Copper is commonly used as an antifouling agent on ship hulls. Alteromonas spp. are early colonizers of copper-based antifouling paint, but their mechanism of tolerance is poorly understood. Sequencing of A. macleodii strains isolated from copper test materials for marine ships indicated the presence of multiple megaplasmids. Plasmids serve as key vectors in horizontal gene transfer and confer traits such as metal resistance, detoxification, ecological interaction, and antibiotic resistance. Bioinformatic analysis identified many metal resistance genes and genes associated with mobility. Understanding the molecular mechanisms and capacity for gene transfer within marine biofilms provides a platform for the development of novel antifouling solutions targeting genes involved in copper tolerance and biofilm formation.
KEYWORDS: Alteromonas macleodii, metal resistance, megaplasmids, copper, antifouling, copA
ABSTRACT
Alteromonas is a widely distributed genus of marine Gammaproteobacteria, with representatives shown to be key players in diverse processes, including biogeochemical cycling and biofouling of marine substrata. While Alteromonas spp. are early colonizers of copper-based antifouling paints on marine vessels, their mechanism of tolerance is poorly understood. PacBio whole-genome sequencing of Alteromonas macleodii strains CUKW and KCC02, isolated from Cu/Ni alloy test coupons submerged in oligotrophic coastal waters, indicated the presence of multiple megaplasmids (ca. 200 kb) in both. A pulsed-field gel electrophoresis method was developed and used to confirm the presence of multiple megaplasmids in these two strains; it was then used to screen additional Alteromonas strains for which little to no sequencing data exist. Plasmids were not detected in any of the other strains. Bioinformatic analysis of the CUKW and KCC02 plasmids identified numerous genes associated with metal resistance. Copper resistance orthologs from both the Escherichia coli Cue and Cus and Pseudomonas syringae Cop systems were present, at times as multiple copies. Metal growth assays in the presence of copper, cobalt, manganese, and zinc performed with 10 Alteromonas strains demonstrated the ability of CUKW and KCC02 to grow at metal concentrations inhibitory to all the other strains tested. This study reports multiple megaplasmids in Alteromonas strains. Bioinformatic analysis of the CUKW and KCC02 plasmids indicate that they harbor elements of the Tra system conjugation apparatus, although their type of mobility remains to be experimentally verified.
IMPORTANCE Copper is commonly used as an antifouling agent on ship hulls. Alteromonas spp. are early colonizers of copper-based antifouling paint, but their mechanism of tolerance is poorly understood. Sequencing of A. macleodii strains isolated from copper test materials for marine ships indicated the presence of multiple megaplasmids. Plasmids serve as key vectors in horizontal gene transfer and confer traits such as metal resistance, detoxification, ecological interaction, and antibiotic resistance. Bioinformatic analysis identified many metal resistance genes and genes associated with mobility. Understanding the molecular mechanisms and capacity for gene transfer within marine biofilms provides a platform for the development of novel antifouling solutions targeting genes involved in copper tolerance and biofilm formation.
INTRODUCTION
Alteromonas is a globally distributed genus of marine Gammaproteobacteria found in a variety of habitats, with representatives that display resilient physiologies able to adapt to a wide range of solutes, temperatures, and environmental challenges (1–5). The genus currently consists of 20 species, with A. flaya and A. facilis being the most recent additions (6) (http://www.bacterio.net/alteromonas.html). Two species in particular, A. macleodii and A. mediterranea, display a global distribution, and there is considerable genomic information available for these species. The combined genomic and environmental distribution of A. mediterranea indicated that this species colonizes large particles that quickly sink to deep waters, while A. macleodii has a greater potential for regulation, as manifested by more two-component systems, and catabolic pathways conducive to transient particle attachment (4). A. macleodii and A. mediterranea are readily isolated by standard cultivation techniques and are accumulating in individual researchers’ collections due to their presence in a range of marine niches, including open ocean and particulate matter within the water column (3, 4, 7), coral reefs (8), and marine sponges (9) and in association with phytoplankton, including both cyanobacteria (10–12) and dinoflagellates (13; K. D. Cusick, unpublished data).
In general, Alteromonas strains are typically classified as copiotrophic, with high specific metabolic capabilities that are able to rapidly respond to environmental changes (14, 15) and degrade a wide range of organic substrates (16–18). They occur at low abundance in oligotrophic systems but flourish under conditions of nutrient enrichment or when particular organic matter becomes available (4, 19, 20). Metatranscriptomic studies have shown Alteromonas to be a key member of global ocean carbon and nitrogen cycling despite its often low representation in metagenomes of various marine habitats, including the oligotrophic open ocean and deep-sea systems (20–22). Alteromonas strains have also been shown to indirectly impact biogeochemical cycling due to their associations with various phytoplankton species, in some cases thriving during the later stages of blooms (12) likely as a result of their strong capability to recycle dissolved organic carbon (23). In addition to their contributions to carbon cycling, Alteromonas strains have also been found to be important in marine iron cycling and transport (22, 24).
Comparative genomic analysis of plasmids and conjugative elements within the Alteromonas genus has revealed a modular and dynamic framework that drives gene flux over a range that potentially overlaps the entire genus and beyond (25). Analysis of many A. macleodii and A. mediterranea isolates has identified the core and flexible genomes of Alteromonas (1, 7). The flexible genome consists of multiple genomic islands (typically 10- to 100-kb regions containing gene clusters with specific functions flanked by elements that facilitate integration into and excision out of the genome [26]) occurring at equivalent positions in the genomes of the different isolates. Gene cassettes found within the chromosomal flexible genomic islands of Alteromonas were also found on their plasmids and integrative conjugative elements, indicating that these mobile elements serve as vectors for the transfer of these genomic islands in Alteromonas, which may contribute to their extensive genetic diversity (25).
Extensive sequencing data exist for bacterial genomes; however, the majority of these genomes were obtained via short-read sequencing technologies. Plasmids typically contain numerous repeats and mobile genetic elements. Recent studies on both environmental and clinical bacterial species demonstrate that plasmid assembly and annotation, including differentiation between chromosome- and plasmid-based genetic elements, is accurately achieved only with long-read technologies (27, 28). While whole-genome sequencing has become the standard for plasmid analysis, short-read sequencing technologies are unable to span plasmid repeat sequences, resulting in a fragmented assembly (29). Comparing draft genomes of A. macleodii strain HOT1A3 generated through various sequencing and assembly approaches with that of the fully closed genome generated with the SMRT PacBio platform revealed a megaplasmid (148 kb) that was identified only via the PacBio platform (28). This megaplasmid possessed an almost complete flexible genomic island and contained many genes involved in metal resistance previously only found in A. mediterranea, indicating the potential for genetic transfer among marine bacterial populations. The recent genomic sequencing of A. macleodii Te101, also using PacBio SMRT sequencing analysis, identified a 237-kb megaplasmid (12).
Our laboratory previously isolated a strain of A. macleodii (CUKW) from Cu/Ni alloy coupons submerged in tropical coastal waters that possesses a very high copper tolerance. A mutant (KCC02) was created via adaptive evolution in which a subculture of CUKW was continuously transferred in sterile Burkholder’s B medium supplemented with 3 mM copper for over 1 year. Whole-genome sequencing using the PacBio platform has indicated the presence of two megaplasmids (∼200 kb) in CUKW and three in KCC02.
Pulsed-field gel electrophoresis (PFGE) was selected as a means to confirm and further analyze these plasmids. However, existing PFGE protocols proved unsuccessful with these strains. A defining characteristic among multiple A. macleodii strains is its production of copious amounts of exopolysaccharide (EPS) (30–34). The strains in our laboratory (CUKW and KCC02) as well as other isolates from individual researchers’ collections have also been noted to produce EPS. As a testament to the importance of genomic plasticity of A. macleodii with respect to flexible genomic islands and mobile genetic elements, analyses of multiple A. macleodii genomes showed strong evidence of recombination events with distantly related bacterial species within genomic islands associated with EPS biosynthesis (35).
The application of existing PFGE methods to confirm plasmid presence, number, and size proved unsuccessful in our Alteromonas strains. Therefore, we developed a new PFGE protocol to confirm the presence of two plasmids in A. macleodii CUKW and three plasmids in A. macleodii KCC02, as indicated by whole-genome sequencing and bioinformatic analysis. This method was then applied to multiple strains of A. macleodii and A. mediterreanea isolated from a variety of marine habitats for which little to no sequence data exist.
RESULTS AND DISCUSSION
Development of a PFGE protocol for sizing multiple megaplasmids in Alteromonas spp.
This study reports Alteromonas strains in which multiple megaplasmids were identified. The genomes of A. macleodii strains CUKW and KCC02 were recently sequenced using the PacBio SMRT cell platform (K. D. Cusick, A. Iturbide, I. Erill, and S. W. Polson, unpublished data). Assembly and bioinformatic analyses indicated the presence of two megaplasmids in CUKW of sizes ca. 212 kb and 178 kb (here referred to as pCUKW-212 and pCUKW-178, respectively) and three in KCC02, sized 258 kb, 243 kb, and 180 kb (here referred to as pKCC02-258, -243, and -180, respectively). Experimental verification of megaplasmid size and number was not achievable using existing PFGE protocols. Successful megaplasmid presence, number, and approximate sizing were achieved by using the PFGE method developed in this study. The presence of two megaplasmids was confirmed in CUKW, with sizes approximate to those determined via bioinformatic analysis. This method also confirmed the presence of three megaplasmids in KCC02 and was sufficient to distinguish between the 258-, 243-, and 180-kb plasmids.
No plasmid bands were ever detected using method 1 (see Materials and Methods). This method yielded smearing throughout the lane in both CUKW and KCC02, very faint chromosomal bands, and in most cases, high fluorescence in the well (data not shown). The faint chromosomal bands and fluorescence indicate insufficient lysis, while the smearing is likely due to lysozyme incubation. Method 2 (slightly modified PulseNet Vibrio protocol) consistently yielded chromosomal bands but faint to no plasmid bands (Fig. 1). Greater fluorescence was seen in the wells with this method, indicating incomplete lysis, and the faint to no plasmid bands indicated that the higher temperature used in the proteinase K and washing steps may influence recovery and integrity of plasmids from Alteromonas. The cell lysis and plug preparation protocol developed in this study yielded chromosomal and plasmid bands, indicating sufficient cell lysis while maintaining plasmid DNA integrity (Fig. 1). Following cell lysis and plug preparation optimization, electrophoresis settings were further optimized. This included the addition of thiourea to the running buffer at a final concentration of 100 μM, which enhanced the detection of plasmid bands (Fig. 2). Optimal voltage settings for achieving plasmid separation were as follows: a gradient of 6 V cm−1, an included angle of 120°, an initial switch time of 1 s, and a final switch time of 30 s, with a linear ramping factor. There were no observable differences in plasmid separation between 22 and 25 h (data not shown), so 24 h was used when additional Alteromonas strains were screened.
FIG 1.
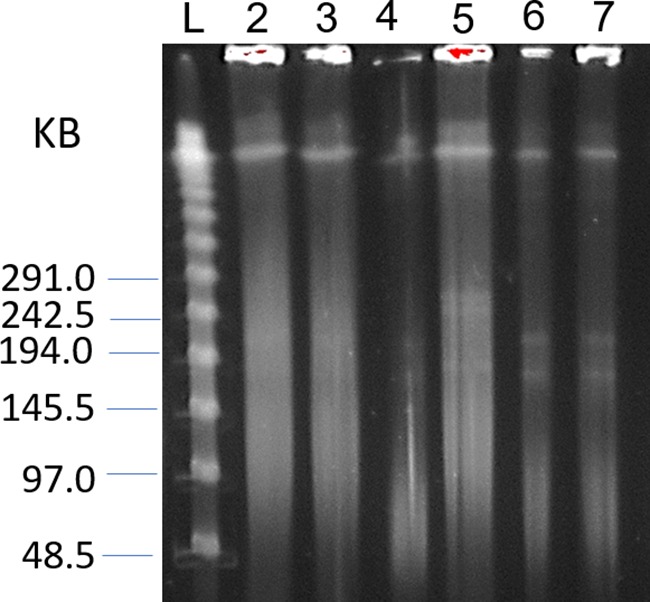
Comparison of PFGE methods 2 and 3. Plugs of Alteromonas macleodii strains CUKW and KCC02 were prepared as described in Materials and Methods and examined on a 1% agarose gel using a clamped homogenous electric field system in 0.5× TBE buffer with 100 μM thiourea. Multiple distinct plasmid bands were evident in A. macleodii CUKW by using the method developed here. Plugs of CUKW in lanes 2 to 4 were prepared using an existing method in which the initial buffer did not contain glucose, and the proteinase K incubation and subsequent wash steps were performed at 55°C (method 2). Plugs in lanes 5 (KCC02) and lanes 6 and 7 (CUKW) were prepared with the cell suspension buffer containing glucose, and the proteinase K incubation and subsequent wash steps were performed at 28°C (method 3). Lane L contains lambda concatemer markers with the sizes of representative bands (in kilobases) shown on the left.
FIG 2.

Optimization of voltage settings and inclusion of thiourea in running buffer enhance plasmid band separation. Optimization consisted of an initial switch time of 1 s and a final switch time of 30 s, with a linear ramping factor, for 24 h. Thiourea was added to the running buffer at a final concentration of 100 μM. These optimizations were sufficient to separate two plasmids of similar size in KCC02 and confirmed sequencing and assembly results with the PacBio platform, which indicated two plasmids in CUKW (lanes 1 and 3), of sizes 212 and 178 kb, and three in strain KCC02 (lanes 4 to 6), of sizes 258, 243, and 180 kb. Both strains were grown to mid-exponential phase (OD600 = 1.2 [CUKW] and 1.45 [KCC02]), and 8 ml of a 10-ml culture was harvested for plug preparation. Lane L, lambda ladder as described in the legend for Fig. 1. The tooling line represents the absence of two gel lanes, which were omitted from the figure since the samples were not included in the current analyses.
Detecting and sizing bacterial megaplasmids is a challenge due to their physical similarities with chromosomal DNA. Detection often requires the development of a new method or optimization of an established protocol. Argandona et al. (36) found it necessary to develop a protocol to successfully identify megaplasmids in the marine genus Halomonas due to the exopolysaccharides secreted by several species. Ultimately, the method of Wheatcroft et al. (37) was modified to include the critical optimizations of changing the resuspension solution from doubly distilled water to one containing 2% (wt/vol) salts to prevent cell lysis and the development of a new medium to inhibit polysaccharide production (36). Barton et al. developed a PFGE-based method that required treatment with S1 nuclease; application of this method led to the discovery of previously undetected plasmids of >100 kb in multiple strains of Klebsiella, Staphylococcus aureus, and Staphylococcus epidermidis (38). The primary modifications to the protocol developed here are the initial centrifugation steps at room temperature rather than at 4°C, inclusion of glucose in the cell suspension buffer, and lysis/proteinase K incubation at 28°C. These modifications were necessary, as multiple Alteromonas strains were found to secrete an exopolymer at some or all steps of standard protocols, which prohibited sufficient cell distribution within the plugs and/or unsuccessful lysis. Some protocols perform the initial centrifugation steps at 4°C (39); cell harvesting at this temperature, regardless of cell volume (5, 10, or 20 ml), stimulated the production of an unidentified exopolymer (results not shown) that affected downstream plug preparation. Plugs prepared from cells harvested at 4°C did not solidify or solidified only minimally, even when placed at 4°C for greater than 30 min. Proteinase K incubation is commonly performed at 54 to 55°C (39–41) but was found to negatively impact the recovery and integrity of plasmids in these Alteromonas strains.
Screening of Alteromonas isolates.
The method developed here was applied to additional Alteromonas strains to screen for the presence of plasmids; these included strains for which little to no sequence data exist. Most data for strains for which draft genome data do exist consist of whole-genome shotgun data, generated as “short reads” of 100 to 500 bp using the Illumina platform. This platform has been shown to present challenges in assembling plasmids from multiple bacterial species due to the repeats and mobile genetic elements characteristic of most large plasmids (27, 29). Short-read technologies proved inadequate for detecting the megaplasmid in A. macleodii HOT1A3 (28) in addition to other bacterial genera (27). The ATCC type strain 27126, for which a fully closed genome exists, was used as a control to confirm the ability to detect chromosomal DNA, indicative of efficient cell lysis (see Fig. S1 in the supplemental material). An additional eight Alteromonas strains, obtained from various researchers’ labs and identified as either A. macleodii or A. mediterranea based on published whole-genome sequencing or 16S rRNA gene sequencing performed here (data not shown), were then screened for the presence of plasmids using the method developed here. These included strains EZ55 (10), isolated from a coculture with the cyanobacterium Prochlorococcus; W12 (42), isolated from the diatom Thalassiosira rotula, and OCN004 (43), isolated from coral; J589, V450 (9), and J192, isolated from mid- to deep-sea marine sponges; and CD34d and MR31d, isolated from coral species (8) (Table 1). No plasmids were detected in any of the strains (Fig. S2 to S4).
TABLE 1.
List of strains, including culture conditions and sequencing details, used in this studya
| Bacterial strain | Medium | OD600 | Vol (ml) | Source or reference | Sequencing level | Sequence information | No. of plasmids | Environment |
|---|---|---|---|---|---|---|---|---|
| A. macleodii CUKW | Burkholder's B | 1.36 | 10 | Cusick et al. (5) | Closed genome | This study | 2 | Copper coupons |
| A. macleodii KCC02 | Burkholder's B | 1.69 | 10 | This study | Closed genome | This study | 3 | Lab mutant maintained for >1 yr with 3 mM copper |
| A. macleodii ATCC 27126T | 2216 | 1.18 | 10 | Baumann et al. (75); Gonzaga et al. (2) | Closed genome | CP003841; 454 GS FLX Titanium and Illumina Genome Analyzer IIx | None | Surface water, HI |
| A. macleodii J912 | 2216 | 0.54 | 10 | Peter McCarthy, HBOI, personal collection | NA | NA | None | Marine sponge (Spongosorites sp.), 573-ft depth |
| A. macleodii J589 | 2216 | 0.72 | 10 | Peter McCarthy, HBOI personal collection | NA | NA | None | Marine sponge (Asteropus simplex), 445-ft depth |
| A. macleodii V450 | 2216 | 0.33 | 10 | Wang et al. (9) | Contigs | Illumina HiSeq; BioSample ID SAMN05931236 | None | Marine sponge (Leiodermatium sp.), 1,288-ft depth |
| Alteromonas strain OCN004 | 2216 | 1.45 | 10 | Ushijima et al. (43) | NA | NA | None | Hawaiian rice coral (Montipora capitata), shallow water, Oahu, HI |
| Burkholder's B | 1.03 | 6 | Ushijima et al. (43) | NA | NA | None | Hawaiian rice coral (Montipora capitata), shallow water, Oahu, HI | |
| A. macleodii EZ55 | YTSS | 1 | 6 | Morris et al. (10) | Incomplete | 454 GS FLX Titanium; BioProject ID PRJNA50031 | None | Cyanobacterium Prochlorococcus coculture |
| Alteromonas strain W12 | Burkholder's B | 1.17 | 6 | Dana Hunt, Duke University Marine Lab | Contigs | Illumina HiSeq; Bioproject ID PRJNA306252 | None | Coculture with algal species Thalassiosira rotula |
| Alteromonas strain C34d | Modified Burkholder's B | 1.06 | 6 | Tran and Hadfield (8) | NA | NA | None | Crustose coralline algae |
| Alteromonas strain MR31d | Modified Burkholder's B | 1.05 | 6 | Tran and Hadfield (8) | NA | NA | None | Montipora coral reef |
NA, not available; HBOI, Harbor Branch Oceanographic Institute.
Bioinformatic analysis of A. macleodii CUKW and KCC02 plasmids.
This study reports multiple megaplasmids within individual Alteromonas strains. Of the seven complete publicly available A. macleodii genomes, three possess a single plasmid (Table 2). Strain AD037 was recently sequenced and was also found to contain a plasmid (25). Of the 15 complete A. mediterranea genomes, eight strains contain a single plasmid, with sizes ranging from 200 to 610 kb (Table 2). Of these strains, six contained the same plasmid (Table 2) (25). Of the four sequenced strains of A. stellipolaris, one contains a single plasmid of 252,173 bp in size. All other Alteromonas species with closed or nearly complete sequenced genomes do not contain plasmids (A. naphthalenivorans, A. australica, Alteromonas strains RW2A1, Mex 14, ALT99, and BS11, A. marina, and Alteromonas strain Nap26).
TABLE 2.
Plasmids identified in Alteromonas genomes
| Species | Strain | Chromosome accession no. | Plasmid accession no. | Description | Plasmid size (bp) |
|---|---|---|---|---|---|
| A. mediterranea | DE | CP001103.3 | |||
| AltDE1 | CP003917.1 | CP003918.1 | pAMDE1-300 | 303,282 | |
| MED64 | CP004848.1 | ||||
| U7 | CP004851.1 | ||||
| U8 | CP004852.1 | ||||
| UM7 | CP004853.1 | CP004854.1 | pAMDE1-300 | 303,282 | |
| UM4b | CP004855.1 | ||||
| UM8 | CP013928.1 | CP013929.1 | pAMDE1-300 | 287,708 | |
| CP48 | CP018024.1 | CP018025.1 | pAMCP48-600 | 603,655 | |
| CP49 | CP018027.1 | CP018028.1 | pAMCP49-600 | 610,127 | |
| RG65 | CP018029.1 | CP018030.1 | pAMRG65-300 | 302,350 | |
| AR43 | CP018026.1 | ||||
| English Channel 615 | CP004846.1 | CP004847.1 | pAMEC615-200 | 200,847 | |
| U10 | CP013930.1 | CP013931.1 | pAMDE1-300 | 303,282 | |
| Ionian Sea U4 | CP004849.1 | CP004850.1 | pAMDE1-300 | 303,282 | |
| A. macleodii | ATCC 27126 | CP003841.1 | |||
| English Channel 673 | CP003844.1 | ||||
| Black Sea 11 | CP003845.1 | ||||
| Balearic Sea AD45 | CP003873.1 | CP003874.1 | pAMBAS45 | 45,406 | |
| D7 | CP014323.1 | ||||
| HOT1A3 | CP012202.1 | CP012203.1 | pAM1A3 | 148,934 | |
| Te101 | CP018321.1 | CP018322.1 | pTE101a | 237,311 | |
| A. stellipolaris | LMG 21861 | CP013926.1 | CP013927.1 | pASTE61-200 | 252,173 |
| LMG 21856 | CP013120.1 | ||||
| PQQ-42 | CP015345.1 | ||||
| PQQ-44 | CP015346.1 |
Plasmids serve as key vectors in horizontal gene transfer, often conferring traits such as heavy metal resistance, detoxification, virulence, ecological interaction, and antibiotic resistance (44). Mobility is a significant trait of plasmid fitness, and plasmids can be classified based on their mobility: conjugative, mobilizable, or nonmobilizable (44). Transmissible plasmids can be classified as conjugative (self-transmissible) or mobilizable. Conjugative plasmids contain all the machinery needed for self-transfer, while mobilizable plasmids “borrow” portions of this machinery from other genetic elements within the cell (44). Plasmids must possess two sets of genes for conjugative transfer: mobility (MOB) genes, and those that code for a type 4 secretion system (T4SS) to form the membrane-associated mating pair formation (MPF) complex. A plasmid is termed self-transmissible or conjugative if it contains its own set of MPF genes; if it relies on the MPF of another genetic element in the cell, it is referred to as mobilizable (44). Nonmobilizable plasmids do not contain these features yet can be mobilized using MOB components from another plasmid in the same cell (45, 46). Orthologs of the type IV secretion system, frequently referred to as the Tra system, have a broad nomenclature, including VirB4, TrbE, TraE, TraC, MpfC, TraB, and TraU (44).
All Alteromonas plasmids of >200 kb identified previously, nearly all of which occurred exclusively within A. mediterranea, were classified as conjugative plasmids (25). Plasmids of <200 kb, all of which were found in A. macleodii, lacked the conjugative modules and were classified as nonmobilizable. The exception to this was the 237-kb pTE101 plasmid from A. macleodii Te101, in which a region coding for conjugal transfer and plasmid maintenance was identified (12).
In general, two of the KCC02 plasmids are highly similar to the two found in CUKW: pKCC02-243 is the functional equivalent of pCUKW-178, and pKCC02-258 is the functional equivalent of pCUKW-212. Bioinformatic analysis of these two plasmid sets indicate that they all harbor elements of the Tra system conjugation apparatus, although their type of mobility remains to be experimentally verified. pCUKW-178 and pKCC02-243 both harbor tra conjugal transfer genes (Tables 3 and 4; Fig. 3), as well genes for the ParAB and Rep systems (Tables 3 and 4; Fig. 3). Both the ParAB and RepABC systems are encoded by many low-copy-number plasmids and help to improve plasmid partitioning (47, 48). pCUKW-212 and pKCC02-243 also contain numerous elements suggestive of conjugation, including replication (RepA) initiation proteins and the RepA replicase, multiple type IV secretion proteins of the Icm family, conjugal transfer/type IV secretion proteins of the Tra/Dot family, and ParA and ParB (Table 5). The third plasmid in strain KCC02, pKCC02-180, also contains genes coding for the ParAB system and a limited number of type IV secretion system genes (Table 6).
TABLE 3.
Metal resistance genes, mobile genetic elements, and conjugation apparatus on pCUKW-178
| Gene ID | Protein description | Classification |
|---|---|---|
| 04298 | ParA family protein | Conjugation/plasmid maintenance |
| 04300 | IS66 insertion sequence | Mobile genetic element |
| 04301 | IS110 transposase | Mobile genetic element |
| 04314 | Efflux RND transporter | Efflux RND transporter |
| 04315 | CusA/Czc heavy metal RND transporter | Copper/metal resistance/transport |
| 04317 | Copper resistance CopC | Copper resistance/transport |
| 04318 | Cu-binding protein | Copper resistance/transport |
| 04348 | Cu-translocating ATPase | Copper resistance/transport |
| 04349 | Cupredoxin protein | Copper resistance/transport |
| 04355 | Transposase | Mobile genetic element |
| 04356 | IS481 transposase | Mobile genetic element |
| 04357 | IS481 transposase | Mobile genetic element |
| 04363 | Heavy metal transporter | Efflux RND transporter |
| 04364 | CusA/Czc heavy metal RND transporter | Copper/metal resistance/transport |
| 04376 | Metal-binding protein | Copper/metal resistance/transport |
| 04377 | Copper resistance CopC | Copper resistance/transport |
| 04378 | Cu export protein | Copper resistance/transport |
| 04384 | Cu-translocating ATPase | Copper resistance/transport |
| 04385 | Cupredoxin protein | Copper resistance/transport |
| 04392 | Cu resistance protein B | Copper resistance/transport |
| 04393 | Cu resistance multicopper oxidase | Copper resistance/transport |
| 04395 | IS3 transposase | Mobile genetic element |
| 04396 | IS3 transposase | Mobile genetic element |
| 04402 | Cd/Pb transcriptional regulator | Heavy metal |
| 04407 | CusA/Czc heavy metal RND transporter | Copper/metal resistance/transport |
| 04411 | IS5 transposase | Mobile genetic element |
| 04413 | Hg transcriptional regulator | Mercury transport/resistance |
| 04414 | Mercury transporter | Mercury transport/resistance |
| 04415 | Mercuric transport | Mercury transport/resistance |
| 04416 | Mercury transporter MerC | Mercury transport/resistance |
| 04417 | Mercury reductase | Mercury transport/resistance |
| 04420 | IS481 transposase | Mobile genetic element |
| 04422 | Integrase | Mobile genetic element |
| 04428 | Transposase | Mobile genetic element |
| 04430 | DDE transposase | Mobile genetic element |
| 04436 | Conjugal transfer TraG | Conjugation/plasmid maintenance |
| 04438 | Integrating conjugal element protein | Conjugation/plasmid maintenance |
| 04439 | Integrating conjugal element protein | Conjugation/plasmid maintenance |
| 04447 | Hg transcriptional regulator | Mercury transport/resistance |
| 04448 | Mercury transporter | Mercury transport/resistance |
| 04449 | Mercury transport | Mercury transport/resistance |
| 04451 | Mercury reductase | Mercury transport/resistance |
| 04454 | CusA/Czc heavy metal RND transporter | Copper/metal resistance/transport |
| 04455 | Efflux RND transporter | Efflux RND transporter |
| 04457 | Chromosome partitioning protein ParA | Conjugation/plasmid maintenance |
| 04458 | Recombinase | Mobile genetic element |
| 04459 | Transposase | Mobile genetic element |
| 04460 | Integrase | Mobile genetic element |
| 04461 | Integrase | Mobile genetic element |
| 04465 | IS91 transposase | Mobile genetic element |
| 04469 | Transposase | Mobile genetic element |
| 04471 | IS3 transposase | Mobile genetic element |
| 04472 | IS5 transposase | Mobile genetic element |
| 04473 | Replication protein A | Conjugation/plasmid maintenance |
| 04374 | MerR | Copper resistance/transport |
| 04383 | MerR | Copper resistance/transport |
TABLE 4.
Metal resistance genes, mobile genetic elements, and conjugation apparatus located on pKCC02-243
| Gene ID | Protein description | Classification |
|---|---|---|
| 04072 | Integrase | Mobile genetic element |
| 04073 | Integrase | Mobile genetic element |
| 04075 | IS3 family transposase | Mobile genetic element |
| 04076 | IS3 family transposase | Mobile genetic element |
| 04080 | IS3 family transposase | Mobile genetic element |
| 04088 | IS3 family transposase | Mobile genetic element |
| 04089 | Tn3 transposase | Mobile genetic element |
| 04091 | Tn3 transposase | Mobile genetic element |
| 04100 | Transposase | Mobile genetic element |
| 04117 | Transposase | Mobile genetic element |
| 04125 | Conjugal transfer protein TraG | Conjugation/plasmid maintenance |
| 04127 | Integrating conjugal element protein | Conjugation/plasmid maintenance |
| 04128 | Integrating conjugal element protein | Conjugation/plasmid maintenance |
| 04129 | Integrating conjugal element protein | Conjugation/plasmid maintenance |
| 04136 | Hg (II) transcriptional regulator | Mercury transport/resistance |
| 04137 | Mercury transporter | Mercury transport/resistance |
| 04138 | Mercuric transport protein | Mercury transport/resistance |
| 04140 | Mercury reductase | Mercury transport/resistance |
| 04143 | CusA/CzcA metal efflux transporter | Copper/metal resistance/transport |
| 04144 | Efflux RND transporter | Efflux RND transporter |
| 04147 | Recombinase | Mobile genetic element |
| 04148 | Transposase | Mobile genetic element |
| 04149 | Integrase | Mobile genetic element |
| 04150 | Phage integrase | Mobile genetic element |
| 04154 | Transposase | Mobile genetic element |
| 04159 | Transposase | Mobile genetic element |
| 04161 | IS3 family transposase | Mobile genetic element |
| 04162 | IS5 family transposase | Mobile genetic element |
| 04168 | IS66 insertion sequence protein | Mobile genetic element |
| 04169 | IS110 transposase | Mobile genetic element |
| 04182 | Efflux RND transporter | Efflux RND transporter |
| 04183 | CusA/CzcA metal efflux transporter | Copper/metal resistance/transport |
| 04185 | Copper resistance CopC | Copper resistance/transport |
| 04186 | Cu-binding protein | Copper resistance/transport |
| 04216 | Cu-translocating P-type ATPase | Copper resistance/transport |
| 04217 | Cupredoxin domain protein | Copper resistance/transport |
| 04223 | Transposase | Mobile genetic element |
| 04224 | IS481 transposase | Mobile genetic element |
| 04225 | IS481 transposase | Mobile genetic element |
| 04231 | Heavy metal transporter | Efflux RND transporter |
| 04232 | CusA/CzcA metal efflux transporter | Copper/metal resistance/transport |
| 04245 | Metal binding protein | Copper/metal resistance/transport |
| 04246 | Copper resistance CopC | Copper resistance/transport |
| 04247 | Copper export protein | Copper resistance/transport |
| 04253 | Cu-translocating P-type ATPase | Copper resistance/transport |
| 04254 | Cupredoxin domain protein | Copper resistance/transport |
| 04261 | Copper resistance CopB | Copper resistance/transport |
| 04262 | Cu resistance multicopper oxidase | Copper resistance/transport |
| 04264 | IS3 family transposase | Mobile genetic element |
| 04265 | IS3 family transposase | Mobile genetic element |
| 04271 | Cd(II)/Pb(II) transcriptional regulator | Copper/metal resistance/transport |
| 04276 | CusA/CzcA metal efflux transporter | Copper/metal resistance/transport |
| 04280 | IS5 family transposase | Mobile genetic element |
| 04282 | Hg(II) transcriptional regulator | Mercury transport/resistance |
| 04283 | Mercury transporter | Mercury transport/resistance |
| 04284 | Mercuric transport protein | Mercury transport/resistance |
| 04285 | Mercury transporter MerC | Mercury transport/resistance |
| 04286 | Mercury reductase | Mercury transport/resistance |
| 04289 | IS481 transposase | Mobile genetic element |
| 04291 | Integrase | Mobile genetic element |
| 04295 | Tn3 transposase | Mobile genetic element |
| 04297 | Transposase | Mobile genetic element |
| 04146 | Chromosome partitioning protein A | Conjugation/plasmid maintenance |
| 04163 | Replication protein A | Conjugation/plasmid maintenance |
| 04166 | ParA family protein | Conjugation/plasmid maintenance |
| 04215 | MerR transcriptional regulator | Copper resistance/transport |
| 04252 | MerR transcriptional regulator | Copper resistance/transport |
FIG 3.
Both Alteromonas macleodii strains CUKW and KCC02 harbor a plasmid on which are located numerous genes for conferring metal, specifically copper, tolerance. Genes from the following categories are illustrated on both pCUKW-178 and pKCC02-243: mobile genetic elements (pink), conjugation and plasmid maintenance (purple), mercury transport/resistance (red), CusA/Czc heavy metal efflux (green), RND transporters (orange), copper resistance (dark blue). Light blue indicates the size and orientation of the remaining genes on the plasmid.
TABLE 5.
Genomic conjugation elements located on pCUKW-212 and pKCC02-258
| Gene ID for pCUKW-212a | Gene ID for pKCC02-258 | Protein description |
|---|---|---|
| 04091 | 04587 | Replication initiation protein |
| 04094 | 04590 | Replication protein A |
| 04095 | 04591 | RepA replicase |
| 04213 | 04770 | Type IV secretion protein IcmB |
| 04220 | 04776 | Type IV secretion protein IcmK |
| 04221 | 04777 | Type IV secretion protein IcmL |
| 04231 | 04787 | Type IV secretion protein IcmO |
| NP | 04786 | Type IV secretion protein IcmP |
| 04239 | 04795 | Conjugal transfer/type IV secretion DotA/TraY family |
| 04245 | 04801 | Type IV secretion system DotC |
| NP | 04802 | Type IV secretion protein DotD |
| 04298 | 04663 | Chromosome partitioning protein ParA |
| 04296 | 04563 | Chromosome partitioning protein B |
| NP | 04796 | Conjugal transfer protein TraG/pilus assembly protein PilL |
NP, not present.
TABLE 6.
Conjugation and plasmid maintenance elements on pKCC02-180
| Gene ID | Protein description |
|---|---|
| 04310 | Chromosome partitioning protein B |
| 04366 | Type IV secretion system TraC |
| 04433 | Tellurite resistance TerB |
| 04481 | Chromosome partitioning protein ParA |
A key feature of plasmids is that they typically carry genes conferring resistance to antibiotics and heavy metals, the ability to utilize various carbon and energy sources, virulence, and symbiotic relationships (49). Genes conferring resistance to many heavy metals, including AsO2−, AsO43−, Cd2+, Co2+, CrO42−, Cu2+, Hg(II)+, Ni2+, Sb3+, TeO32−, Tl+, and Zn2+, have been found on bacterial plasmids (50–54).
Bioinformatic analysis of CUKW and KCC02 demonstrated multiple copies of genes associated with copper resistance situated on one set of plasmids (pCUKW-178 and pKCC02-243), as well as multiple RND (resistance, nodulation, and cell division) transporters, which can function as efflux pumps in heavy metal resistance (55). pCUKW-178 and pKCC02-243 are comparable in gene content, containing the same number of copper/heavy metal resistance genes (Tables 3 and 4; Fig. 3) and other genes associated with metal resistance. Both contain two sets of mercury transport/resistance genes (mer operon), including those coding for an Hg(II)-specific transcriptional regulator, a reductase, and multiple transporters (Tables 3 and 4; Fig. 3). They each also contain genes coding for four CusA/Czc RND transporters. General functions of bacterial RND transporters include heavy metal efflux and multidrug resistance (56). czcA, first identified on a megaplasmid in R. metallidurans, confers resistance to cobalt, zinc, and cadmium (Cd2+), while cus is an RND transporter gene within the Escherichia coli Cus operon (57, 58). In addition to the CusA/Czc-specific RND transporters, both pCUKW-178 and pKCC02-243 contain multiple copies of genes putatively identified as coding for RND transporters (Fig. 3).
The copper tolerance capabilities and genomic analyses of CUKW and KCC02 have been described separately (Cusick et al., unpublished data). However, an analysis of the plasmid-associated copper genes is included here. Four main models exist for bacterial copper tolerance and homeostasis: the chromosome-based Cue and Cus systems of E. coli, the plasmid-based Pco system of E. coli, and the plasmid-based Cop system of Pseudomonas syringae (59–61). In E. coli, Cue is the primary resistance system for alleviating excess copper in the cytoplasm. CueO is a multicopper oxidase involved in copper tolerance, while CopA is a Cu(I)-translocating ATPase (62, 63). Cus is a periplasmic efflux system containing RND transporters. In the plasmid-based Pco system, PcoA is a multicopper oxidase that likely oxidizes Cu(I) to the less toxic Cu(II). The plasmid-based Cop system of P. syringae contains the following: the multicopper oxidase CopA; CopC, CopB, and CopD, which are responsible for copper binding and transport in the periplasm and membranes; and the two-component regulatory system CopRS.
Both pCUKW-178 and pKCC02-243 contain multiple copies of genes encoding orthologs of the Cu-translocating P-type ATPase CopA and multicopper oxidase CueO (Tables 3 and 4; Fig. 3). In E. coli, CueR is a copper-responsive homolog of the transcriptional regulator MerR and regulates both CueO and CopA (64). pCUKW-178 and pKCC02-243 also contain genes encoding MerR orthologs, situated immediately upstream of the Cu-translocating P-type ATPases (Fig. 3). Both plasmids contain a minimum of two copies each of genes encoding orthologs identified in E. coli or P. syringae as being involved in copper resistance, including CopB, CopC and CopD; copper export proteins; and cupredoxin domain (CueO) proteins. In both CUKW and KCC02, genes coding for cupredoxin domain proteins are situated next to other copper resistance (copB, copC) genes (Fig. 3). Increasing evidence indicates that the periplasmic binding protein CopC frequently occurs with only the inner membrane protein CopD, typically as a fusion protein (65). Genomic analysis indicates that this occurs in Alteromonas, as a minimum of two sets of copCD are located on both the pCUKW-178 and pKCC02-243 plasmids (Fig. 3).
The second plasmid set, pCUKW-212 and pKCC02-258, is highly similar in terms of gene content and organization: nearly all genes present on pCUKW-212 are also on pKCC02-258 (Tables S1 and S2). Both plasmids are dominated by open reading frames encoding numerous hypothetical proteins. The additional genes on pKCC02-258 lie in regions flanked by transposable elements and include those coding for AcrB/AcrD/AcrF family proteins and multiple copies of RND transporters and multiple copies of genes coding for ligand-gated channels, all of which are located downstream of a putative two-component signal transduction system. This entire region is then duplicated. Additional genes include those encoding multiple copies of ABC transporters and two copies of an RNA polymerase sigma factor, as well as multiple hypothetical proteins. While the functions of the numerous hypotheticals remain to be defined, no homologs of copper or other metal resistance genes were found on either pCUKW-212 or pKCC02-243.
Metal growth assays.
Plasmid-based metal resistances have been found to be highly specific, i.e., there is no general mechanism for resistance to all heavy metal ions, and the resistance mechanisms are primarily comprised of efflux pumps and/or enzymatic detoxification (50); active extrusion is one of the main mechanisms of copper resistance as well (66). To assess the potential roles of the plasmid-based copper and other metal resistance genes in CUKW and KCC02, the growth of all Alteromonas strains included in the PFGE plasmid analysis was assessed upon exposure to high concentrations of copper, cobalt, manganese, and zinc. All are trace metals that serve as enzyme cofactors in microbially mediated marine biogeochemical cycles (67) but, as with all trace metals, are detrimental at high levels.
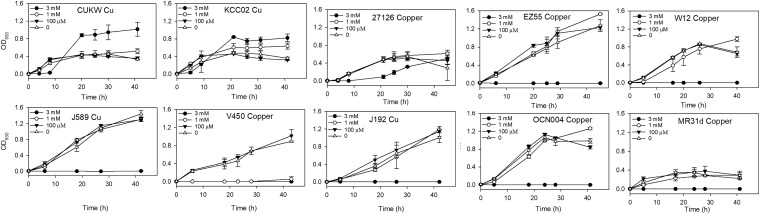
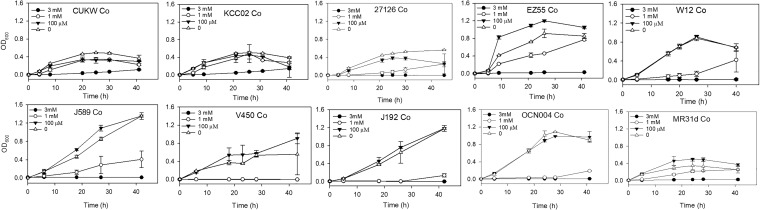
CUKW achieved the highest levels of growth when grown with 3 mM copper. The growth of KCC02 was also enhanced in the presence of 3 mM copper. These were the only strains in which 3 mM copper did not inhibit growth. Growth was delayed nearly 24 h in A. macleodii 27126, at which point minimal growth was observed. No other strains were able to grow at 3 mM copper (Fig. 4). Minimal growth occurred in both CUKW and KCC02 with 3 mM cobalt; for both, this occurred after ca. 30 h. Growth of all other strains was completely inhibited at 3 mM cobalt. Growth was mildly impaired in CUKW at 1 mM cobalt, while growth in KCC02 was the same as for control cultures. Growth was substantially impaired in all other strains and was typically delayed 20 to 24 h. Several strains (J192, V450, OCN004) exhibited no growth at 1 mM cobalt (Fig. 5). When grown with manganese, KCC02 displayed impaired growth at 3 mM, while CUKW displayed minimal growth. EZ55 growth was not inhibited at 3 mM manganese, with EZ55 achieving levels of growth at all concentrations of manganese comparable to those of control cultures. Growth of J192 and 27126 was also not inhibited at 3 mM manganese. All other strains displayed minimal to no growth at 3 mM manganese. Overall, nearly all strains achieved normal growth at 1 mM and 100 μM manganese (Fig. 6). The growth inhibition of CUKW and KCC02 at 3 mM manganese suggests that resistance to this metal may be conferred by chromosomal rather than plasmid-based features. A similar trend was observed upon exposure to zinc, in that growth was inhibited in all strains, including CUKW and KCC02. However, the growth curve of CUKW with 1 mM zinc was comparable to that of the control; while KCC02 did not achieve the same level of growth in 1 mM zinc as in the control, growth was not delayed and displayed a trend similar to that of the control (Fig. 7). Growth in the presence of 1 mM zinc was either completely inhibited (strains V450, OCN004, MR31d, and W12) or delayed ca. 20 to 24 h (strains J589, EZ55, J192, and 27126) (Fig. 7). Collectively, these data demonstrate the ability of strains CUKW and KCC02 to overcome metal challenges inhibitory to other Alteromonas strains. These two strains harbor megaplasmids on which are found numerous genes coding for RND efflux transporters as well as copper- and heavy-metal-associated resistance genes, indicating that the ability of these strains to overcome metal challenges is conferred by plasmid-based genetic elements.
FIG 4.
Growth curves of 10 Alteromonas strains in the presence of copper concentrations ranging from 100 μM to 3 mM.
FIG 5.
Growth curves of 10 Alteromonas strains in the presence of cobalt concentrations ranging from 100 μM to 3 mM.
FIG 6.
Growth curves of 10 Alteromonas strains in the presence of manganese concentrations ranging from 100 μM to 3 mM.
FIG 7.
Growth curves of 10 Alteromonas strains in the presence of zinc concentrations ranging from 100 μM to 3 mM.
Conclusions.
It is highly likely that the presence of megaplasmids among bacterial species is more common than believed due to their poor recovery in plasmid extracts by frequently employed methods and the bias toward chromosomal DNA when extracting environmental samples for metagenomic sequencing. It is critical to determine their presence, as the genes they carry typically encode novel traits (38). Additionally, identifying plasmid diversity and mobility within microbial communities facilitates interpreting how communities respond to environmental challenges (44). The ability of A. macleodii strains CUKW and KCC02 to overcome copper challenges is likely plasmid based. CUKW was isolated from copper coupons, suspended in tropical coastal water, being tested as potential coatings for marine vessels. Thus, the niche from which CUKW was isolated is in keeping with previous findings for Alteromonas spp. shown to be the primary colonizers of many different marine substrata (68), including copper-treated marine vessels (69). The physical nature of the marine biofilm matrix (open channel and pore structure) coupled with the community-level communication among species facilitates genetic exchange (70). Bacterial conjugation occurs within biofilms, and in fact, it has been proposed that conjugative plasmids favor the incorporation of the plasmid-bearing species into a biofilm environment (71).
The presence of conjugal machinery on the CUKW and KCC02 plasmids indicates the potential for these to be transferred within surface-associated marine microbial communities, especially in those environments prone to metal challenges such as are encountered on copper-treated marine vessels.
MATERIALS AND METHODS
Bacterial strains and culture media for PFGE.
Individual bacterial species were grown to mid-exponential phase as determined by measurements of optical density 600 nm (OD600) in broth medium appropriate for the strain (Table 1). Sequencing information and the environment from which the strains were isolated are provided in Table 1. Media used included the following: Burkholder’s formulation B (72), modified Burkholder’s B supplemented with 100 μl glycerol per 100 ml broth, YTSS (4.0 g tryptone, 2.5 g yeast extract, 15 g sea salts per liter), and Difco marine broth 2216 (34.7 g per liter). For all PFGE protocols, cell pellets were collected by aliquoting 6 or 10 ml of culture into 2-ml sterile microcentrifuge tubes using wide-bore pipette tips and centrifuging them for 2 min at 9,200 × g. The supernatant was decanted, and all pellets from each individual culture were combined into a single tube. The same volume of reagents was used whether 6 or 10 ml of culture was harvested. Plugs were prepared from the cell pellets by using one of the methods describe below.
PFGE methods.
(i) Method 1. Pulsed-field gel electrophoresis (PFGE) method 1 was derived from a protocol previously developed for identifying megaplasmids in marine Streptomyces (39). Cell pellets were washed with cell suspension buffer (CSB) (10 mM Tris [pH 7.2], 150 mM NaCl, 50 mM EDTA). Cells were then centrifuged at 9,200 × g, 4°C, for 10 min and resuspended in 500 μl CSB. An equal volume of melted 2% InCert agarose (Cambrex Bioscience), prepared in 10 mM Tris (pH 7.2), 50 mM EDTA (pH 8), was added to each 500-μl aliquot and resuspended using a wide-bore pipette tip. Gel plugs were formed by pipetting ca. 100-μl aliquots into plug molds. The plugs were solidified by holding them at 4°C for 30 min. The plugs were initially held at 4°C for 10 min. However, this was found to be insufficient for the plugs to solidify, and so the time at 4°C was increased to 30 min. In several instances, this extended time was also found to be insufficient for the plugs to solidify (as described in Results). Solidified plugs were placed in a 50-ml sterile centrifuge tube using a sterile loop and incubated in 4 ml of lysozyme solution (10 mM Tris [pH 7.2], 50 mM NaCl, 0.2% sodium deoxycholate [Sigma], 0.5% sodium lauryl sarcosine [Sigma], 1 mg/ml lysozyme [Acros Organics]) at 37°C for 2 h with gentle agitation. The lysozyme solution was discarded, and the plugs were rinsed in sterile deionized water. The plugs were then incubated in 4 ml of proteinase K solution (100 mM EDTA [pH 8], 0.2% Na deoxycholate, 1% Na lauryl sarcosine, with 1 mg/ml proteinase K [Fisher]) at 50°C overnight. The plugs were washed four times for 1 h each at room temperature in wash buffer (20 mM Tris [pH 8], 50 mM EDTA). The second wash included phenylmethylsulfonyl fluoride (PMSF; Sigma) at a final concentration of 1 mM to inactivate the proteinase K. The plugs were then washed once in 0.1× wash buffer and stored in 0.1× wash buffer at 4°C until use.
(ii) Method 2. The protocol for method 2 was a slightly modified version of that used by the Centers for Disease Control and Prevention for PulseNet PFGE analysis of Vibrio species (https://www.cdc.gov/pulsenet/PDF/vibrio_pfge_protocol-508c.pdf). Cell pellets were resuspended in 500 μl cell suspension buffer (100 mM Tris [pH 8], 100 mM EDTA [pH 8]) using wide-bore pipette tips. The single pellet was centrifuged at room temperature for 2 min at 9,200 × g. The supernatant was decanted, and the pellet was gently resuspended in 500 μl CSB. The suspension was immediately combined with 25 μl of 20 mg ml−1 proteinase K (Qiagen) and 500 μl of 2% InCert agarose dissolved in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0), pipetted gently several times, and distributed in ca. 100-μl aliquots into sterile plug molds. The plugs were placed at 4°C for 30 min. The plugs were then placed in a 50-ml sterile centrifuge tube containing 4 ml lysis buffer (50 mM Tris, 50 mM EDTA, 1% sarcosyl) and 20 μl of 20 mg ml−1 proteinase K and incubated at 55°C for 2.5 h with gentle agitation (75 rpm). The plugs were washed twice in sterile deionized water at 55°C with agitation for 10 min, followed by four washes in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) at 55°˚C. The plugs were stored in TE buffer at 4°C until PFGE analysis.
(iii) Method 3. The following PFGE method (method 3) was developed in this study. Cell pellets were resuspended in 500 μl cell suspension buffer (100 mM Tris [pH 8], 100 mM EDTA [pH 8], 50 mM glucose; here referred to as CSBG) using wide-bore pipette tips. The single pellet was centrifuged at room temperature for 2 min at 9,200 × g. The supernatant was decanted, and the pellet was gently resuspended in 500 μl CSBG. The suspension was immediately combined with 25 μl of 20 mg ml−1 proteinase K (Qiagen) and 500 μl of 2% InCert agarose dissolved in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0), pipetted gently several times, and distributed in ca. 100-μl aliquots into sterile plug molds. The plugs were placed at 4°C for 30 min. The plugs were then placed in a 50-ml sterile centrifuge tube containing 4 ml lysis buffer and 20 μl of 20 mg ml−1 proteinase K and incubated at 28°C for 2.5 h with gentle agitation (75 rpm). The plugs were washed twice in sterile deionized water at 28°C with agitation for 10 min, followed by four washes in TE buffer (10 mM Tris, 1 mM EDTA, pH 8.0) at 28°C. The plugs were stored in TE buffer at 4°C until PFGE analysis.
Pulse times and voltage settings.
For gels and running buffer, TBE at a 10× stock concentration (0.89 M Tris, 0.89 M borate, and 0.02 M EDTA; Fisher BioReagents) was diluted in sterile MilliQ water to a 0.5× concentration. Agarose plugs were examined on a 1% agarose gel with 0.5× TBE buffer using a clamped homogeneous electric field system (CHEF DR-III; Bio-Rad, Melville, NY) in 0.5× TBE buffer containing 100 μM thiourea at 14°C. Initial attempts utilized the two-state operating mode, with a velocity of 6 V cm−1, an included angle of 120° (two electric fields whose orientation differs by 120°), and initial and final switch times of 3.51 s and 1 min 6.53 s, respectively, with a linear ramping factor and a total run time of 27 h 23 min. Further optimization in the two-state mode included an initial switch time of 1 s, a final switch time of 30 s, and a linear ramping factor. Run times of 22, 24, and 25 h were tested. Gels were stained with 1× SYBR green I nucleic acid stain (Lonza) for 30 min and imaged under UV transillumination with a Capture GL200. Bacteriophage lambda concatemers (New England Biolabs) served as the ladder for all gels.
Metal growth assays.
Stock solutions were prepared in sterile nuclease-free water of the following salts, all at a concentration of 500 mM with the exception of zinc chloride, which was at 100 mM: copper(II) sulfate (Acros Organics), manganese chloride tetrahydrate (Fisher Chemical), zinc chloride (Fisher Chemical), and cobalt(II) chloride hexahydrate (ACROS Organics). Working solutions were prepared by dilution in sterile nuclease-free water. For metal growth assays, single colonies of each strain were inoculated into 3 ml of the appropriate medium and grown overnight with agitation. Upon reaching late exponential phase, cultures were inoculated at a 1:100 dilution into fresh medium and aliquoted in duplicate into 24-well nontreated tissue culture plates to a final volume of 1 ml. Metals were added to final concentrations of 3 mM, 1 mM, and 100 μM. Controls consisted of sterile nuclease-free water added at the same volume as the metal suspension. Blanks consisted of cell-free medium to which was added each of the metal stock solutions at final concentrations of 3 mM, 1 mM, and 100 μM and water only. Plates were incubated with the covers on with mild agitation (50 rpm) at 26°C. All growth assays for each strain were performed at a minimum in duplicate. Growth was defined as the change in OD600 over time. The optical density was measured approximately every 4 to 6 h for 48 h using the Modulus II multimode microplate reader (Turner Biosystems). Results for samples were normalized to those of the abiotic controls included on each plate.
Bioinformatic analysis.
Plasmids were annotated by the University of Delaware Bioinformatics Core Facility using Prokka (v1.13.3) (73) and a custom annotation database constructed from proteins from 28 Alteromonas species RefSeq genomes. Genome organization was visualized using Artemis (74) v.17.0.1. Additional manual annotation and gene comparisons utilized NCBI tools.
Data availability.
All raw sequence data, genome assemblies, and annotations for KCC02 and CUKW genomes described in this study are deposited in NCBI under BioProject ID PRJNA485824.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the researchers who provided Alteromonas strains from their individual collections: Erik Zinser, University of Tennessee (EZ55); Peter McCarthy, Harbor Branch Oceanographic Institute/Florida Atlantic University (J912, J589, V450); Blake Ushijima, Smithsonian Marine Station (OCN004); Dana Hunt, Duke University Marine Lab (W12); and Michael Hadfield, University of Hawaii (C34d, MR31d). Bioinformatics analysis was performed by the University of Delaware Bioinformatics Core Facility using the Biomix computational cluster supported by Delaware INBRE (NIH NIGMS P20 GM103446).
This research was supported by UMBC internal support to K.D.C.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Lopez-Perez M, Gonzaga A, Martin-Cuadrado AB, Onyshchenko O, Ghavidel A, Ghai R, Rodriguez-Valera F. 2012. Genomes of surface isolates of Alteromonas macleodii: the life of a widespread marine opportunistic copiotroph. Sci Rep 2:696. doi: 10.1038/srep00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzaga A, Lopez-Perez M, Martin-Cuadrado AB, Ghai R, Rodriguez-Valera F. 2012. Complete genome sequence of the copiotrophic marine bacterium Alteromonas macleodii strain ATCC 27126T. J Bacteriol 194:6998. doi: 10.1128/JB.01565-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sass AM, Sass H, Coolen MJ, Cypionka H, Overmann J. 2001. Microbial communities in the chemocline of a hypersaline deep-sea basin (Urania basin, Mediterranean Sea). Appl Environ Microbiol 67:5392–5402. doi: 10.1128/aem.67.12.5392-5402.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivars-Martínez E, D'Auria G, Rodríguez-Valera F, Sânchez-Porro C, Ventosa A, Joint I, Mühling M. 2008. Biogeography of the ubiquitous marine bacterium Alteromonas macleodii determined by multilocus sequence analysis. Mol Ecol 17:4092–4106. doi: 10.1111/j.1365-294x.2008.03883.x. [DOI] [PubMed] [Google Scholar]
- 5.Cusick KD, Dale JR, Fitzgerald LA, Little BJ, Biffinger JC. 2017. Adaptation to copper stress influences biofilm formation in Alteromonas macleodii. Biofouling 33:505–519. doi: 10.1080/08927014.2017.1329423. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Wang C, Han JR, Chen GJ, Du ZJ. 2019. Alteromonas flava sp. nov. and Alteromonas facilis sp. nov., two novel copper tolerating bacteria isolated from a sea cucumber culture pond in China. Syst Appl Microbiol 42:217–222. doi: 10.1016/j.syapm.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Perez M, Gonzaga A, Rodriguez-Valera F. 2013. Genomic diversity of “deep ecotype” Alteromonas macleodii isolates: evidence for pan-Mediterranean clonal frames. Genome Biol Evol 5:1220–1232. doi: 10.1093/gbe/evt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran C, Hadfield MG. 2011. Larvae of Pocillopora damicornis (Anthozoa) settle and metamorphose in response to surface-biofilm bacteria. Mar Ecol Prog Ser 433:85–96. doi: 10.3354/meps09192. [DOI] [Google Scholar]
- 9.Wang G, Barrett NH, McCarthy PJ. 2017. Draft genome sequence of deep-sea Alteromonas sp. strain V450 isolated from the marine sponge Leiodermatium sp. Microbiol Resour Announc 5:e01508-16. doi: 10.1128/genomeA.01508-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris JJ, Kirkegaard R, Szul MJ, Johnson ZI, Zinser ER. 2008. Facilitation of robust growth of Prochlorococcus colonies and dilute liquid cultures by “helper” heterotrophic bacteria. Appl Environ Microbiol 74:4530–4534. doi: 10.1128/AEM.02479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biller SJ, Coe A, Martin-Cuadrado A-B, Chisholm SW. 2015. Draft genome sequence of Alteromonas macleodii strain MIT1002, isolated from an enrichment culture of the marine cyanobacterium Prochlorococcus. Genome Announc 3:e00967-15. doi: 10.1128/genomeA.00967-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou S, Lopez-Perez M, Pfreundt U, Belkin N, Stuber K, Huettel B, Reinhardt R, Berman-Frank I, Rodriguez-Valera F, Hess WR. 2018. Benefit from decline: the primary transcriptome of Alteromonas macleodii str. Te101 during Trichodesmium demise. ISME J 12:981–996. doi: 10.1038/s41396-017-0034-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Law SV, Rodrigues KF, Anton A, Chin G. 2017. Microbial 16S rDNA sequencing of cultivable bacteria associated with toxic dinoflagellate, Pyrodinium bahamense var. compressum. Trans Sci Technol 4:318–323. [Google Scholar]
- 14.Allers E, Gómez-Consarnau L, Pinhassi J, Gasol JM, Simek K, Pernthaler J. 2007. Response of Alteromonadaceae and Rhodobacteriaceae to glucose and phosphorus manipulation in marine mesocosms. Environ Microbiol 9:2417–2429. doi: 10.1111/j.1462-2920.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 15.Nelson CE, Wear EK. 2014. Microbial diversity and the lability of dissolved organic carbon. Proc Natl Acad Sci U S A 111:7166–7167. doi: 10.1073/pnas.1405751111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivars-Martinez E, Martin-Cuadrado A-B, D'Auria G, Mira A, Ferriera S, Johnson J, Friedman R, Rodriguez-Valera F. 2008. Comparative genomics of two ecotypes of the marine planktonic copiotroph Alteromonas macleodii suggests alternative lifestyles associated with different kinds of particulate organic matter. ISME J 2:1194–1212. doi: 10.1038/ismej.2008.74. [DOI] [PubMed] [Google Scholar]
- 17.Hunt DE, Lin Y, Church MJ, Karl DM, Tringe SG, Izzo LK, Johnson ZI. 2013. Relationship between abundance and specific activity of bacterioplankton in open ocean surface waters. Appl Environ Microbiol 79:177–184. doi: 10.1128/AEM.02155-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayali X, Weber PK, Mabery S, Pett-Ridge J. 2014. Phylogenetic patterns in the microbial response to resource availability: amino acid incorporation in San Francisco Bay. PLoS One 9:e95842. doi: 10.1371/journal.pone.0095842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Martínez J, Acinas SG, Massana R, Rodríguez-Valera F. 2002. Prevalence and microdiversity of Alteromonas macleodii-like microorganisms in different oceanic regions. Environ Microbiol 4:42–50. doi: 10.1046/j.1462-2920.2002.00255.x. [DOI] [PubMed] [Google Scholar]
- 20.Pfreundt U, Spungin D, Bonnet S, Berman-Frank I, Hess WR. 2016. Global analysis of gene expression dynamics within the marine microbial community during the VAHINE mesocosm experiment in the southwest Pacific. Biogeosciences 13:4135–4149. doi: 10.5194/bg-13-4135-2016. [DOI] [Google Scholar]
- 21.McCarren J, Becker JW, Repeta DJ, Shi YM, Young CR, Malmstrom RR, Chisholm SW, DeLong EF. 2010. Microbial community transcriptomes reveal microbes and metabolic pathways associated with dissolved organic matter turnover in the sea. Proc Natl Acad Sci U S A 107:16420–16427. doi: 10.1073/pnas.1010732107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker BJ, Sheik CS, Taylor CA, Jain S, Bhasi A, Cavalcoli JD, Dick GJ. 2013. Community transcriptomic assembly reveals microbes that contribute to deep-sea carbon and nitrogen cycling. ISME J 7:1962–1973. doi: 10.1038/ismej.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pedler BE, Aluwihare LI, Azam F. 2014. Single bacterial strain capable of significant contribution to carbon cycling in the surface ocean. Proc Natl Acad Sci U S A 111:7202–7207. doi: 10.1073/pnas.1401887111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Toner BM, Baker BJ, Breier JA, Sheik CS, Dick GJ. 2014. Microbial iron uptake as a mechanism for dispersing iron from deep-sea hydrothermal vents. Nat Commun 5:3192. doi: 10.1038/ncomms4192. [DOI] [PubMed] [Google Scholar]
- 25.López-Pérez M, Ramon-Marco N, Rodriguez-Valera F. 2017. Networking in microbes: conjugative elements and plasmids in the genus Alteromonas. BMC Genomics 18:36. doi: 10.1186/s12864-016-3461-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hacker J, Carniel E. 2001. Ecological fitness, genomic islands and bacterial pathogenicity. A Darwinian view of the evolution of microbes. EMBO Rep 2:376–381. doi: 10.1093/embo-reports/kve097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conlan S, Thomas PJ, Deming C, Park M, Lau AF, Dekker JP, Snitkin ES, Clark TA, Luong K, Song Y, Tsai YC, Boitano M, Dayal J, Brooks SY, Schmidt B, Young AC, Thomas JW, Bouffard GG, Blakesley RW, NISC Comparative Sequencing Program, Mullikin JC, Korlach J, Henderson DK, Frank KM, Palmore TN, Segre JA. 2014. Single-molecule sequencing to track plasmid diversity of hospital-associated carbapenemase-producing Enterobacteriaceae. Sci Transl Med 6:254ra126. doi: 10.1126/scitranslmed.3009845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fadeev E, De Pascale F, Vezzi A, Hubner S, Aharonovich D, Sher D. 2016. Why close a bacterial genome? The plasmid of Alteromonas macleodii HOT1A3 is a vector for inter-specific transfer of a flexible genomic island. Front Microbiol 7:248. doi: 10.3389/fmicb.2016.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schürch AC, Arredondo-Alonso S, Willems RJL, Goering RV. 2018. Whole genome sequencing options for bacterial strain typing and epidemiologic analysis based on single nucleotide polymorphism versus gene-by-gene–based approaches. Clin Microbiol Infect 24:350–354. doi: 10.1016/j.cmi.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 30.Mehta A, Sidhu C, Pinnaka AK, Roy Choudhury A. 2014. Extracellular polysaccharide production by a novel osmotolerant marine strain of Alteromonas macleodii and its application towards biomineralization of silver. PLoS One 9:e98798. doi: 10.1371/journal.pone.0098798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cambon-Bonavita MA, Raguenes G, Jean J, Vincent P, Guezennec J. 2002. A novel polymer produced by a bacterium isolated from a deep-sea hydrothermal vent polychaete annelid. J Appl Microbiol 93:310–315. doi: 10.1046/j.1365-2672.2002.01689.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Zuo Q, Wang Y, Song J, Yang H, Zhang Y, Li B. 2017. Characterization of a novel bioflocculant from a marine bacterium and its application in dye wastewater treatment. BMC Biotechnol 17:11. doi: 10.1186/s12896-017-0404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang ZL, Cai RH, Zhang WH, Fu YN, Jiao NZ. 2017. A novel exopolysaccharide with metal adsorption capacity produced by a marine bacterium Alteromonas sp. JL2810. Mar Drugs 15:E175. doi: 10.3390/md15060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raguenes G, Pignet P, Gauthier G, Peres A, Christen R, Rougeaux H, Barbier G, Guezennec J. 1996. Description of a new polymer-secreting bacterium from a deep-sea hydrothermal vent, Alteromonas macleodii subsp. fijiensis, and preliminary characterization of the polymer. Appl Environ Microbiol 62:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun Y, Luo H. 2018. Homologous recombination in core genomes facilitates marine bacterial adaptation. Appl Environ Microbiol 84:e02545-17. doi: 10.1128/AEM.02545-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Argandona M, Martinez-Checa F, Llamas I, Quesada E, del Moral A. 2003. Megaplasmids in Gram-negative, moderately halophilic bacteria. FEMS Microbiol Lett 227:81–86. doi: 10.1016/S0378-1097(03)00651-7. [DOI] [PubMed] [Google Scholar]
- 37.Wheatcroft R, McRae DG, Miller RW. 1990. Changes in the Rhizobium meliloti genome and the ability to detect supercoiled plasmids during bacteroid development. Mol Plant Microbe Interact 3:9–17. doi: 10.1094/MPMI-3-009. [DOI] [Google Scholar]
- 38.Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240. doi: 10.1006/abio.1995.1220. [DOI] [PubMed] [Google Scholar]
- 39.Ravel J, Schrempf H, Hill RT. 1998. Mercury resistance is encoded by transferable giant linear plamids in two Chesapeake Bay Streptomyces strains. Appl Environ Microbiol 64:3383–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornell CR, Marasini D, Fakhr MK. 2018. Molecular characterization of plasmids harbored by actinomycetes isolated from the Great Salt Plains of Oklahoma using PFGE and next generation whole genome sequencing. Front Microbiol 9:2282. doi: 10.3389/fmicb.2018.02282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iglesias-Torrens Y, Miro E, Guirado P, Llovet T, Munoz C, Cerda-Cuellar M, Madrid C, Balsalobre C, Navarro F. 2018. Population structure, antimicrobial resistance, and virulence-associated genes in Campylobacter jejuni isolated from three ecological niches: gastroenteritis patients, broilers, and wild birds. Front Microbiol 9:1676. doi: 10.3389/fmicb.2018.01676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia NS, Yung C-M, Davis KM, Rynearson T, Hunt DE. 2017. Draft genome sequences of three bacterial isolates from cultures of the marine diatom Thalassiosira rotula. Genome Announc 5:e00316-17. doi: 10.1128/genomeA.00316-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ushijima B, Smith A, Aeby GS, Callahan SM. 2012. Vibrio owensii induces the tissue loss disease Montipora white syndrome in the Hawaiian reef coral Montipora capitata. PLoS One 7:e46717. doi: 10.1371/journal.pone.0046717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smillie C, Garcillan-Barcia MP, Francia MV, Rocha EP, de la Cruz F. 2010. Mobility of plasmids. Microbiol Mol Biol Rev 74:434–452. doi: 10.1128/MMBR.00020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moran RA, Hall RM. 2019. pBuzz: a cryptic rolling-circle plasmid from a commensal Escherichia coli has two inversely oriented oriTs and is mobilised by a B/O plasmid. Plasmid 101:10–19. doi: 10.1016/j.plasmid.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Ramsay JP, Firth N. 2017. Diverse mobilization strategies facilitate transfer of non-conjugative mobile genetic elements. Curr Opin Microbiol 38:1–9. doi: 10.1016/j.mib.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Bignell C, Thomas CM. 2001. The bacterial ParA-ParB partitioning proteins. J Biotechnol 91:1–34. doi: 10.1016/s0168-1656(01)00293-0. [DOI] [PubMed] [Google Scholar]
- 48.Bartosik D, Szymanik M, Wysocka E. 2001. Identification of the partitioning site within the repABC-type replicon of the composite Paracoccus versutus plasmid pTAV1. J Bacteriol 183:6234–6243. doi: 10.1128/JB.183.21.6234-6243.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bañuelos-Vazquez LA, Torres Tejerizo G, Brom S. 2017. Regulation of conjugative transfer of plasmids and integrative conjugative elements. Plasmid 91:82–89. doi: 10.1016/j.plasmid.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 50.Silver S, Phung LT. 1996. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 51.Ben Fekih I, Zhang C, Li YP, Zhao Y, Alwathnani HA, Saquib Q, Rensing C, Cervantes C. 2018. Distribution of arsenic resistance genes in prokaryotes. Front Microbiol 9:2473. doi: 10.3389/fmicb.2018.02473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parsons C, Lee S, Kathariou S. 2019. Heavy metal resistance determinants of the foodborne pathogen Listeria monocytogenes. Genes 10:11. doi: 10.3390/genes10010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Piotrowska M, Popowska M. 2015. Insight into the mobilome of Aeromonas strains. Front Microbiol 6:494. doi: 10.3389/fmicb.2015.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chasteen TG, Fuentes DE, Tantalean JC, Vasquez CC. 2009. Tellurite: history, oxidative stress, and molecular mechanisms of resistance. FEMS Microbiol Rev 33:820–832. doi: 10.1111/j.1574-6976.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- 55.Bruins MR, Kapil S, Oehme FW. 2000. Microbial resistance to metals in the environment. Ecotoxicol Environ Saf 45:198–207. doi: 10.1006/eesa.1999.1860. [DOI] [PubMed] [Google Scholar]
- 56.Ma Z, Jacobsen FE, Giedroc DP. 2009. Coordination chemistry of bacterial metal transport and sensing. Chem Rev 109:4644–4681. doi: 10.1021/cr900077w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Franke S, Grass G, Nies DH. 2001. The product of the ybdE gene of the Escherichia coli chromosome is involved in detoxification of silver ions. Microbiology 147:965–972. doi: 10.1099/00221287-147-4-965. [DOI] [PubMed] [Google Scholar]
- 58.Nies DH. 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol Rev 27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 59.Rensing C, Grass G. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev 27:197–213. doi: 10.1016/S0168-6445(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 60.Bondarczuk K, Piotrowska-Seget Z. 2013. Molecular basis of active copper resistance mechanisms in Gram-negative bacteria. Cell Biol Toxicol 29:397–405. doi: 10.1007/s10565-013-9262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cooksey DA. 1994. Molecular mechanisms of copper resistance and accumulation in bacteria. FEMS Microbiol Rev 14:381–386. doi: 10.1111/j.1574-6976.1994.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 62.Rensing C, Fan B, Sharma R, Mitra B, Rosen BP. 2000. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc Natl Acad Sci U S A 97:652–656. doi: 10.1073/pnas.97.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grass G, Rensing C. 2001. Genes involved in copper homeostasis in Escherichia coli. J Bacteriol 183:2145–2147. doi: 10.1128/JB.183.6.2145-2147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Outten FW, Outten CE, Hale J, O'Halloran TV. 2000. Transcriptional activation of an E. coli copper efflux regulon by the chromosomal MerR homologue, CueR. J Biol Chem 275:31024–31029. doi: 10.1074/jbc.M006508200. [DOI] [PubMed] [Google Scholar]
- 65.Lawton TJ, Kenney GE, Hurley JD, Rosenzweig AC. 2016. The CopC family: structural and bioinformatic insights into a diverse group of periplasmic copper binding proteins. Biochemistry 55:2278–2290. doi: 10.1021/acs.biochem.6b00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grass G, Rensing C, Solioz M. 2011. Metallic copper as an antimicrobial surface. Appl Environ Microbiol 77:1541–1547. doi: 10.1128/AEM.02766-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morel FMM, Price NM. 2003. The biogeochemical cycles of trace metals in the oceans. Science 300:944–947. doi: 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- 68.Dang H, Lovell CR. 2000. Bacterial primary colonization and early succession on surfaces in marine waters as determined by amplified rRNA gene restriction analysis and sequence analysis of 16S rRNA genes. Appl Environ Microbiol 66:467–475. doi: 10.1128/aem.66.2.467-475.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen C-L, Maki JS, Rittschof D, Teo S-M. 2013. Early marine bacterial biofilm on a copper-based antifouling paint. Int Biodeterior Biodegradation 83:71–76. doi: 10.1016/j.ibiod.2013.04.012. [DOI] [Google Scholar]
- 70.Dang H, Lovell CR. 2016. Microbial surface colonization and biofilm development in marine environments. Microbiol Mol Biol Rev 80:91–138. doi: 10.1128/MMBR.00037-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghigo J-M. 2001. Natural conjugative plasmids induce bacterial biofilm development. Nature 412:442–445. doi: 10.1038/35086581. [DOI] [PubMed] [Google Scholar]
- 72.Bidwell JP, Spotte S. 1985. Artificial sea waters: formulas and methods. Jones and Bartlett, Boston, MA. [Google Scholar]
- 73.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 74.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945. doi: 10.1093/bioinformatics/16.10.944. [DOI] [PubMed] [Google Scholar]
- 75.Baumann L, Baumann P, Mandel M, Allen RD. 1972. Taxonomy of aerobic marine eubacteria. J Bacteriol 110:402–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw sequence data, genome assemblies, and annotations for KCC02 and CUKW genomes described in this study are deposited in NCBI under BioProject ID PRJNA485824.