamati A, Pines O. Fumarase: a paradigm of dual targeting and dual localized functions. FEBS J. 2011;278:4230–4242. doi: 10.1111/j.1742-4658.2011.08359.x. [DOI] [PubMed] [Google Scholar]
- [17].Sass E, Blachinsky E, Karniely S, Pines O. Mitochondrial and Cytosolic Isoforms of Yeast Fumarase Are Derivatives of a Single Translation Product and Have Identical Amino Termini. J Biol Chem. 2001;276:46111–46117. doi: 10.1074/jbc.M106061200. http://www.jbc.org/content/276/49/46111.abstract. [DOI] [PubMed] [Google Scholar]
- [18].Dik E, Naamati A, Asraf H, Lehming N, Pines O. Human Fumarate Hydratase Is Dual Localized by an Alternative Transcription Initiation Mechanism. Traffic. 2016;17:720–732. doi: 10.1111/tra.12397. [DOI] [PubMed] [Google Scholar]
- [19].Frezza C. Mitochondrial metabolites: undercover signalling molecules. Interface Focus. 2017;7 doi: 10.1098/rsfs.2016.0100. 20160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].W DT, H RE, M S. Fumaric aciduria a new organic aciduria associated with mental retardation and speech impairment. Clin Chim Acta. 1983;132:301–308. doi: 10.1016/0009-8981(83)90008-6. https://eurekamag.com/research/005/489/005489909.php. [DOI] [PubMed] [Google Scholar]
- [21].Allegri G, Fernandes MJ, Scalco FB, Correia P, Simoni RE, Llerena JC, de Oliveira MLC. Fumaric aciduria: an overview and the first Brazilian case report. J Inherit Metab Dis. 2010;33:411–419. doi: 10.1007/s10545-010-9134-2. [DOI] [PubMed] [Google Scholar]
- [22].Ryder B, Moore F, Mitchell A, Thompson S, Christodoulou J, Balasubramaniam S. In: Fumarase Deficiency: A Safe and Potentially Disease Modifying Effect of High Fat/Low Carbohydrate Diet BT - JIMD Reports. Morava E, Baumgartner M, Patterson M, Rahman S, Zschocke J, Peters V, editors. Vol. 40. Springer Berlin Heidelberg; Berlin, Heidelberg: 2018. pp. 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].A L, MLC. Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- [24].Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. Int J Nephrol Renov Dis. 2014;7:253–260. doi: 10.2147/IJNRD.S42097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Menko FH, Maher ER, Schmidt LS, Middelton LA, Aittomaki K, Tomlinson I, Richard S, Linehan WM. Hereditary leiomyomatosis and renal cell cancer (HLRCC): renal cancer risk, surveillance and treatment. Fam Cancer. 2014;13:637–644. doi: 10.1007/s10689-014-9735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Castro-Vega LJ, Buffet A, De Cubas AA, Cascón A, Menara M, Khalifa E, Amar L, Azriel S, Bourdeau I, Chabre O, Currás-Freixes M, et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum Mol Genet. 2014;23:2440–2446. doi: 10.1093/hmg/ddt639. [DOI] [PubMed] [Google Scholar]
- [27].Clark GR, Sciacovelli M, Gaude E, Walsh DM, Kirby G, Simpson MA, Trembath RC, Berg JN, Woodward ER, Kinning E, Morrison PJ, et al. Germline FH mutations presenting with pheochromocytoma. J Clin Endocrinol Metab. 2014;99:E2046–50. doi: 10.1210/jc.2014-1659. [DOI] [PubMed] [Google Scholar]
- [28].Zhang J, Walsh MF, Wu G, Edmonson MN, Gruber TA, Easton J, Hedges D, Ma X, Zhou X, Yergeau DA, Wilkinson MR, et al. Germline Mutations in Predisposition Genes in Pediatric Cancer. N Engl J Med. 2015;373:2336–2346. doi: 10.1056/NEJMoa1508054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Fieuw A, Kumps C, Schramm A, Pattyn F, Menten B, Antonacci F, Sudmant P, Schulte JH, Van Roy N, Vergult S, Buckley PG, et al. Identification of a novel recurrent 1q42.2-1qter deletion in high risk MYCN single copy 11q deleted neuroblastomas. Int J Cancer. 2011;130:2599–2606. doi: 10.1002/ijc.26317. [DOI] [PubMed] [Google Scholar]
- [30].Ha Y-S, Chihara Y, Yoon H-Y, Kim Y-J, Kim T-H, Woo SH, Yun S-J, Kim IY, Hirao Y, Kim W-J, et al. Downregulation of Fumarate Hydratase Is Related to Tumorigenesis in Sporadic Renal Cell Cancer. Urol Int. 2013;90:233–239. doi: 10.1159/000345608. [DOI] [PubMed] [Google Scholar]
- [31].Hu J, Locasale JW, Bielas JH, O’Sullivan J, Sheahan K, Cantley LC, Vander Heiden MG, Vitkup D. Heterogeneity of tumor-induced gene expression changes in the human metabolic network. Nat Biotechnol. 2013;31:522. doi: 10.1038/nbt.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Frezza C, Pollard PJ, Gottlieb E. Inborn and acquired metabolic defects in cancer. J Mol Med. 2011;89:213–220. doi: 10.1007/s00109-011-0728-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Akram M. Citric Acid Cycle and Role of its Intermediates in Metabolism. Cell Biochem Biophys. 2014;68:475–478. doi: 10.1007/s12013-013-9750-1. [DOI] [PubMed] [Google Scholar]
- [34].Frezza C, Zheng L, Folger O, Rajagopalan KN, MacKenzie ED, Jerby L, Micaroni M, Chaneton B, Adam J, Hedley A, Kalna G, et al. Haem oxygenase is synthetically lethal with the tumour suppressor fumarate hydratase. Nature. 2011;477:225–228. doi: 10.1038/nature10363. [DOI] [PubMed] [Google Scholar]
- [35].Yang Y, Lane AN, Ricketts CJ, Sourbier C, Wei M-H, Shuch B, Pike L, Wu M, Rouault TA, Boros LG, Fan TW-M, et al. Metabolic reprogramming for producing energy and reducing power in fumarate hydratase null cells from hereditary leiomyomatosis renal cell carcinoma. PLoS One. 2013;8:e72179–e72179. doi: 10.1371/journal.pone.0072179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gonçalves E, Sciacovelli M, Costa ASH, Tran MGB, Johnson TI, Machado D, Frezza C, Saez-Rodriguez J. Post-translational regulation of metabolism in fumarate hydratase deficient cancer cells. Metab Eng. 2018;45:149–157. doi: 10.1016/j.ymben.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zorova LD, Popkov VA, Plotnikov EY, Silachev DN, Pevzner IB, Jankauskas SS, Babenko VA, Zorov SD, Balakireva AV, Juhaszova M, Sollott SJ, et al. Mitochondrial membrane potential. Anal Biochem. 2018;552:50–59. doi: 10.1016/j.ab.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Mullen AR, Wheaton WW, Jin ES, Chen PH, Sullivan LB, Cheng T, Yang Y, Linehan WM, Chandel NS, DeBerardinis RJ. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature. 2011;481:385–388. doi: 10.1038/nature10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, Kelleher JK, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ternette N, Yang M, Laroyia M, Kitagawa M, O’Flaherty L, Wolhulter K, Igarashi K, Saito K, Kato K, Fischer R, Berquand A, et al. Inhibition of Mitochondrial Aconitase by Succination in Fumarate Hydratase Deficiency. Cell Rep. 2013;3:689–700. doi: 10.1016/J.CELREP.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zheng L, MacKenzie ED, Karim SA, Hedley A, Blyth K, Kalna G, Watson DG, Szlosarek P, Frezza C, Gottlieb E. Reversed argininosuccinate lyase activity in fumarate hydratase-deficient cancer cells. Cancer Metab. 2013;1:12. doi: 10.1186/2049-3002-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].O’Flaherty L, Adam J, Heather LC, Zhdanov AV, Chung YL, Miranda MX, Croft J, Olpin S, Clarke K, Pugh CW, Griffiths J, et al. Dysregulation of hypoxia pathways in fumarate hydratase-deficient cells is independent of defective mitochondrial metabolism. Hum Mol Genet. 2010;19:3844–3851. doi: 10.1093/hmg/ddq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Adam J, Yang M, Bauerschmidt C, Kitagawa M, O’Flaherty L, Maheswaran P, Özkan G, Sahgal N, Baban D, Kato K, Saito K, et al. A Role for Cytosolic Fumarate Hydratase in Urea Cycle Metabolism and Renal Neoplasia. Cell Rep. 2013;3:1440–1448. doi: 10.1016/j.celrep.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kulkarni RA, Bak DW, Wei D, Bergholtz SE, Briney CA, Shrimp JH, Thorpe AL, Bavari A, Alpsoy A, Levy M, Florens L, et al. A chemoproteomic portrait of the oncometabolite fumarate. bioRxiv. 2018 doi: 10.1038/s41589-018-0217-y. http://biorxiv.org/content/early/2018/03/22/285759.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tyrakis PA, Yurkovich ME, Sciacovelli M, Papachristou EK, Bridges HR, Gaude E, Schreiner A, D’Santos C, Hirst J, Hernandez-Fernaud J, Springett R, et al. Fumarate Hydratase Loss Causes Combined Respiratory Chain Defects. Cell Rep. 2017;21:1036–1047. doi: 10.1016/j.celrep.2017.09.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Toth EA, Yeates TO. The structure of adenylosuccinate lyase, an enzyme with dual activity in the de novo purine biosynthetic pathway. Structure. 2000;8:163–174. doi: 10.1016/s0969-2126(00)00092-7. [DOI] [PubMed] [Google Scholar]
- [47].Adam J, Ramracheya R, Chibalina MV, Ternette N, Hamilton A, Tarasov AI, Zhang Q, Rebelato E, Rorsman NJG, Martin-Del-Rio R, Lewis A, et al. Fumarate Hydratase Deletion in Pancreatic beta Cells Leads to Progressive Diabetes. Cell Rep. 2017;20:3135–3148. doi: 10.1016/j.celrep.2017.08.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Zecchini V, Frezza C. Metabolic synthetic lethality in cancer therapy. Biochim Biophys Acta. 2017;1858:723–731. doi: 10.1016/j.bbabio.2016.12.003. [DOI] [PubMed] [Google Scholar]
- [49].Matthew B, T SR, B JW. Succination of Proteins by Fumarate. Ann N Y Acad Sci. 2008;1126:272–275. doi: 10.1196/annals.1433.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kulkarni RA, Bak DW, Wei D, Bergholtz SE, Briney CA, Shrimp JH, Alpsoy A, Thorpe AL, Bavari AE, Crooks DR, Levy M, et al. A chemoproteomic portrait of the oncometabolite fumarate. Nat Chem Biol. 2019 doi: 10.1038/s41589-018-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Alderson NL, Wang Y, Blatnik M, Frizzell N, Walla MD, Lyons TJ, Alt N, Carson JA, Nagai R, Thorpe SR, Baynes JW. S-(2-Succinyl)cysteine: a novel chemical modification of tissue proteins by a Krebs cycle intermediate. Arch Biochem Biophys. 2006;450:1–8. doi: 10.1016/j.abb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- [52].Blatnik M, Frizzell N, Thorpe SR, Baynes JW. Inactivation of glyceraldehyde-3-phosphate dehydrogenase by fumarate in diabetes: formation of S-(2-succinyl)cysteine, a novel chemical modification of protein and possible biomarker of mitochondrial stress. Diabetes. 2008;57:41–49. doi: 10.2337/db07-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bardella C, El-Bahrawy M, Frizzell N, Adam J, Ternette N, Hatipoglu E, Howarth K, O’Flaherty L, Roberts I, Turner G, Taylor J, et al. Aberrant succination of proteins in fumarate hydratase-deficient mice and HLRCC patients is a robust biomarker of mutation status. J Pathol. 2011;225:4–11. doi: 10.1002/path.2932. [DOI] [PubMed] [Google Scholar]
- [54].Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, Stevens M, et al. Renal Cyst Formation in Fh1-Deficient Mice Is Independent of the Hif/Phd Pathway: Roles for Fumarate in KEAP1 Succination and Nrf2 Signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ooi A, Wong J-C, Petillo D, Roossien D, Perrier-Trudova V, Whitten D, Min BWH, Tan M-H, Zhang Z, Yang XJ, Zhou M, et al. An Antioxidant Response Phenotype Shared between Hereditary and Sporadic Type 2 Papillary Renal Cell Carcinoma. Cancer Cell. 2011;20:511–523. doi: 10.1016/J.CCR.2011.08.024. [DOI] [PubMed] [Google Scholar]
- [56].Kerins MJ, Vashisht AA, Liang BX, Duckworth SJ, Praslicka BJ, Wohlschlegel JA, Ooi A. Fumarate Mediates a Chronic Proliferative Signal in Fumarate Hydratase-Inactivated Cancer Cells by Increasing Transcription and Translation of Ferritin Genes. Mol Cell Biol. 2017;37 doi: 10.1128/MCB.00079-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zheng L, Cardaci S, Jerby L, MacKenzie ED, Sciacovelli M, Johnson TI, Gaude E, King A, Leach JD, Edrada-Ebel R, Hedley A, et al. Fumarate induces redox-dependent senescence by modifying glutathione metabolism. Nat Commun. 2015;6:6001. doi: 10.1038/ncomms7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jin L, Li D, Alesi GN, Fan J, Kang H-B, Lu Z, Boggon TJ, Jin P, Yi H, Wright ER, Duong D, et al. Glutamate Dehydrogenase 1 Signals through Antioxidant Glutathione Peroxidase 1 to Regulate Redox Homeostasis and Tumor Growth. Cancer Cell. 2015;27:257–270. doi: 10.1016/j.ccell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Canning P, Sorrell FJ, Bullock AN. Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med. 2015;88:101–107. doi: 10.1016/j.freeradbiomed.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Adam J, Hatipoglu E, O’Flaherty L, Ternette N, Sahgal N, Lockstone H, Baban D, Nye E, Stamp GW, Wolhuter K, Stevens M, et al. Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell. 2011;20:524–537. doi: 10.1016/j.ccr.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Rojo de la Vega M, Chapman E, Zhang DD. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Anderson CP, Shen M, Eisenstein RS, Leibold EA. Mammalian iron metabolism and its control by iron regulatory proteins. Biochim Biophys Acta - Mol Cell Res. 2012;1823:1468–1483. doi: 10.1016/j.bbamcr.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sullivan LB, Martinez-Garcia E, Nguyen H, Mullen AR, Dufour E, Sudarshan S, Licht JD, Deberardinis RJ, Chandel NS. The Proto-oncometabolite Fumarate Binds Glutathione to Amplify ROS-Dependent Signaling. Mol Cell. 2013;51:236–248. doi: 10.1016/j.molcel.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Reisman D, Glaros S, Thompson EA. The SWI/SNF complex and cancer. Oncogene. 2009;28:1653. doi: 10.1038/onc.2009.4. [DOI] [PubMed] [Google Scholar]
- [65].Islam MS, Leissing TM, Chowdhury R, Hopkinson RJ, Schofield CJ. 2-Oxoglutarate-Dependent Oxygenases. Annu Rev Biochem. 2018;87:585–620. doi: 10.1146/annurev-biochem-061516-044724. [DOI] [PubMed] [Google Scholar]
- [66].Safran M, Kaelin WG. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779–783. doi: 10.1172/JCI18181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5:378–389. doi: 10.1016/j.apsb.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Singh D, Arora R, Kaur P, Singh B, Mannan R, Arora S. Overexpression of hypoxia-inducible factor and metabolic pathways: possible targets of cancer. Cell Biosci. 2017;7:62. doi: 10.1186/s13578-017-0190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- [70].Patrick P, Noel W, Ella B, Afrina A, George E, Sanjiv M, Richard P, Ian T. Evidence of increased microvessel density and activation of the hypoxia pathway in tumours from the hereditary leiomyomatosis and renal cell cancer syndrome. J Pathol. 2004;205:41–49. doi: 10.1002/path.1686. [DOI] [PubMed] [Google Scholar]
- [71].Hewitson KS, Liénard BMR, McDonough MA, Clifton IJ, Butler D, Soares AS, Oldham NJ, McNeill LA, Schofield CJ. Structural and Mechanistic Studies on the Inhibition of the Hypoxia-inducible Transcription Factor Hydroxylases by Tricarboxylic Acid Cycle Intermediates. J Biol Chem. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. http://www.jbc.org/content/282/5/3293.abstract. [DOI] [PubMed] [Google Scholar]
- [72].Kim J, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [73].Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- [74].Xie H, Valera VA, Merino MJ, Amato AM, Signoretti S, Linehan WM, Sukhatme VP, Seth P. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8:626–635. doi: 10.1158/1535-7163.MCT-08-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Suo G, Jiang Y, Cowan B, Wang JY. Platelet-derived growth factor C is upregulated in human uterine fibroids and regulates uterine smooth muscle cell growth. Biol Reprod. 2009;81:749–758. doi: 10.1095/biolreprod.109.076869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].de Velasco G, Munoz C, Sepulveda JM, Castellano D. Sequential treatments in hereditary leiomyomatosis and renal cell carcinoma (HLRCC): Case report and review of the literature. Can Urol Assoc J. 2015;9:E243–6. doi: 10.5489/cuaj.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Rasmussen KD, Helin K. Role of TET enzymes in DNA methylation, development, and cancer. Genes Dev. 2016;30:733–750. doi: 10.1101/gad.276568.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Park SY, Park J-W, Chun Y-S. Jumonji histone demethylases as emerging therapeutic targets. Pharmacol Res. 2016;105:146–151. doi: 10.1016/j.phrs.2016.01.026. [DOI] [PubMed] [Google Scholar]
- [79].Baylln SB, Herman JG, Graff JR, Vertino PM, Issa J-P. In: Alterations in DNA Methylation: A Fundamental Aspect of Neoplasia. Vande Woude GF, G.B.T.-AKlein CR, editors. Academic Press; 1997. pp. 141–196. [DOI] [PubMed] [Google Scholar]
- [80].Linehan WM, Spellman PT, Ricketts CJ, Creighton CJ, Fei SS, Davis C, Wheeler DA, Murray BA, Schmidt L, Vocke CD, Peto M, et al. Comprehensive Molecular Characterization of Papillary Renal-Cell Carcinoma. N Engl J Med. 2015;374:135–145. doi: 10.1056/NEJMoa1505917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ricketts CJ, De Cubas AA, Fan H, Smith CC, Lang M, Reznik E, Bowlby R, Gibb EA, Akbani R, Beroukhim R, Bottaro DP, et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018;23:313–326.e5. doi: 10.1016/j.celrep.2018.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Rayess H, Wang MB, Srivatsan ES. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130:1715–1725. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Sciacovelli M, Goncalves E, Johnson TI, Zecchini VR, da Costa AS, Gaude E, Drubbel AV, Theobald SJ, Abbo SR, Tran MG, Rajeeve V, et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature. 2016;537:544–547. doi: 10.1038/nature19353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer. 2018 doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- [85].Puisieux A, Brabletz T, Caramel J. Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol. 2014;16:488. doi: 10.1038/ncb2976. [DOI] [PubMed] [Google Scholar]
- [86].He X, Yan B, Liu S, Jia J, Lai W, Xin X, Tang C, Luo D, Tan T, Jiang Y, Shi Y, et al. Chromatin Remodeling Factor LSH Drives Cancer Progression by Suppressing the Activity of Fumarate Hydratase. Cancer Res. 2016 doi: 10.1158/0008-5472.CAN-16-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Alfonso C, Lucia A. The Jumonji family: past, present and future of histone demethylases in cancer. Biomol Concepts. 2014;5:209. doi: 10.1515/bmc-2014-0010. [DOI] [PubMed] [Google Scholar]
- [88].Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, Liu L, Liu Y, Yang C, Xu Y, Zhao S, et al. Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gerken T, Girard CA, Tung Y-CL, Webby CJ, Saudek V, Hewitson KS, Yeo GSH, McDonough MA, Cunliffe S, McNeill LA, Galvanovskis J, et al. The Obesity-Associated FTO Gene Encodes a 2-Oxoglutarate-Dependent Nucleic Acid Demethylase. Science (80-. ) 2007;318:1469–1472. doi: 10.1126/science.1151710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G, Vanacova S. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3′-end processing. Nucleic Acids Res. 2017;45:11356–11370. doi: 10.1093/nar/gkx778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Zhao X, Yang Y, Sun B-F, Shi Y, Yang X, Xiao W, Hao Y-J, Ping X-L, Chen Y-S, Wang W-J, Jin K-X, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Tong WH, Sourbier C, Kovtunovych G, Jeong SY, Vira M, Ghosh M, Romero VV, Sougrat R, Vaulont S, Viollet B, Kim YS, et al. The glycolytic shift in fumarate-hydratase-deficient kidney cancer lowers AMPK levels, increases anabolic propensities and lowers cellular iron levels. Cancer Cell. 2011;20:315–327. doi: 10.1016/j.ccr.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sourbier C, Ricketts CJ, Matsumoto S, Crooks DR, Liao PJ, Mannes PZ, Yang Y, Wei MH, Srivastava G, Ghosh S, Chen V, et al. Targeting ABL1-mediated oxidative stress adaptation in fumarate hydratase-deficient cancer. Cancer Cell. 2014;26:840–850. doi: 10.1016/j.ccell.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Xu Y, Taylor P, Andrade J, Ueberheide B, Shuch B, Glazer PM, Bindra RS, Moran MF, Linehan WM, Neel BG. Pathologic Oxidation of PTPN12 Underlies ABL1 Phosphorylation in Hereditary Leiomyomatosis and Renal Cell Carcinoma. Cancer Res. 2018;78:6539–6548. doi: 10.1158/0008-5472.CAN-18-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Boettcher M, Lawson A, Ladenburger V, Fredebohm J, Wolf J, Hoheisel JD, Frezza C, Shlomi T. High throughput synthetic lethality screen reveals a tumorigenic role of adenylate cyclase in fumarate hydratase-deficient cancer cells. BMC Genomics. 2014;15:158. doi: 10.1186/1471-2164-15-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Johnson TI, Costa ASH, Ferguson AN, Frezza C. Fumarate hydratase loss promotes mitotic entry in the presence of DNA damage after ionising radiation. Cell Death Dis. 2018;9:913. doi: 10.1038/s41419-018-0912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Yogev O, Yogev O, Singer E, Shaulian E, Goldberg M, Fox TD, Pines O. Fumarase: A Mitochondrial Metabolic Enzyme and a Cytosolic/Nuclear Component of the DNA Damage Response. PLOS Biol. 2010;8:e1000328. doi: 10.1371/journal.pbio.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48:e245. doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kuhajda FP. AMP-activated protein kinase and human cancer: cancer metabolism revisited. Int J Obes. 2008;32:S36. doi: 10.1038/ijo.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hardie DG. AMPK--sensing energy while talking to other signaling pathways. Cell Metab. 2014;20:939–952. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Li N, Huang D, Lu N, Luo L. Role of the LKB1/AMPK pathway in tumor invasion and metastasis of cancer cells (Review) Oncol Rep. 2015;34:2821–2826. doi: 10.3892/or.2015.4288. [DOI] [PubMed] [Google Scholar]
- [102].Drusian L, Nigro EA, Mannella V, Pagliarini R, Pema M, Costa ASH, Benigni F, Larcher A, Chiaravalli M, Gaude E, Montorsi F, et al. mTORC1 Upregulation Leads to Accumulation of the Oncometabolite Fumarate in a Mouse Model of Renal Cell Carcinoma. Cell Rep. 2018;24:1093–1104.e6. doi: 10.1016/j.celrep.2018.06.106. [DOI] [PubMed] [Google Scholar]
- [103].Fajardo MA, Piazza AG, Tinsley NH. The Role of Cyclic Nucleotide Signaling Pathways in Cancer: Targets for Prevention and Treatment. Cancers (Basel) 2014;6 doi: 10.3390/cancers6010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].O’Connor MJ. Targeting the DNA Damage Response in Cancer. Mol Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- [105].Jiang Y, Qian X, Shen J, Wang Y, Li X, Liu R, Xia Y, Chen Q, Peng G, Lin S-Y, Lu Z. Local generation of fumarate promotes DNA repair through inhibition of histone H3 demethylation. Nat Cell Biol. 2015;17:1158. doi: 10.1038/ncb3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Sulkowski PL, Sundaram RK, Oeck S, Corso CD, Liu Y, Noorbakhsh S, Niger M, Boeke M, Ueno D, Kalathil AN, Bao X, et al. Krebs-cycle-deficient hereditary cancer syndromes are defined by defects in homologous-recombination DNA repair. Nat Genet. 2018;50:1086–1092. doi: 10.1038/s41588-018-0170-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Iommarini L, Porcelli AM, Gasparre G, Kurelac I. Non-Canonical Mechanisms Regulating Hypoxia-Inducible Factor 1 Alpha in Cancer. Front Oncol. 2017;7:286. doi: 10.3389/fonc.2017.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]