Abstract
This study aimed to determine the prevalence of APC-associated familial adenomatous polyposis (FAP) and MUTYH-associated polyposis (MAP) in a large cohort, taking into account factors as adenoma count and year of diagnosis. All application forms used to send patients in for APC and MUTYH variant analysis between 1992 and 2017 were collected (n = 2082). Using the data provided on the application form, the APC and biallelic MUTYH prevalence was determined and possible predictive factors were examined using multivariate multinomial logistic regression analysis in SPSS. The prevalence of disease causing variants in the APC gene significantly increases with adenoma count while MAP shows a peak prevalence in individuals with 50–99 adenomas. Logistic regression analysis shows significant odds ratios for adenoma count, age at diagnosis, and, interestingly, a decline in the chance of finding a variant in either gene over time. Moreover, in 22% (43/200) of patients with FAP-related extracolonic manifestations a variant was identified. The overall detection rates are above 10% for patients with >10 adenomas aged <60 and >20 adenomas aged <70. Patients with variants outside these criteria had FAP-related extracolonic manifestations, colorectal cancer aged <40, somatic KRAS c.34G > T variant in the tumor or a first-degree relative with >10 adenomas. Therefore, APC and MUTYH testing in patients with >10 adenomas aged <60 and with >20 adenomas aged <70 is advised. Almost all FAP and MAP patients not meeting these criteria showed other characteristics that can be used as an indication to prompt genetic testing.
Subject terms: Genetic testing, Colorectal cancer
Introduction
Due to a combination of environmental and low penetrant risk genetic factors [1–3], a large proportion of the general population will develop one or more adenomatous polyps (25% at age 50 and 50% at age 70 [4]). These polyps are possible precursors of colorectal carcinoma (CRC). The most commonly reported polyposis coli syndromes are APC-associated familial adenomatous polyposis (FAP) and MUTYH-associated polyposis (MAP) [5, 6]. Variants affecting the function of these genes are found in 8–10% of all patients with polyposis, depending on age and number of adenomas. Other forms of adenomatous polyposis explaining <1% of polyposis patients include PolE/D- [7], NTHL1- [8], MSH3- [9], MBD4- [10], and MLH3-associated polyposis [11]. Furthermore, mosaic APC variants are found in a substantial proportion of the remaining unexplained polyposis cases [12].
Identifying patients and family members with a genetic predisposition for polyposis is important due to the high CRC risk that carriers face, even at a young age. This risk can be largely circumvented through regular surveillance and adenoma removal. Since adenomas after the age of 50 are common in the general population, offering genetic testing to all patients with adenomatous polyps is not yet cost effective. No clear guidelines for genetic testing existed until recently, and studies on the rates of variant detection have focused on patients with more than 20 adenomas lacking detailed information on the outcome of genetic testing in patients with less than 20 adenomas.
The present cohort consists of Dutch polyposis patients tested for APC and/or MUTYH variants between 1992 and 2017. The primary aim of this study was to determine the prevalence of APC and biallelic MUTYH disease causing variants in individuals referred to the clinical genetics department for DNA testing. Furthermore, we studied the relationship between the APC and MUTYH variants and several covariates. Based on these outcomes, guidelines were developed regarding the indications for referral to a clinical geneticist for DNA analyses.
Methods
Study population
This cross-sectional study was conducted amongst probands referred to a clinical geneticist (1992–2017) based on an individual’s phenotype and/or family history of cancer and polyps. After consultation at centers across the Netherlands, blood samples and prespecified application forms were sent to the LUMC Laboratory of Diagnostic Genome Analysis (LDGA) for diagnostic analysis of the APC and MUTYH genes. The prespecified application form included age at testing, age at diagnosis of colorectal adenomas and/or CRC, personal history of other cancers, and a pedigree with relevant family information. The clinical information of the majority of the patients had been collected in databases developed for other studies [3, 13, 14]. These databases were merged and any required additional information was added. In total 2082 patients were included, exclusion criteria are listed in Fig. S1.
Although no clear guidelines existed, the presence of >10 adenomatous polyps was generally considered a reason for referral, as also advised by the American College of Gastroenterology (ACG) [15]. Moreover, FAP-related extracolonic manifestations were considered an indication for genetic APC testing and a somatic NM_033360.3 (KRAS): c.34G > T in tumor for MUTYH testing [16, 17].
Clinical genetic testing was performed with full gene Sanger sequencing and rearrangements were analyzed using multiplex ligation dependent probe amplification for the APC and MUTYH genes. MUTYH clinical diagnostics became available in 2004 [6], individuals suspicious for MAP but tested before 2004 were analyzed retrospectively.
Missing data
Due to incompletely filled in application forms, 26 patients were included with missing values for the age at first adenoma, 9 missed age at first CRC, and 164 patients missed family history. Possible explanations for a missing or incomplete pedigree information on the application form were adoption and no contact with family members.
Both the APC and MUTYH gene were sequenced in the majority of patients. However, in 387 (19%) and 339 (16%) patients only the MUTYH or the APC gene, respectively, was tested. The reasons for not testing these genes are summarized in Table S1.
Definitions
The terms “polyp” and “adenoma” were both used to describe patient samples sent for analysis. If no histology was mentioned, “polyps” were assumed to be adenomatous. After 2004, patients with hyperplastic/serrated polyps were occasionally sent for specifically MUTYH analysis [18]. Patients with exclusively serrated/hyperplastic type (n = 19) were treated separately in this study. Patients with other types of polyps such as hamartomatous or juvenile polyps were excluded.
Patients with a phenotype described as “FAP” (n = 170) or “polyposis” (n = 19) were considered to have >100 adenomas, “multiple adenomas/polyps” (n = 206) and “polyps” (n = 14) were categorized as 20–49 adenomas, and “some polyps” (n = 11) as less than 10 adenomas, as described previously [3]. Individuals without information on polyp history were excluded. Moreover, family members with 10 polyps or ‘some’ polyps were labeled as having <10 polyps, descriptions such as “FAP,” “AFAP,” and “multiple” were considered to have >10, and the bare description “polyps” as number unknown. When more than one first-degree relative (FDR) were reported with polyps, the highest number of polyps was used. Whenever multiple family members were diagnosed with CRC, the youngest was defined as the age of CRC in that family.
An APC de novo variant was assumed whenever the patients parents tested negative for the APC variant (n = 10) or whenever the pedigree showed no relevant cancers or polyps (n = 69).
Statistical methods
Multivariable logistic regression analysis was used to assess associations between variant status (yes/no) and covariates of interest. These covariates included cumulative polyp count (<10, 10–19, 20–49, 50–99, and >100), age at diagnosis (<30, 30–39, 40–49, 50–59, and >60), history of CRC (no, <40, 40–50, and >50 (when multiple CRC, youngest age of diagnosis was used)), FDR with polys (no, yes <10, yes >10, and yes number unknown), with CRC (no, yes <50 years, yes >50 years, and yes age unknown), and year of analysis (<1995–1999, 2000–2005, 2006–2011, and 2012–2017). The patients without any adenomas were treated as a separate group.
Patients in whom APC or MUTYH was not tested were not included in the logistic regression analysis of the APC or MUTYH variant, respectively. All these patients were excluded from the analysis for overall variant detection.
Results were reported as odds ratios, with a 95% confidence interval, and a p-value < 0.05 was considered statistically significant. The statistical analyses were performed using SPSS statistics 23.
Results
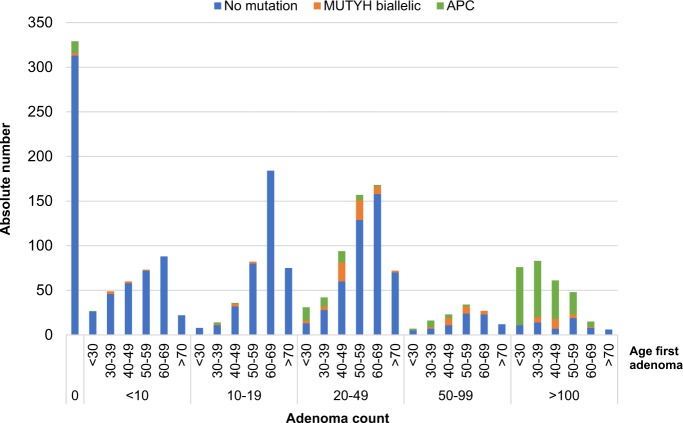
Of the 2082 individuals included in the study (Table 1), in total, 14% (n = 293) carried an APC variant, 6% (n = 119) a biallelic MUTYH variant, and 2% (n = 39) a monoallelic MUTYH variant. Overall, a personal history of CRC was reported in 36% (n = 746) of patients. Notably, 16% (n = 336) had no history of adenomas whatsoever. In the overall cohort, variant detection rate is highest in patients with more than 20 adenomas (Fig. 1) and increases with younger ages (Fig. 2).
Table 1.
Cohort characteristics
| Total (n = 2082) | APC | Biallelic MUTYH | Monoallelic MUTYH | |
|---|---|---|---|---|
| Male—n (%) | 1202 (58%) | 147 (50%) | 64 (54%) | 27 (69%) |
| Adenoma count | ||||
| 0 | 336 | 13 (3.9%) | 3 (0.9%) | 7 (2.1%) |
| 1–9 | 328 | 1 (0.3%) | 6 (1.8%) | 7 (2.1%) |
| 10–19 | 406 | 3 (0.7%) | 6 (1.5%) | 7 (1.7%) |
| 20–49 | 590 | 50 (8.5%) | 60 (10%) | 15 (2.5%) |
| 50–99 | 122 | 15 (12%) | 22 (18%) | 3 (2.5%) |
| >100 | 300 | 211 (70%) | 22 (7.3%) | 0 (0%) |
| Mean age at adenoma diagnosis (min–max) | 53 (4–84) | 36 (9–68) | 49 (21–75) | 54 (23–77) |
| CRC, yes | 746 (36%) | 57 (7.6%) | 82 (11%) | 15 (2.0%) |
| Mean age at (first) CRC diagnosis (min–max) | 53 (12–91) | 41 (21–58) | 49 (21–76) | 57 (28–91) |
| FAP extracolonic manifestations, yes | 200 (10%) | 43 (22%) | 8 (4.0%) | 4 (2.0%) |
| FDR with polyps | 728 (38%) | 156 (21%) | 46 (6.3%) | 18 (2.5%) |
| Missing | 164 (8%) | 12 (7.3%) | 4 (2.4%) | 1 (0.6%) |
| FDR with CRC | 811 (42%) | 76 (9.7%) | 42 (5.1%) | 26 (3.2%) |
| Missing | 164 (8%) | 12 (7.3%) | 4 (2.4%) | 1 (0.6%) |
Fig. 1.
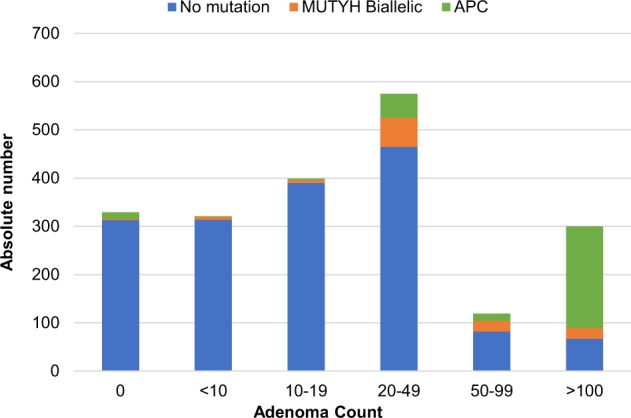
Absolute numbers of patients sent in for genetic testing among the different adenoma count groups. APC and MUTYH variant detection depicted in green and yellow, respectively
Fig. 2.
Variant detection in different adenoma count groups, specified by age group
Association between phenotypic characteristics and a variant in APC and/or MUTYH
Multivariable logistic regression analysis (Table 2) shows that the odds of identifying a variant in either gene steadily increases with adenoma count. The odds of APC variant detection are highest in patients with >100 adenomas (OR 289.9; 95% CI 35.2–2385.2), while the odds ratio for biallelic MUTYH variants was highest for the 50–99 adenoma count (OR 10.8; 95% CI 4.0–29.1).
Table 2.
Multivariate analysis
| APC or Biallelic MUTYH | APC | Biallelic MUTYH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | OR (95% CI) | P-value | Na | OR (95% CI) | P-value | Na | OR (95% CI) | P-value | |
| Adenoma count | |||||||||
| <10 | 188 | Ref | <0.001 | 205 | Ref | <0.001 | 296 | Ref | <0.001 |
| 10–19 | 292 | 2.6 (0.8–8.0) | 296 | 8.5 (0.8–88.9) | 367 | 1.5 (0.5–4.7) | |||
| 20–49 | 486 | 12.9 (5.0–33.3) | 502 | 39.2 (4.7–324.2) | 515 | 6.3 (2.6–15.1) | |||
| 50–99 | 114 | 15.5 (5.5–43.8) | 115 | 32.5 (3.6–289.9) | 116 | 10.8 (4.0–29.1) | |||
| >100 | 264 | 59.4 (22.4–157.2) | 275 | 289.9 (35.2–2385.2) | 269 | 3.5 (1.3–9.5) | |||
| Age at adenoma diagnosis | |||||||||
| >60 | 486 | Ref | <0.001 | 502 | Ref | <0.001 | 608 | Ref | <0.001 |
| 50–59 | 328 | 5.1 (2.7–9.5) | 335 | 3.3 (1.3–8.4) | 370 | 3.9 (2.0–7.6) | |||
| 40–49 | 235 | 14.5 (6.8–31.0) | 248 | 12.0 (4.6–31.4) | 260 | 5.5 (2.3–13.5) | |||
| 30–39 | 164 | 11.4 (5.3–24.8) | 172 | 12.6 (4.9–32.7) | 188 | 3.9 (1.4–10.6) | |||
| <30 | 131 | 18.7 (8.4–41.4) | 136 | 33.1 (12.5–87.5) | 137 | 0.9 (0.2–3.7) | |||
| CRC (age) | |||||||||
| No | 920 | Ref | 0.006 | 951 | Ref | 0.003 | 1065 | Ref | <0.001 |
| <40 | 56 | 1.3 (0.6–3.0) | 58 | 0.6 (0.3–1.3) | 63 | 3.9 (1.5–10.0) | |||
| 40–50 | 111 | 1.3 (0.7–2.5) | 115 | 0.3 (0.1–0.6) | 123 | 4.5 (2.2–9.0) | |||
| >50 | 257 | 2.6 (1.5–4.5) | 269 | 0.5 (0.2–1.1) | 312 | 5.1 (2.8–9.2) | |||
| FDR with polyps | |||||||||
| No | 809 | Ref | <0.001 | 834 | Ref | <0.001 | 945 | Ref | 0.098 |
| Yes, ≤10 polyps | 140 | 0.5 (0.2–1.0) | 153 | 1.5 (0.7–3.3) | 191 | 0.3 (0.1–0.8) | |||
| Yes, >10 polyps | 184 | 4.5 (2.6–8.0) | 191 | 4.5 (2.5–8.4) | 194 | 1.2 (0.6–2.3) | |||
| Yes, number unknown | 211 | 0.4 (0.2–0.7) | 215 | 0.4 (0.2–0.8) | 233 | 0.8 (0.5–1.5) | |||
| FDR with CRC | |||||||||
| No | 812 | Ref | 0.007 | 842 | Ref | 0.155 | 923 | Ref | 0.193 |
| Yes, ≤50 y | 132 | 1.3 (0.7–2.4) | 140 | 1.0 (0.5–2.1) | 157 | 1.5 (0.8–2.9) | |||
| Yes, >50 y | 349 | 0.5 (0.3–0.8) | 358 | 0.6 (0.3–1.0) | 426 | 0.7 (0.4–1.3) | |||
| Yes, age unknown | 51 | 1.6 (0.6–4.3) | 53 | 2.0 (0.7–6.0) | 57 | 0.7 (0.2–2.3) | |||
| Year of DNA testing | |||||||||
| 2012–2017 | 401 | Ref | <0.001 | 415 | Ref | <0.001 | 511 | Ref | 0.006 |
| 2006–2011 | 511 | 1.7 (1.0–3.0) | 521 | 2.0 (1.0–4.3) | 591 | 1.1 (0.5–2.7) | |||
| 2000–2005 | 266 | 3.9 (2.2–7.2) | 280 | 2.5 (1.2–5.4) | 291 | 2.3 (1.2–4.7) | |||
| <1995–1999 | 166 | 9.8 (4.7–20.3) | 177 | 9.6 (4.1–22.2) | 170 | 1.0 (0.5–2.0) | |||
aNumbers are without cases with missing information
A personal history of CRC increased the likelihood of detecting biallelic MUTYH variants (<40: OR 3.9 [95% CI 1.5–10.0], 40–50: OR 4.5 [95% CI 2.2–9.0], and >50 OR 5.1 [95% CI 2.8–9.2]). However, no effect was found for the detection of an APC variant.
The chance of finding a MUTYH or APC variant was not increased in patients with a FDR with CRC. Conversely, a FDR with >10 polyps did increase the odds of detecting an APC variant significantly (OR 4.5 [2.5–8.4]).
Variant detection rate trends over time
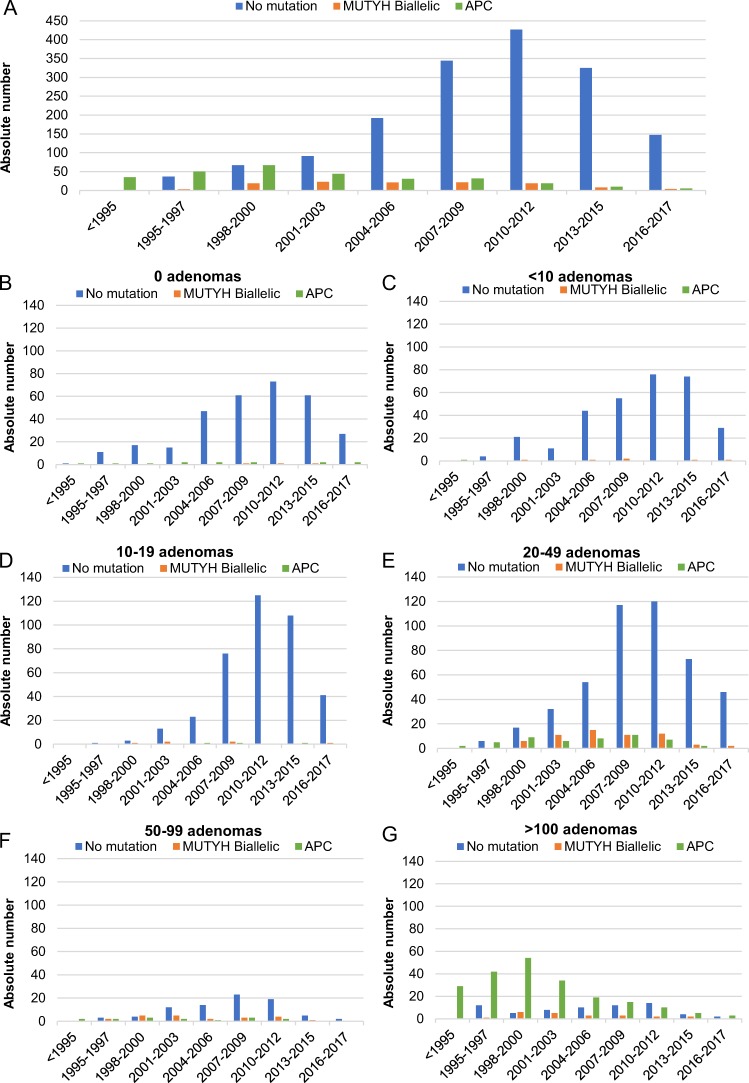
Also, the chance of finding a variant decreased over the last 20 years (<1995–1999: OR 9.8 [4.7–20.3], 2000–2005: OR 3.9 [2.2–7.2], and 2006–2011: OR 1.7 [1.0–3.0]). However, the odds of finding a biallelic MUTYH variant were highest between 2000 and 2005. Possibly explained by the introduction of MUTYH diagnostics in 2004, also attributing to the increase in number of patients sent in for DNA testing in general (Fig. 3).
Fig. 3.
Trends in variant detection. a APC/MUTYH variant detection in all adenoma groups. b Detection in patients without adenomas, c 1–9 adenomas, d 10–19 adenomas, e 20–49 adenomas, f 50–99 adenomas, and g more than 100 adenomas
APC and MUTYH detection rates in patients with less than 20 adenomas
Since a large number of patients with less than 20 adenomas underwent genetic testing (n = 1070, 51%), these categories are described in more detail.
No adenomas
The majority of patients without adenomas underwent testing due to CRC (n = 176), FAP-related extracolonic manifestations (n = 75), or both (n = 11). Nineteen had hyperplastic polyposis, while the rest were tested based on a positive family history. APC was tested in 203 and MUTYH was tested in 259 of these patients. Thirteen FAP and three MAP patients were detected in this group (Table S2). Nine of the APC variant carriers had extracolonic manifestations (mean age ~13, range 1.5–38). In addition, four had experienced CRC (two aged <40, one <50, and one >50). Of the MAP patients, all three had CRCs (<50 years old) with a KRAS c.34G > T transversion.
Of the 52 patients with solely CRC aged <40, 8% (2/24) had FAP and 4% (2/50) had MAP. In patients with CRC between age 40 and 50 years this was, respectively, 4% (1/24) and 2% (1/41).
1–9 adenomas
In patients with 1–9 adenomas (n = 328; APC tested n = 217 and MUTYH tested n = 309), one APC and six biallelic MUTYH variants were identified (2% variant detection rate). In this group the APC variant carrier already developed adenomas by the age of 20 and had a FDR with >100 polyps. Of the MAP patients, four were affected with CRC between the ages 39 and 53. Information on KRAS status in tumor DNA was available for one patient, showing a somatic KRAS c.34G > T transversion.
10–19 adenomas
Finally, in the group with 10–19 adenomas (n = 406; APC tested n = 324 and MUTYH tested n = 401) three FAP and six MAP patients were diagnosed who all developed adenomas aged under 60.
Aged above 70
No MUTYH or APC variants were found in patients with fewer than 20 adenomas aged above 70 years (n = 82). In the patients with more than 20 adenomas aged over 70 years, one MAP patient was found (1/90, 1.1%).
The prevalence of APC or biallelic MUTYH variants in different clinical phenotypes in patients with <20 adenomas is depicted in Table S3 (as adapted from Grover et al. [19]).
APC de novo
Based on family history, we surmise that a de novo variant has arisen in 24% of all APC variant carriers (69/292), which is comparable to the prevalence described previously [20]. This is also a plausible explanation for a negative family history in a number of FAP patients (Table S3).
Discussion
This study reports on 2082 individuals who underwent APC and MUTYH analysis at the LDGA between 1992 and 2017. The variant detection rates in patients with classic polyposis for FAP (70%) and MAP (7%) were comparable to previous studies [5, 21–24]. As expected, MAP showed a greater prevalence than FAP among individuals with 20–49 adenomas (FAP 9% vs. MAP 10%) and 50–99 adenomas (FAP 12% vs. MAP 18%). Notably, a recent study reported lower variant rates in all adenoma groups, possibly explained by the differences in clinical background (i.e., older age) and more recent years of diagnosis (2012–2016) [25].
Although most patients undergoing DNA analysis nowadays have fewer than 20 adenomas, clinical factors associated with the presence of a germline APC or biallelic MUTYH variants in this group are still poorly understood. A study by Grover et al. [19] reported a low variant detection rate, but no clinical description of the variant carriers was provided. The study from Stanich et al. [25] analyzed a large cohort of patients with 10–20 polyps, however no patients with less than ten polyps were included. In our cohort, a large group of individuals without adenomas (n = 336) was included.
Except for four MAP patients (Table S2), all patients with APC or biallelic MUTYH variants presented with >10 adenomas aged <60, >20 adenomas aged <70, CRC below age 40, a typical KRAS c.34G > T variant, a FDR relative with >10 polyps, or FAP-related extracolonic manifestations explaining their referral.
Since KRAS was not systematically analyzed in CRC cases, no variant detection rate could be determined for this cohort. Previous studies showed in 10–25% of the CRC cases with the KRAS c.34G > T variant a biallelic MUTYH variant. KRAS analysis in CRC is often performed because of the prognostic and therapeutic value [16, 17].
To analyze the impact on detection rates of several factors regression analysis was performed. While a younger age of first adenoma was associated with an increasing odds ratio of finding a variant in either gene, a personal history of CRC only increased the odds of finding a biallelic MUTYH variant, as also reported by Grover et al. [19]. This can possibly be explained by the fact that known FAP patients undergo a (sub)total colectomy at an early age, effectively preventing the development of CRC. A family history of CRC did not influence the chance of finding either an APC or MUTYH variant. On the other hand, having a FDR with more than ten polyps clearly increased the chance of detecting an APC variant (OR 4.5, 2.5–8.4).
Increasing numbers of patients undergo DNA analysis while variant detection rate has steadily declined over the years. This resulted in an avoidable burden and expense for family cancer clinics and emphasizes the need for more stringent guidelines. One plausible explanation for the increase is the introduction of MUTYH gene testing in 2004, allowing milder phenotypes to be tested and thus increasing the number of patients with fewer than 20 adenomas. An alternative explanation is the introduction of population screening in the Netherlands in 2014 leading to increasing numbers of patients aged >55, with <10–20 adenomas. However, the total number of individuals declined after 2013, possibly due to other Dutch laboratories offering MUTYH and APC testing themselves. Finally, the introduction of more sensitive techniques, such as chromoendoscopy, improvement of endoscopy equipment, implementation of adenoma detection rate as a quality measure, and better bowel preparation, has led to improved adenoma detection, particularly of low stage and small adenomas (i.e., <0.5 mm) [26–28]. Moreover, a gradual incline in the percentage of de novo APC variants was seen over the years (<1995–1999: 14%, 2000–2005: 28%, 2006–2011: 36%, and 2012–2017: 29%), likely indicating that the majority of Dutch FAP families have been identified.
In 2015, the ACG issued guidelines for APC and MUTYH genetic testing in individuals with >10 cumulative colorectal adenomas, FAP-related extracolonic manifestations, or a family history of an adenomatous polyposis syndrome [15]. Based on our data, these guidelines may result in unnecessary testing, especially above the age of 60. On the other hand, Dutch guidelines also formulated in 2015 advise patients with either ten or more adenomas <60 years (cumulative) or 20 or more adenomas <70 years (cumulative) to be referred for genetic testing. The most recent NCCN guidelines [29] suggest genetic testing for all patients with >20 adenomas or a personal history of desmoid tumors, hepatoblastoma, cribriform-morular papillary thyroid cancer, and CHRPE, or patients with 10–20 adenomas with specific features such as age of onset influencing whether testing should be offered. Both these guidelines are supported by our data.
Stanich et al. [25] suggest testing in all patients with >10 polyps, regardless of histology or age despite their observation of declining variant rates with increasing age. Their reason is the observed detection rate in nonpolyposis related genes of around 5%. However, the 1% CHEK2 variants reflects the prevalence in the general population [30] and does, in our opinion, not explain the polyposis phenotype. Furthermore, we excluded patients with MMR gene variants since further research is needed to draw firm conclusions about the association with polyposis.
CRC < 40 years in patients without adenomas might be a reason for testing, since variants were found in 9% and 4% of our cohort in, respectively, APC and MUTYH. Testing patients with adenomas above the age of 70 should on the other hand be undertaken with caution, since the variant detection rate was 1%. Of course, other more specific circumstances might warrant testing, such as polyps below age 20 and numerous primary CRC (≥2).
One weakness of this study was that not all patients with low adenoma counts were tested for both APC and MUTYH. We detected 4% APC and 1% biallelic MUTYH variants in 0 adenoma patients, <1% APC and 2% MUTYH in 1–9 adenoma patients, and 1% APC and 2% MUTYH in 10–19 adenomas patients. Based on the variant detection rate found in other studies, we anticipate that few or no cases were missed in our cohort [19, 31].
Moreover, variants in other genes were not taken into account. Many of the patients were tested for PolE/D [32], MSH3, and NTHL1 on a research basis, the proven variant carriers were excluded in this study. Possible variants in other genes such as SMAD4, BMPR1A, and PTEN might be present, albeit in a small percentage of our cohort. In many labs, these genes have been included in NGS panels over the recent years, but, due to their rarity and often distinct phenotype, they do not justify lowering the suggested testing threshold. Nonetheless, in the near future the NGS panels will become more extensive, including more of other polyposis and colorectal cancer related genes as already proposed by the NCCN guidelines [29]. This will increase the yield of genetic testing also for other genes than APC and MUTYH.
The 2% heterozygote MUTYH carriers detected in this study is higher than expected based on the 1% prevalence reported in the Exome Aggregation Consortium database but similar to what Grover et al. [19] found in patients with <20 adenomas. It is possible that some monoallelic MUTYH carriers have other genetic factors, which combined with MUTYH explains adenoma development. As illustrated by two of the APC variant carriers also carrying a monoallelic MUTYH variant.
APC mosaicism was recently identified in 25–50% of unexplained patients with >20 adenomas [12]. In most of these cases, the mosaicism was undetectable in leukocyte-derived DNA and required testing of DNA isolated from >2 adenomas. Tumor testing is still logistically challenging and not performed in the current cohort. However, it might be an efficient approach in the future, especially for low adenoma count patients.
Conclusion
Adenoma count, age at adenoma diagnosis, and year of analysis are important predictive factors for APC and MUTYH variants. In view of the decline in variant detection, careful consideration for gene testing, especially in patients with lower polyp counts, is advised. Nevertheless, APC and MUTYH testing seems indicated in patients with >10 adenomas aged <60 and >20 adenomas aged <70. Other indications for referral are FAP-related extracolonic manifestations, CRC aged <40, a somatic KRAS c.34G > T transversion, or a FDR with >10 adenomas.
Supplementary information
Acknowledgments
Funding
Funding for assistance in English writing was provided by the Department Of Clinical Genetics, LUMC.
Author contributions
DT had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: SWtB, MN. Acquisition of data: CMT, SSS, and SWtB. Analysis and interpretation of the data: DT, MS, SWtB, MN, and SSS. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: DT, SWtB, and MN.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics
The study was approved by local ethics review boards (P01.019).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41431-019-0509-z) contains supplementary material, which is available to authorized users.
References
- 1.Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science. 2018;359:592–7. doi: 10.1126/science.aah3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, Kulldorff M, et al. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet. 2003;361:1491–5. doi: 10.1016/S0140-6736(03)13173-X. [DOI] [PubMed] [Google Scholar]
- 3.Hes FJ, Ruano D, Nieuwenhuis M, Tops CM, Schrumpf M, Nielsen M, et al. Colorectal cancer risk variants on 11q23 and 15q13 are associated with unexplained adenomatous polyposis. J Med Genet. 2014;51:55–60. doi: 10.1136/jmedgenet-2013-102000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Hees F, Saini SD, Lansdorp-Vogelaar I, Vijan S, Meester RG, de Koning HJ, et al. Personalizing colonoscopy screening for elderly individuals based on screening history, cancer risk, and comorbidity status could increase cost effectiveness. Gastroenterology. 2015;149:1425–37. doi: 10.1053/j.gastro.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–5. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 6.Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, et al. Inherited variants of MYH associated with somatic G:C—>T:A mutations in colorectal tumors. Nat Genet. 2002;30:227–32. doi: 10.1038/ng828. [DOI] [PubMed] [Google Scholar]
- 7.Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45:136–44. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weren RD, Ligtenberg MJ, Kets CM, de Voer RM, Verwiel ET, Spruijt L, et al. A germline homozygous mutation in the base-excision repair gene NTHL1 causes adenomatous polyposis and colorectal cancer. Nat Genet. 2015;47:668–71. doi: 10.1038/ng.3287. [DOI] [PubMed] [Google Scholar]
- 9.Adam R, Spier I, Zhao B, Kloth M, Marquez J, Hinrichsen I, et al. Exome sequencing identifies biallelic MSH3 germline mutations as a recessive subtype of colorectal adenomatous polyposis. Am J Hum Genet. 2016;99:337–51. doi: 10.1016/j.ajhg.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanders MA, Chew E, Flensburg C, Zeilemaker A, Miller SE, Al Hinai AS, et al. MBD4 guards against methylation damage and germ line deficiency predisposes to clonal hematopoiesis and early-onset AML. Blood. 2018;132:1526–34. doi: 10.1182/blood-2018-05-852566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olkinuora A, Nieminen TT, Martensson E, Rohlin A, Ristimaki A, Koskenvuo L, et al. Biallelic germline nonsense variant of MLH3 underlies polyposis predisposition. Genet Med. 2018;21:1868–73. doi: 10.1038/s41436-018-0405-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen AM, Crobach S, Geurts-Giele WR, van den Akker BE, Garcia MV, Ruano D, et al. Distinct patterns of somatic mosaicism in the APC gene in neoplasms from patients with unexplained adenomatous polyposis. Gastroenterology. 2017;152:546–9 e3. doi: 10.1053/j.gastro.2016.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Hes FJ, Nielsen M, Bik EC, Konvalinka D, Wijnen JT, Bakker E, et al. Somatic APC mosaicism: an underestimated cause of polyposis coli. Gut. 2008;57:71–6. doi: 10.1136/gut.2006.117796. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen M, Franken PF, Reinards TH, Weiss MM, Wagner A, van der Klift H, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP) J Med Genet. 2005;42:e54. doi: 10.1136/jmg.2005.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, et al. ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol. 2015;110:223–62. doi: 10.1038/ajg.2014.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Puijenbroek M, Nielsen M, Tops CM, Halfwerk H, Vasen HF, Weiss MM, et al. Identification of patients with (atypical) MUTYH-associated polyposis by KRAS2 c.34G > T prescreening followed by MUTYH hotspot analysis in formalin-fixed paraffin-embedded tissue. Clin Cancer Res. 2008;14:139–42. doi: 10.1158/1078-0432.CCR-07-1705. [DOI] [PubMed] [Google Scholar]
- 17.Aime A, Coulet F, Lefevre JH, Colas C, Cervera P, Flejou JF, et al. Somatic c.34G>T KRAS mutation: a new prescreening test for MUTYH-associated polyposis? Cancer Genet. 2015;208:390–5. doi: 10.1016/j.cancergen.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Boparai KS, Dekker E, Van Eeden S, Polak MM, Bartelsman JF, Mathus-Vliegen EM, et al. Hyperplastic polyps and sessile serrated adenomas as a phenotypic expression of MYH-associated polyposis. Gastroenterology. 2008;135:2014–8. doi: 10.1053/j.gastro.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 19.Grover S, Kastrinos F, Steyerberg EW, Cook EF, Dewanwala A, Burbidge LA, et al. Prevalence and phenotypes of APC and MUTYH mutations in patients with multiple colorectal adenomas. JAMA. 2012;308:485–92. doi: 10.1001/jama.2012.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisgaard ML, Fenger K, Bulow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat. 1994;3:121–5. doi: 10.1002/humu.1380030206. [DOI] [PubMed] [Google Scholar]
- 21.Powell SM, Petersen GM, Krush AJ, Booker S, Jen J, Giardiello FM, et al. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993;329:1982–7. doi: 10.1056/NEJM199312303292702. [DOI] [PubMed] [Google Scholar]
- 22.Miyoshi Y, Ando H, Nagase H, Nishisho I, Horii A, Miki Y, et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc Natl Acad Sci USA. 1992;89:4452–6. doi: 10.1073/pnas.89.10.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sieber OM, Lipton L, Crabtree M, Heinimann K, Fidalgo P, Phillips RK, et al. Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N Engl J Med. 2003;348:791–9. doi: 10.1056/NEJMoa025283. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Baudhuin LM, Boardman LA, Steenblock KJ, Petersen GM, Halling KC, et al. MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology. 2004;127:9–16. doi: 10.1053/j.gastro.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 25.Stanich PP, Pearlman R, Hinton A, Gutierrez S, LaDuca H, Hampel H, et al. Prevalence of germline mutations in polyposis and colorectal cancer-associated genes in patients with multiple colorectal polyps. Clin Gastroenterol Hepatol. 2018;17:2008–15.e3. doi: 10.1016/j.cgh.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Brown SR, Baraza W, Din S, Riley S. Chromoscopy versus conventional endoscopy for the detection of polyps in the colon and rectum. Cochrane Database Syst Rev. 2016;4:CD006439. doi: 10.1002/14651858.CD006439.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aranda-Hernandez J, Hwang J, Kandel G. Seeing better—evidence based recommendations on optimizing colonoscopy adenoma detection rate. World J Gastroenterol. 2016;22:1767–78. doi: 10.3748/wjg.v22.i5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner H, Altenhofen L, Kretschmann J, Rosch T, Pox C, Stock C, et al. Trends in adenoma detection rates during the first 10 years of the german screening colonoscopy program. Gastroenterology. 2015;149:356–66 e1. doi: 10.1053/j.gastro.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 29.Gupta S, Provenzale D, Regenbogen SE, Hampel H, Slavin TP, Hall MJ, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 3.2017. J Natl Compr Cancer Netw. 2017;15:1465–75. doi: 10.6004/jnccn.2017.0176. [DOI] [PubMed] [Google Scholar]
- 30.Huijts PE, Hollestelle A, Balliu B, Houwing-Duistermaat JJ, Meijers CM, Blom JC, et al. CHEK2*1100delC homozygosity in the Netherlands—prevalence and risk of breast and lung cancer. Eur J Hum Genet. 2014;22:46–51. doi: 10.1038/ejhg.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knopperts AP, Nielsen M, Niessen RC, Tops CM, Jorritsma B, Varkevisser J, et al. Contribution of bi-allelic germline MUTYH mutations to early-onset and familial colorectal cancer and to low number of adenomatous polyps: case-series and literature review. Fam Cancer. 2013;12:43–50. doi: 10.1007/s10689-012-9570-2. [DOI] [PubMed] [Google Scholar]
- 32.Elsayed FA, Tops CMJ, Nielsen M, Ruano D, Vasen HFA, Morreau H, et al. Low frequency of POLD1 and POLE exonuclease domain variants in patients with multiple colorectal polyps. Mol Genet Genom Med. 2019;7:e00603. doi: 10.1002/mgg3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.