Abstract
Significant advances in the potency and tolerability of antiretroviral therapy (ART) have led to very high rates of virologic success for most who remain adherent to therapy. As a result, the life expectancy of people living with HIV (PLWH) has increased significantly. PLWH do, however, continue to experience a significantly higher risk of noninfectious comorbidities and chronic age-related complications, including cardiovascular disease and malignancies, which are now the biggest drivers of this excess morbidity and mortality. Therefore, in addition to virologic failure, the management of the treatment-experienced patient increasingly requires optimization of ART to enhance tolerability, avoid drug–drug interactions, and mitigate non-AIDS complications and comorbid conditions. This article will present principles of the management of virologic failure, poor immunologic recovery, and strategies for optimizing ART in the setting of virologic suppression.
Keywords: adherence, comorbidities, HIV, virologic failure
Introduction
Modern antiretroviral regimens achieve a very high level of virologic success, and rates of virologic failure have declined significantly over the past decade. As a result, the life expectancy of people living with human immunodeficiency virus (HIV) (PLWH) has increased significantly in the modern antiretroviral therapy (ART) era, and the survival gap between PLWH and the general population has decreased significantly.1 The remaining excess morbidity and mortality are now driven mostly by noninfectious comorbidities, including cardiovascular and metabolic complications, liver disease, including chronic hepatitis C, and non-AIDS malignancies.2 These occur at much higher rates in people living with HIV than in the general population, and their pathogenesis likely involves patient factors (sociodemographic and behavioral), viral factors [HIV-associated chronic inflammation and immune activation, as well as likely viral copathogens such as hepatitis C virus (HCV) and cytomegalovirus (CMV)] and treatment factors (potential toxicities from antiretroviral drugs and other concomitant medications).
Given these trends, the management of treatment-experienced patients has shifted from a focus on the management of virologic failure in patients receiving ART towards more focus on optimization of ART to enhance tolerability and avoid drug–drug interactions, and identification of potential non-AIDS complications and comorbid conditions that might require modification of antiretroviral regimens in the setting of virologic suppression.
In this article, we will first review the management of virologic failure and poor immunologic recovery on ART. Then, we will discuss the rationale, general principles, and recommended strategies for optimization of ART in the setting of virologic suppression.
Management of patients with virologic failure
The risk of virologic failure has declined significantly in the past decade. This is likely due to a combination of more potent antiretroviral regimens and ease of their administration with multiple single-tablet regimen (STR) options.
We propose the following steps in the assessment and management of patients with virologic failure:
define the problem: operational definition of virologic failure;
analyze the cause(s), consider patient and viral factors;
establish goals;
determine strategy.
Definition of virologic failure
The goal of ART is to achieve and maintain HIV-1 plasma RNA levels below lower limits of detection (LLOD) with the assays currently used in clinical settings (between 20 and 50 copies/ml).3 Virologic suppression below these levels prevents drug resistance emergence,4 and is strongly predictive of lower rates of progression to acquired immunodeficiency syndrome (AIDS) and death.5 Common terminology and definitions used in defining virologic suppression and failure in HIV treatment guidelines and literature are shown in Table 1.
Table 1.
Virologic response definitions.
| Virologic response definitions (adapted from DHHS HIV guidelines) |
|---|
|
Virologic suppression: Confirmed HIV-1 RNA level below
LLOD of available clinical
assays. Virologic failure: Failure to achieve or maintain HIV-1 RNA level <200 copies/ml. Incomplete virologic response: Two consecutive HIV-1 RNA levels ⩾200 copies/ml after 24 weeks on an ART regimen in a patient who has not yet achieved virologic suppression. Virologic rebound: Confirmed HIV-1 RNA level ⩾200 copies/ml after virologic suppression. Virologic blip: After virologic suppression, an isolated detectable HIV-1 RNA level <200 copies/ml that is immediately followed by virologic suppression again. Low-level viremia: Confirmed (repeated) detectable HIV-1 RNA level <200 copies/ml. |
ART, antiretroviral therapy; HIV, human immunodeficiency virus; LLOD, Lower limit of detection.
The most important clinical question is what threshold of low-level viremia is predictive of worse clinical outcomes, whether future risk of virologic failure or development of AIDS or non-AIDS complications. Large retrospective HIV cohorts shed some light on this question. Virologic blips, defined as isolated detectable viremia between 20 and 200 copies/ml followed by a return to viral suppression, have not been demonstrated to predict subsequent virologic failure.6 Conversely, multiple recent studies show that persistent low-level viremia, less than 500 copies/ml, particularly in the range between 200 and 500 copies/ml, is strongly predictive of development of AIDS event, death, or subsequent virologic failure, but a low-level viremia of <200 copies/ml is not strongly predictive of worse outcomes.7–9 Based on this data, the United States Department of Health and Human Services (DHHS) guidelines adopted a definition of virologic failure as ‘confirmed’ viral load above 200 copies/ml, which should be confirmed in two consecutive assays.3 However, even patients with persistent detectable viremia below the 200 copies/ml threshold should be counseled on adherence and monitored closely, since this has been shown in some studies to be associated with future virologic failure.10,11
Analyzing the causes of virologic failure
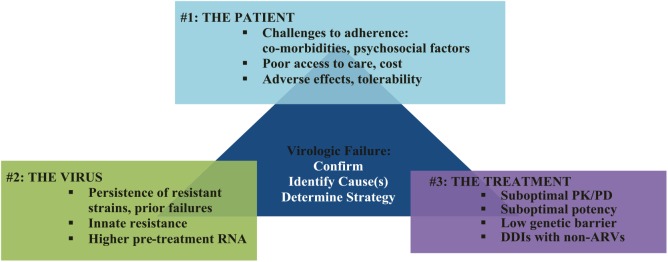
Virologic failure could be assessed as resulting from one or a combination of three groups of factors: patient factors; viral factors; and drug-related factors (Figure 1).
Figure 1.
Analyzing the causes of virologic failure.
ARV, antiretroviral; DDI, drug–drug interactions; PK/PD, pharmacokinetics/pharmacodynamics.
Patient factors: challenges to treatment adherence
The most common cause of virologic failure is suboptimal adherence to ART. Before considering other causes, a thorough investigation of barriers to ART adherence should be undertaken. Potential barriers may include comorbid mental health disorders or active substance abuse; psychosocial factors, such as housing instability, poor access to care, or issues related to drug adverse effects; tolerability; costs; pill burden; or dosing frequency. Evidence-based guidelines for improving retention in HIV care and antiretroviral adherence for PLWH are available,12 and specific strategies for optimizing ART adherence will be discussed in more detail below.
Viral factors: drug resistance
A second important cause of virologic failure is the development of drug resistance mutations. The HIV-1 reverse transcriptase (RT) is inherently error-prone, and lacks proof-reading in its activity, leading to high mutation rates and likelihood of evolution of drug-resistant viral strains if viral replication is ongoing.13 In newly acquired infections, there is also the possibility of transmitted drug resistance at the time of infection, which has been reported in up to 16% of treatment-naïve patients.3 In treatment-experienced patients, ongoing replication leading to drug resistance is more likely, due to either suboptimal drug exposure from poor adherence (see patient factors above) or failure of the ART regimen to achieve viral suppression despite good adherence (see treatment factors below). An example of the latter was seen in the SWITCHMRK 1 and 2 clinical trials, in which patients with a history of prior virologic failure were more likely to experience virologic failure when switched to a raltegravir (RAL)-based regimen than continuation of a boosted protease inhibitor (PI) regimen, likely from unmasking of pre-existing drug resistance.14
Risk factors associated with virologic failure due to drug resistance include higher pretreatment viral loads, and, in some cases, lower pretreatment CD4 counts, especially for certain less potent regimens.3 Innate resistance to certain ART classes based on viral tropism (e.g. CCR5 antagonists in patients with X4 or dual tropic virus) or HIV-2 coinfection may also explain virologic failure. In treatment-experienced patients, inadequate accounting for prior ART history, resistance testing, or archived drug resistance mutations can lead to a switch to a regimen that fails to suppress viral replication and leads to more resistance.
Treatment (drug-related) factors
The final cause of virologic failure are drug-related or pharmacologic factors. Often, this category of failure is caused by suboptimal pharmacokinetics of the ART regimen related to drug–drug interactions or drug–food interactions that prevent adequate serum concentrations of the antiretroviral agent (See resources in Table 2). Genetic polymorphisms have been shown to affect antiretroviral drug metabolism (e.g. cytochrome P450 isoenzymes) or drug transport (e.g. P-glycoproteins), leading to altered drug concentrations that compromise ART efficacy and safety.15 Examples of how to assess and optimize these drug-related factors are discussed in detail below (see Table 2 for additional HIV pharmacologic resources). Medical conditions, such as chronic diarrhea or intestinal malabsorption, can also impact treatment efficacy. Errors in medication prescribing or administration, especially during transitions of care, can jeopardize virologic suppression. Finally, certain antiretroviral medications may require fewer mutations before resistance can occur, often referred to as a ‘low genetic barrier to resistance,’ which might jeopardize future treatment options.16 Examples of such medications include lamivudine (3TC), emtricitabine (FTC), raltegavir (RAL), Elvitegravir (EVG), and efavirenz (EFV). Fortunately, the development of well-tolerated, single-tablet or once-daily, ART regimens, based mostly on integrase inhibitors (INSTI) with a higher genetic barrier to resistance can help mitigate some of these treatment-related factors contributing to virologic failure.17
Table 2.
HIV drug interaction resources.
| General | Disease specific |
|---|---|
| Micromedex (version 2.0) (https://www.micromedexsolutions.com/home/dispatch) |
University of Liverpool (https://www.hiv-druginteractions.org/) |
| Epocrates (https://www.epocrates.com/) |
HIV In Site (UCSF) (http://hivinsite.ucsf.edu/) |
|
Clinical Pharmacology
https://www.clinicalpharmacology.com/ Medscape Drug Reference https://reference.medscape.com/ Facts and Comparisons 4.0/Lexicomp https://fco.factsandcomparisons.com/lco/action/home https://online.lexi.com/lco/action/login |
Toronto HIV Clinic (https://hivclinic.ca/) |
HIV, human immunodeficiency virus.
Establishing the goals of antiretroviral therapy in a patient with virologic failure
Once the factors underlying virologic failure have been investigated, the provider and patient must establish the goals of ART moving forward. In the modern ART era, the vast majority of patients will achieve virologic suppression if adherent to their medications. The current guidelines recommend that, in designing a new ART regimen for a patient with virologic failure, the goal is to include at least two, and preferably three, medications predicted to be fully active based on prior ART history, resistance testing, and mechanisms of action.3 In some cases, drugs with partial activity against the patient’s virus, such as nucleoside RT inhibitors (NRTI) or PI, may be retained in the regimen in order to provide immunologic and virologic benefits associated with maintaining a viral population that has reduced replicative capacity.18 However, there will be rare patients who are not able to achieve maximal virologic suppression with currently available agents due to toxicities or acquired resistance to most available drugs. In these patients, the goals are to prevent clinical progression of HIV disease, preserve immunologic function, and minimize development of further resistance that could compromise future ART.3 Cohort studies in this patient population with multidrug ART resistance demonstrate that even modest reductions in HIV RNA levels may translate into meaningful clinical benefits,19,20 although at the risk of potential further resistance.
Determining the antiretroviral treatment strategy moving forward
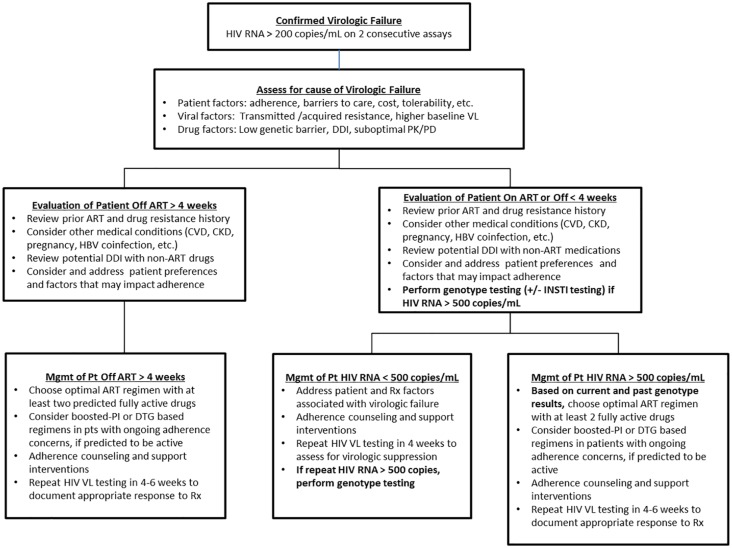
The final step in addressing virologic failure is to identify the best antiretroviral treatment strategy moving forward. The factors considered in choosing a future ART regimen include assessment of adherence as above, consideration of prior ART treatment history, and, importantly, HIV drug-resistance testing results at present and in the past. Another important factor is the patient’s hepatitis B virus (HBV) coinfection status because drugs active against HBV must be continued in the new regimen to avoid HBV reactivation, which can result in fulminant hepatic failure.21 Figure 2 outlines a suggested management approach to selecting a new ART regimen.
Figure 2.
Suggested management approach to selecting a new ART regimen.
For more details, please refer to Department of Health and Human Services ART guidelines (https://aidsinfo.nih.gov/guidelines/) or European AIDS Clinical Society guidelines (http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html).
AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CKD, chronic kidney disease; CVD, cardiovascular disease; DDI, drug–drug interactions; DTG, Dolutegravir; HBV, hepatitis B virus; HIV, human immunodeficiency virus; INSTI, integrase inhibitors; Mgmt, management; PK/PD, pharmacokinetics/pharmacodynamics; Pt, patient; Rx, prescribed regimen; VL, viral load.
Performing HIV drug-resistance testing
All patients with virologic failure with HIV RNA levels >1000 copies/ml should have drug resistance testing performed, while resistance testing can be considered between 500 and 1000 copies/ml though it may be unsuccessful. Drug resistance ideally is performed while the patient is still on the failing ART regimen, or within 4 weeks of discontinuation of the regimen. Resistance testing done after a longer period off therapy may still provide useful information, but drug-resistant virus strains can decline rapidly below the LLOD as the wild-type virus returns as the dominant virus off ART.22 These archived drug-resistant strains can still re-emerge once ART is reintroduced.
Drug-resistance testing is divided into genotypic and phenotypic assays. Genotypic assays typically use Sanger sequencing to detect mutations in the RT, protease (PR), and integrase (IN) genes. IN resistance testing may be included in standard assays, or may require separate testing depending on the commercial assay used. Currently available commercial assays will generally detect mutations present in >10–20% of the circulating virus population. The International AIDS-USA society maintains an updated list of clinically significant resistance mutations in the RT, PR, and IN genes. Other resources, such as the online Stanford University HIV Drug Resistance Database (https://hivdb.stanford.edu/) provide guidance on how to interpret genotype resistance data. Phenotypic assays evaluate the ability of a patient’s virus to grow in different concentrations of ART drugs compared with a reference HIV strain. The drug concentration that inhibits viral replication by 50% (IC50) is calculated, and the ratio of IC50 for the patient and reference viruses is reported to give a relative fold increase in IC50 or fold resistance.
Genotypic assays are preferred in first- and second-line regimen failure when resistance profile is not expected to be complex due to more widespread availability, quicker turnaround time, lower cost, and generally clearer interpretation of results.
Although phenotypic assays are more costly and labor-intensive, they can augment the results of genotypic resistance testing in the setting of known or suspected complex or extensive genotypic drug resistance mutations in patients with multiple treatment failures. Randomized clinical trials have demonstrated a consistent clinical benefit to resistance testing over expert clinician judgment without resistance testing in patients with virologic failure,23,24 although the best scenario is resistance testing combined with expert physician interpretation.
Using HIV drug-resistance results
Failure to detect drug resistance in the context of virologic failure on ART almost always indicates poor adherence. The focus should be directed to identifying and mitigating the patient and treatment factors related to adherence discussed above. Any available efforts to reduce toxicity, simplify dosing, or address behavioral barriers to adherence should be pursued, which may require modifications in the ART regimen. If suboptimal adherence remains a concern, choosing a regimen with a higher barrier to resistance, such as one containing either a boosted PI or Dolutegravir (DTG), can reduce the likelihood of selection for drug resistance.
In patients with virologic failure with detected resistance, a change in the ART regimen will be required, with the goal of constructing a new regimen with at least two, and preferably three, fully active drugs based on the patient’s ART history and current and past drug-resistance testing.3 The addition of a single new active drug to a failing regimen is not recommended due to the risk of resistance development and loss of that agent as a part of future treatment regimens. Decisions regarding whether to continue or stop specific drugs should be based on resistance testing results, but some general principles apply. Drugs from the non-nucleoside reverse transcriptase inhibitor (NNRTI) class, and the early generation INSTI RAL and EVG, should generally be discontinued to avoid further resistance that may jeopardize future options in the same class. However, drugs from the NRTI class and boosted PIs may retain partial activity and could be continued. For example, in patients with the M184V mutation, continuation of 3TC or FTC has proven beneficial in partial virologic suppression, likely by selecting for drug-resistant strains that are less fit or replication competent than wild-type strains and are hypersusceptible to other agents such as tenofovir and zidovudine.25 Finally, some specific drugs such as DTG and boosted darunavir (DRV) may be started or continued in the presence of known or suspected INSTI or PI class resistance, but these agents should be given twice daily rather than daily.26,27
Strategy based on failure of initial ART regimen
For patients who develop virologic failure on their initial ART regimen, the nature of the failed regimen can provide some clues as to potentially effective salvage regimens.
Patients failing an initial regimen of NNRTI + NRTIs often develop resistance-associated mutations to the NNRTI class (such as K103N) and to 3TC/FTC (M184V). Recommended second-line regimens shown to be efficacious in clinical trials (such as the Second Line, EARNEST, and the DAWNING trials) include a boosted PI + NRTIs or an INSTI + NRTIs.28–31
Patients failing an initial regimen of boosted PI + NRTIs often have no drug resistance or only isolated M184V/I resistance. The most common reasons for virologic failure in this scenario are poor adherence, drug–drug interactions, or poor tolerability.3 If well-tolerated, the same regimen can be continued with concerted efforts to improve adherence and close virologic monitoring. However, if drug–drug interactions or poor tolerability are a concern, a change to a different boosted PI + two NRTIs or to an INSTI + two NRTIs is warranted.
Patients failing an initial regimen of INSTI + NRTIs often develop 3TC/FTC resistance, but resistance to the INSTI is more variable. Patients who fail RAL- or EVG-containing regimens may develop resistance to these drugs, but remain susceptible to DTG.32 Conversely, patients who fail DTG or bictegravir (BIC)-containing regimens rarely develop resistance to these drugs.33,34 Although there is a paucity of clinical trial evidence, the preferred second-line regimens in this scenario include either a boosted PI or DTG-based regimen.3 For those patients with INSTI resistance, the recommended DTG dose is 50 mg twice daily.
Strategy based on failure of multiple ART regimens
For patients who have failed multiple ART regimens, the construction of a new ART regimen should be done in consultation with an expert in managing treatment-experienced patients.3 In general, the anchor of the ART regimen, if available, should include either a fully active INSTI, such as DTG, or a fully active boosted PI, such as DRV, paired with two NRTIs, at least one of which is fully active. If no active NRTI exists, then an INSTI plus a boosted PI regimen can be considered. In some of these cases, additional drugs could include the NNRTI etravirine, the fusion inhibitor T-20, or a CCR5 antagonist if an assay confirms CCR5 viral tropism. Careful attention to drug–drug interactions and appropriate dosing of agents is paramount as the complexity of ART regimens increases. The monoclonal antibody ibalizumab is a recently approved CD4 postattachment inhibitor that can be considered in heavily treatment-experienced patients without other options, and is administered by intravenous infusions every 2 weeks.35 Finally, the first-in-class, investigational gp120 attachment inhibitor fostemsavir has phase III efficacy and safety data at 48 weeks in heavily treatment-experienced patients, and could be a treatment option if it receives regulatory approval.36 Such patients should also be considered for inclusion into trials of other new and investigational agents.
Management of patients with poor CD4 count recovery
Most patients who achieve sustained virologic suppression with ART will experience a steady increase in peripheral blood CD4 cell recovery, usually into the normal range (>500 cells/mm3). However, about 15–20% of patients who start at very low CD4 counts (<200 cells/mm3) will fail to have the desired immunologic recovery but will plateau at a lower CD4 count, usually defined as either <200 or <350 cells/mm3.37,38 The major risk factors for poor CD4 cell recovery include older age, lower nadir CD4 count, and lower CD4 count at ART initiation.39,40 Conversely, earlier ART initiation can enhance maximal CD4 cell recovery.41
Unfortunately, those who have poor CD4 recovery, sometimes called ‘immunologic nonresponders,’ have increased risk of mortality and morbidity. For example, Engsig and colleagues found a 2.6-fold increased mortality in those whose CD4 counts remained <200 cells/mm3 despite at least 3 years of suppressive ART, compared with their counterparts with immunologic recovery.40 PLWH with poor immunologic response despite virologic suppression have been found to be at increased risk for cardiovascular events, osteoporotic fractures, chronic liver disease-related mortality, and infection-related non-AIDS malignancies in observational studies.42–45 While these increased mortality and morbidity risks are most apparent at CD4 cell counts <500 cells/mm3, there is likely a mortality benefit to higher CD4 cell recovery across the full spectrum of normal values.46
Despite the strong prognostic importance of CD4 cell count recovery, no adjunctive therapies that increase CD4 cell recovery beyond what is achieved with suppressive ART have succeeded at mitigating these mortality and morbidity risks. ART intensification (adding additional ART drugs), or ART class switching in patients who already are virologically suppressed, do not improve outcomes and are not recommended.47,48 Two large randomized controlled trials of interleukin-2 adjunctive therapy demonstrated increased CD4 cell counts but no discernible clinical benefit to warrant its use.49 Additional clinical trials are ongoing to evaluate other immune-based therapies to increase CD4 cell counts (such as interleukin-7), but none are proven to be beneficial, and they should not be used outside a clinical trial setting.
Therefore, at present, clinicians’ approach to immunologic nonresponse should focus on prevention (early initiation of ART before significant CD4 decline); ruling out alternative causes of lymphopenia (drug toxicities, viral coinfections, malignancies); and mitigation of downstream consequences of immunosuppression. Fortunately, emerging consensus based on large observational studies also shows that primary prophylaxis against Pneumocystis jirovecii pneumonia can be safely stopped in patients with a CD4 cell count between 101 and 200 cells/mm3 who were virologically suppressed on ART.50
Optimizing antiretroviral therapy in setting of virologic suppression
Rationale for optimizing antiretroviral therapy in the setting of virologic suppression
Simplification of antiretroviral therapy to enhance adherence
Since 2006, the advent of STRs has allowed a significant simplification of ART regimens in most treatment-naïve and -experienced patients51; their use has increased significantly in recent years and has been associated with increased adherence and a trend toward lower rates of discontinuation.52,53 However, disadvantages of STRs may include lack of flexibility with dosing of individual components if adjustment is required due to renal function or drug–drug interactions.51 Additionally, as most STRs are available only as brand products, cost may be a limiting factor, which will be discussed below.
Novel strategies, including long-acting HIV parenteral drugs or implants, will likely soon further improve simplification of ART administration.54 Monthly injections of two long-acting agents Cabotegravir (an INSTI) and Rilpivirine (RPV; an INSTI) have been shown to be safe, effective, and well tolerated (despite a high rate of mild injection site reactions) in trials.55,56
Improvement of tolerability
Adverse effects are possible with all antiretroviral agents, and are one of the leading reasons for switching regimens. Newer ARVs are associated with fewer serious and intolerable adverse effects, as noted by low discontinuation rates in randomized clinical trials, but long-term or rare side effects in special populations will likely not be evident until years into clinical practice, requiring continued vigilance by the healthcare providers, patients, industry, and regulators. Examples of ARV switches to newer agents within the same class or to a different class of ARV for improved tolerability are presented below. As ART is now recommended in all PLWH and needs to be continued indefinitely, the major focus has shifted from common, short-term adverse effects, such as gastrointestinal upset, to increased attention on the mitigation of long-term effects such as renal, bone, and cardiovascular toxicities. DHHS guidelines include comprehensive tables of adverse effects and their recommended management.3
Prevention or mitigation of drug–drug interactions
ARV agents may interact with a number of medications, necessitating change in therapy to avoid toxicities or impact on the therapeutic response. Whether to alter the ARV or the non-ARV agent will often depend on clinical stability of the patient’s condition and available alternatives. Care should be taken to review potential interactions with non-ARVs when adding or switching to a new ARV. The interactions may occur during absorption, distribution, metabolism, or elimination, and should be considered carefully when readjusting ARV regimens.57
The following are examples of drug–drug interactions between ARVs and non-ARVs that should be considered:
Polyvalent cations (aluminum, magnesium and calcium containing drugs): they decrease INSTI exposure. It is therefore recommended to temporally space their administration from that of the INSTI. Avoid coadministration of magnesium/aluminum hydroxide-containing antacids with once-daily RAL.58
Direct-acting anticoagulants: exercise caution as their exposure can be increased by coadministration with EVG/cobicistat.
Anti-seizure medications: Carbamazepine and Phenytoin could decrease INSTI exposure; twice daily DTG can be used with Carbamazepine
Metformin: BIC and DTG administration blocks metformin excretion, increasing metformin exposure. Monitor for metformin adverse effects and when initiating metformin start at lower dose and titrate based on glycemic control.
Rifamycins: they decrease INSTI exposure. It is OK to use Rifabutin with DTG.
Steroids: PIs and EVG/c can increase their serum levels. This can occur even with inhaled formulations (inhaled Beclomethasone appears to be safe).
Proton pump inhibitors: they decrease exposure to Atazanavir (ATV) and RPV.
HMG CoA reductase inhibitors (statins): their metabolism can be impaired by PIs leading to significantly increased serum levels.
Accommodating food requirements
Several ARVs require coadministration with food for optimal absorption; it is therefore imperative to assess each patient for food insecurity when selecting a regimen. Examples include RPV and DRV: RPV exposure is lower in fasted state, requiring administration with a protein-rich meal or drink.59 DRV exposure is significantly increased when DRV/cobicistat is given with a high fat meal.60
Occasionally, patients are unable to take oral medications, requiring the use of enteral feeding tubes. This may be due to opportunistic infections (i.e. Candida esophagitis), or medical conditions such as small bowel obstruction, need for surgery, or dysphagia from a stroke. As most ARV are available as a tablet or a capsule, it is important to explore which drugs can be safely crushed (for example for administration with a feeding tube) and which have an available liquid formulation. The data in this area is limited but emerging.61–63 Providers should consult with their clinical pharmacists and the manufacturer, or perform a literature search for the most recent data. The University of Toronto also maintains a summary of data on their website, which may be a useful resource (https://hivclinic.ca/).
Pregnancy-related concerns
ART is recommended in all PLWH, including those planning a pregnancy or who are pregnant.
Certain agents should be avoided in PLWH who are pregnant or contemplating pregnancy due to potential fetal toxicity, or because of altered pharmacokinetics, especially in the second or third trimester, leading to subtherapeutic levels. Several commonly prescribed antiretrovirals have no safety data in pregnancy and should be avoided.
At this time, and pending additional data, the DHHS guidelines recommend against the use of DTG in females contemplating pregnancy, or those who are in the first trimester, due to the early signal of increased neural tube defects from a Botswana cohort.64 The current preferred regimens, if no contraindications exist, include abacavir/lamivudine or tenofovir disoproxil fumarate (TDF) with either lamivudine or emtricitabine as the backbone, with one of the following: twice daily RAL, DTG after the first trimester, ritonavir-boosted ATV, or twice daily boosted DRV.
Cost considerations
ARV cost remains a controversial and dynamic topic due to variability in medical coverage, insurance plans, or pharmacy benefits, which may include hidden rebates, discounts, or reimbursements. Often, such practices are confidential, and, therefore, difficult to evaluate or compare. Nevertheless, despite the generally high cost of ARVs, cost-effectiveness of HIV treatment has been well established.65,66 One mechanism for potential cost reduction is the introduction of generic medications that can drive down cost and expand access.66,67 This advantage might have to be balanced against the use of older drugs that are available in generic formulations but have higher rates of toxicity (such as EFV or TDF).
General principles for antiretroviral therapy optimization
Regardless of the reasons for antiretroviral therapy changes, it is important to remember that the primary objective for all patients remains maintenance of virologic suppression. Incorporating antiretrovirals in the new regimen that had been part of a previously failing regimen could potentially jeopardize the success of the new regimen, as ‘archived’ resistance mutations might resurface. It is therefore important to account for prior ART history, and results of prior resistance testing before any antiretroviral switch. In the absence of resistance testing, it is safe to assume resistance to EFV, 3TC, FTC, RAL, or EVG, if these agents were part of a previously failing regimen since they have a relatively low genetic barrier to resistance. In virologically suppressed patients with a history of multiple failures or prior regimens, proviral DNA genotypic testing may be useful.
Finally, in patients with HIV/HBV coinfection, it is important to maintain two HBV-active drugs in new regimen. The use of 3TC or FTC as sole HBV-active drug is NOT recommended, as HBV resistance to these will likely develop rapidly.68
Examples of recommended ARV switch strategies
Based on results of previously published trials, the following are examples of antiretroviral therapy changes that could achieve simplification, improve tolerability, address drug–drug or drug–food interactions or be optimal in special situations such as pregnancy are presented below.
Switches within the same ARV class:
EFV to RPV or doravirine (DOR): switching from EFV/FTC/TDF to RPV/FTC/ TDF might mitigate EFV intolerance, including neuropsychiatric side effects.69 The newly approved NNRTI DOR, either in the fixed drug combination of DOR/3TC/TDF, or paired with another NRTI backbone, can also avoid the neuropsychiatric side effects of EFV or food–drug interactions with RPV.70
TDF to TAF: switches from TDF/FTC to TAF/FTC with any third agent,71 or with RPV as third agent,72 have been associated with improved bone mineral density and markers of renal dysfunction.
ABC to TAF: this switch was associated with no difference in renal or bone safety profile.73 It could be considered in patients with high cardiovascular disease risk.
RAL to EVG/cobicistat. A twice-daily RAL was simplified to a once-daily EVG/cobicistat regimen.74 While a once-daily RAL option now does exist, one could consider that a switch from RAL to DTG or BIC might offer additional benefit of higher genetic barrier to resistance of the latter two INSTIs.
Switches to different ARV classes:
Boosted PI to INSTI (BIC, DTG, or EVG/c).75–77 Switch from PI to INSTI has been associated with improved GI tolerability and metabolic profiles, including lower lipid levels with switch to DTG in patients with high cardiovascular risk.75 However, recent concerns emerge that the switch might also be associated with increased weight.78
Boosted PI to RPV: this switch has been associated with improved lipid profiles.79
Switches from triple ARV to dual ARV therapy:
DTG/RPV: Switch to a coformulated DTG/RPV has been shown to be safe and effective.80 This can be considered in patients in whom NRTI exposure needs to be avoided (e.g. with significant renal impairment and high cardiovascular risk rendering administration of TDF, TAF, and ABC difficult).
DTG/3TC: a switch to the two-drug combination DTG/3TC, a regimen shown to be effective as initial therapy,81 was also recently shown to be non-inferior to continuing a TAF-based three-drug regimen in maintaining virologic suppression in HIV-1-infected ART-experienced adults.82 This regimen may be considered for patients with significant metabolic comorbidities, such as cardiovascular, renal, or bone disease.
Boosted PI + 3TC: switch to LPV/ritonavir + 3TC,83 boosted ATV + 3TC,84 or boosted DRV + 3TC,85 have also been shown to be safe and effective. These could also be considered if there is a need to avoid NRTI exposure.
Discontinuation or interruption of antiretroviral therapy
The planned discontinuation or interruption of antiviral therapy is not recommended.3 The Strategies for Management of Antiretroviral Therapy (SMART) study showed CD4-guided interruption of ART is associated with increased risk of AIDS and non-AIDS complications.86
Short-term, unanticipated treatment interruptions due to acute medical issues or drug availability can occur, but should be avoided or minimized when possible. Even in these cases, antivirals should be resumed as soon as medically practical.3
Conclusion
As we have reviewed, the current era of modern ART has introduced both great advances in the care of PLWH, but also new challenges, with the increasing life expectancy and accumulation of comorbid medical conditions in this population. The overarching goal of ART is still to achieve and maintain virologic suppression in all patients, but simplification, especially with potent single tablet regimens, and optimization of ART to reduce toxicities and ensure long-term tolerability has become a more important focal point in ongoing HIV management. The prospects of long-acting injectable HIV ARVs soon to be available represent the next horizon in the maintenance of virologic suppression in PLWH. As has been the case since the advent of ART, experienced HIV clinicians, working in multidisciplinary, collaborative teams with clinical pharmacists and other healthcare workers, can significantly impact the survival and quality of life of their patients by expertly deploying these treatment strategies in a patient-centered approach.
Footnotes
Author contributions: Roger Bedimo was responsible for the conception and overall organization of the manuscript, and also wrote substantial portions of the manuscript. Tomasz Jodlowski and James Cutrell contributed substantial portions of the manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: this work was done as part of the corresponding author’s functions at the VA North Texas Health Care System (United States government employee). This study was funded by VA MERIT grant I01 CX000418.
Conflict of interest statement: Roger Bedimo has received research funding from ViiV Healthcare and Merck and Co. He has also served in ad hoc scientific advisory boards for ViiV Healthcare and Merck and Co.
Tomasz Jodlowski and James Cutrell declare no financial conflicts of interest.
Ethical statement: Our study did not require ethical board approval because it did not contain human or animal trials.
ORCID iD: Roger Bedimo  https://orcid.org/0000-0001-9794-0389
https://orcid.org/0000-0001-9794-0389
Contributor Information
James Cutrell, Department of Medicine, University of Texas Southwestern Medical Center, Dallas, USA.
Tomasz Jodlowski, Department of Pharmacy, VA North Texas Health Care System, Dallas, USA.
Roger Bedimo, Department of Medicine, VA North Texas Health Care System and the University of Texas Southwestern Medical Center, 4500 South Lancaster Road, 111-D, Dallas, TX 75216, USA.
References
- 1. Legarth RA, Ahlstrom MG, Kronborg G, et al. Long-term mortality in hiv-infected individuals 50 years or older: a nationwide, population-based cohort study. J Acquir Immune Defic Syndr 2016; 71: 213–218. [DOI] [PubMed] [Google Scholar]
- 2. Smit M, Brinkman K, Geerlings S, et al. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15: 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Department of Health and Human Services; Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. (accessed 1 May 2019). [Google Scholar]
- 4. Kieffer TL, Finucane MM, Nettles RE, et al. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J Infect Dis 2004; 189: 1452–1465. [DOI] [PubMed] [Google Scholar]
- 5. Thiebaut R, Morlat P, Jacqmin-Gadda H, et al. Clinical progression of HIV-1 infection according to the viral response during the first year of antiretroviral treatment. Groupe d’Epidemiologie du SIDA en Aquitaine (GECSA). AIDS 2000; 14: 971–978. [DOI] [PubMed] [Google Scholar]
- 6. Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA 2005; 293: 817–829. [DOI] [PubMed] [Google Scholar]
- 7. Antiretroviral Therapy Cohort Collaboration (ART-CC), Vandenhende MA, Ingle S, et al. Impact of low-level viremia on clinical and virological outcomes in treated HIV-1-infected patients. AIDS 2015; 29: 373–383. [DOI] [PubMed] [Google Scholar]
- 8. Bernal E, Gomez JM, Jarrin I, et al. Low-level viremia is associated with clinical progression in HIV-infected patients receiving antiretroviral treatment. J Acquir Immune Defic Syndr 2018; 78: 329–337. [DOI] [PubMed] [Google Scholar]
- 9. Elvstam O, Medstrand P, Yilmaz A, et al. Virological failure and all-cause mortality in HIV-positive adults with low-level viremia during antiretroviral treatment. PLoS One 2017; 12: e0180761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joya C, Won SH, Schofield C, et al. Persistent low-level viremia while on antiretroviral therapy is an independent risk factor for virologic failure. Clin Infect Dis 2019; 69: 2145–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis 2018; 18: 188–197. [DOI] [PubMed] [Google Scholar]
- 12. Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an international association of physicians in AIDS care panel. Ann Intern Med 2012; 156: 817–833, W-284, W-285, W-286, W-287, W-288, W-289, W-290, W-291, W-292, W-293, W-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts JD, Bebenek K, Kunkel TA. The accuracy of reverse transcriptase from HIV-1. Science 1988; 242: 1171–1173. [DOI] [PubMed] [Google Scholar]
- 14. Eron JJ, Young B, Cooper DA, et al. Switch to a raltegravir-based regimen versus continuation of a lopinavir-ritonavir-based regimen in stable HIV-infected patients with suppressed viraemia (SWITCHMRK 1 and 2): two multicentre, double-blind, randomised controlled trials. Lancet 2010; 375: 396–407. [DOI] [PubMed] [Google Scholar]
- 15. Calcagno A, Cusato J, D’Avolio A, et al. Genetic polymorphisms affecting the pharmacokinetics of antiretroviral drugs. Clin Pharmacokinet 2017; 56: 355–369. [DOI] [PubMed] [Google Scholar]
- 16. Luber AD. Genetic barriers to resistance and impact on clinical response. MedGenMed 2005; 7: 69. [PMC free article] [PubMed] [Google Scholar]
- 17. Llibre JM, Pulido F, Garcia F, et al. Genetic barrier to resistance for dolutegravir. AIDS Rev 2015; 17: 56–64. [PubMed] [Google Scholar]
- 18. Deeks SG, Wrin T, Liegler T, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. N Engl J Med 2001; 344: 472–480. [DOI] [PubMed] [Google Scholar]
- 19. Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet 2004; 364: 51–62. [DOI] [PubMed] [Google Scholar]
- 20. Raffanti SP, Fusco JS, Sherrill BH, et al. Effect of persistent moderate viremia on disease progression during HIV therapy. J Acquir Immune Defic Syndr 2004; 37: 1147–1154. [DOI] [PubMed] [Google Scholar]
- 21. Dore GJ, Soriano V, Rockstroh J, et al. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 2010; 24: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Devereux HL, Youle M, Johnson MA, et al. Rapid decline in detectability of HIV-1 drug resistance mutations after stopping therapy. AIDS 1999; 13: F123–F127. [PubMed] [Google Scholar]
- 23. Durant J, Clevenbergh P, Halfon P, et al. Drug-resistance genotyping in HIV-1 therapy: the VIRADAPT randomised controlled trial. Lancet 1999; 353: 2195–2199. [DOI] [PubMed] [Google Scholar]
- 24. Cohen CJ, Hunt S, Sension M, et al. A randomized trial assessing the impact of phenotypic resistance testing on antiretroviral therapy. AIDS 2002; 16: 579–588. [DOI] [PubMed] [Google Scholar]
- 25. Campbell TB, Shulman NS, Johnson SC, et al. Antiviral activity of lamivudine in salvage therapy for multidrug-resistant HIV-1 infection. Clin Infect Dis 2005; 41: 236–242. [DOI] [PubMed] [Google Scholar]
- 26. TIVICAY (dolutegravir) [package insert]. Research Triangle Park, NC: ViiV Healthcare, 2013. [Google Scholar]
- 27. Prezista (darunavir) [package insert]. Beerse, Belgium: Janssen Pharmaceutica, N.V, 2006. [Google Scholar]
- 28. Group SLS, Boyd MA, Kumarasamy N, et al. Ritonavir-boosted lopinavir plus nucleoside or nucleotide reverse transcriptase inhibitors versus ritonavir-boosted lopinavir plus raltegravir for treatment of HIV-1 infection in adults with virological failure of a standard first-line ART regimen (SECOND-LINE): a randomised, open-label, non-inferiority study. Lancet 2013; 381: 2091–2099. [DOI] [PubMed] [Google Scholar]
- 29. Paton NI, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med 2014; 371: 234–247. [DOI] [PubMed] [Google Scholar]
- 30. Hakim JG, Thompson J, Kityo C, et al. Lopinavir plus nucleoside reverse-transcriptase inhibitors, lopinavir plus raltegravir, or lopinavir monotherapy for second-line treatment of HIV (EARNEST): 144-week follow-up results from a randomised controlled trial. Lancet Infect Dis 2018; 18: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19: 253–264. [DOI] [PubMed] [Google Scholar]
- 32. Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis 2014; 210: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orrell C, Hagins DP, Belonosova E, et al. Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): week 48 results from a randomised, open-label, non-inferiority, phase 3b study. Lancet HIV 2017; 4: e536–e546. [DOI] [PubMed] [Google Scholar]
- 34. Stellbrink HJ, Arribas JR, Stephens JL, et al. Co-formulated bictegravir, emtricitabine, and tenofovir alafenamide versus dolutegravir with emtricitabine and tenofovir alafenamide for initial treatment of HIV-1 infection: week 96 results from a randomised, double-blind, multicentre, phase 3, non-inferiority trial. Lancet HIV 2019; 6: e364–e372. [DOI] [PubMed] [Google Scholar]
- 35. Emu B, Fessel J, Schrader S, et al. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med 2018; 379: 645–654. [DOI] [PubMed] [Google Scholar]
- 36. Cahn P, Fink V, Patterson P. Fostemsavir: a new CD4 attachment inhibitor. Curr Opin HIV AIDS 2018; 13: 341–345. [DOI] [PubMed] [Google Scholar]
- 37. Kelley CF, Kitchen CM, Hunt PW, et al. Incomplete peripheral CD4+ cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009; 48: 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lok JJ, Bosch RJ, Benson CA, et al. Long-term increase in CD4+ T-cell counts during combination antiretroviral therapy for HIV-1 infection. AIDS 2010; 24: 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Florence E, Lundgren J, Dreezen C, et al. Factors associated with a reduced CD4 lymphocyte count response to HAART despite full viral suppression in the EuroSIDA study. HIV Med 2003; 4: 255–262. [DOI] [PubMed] [Google Scholar]
- 40. Engsig FN, Zangerle R, Katsarou O, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis 2014; 58: 1312–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med 2013; 368: 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lichtenstein KA, Armon C, Buchacz K, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis 2010; 51: 435–447. [DOI] [PubMed] [Google Scholar]
- 43. Yong MK, Elliott JH, Woolley IJ, et al. Low CD4 count is associated with an increased risk of fragility fracture in HIV-infected patients. J Acquir Immune Defic Syndr 2011; 57: 205–210. [DOI] [PubMed] [Google Scholar]
- 44. Weber R, Sabin CA, Friis-Moller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med 2006; 166: 1632–1641. [DOI] [PubMed] [Google Scholar]
- 45. Bedimo RJ, McGinnis KA, Dunlap M, et al. Incidence of non-AIDS-defining malignancies in HIV-infected versus noninfected patients in the HAART era: impact of immunosuppression. J Acquir Immune Defic Syndr 2009; 52: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Drechsler H, Zhang S, Holodniy M, et al. CD4 counts and mortality in virologically suppressed US veterans. J Int Assoc Provid AIDS Care 2014; 13: 120–126. [DOI] [PubMed] [Google Scholar]
- 47. Gandhi RT, Zheng L, Bosch RJ, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med 2010; 7: pii: e1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cuzin L, Trabelsi S, Delobel P, et al. Maraviroc intensification of stable antiviral therapy in HIV-1-infected patients with poor immune restoration: MARIMUNO-ANRS 145 study. J Acquir Immune Defic Syndr 2012; 61: 557–564. [DOI] [PubMed] [Google Scholar]
- 49. Group IES, Committee SS, Abrams D, et al. Interleukin-2 therapy in patients with HIV infection. N Engl J Med 2009; 361: 1548–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Opportunistic Infections Project Team of the Collaboration of Observational HIVERiE, Mocroft A, Reiss P, et al. Is it safe to discontinue primary Pneumocystis jiroveci pneumonia prophylaxis in patients with virologically suppressed HIV infection and a CD4 cell count <200 cells/microL? Clin Infect Dis 2010; 51: 611–619. [DOI] [PubMed] [Google Scholar]
- 51. Cutrell J, Bedimo R. Single-tablet regimens in the treatment of HIV-1 infection. Fed Pract 2016; 33: 24S–30S. [PMC free article] [PubMed] [Google Scholar]
- 52. Clay PG, Yuet WC, Moecklinghoff CH, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther 2018; 15: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nachega JB, Parienti JJ, Uthman OA, et al. Lower pill burden and once-daily antiretroviral treatment regimens for HIV infection: a meta-analysis of randomized controlled trials. Clin Infect Dis 2014; 58: 1297–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gulick RM, Flexner C. Long-acting HIV drugs for treatment and prevention. Annu Rev Med 2019; 70: 137–150. [DOI] [PubMed] [Google Scholar]
- 55. Swindells S, Andrade-Villanueva JF, Richmond G, et al. Long-acting cabotegravir + rilpivirine as maintenance therapy: atlas week 48 results. In: Conference on Retroviruses and Opportunistic Infections Seattle, WA, March 4–7, 2019. [Google Scholar]
- 56. Orkin C, Molina JM, Lombaard J, et al. Long-acting cabotegravir + rilpivirine for hiv maintenance: flair week 48 results. In: Conference on retroviruses and opportunistic infections, Seattle, WA, 4–7 March 2019. [Google Scholar]
- 57. Devanathan AS, Anderson DJC, Cottrell ML, et al. Contemporary drug-drug interactions in HIV treatment. Clin Pharmacol Ther 2019; 105: 1362–1377. [DOI] [PubMed] [Google Scholar]
- 58. Krishna R, East L, Larson P, et al. Effect of metal-cation antacids on the pharmacokinetics of 1200 mg raltegravir. J Pharm Pharmacol 2016; 68: 1359–1365. [DOI] [PubMed] [Google Scholar]
- 59. Edurant® (rilpivirine) [package insert]. Latina, Italy: Janssen-Cilag SpA, 2011. [Google Scholar]
- 60. PREZCOBIX® (darunavir and cobicistat) [package insert]. Gurabo, PR: Janssen Pharmaceuticals, Inc, 2015. [Google Scholar]
- 61. Huesgen E, DeSear KE, Egelund EF, et al. Practical considerations with crushing and enteral tube scenarios. Pharmacotherapy 2016; 36: 1145–1165. [DOI] [PubMed] [Google Scholar]
- 62. Brooks KM, Garrett KL, Kuriakose SS, et al. Decreased absorption of dolutegravir and tenofovir disoproxil fumarate, but not emtricitabine, in an HIV-infected patient following oral and jejunostomy-tube administration. Pharmacotherapy 2017; 37: e82–e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Chrdle A, Jerhotova Z, Vacik M, et al. Crushed dolutegravir/abacavir/lamivudine given via nasogastric tube in gastric outlet obstruction caused by cancer resulted in rapid viral load suppression. Int J STD AIDS 2019; 30: 94–98. [DOI] [PubMed] [Google Scholar]
- 64. Zash R, Makhema J, Shapiro RL. Neural-tube defects with dolutegravir treatment from the time of conception. N Engl J Med 2018; 379: 979–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Freedberg KA, Losina E, Weinstein MC, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 2001; 344: 824–831. [DOI] [PubMed] [Google Scholar]
- 66. Walensky RP, Sax PE, Nakamura YM, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med 2013; 158: 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Krentz HB, Campbell S, Lahl M, et al. De-simplifying single-tablet antiretroviral treatments: uptake, risks and cost savings. HIV Med 2019, 20:214–221. [DOI] [PubMed] [Google Scholar]
- 68. Jia HH, Li KW, Chen QY, et al. High prevalence of HBV lamivudine-resistant mutations in HBV/HIV co-infected patients on antiretroviral therapy in the area with the highest prevalence of HIV/HBV co-infection in China. Intervirology 2018; 61: 123–132. [DOI] [PubMed] [Google Scholar]
- 69. Mills AM, Cohen C, Dejesus E, et al. Efficacy and safety 48 weeks after switching from efavirenz to rilpivirine using emtricitabine/tenofovir disoproxil fumarate-based single-tablet regimens. HIV Clin Trials 2013; 14: 216–223. [DOI] [PubMed] [Google Scholar]
- 70. Thompson M, Orkin C, Molina JM, et al. Once-daily doravirine for initial treatment of adults living with HIV-1: an integrated safety analysis. Clin Infect Dis. Epub ahead of print 23 May 2019. DOI: 10.1093/cid/ciz423. [DOI] [PubMed] [Google Scholar]
- 71. Gallant JE, Daar ES, Raffi F, et al. Efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate given as fixed-dose combinations containing emtricitabine as backbones for treatment of HIV-1 infection in virologically suppressed adults: a randomised, double-blind, active-controlled phase 3 trial. Lancet HIV 2016; 3: e158–e165. [DOI] [PubMed] [Google Scholar]
- 72. Hagins D, Orkin C, Daar ES, et al. Switching to coformulated rilpivirine (RPV), emtricitabine (FTC) and tenofovir alafenamide from either RPV, FTC and tenofovir disoproxil fumarate (TDF) or efavirenz, FTC and TDF: 96-week results from two randomized clinical trials. HIV Med 2018; 19: 724–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Winston A, Post FA, DeJesus E, et al. Tenofovir alafenamide plus emtricitabine versus abacavir plus lamivudine for treatment of virologically suppressed HIV-1-infected adults: a randomised, double-blind, active-controlled, non-inferiority phase 3 trial. Lancet HIV 2018; 5: e162–e171. [DOI] [PubMed] [Google Scholar]
- 74. Mills A, Crofoot G, Ortiz R, et al. Switching from twice-daily raltegravir plus tenofovir disoproxil fumarate/emtricitabine to once-daily elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate in virologically suppressed, HIV-1-infected subjects: 48 weeks data. HIV Clin Trials 2014; 15: 51–56. [DOI] [PubMed] [Google Scholar]
- 75. Gatell JM, Assoumou L, Moyle G, et al. Switching from a ritonavir-boosted protease inhibitor to a dolutegravir-based regimen for maintenance of HIV viral suppression in patients with high cardiovascular risk. AIDS 2017; 31: 2503–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Daar ES, DeJesus E, Ruane P, et al. Efficacy and safety of switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from boosted protease inhibitor-based regimens in virologically suppressed adults with HIV-1: 48 week results of a randomised, open-label, multicentre, phase 3, non-inferiority trial. Lancet HIV 2018; 5: e347–e356. [DOI] [PubMed] [Google Scholar]
- 77. Arribas JR, DeJesus E, van Lunzen J, et al. Simplification to single-tablet regimen of elvitegravir, cobicistat, emtricitabine, tenofovir DF from multi-tablet ritonavir-boosted protease inhibitor plus coformulated emtricitabine and tenofovir DF regimens: week 96 results of STRATEGY-PI. HIV Clin Trials 2017; 18: 118–125. [DOI] [PubMed] [Google Scholar]
- 78. Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad 2019; 5: 41–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Palella FJ, Jr, Fisher M, Tebas P, et al. Simplification to rilpivirine/emtricitabine/tenofovir disoproxil fumarate from ritonavir-boosted protease inhibitor antiretroviral therapy in a randomized trial of HIV-1 RNA-suppressed participants. AIDS 2014; 28: 335–344. [DOI] [PubMed] [Google Scholar]
- 80. Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391: 839–849. [DOI] [PubMed] [Google Scholar]
- 81. Nyaku AN, Zheng L, Gulick RM, et al. Dolutegravir plus lamivudine for initial treatment of HIV-1-infected participants with HIV-1 RNA <500 000 copies/mL: week 48 outcomes from ACTG 5353. J Antimicrob Chemother 2019; 74: 1376–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van Wyk J, Ajana F, Bissop F, et al. Switching to DTG+3TC fixed dose combination (FDC) is non-inferior to continuing a TAF-based regimen (TBR) in maintaining virologic suppression through 24 weeks (TANGO Study). In: International AIDS Society Mexico City, 21–24 July 2019. [Google Scholar]
- 83. Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, non-inferiority trial. Lancet Infect Dis 2015; 15: 785–792. [DOI] [PubMed] [Google Scholar]
- 84. Fabbiani M, Gagliardini R, Ciccarelli N, et al. Atazanavir/ritonavir with lamivudine as maintenance therapy in virologically suppressed HIV-infected patients: 96 week outcomes of a randomized trial. J Antimicrob Chemother 2018; 73: 1955–1964. [DOI] [PubMed] [Google Scholar]
- 85. Pulido F, Ribera E, Lagarde M, et al. Dual therapy with darunavir and ritonavir plus lamivudine vs triple therapy with darunavir and ritonavir plus tenofovir disoproxil fumarate and emtricitabine or abacavir and lamivudine for maintenance of human immunodeficiency virus type 1 viral suppression: randomized, open-label, noninferiority DUAL-GESIDA 8014-RIS-EST45 trial. Clin Infect Dis 2017; 65: 2112–2118. [DOI] [PubMed] [Google Scholar]
- 86. Strategies for Management of Antiretroviral Therapy Study Group, El-Sadr WM, Lundgren J, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 2006; 355: 2283–2296. [DOI] [PubMed] [Google Scholar]