Introduction
Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease characterized by inflammatory nodules, abscesses, and fistulae. Severe disease is frequently treated with adalimumab or infliximab, but limited research and insurance coverage confine treatment options. Often, aggressive multifaceted therapeutic regimens fail in severe cases.
The Janus kinase (JAK) inhibitor, tofacitinib, acts through the JAK-STAT pathway, suppressing inflammatory cytokines implicated in HS pathogenesis, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α.1 JAK inhibitors may benefit HS patients who lack response to targeted biologic therapy. We report on 2 patients with a rare, recalcitrant, ulcerating HS phenotype who, despite not responding to numerous treatments, including biologics (Table I), exhibited prolonged response to a tofacitinib multimodal regimen.
Table I.
Baseline patient characteristics
| Patient 1 | Patient 2 | |
|---|---|---|
| Age | 26 | 24 |
| Gender | M | F |
| Employment status | On disability | On disability |
| Hurley stage | III | III |
| Anatomic region | Bilateral axillae, abdomen, bilateral inguinal folds | Inframammary, abdominal pannus, bilateral inguinal folds, perineum, buttocks, intergluteal cleft, posterior thighs |
| Appearance | >10 draining abscesses and ulcerations | 10-20 ulcerations |
| Comorbidities | Anemia of chronic disease, depression, Obesity, paradoxical psoriasis | Anemia of chronic disease, diabetes mellitus type II, endometriosis, gastroesophageal reflux disorder, hypothyroidism, migraines, morbid obesity, osteoporosis, Polycystic ovarian syndrome, pyoderma gangrenosum |
| HS maintenance regimen | Tofactinib 5 mg 2x daily; amoxicillin 500 mg 2x daily; cyclosporine 300 mg (∼5 mg/kg) daily | Tofacitinib 5 mg 2× daily; 4 week antibiotic cycle of amoxicillin + amoxicllin-clavulanate ×3 weeks and azithromycin ×1 week; 1250 mg MMF 2× daily; triamcinolone ointment |
| Prior therapies | Adalimumab, infliximab, methotrexate, oral amoxicillin, oral amoxicillin-clavulanate, oral doxycycline, oral minocycline, acitretin | Adalimumab, etanercept, infliximab, ustekinumab, anakinra, azathioprine, cyclosporine, methotrexate, oral steroids, tacrolimus, oral contraceptive pills |
Case 1
A 26-year-old man with a 10-year HS history and resultant microcytic anemia requiring blood transfusion reported a sedentary, restricted lifestyle secondary to HS-related pain. Wound cultures 8 days before starting tofacitinib showed mixed bacterial flora, coagulase-positive Staphylococcus aureus, β-hemolytic Streptococcus group B, and rare Pseudomonas aeruginosa growth. Treatment included infliximab, 400 mg (∼6 mg/kg) every 4 weeks, and amoxicillin, 500 mg twice daily. Infliximab was discontinued after inadequate response and a paradoxical psoriasiform reaction covering about 20% of his body. At tofacitinib initiation, his disease was Hurley stage III, with a very severe Hidradenitis Suppurativa Physician's Global Assessment (HS PGA) and more than 10 abscesses and large ulcerations with undermined borders on a base of excess granulation tissue in the axillae, abdomen, and inguinal folds (Table I, Fig 1). Tofacitinib, 5 mg twice a day, cyclosporine (∼5 mg/kg), and amoxicillin were initiated (Table I).
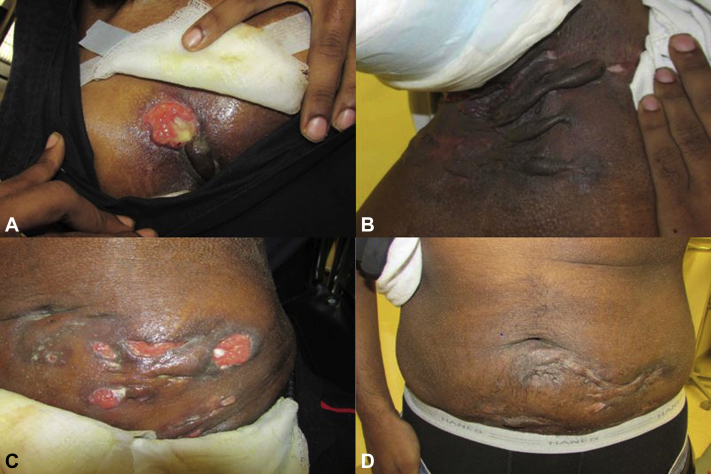
Fig 1.
Improvement in axillary and abdominal HS lesions in case 1 from baseline (A and C) to 11 months of treatment (B and D).
Erythrocyte sedimentation rate decreased from 84 to 50 two months into tofacitinib treatment. At 4 months, he had healing ulcerations and decreased drainage and pain. Cyclosporine was tapered at 8 months due to improvement. He continued tofacitinib and amoxicillin. At 11 months, he was pain and drainage free. Examination found inactive disease and nearly healed wounds (Fig 1). He reported improved quality of life, could shower, and sought employment. He experienced no adverse events, and at 12 months he self-discontinued tofacitinib given improvement. After a period of quiescence, modest disease activity returned, tofacitinib was re-initiated, and he is in remission again.
Case 2
A 23-year-old morbidly obese woman with a 6-year HS history and multiple HS-related hospitalizations reported HS that profoundly impacted her life: she received disability benefits and slept in a wheelchair because of pain. A previous infliximab regimen was discontinued because of transformation from a classic abscess phenotype to ulcerating phenotype. Treatment before tofacitinib included 1250 mg of mycophenolate mofetil (MMF) twice a day for 5 months, amoxicillin and amoxicillin-clavulanate, and triamcinolone ointment, offering minimal improvement. At tofacitinib initiation, her disease was Hurley stage III with minimal HS PGA but greater than 20 ulcerations on the neck, inframammary skin, abdomen, inguinal creases, and posterior thighs (Table I, Fig 2). These ulcerations were deep with varying degrees of granulation tissue. Tofacitinib, 5 mg twice a day, was added with MMF, topical steroids, and oral antibiotics (Table I).
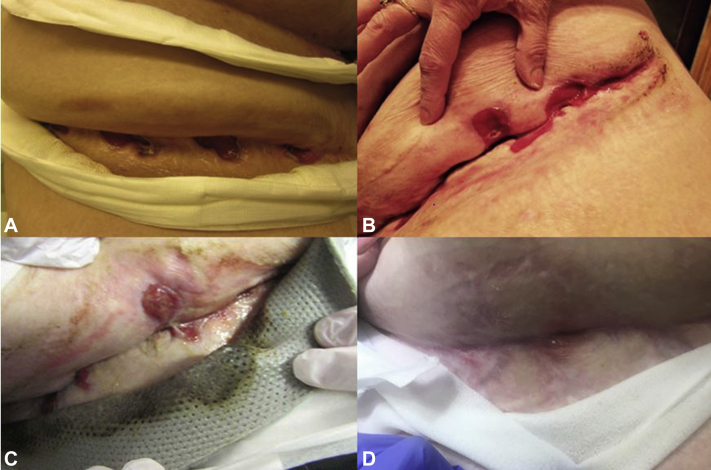
Fig 2.
Improvement in abdominal wounds in case 2 from (A) 2013, (B) June 2016, (C) January 2017, and (D) March 2019.
She experienced gradual improvement over 3 years with normalization of her anemia, leukocytosis, and thrombocytosis and avoided HS-related inpatient hospitalizations. Wound cultures 1 year into treatment found mixed skin flora. Her ulcerations markedly healed and flattened (Fig 2). Complex psychosocial factors complicated discontinuation of any therapies listed in Table I, but at a recent visit, tapering of MMF was suggested. She is now sleeping in bed and ambulating more frequently. No tofacitinib adverse events were experienced until localized herpes zoster development 3 years into treatment. This event required inpatient admission with a 3-day intravenous valacyclovir course due to the patient's immunosuppressive regimen, which was continued throughout hospitalization to prevent disease relapse.
Discussion
Severe HS often necessitates creative management strategies. Our patients' adverse reactions to biologics suggested immunologic volatility, warranting other therapies. Their phenotypes extend beyond the traditional HS phenotype of abscesses and tunneling, exhibiting propensity toward ulceration and pathergy. Rheumatoid arthritis (RA) reports describe paradoxical pyoderma gangrenosum (PG) during anti-TNF therapy similar to the transformation in case 2.2 In both cases, the ulcerations observed were likely a variant HS presentation rather than true PG. Although the patient in case 1 did not have biopsies performed, the patient in case 2 had several biopsies indicating HS before starting tofacitinib. Successful PG treatment reports with tofacitinib may relate to the improvement seen.3,4
Tofacitinib is a small molecule inhibitor of JAK proteins involved in the pathogenesis of several inflammatory conditions.1 Its use is documented in the RA literature but is previously undescribed in HS. Tofacitinib blocks IL-6 signaling through the JAK-STAT pathway. IL-6, IL-1β, and TNF-α are implicated in an inflammatory feedback loop involving STAT3 in RA development.1 These are also implicated in HS.5 We hypothesize tofacitinib's modulation of STAT3 dampens immune responses in HS.
Immunosuppression risk was weighed against the risk inherent to our patients' inflammatory states and sedentary lifestyles. Aggressive intervention was pursued to avoid further decompensation. Tofacitinib with cyclosporine or MMF was initiated with antibiotics for long-term management. Cyclosporine and MMF served as bridge therapy because of tofacitinib's slow onset of action and the patients' severe disease. This combination was partially reinforced by observations in alopecia areata in which tofacitinib nonresponders treated with pulsed prednisone demonstrated stronger responses.6 To our knowledge, no published data on MMF's efficacy in HS exists; limited data suggest cyclosporine in HS may be effective.7
Consideration was given to the safety profile of combined immunosuppressants—no data on tofacitinib and cyclosporine were found. High-intensity tofacitinib with MMF in renal transplant patients shows increased serious infection risk compared with that of cyclosporine and MMF-treated controls.8 Varicella zoster reactivation is a concern when using JAK inhibitors.9 We forewent vaccination given our patients' baseline severities and immunosuppression. Both patients had recommended annual tuberculosis screening.10 Increased immunosuppression risk necessitated increased laboratory monitoring. The patient in case 1 had a complete blood count and comprehensive metabolic panel monthly the first 3 months, then every 4 to 6 months. The patient in case 2 had a complete blood count and comprehensive metabolic panel monthly for 2.5 years followed by every 3 months.
The improved clinical status and quality of life in our patients shows tofacitinib's potential value in HS, especially in treatment resistance. Obtaining tofacitinib coverage proved difficult; both relied on Pfizer's assistance program. Further investigations on JAK inhibitors in HS may open therapeutic avenues, which are critical given the striking morbidity and limited therapies of HS.
Footnotes
Funding sources: None.
Conflicts of interest: Dr Alexa B. Kimball serves as a consultant and investigator for Novartis, Abbvie, UCB, Janssen, and Pfizer. Her fellowship program receives funding from Janssen and Abbvie. Dr Martina Porter serves as a consultant to AbbVie and Novartis. Dr Kelsey Flood has received fellowship funding from AbbVie and Janssen that went directly to her institution. Dr Monica Rosales Santillan has received fellowship funding from AbbVie and Janssen that went directly to her institution. Mr Savage and Dr Charrow have no conflicts to disclose.
References
- 1.Mori T., Miyamoto T., Yoshida H. IL-1β and TNFα-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol. 2011;23(11):701–712. doi: 10.1093/intimm/dxr077. [DOI] [PubMed] [Google Scholar]
- 2.Vandevyvere K., Luyten F.P., Verschueren P., Lories R., Segaert S., Westhovens R. Pyoderma gangrenosum developing during therapy with TNF-alpha antagonists in a patient with rheumatoid arthritis. Clin Rheumatol. 2007;26(12):2205–2206. doi: 10.1007/s10067-007-0733-8. [DOI] [PubMed] [Google Scholar]
- 3.Kochar B., Herfarth N., Mamie C., Navarini A.A., Scharl M., Herfarth H.H. Tofacitinib for the treatment of pyoderma gangrenosum. Clin Gastroenterol Hepatol. 2019;17(5):991–993. doi: 10.1016/j.cgh.2018.10.047. [DOI] [PubMed] [Google Scholar]
- 4.Gregory M.H., Ciorba M.A., Deepak P., Christophi G.P. Successful treatment of pyoderma gangrenosum with concomitant tofacitinib and infliximab. Inflamm Bowel Dis. 2019;25(7):e87–e88. doi: 10.1093/ibd/izz015. [DOI] [PubMed] [Google Scholar]
- 5.Witte-Händel E., Wolk K., Tsaousi A. The IL-1 pathway is hyperactive in Hidradenitis suppurativa and contributes to skin infiltration and destruction. J Invest Dermatol. 2019;139(6):1294–1305. doi: 10.1016/j.jid.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Liu L.Y., Craiglow B.G., Dai F., King B.A. Tofacitinib for the treatment of severe alopecia areata and variants: a study of 90 patients. J Am Acad Dermatol. 2017;76(1):22–28. doi: 10.1016/j.jaad.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi L., Hansel K., Stingeni L. Recalcitrant severe hidradenitis suppurativa successfully treated with cyclosporine A. J Am Acad Dermatol. 2012;67(6):e278–e279. doi: 10.1016/j.jaad.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Vincenti F., Tedesco Silva H., Busque S. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant. 2012;12(9):2446–2456. doi: 10.1111/j.1600-6143.2012.04127.x. [DOI] [PubMed] [Google Scholar]
- 9.Winthrop K.L., Yamanaka H., Valdez H. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66(10):2675–2684. doi: 10.1002/art.38745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.FDA . 2018. XELJANZ (tofacitinib) [Package Insert] p. 52. [Google Scholar]