Abstract
Wastewater rich in organic carbon, nitrogen and phosphorus may serve as a convenient source of carbon and nutrients for a year-long microalgae production. Scientific reports indicate that some single-cell microalgae such as Chlorella and Scenedesmus, are highly tolerant to wastewater environments and efficiently remove biogenic compounds. The aim of this study was to determine the possibility of using the effluent produced in the process of anaerobic degradation of whey as a culture medium for the multiplication of Chlorella vulgaris algae biomass and to characterise their growth efficiency and rate. The content of nitrogen and phosphorus in wastewater was sufficient for conducting an effective culture of algae. The efficiency of nitrogen removal in the flow system was 15.61 ± 1.38 mg N/dm3/day.
Keywords: Chlorella vulgaris, Post-fermentation effluent, Microalgae, Biomass
Introduction
In recent years, anaerobic wastewater treatment originating from a variety of industries has been regarded as an economically and technologically justified process (Tiwary et al. 2015). The anaerobic microbiological process, where complex organic substances are transformed into methane and carbon dioxide, is a widely applied technology for the stabilisation of waste with a concurrent generation of energy in the form of biogas (Niu et al. 2014). Although the process of methane fermentation offers high efficiency in the removal of organic compounds, nutrients such as nitrogen and phosphorus are removed to a small degree.
Despite many advantages, anaerobic technologies of wastewater treatment also feature certain imperfections, which limit the possibilities of common application (Chan et al. 2009). One of the drawbacks of the fermentation process is the removal of biogenic compounds (nitrogen and phosphorus) solely through sludge biomass growth. These compounds are removed in low amounts which usually do not exceed 10% (Jędrzejewska-Cicińska and Krzemieniewski 2010). Therefore, anaerobic reactors are systems which do not ensure a comprehensive removal of contamination. Wastewater treated in this way does not meet the criteria for being discharged directly to a recipient. Therefore, it requires additional technological treatment which generates further exploitation costs. For this reason, it is necessary to search for solution to improve the efficiency of anaerobic technologies and make them more universal. One such solution could be phytoremediation, which uses plants to neutralise contaminations (Oswald 2003). Wastewater rich in organic carbon, nitrogen and phosphorus may serve as a convenient source of carbon and nutrients for a year-long microalgae production (Schenk et al. 2008). Scientific reports indicate that some single-cell microalgae such as Chlorella and Scenedesmus are highly tolerant to wastewater environments and efficiently remove biogenic compounds (Ruiz-Marin et al. 2010).
Microalgae constitute a diversified group of eukaryotic photosynthetic microorganisms which colonise both the marine and freshwater environments. Microalgae are among the fastest developing photosynthetic organisms. Their photosynthetic mechanism is similar to that of terrestrial plants. Microalgae do not compete for cultivated land. With access to water, carbon dioxide and biogenic compounds such as nitrogen and phosphorus, offer higher biomass yields than terrestrial plants. Algae have the ability to produce 50 times more biomass than higher plants (Li et al. 2008; Apt and Behrens 1999). Different varieties of algae are able to develop in a wide variety of environments, even in degraded or contaminated areas (Mata et al. 2010). Such cultures bring a positive effect to the natural environment because algae may be produced using communal, agricultural or industrial waste-water containing carbon dioxide which is required for their growth CO2 (Chisti 2007). The molar ratio of the algae biomass main components proposed by Grobbelaar (2003) is as follows: CO0.48H1.83N0.11P0.01. In the course of microalgae population development, four following phases can be distinguished: adaptation phase, growth phase, stationary phase and decline phase (Barsanti and Gualtieri 2006; Singh et al. 2014).
The importance and interest in algae has been increasing with time. More and more frequently algae cultivations of high purity are carried out (Lorenz and Cysewski 2000). Substances obtained from algae may constitute a source of nutritional value and a diet component for both humans and animals (Dallaire et al. 2007). Microalgae have already been used as diet supplements, an addition to cosmetics, for wastewater treatment and as a potential biomass source in the production of biofuels (Aslan and Kapdan 2006; Feng et al. 2011; Gellenbeck 2012). The benefits of using wastewater as a culture medium for microalgae production include a reduction in water use and costs of nutritional components added to cultures and the removal of nitrogen and phosphorus from wastewater (Pittman et al. 2011). There are few reports regarding the use of anaerobic fermentation effluents for cultivation of Chlorella vulgaris. These studies have focused mainly on the production of algae biomass in the static conditions. In such an arrangement, the researchers added a single dose of the anaerobic effluent and performed an algae cultivation.
There are no reports on algae cultivation with the use of anaerobic effluents in dynamic conditions. Therefore, the author of this publication performed an experiment on algae cultivation in dynamic conditions with simultaneous inflow of anaerobic effluent and biomass collection.
The aim of this study was to determine the possibility of using the effluent produced in the process of anaerobic degradation of whey, as a culture medium for the multiplication of C. vulgaris algae biomass and to characterise their growth efficiency and rate.
Materials and methods
Microorganism and culture medium
The study used C. vulgaris microalgae originating from a culture of the Collection of Baltic Algae of the Institute of Oceanography at the University of Gdańsk (Fig. 1).
Fig. 1.
Chlorella vulgaris seen under microscopic magnification: a ×100; b ×40
Research station
The culture was conducted in 1.0 dm3 (active volume) glass photobioreactors (SIMAX) placed on magnetic agitators with a set of lamps (NARVA, 2·36 W). The culture temperature was maintained at 25 ± 2 °C. Each reactor was equipped with an aeration system consisting of membrane pumps and diffusers distributing the supplied air (Fig. 2). The photobioreactors were illuminated at 3000 lx and aeration intensity was 0.6 vvm. Initial concentrations of microalgae biomass, characterised by the content of dry matter in the bioreactors, were prepared at the level of about 50 mg TS/dm3. Effluent dosing and the receipt of inoculum was carried out using peristaltic pumps (MasterFlex, 7525-20).
Fig. 2.
Research station
Post-fermentation effluent
The experiment tested the effluent obtained from a UASB-type model anaerobic bioreactor, supplied with wastewater prepared based on acidic whey. The anaerobic bioreactor of a labyrinth flow and active volume of 70 dm3 worked under mesophilic conditions, at the load maintained at A = 2.6 kg BOD5/m3, hydraulic stop time of 15 days and the process temperature of 35 ± 2 °C. The values of the basic indices of the effluent and the synthetic culture medium are presented in Table 1. Post-fermentation effluent was subjected to vacuum microfiltration each time to remove the suspension which hindered light access to the culture. In the series control algae were cultured in Bold’s basal medium with threefold nitrogen and vitamins; modified (3N-BBM+V). The 3N-BBM+V compositions were as follows: NaNO3 750 mg/dm3, CaCl2·2H2O 25 mg/dm3, MgSO4·7 H2O 75 mg/dm3, K2HPO4·3 H2O 11.5 mg/dm3, KH2PO4 26.9 mg/dm3, NaCl 25 mg/dm3, Na2EDTA 0.0045 mg/dm3, FeCl3·6 H2O 0.582 mg/dm3, MnCl2·4 H2O 0.26 mg/dm3, ZnCl2 0.03 mg/dm3, CoCl2·6 H2O 0.012 mg/dm3, NaMoO4·2 H2O 0.24 mg/dm3, B12 1 ml/dm3 and B1 1 ml/dm3.
Table 1.
Characteristics of a post-fermentation effluent and synthetic medium
| Index | Unit | Post-fermentation effluent | Synthetic medium |
|---|---|---|---|
| COD | mg O2/dm3 | 825 | – |
| TN | mg/dm3 | 168.2 ± 5.5 | 109.6 ± 0.89 |
| PO43− − Ptotal | mg/dm3 | 10.08 ± 0.15 | 8.29 ± 0.15 |
| Mn | mg/dm3 | 0.931 ± 0.02 | < 0.02 |
| Zn | mg/dm3 | 0.134 ± 0.01 | < 0.005 |
| Fe | mg/dm3 | 1.06 ± 0.02 | < 0.1 |
| SO42− | mg/dm3 | 55.45 ± 2.1 | 7.31 ± 0.63 |
| Reaction | – | 8.5 | 7.79 |
Experiment
The experimental research was divided into two phases: an adaptation phase and a flow culture. The cultivation was performed in three repetitions. At the same time, a control was carried out using a synthetic culture medium.
Adaptation phase
The adaptation phase was carried out in a photobioreactor filled with a culture medium and an addition of inoculum (50 mg/dm3). This adaptation culture was conducted in a stationary mode. Undiluted post-fermentation effluent was used as the culture medium. The aim of this phase was the adaptation of microalgae to the applied culture medium and biomass multiplication. This phase lasted until the maximum value of the biomass growth index was obtained. The culture was then continued in a flow culture mode (second phase). During the adaptation, the maximum daily total nitrogen removal was determined, which was the basis for determination of the daily dose of post-fermentation effluent supplied to the system. The maximum daily consumption of nitrogen compounds was from 16.55 ± 0.15 mg N/dm3/day. In the control series, the maximum daily consumption of nitrogen compounds was from 15.91 ± 0.99 mg N/dm3/day.
Flow culture
The second experimental phase involved the operation of the culture with dosing portions of post-fermentation effluent and the removal of the culture medium. The amount of nitrogen compounds introduced to the system with the culture medium was adjusted to be approximate to the daily nitrogen consumption in the adaptation culture (16.55 ± 0.15 mg N/dm3/day). Considering the content of the total nitrogen in the post-fermentation effluent, which was 168.2 ± 5.5 mg N/dm3, the daily dose was set at 96 cm3/day (2 cm3/0.5 h), which corresponded to the nitrogen amount at the level of 16.15 ± 0.53 mg N/dm3/day. In the control series, the flow was set at 145 cm3/day (3 cm3/0.5 h).
Measurement of biomass concentration
The dry matter content (TS) was determined by filtering 20-ml samples of the culture through a 90 mm in diameter hard cellulose filter. Following the filtration process, the filter was dried in a laboratory dryer (Binder, Germany) until a stable mass was obtained. In order to determine the content of dry matter, we get the difference in mass between a dry filter before filtration and a dry filter after filtration. Measurements of dry matter content in the adaptation phase were done every 24 h and every 48 h during the flow culture phase.
Determination of growth parameters and nitrogen removal parameter
The biomass productivity (Pbiomass, g TS/dm3/day) and nitrogen removal efficiency (Pnitrogen, mg N/dm3/day) were calculated based on the following equation:
where ΔX is the difference in biomass concentration (g TS/dm3) or nitrogen concentration (mg N/dm3) over a cultivation time of Δt (d).
Taxonomic analysis
A taxonomic analysis of algae biomass was carried out under microscopic magnifications: ×1.25 ×10 ×40 or ×1.25 ×10 ×10 of an MF 346 (OPTA-TECH) biological microscope with 3-MP camera (Opta-Tech).
Measurement of nutrient concentrations
To determine the biogenic compound removal efficiency in the flow culture of algae, a daily analysis was made of the total nitrogen and phosphorus at the discharge from the photobioreactors. The samples were pre-filtered through cellulose filters to remove solids. The filtered samples were subject to analyses using LCK cuvette tests (Hach Lange, USA).
Statistical analysis
Statistical analysis was performed using Statistica software. The results are presented in the form of mean values ± standard deviation from using one-way analysis of variance (ANOVA). A difference was considered statistically significant at p < 0.05.
Results and discussion
Cell growth
Scientific references provide many examples of algae-based systems in wastewater treatment. Sawayama et al. (1995) used algae cultures of Botryococcus braunii species as the third phase of wastewater treatment in closed systems. Such a technological solution allowed for efficient removal of both nitrogen and phosphorus from communal wastewater discharged from activated sludge reservoirs. The use of wastewater resulted in algae biomass production with a high concentration of carbohydrates. Chiu et al. (2015) presented data and a detailed description of the application of varied waste-water in Chlorella sp. algae culture. They distinguish three main wastewater sources: communal, agricultural and industrial, containing a wide variety of components. Researchers indicate that nutrients such as nitrogen and phosphorus contained in wastewater may successfully be used as a culture medium for an intensive biomass culture.
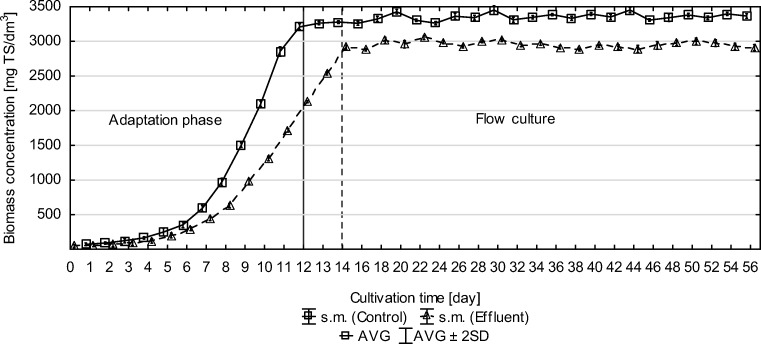
In the present experiment, the C. vulgaris microalgae culture was conducted with the use of post-fermentation effluent as a culture medium. An undiluted post-methane fermentation effluent from the dairy industry was used. The experiment was divided into two phases. In the adaptation phase, the biomass was cultured in a culture medium in stationary mode until the maximum biomass productivity was obtained. In the flow phase, portions of culture medium were fed and portions of the effluent were removed. The initial concentration of biomass in the adaptation phase was 50 ± 12 mg TS/dm3. The maximum biomass productivity was obtained on day 12 of the culture and it was 413.67 ± 4.51 mg TS/dm3/day. On day 13, the productivity remained at a similar level (409.33 ± 3.51 mg TS/dm3/day), while on day 14, it dropped to 372 ± 4.51 mg TS/dm3/day. At that time, the experiment passed to the second phase (flow culture). The maximum biomass concentration, which was obtained in the adaptation phase, was 2.915 ± 17 mg TS/dm3. In the flow phase, the biomass concentration at the discharge from a photobioreactor remained at a similar level as in the adaptation phase, which confirms that the retention time of the effluent in the photobioreactor was well adjusted. The biomass content in the flow system ranged from 2.863 to 3.065 mg TS/dm3 at an average concentration of 2.953 ± 49 mg TS/dm3.
In the control series, the initial concentration of biomass in the adaptation phase was 56.3 ± 11 mg TS/dm3. The maximum biomass productivity was obtained on day 11 of the culture and it was 757.67 ± 15.3 mg TS/dm3/day. The maximum biomass concentration, which was obtained in the adaptation phase was 3285 ± 9 mg TS/dm3. In the control series, the biomass content in the flow system ranged from 3254 to 3474 mg TS/dm3 at an average concentration of 3355 ± 52 mg TS/dm3 (Fig. 3).
Fig. 3.
Biomass concentration in the adaptation phase and flow phase
Zhou et al. (2018) studied the possibility of culturing Chlorella zofingiensis using communal wastewater and post-methane fermentation effluent (swine slurry), mixed in varied proportions, as a culture medium. For the culture medium consisting of only communal wastewater, the biomass productivity was 280 mg/dm3/day. The maximum biomass productivity was obtained by adding 8% of anaerobic effluent to communal wastewater, which was used as a culture medium. This productivity was 630 mg/dm3/day. Zhu et al. (2013) carried out C. zofingiensis microalgae culture using diluted wastewater from swine production. The maximum biomass productivity achieved by them was 296.16 ± 19.16 mg/dm3/day. On the other hand, Sepúlveda et al. (2015) used effluent from anaerobic treatment of municipal waste at different dilution degrees (0–80%). The maximum biomass growth in this study reached 400 mg/dm3/day.
Nutrient concentrations
The initial content of the total nitrogen in the culture medium in the adaptation phase was 168.2 ± 5.5 mg N/dm3. The highest daily consumption of nitrogen compounds in the adaptation phase was 16.55 ± 0.15 mg N/dm3/day. The dose of the supplied effluent was determined based on the daily consumption of nitrogen compounds, at the maximum biomass productivity, and was 96 cm3/day (2 cm3/0.5 h), which corresponded to the load of 16.15 ± 0.53 mg N/dm3/day. The total nitrogen concentration at the transition to the flow culture phase of the experiment was 10.68 ± 0.75 mg N/dm3. The total nitrogen concentration at the discharge from the photobioreactor during the flow phase ranged from 5.32 to 13.14 mg N/dm3 at an average concentration of 9.85 ± 1.49 mg N/dm3. The average efficiency of nitrogen compound removal in the flow culture was 15.66 ± 1.15 mg N/dm3/day.
In the control series, the initial content of the total nitrogen in the culture medium in the adaptation phase was 109.60 ± 0.89 mg N/dm3. The highest daily consumption of nitrogen compounds in the adaptation phase was 15.91 ± 0.99 mg N/dm3/day. In the control, the total nitrogen concentration at the discharge from the photobioreactor during the flow phase ranged from 0.92 to 3.75 mg N/dm3 at an average concentration of 2.05 ± 0.73 mg N/dm3. The average efficiency of nitrogen compound removal in the flow culture was 15.78 ± 0.50 mg N/dm3/day (Fig. 4).
Fig. 4.
Total nitrogen and total phosphorus concentration in the adaptation phase and flow phase
The initial content of the total phosphorus in the culture medium in the adaptation phase was 10.08 ± 0.15 mg P/dm3. The total phosphorus concentration in the transition to the flow culture was 3.42 ± 0.08 mg P/dm3. The average efficiency of phosphorus compound removal in the flow phase was 0.84 ± 0.12 mg P/dm3/day.
In the control series, the initial content of the total phosphorus in the culture medium in the adaptation phase was 8.28 ± 0.15 mg P/dm3. The average efficiency of phosphorus compound removal in the flow phase was 1.15 ± 0.08 mg P/dm3/day (Fig. 4).
Zhou et al. (2018) obtained the highest degree of total nitrogen removal using only communal wastewater as the culture medium (21 mg N/dm3/day). The removal degree of phosphorus was 4.6 mg P/dm3/day. Sepúlveda et al. (2015), in their experiment involving post-methane fermentation effluent from communal wastewater obtained the maximum degree of nitrogen removal of 35 mg N/dm3/day. The efficiency of phosphorus removal was 5.7 mg P/dm3/day. Cabanelas et al. (2013) decided to use effluents originating from varied phases of treatment of the municipal wastewater treatment station to culture C. vulgaris. As the result of the experiment, they obtained the maximum nitrogen and phosphorus removal index of 9.8 mg N/dm3/day and 3.0 mg P/dm3/day, respectively. However, Sevrin-Reyssac (1998) used swine slurry to grow a polyculture consisting of Scenedesmus falcatus, Scenedesmus quadricauda, and Chlorella sp. and the maximum degree of nitrogen removal was 12.0 mg N/dm3/day.
Effluent from anaerobic fermentation of acid whey was used in the study. The study proved that cultivation of C. vulgaris with a capacity of 1 m3 annually will allow managing of 35 m3 of acid whey.
Conclusions
High efficiency in biogenic compound removal had a positive effect on the final biomass content of the tested microalgae. The application of the tested wastewater considerably reduced the necessity of using chemical reagents. The content of nitrogen and phosphorus in wastewater was sufficient for conducting an effective culture of algae. The efficiency of nitrogen removal in the flow system was 15.61 ± 1.38 mg N/dm3/day.
Funding
This work was supported by the National Science Center, Poland, Project No. 2016/21/N/ST8/01879
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Karolina Szwarc, Email: karolina.kupczyk@uwm.edu.pl.
Dawid Szwarc, Email: dawid.szwarc@uwm.edu.pl.
Marcin Zieliński, Email: marcin.zielinski@uwm.edu.pl.
References
- Apt KE, Behrens PW. Commercial developments in microalgal biotechnology. J Phycol. 1999;35(2):215–226. doi: 10.1046/j.1529-8817.1999.3520215.x. [DOI] [Google Scholar]
- Aslan S, Kapdan IK. Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng. 2006;28(1):64–70. doi: 10.1016/j.ecoleng.2006.04.003. [DOI] [Google Scholar]
- Barsanti L, Gualtieri P. Algal culturing. Algae: anatomy, biochemistry and biotechnology. Boca Ranton: CRC Press; 2006. pp. 209–250. [Google Scholar]
- Cabanelas ITD, Ruiz J, Arbib Z, Chinalia FA, Garrido-Pérez C, Rogalla F, Perales JA. Comparing the use of different domestic wastewaters for coupling microalgal production and nutrient removal. Bioresour Technol. 2013;131:429–436. doi: 10.1016/j.biortech.2012.12.152. [DOI] [PubMed] [Google Scholar]
- Chan YJ, Chong MF, Law CL, Hassell DG. A review on anaerobic–aerobic treatment of industrial and municipal wastewater. Chem Eng J. 2009;155(1-2):1–18. doi: 10.1016/j.cej.2009.06.041. [DOI] [Google Scholar]
- Chisti Y. Biodiesel from microalgae. Biotechnol Adv. 2007;25(3):294–306. doi: 10.1016/j.biotechadv.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Chiu SY, Kao CY, Chen TY, Chang YB, Kuo CM, Lin CS. Cultivation of microalgal Chlorella for biomass and lipid production using wastewater as nutrient resource. Bioresour Technol. 2015;184:179–189. doi: 10.1016/j.biortech.2014.11.080. [DOI] [PubMed] [Google Scholar]
- Dallaire V, Lessard P, Vandenberg G, de la Noüe J. Effect of algal incorporation on growth, survival and carcass composition of rainbow trout (Oncorhynchus mykiss) fry. Bioresour Technol. 2007;98(7):1433–1439. doi: 10.1016/j.biortech.2006.05.043. [DOI] [PubMed] [Google Scholar]
- Feng Y, Li C, Zhang D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour Technol. 2011;102(1):101–105. doi: 10.1016/j.biortech.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Gellenbeck K. Utilization of algal materials for nutraceutical and cosmeceutical applications—what do manufacturers need to know? J Appl Phycol. 2012;24(3):309–313. doi: 10.1007/s10811-011-9722-z. [DOI] [Google Scholar]
- Grobbelaar JU (2003) Algal nutrition–mineral nutrition. Handbook of microalgal culture: biotechnology and applied phycology. 95–115
- Jędrzejewska-Cicińska M, Krzemieniewski M. Effect of corrosion of steel elements on the treatment of dairy wastewater in a UASB reactor. Environ Technol. 2010;31(6):585–589. doi: 10.1080/09593331003616821. [DOI] [PubMed] [Google Scholar]
- Li Y, Horsman M, Wu N, Lan CQ, Dubois-C Alero N. Biofuels from microalgae. Biotechnol Prog. 2008;24(4):815–820. doi: 10.1021/bp070371k. [DOI] [PubMed] [Google Scholar]
- Lorenz RT, Cysewski GR. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000;18(4):160–167. doi: 10.1016/S0167-7799(00)01433-5. [DOI] [PubMed] [Google Scholar]
- Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev. 2010;14:217–232. doi: 10.1016/j.rser.2009.07.020. [DOI] [Google Scholar]
- Niu Q, Hojo T, Qiao W, Qiang H, Li YY. Characterization of methanogenesis, acidogenesis and hydrolysis in thermophilic methane fermentation of chicken manure. Chem Eng J. 2014;244:587–596. doi: 10.1016/j.cej.2013.11.074. [DOI] [Google Scholar]
- Oswald WJ. My sixty years in applied algology. J Appl Phycol. 2003;15(2):99–106. doi: 10.1023/A:1023871903434. [DOI] [Google Scholar]
- Pittman JK, Dean AP, Osundeko O. The potential of sustainable algal biofuel production using wastewater resources. Bioresour Technol. 2011;102(1):17–25. doi: 10.1016/j.biortech.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Ruiz-Marin A, Mendoza-Espinosa LG, Stephenson T. Growth and nutrient removal in free and immobilized green algae in batch and semi-continuous cultures treating real wastewater. Bioresour Technol. 2010;101(1):58–64. doi: 10.1016/j.biortech.2009.02.076. [DOI] [PubMed] [Google Scholar]
- Sawayama S, Inoue S, Dote Y, Yokoyama SY. CO2 fixation and oil production through microalgae. Energy Convers Manag. 1995;36(69):729–731. doi: 10.1016/0196-8904(95)00108-P. [DOI] [Google Scholar]
- Schenk PM, Thomas-Hall SR, Stephens E, Marx UC, Mussgnug JH, Posten C, Hankamer B. Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg Res. 2008;1(1):20–43. doi: 10.1007/s12155-008-9008-8. [DOI] [Google Scholar]
- Sepúlveda C, Acién FG, Gómez C, Jiménez-Ruíz N, Riquelme C, Molina-Grima E. Utilization of centrate for the production of the marine microalgae Nannochloropsis gaditana. Algal Res. 2015;9:107–116. doi: 10.1016/j.algal.2015.03.004. [DOI] [Google Scholar]
- Sevrin-Reyssac J. Biotreatment of swine manure by production of aquatic valuable biomasses. Agric Ecosyst Environ. 1998;68(3):177–186. doi: 10.1016/S0167-8809(97)00070-4. [DOI] [Google Scholar]
- Singh B, Guldhe A, Rawat I, Bux F. Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew Sust Energ Rev. 2014;29:216–245. doi: 10.1016/j.rser.2013.08.067. [DOI] [Google Scholar]
- Tiwary A, Williams ID, Pant DC, Kishore VVN. Emerging perspectives on environmental burden minimisation initiatives from anaerobic digestion technologies for community scale biomass valorisation. Renew Sust Energ Rev. 2015;42:883–901. doi: 10.1016/j.rser.2014.10.052. [DOI] [Google Scholar]
- Zhou W, Wang Z, Xu J, Ma L. Cultivation of microalgae Chlorella zofingiensis on municipal wastewater and biogas slurry towards bioenergy. J Biosci Bioeng. 2018;126(5):644–648. doi: 10.1016/j.jbiosc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- Zhu L, Wang Z, Shu Q, Takala J, Hiltunen E, Feng P, Yuan Z. Nutrient removal and biodiesel production by integration of freshwater algae cultivation with piggery wastewater treatment. Water Res. 2013;47(13):4294–4302. doi: 10.1016/j.watres.2013.05.004. [DOI] [PubMed] [Google Scholar]