Abstract
Hypertension is one of the most common chronic diseases as well as the leading risk factor for cardiovascular disease (CVD). Efficient screening and accurate blood pressure (BP) monitoring are the basic methods of detection and management. However, with developments in electronic technology, BP measurement and monitoring are no longer limited to the physician’s office. Epidemiological and clinical studies have documented strong evidence for the efficacy of out-of-office BP monitoring in multiple fields for managing hypertension and CVD. This review discusses applications for out-of-office BP monitoring, including home blood pressure monitoring (HBPM) and ambulatory blood pressure monitoring (ABPM), based on recent epidemiological data and clinical studies regarding the following factors: the detection of abnormal BP phenotypes, namely, white coat hypertension and masked hypertension; stronger ability to determine the prognosis for target organ damage and mortality; better BP control; screening for hypotension; and unique approaches to identifying circadian BP patterns and BP variability.
Keywords: hypertension, blood pressure, home blood pressure monitoring, ambulatory blood pressure monitoring, white coat hypertension, masked hypertension
Introduction
Hypertension has long been the most common disease worldwide, with an estimated global prevalence of 1.13 billion people in 2015.1 In China, according to the latest data from a nationwide survey on hypertension from 2012 to 2015, the adult age-weighted prevalence of hypertension was 23.2%.2 The 2017 American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the prevention, detection, evaluation, and management of high blood pressure (BP) in adults revised the cutoff for diagnosing hypertension to ⩾130/80 mm Hg; this means that the prevalence of hypertension will increase to 46% in the USA,3 and to 46.4% in China, which is twice as high as that based on current Chinese guidelines.4 Studies show that control of high BP can result in significant risk reductions in cardiovascular morbidity and mortality.5 Office BP measurement (OBPM) has been the standard for diagnosing and managing hypertension; however, results may be confounded by several factors and are limited by time and location. To improve the rates of detection and control of high BP in the general population, guidelines from different countries and organizations have proposed several recommendations. These academic statements concur in their recommendations for using out-of-office BP monitoring in patients with hypertension or in high-risk populations.3,4,6–8 For example, the 2017 ACC/AHA guidelines included an IA (Class of Recommendation and Level of Evidence) recommendation for out-of-office BP measurement to confirm the diagnosis of hypertension and to titrate BP-lowering medications.3 The 2018 European Society of Cardiology/European Society of Hypertension guidelines for the management of arterial hypertension also recommended that out-of-office BP measurements should be used to confirm a diagnosis of hypertension,6 and the 2018 Chinese guidelines for the prevention and treatment of hypertension recommended that, if available, out-of-office BP monitoring should be implemented to diagnose white coat hypertension (WCH) and masked hypertension (MH), or to evaluate the effects of antihypertensive treatment.4
In addition to its use for diagnosing and managing hypertension, out-of-office BP may be practical in screening for hypotension, identifying circadian BP patterns and BP variability, and risk stratification for BP-related target organ damage and mortality. This review aims to summarize the current evidence for the applications of out-of-office BP monitoring, including home blood pressure monitoring (HBPM) and ambulatory blood pressure monitoring (ABPM), with regard to current studies on cardiovascular disease (CVD).
General characteristics of OBPM, HBPM, and ABPM
OBPM is measured by a nurse or doctor with a conventional calibrated mercury sphygmomanometer or an electronic device in the clinic. HBPM, also called self-BP monitoring, is performed by the patient or a family member in a familiar environment, providing at-home long-term BP values. ABPM offers abundant information on 24-h profiles including nocturnal BP and short-term BP variability. The general characteristics of OBPM, HBPM, and ABPM are listed in Table 1.
Table 1.
The general characteristics of OBPM, HBPM, and ABPM.
| Characteristics | OBPM | HBPM | ABPM |
|---|---|---|---|
| Popularity of device | Wider use in office | General use at home | Less use in office |
| Reproducibility | Poor | Better | Poor |
| Diagnostic threshold for hypertension (mmHg) | 140/90(130/80a) | 135/85(130/80a) | Mean day time 135/85(130/80a) Mean night time 120/70(110/65a) Mean 24-h 130/80(125/75a) |
| Detection of BP variability | Provide visit-to-visit BP variability | Provide day to day BP variability | Provide 24-h BP variability |
| Evaluation of nocturnal BP | Not applicable | Not applicable | Applicable |
| Evaluation of morning surge | Not applicable | Applicable | Applicable |
| White coat effect | Present | Absent | Absent |
The diagnostic threshold in America based on the 2017 ACC guideline.
ABPM, ambulatory blood pressure measurement; ACC, American College of Cardiology; BP, blood pressure; HBPM, home blood pressure measurement; OBPM, office blood pressure measurement.
Applications for out-of-office BP monitoring
Detecting WCH and MH
Combining OBPM and out-of-office BP readings, BP phenotypes can be divided into normotension, WCH, MH, and sustained hypertension. WCH is defined as an elevated in-office BP and normal out-of-office BP, while MH defines patients with normal in-office BP but with elevated out-of-office BP measurements (Figure 1).
Figure 1.

Classification of BP subtypes by combination of clinic office BP and out-of-office BP.
*The diagnostic threshold in the United States based on the 2017 ACC/AHA guideline.
ABPM, ambulatory blood pressure measurement; ACC/AHA, American College of Cardiology/American Heart Association; BP, blood pressure; HBPM, home blood pressure measurement.
Studies report that overall prevalence of WCH in the general population is 9–23%,9–13 and that WCH accounts for 30–40% of patients with elevated OBPM.6 WCH has long been considered a benign phenomenon because numerous epidemiological studies found no association between WCH and target organ damage.14,15 Although some observational studies and meta-analyses showed that WCH was associated with subclinical target organ damage, the cross-sectional design of these studies failed to prove causality.16,17 Recently, our large-scale meta-analysis found that, after multivariate adjustment, WCH was associated with an increased risk of CVD and all-cause mortality in individuals without antihypertensive treatment at baseline compared with normotensive counterparts. It is interesting that the risks of CVD and mortality are similar in patients with WCH who receive antihypertensive therapy (‘treated’) versus patients with normal BP both in- and out-of-office.18 These results provided robust evidence that WCH is not ‘innocent,’ and, on the contrary, adversely affects long-term prognosis. It should be noted that, in our study, the risk of CVD was consistently increased in patients with untreated WCH detected by ABPM or HBPM. A Spanish cohort study further confirmed that WCH is not a benign phenomenon. The study was a registry-based, multicenter cohort evaluating 63,910 adults with OBPM and 24-h ABPM data. After a median follow-up of 4.7 years, WCH was associated with a significantly increased risk of all-cause mortality [hazard ratio (HR): 1.79; 95% confidence interval (CI): 1.38–2.32] and cardiovascular mortality (HR: 1.96; 95% CI: 1.22–3.15) after multivariate adjustment.19 Similarly, the Ohasama study enrolled 1464 participants, and, with a mean follow-up of 17.1 years, concluded that partial WCH (either home or ambulatory normotension with office hypertension) was associated with a long-term risk of stroke.20 Most recently, an updated meta-analysis that included the Spanish ABPM cohort study also showed that untreated WCH was associated with a significantly increased risk of cardiovascular mortality (HR: 2.09; 95% CI: 1.23–4.48), all-cause mortality (HR: 1.33; 95% CI: 1.07–1.67), and CVD (HR: 1.36; 95% CI: 1.03–2.00).21
MH is defined as elevated out-of-office BP and normal office BP, and has been generally considered harmful and to require appropriate treatment. The prevalence of MH ranges from 6.7% to 20% in different reports.13,22,23 In the Spanish cohort study, after adjusting for multiple risk factors, MH defined by ABPM was strongly associated with all-cause mortality (HR: 2.83; 95% CI: 2.12–3.79) and cardiovascular mortality (HR: 2.85; 95% CI: 1.66–4.90).19 Another cohort study including 4261 Japanese patients also concluded that MH based on HBPM was associated with an increased risk of stroke (HR: 2.66; 95% CI: 1.15–6.13).24
It should be noted that in individuals with prehypertension defined according to office BP (120–139/80–89 mm Hg), a large proportion would be classified as MH if out-of-office BP monitoring was performed. The International Database on Ambulatory BP in relation to Cardiovascular Outcomes (IDACO) study revealed that, in people with prehypertension, 29.3% would be diagnosed as having MH if ABPM was performed. The Masked Hypertension Study also showed that 34.1% of participants defined as prehypertensive were confirmed to be MH under ABPM.25 In addition, the International Database of HOme BP in relation to Cardiovascular Outcome (IDHOCO) study revealed similar findings by HBPM, stating that MH accounted for 18.4% and 30.4% of patients with low-range (120–129/80–84 mm Hg) and high-range (130–139/85–89 mm Hg) prehypertension, respectively. Without out-of-office measurements, a large number of patients with MH could be misdiagnosed, which might result in inappropriate treatment. Our series studies showed that prehypertension is associated with an increased risk of all-cause mortality, CVD, and end-stage renal disease.26–32 However, whether this target organ damage is caused by undetected MH or prehypertension is unclear and requires further studies. Based on data from the studies cited previously, out-of-office BP measurement, either HBPM or ABPM, is highly recommended for the detection of WCH or MH in suspected individuals.
Prognostic predictive power of out-of-office BP for target organ damage and mortality
Given the limitations of OBPM, the prognostic predictive power for target organ damage of in-office BP may be lower than that of out-of-office BP. Because of rapid developments in electronic technology, numerous studies comparing the prognostic predictive power of out-of-office BP and office BP have been performed. The Finn-Home study showed that HBPM is strongly correlated with cardiovascular risk,33 and is a stronger predictor of left ventricular hypertrophy and atherosclerosis than office BP.34–36 The Spanish cohort study demonstrated that 24-h systolic BP was a better predictor of all-cause mortality (HR: 1.58 per 1-standard deviation increase in ABPM; 95% CI: 1.56–1.60, after adjusting for OBPM) than office systolic BP (HR: 1.02; 95% CI: 1.00–1.04, after adjusting for 24-h ambulatory BP).19 Other studies drew a similar conclusion, namely that HBPM or ABPM can better predict cardiovascular events or other target organ damage.37–46 Therefore, people who are at high risk, or have elevated BP, should consider out-of-office measurements, which are stronger predictors.
However, it should be noted that currently there are limited data to support the proposal of whether, in the general population, the use of out-of-office BP instead of office BP in CVD risk scoring systems can provide further incremental value for CVD prediction. Data from a Swedish cohort study showed that, although ambulatory systolic BP was an independent risk factor for CVD, addition to the Framingham Risk Score led to only small increases to the overall model fit, discrimination (a 1% increase in the area under the receiver-operating characteristic curve), calibration, and reclassification.47 Recently, another study also showed that using BP measurements obtained through ABPM or HBPM instead of OBPM may have little effect on CVD risk estimates obtained from the Framingham, QRISK2 (risk of developing a heart attack or stroke over the next 10 years), or SCORE (Systematic COronary Risk Evaluation) risk equations.48 Therefore, current cardiovascular risk assessment systems, including China-PAR (Prediction for Atherosclerotic CVD [ASCVD] Risk in China),49 European SCORE,50 ASCVD-PCE (Pooled Cohorts Equations) of the USA,51 the Q risk score (QRISK) model of Great Britain,52 and the Framingham Risk Score,53 include only clinic BP in the model. However, all of these cardiovascular risk assessment systems were developed in the general population. In patients with abnormal BP phenotypes, such as WCH or MH, current CVD risk assessment systems may over- or underestimate the risk. Further studies are needed to explore whether incorporation of out-of-office BP has incremental value for CVD prediction in people with abnormal BP phenotypes.
Better BP control
The Telemonitoring or Self-Monitoring of BP in Hypertension (TASMINH4) trial is a parallel randomized controlled trial that was performed in the United Kingdom that aimed to evaluate the efficacy of self-BP monitoring, telemonitoring, and usual care in BP control. In this trial, participants were assigned randomly (1:1:1) to a self-monitoring group, a telemonitoring group, or a usual care group. After 12 months, both self-monitoring and telemonitoring groups had a lower BP level than the usual care group (137.0 ± 16.7 and 136.0 ± 16.1, versus 140.4 ± 16.5 mm Hg).54 Good adherence to therapy is a prerequisite to achieving better BP control. A more recent randomized controlled trial showed that short-term HBPM can significantly improve medication adherence and result in greater reduction in office BP.55 Similarly, a meta-analysis by Duan and colleagues including 46 randomized controlled trials confirmed that HBPM can improve BP control.56 Other controlled studies and meta-analyses draw similar conclusions, namely, that out-of-office BP monitoring could result in better BP control compared with OBPM alone.57–62 Although it is generally accepted that HBPM is useful for better BP control in patients with hypertension, a systematic review showed that HBPM alone is not associated with better BP control, but, with cointerventions, leads to significant BP reduction.63 To date, it remains uncertain whether HBPM can improve BP control over the longer term. Furthermore, few data on the association of ABPM and BP control have been reported. Large-scale studies with longer follow up are required to address these issues.
Good BP control plays a pivotal role in reducing the prevalence of CVD. A meta-analysis showed that every 5-mm Hg decrease in systolic BP was associated with a 13% lower risk of cardiovascular events, and a 2-mm Hg decrease in diastolic BP was associated with a 12% lower risk of cardiovascular events.64 However, evaluation of the association between BP reduction and CVD was based on office BP values; studies on BP treatment goals based on HBPM or ABPM are limited. Current academic guidelines recommending initiating treatment and determining BP goals for managing hypertension are still based on OBPM.3,4,6,7 Several ongoing studies are evaluating the use of out-of-office BP monitoring to guide hypertension control. The TELEBPMET (a randomized controlled study based on home BP telemonitoring versus conventional management and assessment of psychological determinants of adherence) study will include a total of 252 patients and randomize them to usual care or home BP telemonitoring.65 The primary study endpoint will be the rate among subjects of achieving normal daytime ambulatory BP targets. The GYMNs (Guiding Hypertension Management Using Different Blood Pressure Monitoring Strategies) study is a prospective double-blind randomized trial, which is planned to enroll a total of 252 patients and allocate them into three arms: home BP, unattended automated BP, and central BP-guided treatment.66 The primary outcome is the change in 24-h mean ambulatory systolic BP at 3 months, and the decrease in left ventricular mass will be evaluated at 12 months. However, both the TELEBPMET and GYMNs studies are not designed to evaluate the risk of CVD according to different BP management strategies. The MASTER (MASked-unconTrolled hypERtension management based on OBPM or ABPM) study is a 4-year prospective, randomized, open-label, blinded-endpoint investigation, which included 1240 patients with masked uncontrolled hypertension (MUCH) and randomized to a management strategy based on OBPM or on ABPM.67 The effects of the MUCH management strategy based on OBPM or ABPM on CVD will be assessed at 4 years. The results of these studies will provide more information about the effects of different BP-guiding management strategies on the prevention of CVD.
Out-of-office BP monitoring for screening hypotension
Hypotension is usually defined as OBPM < 110/70 mm Hg, daytime ABPM < 105/65 mm Hg, or 24-h ABPM < 100/60 mm Hg.68 Although the ‘J curve’ phenomenon is still controversial,69–71 lower BP readings are undoubtedly not an improvement.68,71 The Systolic Blood Pressure Intervention Trial showed that patients with hypertension and high CVD risk assigned to an intensive BP treatment goal (systolic BP < 120 mm Hg) experienced lower CVD risk but also increased risk of severe hypotension and syncope.72 In a study including 70,997 patients with hypertension receiving antihypertensive treatment, one in eight patients was at risk of hypotension, and ABPM could better screen for hypotension than OBPM.68
Orthostatic hypotension, a usually asymptomatic condition whereby BP drops when rising to a standing position, has been confirmed to be associated with cardiovascular events and dementia.73–77 Although it is not difficult to diagnose, orthostatic hypotension is rarely screened using OBPM unless obvious symptoms develop. A study by Cremer and colleagues showed that HBPM is better than OBPM in screening for orthostatic hypotension.78 Another study showed that ABPM can predict autonomic dysfunction in orthostatic hypotension,79 and may be useful when assessing patients for orthostatic hypotension.80 Postprandial hypotension occurs when systolic BP decreases by more than 20 mm Hg within 2 h after a meal. Some studies have shown that this condition also results in an increased risk of stroke, cerebrovascular damage, syncope, and mortality,81–83 and that HBPM is a suitable screening method.83
Based on these findings, out-of-office BP monitoring is a good choice for screening for hypotension (including orthostatic and postprandial hypotension) in patients with hypertension receiving antihypertensive treatment (especially older patients) or with normal BP with autonomic dysfunction. If hypotension is documented, modifying a patient’s antihypertensive medication or other interventions should be considered. In addition, future studies should focus on individual therapy and optimal thresholds for patients with hypertension in order to avoid undetected hypotension.
Identifying BP variability, circadian BP patterns, and other special BP phenotypes
Variability is an intrinsic property of BP. In patients with regular follow-up, long-term visit-to-visit BP variability is associated more strongly with cardiovascular and all-cause mortality than mean BP.84 HBPM may be more suitable for determining long-term BP variability because it can improve patients’ adherence to BP monitoring. The Didima study showed that, in the general population with a 19-year follow up, systolic home BP variability exhibited superior prognostic ability compared with office BP.85
Although long-term BP variability is not available through ABPM, ABPM can provide short-term variability data when variability is defined as the average variation of BP throughout the day and the circadian rhythm. Based on 24-h BP profiles, normal BP circadian rhythm is defined as dipping BP with a decrease of 10–20% at night compared with daytime. Accordingly, other circadian BP patterns, including extreme dipping (>20% drop), nondipping (<10% drop), and reverse dipping (reversely increased) BP, are also defined by night-time mean BP compared with daytime BP.86 Furthermore, ABPM is useful for detecting other special BP phenotypes, such as isolated nocturnal hypertension and morning BP surge. Nondipping BP,87–89 reverse dipping BP,90,91 nocturnal hypertension,92,93 and morning BP surge88,89,94,95 are associated with an increased risk of target organ damage.
Compared with ABPM, HBPM has been criticized as being inconvenient for the monitoring of circadian BP patterns and nocturnal hypertension. However, recent studies showed that HBPM provided similar values and had close agreement in detecting nondipping BP, as well as target organ damage, compared with ABPM.96,97 However, because of limited available data, additional studies focusing on circadian BP patterns, BP variability, and special phenotypes detected by HBPM are required to determine the optimal strategies for managing hypertension based on out-of-office measurements, and thus reduce adverse outcomes.
Conclusion
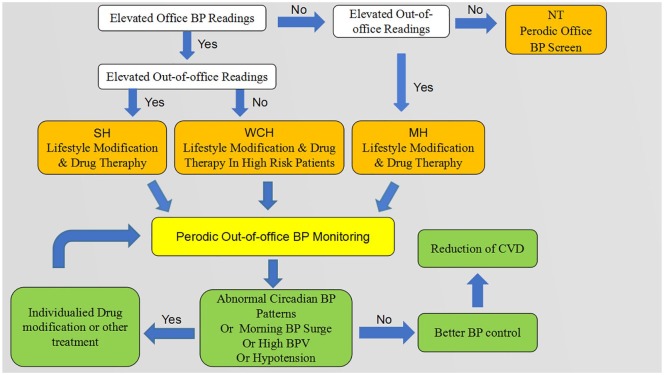
Although out-of-office BP monitoring is beneficial, it is not as widely used as we propose. Regarding ABPM, the relatively higher expense, interference with daily activities, and inaccurate readings due to incorrect measuring position are the main reasons for its low rate of use. HBPM, moreover, requires formal skills training and is limited by nocturnal BP detection, recording bias, and arbitrary self-modification of treatment by anxious patients. Given their advantages and disadvantages, HBPM and ABPM should be considered to be complementary rather than competitive in managing hypertension. Our proposed clinical procedure for using out-of-office BP monitoring is presented in Figure 2.
Figure 2.
Proposed clinical procedure for application of out-of-office BP monitoring.
BP, blood pressure; BPV, blood pressure variability; CVD, cardiovascular disease; MH, masked hypertension; NT, normotension; SH, sustained hypertension; WCH, white coat hypertension.
To better incorporate out-of-office BP monitoring into clinical practice, initiatives should also be taken regarding the following. First, physicians need to be aware of the indications and limitations of out-of-office BP measurement. Second, patients should receive clearer instructions, training, and education regarding BP monitoring. Third, ideally, governments and public health researchers will engage in efforts to address cost-effective methods of out-of-office BP monitoring. Fourth, combining remote data transmission with clinic-centered monitoring is helpful in avoiding the drawbacks of HBPM, such as recording bias and arbitrary self-modification of treatment, in further improving BP control. We are currently performing the Home Blood Pressure Monitoring Cohort Study Based On Intelligent Cloud Platform (HBPM-iCloud) trial to evaluate HBPM for predicting the risk of CVD and mortality in a large Chinese population.
In conclusion, based on recent clinical studies, out-of-office BP measurements have benefits with regard to the following areas: detecting certain abnormal BP phenotypes, namely, WCH and MH; stronger prediction of determining the prognosis regarding target organ damage and mortality; better BP control; hypotension screening; and offering a unique approach to identifying circadian BP patterns and BP variability.
Acknowledgments
We thank Jane Charbonneau from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Author contributions: HZ, HZ, WM, and YH contributed to the conception or design of the work. XL, WM, and YH contributed to the acquisition, analysis, or interpretation of data for the work. HZ and HZ drafted the manuscript. WM and YH critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: The project was supported by the National Natural Science Foundation of China (No: 81600239), the Science and Technology Innovation Project from Foshan, Guangdong (FS0AA-KJ218-1301-0006), Scientific research start-up plan of Southern Medical University (CX2018N202), and the Clinical Research Startup Program of Shunde Hospital, Southern Medical University (CRSP2019001).
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iD: Yuli Huang  https://orcid.org/0000-0001-5104-567X
https://orcid.org/0000-0001-5104-567X
Contributor Information
Hailan Zhu, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, Guangdong, China.
Haoxiao Zheng, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, Guangdong, China.
Xinyue Liu, Department of Cardiology, Shunde Hospital, Southern Medical University, Foshan, Guangdong, China.
Weiyi Mai, Department of Cardiology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
Yuli Huang, Department of Cardiology, Shunde Hospital, Southern Medical University, Jiazi Road 1, Lunjiao Town, Shunde District, Foshan, Guangdong 523808, China; The George Institute for Global Health, NSW, Australia.
References
- 1. Zhou B, Bentham J, Bixby H, et al. Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet 2017; 389: 37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang Z, Chen Z, Zhang L, et al. Status of hypertension in China. Circulation 2018; 137: 2344–2356. [DOI] [PubMed] [Google Scholar]
- 3. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018; 138: e426–e483. [DOI] [PubMed] [Google Scholar]
- 4. Liu LS, Wu ZS, Wang JG, et al. 2018 Chinese guidelines for prevention and treatment of hypertension-A report of the revision committee of Chinese Guidelines for Prevention and Treatment of Hypertension. J Geriatr Cardiol 2019; 16: 182–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387: 957–967. [DOI] [PubMed] [Google Scholar]
- 6. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. J Hypertens 2018; 36: 1953–2041. [DOI] [PubMed] [Google Scholar]
- 7. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for blood pressure measurement, diagnosis, assessment of risk, prevention, and treatment of hypertension. Can J Cardiol 2016; 32: 569–588. [DOI] [PubMed] [Google Scholar]
- 8. National Institute for Health and Care Excellence. Hypertension in adults: diagnosis and management. Clinical Guidelines. London: National Institute for Health and Care Excellence, 2019. [Google Scholar]
- 9. de la Sierra A, Vinyoles E, Banegas JR, et al. Short-term and long-term reproducibility of hypertension phenotypes obtained by office and ambulatory blood pressure measurements. J Clin Hypertens (Greenwich) 2016; 18: 927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Omboni S, Aristizabal D, De la Sierra A, et al. Hypertension types defined by clinic and ambulatory blood pressure in 14 143 patients referred to hypertension clinics worldwide. Data from the ARTEMIS study. J Hypertens 2016; 34: 2187–2198. [DOI] [PubMed] [Google Scholar]
- 11. Tocci G, Presta V, Figliuzzi I, et al. Prevalence and clinical outcomes of white-coat and masked hypertension: analysis of a large ambulatory blood pressure database. J Clin Hypertens (Greenwich) 2018; 20: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sung SH, Cheng HM, Wang KL, et al. White coat hypertension is more risky than pre-hypertension: important role of arterial wave reflections. Hypertension 2013; 61: 1346–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noubiap JJ, Nansseu JR, Nkeck JR, et al. Prevalence of white coat and masked hypertension in Africa: a systematic review and meta-analysis. J Clin Hypertens (Greenwich) 2018; 20: 1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Myers MG, Stergiou GS. White coat phenomenon: removing the stigma of hypertension. Hypertension 2016; 67: 1111–1113. [DOI] [PubMed] [Google Scholar]
- 15. Briasoulis A, Androulakis E, Palla M, et al. White-coat hypertension and cardiovascular events: a meta-analysis. J Hypertens 2016; 34: 593–599. [DOI] [PubMed] [Google Scholar]
- 16. Cuspidi C, Rescaldani M, Tadic M, et al. White-coat hypertension, as defined by ambulatory blood pressure monitoring, and subclinical cardiac organ damage: a meta-analysis. J Hypertens 2015; 33: 24–32. [DOI] [PubMed] [Google Scholar]
- 17. Cuspidi C, Sala C, Tadic M, et al. Is white-coat hypertension a risk factor for carotid atherosclerosis? a review and meta-analysis. Blood Press Monit 2015; 20: 57–63. [DOI] [PubMed] [Google Scholar]
- 18. Huang Y, Huang W, Mai W, et al. White-coat hypertension is a risk factor for cardiovascular diseases and total mortality. J Hypertens 2017; 35: 677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Banegas JR, Ruilope LM, de la Sierra A, et al. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 2018; 378: 1509–1520. [DOI] [PubMed] [Google Scholar]
- 20. Satoh M, Asayama K, Kikuya M, et al. Long-term stroke risk due to partial white-coat or masked hypertension based on home and ambulatory blood pressure measurements: the Ohasama Study. Hypertension 2016; 67: 48–55. [DOI] [PubMed] [Google Scholar]
- 21. Cohen JB, Lotito MJ, Trivedi UK, et al. Cardiovascular events and mortality in white coat hypertension: a systematic review and meta-analysis. Ann Intern Med 2019; 170: 853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tocci G, Presta V, Figliuzzi I, et al. Prevalence and clinical outcomes of white-coat and masked hypertension: analysis of a large ambulatory blood pressure database. J Clin Hypertens (Greenwich) 2018; 20: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang L, Li Y, Wei FF, et al. Strategies for classifying patients based on office, home, and ambulatory blood pressure measurement. Hypertension 2015; 65: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 24. Fujiwara T, Yano Y, Hoshide S, et al. Association of Cardiovascular Outcomes With Masked Hypertension Defined by Home Blood Pressure Monitoring in a Japanese General Practice Population. JAMA Cardiol 2018; 3: 583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimbo D, Newman JD, Schwartz JE. Masked hypertension and prehypertension: diagnostic overlap and interrelationships with left ventricular mass: the Masked Hypertension Study. Am J Hypertens 2012; 25: 664–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang Y, Su L, Cai X, et al. Association of all-cause and cardiovascular mortality with prehypertension: a meta-analysis. Am Heart J 2014; 167: 160–168. [DOI] [PubMed] [Google Scholar]
- 27. Huang Y, Wang S, Cai X, et al. Prehypertension and incidence of cardiovascular disease: a meta-analysis. BMC MED 2013; 11: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang Y, Cai X, Zhang J, et al. Prehypertension and Incidence of ESRD: a systematic review and meta-analysis. Am J Kidney Dis 2014; 63: 76–83. [DOI] [PubMed] [Google Scholar]
- 29. Huang Y, Cai X, Liu C, et al. Prehypertension and the risk of coronary heart disease in Asian and Western populations: a meta-analysis. J Am Heart Assoc 2015; 4: e001519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang Y, Cai X, Li Y, et al. Prehypertension and the risk of stroke: a meta-analysis. Neurology 2014; 82: 1153–1161. [DOI] [PubMed] [Google Scholar]
- 31. Chen Y, Huang YL, Mai WY. Prehypertension or masked hypertension-which is responsible for target-organ damage? Nat Rev Cardiol 2015; 12: 497. [DOI] [PubMed] [Google Scholar]
- 32. Huang Y, Qiu W, Liu C, et al. Prevalence and risk factors associated with prehypertension in Shunde District, southern China. BMJ Open 2014; 4: e6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Niiranen TJ, Hänninen M, Johansson J, et al. Home-Measured Blood Pressure Is a Stronger Predictor of Cardiovascular Risk Than Office Blood Pressure. Hypertension 2010; 55: 1346–1351. [DOI] [PubMed] [Google Scholar]
- 34. Niiranen TJ, Jula AM, Kantola IM, et al. Home-measured blood pressure is more strongly associated with electrocardiographic left ventricular hypertrophy than is clinic blood pressure: the Finn-HOME study. J Hum Hypertens 2007; 21: 788–794. [DOI] [PubMed] [Google Scholar]
- 35. Siven SS, Niiranen TJ, Langen VL, et al. Home versus office blood pressure: longitudinal relations with left ventricular hypertrophy: the Finn-Home study. J Hypertens 2017; 35: 266–271. [DOI] [PubMed] [Google Scholar]
- 36. Niiranen T, Jula A, Kantola I, et al. Home-measured blood pressure is more strongly associated with atherosclerosis than clinic blood pressure: the Finn-HOME Study. J Hypertens 2007; 25: 1225–1231. [DOI] [PubMed] [Google Scholar]
- 37. Shimada K, Kario K, Kushiro T, et al. Prognostic significance of on-treatment home and clinic blood pressure for predicting cardiovascular events in hypertensive patients in the HONEST study. J Hypertens 2016; 34: 1520–1527. [DOI] [PubMed] [Google Scholar]
- 38. Murakami K, Asayama K, Satoh M, et al. Home blood pressure predicts stroke incidence among older adults with impaired physical function: the Ohasama study. J Hypertens 2017; 35: 2395–2401. [DOI] [PubMed] [Google Scholar]
- 39. Ward AM, Takahashi O, Stevens R, et al. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens 2012; 30: 449–456. [DOI] [PubMed] [Google Scholar]
- 40. Fuchs SC, Mello RG, Fuchs FC. Home blood pressure monitoring is better predictor of cardiovascular disease and target organ damage than office blood pressure: a systematic review and meta-analysis. Curr Cardiol Rep 2013; 15: 413. [DOI] [PubMed] [Google Scholar]
- 41. Kollias A, Ntineri A, Stergiou GS. Association of night-time home blood pressure with night-time ambulatory blood pressure and target-organ damage: a systematic review and meta-analysis. J Hypertens 2017; 35: 442–452. [DOI] [PubMed] [Google Scholar]
- 42. Ushigome E, Matsumoto S, Oyabu C, et al. Prognostic significance of day-by-day variability of home blood pressure on progression to macroalbuminuria in patients with diabetes. J Hypertens 2018; 36: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 43. Shen J, Li ZM, He LZ, et al. Comparison of ambulatory blood pressure and clinic blood pressure in relation to cardiovascular diseases in diabetic patients. Medicine (Baltimore) 2017; 96: e7807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cai A, Liu C, Zhou D, et al. Ambulatory blood pressure is superior to clinic blood pressure in relation to ischemic stroke in both diabetic and nondiabetic patients. Blood Press Monit 2017; 22: 314–321. [DOI] [PubMed] [Google Scholar]
- 45. Salles GF, Leite NC, Pereira BB, et al. Prognostic impact of clinic and ambulatory blood pressure components in high-risk type 2 diabetic patients: the Rio de Janeiro Type 2 Diabetes Cohort Study. J Hypertens 2013; 31: 2176–2186. [DOI] [PubMed] [Google Scholar]
- 46. Kakaletsis N, Ntaios G, Milionis H, et al. Prognostic value of 24-h ABPM in acute ischemic stroke for short-, medium-, and long-term outcome: a systematic review and meta-analysis. Int J Stroke 2015; 10: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 47. Bell KJ, Beller E, Sundstrom J, et al. Ambulatory blood pressure adds little to Framingham Risk Score for the primary prevention of cardiovascular disease in older men: secondary analysis of observational study data. BMJ Open 2014; 4: e006044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lay-Flurrie S, Stevens R, de Leeuw P, et al. Using out-of-office blood pressure measurements in established cardiovascular risk scores: a secondary analysis of data from two blood pressure monitoring studies. Br J Gen Pract 2019; 69: e381–e388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cao J, Chen JC, Chen SF, et al. Guideline on the assessment and management of cardiovascular risk in China. Zhonghua Yu Fang Yi Xue Za Zhi 2019; 53: 13–35. [DOI] [PubMed] [Google Scholar]
- 50. Conroy RM, Pyorala K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003; 24: 987–1003. [DOI] [PubMed] [Google Scholar]
- 51. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 Suppl. 2): S49–S73. [DOI] [PubMed] [Google Scholar]
- 52. Hippisley-Cox J, Coupland C, Robson J, et al. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ 2010; 341: c6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117: 743–753. [DOI] [PubMed] [Google Scholar]
- 54. McManus RJ, Mant J, Franssen M, et al. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet 2018; 391: 949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Muhammad J, Jamial MM, Ishak A. Home blood pressure monitoring has similar effects on office blood pressure and medication compliance as usual care. Korean J Fam Med 2019; 40: 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duan Y, Xie Z, Dong F, et al. Effectiveness of home blood pressure telemonitoring: a systematic review and meta-analysis of randomised controlled studies. J Hum Hypertens 2017; 31: 427–437. [DOI] [PubMed] [Google Scholar]
- 57. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013; 310: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ciemins EL, Arora A, Coombs NC, et al. Improving blood pressure control using smart technology. Telemed J E Health 2018; 24: 222–228. [DOI] [PubMed] [Google Scholar]
- 59. Kim Y, Shin DG, Park S, et al. Randomized clinical trial to assess the effectiveness of remote patient monitoring and physician care in reducing office blood pressure. HypertensRes 2015; 38: 491–497. [DOI] [PubMed] [Google Scholar]
- 60. Stoddart A, Hanley J, Wild S, Pagliari C, et al. Telemonitoring-based service redesign for the management of uncontrolled hypertension (HITS): cost and cost-effectiveness analysis of a randomised controlled trial. BMJ Open 2013; 3: e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McManus RJ, Mant J, Haque MS, et al. Effect of self-monitoring and medication self-titration on systolic blood pressure in hypertensive patients at high risk of cardiovascular disease: the TASMIN-SR randomized clinical trial. JAMA 2014; 312: 799–808. [DOI] [PubMed] [Google Scholar]
- 62. Lee HY, Kim JY, Na KY, et al. The role of telehealth counselling with mobile self-monitoring on blood pressure reduction among overseas Koreans with high blood pressure in Vietnam. J Telemed Telecare 2019; 25: 241–248. [DOI] [PubMed] [Google Scholar]
- 63. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med 2017; 14: e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Verdecchia P, Gentile G, Angeli F, et al. Influence of blood pressure reduction on composite cardiovascular endpoints in clinical trials. J Hypertens 2010; 28: 1356–1365. [DOI] [PubMed] [Google Scholar]
- 65. Parati G, Omboni S, Compare A, et al. Blood pressure control and treatment adherence in hypertensive patients with metabolic syndrome: protocol of a randomized controlled study based on home blood pressure telemonitoring vs. conventional management and assessment of psychological determinants of adherence (TELEBPMET Study). Trials 2013; 14: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cheng HM, Sung SH, Chen CH, et al. Guiding Hypertension Management Using Different Blood Pressure Monitoring Strategies (GYMNs study): comparison of three different blood pressure measurement methods: study protocol for a randomized controlled trial. Trials 2019; 20: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Parati G, Agabiti-Rosei E, Bakris GL, et al. MASked-unconTrolled hypERtension management based on office BP or on ambulatory blood pressure measurement (MASTER) Study: a randomised controlled trial protocol. BMJ Open 2018; 8: e021038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Divison-Garrote JA, Banegas JR, De la Cruz JJ, et al. Hypotension based on office and ambulatory monitoring blood pressure. Prevalence and clinical profile among a cohort of 70,997 treated hypertensives. J Am Soc Hypertens 2016; 10: 714–723. [DOI] [PubMed] [Google Scholar]
- 69. Rahman F, McEvoy JW. The J-shaped curve for blood pressure and cardiovascular disease risk: historical context and recent updates. Curr Atheroscler Rep 2017; 19: 34. [DOI] [PubMed] [Google Scholar]
- 70. Verdecchia P, Angeli F, Reboldi G. The tale of an innocent: intensive treatment and the J-curve in the SPRINT trial (Systolic Blood Pressure Intervention Trial). Circulation 2017; 136: 2230–2232. [DOI] [PubMed] [Google Scholar]
- 71. Bangalore S, Messerli FH, Wun CC, et al. J-curve revisited: an analysis of blood pressure and cardiovascular events in the Treating to New Targets (TNT) Trial. Eur Heart J 2010; 31: 2897–2908. [DOI] [PubMed] [Google Scholar]
- 72. SPRINT Research Group, Wright JT, Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373: 2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fedorowski A, Wahlstrand B, Hedner T, et al. Systolic and diastolic component of orthostatic hypotension and cardiovascular events in hypertensive patients: the Captopril Prevention Project. J Hypertens 2014; 32: 75–81. [DOI] [PubMed] [Google Scholar]
- 74. Fleg JL, Evans GW, Margolis KL, et al. Orthostatic hypotension in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension 2016; 68: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Juraschek SP, Daya N, Appel LJ, et al. Orthostatic hypotension and risk of clinical and subclinical cardiovascular disease in middle-aged adults. J Am Heart Assoc 2018; 7: e008884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J 2015; 36: 1609–1617. [DOI] [PubMed] [Google Scholar]
- 77. Cremer A, Soumaré A, Berr C, et al. Orthostatic hypotension and risk of incident dementia : results from a 12-year follow-up of the three-city study cohort. Hypertension 2017; 70: 44–49. [DOI] [PubMed] [Google Scholar]
- 78. Cremer A, Rousseau A, Boulestreau R, et al. Screening for orthostatic hypotension using home blood pressure measurements. J Hypertens 2019; 37: 923–927. [DOI] [PubMed] [Google Scholar]
- 79. Alquadan KF, Singhania G, Koratala A, et al. Office orthostatic blood pressure measurements and ambulatory blood pressure monitoring in the prediction of autonomic dysfunction. Clin Hypertens 2017; 23: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Ejaz AA, Kazory A, Heinig ME. 24-hour blood pressure monitoring in the evaluation of supine hypertension and orthostatic hypotension. J Clin Hypertens 2007; 9: 952–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Son JT, Lee E. Postprandial hypotension among older residents of a nursing home in Korea. J Clin Nurs 2012; 21: 3565–3573. [DOI] [PubMed] [Google Scholar]
- 82. Tabara Y, Okada Y, Uetani E, et al. Postprandial hypotension as a risk marker for asymptomatic lacunar infarction. J Hypertens 2014; 32: 1084–1090. [DOI] [PubMed] [Google Scholar]
- 83. Alfie J. Utility of home blood pressure monitoring to evaluate postprandial blood pressure in treated hypertensive patients. Ther Adv Cardiovasc Dis 2015; 9: 133–139. [DOI] [PubMed] [Google Scholar]
- 84. Stevens SL, Wood S, Koshiaris C, et al. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. BMJ 2016; 354: i4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ntineri A, Kalogeropoulos PG, Kyriakoulis KG, et al. Prognostic value of average home blood pressure and variability: 19-year follow-up of the Didima study. J Hypertens 2018; 36: 69–76. [DOI] [PubMed] [Google Scholar]
- 86. Bowles NP, Thosar SS, Herzig MX, et al. Chronotherapy for hypertension. Curr Hypertens Rep 2018; 20: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Chen Y, Liu J, Zhen Z, et al. Assessment of left ventricular function and peripheral vascular arterial stiffness in patients with dipper and non-dipper hypertension. J Investig Med 2018; 66: 319–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mokwatsi GG, Schutte AE, Mels C, et al. Morning blood pressure surge in young black and white adults: the African-PREDICT study. J Hum Hypertens 2019; 33: 22–33. [DOI] [PubMed] [Google Scholar]
- 89. Pierdomenico SD, Pierdomenico AM, Coccina F, et al. Prognostic value of nondipping and morning surge in elderly treated hypertensive patients with controlled ambulatory blood pressure. Am J Hypertens 2017; 30: 159–165. [DOI] [PubMed] [Google Scholar]
- 90. Kim BK, Kim Y, Lee Y, et al. A reverse dipping pattern predicts cardiovascular mortality in a clinical cohort. J Korean Med Sci 2013; 28: 1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yan B, Sun L, Gao Y, et al. Blood pressure reverse dipping may associate with stable coronary artery disease in patients with essential hypertension: a cross-sectional study. Sci Rep 2016; 6: 25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang C, Deng W, Gong W, et al. Nocturnal hypertension correlates better with target organ damage in patients with chronic kidney disease than a nondipping pattern. J Clin Hypertens 2015; 17: 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kario K, Hoshide S, Haimoto H, et al. Sleep blood pressure self-measured at home as a novel determinant of organ damage: Japan Morning Surge Home Blood Pressure (J-HOP) study. J Clin Hypertens (Greenwich) 2015; 17: 340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kawauchi D, Hoshide S, Kario K. Morning home blood pressure and cardiovascular events in a Japanese general practice population over 80 years old: the J-HOP study. Am J Hypertens 2018; 31: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 95. Wanthong S, Kabutoya T, Hoshide S, et al. Early morning-Best time window of hourly 24-hour ambulatory blood pressure in relation to hypertensive organ damage: The Japan Morning Surge-Home Blood Pressure study. J Clin Hypertens (Greenwich) 2019; 21: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kollias A, Ntineri A, Stergiou GS. Association of night-time home blood pressure with night-time ambulatory blood pressure and target-organ damage: a systematic review and meta-analysis. J Hypertens 2017; 35: 442–452. [DOI] [PubMed] [Google Scholar]
- 97. Kollias A, Andreadis E, Agaliotis G, et al. The optimal night-time home blood pressure monitoring schedule: agreement with ambulatory blood pressure and association with organ damage. J Hypertens 2018; 36: 243–249. [DOI] [PubMed] [Google Scholar]