Abstract
Dietary fish oil restores ovarian function in subfertile rats, which is thought to be associated with decreased transcription of follicle-stimulating hormone (FSH) β-subunit. We have previously demonstrated a reduction in early follicular serum FSH levels in normal weight but not obese women after treatment with omega-3 polyunsaturated fatty acids (PUFA). Herein, we report the effect of supplementation with omega-3 PUFA on urinary reproductive hormones across the whole menstrual cycle. This interventional study included 17 eumenorrheic women, aged 24-41 years. One month of daily morning urine was collected before and after 1 month of omega-3 PUFA supplementation with 4 g of eicosapentaenoic acid and docosahexaenoic acid daily. Measurements included urinary FSH, luteinizing hormone (LH) and estrogen and progesterone metabolites, plasma fatty acid composition, and markers of endoplasmic reticulum stress. Compliance with dietary supplementation was verified by significantly reduced ratios of omega-6 to omega-3 PUFA for all subjects after treatment (P < .01). After 1 month of omega-3 PUFA supplementation, urinary FSH was significantly decreased in normal weight, but not obese women, in both follicular and luteal phases (−28.4% and −12.6%, respectively, both P = .04). No significant changes were seen in LH or sex steroids for either weight group. The selective and specific decrease in FSH suggests that omega-3 PUFA supplementation merits further investigation in normal weight women with decreased fertility and/or diminished ovarian reserve.
Keywords: omega-3 polyunsaturated fatty acids, FSH, LH, obesity, fertility
Introduction
Consumption of omega-3 polyunsaturated fatty acids (PUFA) is increasing among women of reproductive age, and about 19 million US adults (roughly 8% of the US population) reported taking fish oil in 2012,1 making it the second most common fertility supplement, after multivitamins.2 Diets enriched with omega-3 PUFA restore fertility in aged mice by improving oocyte quality and enhance early embryonic development.3 However, studies of preconception omega-3 PUFA supplementation in women seeking pregnancy have produced conflicting results. Some report favorable effects, including increased progesterone secretion, decreased anovulation, and improved embryo quality and pregnancy outcomes,4-8 while other studies report no difference.9 We noted that prior studies did not report body weight or involved an appropriate consideration for the potential modifying effect of obesity in evaluating the impact of omega-3 PUFA on fertility. Maternal obesity has a major impact on childbearing and may introduce an effect modification (statistical interaction) on fertility outcomes.10,11 Abnormal body mass index (BMI) differentially affects reproduction, as high BMI reduces the chance of conception in women12 and affects the efficacy of contraceptives13; yet, low BMI may also decrease fecundability and assisted reproduction success.14 We hypothesize that failure to account for body mass in fertility studies may obscure a true effect of a potentially favorable intervention.
We have previously demonstrated, based on a single day assessment in the follicular phase, that treatment with omega-3 PUFA reduced serum follicle-stimulating hormone (FSH) levels in normal weight (NW), but not obese women.15 Accordingly, transcription of FSH β-subunit is decreased in response to omega-3 PUFA supplementation,16 which may contribute to the restoration of ovarian function in subfertile rats17 and mice.3 Variations in diurnal cyclic reproductive hormone profiles, in response to an intervention, are optimally captured using longitudinal daily urine collection.18 The objective of the current report was to evaluate the impact of supplementation with omega-3 PUFA on daily urinary gonadotropins and estrogen and progesterone metabolites, in both obese and NW women, across the entire menstrual cycle.
Cellular response to endoplasmic reticulum (ER) stress has been shown to be important for hypothalamic–pituitary–ovarian (HPO) hormonal response to obesity.19 Based on evidence that omega-3 dietary supplementation can affect the expression and regulation of ER stress markers,20 which can be detected in the extracellular space,21 we undertook an exploratory examination of the most prevalent ER stress markers: glucose-regulated protein, 78kD (GRP78; also referred to as BiP/HSP5A) and CCAAT-enhancer-binding homologous protein (CHOP). GRP78 is an ER heat-shock chaperone protein critical for protein quality control and functions as a repressor of the unfolded protein response and sensor of ER stress. CCAAT-enhancer-binding homologous protein is an ER stress–induced transcription factor best known for its mediation of apoptosis.22
Materials and Methods
Participants
The study was approved by the Colorado Multiple Institutional Review Board (13-1420); a signed informed consent was obtained from each participant before participation. Study criteria included: (1) age, 18-42 years; (2) BMI: ≥ 30 kg/m2 (obese), or 18-25 kg/m2 (NW); (3) history of regular menses every 25 to 40 days; and (4) normal baseline prolactin, thyroid-stimulating hormone (TSH), and blood count. Overweight women (BMI >25 to <30) were intentionally excluded to maximize potential differences seen between the phenotypes being compared (NW and obese). All participants were nonsmokers. Polycystic ovary syndrome (PCOS) was prospectively ruled out using the National Institutes of Health definition, which includes oligomenorrhea as a central criterion. Thus, all participants were required to have regular menstrual cycles and were not hirsute. Participants were excluded if they had allergies to seafood, used medications known to affect reproductive hormones, took supplemental PUFA, used exogenous sex steroids within the last 3 months, exercised vigorously more than 4 hours weekly, were attempting pregnancy, or were diagnosed with diabetes mellitus. Participants meeting the criteria for the study were asked not to alter their diet during the study period. All participants had a baseline physical examination by study personnel and underwent all sampling at the Clinical and Translational Research Center of the Colorado Clinical and Translational Sciences Institute. A total of 17 women (7 NW and 10 obese) provided complete urinary collection data for analysis (Table 1).
Table 1.
Biometric and Endocrine Characteristics in Normal Weight and Obese Women at Baseline.a
| Parameters | NW Women (SEM) | Obese Women (SEM) | P value |
|---|---|---|---|
| N | 7 | 10 | |
| Age (years) | 30.3 (3.8) | 35.5 (4.7) | <.01 |
| BMI (kg/m2) | 21.8 (1.4) | 35.5 (3.6) | <.01 |
| Weight (kg) | 57.8 (2.4) | 95.0 (14.0) | <.01 |
| Waist circumference (cm) | 74.4 (14.9) | 106.2 (10.5) | <.01 |
| Waist–hip ratio | 0.87 (0.1) | 0.93 (0.04) | .03 |
| AMH (ng/mL) | 4.8 (2.5) | 4.4 (3.0) | .38 |
| Inhibin B (pg/mL) | 70.2 (17.7) | 60.5 (18.1) | .14 |
| Fasting glucose (mg/dL) | 75.6 (26.0) | 88.8 (6.9) | .07 |
| Fasting insulin (mIU/L) | 8.4 (4.0) | 16.7 (8.3) | .01 |
Abbreviations: AMH, anti-Müllerian hormone; BMI, body mass index; NW, normal weight.
aValues represent mean (standard error of the mean, SEM). P values represent 2-sample t test.
Protocol
All women collected urine for 2 months: before and after one full menstrual cycle of dietary omega-3 PUFA supplementation (Supplemental Figure S1). Daily, first-morning voided urine was collected over the entire menstrual cycle using previously described methodology.15 Collection began with the first day of menstrual bleeding following enrollment (month 1). Starting with day 1 of the next menstrual cycle after baseline (month 2), study participants were instructed to take 4 g daily of Lovaza (GlaxoSmithKline, Philadelphia, PA), a concentrated and purified omega-3 PUFA preparation, approved by the US Food and Drug Administration for treatment of hypertriglyceridemia. One gram of Lovaza contains approximately 465 mg of eicosapentaenoic acid (EPA) and 375 mg of docosahexaenoic acid (DHA) as ethyl esters. This corresponds to an effective human dose used in clinical practice that is also roughly equivalent to amounts used for reproductive outcomes in animals.23 Blood fatty acid levels were determined to monitor compliance and verify the effectiveness of the treatment. Urine was not collected during month 2. On day 1 of the third menses (month 3, post-omega-3), participants again collected daily morning urine for the entire menstrual cycle and omega-3 PUFA supplementation continued until the end of Month 3. Levels of luteinizing hormone (LH), FSH, and estrogen and progesterone metabolites were determined in all urinary samples.
Hormone Assays
Urinary LH and FSH were measured by a competitive immunoassay using direct chemiluminescent technology (Centaur XP; Siemens Healthcare Diagnostics, Tarrytown, NY). Inter- and intra-assay coefficients of variation were 4.8% and 3.4% for LH, respectively, and 6.6% and 5.0% for FSH, respectively. Serum inhibin B and anti-Müllerian hormone (AMH) were assayed using commercial ELISA (Beckman Coulter, Brea, CA) at Ligand Assay and Analysis Core Services (University of Virginia). Functional sensitivity (defined as the lowest concentration reliably measured, or the lowest concentration with an inter-assay coefficient of variation 20%) was 10 pg/mL for inhibin B and 0.16 ng/mL for AMH. The inter- and intra-assay coefficients of variation were 5.2% and 4.4%, respectively, for inhibin B, and 7.0% and 3.0%, respectively, for AMH.
Daily urine samples were assayed for estrone conjugates (E1c) and pregnanediol glucuronide (Pdg), using immunoassays adapted for use on the Siemens Centaur CP. Hormone concentrations were adjusted for urinary creatinine and normalized to a 28-day cycle. The intra- and inter-assay CVs were 1.9% and 4.1% for E1c and 2.1% and 3.9% for Pdg, respectively.
Endoplasmic Reticulum Stress Marker Assays
Levels of CHOP and GRP78/BiP/HSP5A proteins in the participants pooled serum (from frequent blood-sampling visits) were measured by ELISA using commercially available kits (GRP78/BiP: Enzo Life Sciences, Farmingdale, NY and CHOP: LifeSpan BioSciences, Inc, Seattle, WA) conducted according to the manufacturer’s instructions.
Fatty Acid Composition
Phospholipid PUFA composition analysis, a validated assessment of dietary PUFA intake, was obtained for all subjects at baseline and after dietary supplementation. Whole blood samples were separated, and plasma was frozen and stored at −80°C for later analysis. Plasma phospholipids were extracted in methanol and directly methylated with sodium methoxide (25% wt/vol) as described previously.15 Fatty acid methyl esters (FAMEs) were extracted in hexanes and separated using microcapillary gas liquid chromatography with flame ionization detection. Individual FAMEs were identified by comparison of retention times with known standards. Plasma PUFA composition was expressed as a percentage of total PUFA. Response to intervention was evaluated by changes in the plasma ratios of omega-6 (sum of percentages for linolenic acid, 18:2n-6; dihomo-gamma-linolenic acid, 20:3n6; and arachidonic acid, 20:4n6) to omega-3 (sum of percentages for EPA, 20:5n3; DHA, 22:6n3; and alpha-linolenic acid [ALA], 18:2n3) fatty acids.
Statistical Analysis
Daily urinary LH, FSH, E1c, and Pdg concentrations were evaluated using area under the curve (AUC) and medians for the follicular and luteal phases. Cycles were adjusted to 28 days centered on the day of luteal transition. Luteal activity and day of luteal transition were calculated using a previously validated algorithm to determine whether ovulation had occurred.24
Biometric and endocrine characteristics at baseline were compared using 2 sample t tests to assess whether average change in hormonal and demographic measures differed between obese and NW women. Baseline characteristics for NW and obese groups were compared for all steroid hormones, gonadotropins, and individual PUFA composition using Mann–Whitney tests. The Wilcoxon rank-sum test was used to assess whether the hormonal parameters differed before versus after the omega-3 PUFA intervention. Unequal variances t tests were used to compare serum ER stress markers before and after omega-3 PUFA intervention. Two sample t tests were used to compare ER stress markers between the NW and obese group; however, paired t tests were used to compare the groups before and after intervention. A P value <.05 was used to denote statistical significance.
Results
Biometric and Endocrine Characteristics
A total of 17 women (7 NW controls and 10 obese) provided complete urinary collection data for analysis (Table 1). Although the obese women were older (P < .01), there was no significant difference in the ovarian reserve markers AMH or inhibin B, between the groups. As expected by the study design, obese women had significantly higher BMI, weight, waist circumference, and waist–hip ratio. Additionally, the obese group had significantly higher fasting insulin levels compared to NW controls (16.7 ± 8.3 mIU/L vs 8.43 ± 4.0 mIU/L; P = .01) but no difference in fasting serum glucose (88.8 ± 6.9 mg/dL vs 75.6 ± 26.0 mg/dL; P = .07), indicating appropriate selection of non-diabetic study participants. Average menstrual cycle length was similar between the groups (28.5 days for obese vs 27.6 days for NW; P = .27) and did not change after dietary intervention (28.6 days for obese vs 27.6 days for NW; P = .23). Omega-3 treatment did not significantly change any ovarian reserve parameters or insulin resistance determinants in either group (Data not shown). All subjects were ovulatory before and after intervention, as determined by objective a priori criteria.24
Fatty Acid Composition
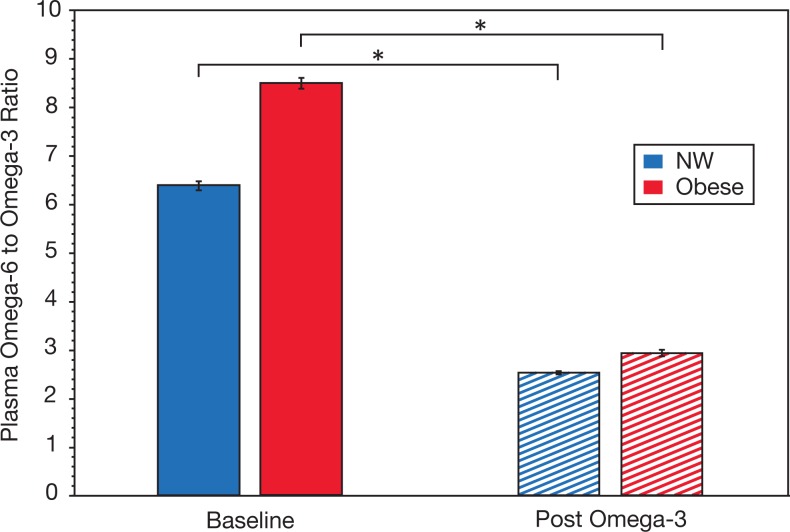
At baseline, the ratio of omega-6 to omega-3 PUFA in plasma was similar between the 2 groups (Figure 1). Following omega-3 PUFA supplementation, the plasma omega-6/omega-3 PUFA ratio was significantly decreased in both NW and obese women, thus verifying compliance with the study intervention. There was an approximately 3-fold reduction for both NW (7.4 ± 3.0 pre vs 2.8 ± 1.0 post) and obese groups (8.9 ± 1.8 pre vs 3.0 ± 0.6 post), both P < .01.
Figure 1.
Impact of omega-3 polyunsaturated fatty acid (PUFA) supplementation on plasma omega-6 to omega-3 ratio. Levels in normal weight (NW; blue) and obese (red) women both at baseline (solid bars) and after 1 month of omega-3 supplementation (hashed bars) are shown. Differences between NW and obese women were not significant both at baseline and post omega-3 supplementation. Data represent mean ± standard error of the mean. *P < .01.
Gonadotropins
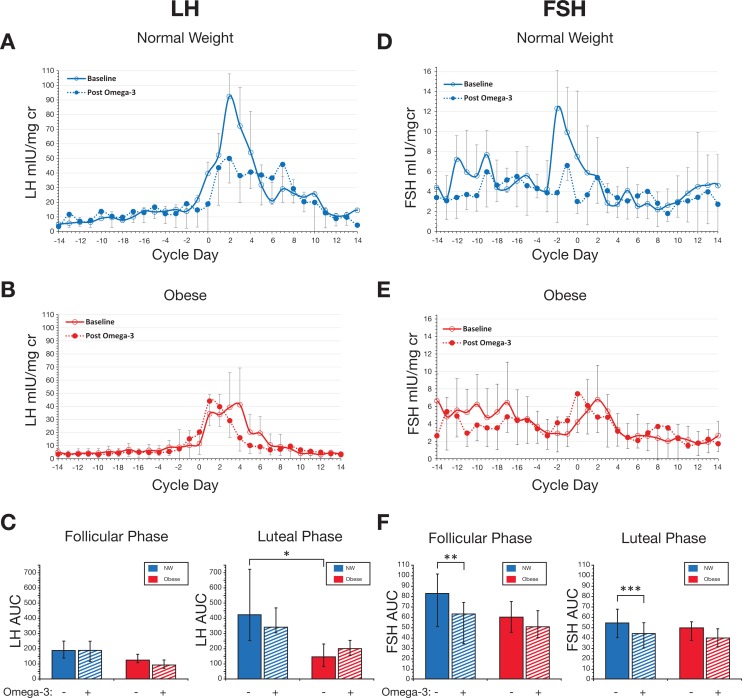
At baseline, obese women produced significantly less luteal phase LH as compared to their NW counterparts (Figure 2A-C). The median luteal LH AUC was 426.4 (interquartile range [IQR]: 252.6-722.9) for NW versus 149.2 (IQR: 83.0-232.4) for obese women, P = .02. Baseline FSH measurements were similar for both groups (Figure 2D-F).
Figure 2.
Effect of omega-3 polyunsaturated fatty acid (PUFA) supplementation on urinary gonadotropins. Daily morning urine was assayed for luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and normalized to creatinine. Cycles were aligned based on the Day of Luteal Transition (Day 0). Data are from normal weight (NW) women (blue; n = 7) and obese women (red; n = 10). For each hormone, NW subjects are compared at baseline (open circles, solid line) and after omega-3 supplementation (closed circles, dashed line; panel A for LH; panel D for FSH). Obese subjects are compared at baseline (open circles, solid line) and after omega-3 supplementation (closed circles, dashed line; panel B for LH; panel E for FSH). Error bars indicate standard error of the mean for group composites. Panels C and F represent data for area under the curve (AUC) at baseline (solid bars) and post omega-3 supplementation (hashed bars) by menstrual cycle phase. All significant differences between LH and FSH measurements are indicted by asterisk, as follows. All other comparisons performed on the LH and FSH measurements were not significant *P = .02. **P = .04. ***P = .01. Data are medians; error bars indicate interquartile range (IQR).
Following omega-3 supplementation, we observed a significant reduction in FSH, in both follicular and luteal phases, for NW women only. The median follicular FSH AUC was 83.3 (IQR: 51.0-101.7) at baseline versus 63.6 (IQR: 34.7-74.5) after intervention, P = .04. Similarly, the median luteal FSH AUC showed a comparable decrease in NW women, 54.5 (IQR: 40.1-67.7) at baseline vs 44.3 (IQR: 30.1-54.6) after intervention, P = .04. The absolute magnitude of FSH reduction in NW women was −28.4 and −12.6% for follicular and luteal phases, respectively. Follicle-stimulating hormone response to omega-3 PUFA in NW women was the only notable finding; obese women did not change their FSH parameters after intervention. Likewise, no LH measures exhibited any significant differences in response to intervention for either weight group.
Steroid Hormones
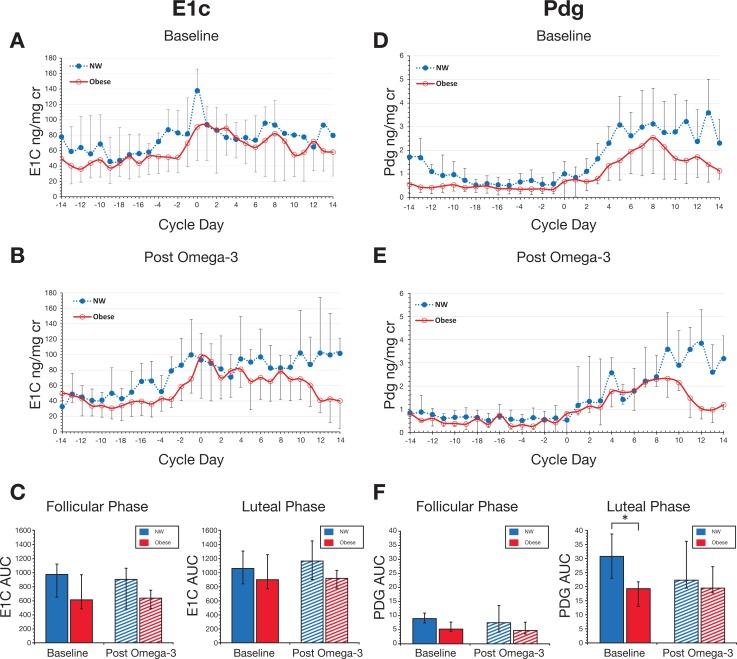
At baseline, obese women produced significantly less luteal Pdg as compared to NW women (Figure 3D-F). The median luteal AUC for Pdg was 30.9 (IQR: 23.0-38.8) for NW women versus 19.4 (IQR: 12.9-21.7) for obese women, P = .03. Baseline E1c measurements were not significantly different between the groups (Figure 3A-C) with a trend for lower production for obese women in the follicular phase; median follicular E1c was 978.8 (IQR: 574.8-1180.2) for NW versus 618.2 (IQR: 484.9-1015.7) for obese, P = .06.
Figure 3.
Effect of omega-3 polyunsaturated fatty acid (PUFA) supplementation on urinary estrone conjugates (E1c) and pregnanediol-3-glucuronide (Pdg). Daily morning urine was assayed for E1c and Pdg, normalized to creatinine. Cycles were aligned based on the day of luteal transition (Day 0). Data are from normal weight (NW) women (blue; n = 7) and obese women (red; n = 10). For each hormone, NW and obese subjects are compared in the baseline month (A and D, respectively) and post omega-3 supplementation (B and E, respectively). Error bars indicate standard error of the mean for group composites. Panels C and F represent data for area under the curve (AUC) depicted as area at baseline (solid bars) and post omega-3 supplementation (hashed bars) by menstrual cycle phase. *P = .03. Data are medians; error bars indicate interquartile range (IQR).
Following omega-3 supplementation, no significant effect on E1c or Pdg production was observed for either weight group. Postintervention increases in E1c for both groups during the luteal phase (Figure 3C) and for Pdg in obese women in the luteal phase (Figure 3F) were not statistically significant.
Endoplasmic Reticulum Stress Markers
At baseline, there was no significant difference in mean serum CHOP expression between NW and obese groups (62.60 and 65.64 ng/mL, respectively, P = .21, Supplemental Figure S2). However, omega-3 supplementation induced a small increase in CHOP in NW women and a corresponding decrease in the obese cohort, such that the difference in mean serum CHOP expression between NW and obese groups (71.12 and 57.73 ng/mL, respectively) approached statistical significance (P = .06). BiP/GRP78 did not show any significant differences in mean serum expression during the baseline month for NW and obese groups (2074.9 and 2396.5 ng/mL, respectively, P = .18, Supplemental Figure S2), and unlike CHOP, there were no significant differences between NW and obese women after omega-3 supplementation (NW = 3143.21; obese = 3608.4; both ng/mL, P = .21). The postomega-3 treatment increase in GRP78 in both groups (Supplemental Figure S2) was not statistically significant.
Discussion
We have previously demonstrated a reduction in early follicular serum FSH levels in NW but not obese women after treatment with omega-3 PUFA.15 In this study, we evaluated the impact of supplementation with omega-3 PUFA on urinary gonadotropins, in obese and NW women, across the whole menstrual cycle. We report a significant and consistent reduction in FSH levels for NW but not obese women after omega-3 PUFA supplementation. The magnitude of FSH reduction was almost 30% in the follicular phase. We observed no changes in any other hormonal parameters in response to omega-3 PUFA. Our findings are consistent with the previously published analysis of serum frequent blood sampling performed during early follicular phase from the same cohort.15 Follicle-stimulating hormone reduction in NW women was seen throughout the entire menstrual cycle, both follicular and luteal phases. To the best of our knowledge, this is the first comprehensive analysis of the impact of omega-3 PUFA in NW women, followed by daily urinary collection. Our findings add to and enhance the emerging interest in the use of omega-3 PUFA supplementation for improving female fertility and suggest that body weight differences may explain some of the conflicting human data on reproductive outcomes.
In women undergoing assisted reproductive treatments, the preponderance of studies has demonstrated favorable effects of preconception PUFA supplementation and/or higher intake. Hammiche et al6 reported a significant benefit of higher preconception PUFA intake on embryo morphology during in vitro fertilization (IVF) while Moran et al7 showed improved pregnancy rates after IVF in women with higher preconception intake compared to those with lower intake. In contrast, Jungheim et al25 demonstrated that women with elevated serum ALA, an 18-carbon omega-3 PUFA, had a decreased chance of pregnancy after IVF compared to women with lower serum levels; however, the sample size was relatively modest. Additionally, ALA is about 6-fold less potent than EPA or DHA, and thus, theoretically, correspondingly higher ALA concentrations would be required to achieve the same effect. More recently, the PREPARE trial demonstrated that, among patients undergoing IVF without intracytoplasmic sperm injection, those receiving omega-3 and vitamin D supplements produced embryos with higher embryo scores, as compared to control couples.26 Similarly, subgroup analysis for the EARTH prospective cohort reported that higher levels of serum omega-3 PUFAs were associated with higher probability of clinical pregnancy and live birth rate.27 Taken together, this evidence suggests a benefit of omega-3 PUFA supplementation to couples utilizing assisted reproductive technology.
Data from animal models show a more consistent benefit of omega-3 PUFA supplementation on fertility outcomes and potential mechanistic pathways. In a rodent model, dietary fish oil, rich in omega-3 fatty acids, restores ovarian function in subfertile rats, which is thought to be due to decreased transcription of FSH β-subunit.16 Similarly, oral supplementation with fish oil preserved ovarian function in a diabetic rat model known to be associated with profound ovarian insufficiency.17 Finally, consumption of a diet rich in omega-3 PUFA prolonged reproductive life span in mice over several generations.3 A dramatic improvement in egg quality and ovarian follicle counts was achieved resulting in a reversal of diminished ovarian reserve in advanced maternal age mice.
Evidence suggests that ER stress, a critical pathway in the pathogenesis of obesity,28 may impact both the pituitary and ovary to modulate gonadotropin secretion, folliculogenesis, and oocyte quality.19,29-31 Induction of ER stress in pituitary gonadotropes suppresses LH and FSH secretion and may underlie the hypogonadotropism of obesity.30 In the ovary, ER stress impairs ovarian function and reduces FSH responses, potentially resulting in an increase in FSH, characteristic of ovarian aging.29 ,31 Additionally, administration of DHA has been shown to reduce ER stress following traumatic brain injury32 and omega-3 PUFA treatment attenuated ER stress in patients with nonalcoholic steatohepatitis.33 The isolated decrease in FSH in response to omega-3 supplementation is consistent with a reduction in ovarian ER stress, increased sensitivity to FSH and/or modulation of HPO feedback.15,31 Thus, in hopes of identifying a potential mechanism of action for our findings, we postulated that serum markers of ER stress (GRP78/BiP and CHOP) might be altered by omega-3 PUFA supplementation.
Examination of systemic, serum levels of GRP78 and CHOP did not reveal any significant differences with respect to obesity or omega-3 PUFA supplementation; although a decrease in CHOP levels in obese subjects treated with omega-3 PUFA did approach significance (P = .06). Increased CHOP expression in response to ER stress promotes apoptosis22 and has been linked to impaired granulosa cell function and follicular atresia.29 Thus, a reduction in CHOP expression in response to omega-3 PUFA supplementation is consistent with improved ovarian function and the observed decrease in FSH. We acknowledge that the serum levels of CHOP and GRP78 may not reflect tissue-specific ER stress in the ovary or pituitary, but the effects of dietary omega-3 PUFA and obesity on such markers, in follicular fluid and granulosa cells from oocyte retrievals, merit further investigation.
We have considered certain limitations of the current study. While the intervention was shown to alter blood levels of target fatty acids, indicating compliance and efficacy, the period of intervention may have been too short to significantly impact other steroid hormones and gonadotropins. In addition, our relatively small sample size may have impacted our ability to detect statistical significance for some of our parameters. However, we note that significant effects were observed in the smaller NW cohort. We note that the obese women were older than their lean counterparts (mean 30.3 vs 35.5); however, age-related increases in FSH are typically not significant in this age range,34-36 and levels of inhibin and AMH were not different (Table 1). Additionally, while all women had normal AMH levels and regular menstrual cycles and were clinically not hirsute, the use of NIH criteria to exclude women with PCOS may miss identifying women with a milder Rotterdam PCOS phenotype (2 of 3 of ovulatory dysfunction with regular cycles, polycystic ovarian morphology or biochemical hyperandrogenism) and thus limit the generalizability of our study. Lastly, we recognize our discussion of the effects of omega-3 PUFA supplementation on reproductive function is limited to the endocrine HPO axis, as we did not include pregnancy as a defined outcome. The strengths of our study include that our urinary data spanned the entire menstrual cycle and therefore provided a more comprehensive analysis of how urinary steroid hormones and gonadotropins change over both follicular and luteal phases in response to PUFA supplementation. We also provide evidence that, even in normally cycling, ovulatory women, obesity is a significant modulator of the effects of dietary omega-3 PUFA supplementation on the reproductive axis.
Conclusion
In summary, as elevated FSH is a hallmark of both the menopausal state and diminished ovarian reserve, future studies are well positioned to examine how omega-3 PUFA supplementation, which we have found to selectively and specifically decrease FSH, might benefit women. An investigation of women with decreased fertility should be undertaken with a goal of evaluating a potential impact on fecundity, chances of success with fertility treatments and assisted reproductive techniques, as well as to gain further mechanistic insights in terms of overall fertility.
Supplemental Material
Supplemental Material, Bauer_Supplemental_Fig_1,2 for Reduction in FSH Throughout the Menstrual Cycle After Omega-3 Fatty Acid Supplementation in Young Normal Weight but not Obese Women by Jessica L. Bauer, Katherine Kuhn, Andrew P. Bradford, Zain A. Al-Safi, Mary A. Harris, Robert H. Eckel, Celeste Y. Robledo, Anahit Malkhasyan, Joshua Johnson, Nancy R. Gee and Alex J. Polotsky in Reproductive Sciences
Acknowledgments
The authors acknowledge the assistance of Bill Lasley, PhD, and the Endocrine Core at the California National Primate Research Center, for the urinary steroid assays. The authors also acknowledge the University of Virginia, Center for Research in Reproduction Ligand Assay and Analysis Core for assistance with hormonal assays and thank Jack Dunker for assistance with formatting the figures and tables.
Authors’ Note: ClinicalTrials.gov Identifier: NCT01894581
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge support for this study from NICHD (R01 HD081162 and U54 HD058155) and Colorado Clinical Translational Sciences Institute NIH 1UL1 RR025780 (University of Colorado CTRC).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015;79:1–16. [PMC free article] [PubMed] [Google Scholar]
- 2. Palmsten K, Flores KF, Chambers CD, Weiss LA, Sundaram R, Buck Louis GM. Most frequently reported prescription medications and supplements in couples planning pregnancy: the LIFE study. Reprod Sci (Thousand Oaks, Calif). 2018;25(1):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nehra D, Le HD, Fallon EM, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11(6):1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mumford SL, Chavarro JE, Zhang C, et al. Dietary fat intake and reproductive hormone concentrations and ovulation in regularly menstruating women. Am J Clin Nutr. 2016;103(3):868–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin JC, Zhou SJ, Flynn AC, Malek L, Greco R, Moran L. The assessment of diet quality and its effects on health outcomes pre-pregnancy and during pregnancy. Semin Reprod Med. 2016;34(2):83–92. [DOI] [PubMed] [Google Scholar]
- 6. Hammiche F, Vujkovic M, Wijburg W, et al. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil Steril. 2011;95(5):1820–1823. [DOI] [PubMed] [Google Scholar]
- 7. Moran LJ, Tsagareli V, Noakes M, Norman R. Altered preconception fatty acid intake is associated with improved pregnancy rates in overweight and obese women undertaking in vitro fertilisation. Nutrients. 2016;8(1):pii: E10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wonnacott KE, Kwong WY, Hughes J, et al. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction (Cambridge, England). 2010;139(1):57–69. [DOI] [PubMed] [Google Scholar]
- 9. Wise LA, Wesselink AK, Tucker KL, et al. Dietary fat intake and fecundability in two preconception cohort studies. Am J Epidemiol. 2017;187(1):60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polotsky AJ, Hailpern SM, Skurnick JH, Lo JC, Sternfeld B, Santoro N. Association of adolescent obesity and lifetime nulliparity--the Study of Women’s Health Across the Nation (SWAN). Fertil Steril. 2010;93(6):2004–2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brunner Huber LR, Hogue CJ. The association between body weight, unintended pregnancy resulting in a livebirth, and contraception at the time of conception. Matern Child Health J. 2005;9(4):413–420. [DOI] [PubMed] [Google Scholar]
- 12. Lan L, Harrison CL, Misso M, et al. Systematic review and meta-analysis of the impact of preconception lifestyle interventions on fertility, obstetric, fetal, anthropometric and metabolic outcomes in men and women. Hum Reprod (Oxford, England). 2017;32(9):1925–1940. [DOI] [PubMed] [Google Scholar]
- 13. Edelman AB, Cherala G, Munar MY, McInnis M, Stanczyk FZ, Jensen JT. Correcting oral contraceptive pharmacokinetic alterations due to obesity: a randomized controlled trial. Contraception. 2014;90(5):550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawwass JF, Kulkarni AD, Hipp HS, Crawford S, Kissin DM, Jamieson DJ. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertil Steril. 2016;106(7):1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al-Safi ZA, Liu H, Carlson NE, et al. Omega-3 fatty acid supplementation lowers serum FSH in normal weight but not obese women. J Clin Endocrinol Metab. 2016;101(1):324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moriyama R, Yamazaki T, Kato T, Kato Y. Long-chain unsaturated fatty acids reduce the transcriptional activity of the rat follicle-stimulating hormone beta-subunit gene. J Reprod Dev. 2016;62(2):195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khedr NF. Fish oil and wheat-germ oil supplementation restores ovarian function in streptozotocin-diabetic rats. Reprod Fertil Dev. 2017;29(9):1689–1698. [DOI] [PubMed] [Google Scholar]
- 18. Meyer PM, Zeger SL, Harlow SD, et al. Characterizing daily urinary hormone profiles for women at midlife using functional data analysis. Am J Epidemiol. 2007;165(8):936–945. [DOI] [PubMed] [Google Scholar]
- 19. Wu LL, Russell DL, Wong SL, et al. Mitochondrial dysfunction in oocytes of obese mothers: transmission to offspring and reversal by pharmacological endoplasmic reticulum stress inhibitors. Development (Cambridge, England). 2015;142(4):681–691. [DOI] [PubMed] [Google Scholar]
- 20. Fu Z, Lofqvist CA, Shao Z, et al. Dietary omega-3 polyunsaturated fatty acids decrease retinal neovascularization by adipose-endoplasmic reticulum stress reduction to increase adiponectin. Am J Clin Nutr. 2015;101(4):879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aksoy MO, Kim V, Cornwell WD, et al. Secretion of the endoplasmic reticulum stress protein, GRP78, into the BALF is increased in cigarette smokers. Respir Res. 2017;18(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guzel E, Arlier S, Guzeloglu-Kayisli O, et al. Endoplasmic reticulum stress and homeostasis in reproductive physiology and pathology. Int J Mol Sci. 2017;18(4):pii: E792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Billman G. Effect of dietary omega-3 polyunsaturated fatty acids on heart rate and heart rate variability in animals susceptible or resistant to ventricular fibrillation. Front Physiol. 2012;3(71):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kassam A, Overstreet JW, Snow-Harter C, De Souza MJ, Gold EB, Lasley BL. Identification of anovulation and transient luteal function using a urinary pregnanediol-3-glucuronide ratio algorithm. Environ Health Perspect. 1996;104(4):408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum alpha-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil Steril. 2011;96(4):880–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kermack AJ, Calder PC, Houghton FD, Godfrey KM, Macklon NS. A randomised controlled trial of a preconceptional dietary intervention in women undergoing IVF treatment (PREPARE trial). BMC Womens Health. 2014;14:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Messerlian C, Williams PL, Ford JB, et al. The Environment and Reproductive Health (EARTH) study: a prospective preconception cohort. Hum Reprod Open. 2018;2018(2):pii: hoy001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cnop M, Foufelle F, Velloso LA. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol Med. 2012;18(1):59–68. [DOI] [PubMed] [Google Scholar]
- 29. Huang N, Yu Y, Qiao J. Dual role for the unfolded protein response in the ovary: adaption and apoptosis. Protein Cell. 2017;8(1):14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li S, Mbong EF, John DT, Terasaka T, Li D, Lawson MA. Induction of stress signaling in vitro and suppression of gonadotropin secretion by free fatty acids in female mouse gonadotropes. Endocrinology. 2018;159(2):1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Babayev E, Lalioti MD, Favero F, Seli E. Cross-talk between FSH and endoplasmic reticulum stress: a mutually suppressive relationship. Reprod Sci (Thousand Oaks, Calif). 2016;23(3):352–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Begum G, Yan HQ, Li L, Singh A, Dixon CE, Sun D. Docosahexaenoic acid reduces ER stress and abnormal protein accumulation and improves neuronal function following traumatic brain injury. J Neurosci. 2014;34(10):3743–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Okada L, Oliveira CP, Stefano JT, et al. Omega-3 PUFA modulate lipogenesis, ER stress, and mitochondrial dysfunction markers in NASH - proteomic and lipidomic insight. Clin Nutr. 2017;37(5):1474–1484. [DOI] [PubMed] [Google Scholar]
- 34. Kiranmayee D, Praveena T, Himabindu Y, Sriharibabu M, Kavya K, Mahalakshmi M. The effect of moderate physical activity on ovarian reserve markers in reproductive age women below and above 30 years. J Hum Reprod Sci. 2017;10(1):44–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reame NE, Kelche RP, Beitins IZ, Yu MY, Zawacki CM, Padmanabhan V. Age effects of follicle-stimulating hormone and pulsatile luteinizing hormone secretion across the menstrual cycle of premenopausal women. J Clin Endocrinol Metab. 1996;81(4):1512–1518. [DOI] [PubMed] [Google Scholar]
- 36. van Rooij IA, Broekmans FJ, Scheffer GJ, et al. Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril. 2005;83(4):979–987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Bauer_Supplemental_Fig_1,2 for Reduction in FSH Throughout the Menstrual Cycle After Omega-3 Fatty Acid Supplementation in Young Normal Weight but not Obese Women by Jessica L. Bauer, Katherine Kuhn, Andrew P. Bradford, Zain A. Al-Safi, Mary A. Harris, Robert H. Eckel, Celeste Y. Robledo, Anahit Malkhasyan, Joshua Johnson, Nancy R. Gee and Alex J. Polotsky in Reproductive Sciences