Staphylococcus aureus is an important pathogen that can be widely distributed in the community and clinical settings. The emergence of S. aureus with multiple-antibiotic resistance has complicated staphylococcal infection control. The development of alternative strategies with powerful bactericidal effects is urgently needed. Cold atmospheric plasma (CAP) is a promising strategy for microorganism inactivation. Nevertheless, the underlying mechanisms of microbial inactivation or resistance are not completely illustrated. In this study, we validated the bactericidal effects of CAP on S. aureus, including antibiotic-resistant strains. We also found that the golden staphyloxanthin, as well as its yellow pigment intermediates, protected S. aureus against CAP, and blocking the staphyloxanthin synthesis pathway at the early steps could strengthen the sensitivity of S. aureus to CAP treatment. These data provide insights into the germicidal mechanism of CAP from the aspect of bacteria and suggest new targets against S. aureus infections.
KEYWORDS: Staphylococcus aureus, cold atmospheric plasma, bactericidal effect, staphyloxanthin biosynthetic pathway, crtOPQMN operon
ABSTRACT
Staphylococcus aureus infection poses a serious threat to public health, and antibiotic resistance has complicated the clinical treatment and limited the solutions available to solve this problem. Cold atmospheric plasma (CAP) is a promising strategy for microorganism inactivation. However, the mechanisms of microbial inactivation or resistance remain unclear. In this study, we treated S. aureus strains with a self-assembled CAP device and found that CAP can kill S. aureus in an exposure time-dependent manner. In addition, the liquid environment can influence the survival rate of S. aureus post-CAP treatment. The S. aureus cells can be completely inactivated in normal saline and phosphate-buffered saline but not in tryptic soy broth culture medium. Scanning and transmission electron microscopy revealed that the CAP-treated S. aureus cells maintained integrated morphological structures, similar to the wild-type strain. Importantly, the CAP-treated S. aureus cells exhibited a reduced pigment phenotype. Deletion of the staphyloxanthin biosynthetic genes crtM and crtN deprived the pigmentation ability of S. aureus Newman. Both the Newman-ΔcrtM and Newman-ΔcrtN mutants presented high sensitivity to CAP treatment, whereas Newman-ΔcrtO exhibited a survival rate comparable to wild-type Newman after CAP treatment. Our data demonstrated that the yellow pigment intermediates of the staphyloxanthin biosynthetic pathway are responsible for the protection of S. aureus from CAP inactivation. The key enzymes, such as CrtM and CrtN, of the golden staphyloxanthin biosynthetic pathway could be important targets for the design of novel sterilization strategies against S. aureus infections.
IMPORTANCE Staphylococcus aureus is an important pathogen that can be widely distributed in the community and clinical settings. The emergence of S. aureus with multiple-antibiotic resistance has complicated staphylococcal infection control. The development of alternative strategies with powerful bactericidal effects is urgently needed. Cold atmospheric plasma (CAP) is a promising strategy for microorganism inactivation. Nevertheless, the underlying mechanisms of microbial inactivation or resistance are not completely illustrated. In this study, we validated the bactericidal effects of CAP on S. aureus, including antibiotic-resistant strains. We also found that the golden staphyloxanthin, as well as its yellow pigment intermediates, protected S. aureus against CAP, and blocking the staphyloxanthin synthesis pathway at the early steps could strengthen the sensitivity of S. aureus to CAP treatment. These data provide insights into the germicidal mechanism of CAP from the aspect of bacteria and suggest new targets against S. aureus infections.
INTRODUCTION
Staphylococcus aureus infection and the corresponding infectious diseases pose a serious threat to public health (1–3). The chief strategy used to deal with S. aureus infections is antibiotic application. Nevertheless, the emergence and prevalence of drug-resistant S. aureus, such as methicillin-resistant S. aureus (MRSA) and vancomycin-intermediate S. aureus (VISA), have increased the difficulty of S. aureus infection control (4, 5). One reasonable solution to cope with this concern is the development of new drugs, but this developmental process is expensive and time- and labor-consuming. Furthermore, bacteria can rapidly develop resistance to a certain antibiotic. For instance, penicillin-resistant S. aureus was isolated only 2 years after the clinical introduction of penicillin in 1942 (6, 7), and MRSA emerged only 1 year after the first usage of methicillin in 1959 (8). Therefore, the development of alternative strategies as a complement of antibiotic treatments to conquer clinical infections is urgently needed.
Cold atmospheric plasma (CAP) is a new concept of disinfection that presents promising results for inactivation of microorganisms (9, 10). CAP, as ionized gas, is mainly generated by high-energy electrons colliding with atmospheric molecules under low temperature and consists of various particles, including UV photons, charged particles, reactive oxygen species (ROS), and reactive nitrogen species (RNS) (11). Given its rich and diverse components, CAP is considered an ideal tool with promising application prospects and has been applied in various fields, such as food production (9, 12), wound therapy (13), and oncotherapy (14). However, the mechanism underlying bacterial inactivation or resistance remains unclear because of the complex ingredients in CAP. Han et al. demonstrated that CAP kills Gram-negative and Gram-positive bacteria via different mechanisms (15). Escherichia coli strains are mainly killed by cell leakage, whereas S. aureus is chiefly inactivated by intracellular DNA damage after CAP treatment (15–18). Other studies proposed that the ROS of CAP play pivotal roles in bacterial inactivation (19–21). However, accurate targets within the bacterial cells have yet to be reported.
Staphyloxanthin, the golden carotenoid pigment synthesized by S. aureus, is an important virulence factor owing to its antioxidant property (22, 23). It can react with and thus deactivate ROS and help bacteria evade host neutrophil-based killing. Liu et al. reported that S. aureus mutants with disrupted carotenoid biosynthesis are susceptible to oxidant killing (24). Nevertheless, whether the staphyloxanthin biosynthetic pathway can promote resistance to CAP-derived reactive species remains unclear. Herein, we present the bactericidal effects of a self-assembled small portable CAP device on S. aureus. The CAP-treated S. aureus exhibited negligible changes in cell integrity and internal structure but reduced pigmentation. The biosynthesis genes of staphyloxanthin are organized in the crtOPQMN operon. Gene deletion increased the susceptibility of S. aureus Newman-ΔcrtM and Newman-ΔcrtN to CAP treatment, whereas Newman-ΔcrtO presented comparable sensitivity to the wild-type Newman upon CAP exposure. These data illustrate that the yellow pigment intermediates of the staphyloxanthin biosynthetic pathway are sufficient and responsible for the protection of S. aureus from CAP inactivation. The CrtM and CrtN enzymes could be important targets for the design of novel sterilization strategies against S. aureus infections.
RESULTS
Survival of S. aureus strains after CAP treatment.
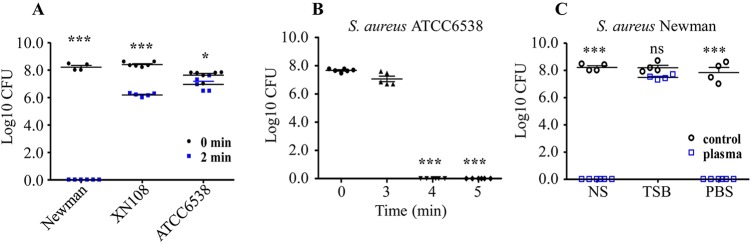
To evaluate the antibacterial efficacy of a self-assembled CAP device, we selected different S. aureus strains, including Newman, XN108, and ATCC 6538, for CAP treatment. After a preliminary 2-min treatment, the survival of all three S. aureus strains in sterilized normal saline (NS) solution was significantly reduced compared with that of the untreated strains (Fig. 1A). However, the survival rate of S. aureus after CAP treatment was strain dependent. The S. aureus Newman was more sensitive to CAP treatment, followed by XN108 (a VISA strain), whereas S. aureus ATCC 6538, a typical Gram-positive strain commonly used for disinfection evaluation (25), was relatively resistant to the 2-min CAP treatment (see Fig. S1 in the supplemental material). When the CAP treatment time for ATCC 6538 was prolonged, the number of surviving cells decreased gradually. The ATCC 6538 cells were completely killed after 4 min of CAP treatment (Fig. 1B). In addition, the S. aureus Newman in NS and phosphate-buffered saline (PBS) was effectively killed, whereas the common S. aureus culture medium tryptic soy broth (TSB) protected S. aureus from CAP killing (Fig. 1C). However, the underlying mechanism remains unclear and needs further investigation.
FIG 1.
Evaluation of the antibacterial efficacy of CAP treatment. (A) Different S. aureus strains resuspended in NS were exposed to CAP for 2 min. The surviving cells were counted using the plate dilution method. (B) The S. aureus strain ATCC 6538 was resuspended in NS and was exposed to CAP for up to 5 min. The surviving cells were counted using the plate dilution method. (C) The S. aureus strain Newman was diluted in NS, TSB, or PBS, and then the mixtures were exposed to CAP for 2 min. The surviving cells were counted using the plate dilution method. The solid symbols in panels A and B represent bacterial samples treated by CAP for different lengths of time. The hollow symbols in panel C represent CAP-treated or -untreated bacterial samples. All experiments were repeated four or five times. The numbers of surviving cells are presented as mean ± SD. One-way ANOVA was used for testing multiple groups. ns, no statistical significance; *, P < 0.05; ***, P < 0.001.
The CAP treatment did not affect the morphological characteristics of S. aureus but impaired the golden pigment phenotype.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were performed to determine whether CAP treatment affects the morphology of S. aureus. The SEM observation revealed that the surface structure of the CAP-treated Newman was similar to that of untreated S. aureus (Fig. 2A and B). Further TEM observation demonstrated that most CAP-treated Newman cells presented normal internal structures, although a few ruptured cells were found in the CAP-treated samples. The difference in morphological characteristics between the CAP-treated and untreated S. aureus cells was negligible (Fig. 2C and D).
FIG 2.
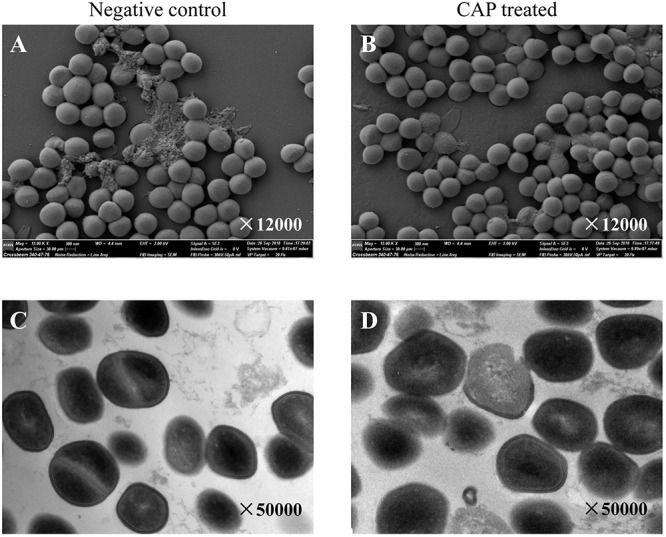
The CAP treatment did not influence the morphological phenotypes of S. aureus. The S. aureus strain Newman was exposed to CAP for 2 min. The untreated Newman strain served as a negative control. Then, the S. aureus cells were harvested and observed under SEM (A, B) and TEM (C, D). The magnification is indicated in each panel.
The CAP treatment significantly weakened the color of the S. aureus Newman suspension and cell pellets (Fig. 3A and B). The golden carotenoid pigment staphyloxanthin is the eponymous feature of the human pathogen S. aureus and is recognized as a potent antioxidant because of its free radical-scavenging properties (24). We then extracted staphyloxanthin from the CAP-treated Newman with methanol. Spectrophotometric results revealed that the optical density at 462 nm (OD462) value of the CAP-processed Newman-derived solution notably declined compared with that of the untreated control (Fig. 3C).
FIG 3.
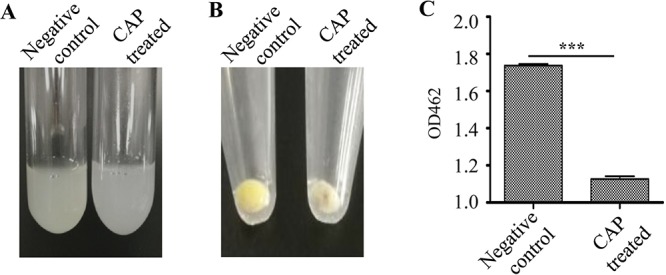
The CAP treatment impaired the pigment phenotype of S. aureus. S. aureus Newman was exposed to CAP for 2 min. The pigment phenotypes of bacterial cells in NS solution (A) and in pellets (B) were observed. (C) The staphyloxanthin in pelleted cells was extracted using methanol, and the OD462 value was determined. Experiments were repeated in triplicate. Statistical significances were calculated using Student’s t test. ***, P < 0.001.
Similar results were achieved when S. aureus strain ATCC 6538 was used (Fig. S2). Taken together, these data indicate that CAP treatment kills S. aureus via a cell damage-independent pathway, and the golden pigment staphyloxanthin of S. aureus might be an important target for CAP killing.
Biosynthetic pathway of staphyloxanthin involved in the protection of S. aureus from CAP treatment.
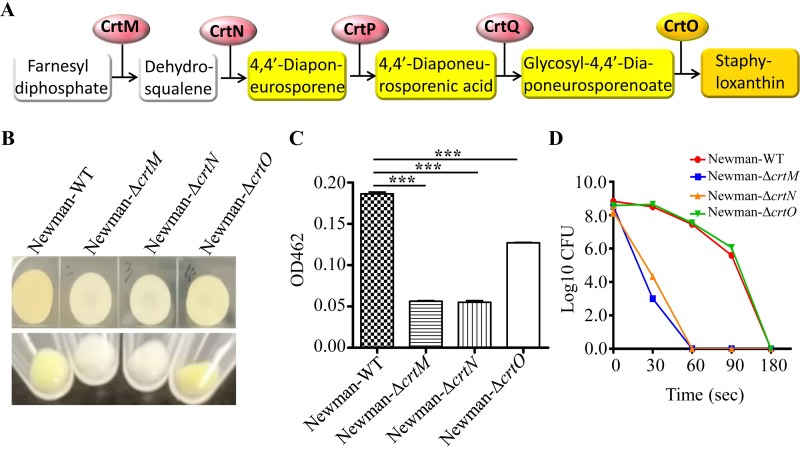
The biosynthesis of golden carotenoid staphyloxanthin in S. aureus is directly controlled by the crtOPQMN operon, which encodes five enzymes to gradually complete the whole process (26, 27). The first step in staphyloxanthin biosynthesis is the head-to-head condensation of two farnesyl diphosphate molecules to form colorless dehydrosqualene (4,4′-diapophytoene), catalyzed by the synthase CrtM (Fig. 4A). Then, the desaturase CrtN dehydrogenates dehydrosqualene to form the deep-yellow pigment intermediate 4,4′-diaponeurosporene. The CrtP enzyme catalyzes the oxidation of the terminal methyl group of 4,4′-diaponeurosporene to yield 4,4′-diaponeurosporenic acid. Then, the CrtQ enzyme esterifies glucose at the C-1′ position with the carboxyl group of 4,4′-diaponeurosporenic acid to produce glycosyl-4,4′-diaponeurosporenoate. Finally, the orange-pigmented acyl compound staphyloxanthin is formed by esterification of glucose at the C-6′ position, catalyzed by the acyltransferase CrtO (28).
FIG 4.
The biosynthetic pathway of staphyloxanthin was responsible for the protection of S. aureus from CAP treatment. (A) Biochemical pathway for staphyloxanthin biosynthesis in S. aureus. The colors of staphyloxanthin and its intermediates are indicated by the background colors. (B) Surface pigmentation of Newman and its derivatives in S. aureus plaques and pellets. (C) Corresponding carotenoid pigments of S. aureus Newman and its derivatives were extracted and the OD462 values were determined. Experiments were repeated in triplicate. Statistical significances were calculated using Student’s t test. ***, P < 0.001. (D) Survival of strain Newman and its derivatives after different times of CAP treatment. The solid symbols with different colors represent S. aureus Newman and its derivatives. The experiments were repeated five times. The numbers of surviving cells in one experiment are presented.
To identify which pigment intermediates are responsible for the resistance of S. aureus to CAP treatment, we subjected certain enzyme deletion mutants, including Newman-ΔcrtM, Newman-ΔcrtN, and Newman-ΔcrtO, to CAP-treated evaluation. Phenotypically, Newman-ΔcrtM and Newman-ΔcrtN were colorless, while Newman-ΔcrtO was yellow pigmented compared with wild-type Newman (Fig. 4B). A pigment extraction assay showed that all three mutants exhibited lower pigmentation than wild-type Newman (Fig. 4C). Upon exposure to CAP treatment, the colorless Newman-ΔcrtM and Newman-ΔcrtN were completely killed after 180 s of CAP treatment, whereas the yellow-pigmented Newman-ΔcrtO presented CAP resistance capability similar to Newman (Fig. 4D). These data suggest that the yellow pigment intermediates, not only the golden compound staphyloxanthin, are responsible for the protection of S. aureus from CAP treatment.
DISCUSSION
Staphylococcus aureus possesses a strong adaptive ability to numerous environments and can quickly develop resistance to routine antibiotics, which makes the control of S. aureus infection very complicated (29). CAP is a new type of bactericidal method composed of various active particles, such as UV photons, energetic ions, charged particles, and reactive species (ROS and RNS), and each component might contribute to killing bacteria via an independent or synergetic pathway (30–32). The UV radiation is clearly elucidated to inactivate bacteria by inducing the dimerization of thymine bases in DNA strands and thus interrupting DNA replication. Nevertheless, the actual role of UV in CAP is questionable. Kelly-Wintenberg et al. showed that UV is not a significant contributor to the bacterial lethality because much UV in CAP is blocked by the packing of samples (33). Other researchers reported that UV alone produced by the plasma does not have any bactericidal effect (34). The charged particles in CAP, including positive and negative ions, are supposed to inactivate bacteria either by directly bombarding the cell wall to cause lesions and openings in the membranes or by accumulating charged particles on the cell surface to cause electrostatic stress and change the tensile strength of the cell membrane (16, 35–37). This type of inactivation more frequently occurs on Gram-negative bacteria because of their vulnerable cell walls. Gram-positive bacteria, such as S. aureus, possess thick cell walls. Thus, Gram-positive bacteria are more likely to be inactivated by intracellular damage caused by reactive species. Studies have confirmed the existence of intracellular ROS and RNS (18, 20), which could result in DNA damage in S. aureus (38, 39).
Although many efforts have been made to uncover the mechanisms underlying CAP inactivation of bacteria and determine which component in CAP plays a dominant role, the actual targets in bacteria during CAP treatment are completely unknown and have rarely been investigated. In the present study, we showed that a self-assembled CAP device can inactivate different S. aureus strains, including those strains exhibiting high-level antibiotic resistance (strain XN108, a VISA strain). In addition, we observed that CAP disinfection was influenced by the liquid environment. The S. aureus cells could be inactivated in NS solution and PBS solution but not in TSB medium. A reasonable explanation is that TSB medium (casein soya bean digest broth) contains abundant proteins/peptides, some of which may have antioxidant capacity. During CAP treatment, the produced ROS and RNS might be quickly neutralized by certain components in TSB broth, resulting in a reduced oxidative killing effect of CAP treatment on bacterial cells.
In order to illustrate the inactivation of CAP treatment on bacterial cells, the morphologies and structures of CAP-treated S. aureus cells were observed. The SEM and TEM revealed limited changes in CAP-treated S. aureus morphology and structure compared with the wild-type strain, whereas the degradation of DNA increased after CAP exposure (data not shown). Interestingly, we observed that CAP-treated S. aureus cells exhibited a significantly weakened pigmentation phenotype. The CAP treatment has been suggested to kill Gram-positive bacteria mainly through ROS and RNS, and the gold pigment of S. aureus is an important factor that protects bacterial cells from oxidative sterilization (22–24). Therefore, we supposed that CAP treatment can target the golden pigment biosynthetic pathway and that the absence of staphyloxanthin or its synthetic precursors would decrease the survival of S. aureus under CAP exposure. The biosynthesis of staphyloxanthin in S. aureus is directly controlled by the crtOPQMN operon, and each gene within this operon gradually contributes to the biosynthesis of the golden pigment (27, 28). Using S. aureus mutants with deletions of CrtM and CrtN, which are responsible for the first and second steps of staphyloxanthin biosynthesis, we found that both Newman-ΔcrtM and Newman-ΔcrtN presented as colorless and became sensitive to CAP treatment. Deletion of crtO, which is in charge of the final step of staphyloxanthin synthesis, resulted in an attenuated golden pigmentation. However, Newman-ΔcrtO exhibited comparable sensitivity to wild-type Newman upon CAP exposure. These data suggest that the golden staphyloxanthin and its corresponding synthetic intermediates, including the deep-yellow pigments 4,4′-diaponeurosporene, 4,4′-diaponeurosporenic acid, and glycosyl-4,4′-diaponeurosporenoate, play crucial roles in the protection of S. aureus from CAP treatment. The golden staphyloxanthin biosynthetic pathway is responsible for S. aureus survival under CAP treatment, and disruption of pigment biosynthesis enhances the sterilization effects on S. aureus.
In conclusion, the CAP treatment can effectively inactivate Gram-positive S. aureus. During treatment, the golden pigment acted as a protective factor. Blocking the staphyloxanthin biosynthesis by deleting the crtM and crtN genes but not the crtO gene strengthened the sensitivity of S. aureus to the CAP treatment. These data provide insights into the germicidal mechanism of CAP from the aspect of bacteria and suggest new targets for the design of novel sterilization strategies against S. aureus infections.
MATERIALS AND METHODS
Bacterial strains.
Staphylococcus aureus strains Newman and RN4220, a human clinical isolate and a standard laboratory strain, respectively, are kept in our laboratory. The S. aureus pigment mutant strains, Newman-ΔcrtM and Newman-ΔcrtN, were kindly provided by Lefu Lan (Shanghai Institute of Materia Medica, Chinese Academy of Sciences). The S. aureus XN108 strain is a VISA strain and is kept in our laboratory. The S. aureus strain ATCC 6538, as an indicator of environment disinfection, was kindly provided by Jinfeng Tie (Institute of Disease Prevention and Control, Academy of Military Medical Sciences of the People’s Liberation Army of China). All S. aureus strains were cultured in TSB medium with shaking at 200 rpm, at 37°C. Escherichia coli DH5α was cultured in Luria-Bertani (LB) medium and used for recombinant plasmid manipulation. The E. coli-S. aureus shuttle vector pBT2 was a gift from Baolin Sun (University of Science and Technology of China).
All S. aureus strains in 80% TSB plus 20% glycerol were stored as frozen stocks and kept at –80°C. During preparation of the working cultures, S. aureus strains of the stocks were first streaked onto the TSB agar plates and grown at 37°C overnight. Then, a single colony of each strain was transferred into 3 ml TSB medium and grown at 37°C with shaking at 200 rpm for 16 h, and the overnight culture was used in the following experiments. The E. coli DH5α in 80% LB medium plus 20% glycerol was stored at –80°C, and the working culture was prepared from the stock as described for S. aureus with LB agar plate or LB medium.
Construction of the crtO deletion S. aureus mutant.
Another pigment mutant strain, Newman-ΔcrtO, was generated using the allelic replacement strategy as previously described (40). Briefly, PCR was used to amplify ∼800 bp upstream of the crtO gene with primers crtO-up-1 (5′-CGCGAATTCGTATACAATTGATGCACAAG-3′) and crtO-up-2 (5′-CGCGGTACCGGTTTTCATCTAAATTGAAT-3′), and ∼800 bp of sequence immediately downstream of crtO with primers crtO-down-1 (5′-CGCGGATCCACATATCATCGTTATTGGT-3′) and crtO-down-2 (5′-GCGTCGACCACCTAAATGCATCACATATC-3′). The upstream and downstream PCR products were then subcloned into the pBT2 plasmid to construct pBT2-crtO with proper restriction enzymes. The pBT2-crtO plasmid was transformed into S. aureus RN4220 for restriction modification and then electro-transformed into the Newman strain. After being cultured overnight at 30°C, the plasmid-carried Newman was then cultured at 42°C to induce the integration of the pBT2-crtO plasmid into the bacterial genome, and the plasmid was then eliminated by culturing strains at 25°C to achieve crtO gene deletion. The final Newman-ΔcrtO mutant was verified using PCR amplification with primers within the crtO gene crtO-in-1 (5′-TAGAGACAAAGAGGGCAGAGTTGATTC-3′) and crtO-in-2 (5′-TGCGTAGTAACTGCGTTAATCTCGG-3′), and primers flanking the crtO gene crtO-out-1 (5′-TCGCTACAGCAGGACTTGCCACTA-3′) and crtO-out-2 (5′-GATAAATGGTCGGATCATCTGGCAA-3′). The PCR products were further DNA sequenced.
The CAP device.
The CAP device used in this study was specially designed for the test-tube treatment, which meets the requirement of simplification and flexibility. It was assembled based on the principle of dielectric barrier discharge (DBD) due to the existence of the nonconducting tube (41) (Fig. 5). This configuration facilitates steady generation of CAP in the test tube and easy plasma treatment in the laboratory.
FIG 5.
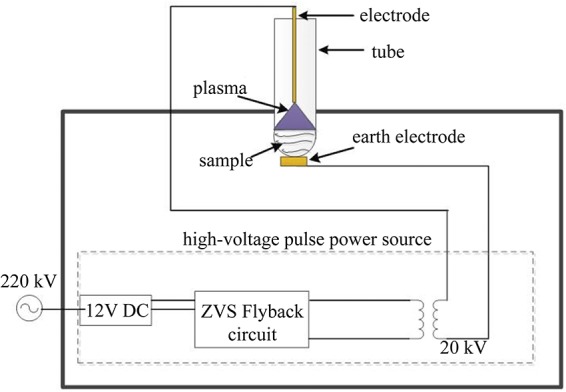
Schematic of the self-assembled small CAP device.
The voltage source consists of a direct-current-regulated power supply (Gophert CPS6003), a zero-voltage switching (ZVS) flyback circuit, and a pulse transformer. It generated a repetitive pulsed high voltage with a maximum amplitude of 20 kV to break down the air gap between the high-voltage electrode and the earth electrode, and then plasma was immediately generated inside the test tube. In order to lower the required high-voltage level, a stainless-steel electrode with a sharp tip was selected as the high-voltage electrode.
A 20-mm-diameter test tube for holding samples was placed on the CAP device. Since it was equivalent to a capacitive load in the circuit, several parameters of the tube were carefully chosen as follows: the volume of liquid samples was kept at 0.5 ml, the gap between the electrode tip and the liquid surface was approximately 5 mm, and the tube was in good contact with the earth electrode to minimize possible electrical dispersibility.
During the experiment, the input voltage of the power supply was held at 15 V, and the treatment time ranged from 0 to 5 min. Plasma generated around the sharp-tip electrode over the sample inside the test tube could be observed by the naked eye in a dark room. The high-voltage and discharge current of the CAP device were measured at Tsinghua University but were occasionally monitored during usage in case a mistake occurred.
The CAP treatment.
To detect the bactericidal effect and optimal germicidal condition of the CAP device, the overnight-cultured S. aureus cells were harvested by centrifugation at 10,000 rpm for 1 min at 25°C. The pelleted cells were suspended in fresh TSB, sterilized PBS, or sterilized NS. Samples were then treated using the CAP device for different exposure times (0 to 5 min). The treatment work was performed in an air-conditioned room with a relative air humidity of 40% ± 1% at 6°C. After treatment, the samples were kept at 25°C for 1 h, and the surviving bacteria were counted via the plate dilution method. Briefly, the treated samples were 10-fold diluted (approximately to 10−1, 10−2, 10−3, 10−4, 10−5, and 10−6). Then, 10 μl of each diluted suspension was plated in triplicate on TSB agar plates. After 16 h of incubation at 37°C, the bacterial colonies on the inoculated plates were counted, and the survival curves were drawn with GraphPad Prism 5 software.
To test the influence of pigment phenotypes on CAP treatment, the overnight-incubated S. aureus cells were harvested using centrifugation at 10,000 rpm for 1 min at 25°C, washed once and resuspended in NS, and then adjusted to 0.5 McFarland density (about 2 × 108 CFU/ml). The cell suspensions were then treated with CAP for different times (0.5 to 3 min) under the conditions described above. After that, the cell suspensions were held at 25°C for 1 h, and then the bacterial cells were counted using the plate dilution method as described above.
Detection of S. aureus carotenoids.
Equivalent volumes of S. aureus overnight cultures were collected, and the cells were harvested using centrifugation at 10,000 rpm for 1 min at 25°C. Then the pelleted cells were washed twice with sterilized water, resuspended in 200 μl of methanol, and heated for 3 min at 55°C. After being centrifuged for 1 min, the supernatant was transferred to a 96-well plate, and the OD462 value was read using a Bio-Tek microplate reader. All experiments were repeated three times.
Scanning electron microscopy (SEM).
The SEM analysis was performed to observe the cell surface integrity. In brief, the S. aureus samples treated with CAP for 5 min were dropped onto a clean cover glass, which was then soaked in 2.5% glutaraldehyde solution overnight to fix the bacterial cells. The fixed cells were washed twice with NS solution, followed by dehydration in gradient ethanol (30%, 50%, 70%, 90%, and 100%), 5 min for each step, and dehydration again in 100% ethanol for 5 min. Then, the cells were continuously treated with tert-butanol of different concentrations (30%, 50%, 70%, 90%, and 100%) for replacement of ethanol. After that, samples were air-dried at 4°C for no more than 30 min and sputter-coated with platinum (Pt) for 100 s prior to visualization. The samples were examined using an S-3400N II electron microscope (Hitachi, Japan). Bacterial cells without CAP treatment served as a negative control.
Transmission electron microscopy (TEM).
The TEM analysis was performed to detect the influence of CAP treatment on the internal cell structure. The S. aureus cells treated with CAP for 5 min were subjected to TEM analysis, and the untreated bacteria served as a control. All samples were prepared as previously described (42). Briefly, bacterial cells were collected using centrifugation at 10,000 rpm for 1 min and washed once with PBS. The pelleted cells were fixed with 2.5% glutaraldehyde solution overnight at 4°C and then washed three times with PBS (15 min for each). Next, the cells were further fixed with 1% OsO4 for 2 h and washed with PBS another three times. After that, the cells were dehydrated in gradient ethanol (50% × 1, 70% × 1, 90% × 1, and 100% × 2) and washed with absolute acetone twice (10 min for each). The dehydrated samples were embedded in a resin for 4 h at 25°C, followed by incubation at 65°C for 48 h to polymerize. Samples were sliced with an EM UC7 ultramicrotome device (Leica, Germany), stained with uranyl acetate for 20 min, and then stained with alkaline lead citrate for 10 min. Finally, the prepared samples were observed under a TECNAI 10 transmission electron microscope (Philips, The Netherlands).
Statistical analysis.
Data analysis was performed using GraphPad Prism 5 software. Unpaired two-tailed Student’s t test was used to compare samples between two groups, and one-way analysis of covariance (ANOVA) was used for testing multiple groups. Results are presented as the mean ± the standard deviation (SD), and a P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Key Biosafety Technology Research and Development Program of China (2017YFC1200404-4) and the National Natural Science Foundation of China (81672071). The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
We declare that we have no competing financial interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Reddy PN, Srirama K, Dirisala SV. 2017. An update on clinical burden, diagnostic tools, and therapeutic options of Staphylococcus aureus. Infect Dis 10:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabbagh P, Riahi SM, Gamble HR, Rostami A. 2019. The global and regional prevalence, burden, and risk factors for methicillin-resistant Staphylococcus aureus colonization in HIV-infected people: a systematic review and meta-analysis. Am J Infect Control 47:323–333. doi: 10.1016/j.ajic.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Raz A, Serrano A, Thaker M, Alston T, Fischetti VA. 2018. Lysostaphin lysibody leads to effective opsonization and killing of methicillin-resistant Staphylococcus aureus in a murine model. Antimicrob Agents Chemother 62:e01056-18. doi: 10.1128/AAC.01056-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lakhundi S, Zhang K. 2018. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev 31:e00020-18. doi: 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu Q, Peng H, Rao X. 2016. Molecular events for promotion of vancomycin resistance in vancomycin intermediate Staphylococcus aureus. Front Microbiol 7:1601. doi: 10.3389/fmicb.2016.01601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rammelkamp CH, Maxon T. 1942. Resistance of Staphylococcus aureus to the action of penicillin. Exp Biol Med 51:386–389. doi: 10.3181/00379727-51-13986. [DOI] [Google Scholar]
- 7.Lowy FD. 2003. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest 111:1265–1273. doi: 10.1172/JCI18535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eriksen KR. 1961. “Celbenin”-resistant Staphylococci. Ugeskrift Laeger 1:113–114. [PubMed] [Google Scholar]
- 9.Mir SA, Shah MA, Mir MM. 2016. Understanding the role of plasma technology in food industry. Food Bioprocess Technol 9:734–750. doi: 10.1007/s11947-016-1699-9. [DOI] [Google Scholar]
- 10.Mai-Prochnow A, Murphy AB, McLean KM, Kong MG, Ostrikov KK. 2014. Atmospheric pressure plasmas: infection control and bacterial responses. Int J Antimicrob Agents 43:508–517. doi: 10.1016/j.ijantimicag.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 11.Scholtz V, Pazlarova J, Souskova H, Khun J, Julak J. 2015. Nonthermal plasma: a tool for decontamination and disinfection. Biotechnol Adv 33:1108–1119. doi: 10.1016/j.biotechadv.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Pankaj SK, Wan Z, Keener KM. 2018. Effects of cold plasma on food quality: a review. Foods 7:E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohd Nasir N, Lee BK, Yap SS, Thong KL, Yap SL. 2016. Cold plasma inactivation of chronic wound bacteria. Arch Biochem Biophys 605:76–85. doi: 10.1016/j.abb.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 14.Dai X, Bazaka K, Richard DJ, Thompson ERW, Ostrikov KK. 2018. The emerging role of gas plasma in oncotherapy. Trends Biotechnol 36:1183–1198. doi: 10.1016/j.tibtech.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Han L, Patil S, Boehm D, Milosavljević V, Cullen PJ, Bourke P. 2016. Mechanisms of inactivation by high-voltage atmospheric cold plasma differ for Escherichia coli and Staphylococcus aureus. Appl Environ Microbiol 82:450–458. doi: 10.1128/AEM.02660-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laroussi M, Mendis DA, Rosenberg M. 2003. Plasma interaction with microbes. New J Phys 5:41. doi: 10.1088/1367-2630/5/1/341. [DOI] [Google Scholar]
- 17.Stoffels E, Sakiyama Y, Graves DB. 2008. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans Plasma Sci 36:1441–1457. doi: 10.1109/TPS.2008.2001084. [DOI] [Google Scholar]
- 18.Guo L, Xu R, Zhao Y, Liu D, Liu Z, Wang X, Chen H, Kong MG. 2018. Gas plasma pre-treatment increases antibiotic sensitivity and persister eradication in methicillin-resistant Staphylococcus aureus. Front Microbiol 9:537. doi: 10.3389/fmicb.2018.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z, Xu Z, Cheng C, Wei J, Lan Y, Ni G, Sun Q, Qian S, Zhang H, Xia W, Shen J, Meng Y, Chu PK. 2017. Bactericidal effects of plasma induced reactive species in dielectric barrier gas-liquid discharge. Plasma Chem Plasma Process 37:415–431. doi: 10.1007/s11090-017-9784-z. [DOI] [Google Scholar]
- 20.Xu Z, Cheng C, Shen J, Lan Y, Hu S, Han W, Chu PK. 2018. In vitro antimicrobial effects and mechanisms of direct current air-liquid discharge plasma on planktonic Staphylococcus aureus and Escherichia coli in liquids. Bioelectrochemistry 121:125–134. doi: 10.1016/j.bioelechem.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Alshraiedeh NH, Alkawareek MY, Gorman SP, Graham WG, Gilmore BF. 2013. Atmospheric pressure, nonthermal plasma inactivation of MS2 bacteriophage: effect of oxygen concentration on virucidal activity. J Appl Microbiol 115:1420–1426. doi: 10.1111/jam.12331. [DOI] [PubMed] [Google Scholar]
- 22.Chen F, Di H, Wang Y, Cao Q, Xu B, Zhang X, Yang N, Liu G, Yang CG, Xu Y, Jiang H, Lian F, Zhang N, Li J, Lan L. 2016. Small-molecule targeting of a diapophytoene desaturase inhibits S. aureus virulence. Nat Chem Biol 12:174–179. doi: 10.1038/nchembio.2003. [DOI] [PubMed] [Google Scholar]
- 23.Liu CI, Liu GY, Song Y, Yin F, Hensler ME, Jeng WY, Nizet V, Wang AH, Oldfield E. 2008. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science 319:1391–1394. doi: 10.1126/science.1153018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. 2005. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J Exp Med 202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu B, Wei Q, Mettetal MR, Han J, Rau L, Tie J, May RM, Pathe ET, Reddy ST, Sullivan L, Parker AE, Maul DH, Brennan AB, Mann EE. 2017. Surface micropattern reduces colonization and medical device-associated infections. J Med Microbiol 66:1692–1698. doi: 10.1099/jmm.0.000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leejae S, Hasap L, Voravuthikunchai SP. 2013. Inhibition of staphyloxanthin biosynthesis in Staphylococcus aureus by rhodomyrtone, a novel antibiotic candidate. J Med Microbiol 62:421–428. doi: 10.1099/jmm.0.047316-0. [DOI] [PubMed] [Google Scholar]
- 27.Lan L, Cheng A, Dunman PM, Missiakas D, He C. 2010. Golden pigment production and virulence gene expression are affected by metabolisms in Staphylococcus aureus. J Bacteriol 192:3068–3077. doi: 10.1128/JB.00928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pelz A, Wieland KP, Putzbach K, Hentschel P, Albert K, Götz F. 2005. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem 28:32493–32498. doi: 10.1074/jbc.M505070200. [DOI] [PubMed] [Google Scholar]
- 29.Pannu MK, Hudman DA, Sargentini NJ, Singh VK. 2019. Role of SigB and staphyloxanthin in radiation survival of Staphylococcus aureus. Curr Microbiol 76:70–77. doi: 10.1007/s00284-018-1586-x. [DOI] [PubMed] [Google Scholar]
- 30.Bourke P, Ziuzina D, Boehm D, Cullen PJ, Keener K. 2018. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol 36:615–626. doi: 10.1016/j.tibtech.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor N, Cahill O, Daniels S, Galvin S, Humphreys H. 2014. Cold atmospheric pressure plasma and decontamination. Can it contribute to preventing hospital-acquired infections? J Hosp Infect 88:59–65. doi: 10.1016/j.jhin.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 32.Liao X, Xiang Q, Liu D, Chen S, Ye X, Ding T. 2017. Lethal and sublethal effect of a dielectric barrier discharge atmospheric cold plasma on Staphylococcus aureus. J Food Prot 80:928–932. doi: 10.4315/0362-028X.JFP-16-499. [DOI] [PubMed] [Google Scholar]
- 33.Kelly-Wintenberg K, Montie TC, Brickman C, Roth JR, Carr AK, Sorge K, Wadsworth LC, Tsai PP. 1998. Room temperature sterilization of surfaces and fabrics with a one atmosphere uniform glow discharge plasma. J Ind Microbiol Biotechnol 20:69–74. doi: 10.1038/sj.jim.2900482. [DOI] [PubMed] [Google Scholar]
- 34.Maisch T, Shimizu T, Li YF, Heinlin J, Karrer S, Morfill G, Zimmermann JL. 2012. Decolonisation of MRSA, S. aureus and E. coli by cold-atmospheric plasma using a porcine skin model in vitro. PLoS One 7:e34610. doi: 10.1371/journal.pone.0034610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourke P, Ziuzina D, Han L, Cullen PJ, Gilmore BF. 2017. Microbiological interactions with cold plasma. J Appl Microbiol 123:308–324. doi: 10.1111/jam.13429. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher MJ, Vaze N, Gangoli S, Vasilets VN, Gutsol AF, Milovanova TN, Anandan S, Murasko DM, Fridman AA. 2007. Rapid inactivation of airborne bacteria using atmospheric pressure dielectric barrier grating discharge. IEEE Trans Plasma Sci 35:1501–1510. doi: 10.1109/TPS.2007.905209. [DOI] [Google Scholar]
- 37.Mendis DA, Rosenberg M, Azam F. 2000. A note on the possible electrostatic disruption of bacteria. IEEE Trans Plasma Sci 28:1304–1306. doi: 10.1109/27.893321. [DOI] [Google Scholar]
- 38.Arjunan KP, Sharma VK, Ptasinska S. 2015. Effects of atmospheric pressure plasmas on isolated and cellular DNA: a review. Int J Mol Sci 16:2971–3016. doi: 10.3390/ijms16022971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boehm D, Bourke P. 2018. Safety implications of plasma-induced effects in living cells: a review of in vitro and in vivo findings. Biol Chem 400:3–17. doi: 10.1515/hsz-2018-0222. [DOI] [PubMed] [Google Scholar]
- 40.Peng H, Hu Q, Shang W, Yuan J, Zhang X, Liu H, Zheng Y, Hu Z, Yang Y, Tan L, Li S, Hu X, Li M, Rao X. 2017. WalK(S221P), a naturally occurring mutation, confers vancomycin resistance in VISA strain XN108. J Antimicrob Chemother 72:1006–1013. doi: 10.1093/jac/dkw518. [DOI] [PubMed] [Google Scholar]
- 41.Wang X-X. 2009. Dielectric barrier discharge and its applications. High Voltage Eng 35:1–11. [Google Scholar]
- 42.Yuan W, Hu Q, Cheng H, Shang W, Liu N, Hua Z, Zhu J, Hu Z, Yuan J, Zhang X, Li S, Chen Z, Hu X, Fu J, Rao X. 2013. Cell wall thickening is associated with adaptive resistance to amikacin in methicillin-resistant Staphylococcus aureus clinical isolates. J Antimicrob Chemother 68:1089–1096. doi: 10.1093/jac/dks522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.