Abstract
Background:
Intrathecal administration of nusinersen in adult spinal muscular atrophy (SMA) patients presents challenges owing to severe scoliosis and previous spinal surgery with metal implantation. In patients with a complex spinal situation, the potential risks of the intrathecal administration may lead to delayed treatment initiation.
Methods:
In this study, we analyzed 53 CT-guided lumbar punctures of 11 adult nonambulatory SMA type 2 and 3 patients. All patients had scoliosis and six patients had previously undergone metal implantation.
Results:
Drug administration was successful in 100% of the patients and none of the patients opted for treatment discontinuation. Complete osseous fusion precluded conventional posterior interlaminar access in eight lumbar punctures in four patients, which required alternative routes including transforaminal punctures and translaminar drilling. Median duration of all lumbar punctures was 9 min and median radiation exposure was 100 mGy* cm. The most common adverse event was post-lumbar puncture syndrome that occurred in five lumbar punctures (9.4%).
Conclusions:
Our data demonstrate that nusinersen can be successfully, safely, and rapidly administered in adult SMA patients with complex spinal conditions and suggest the translaminar drilling technique as an alternative delivery route. Therefore, intrathecal nusinersen treatment should not be withheld from patients because of severe spine deformities, however, drug efficacy in adult SMA patients needs to be investigated in further studies.
Keywords: computed tomography, radiation exposure, scoliosis, transforaminal, translaminar drill
Introduction
Spinal muscular atrophy (SMA) is a monogenetic, autosomal-recessive neurodegenerative disease caused by a mutation in the SMN1 gene.1 The lack of SMN protein and subsequent degeneration of anterior horn cells results in progressive muscle weakness and atrophy. Nusinersen was approved as the first drug for the treatment of SMA in December 2016 by the US Food and Drug Administration and in June 2017 by the European Medicines Agency. The clinical trials prior to approval were conducted in young SMA type 1 and 2 children and demonstrated a remarkable increase of motor function and survival in SMA type 1. 2,3 However, the drug was approved for all subtypes of SMA patients, including adults. Intrathecal administration of nusinersen in adult SMA patients presents challenges owing to severe neuromuscular scoliosis and previous spinal surgery. Recent studies showed that intrathecal treatment with nusinersen in adult patients was feasible and safe when using image-guidance in patients with scoliosis.4,5 However, complete osseous fusion of the posterior elements often precludes posterior interlaminar access.6 In patients with a complex spinal situation, the unclear extent of the therapeutic effect of the drug combined with the potential risks of its application might lead to delayed treatment initiation. Several SMA patients with severe scoliosis have been referred to our institution for intrathecal nusinersen treatment. We analyzed all 53 CT-guided lumbar punctures performed in 11 SMA patients with regard to access to intrathecal compartment, complications, duration of procedure, and radiation exposure.
Methods
The study was performed according to the Declaration of Helsinki and approved by the ethical committee of the Technical University Munich. Written consent was obtained from all participants. Requirements for potential treatment were a documented mutation of SMN1, the absence of contraindications for lumbar punctures, and no conditions that could affect cerebrospinal fluid (CSF) circulation. Treatment was performed in our outpatient clinic for neuromuscular disorders. Demographic and clinical data were collected before the therapy started. Functional scores including the Revised Upper Limb Module (RULM), the Hammersmith Functional Motor Scale Expanded (HFMSE), and the Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised (ALSFRS-R) were assessed. Prior to treatment initiation, an individual benefit–risk assessment was performed. Regarding the potential benefits, realistic treatment goals for our adult SMA patients were stated with a special focus on the remaining motor function and individual disease progression over the previous few years. Likewise, all treatment risks were discussed, including intrathecal application, the reported side effects of the drug, and the need for CT scans with radiation exposure in patients with scoliosis. Finally, each patient defined individual treatment goals and the potential reasons for treatment discontinuation, supported by the physicians and modified at each visit if necessary.
Nusinersen was administered according to drug approval via lumbar puncture on days 1, 14, 28, and 63 followed by a repetitive application every 4 months. CT-guided lumbar punctures were performed by an experienced neuroradiologist using the following CT parameters: tube voltage 120 kV, tube current 100 mAs for planning scans and 30 mAs for interventional scans. During lumbar punctures, patients were positioned in a lateral decubitus position. Premedication with a benzodiazepine (lorazepam) was offered to agitated and anxious patients before the procedure. Local anesthetic infiltration with mepivacaine 2% on the injection site was offered to all patients. Lumbar punctures were performed using 18–22 gauge (G) needles. The translaminar drilling was performed by experienced neuroradiologists in the CT suite using ARROW® OnControl Bone Biopsy System Tray (Teleflex, Morrisville, NC, USA). The diameter of the bone biopsy needle was 11 G (3.0 mm) with a length of 102 mm. A deep infiltration near the periosteum with 5 ml mepivacaine 2% was performed in all cases requiring a translaminar drill. After removal of 5 ml CSF, 12 mg nusinersen was administered intrathecally. Following a clinical observation period of at least 2 hours after lumbar puncture, patients were discharged.
We monitored access to the intrathecal space, medication for sedation, number of lumbar puncture attempts, adverse events, duration of the procedure, and the associated radiation exposure.
Using SPSS (version 25.0; IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA) statistical software, the Mann–Whitney U test was used in metric and not normally distributed variables to determine statistical significance. p values <0.05 were considered statistically significant.
Results
Patients
We analyzed treatment with nusinersen in 11 genetically confirmed patients with SMA type 2 and 3. Patients were aged 16–46 years (mean 33 years) at first lumbar puncture. None of the patients were ambulatory. A total of five patients needed ventilatory support, four of which used noninvasive ventilation overnight. None of our SMA patients had a feeding tube, but swallowing problems were present in three patients. All patients had scoliosis of varying severity. Spinal surgery had been performed in six patients, including extensive posterior spinal fusion in four patients and growing rods in three patients (one patient with both spinal fusion and growing rods). Mean scores on motor function scales before treatment (baseline) were 1.5 points for HFMSE (max. 66), 9.5 points for RULM (max. 37), and 22.9 points for ALS-FRS-R (max. 48). Further scores during treatment are listed in Table 1.
Table 1.
Demographics and clinical data of patients with CT-guided nusinersen treatment.
| SMA type 2 | SMA type 3 | Total | |
|---|---|---|---|
| Number of patients | 6 (54.5%) | 5 (45.5%) | 11 (100%) |
| Demographic data | |||
| Mean age at first injection (range), years | 30 (16–45) | 36 (29–46) | 33 (16–46) |
| Male:Female | 2:4 | 4:1 | 6:5 |
| Clinical data | |||
| Ambulatory | 0 | 0 | 0 |
| Swallowing problems | 2 (66.7%) | 1 (33.3%) | 3 (27.3%) |
| Noninvasive ventilation | 3 (60.0%) | 2 (40.0%) | 5 (45.5%) |
| Scoliosis | 6 (100%) | 5 (100%) | 11 (100%) |
| Spondylodesis | 4 (66.7%) | 2 (33.3%) | 6 (54.5%) |
|
Treatment goals
(stabilization/ improvement of) | |||
| Hand strength | 5 (62.5%) | 3 (37.5%) | 8 (72.7%) |
| Arm strength | 2 (50.0%) | 2 (50.0%) | 4 (36.4%) |
| Respiratory function | 4 (66.7%) | 2 (33.3%) | 6 (54.5%) |
| Swallowing | 2 (100%) | 0 | 2 (18.2%) |
| Sitting stability | 0 | 1 (100%) | 1 (9.1%) |
| Functional scales | |||
| HFMSE score, mean (± SD) | |||
| Baseline | 0.2 (0.4), n = 6 | 3.2 (2.7), n = 5 | 1.5 (2.4), n = 11 |
| 2 months | 0 (0), n = 4 | 2.5 (2.6), n = 4 | 1.3 (2.0), n = 8 |
| 6 months | 0.3 (0.4), n = 4 | 0.0 (0.0), n = 2 | 0.2 (0.4), n = 6 |
| 10 months | 0.5 (0.5), n = 2 | n = 0 | 0.5 (0.5), n = 2 |
| 14 months | 0.5 (0.5), n = 2 | 4.0 (0.0), n = 1 | 1.7 (1.7), n = 3 |
| RULM score, mean (± SD) | |||
| Baseline | 7.1 (6.2), n = 6 | 12.5 (12.1), n = 5 | 9.5 (9.7), n = 11 |
| 2 months | 6.4 (6.2), n = 4 | 7.9 (7.7), n = 4 | 7.1 (7.0), n = 8 |
| 6 months | 10.3 (6.2), n = 4 | 1.0 (1.0), n = 2 | 7.2 (6.7), n = 6 |
| 10 months | 8.0 (8.0), n = 2 | n = 0 | 8.0 (8.0), n = 2 |
| 14 months | 8.0 (8.0), n = 2 | 7.0 (0.0), n = 1 | 7.7 (6.5), n = 3 |
| ALS-FRS-R score, mean (± SD) | |||
| Baseline | 22.0 (6.4), n = 6 | 24.0 (4.9), n = 5 | 22.9 (5.9), n = 11 |
| 2 months | 19.8 (6.8), n = 4 | 24.0 (5.5), n = 4 | 21.9 (6.6), n = 8 |
| 6 months | 20.5 (7.4), n = 4 | 19.5 (4.5), n = 2 | 20.2 (6.6), n = 6 |
| 10 months | 18.0 (9.0), n = 2 | n = 0 | 18.0 (9.0), n = 2 |
| 14 months | 18.0 (9.0), n = 2 | 28.0 (0.0), n = 1 | 21.3 (8.7), n = 3 |
Percentages with respect to total number in subgroup. ALS-FRS-R, amyotrophic lateral sclerosis functional rating scale-revised; HFMSE, Hammersmith functional motor scale expanded; RULM, revised upper limb module.
Many patients were referred to our center when no treatment could be offered to them by other centers for neuromuscular diseases owing to a lack of capacity or experience in image-guided intrathecal treatment. Mean duration from drug approval to treatment initiation was 16.0 months (range 3–23 months). Mean duration from patients’ first consultation at our institution to the first day of treatment was 2.8 months (range 6–326 days). Treatment initiation could be offered to our patients within 2 months of their first consultation. Further delays in treatment initiation were due to patients’ need for reflection, decision-making, and logistical preparation.
Lumbar puncture procedures
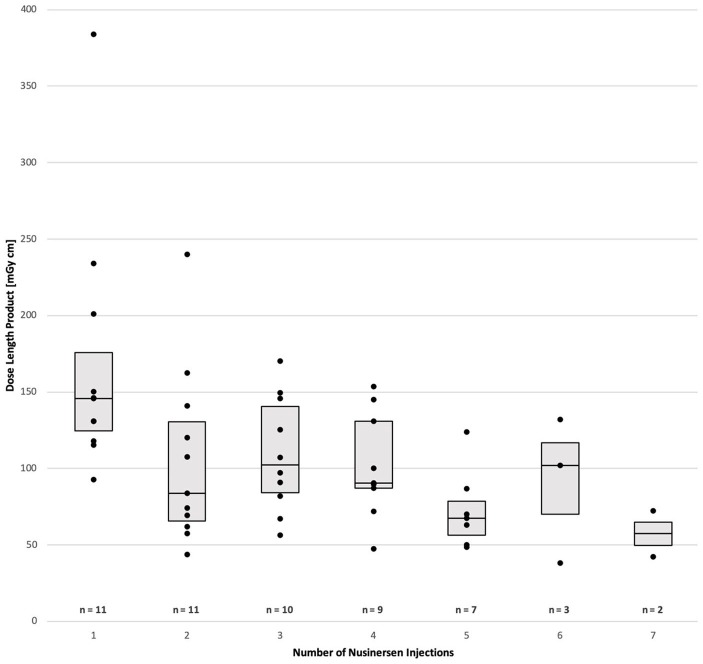
We analyzed all 53 lumbar punctures in our 11 SMA patients (Table 2). Patients had 7 (n = 2), 6 (n = 1), 5 (n = 4), 4 (n = 2), 3 (n = 1), or 2 (n = 1) lumbar punctures. CT-guided drug administration was required in all patients owing to scoliosis, previous spinal surgery, or both. One patient regularly received premedication with lorazepam (0.5–1 mg) owing to anxiety. No sedation was required in any other patient. A local subcutaneous anesthetic with mepivacaine 2% was used for all lumbar punctures and no other anesthetics were required in any of the patients. Summarizing all interventions, two attempts to reach the intrathecal space were required in two lumbar punctures in two patients; in all other punctures, only one attempt was necessary (mean 1.04 attempts). Drug administration was successful in 100% of patients. Classic posterior interlaminar access was possible in 45 lumbar punctures. Alternative routes were necessary in eight lumbar punctures in four patients. A neuroforaminal approach was used in four lumbar punctures in three patients. When a transforaminal puncture was performed, the posterior neural foramen was targeted whenever anatomically possible. In two patients, a neuroforaminal approach was necessary in one lumbar puncture each, followed by conventional interlaminar punctures after re-positioning of the patient. In one patient, neuroforaminal drug administration was assessed in the first and second lumbar puncture, as complete osseous interlaminar fusion precluded the standard posterior lumbar techniques. In this SMA type 2 patient, a translaminar hole was drilled into the bone for the third nusinersen administration (Figure 1) and was used for all subsequent treatments. A translaminar drill was initially used in another SMA type 2 patient with a very challenging spinal anatomy owing to complete lumbar fusion and extensive interlaminar ossification postoperatively (Figure 2). The new osseous canal was used for the following injections. Median duration of CT-guided lumbar puncture was 9.0 min (mean 10.6 min), measured from acquisition of the CT scout to injection of the drug. The median radiation dose of all lumbar punctures indicated as dosage length product (DLP) was 100 mGy* cm (mean 111.6 mGy* cm. Duration and DLP were highest at the first intervention. The Mann–Whitney U test revealed that median DLP at the first intervention was significantly higher compared with subsequent punctures (145.7 mGy* cm at first treatment versus 88.4 mGy* cm from second to seventh treatment, p = 0.002; see also Figure 3).
Table 2.
Details of CT-guided lumbar punctures in 11 SMA patients.
| Posterior interlaminar | Transforaminal | Translaminar | Total | |
|---|---|---|---|---|
| Number of lumbar punctures | 45 (84.9%) | 4 (7.5%) | 4 (7.5%) | 53 (100%) |
| Mean attempts | 1.02 | 1.25 | 1.00 | 1.04 |
| Median duration in min (IQR) | 8.0 (3.0) | 11.0 (4.0) | 18.0 (10.5) | 9.0 (3.0) |
| Median radiation dose in mGy ⋅ cm2 (IQR) | 92.9 (61.8) | 148.05 (19.8) | 103.0 (44.3) | 100.0 (70.8) |
Percentages with respect to total number in subgroup.
IQR, interquartile range.
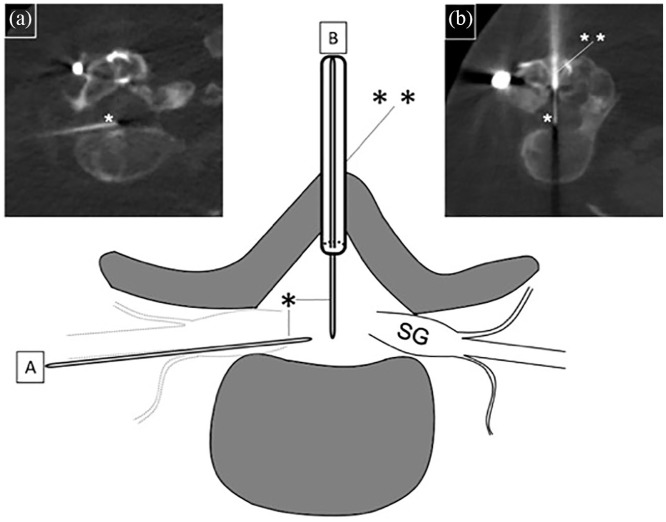
Figure 1.
Illustration of two consecutive nusinersen injections in a 25-year-old woman with SMA type 2. Owing to complete osseous fusion of the dorsal parts of the lumbar spine, there was initially no access except for the transforaminal route (a), which was accessed by a 20 G spinal needle (*). For anatomical reasons, the ventral part of the neural foramen was targeted in this patient. Because this puncture caused lumbar pain and post-puncture headache, in the next session (b) a dorsal 11 G (**) cavity was drilled transosseously at level L2/3. Coaxial to the osseous needle, an 18 G spinal needle (*) was advanced into the spinal canal. At the next intervention, the new osseous canal was used for easy access using a conventional 20 G spinal needle (not shown).
SG, spinal ganglion.
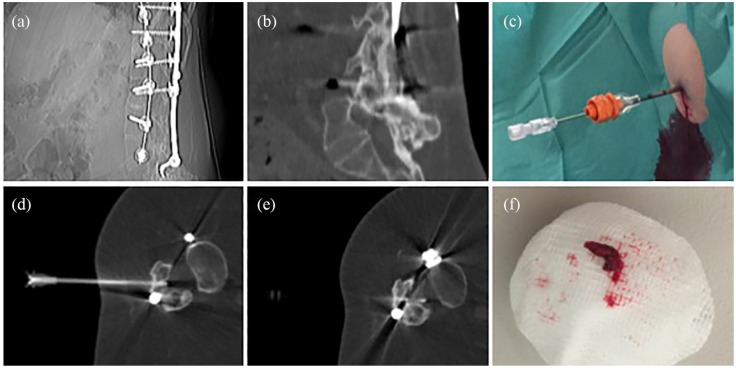
Figure 2.
CT imaging showing a 45-year-old SMA type 2 patient with complete dorsal fusion of the bony spine after dorsal stabilization (a, b). During the first intervention, a bone canal was drilled at level L3/4 (c)–(e), through which a spinal needle could be inserted during the first and subsequent interventions. A 3 mm bone cylinder was removed during the first procedure (f).
Figure 3.
Radiation exposure for first to last CT-guided intervention during intrathecal treatment with nusinersen, shown as a boxplot with data points. Given numbers (n) refer to SMA patients included in the subgroups of first to last intervention.
Complications
No patient discontinued treatment and no major complications occurred during the interventions. However, at least one adverse event during or after treatment was reported in 11 of 53 lumbar punctures (20.8%). The most common adverse event related to lumbar puncture was a post-lumbar puncture syndrome with positional headache as the main symptom in five lumbar punctures (9.4%) in five patients. Temporary back pain lasting for several days after the injection occurred after four lumbar punctures (7.5%) in three patients. Following three out of four punctures (75%) in the three patients with transforaminal access, post-lumbar puncture complications occurred: two of the patients reported a root irritation syndrome, one of whom showed signs of a subarachnoid hemorrhage in the CSF (xanthochromia and increased ferritin level of 1420 µg/l) after 2 weeks; the third patient developed severe headache and lumbar back pain lasting for 7 days after the injection and requiring hospitalization. The two patients with a translaminar access reported no relevant pain or other adverse events.
Discussion
We analyzed 53 CT-guided lumbar punctures performed in 11 nonambulatory SMA patients. Although these patients presented spine deformities, implanted instrumentation, or both, all punctures were successful and no patients opted for treatment discontinuation.
A recent study demonstrated that CT-guided nusinersen treatment should be performed in a prone position4 and another study even recommended a tracheal intubation anesthesia for prone positioning.7 Our study demonstrated that a lateral decubitus position is a viable alternative and we recommend this position over the prone position, which can cause discomfort owing to significant joint contractures and the risk of joint dislocations in SMA patients.
In patients with scoliosis and previous spinal surgery, alternative routes are sometimes required for intrathecal drug administration.7 Image-guided cervical puncture was described as a viable option for intrathecal administration of nusinersen, but patient numbers, particularly in adult SMA patients, were small7–10 and complications need to be considered. A transforaminal approach was recently reported to be an effective and safe alternative to classic interlaminar lumbar puncture in a limited number of patients.6,11,12 With regard to associated complications, one group reported a postprocedural headache rate of 15%, but radicular pain was only reported in one patient.6 In our study, a high rate of complications (75%) occurred after the transforaminal approach, including radicular pain without neurological deficits and signs of spinal bleeding, postprocedural headache, and lumbar pain leading to hospitalization. Although transforaminal access is technically feasible, we assume that minor bleeding frequently occurre due to the injury of blood vessels in close proximity to the spinal nerve leading to pain syndromes. Because the number of patients with transforaminal access in our study was too low to draw general conclusions, this needs to be further evaluated in future studies. Alternative approaches to gain intrathecal access for nusinersen treatment in patients with complex anatomy include lumbar bone laminectomy with or without indwelling lumbar catheter placement.13,14 However, these approaches involve risks because the operation requires general anesthesia and tracheal intubation and the catheter may be a potential site for infection. In this study, we have demonstrated that the translaminar drill is a feasible alternative technique to gain access to the intrathecal space. This minimally invasive intervention, regularly performed for bone biopsies,15 was shown to be well tolerated during and after the procedure in our patients. The deep infiltration with local anesthesia near the periosteum prevented patients experiencing pain during drilling. However, the number of patients in our study was too low to allow reliable conclusions about safety and tolerability. We believe that the repetitive puncture through the drill cavity prevented ossification, but more experience is needed to evaluate the osseous canal in the long term.
Compared with a recent study, which reported 42 min as the mean duration of CT-guided lumbar puncture, the duration of lumbar punctures in our study was significantly shorter with a mean of 10.6 min. However, comparison was impaired owing to the different definition of time measurement (positioning of patients versus planning CT as the starting point). Our results confirm that intrathecal treatment with nusinersen is fast, despite the use of image guidance and the severely impaired patients in our study. Because positioning on the CT table might be uncomfortable, stressful, and painful, particularly for patients with joint contractures, the expected procedural duration represents a relevant factor for patients’ decision-making prior to treatment.
In addition, it is important to investigate the radiation exposure necessary for the intrathecal drug administration, in particular, because patients are generally young and the therapy is potentially chronic. Median radiation dose indicated as DLP in patients with CT-assisted procedures in our study was 100 mGy* cm. A comparison of radiation dosages between studies was limited because of the different units (mSv versus mGy* cm) and different statistical values (median versus mean) used.4,5,16 A recent monocentric study described a mean exposure of 89 mGy* cm (range 9–892 mGy* cm).4 Another group reported median DLP that was higher in patients with spinal fusion (246.5 mGy* cm) than in patients without prior spinal operation (90.4 mGy* cm).16 One reason for the relatively high dose during our interventions could be our preselected patient cohort, because most of our patients were referred after being rejected by other established centers for neuromuscular diseases owing to difficult anatomical conditions. However, efforts must be made to ensure radiation exposure is as low as possible. Possible targets could include low-dose CT protocols with iterative reconstructions and fluoroscopy-assisted examinations. The latter, in particular, was demonstrated to be a potential alternative for image-guided nusinersen treatment in adult SMA type 3 patients without spondylodesis.5 However, it can only be considered for a small proportion of our patients owing to their extremely challenging anatomical conditions (see also Figures 1 and 2). Another option for dose reduction could be the implantation of an intrathecal port-like catheter, for example, with an Ommaya reservoir, that would need only one single image-guided intervention,15 but associated risks including catheter infections need to be considered. We detected a significant decline of median radiation dose and a reduction in duration of procedures when comparing first treatment with subsequent injections. This decrease could be explained by the fact that no imaging prior to treatment was performed, therefore, the CT scan on the first treatment day was the most time-consuming and important because it was required to understand the spinal anatomy of patients.
Owing to the recurrent radiation exposure during nusinersen treatment, the additional cancer risk should be considered and needs to be discussed with the patients. The small intestine, large intestine, ovaries, kidney, uterus, skin, and red bone were identified as the most exposed organs in the radiation field during CT-guided nusinersen injections. When evaluating the lifetime risk of radiation-induced cancer, the type of organ is important, but so are the age and gender of the patient. The highest risk during a single CT-guided injection of nusinersen was demonstrated for young male patients in the large intestine (0.0093%), whereas the ovaries achieved a lower risk (0.0006–0.0011%), bearing in mind that the median DLP of 120.1 mGy ⋅ cm was higher than in our study.16 Therefore, the cancer risk for a single injection was shown to be relatively small, but it must be stressed that the lifetime risk of cancer due to radiation will stochastically increase with the number of interventions. In addition to the risk of cancer, ionizing radiation might affect gonadal function and fertility, but data on this topic are scarce and mainly limited to high-dose radiation therapy.17,18
Analysis of delay in treatment initiation measured as time between drug approval and first day of treatment revealed a mean duration of 16 months, although treatment initiation could be offered to our patients within 2 months following their first consultation. One reason for this delay is the necessity to identify a center with experienced neuroradiologists that allows intrathecal treatment in patients with difficult spinal anatomy. These centers might be located long distances from patients’ home towns or have limited capacities. However, early treatment should be considered. Although natural history studies highlight that progression in SMA type 2 and 3 might be indolent, motor functions decline over time, particularly beyond 1 year of follow-up.19–21 Therapeutic response might be limited when motoric functions are lost and even contractures or skeletal deformities can occur.22 In order to realize early treatment initiation in patients with challenging access, we emphasize the need to evaluate the different options for intrathecal drug delivery shown in this and other studies and refer patients quickly to specialized centers.
One limitation of this study is the low number of patients, in particular, regarding transforaminal or translaminar approaches. In addition, the observation time of treatment was short with only nine patients having completed the induction phase of four doses of nusinersen. Therefore, further experience, in particular with alternative access routes for intrathecal drug administration should be gained in larger cohorts of patients with a special focus on chronic administrations.
Our study was not powered to assess drug efficacy in adult SMA patients. In particular, in the groups at 10 and 14 months after treatment patient numbers were too low to draw conclusions about a change in functional outcome scores. The patients in our study were severely affected, most patients had only minimal remaining movement of their fingers, resulting in a score of 0 points on the HFMSE in many patients (mean score 0.2 points for SMA type 2) and low scores on the RULM at baseline performance (Table 1). This baseline effect is a well-known shortcoming for the widely used HFMSE, hindering the evaluation of drug efficacy in this subgroup of patients.23 In particular, with regard to the potential risks encountered with recurrent lumbar punctures and the high costs of nusinersen, we emphasize the need for further studies with larger patient populations to investigate how this treatment might benefit adult SMA patients. However, it should be considered that a lack of disease progression or small improvements in, for example, finger movements should be considered as a response to treatment and might have a huge effect on the daily life of these patients.
Conclusion
We have demonstrated that nusinersen can successfully and quickly be administered by an experienced neuroradiologist in SMA patients with complex spinal conditions. The only approved treatment for adult SMA should, therefore, not be withheld from patients because of a challenging access route. We introduced the translaminar drill as a feasible alternative technique to classic and transforaminal approaches in patients with complex anatomies.
Acknowledgments
We would like to thank the patients and their families for participating in our study. We are grateful to Dr Kornelia Kreiser for her backup in CT-guided lumbar punctures. The training of clinical scores of some clinicians and study nurses was sponsored by Biogen. We acknowledge support by the German Research Foundation (DFG) and the Technical University of Munich within the funding programme Open Access Publishing.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: IC and MD received a travel grant from Biogen. PL received support for the organization of an independent symposium from Biogen. BF, VP, CZ, and CM report no disclosures.
ORCID iD: Isabell Cordts  https://orcid.org/0000-0002-2078-1997
https://orcid.org/0000-0002-2078-1997
Contributor Information
Isabell Cordts, Department of Neurology, Klinikum rechts der Isar, Technical University Munich, Ismaninger Straße 22, Munich, 81675, Germany.
Paul Lingor, Department of Neurology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Benjamin Friedrich, Department of Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Verena Pernpeintner, Department of Neurology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Claus Zimmer, Department of Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Marcus Deschauer, Department of Neurology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
Christian Maegerlein, Department of Diagnostic and Interventional Neuroradiology, Klinikum rechts der Isar, Technical University Munich, Munich, Germany.
References
- 1. Lefebvre S, Bürglen L, Reboullet S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 1995; 80: 155–165. [DOI] [PubMed] [Google Scholar]
- 2. Finkel RS, Mercuri E, Darras BT, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med 2017; 377: 1723–1732. [DOI] [PubMed] [Google Scholar]
- 3. Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med 2018; 378: 625–635. [DOI] [PubMed] [Google Scholar]
- 4. Wurster CD, Winter B, Wollinsky K, et al. Intrathecal administration of nusinersen in adolescent and adult SMA type 2 and 3 patients. J Neurol 2019; 266: 183–194. [DOI] [PubMed] [Google Scholar]
- 5. Stolte B, Totzeck A, Kizina K, et al. Feasibility and safety of intrathecal treatment with nusinersen in adult patients with spinal muscular atrophy. Ther Adv Neurol Disord 2018; 11: 1756286418803246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nascene DR, Ozutemiz C, Estby H, et al. Transforaminal lumbar puncture: an alternative technique in patients with challenging access. Am J Neuroradiol 2018; 39: 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mousa MA, Aria DJ, Schaefer CM, et al. A comprehensive institutional overview of intrathecal nusinersen injections for spinal muscular atrophy. Pediatr Radiol 2018; 48: 1797–1805. [DOI] [PubMed] [Google Scholar]
- 8. Veerapandiyan A, Pal R, D’Ambrosio S, et al. Cervical puncture to deliver nusinersen in patients with spinal muscular atrophy. Neurology 2018; 91: e620–e624. [DOI] [PubMed] [Google Scholar]
- 9. Gibbs W, Skalski M, Kim P, et al. C1-2 Puncture: a safe, efficacious, and potentially underused technique. Neurographics 2017; 7: 1–8. [Google Scholar]
- 10. Ortiz CB, Kukreja KU, Lotze TE, et al. Ultrasound-guided cervical puncture for nusinersen administration in adolescents. Pediatr Radiol 2019; 49: 136–140. [DOI] [PubMed] [Google Scholar]
- 11. Geraci AP, Black K, Jin M, et al. Transforaminal lumbar puncture for intrathecal nusinersen administration. Muscle Nerve 2018; 58: E4–E5. [DOI] [PubMed] [Google Scholar]
- 12. Weaver JJ, Natarajan N, Shaw DWW, et al. Transforaminal intrathecal delivery of nusinersen using cone-beam computed tomography for children with spinal muscular atrophy and extensive surgical instrumentation: early results of technical success and safety. Pediatr Radiol 2018; 48: 392–397. [DOI] [PubMed] [Google Scholar]
- 13. Moshe-Lilie O, Nizar C, Visser A, et al. Nusinersen in adults with spinal muscular atrophy, a single center experience. Neurology 2019; 92(15 Suppl): P4.4-015. [Google Scholar]
- 14. Lakhotia A, Bhalla S, Doll E, et al. Use of Ommaya reservoir with a thoracic spinal catheter for intrathecal delivery of nusinersen in a patient with spinal muscular atrophy type 2. Neurology. 2018; 90(15 Suppl): P4.464. [Google Scholar]
- 15. Lee RK, Ng AW, Griffith JF. CT-guided bone biopsy with a battery-powered drill system: preliminary results. Am J Roentgenol 2013; 201: 1093–1095. [DOI] [PubMed] [Google Scholar]
- 16. Oldenburg D, Guberina N, Stolte B, et al. Radiation exposure of image-guided intrathecal administration of nusinersen to adult patients with spinal muscular atrophy. Neuroradiology 2019: 1–10. [DOI] [PubMed] [Google Scholar]
- 17. Marci R, Mallozzi M, Di Benedetto L, et al. Radiations and female fertility. Reprod Biol Endocrinol 2018; 16: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ahmad G, Agarwal A. Ionizing radiation and male fertility. In Gunasekaran K, Pandiyan N. (eds) Male infertility. New Delhi: Springer, 2017, pp.185–196. [Google Scholar]
- 19. Zerres K, Rudnik-Schöneborn S. Natural history in proximal spinal muscular atrophy: clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch Neurol 1995; 52: 518–523. [DOI] [PubMed] [Google Scholar]
- 20. Kaufmann P, McDermott MP, Darras BT, et al. Prospective cohort study of spinal muscular atrophy types 2 and 3. Neurology 2012; 79: 1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Swoboda KJ, Prior TW, Scott CB, et al. Natural history of denervation in SMA: relation to age, SMN2 copy number, and function. Ann Neurol 2005; 57: 704–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chiriboga CA. Nusinersen for the treatment of spinal muscular atrophy. Expert Rev Neurother 2017; 17: 955–962. [DOI] [PubMed] [Google Scholar]
- 23. Wadman R, Wijngaarde C, Stam M, et al. Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with spinal muscular atrophy types 1c–4. Euro J Neurol 2018; 25: 512–518. [DOI] [PubMed] [Google Scholar]