Abstract
Objective:
To explore the role of microRNA (miR-21) in new bone formation in ankylosing spondylitis (AS) as mediated by different concentration of tumor necrosis factor-α (TNF-α).
Methods:
Fibroblasts isolated from the hips of patients with AS were induced to osteogenesis. These cells were then stimulated with varying concentrations of TNF-α. MicroRNA-21 expressions were evaluated using reverse transcription–polymerase chain reaction (RT-PCR) and osteogenesis was detected via Alizarin Red S (ARS) staining and measurement of alkaline phosphatase (ALP) activity. Relative expressions of p-STAT3, Nuclear STAT3, cytoplasm STAT3, Runx2, BMP2, osteopontin, osteocalcin, and LC3B in AS fibroblasts were measured after exposure to different concentrations of TNF-α. The STAT3-inhibiting small interfering RNA allowed further exploration on its impact on miR-21 and primary miR-21 expressions. A proteoglycan-induced arthritis (PGIA) Balb/c mouse model was established in order to monitor sacroiliac joint (SIJ) inflammation and subsequent damage through magnetic resonance image. Serum miR-21 and TNF-α expressions were evaluated using RT-PCR and enzyme-linked immunosorbent assay. At week 16, mice models were transfected intravenously with miR-21 overexpressing agomir and miR-21 inhibiting antagomir for 7 successive days. The rate of abnormal bone formation at SIJ was evaluated using microcomputed tomography and hematoxylin and eosin staining at week 24. Western blot analysis enabled quantification of STAT-3, JAK-2, and interleukin (IL)-17A expressions present in the SIJ.
Results:
The in vitro miR-21 expression and osteogenesis activity were noted to be augmented in the setting of low TNF-α concentrations (0.01-0.1 ng/mL) while they were depressed in settings with higher TNF-α concentrations (1-10 ng/mL). Samples with the most distinct ARS manifestation and ALP activity as well as the highest miR-21 expressions were those who received 0.1 ng/mL of TNF-α. Primary miR-21 was found to be notable raised by Si-STAT3, while the converse effect was seen in mature miR-21 expressions. Intravenous injection of exogenous miR-21 contributed to new bone formation and significantly elevated expressions of STAT3, JAK2, and IL-17 in PGIA mice.
Conclusions:
The results revealed that miR-21 may act as a potential mediator between new bone formation and inflammation in AS.
Keywords: ankylosing spondylitis, miR-21, TNF-α, JAK/STAT-3 signaling
Introduction
Ankylosing spondylitis (AS) represents a chronic, progressive inflammatory arthritis which primarily affects the sacroiliac joint (SIJ) and the spine.1 In addition to axial inflammation, abnormal syndesmophyte formation leading to spinal fusion is a significant disease hallmark in this condition, a phenomenon that ultimately limits spinal mobility in patients with AS.2 Reduced quality of life in the initial stages of disease is thought to result from abnormal inflammation. However, as the disease progresses, AS prognosis become more dependent on abnormal bone formation.3,4
MicroRNAs (miRNA) are a class of tiny noncoding RNA molecules that are estimated to be about 22 nucleotides in length. These molecules bind to various sites on the 3′ untranslated region of messenger RNAs (mRNAs) and significantly alter genetic activity.5 They are crucial regulators of genetic expression after the transcriptional process.6 Both the pathology of autoimmunity and immunologic function regulation have been thought to be dependent on miRNA function.7 Existing literature has documented altered miRNA expression to be present in AS.8
MicroRNA-21 is an mRNA that is highly expressed across various cell types and is related to a myriad of biological phenomena that includes cell cycle regulation, differentiation, cellular growth, apoptosis, inflammation, and immune responses.9 MicroRNA-21 molecule has a dual role on bone metabolism that has been found to be strongly linked to inflammation. Both osteoclastic activities, osteogeneic differentiation and osteoclastogenesis have been demonstrated to involve high expressions of miR-21.10-12
Tumor necrosis factor-α (TNF-α) is a well-known inflammatory mediator that is vital in the pathogenesis of AS pathogenesis. A number of investigations have outlined a link between miR-21 and TNF-α. Tumor necrosis factor-α exposure appears to contribute to dysfunctional bony formation in in vitro and in vivo models in settings of depressed miR-21 levels.13 Moreover, anti-TNF-α inoculation in mice without ovaries demonstrate heightened bone formation through miR-21 upregulation.13 MicroRNA-21 expressions were noted to be depressed in higher TNF-α concentrations and augmented in lower TNF-α concentrations.14 Existing literature indicates that miR-21 may play a pivotal role in inflammation resolution and is capable of deescalating the inflammatory response spurred by various triggers that promote miR-21 production.15
The potential role miR-21 has also been explored in AS. Previous studies have demonstrated that Th17 cells possess higher miR-21 levels and that mice that have a miR-21 deficit also have dysregulated Th17 differentiation.16 In addition, miR-21 enhances Th17 cell differentiation via induction of interleukin (IL)-17,16 a process that is now recognized as a critical factor in enhancing abnormal osteogenesis in AS.17,18 Patients with AS were found to have elevated levels of serum mir-21 when compared to healthy controls.19 Our research group has previously discovered a positive relationship between expressions of serum miR-21 and lower bone mineral density as well as radiographic progression in patients with AS.20
Taken together, we hypothesize that miR-21 functions as the mediator between inflammation and abnormal bone formation. Thus, this series of investigations are carried out to explore whether miR-21 contributes to new bone formation mediated by TNF-α.
Materials and Methods
Tissue Samples and Primary Culture of Hip Capsule Fibroblasts
Hip capsule tissues were extracted from 6 patients with AS planned for total hip replacement in our clinical facility. Primary AS fibroblasts from hip capsules were obtained for analysis according to our published protocol.21 When a confluence of 80% was achieved, transfection medium was substituted with osteogenic medium which was a α-minimum essential medium containing 2% fetal bovine serum supplemented with 10 nM dexamethasone, 10 mM β-glycerophosphate, and 50 μg/mL ascorbic acid for osteogenic induction. Cells were allowed to reach the third passage prior to inclusion in our experiments. TNF-α (Sigma, St. Louis, Missouri) at concentrations of 0to 10 ng/mL were used to stimulate a pro-inflammatory microenvironment. Informed consent was gathered from all patients while ethical approval was granted by the local ethics committee.
Real-Time PCR for MiR-21 Expression
TRIzol was used to extract total RNA from the cells. Standard spectrophotometric and electrophoresis methods were used to assess the quality and quantity of the RNA samples. Mature miR-21 levels were quantified with the TaqMan MicroRNA Assay protocol (Applied Biosystems, CA, US) in quantitative reverse transcription–polymerase chain reaction (qRT-PCR). These results were normalized based on housekeeping gene U6 for miR-21 and 18srRNA for pri-miR-21 using the 2−ΔCt method. The relative expression ratio of miR-21 in each sample was derived from the 2−ΔΔCt method. The primer sequences used are as follows: miR-21 F: 5′ ACACTCCAGCTGGGTAGCTTATCAGACTGATG hsa-miR-21 R: 5′ CTCAACTGGTGTCGTGGA U6-F: 5′ CTCGCTTCGGCAGCACA; U6-R: 5′ AACGCTTCACGAATTTGCGT; pri-miR-21-F1: 5′ ATGGCTGTACCACCTTGTCG; pri-miR-21-R1: 5′ GTGCCACTAGACCTAAGGACC; 18srRNA-F: 5′ CCTGGATACCGCAGCTAGGA; 18srRNA-R: 5′ GCGGCGCAATACGAATGCCCC.
Alizarin Red S Staining
A 12-well-plate was used to seed cells at a number of 5 × 104 cells per milliliter before they were left to incubate for 4 weeks. Subsequently, the cells were rinsed twice with 1 × phosphate buffer saline (PBS), fixed for 30 minutes with 4% paraformaldehyde, rinsed twice with deionized water and finally underwent a 15 minute staining period with 2% Alizarin Red S (pH 4.2; Sigma-Aldrich, St. Louis, MO, USA) at room temperature. The final product was then photographed. The staining was quantified by first eluting it with 10% cetylpyridinium chloride (Sigma-Aldrich) and allowing it to incubate for 10 minutes. This process dissolved the stain, and its concentration was quantified with a microplate reader (Power Wave 340; Bio-TEK, Vermont, VT, USA) at an absorbance of 562 nm.
ALP Activity
Of all, 5 × 103 cells AS fibroblasts were plated per well in a 96-well plate before being left in a 5% CO2 microenvironment to incubate overnight at 37°C. Cells were grouped into 5 cohorts as previously described. An ALP kit (Jiancheng Bioengineering Institute, Nanjing, China) was used to quantify ALP activity. Briefly, samples were rinsed thrice with PBS before being lysed with 0.3% Triton X-100 at 4°C for 24 hours. A working solution was added to the cell lysate and left to incubate for 15 minutes. An automatic microplate reader was then used to measure the optical density at 520 nm (OD520)
Western Blot Analysis
Western blot analysis to determine markers of osteogenesis in AS fibroblasts was carried out in accordance to previously documented protocols. Total protein was extracted at day 14 following the induction of osteogenesis. Nuclear and cytoplasmic proteins were also extracted according to the previous protocol.14 Following protein quantification, protein samples were electrophoresed via a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel. The proteins were then immunoblotted onto a nitrocellulose membrane and incubated overnight at 4°C with primary antibodies: anti-p-STAT3 (1:1000 dilution, CST, MA, US), anti-STAT3 (1:1000 dilution, Abcam, Cambridge, UK), anti-Runx2 (1:1000 dilution, Abcam, Cambridge, UK), anti-BMP2 (1:1000 dilution, Bioworld, MN, US), anti-osteopontin (OPN; 1:800 dilution, Abcam, Cambridge, UK), anti-osteocalcin (OCN; 1:5000 dilution, Abcam, Cambridge, UK), anti-LC3B (1:1000 dilution, CST, MA, US), and anti-GAPDH (1:1000 dilution, Abcam, Cambridge, UK). A second incubation period took place the next morning for 1 hour with anti-rabbit secondary antibodies (Santa Cruz, California, US) at room temperature. The resultant images were viewed using enhanced chemiluminescence (Google Biotech, Wuhan, China).
Immunofluorescence Analysis for STAT3
Cells in 0.1 ng/mL TNF-α group were harvested each day from day 1 to 14 using trypsin and smeared onto glass slides (Thermo Scientific, CA, US) at a number of 2.5 × 103. Each slide was left to incubate for 48 hours before undergoing fixation for 15 minutes with 4% paraformaldehyde and an overnight incubation period with primary antibodies for STAT3 (Abcam,Cambridge, UK 1:50) at 4°C. The next day, the slides were exposed to secondary Alexa fluor 488-labeled (green) anti-IgG. Nuclear staining was done with 4',6-diamidino-2-phenylindole (DAPI). The fluorescent sections were done using confocal fluorescence microscopy.
Small Interfering RNA and Transfection
A Genechem kit was used for small interfering RNA (siRNA) transfection. For siRNA inhibition studies, siRNA transfection medium was used to wash AS fibroblasts before a 12-hour incubation period in 5% CO2 at 37°C. The transfection medium contained the transfection reagent and either 50 nM STAT3 siRNA (5′-CCACUUUGGUGUUUCAUAATT-3′) or control siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′). All cells were left to incubate in this medium for 72 hours before protein or RNA extractions were carried out.
Proteoglycan-Induced Arthritis Model
Sixty female Balb/c mice were injected intraperitoneally at weeks 0, 3, and 6 with 100 µg of cartilage proteoglycans (Sigma-Aldrich). The first and third injections of proteoglycan were administered with complete Freund’s adjuvant (Difco, Detroit, Michigan) and the second injection of proteoglycan was administered with incomplete Freund’s adjuvant (Difco) as has been previously described.22
Serum MiR-21 and TNF-α Expressions
Serum miR-21 and TNF-α expressions were detected at 6, 10, 16, 20, 22, and 24 weeks by RT-PCR and enzyme-linked immunosorbent assay (ELISA) respectively. So far, no housekeeping miRNA has been established and validated to normalize for the miRNA content in serum/plasma. Therefore, after addition of miRNeasy, we supplemented the samples with 5 nmol/L U6 RNA as the spiked-in RNA. Serum from mice was collected, and TNF-α levels were determined by ELISA assay, following the manufacturer’s instructions (eBioscience).
Magnetic Resonance Image
Magnetic resonance image (MRI) was used to assess the degree of inflammatory lesions in the SIJ in order to confirm the viability of the model. A PharmaScan small animal system was used to image the rodents at 16 weeks. Prior to imaging, the mice were anesthetized with 2% isoflurane and a laser control system was used to position the mice onto the animal cradles. Vital signs such as the respiratory rate and body temperature were monitored throughout the experiment, with the latter maintained using a water heating pad.
MiR-21 Administration in PGIA Mice Models
At 16 weeks, 18 successfully established proteoglycan-induced arthritis (PGIA) mice were randomly grouped into 3 cohorts that contained 6 rats each: miR-21 mimics (agomir), miR-21 inhibitor (antagomir), and miR-21 agomir control. The control group consisted of PGIA mice that did not receive miR-21 treatment. As documented in other experiments, miR-21 oligomers (RiboBio, Guangzhou, China) first diluted to a final concentration of 50 mM (within the recommended concentration of 1-100 mM). These miR-21 concoctions were then incubated for 20 minutes with Lipofectamine-2000 (Invitrogen, Carlsbad, California) at room temperature before injecting it into the mice tail veins.
Hematoxylin and Eosin Staining
At 24 weeks, 3 animals from each group were killed using the cervical spine dislocation method. Tissues surrounding the SIJ were carefully dissected in order to preserve its integrity. The sample was fixed in 4% paraformaldehyde before being decalcified in EDTA solution for 4 weeks, dehydrated in ethanol, and paraffin-embedded. Blocks of SIJ tissue were sectioned prior to hematoxylin and eosin (HE) staining.
Micro CT-Scan Analysis
At 24 weeks, 3 mice were randomly selected for microcomputed tomography (CT) analysis. A Skyscan 1176 micro-CT instrument (Bruker microCT, Kontich, Belgium) was used to scan the SIJ with the following settings: AI 0.5-mm filter; source current, 500 μA; source voltage, 50 kV; rotation step, 0.4°; and pixel size 9 μm. The NRecon software (Bruker microCT, Kontich, Belgium) was then used to reconstruct the images using the following settings: beam hardening correction 40%; smoothing 2; and ring artefact correction 7.
Western Blot for SIJ IL-17, JAK2, and STAT3
At 24 weeks, another batch of SIJs was harvested and exposed to a mixture of phosphatase and protease inhibitors (Roche) as well as modified RIPA buffer (Sigma). Western blotting was then carried out as previously described. Primary antibodies were purchased as follows: Anti IL-17 (1:1000, Abcam), Anti JAK2 (1:1000, Abcam), and Anti-STAT3 (1:1000 Abcam) and Anti-GAPDH (1:1000 dilution, Abcam). Anti-rabbit secondary antibodies (Santa Cruz, California) were used. The resultant images were viewed using enhanced chemiluminescence (Google Biotech).
Statistical Analysis
The Graphpad Prism version 6.0 was used to perform all statistical analysis. Data were depicted in terms of mean ± standard deviation. Dual-group comparisons were performed using the Student t tests while multiple-group analyses were done using one-way analysis of variance. Statistical significance was determined when P < .05.
Results
Tumor Necrosis Factor-α Influenced MiR-21 Relative Expression and Osteogenic Activity of AS Fibroblasts
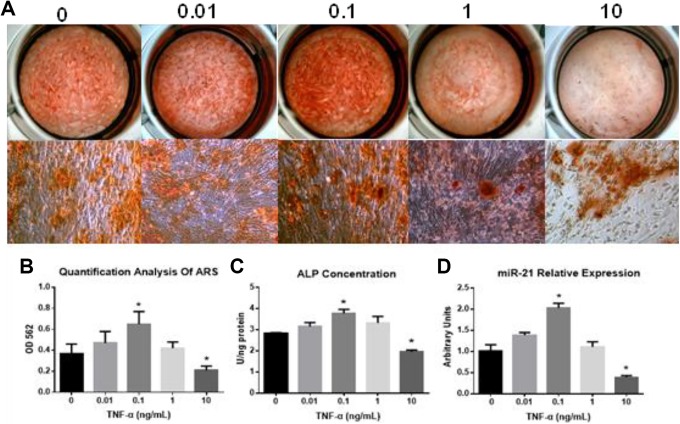
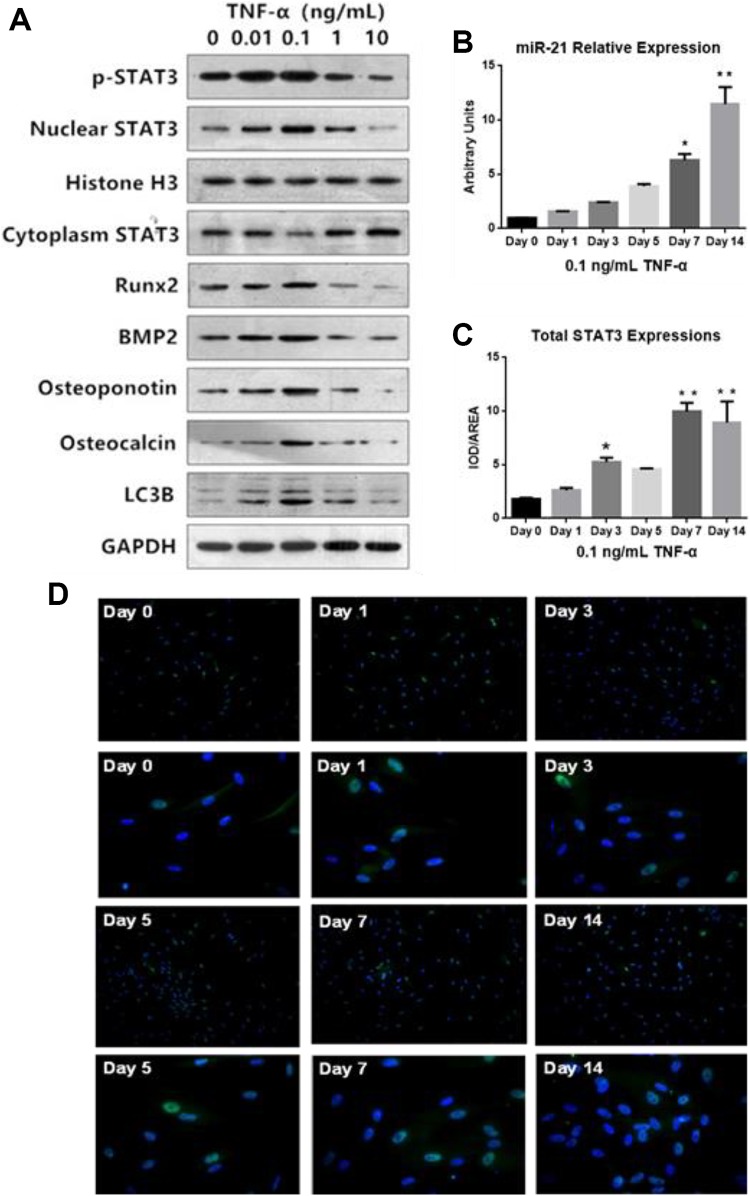
MicroRNA-21 expression gradually increased with progressively higher exposures to TNF-α concentrations (0.01 and 0.1 ng/mL), with the highest miR-21 concentrations seen at TNF-α concentrations of 0.1 ng/mL (Figure 1D). However, miR-21 expression was suppressed at TNF-α concentration of 1 ng/mL and 10 ng/mL Figure 1D. In addition, we found that miR-21 relative expressions in AS fibroblasts gradually increased from day 0 to day 14 (Figure 2B). Tumor necrosis factor-α also promoted the expressions of osteogenesis markers Runx2, BMP2, OPN, and OCN at low concentrations (0.01 and 0.1 ng/mL). Higher concentrations of TNF-α 10 ng/mL markedly suppressed the levels of these markers (Figure 2A). These findings were mirrored in experiments involving alizarin red S staining and quantification of ALP activity (Figure 1A-C). The optimal TNF-α concentration for osteogenesis was 0.1 ng/mL. This value was then used for all subsequent experiments as it proved to be the concentration that provided the best pro-inflammatory environment for inducing AS fibroblast osteogenesis.
Figure 1.
A, Alizarin Red S (ARS) and alkaline phosphatase (ALP) activity during osteogenesis of AS fibroblasts under different concentration of TNF-α. B, Quantification analysis of ARS. C, Quantification analysis of ALP concentration. D, Time dependent miR-21 relative expression under stimulation in AS fibroblasts during osteogenesis. AS indicates ankylosing spondylitis; miR, MicroRNA; TNF-α, tumor necrosis factor-α.
Figure 2.
A, Relative Expression of p-STAT3, Nuclear STAT3, cytoplasm STAT3, Runx2, BMP2, OPN, OCN, and LC3B in AS fibroblasts treatment with different concentrations of TNF-α (ng/mL) B, miR-21 relative expressions under 0.1 ng/mL TNF-α stimulation (*P < .05 compared to 0 ng/mL). C, Quantitative analysis of total STAT3 was conducted for representative capture figures expressed as integrated optical density (IOD)/Area. D, Immunofluorescence analysis of STAT3 expressions in AS fibroblasts treatment with 0.1 ng/mL TNF-α from day 0 to day 14. AS indicates ankylosing spondylitis; miR, microRNA; OCN, osteocalcin; OPN, osteopontin; TNF-α, tumor necrosis factor-α.
STAT3 Activation and Nuclear Translocation During Osteoblasts Differentiation of AS Fibroblasts was Stimulated by TNF-α
Higher nuclear expressions of p-STAT3 and STAT3 were observed in groups with low TNF-α concentrations (0.01, 0.1 ng/mL), while the converse was seen in cytoplasmic STAT3 expressions (Figure 2A). The expression of nuclear STAT3 in the 0.1 ng/mL TNF-α concentration group was also highest compared with others (Figure 2A). In addition, we found that total STAT-3 expressions in AS fibroblasts gradually increased from day 0 to day 14, as evidenced by immunofluorescence analysis (Figure 2C and D). Our findings support the fact that TNF-α is responsible for STAT 3 activation and nuclear translocation during the process of osteoblasts differentiation of AS fibroblasts.
A Positive Feedback Loop Between STAT3 and MiR-21
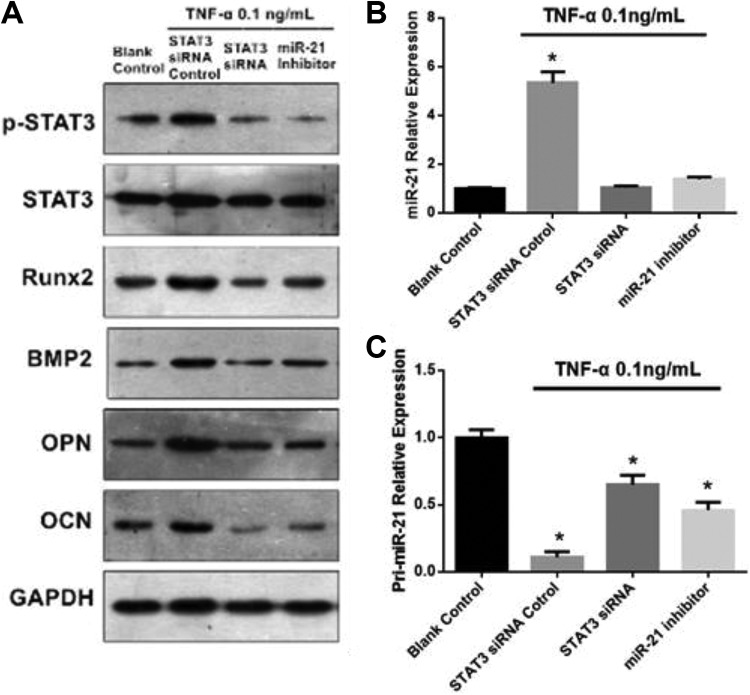
Small interfering RNA interference was used to modulate STAT3 expression in order to ascertain if STAT3 is responsible for miR-21 expressions under 0.1 ng TNF-α. Small interfering RNA-facilitated STAT3 silencing efficiency was confirmed with Western blot (Figure 3A). Primary miR-21 (pri-miR-21) expression was found to increase markedly in cells transfected with siSTAT3 in comparison to nontransfected cells (Figure 3B and C). The converse was observed in mature miR-21 expressions (Figure 3B and C). Markers of osteogenesis were significantly decreased in cells that were transfected with siSTAT3 (Figure 3A). Ankylosing spondylitis fibroblasts that had reduced miR-21 expression (achieved via gene knockdown with anti-miR-21 oligonucleotides) were also found to have suppressed STAT3 activation and lower levels of bone formation markers (Figure 4A).
Figure 3.
A, The effect of STAT3 siRNA and miR-21 inhibitor transfection on the protein expressions of p-STAT3, STAT3, and osteogenesis markers in AS fibroblasts exposed to 0.1 ng/mL TNF-α. B, The effect of STAT3 siRNA and miR-21 inhibitor on the miR-21 relative expression in AS fibroblasts exposed to 0.1 ng/mL TNF-α. C, The effect of STAT3 siRNA and pri-miR-21 inhibitor on the miR-21 relative expression in AS fibroblasts exposed to 0.1 ng/mL TNF-α. AS indicates ankylosing spondylitis; miR, MicroRNA; siRNA, small interfering RNA; TNF-α, tumor necrosis factor-α.
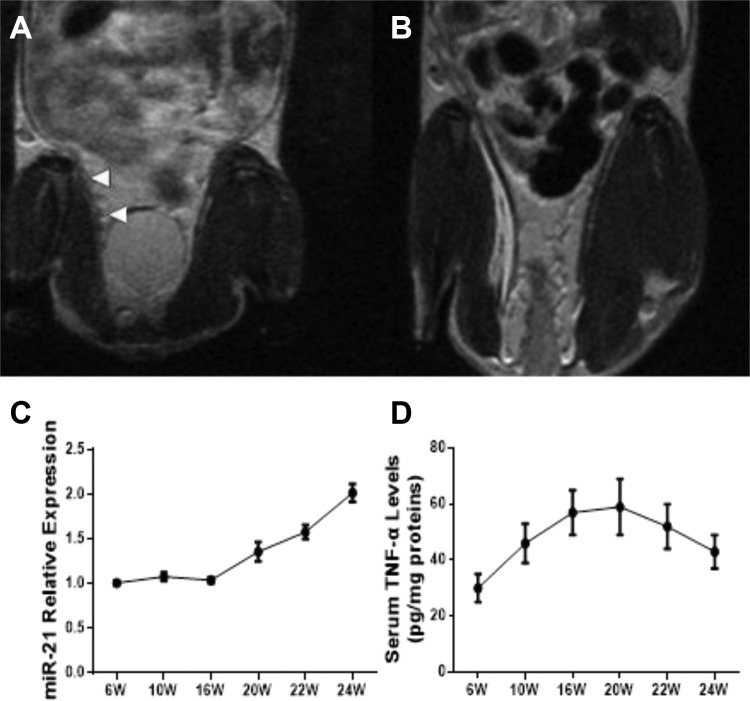
Figure 4.
A and B, Comparison of sacroiliac joint damage in PGIA mice (A) with naive control (B) based on MRI findings. C, Relative expressions of serum miR-21 in PGIA mice from 6 to 24 weeks. D, Expressions of serum TNF-α levels in PGIA mice from 6 to 24 weeks (6 mice for each group). miR indicates MicroRNA; MRI, magnetic resonance image; PGIA, proteoglycan-induced arthritis; TNF-α, tumor necrosis factor-α.
Exogenous MiR-21 Led to SIJ New Bone Formation and Joint Ankylosis Though Regulating JAK2/STAT3 Pathway
Proteoglycan-induced arthritis mice models were successfully established, as confirmed through MRI. Inflammation and SIJ lesion were present in the PGIA model in comparison to naive controls (Figure 4A and B). We examined the change of serum miR-21 expressions and TNF-α levels across 6 to 24 weeks and found a gradually increasing level of serum miR-21. On the other hand, TNF-α levels achieved its peak at 20 weeks and gradually tapered down after that (Figure 4C and D).
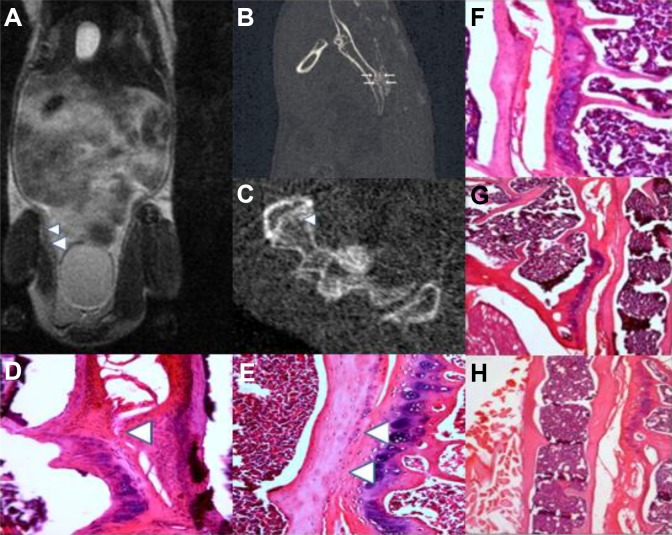
Based on HE staining, MRI and micro-CT examination, miR-21 agomir was noted to investigate a significant response in bony proliferation resulting in fusion of the SIJ by the 24th week (Figure 5A-E), compared to miR-21 antagomir and the control groups (Figure 5F and G). New bony formation was localized in the SIJ and did not extend to neighboring structures, such as enthesial sites along the SIJ. These findings were not observed in naive control mice (Figure 5H), indicating that miR-21’s effects on bone formation are dependent on a pro-inflammatory environment.
Figure 5.
Exogenous miR-21 agomir caused abnormal new bone formation in the SIJ. A, Exogenous miR-21 caused SIJ bony ankylosis as evidenced by MRI (White arrows indicating fusions). B and C, Exogenous miR-21 caused bony ankylosis and new bone formation as detected via Micro-CT and HE staining (White arrows indicating fusions). D, Exogenous miR-21 agomir caused new bone formation by HE staining (white arrows indicating new bone formation). E, Exogenous miR-21 agomir caused SIJ fusion as evidenced by HE staining(white arrows indicating fusions). F, miR-21 antagomir did not cause SIJ new bone formation. G, miR-21 antagomir negative control did not cause SIJ new bone formation. H, Exogenous miR-21 agomir did not cause SIJ new bone formation in naïve control (6 mice for each group). CT indicates computed tomography; HE, hematoxylin and eosin; miR, MicroRNA; MRI, magnetic resonance imaging; SIJ, sacroiliac joint.
MiR-21 Caused Abnormal Bone Formation by Interactive With JAK2/STAT3 Signal Pathway in PGIA Mice
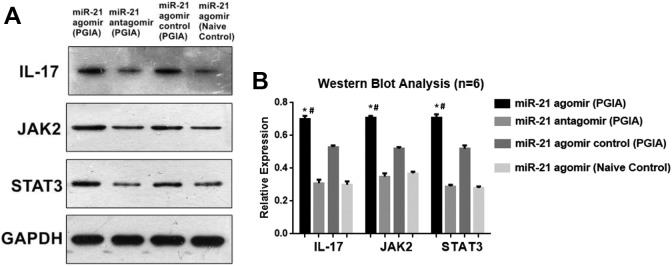
A Western blot analysis of IL-17, JAK2, and STAT3 expressions was done after miR-21 intervention. We found that the miR-21 agomir transfection into PGIA mice caused significantly elevated IL-17, JAK2, and STAT3 expressions in contrast to those seen in the miR-21 antagomir-transfected PGIA mice and naive control mice. On the other hand, although the differences of IL-17, JAK2, and STAT3 expressions between miR-21 agomir and miR-21 agomir control group had no differences, miR-21 agomir group still demonstrated incremental trends of IL-17, JAK2, and STAT3 expressions compared with miR-21 agomir control group (Figure 6A and B).
Figure 6.
A and B, Western blot analysis of IL-17, JAK2, and STAT3 expressions after miR-21 interventions (*P < .05 vs miR-21 antagomir; # P < .05 vs miR-21 agomir naïve control). IL-17 indicates interleukin-17; miR, MicroRNA.
Discussion
This study suggests that miR-21 may serve as a potential biological trigger in instigating osteogenesis in AS which works in a TNF-α dependent manner. There appears to be a positive feedback loop between miR-21 and STAT3. Upon exposure to a pro-inflammatory agent TNF-α at a concentration of 0.1 ng/mL, there was a marked elevation in osteogenic activity as determined by significantly increased levels of ALP, ARS, Runx2, BMP2, and OPN compared to other doses of TNF-α concentration. The miR-21 levels were decreased while pri-miR-21 levels were raised in cells transfected with STAT3-silencing siRNA. Additionally, miR-21 suppression resulted in lower STAT3 activation and subsequently lower levels of protein markers of osteogenesis. Importantly, this process was observed to take place in a pro-inflammatory microenvironment. Finally, exogenous miR-21 agomir injection caused new bone formation and SIJ fusion through interaction in the JAK2/STAT3 pathway in PGIA mice, a process that was not observed in naive controls. When interpreted as a whole, our results suggest that miR-21 is a potential link between inflammation and abnormal bony formation.
Our experiments reveal that miR-21 levels and osteogenic activity are highest only at certain TNF-α expressions (0.1 ng/mL) and are suppressed at higher TNF-α levels (1-10 ng/mL). Upon exposure to 0.1 ng/mL of TNF-α, we observed the highest miR-21 expressions and ALP activity as well as the most distinct ARS manifestation. Research over the years have repeatedly demonstrated TNF-α to play a central role in the development and progression of AS.23 Biological agents TNF-α have been widely integrated into AS treatment regimens.24 Nevertheless, although TNF-α inhibitors are able to suppress the clinical and laboratory manifestations of inflammation, their effect on abnormal bone formation is unclear and contradictory across several studies.25,26 A clinical quality management cohort study demonstrated that the progression of AS, as measured through spinal radiographs, could be inhibited by TNF blockers.27 Jeong et al suggests that a delay in initiating TNF inhibitor therapy may result in deterioration while early and long-term use may slow down spinal radiographic progression.28 This finding that is consistent with another study which shows that a reduction in spinal radiographic progression in patients who used TNF inhibitor for 4 years.29 Conversely, 3 registry studies failed to demonstrate the clinical benefits of isolated use of anti-TNF-α on AS radiographic progression.30-32 Moreover, other studies suggest that TNF inhibitor-facilitated resolution of inflammation resulted in higher syndesmophyte formation, a phenomenon that contributed to worsening SIJ bony abnormalities.33 It has been postulated that lower degrees of systemic inflammation are linked to worsening radiographic progression.34 These controversial results raise questions regarding the efficacy of TNF-α inhibitor on AS radiographic progression.
Therefore, it is important to clarify the relationship between TNF-α concentration levels and the rate of new bone formation. Osteogenic differentiation appears to be augmented in lower TNF-α concentrations, while higher, chronic doses of TNF-α lead to bony loss.35,36 Twenty-four hours low dose treatment with TNF-α markedly enhanced osteogenic differentiation of human primary osteoblasts through stimulation of a paracrine BMP2 loop.37 Osteogenic differentiation of murine MSCs was also found to be enhanced by low levels of TNF-α treatment.38 Exposing bone fracture sites to low doses of TNF-α accelerated bone repair.39
We also found exogenous miR-21 could cause SIJ joint fusion in a pro-inflammatory environment through activation of JAK2/STAT3 signaling as well as elevation of IL-17 levels. The JAK2/STAT3 signaling molecules are tightly related in DNA transcription-related pathways.40 Additionally, STAT3 and JAK2 are both known to modulate the Th17 subset of CD4T cells in several immune and inflammatory diseases.41
Bone metabolism is highly dependent on JAK2/STAT3-mediated osteoblast regulation.42 STAT3 functions as a novel transcription factor that interacts with polypeptide cell surface membrane receptors in order to trigger extracellular signaling such as growth factors and cytokines.43 Interleukin-23 appears to be the dominant STAT3 activator, triggering subsequent activation of JAK and STAT signaling molecules, which ultimately promotes secretion of IL-22, IL-17F, and IL-17A that stabilize Th17 cells.44 The STAT3 signaling inhibition suppresses the proliferation and ostegenic differentiation of BMSC.45 Previous studies have confirmed that genetic polymorphisms in STAT3 are associated with AS.46,47 Another study also found that a JAK2 genetic variant is associated with the incidence of AS among the Chinese population.48
Likewise, our results demonstrated a similar positive feedback loop that appeared to be responsible for abnormal osteogenesis in a pro-inflammatory environment. Previous studies show a miR-21-STAT3 interaction across several disease models such as cancer,49 angiogenesis,50 and fibrosis.51 To the best of our knowledge, this is the first study that documents the involvement of miR-21 and STAT3 in abnormal bone formation in AS. Interleukin-17 was noted to be augmented upon administration of exogenous miR-21 injection. This molecule has a dual role in AS, as it complements the pro-inflammatory effects of TNF-α, promotes bony destruction52 as well as bone formation at sites exposed to mechanical stress or inflammation.52
In conclusion, our study demonstrates that TNF-α exposure activated JAK2/STAT3 signaling which in turn served to modulate miR-21 expression. This signal cascade of miR-21/JAK2/STAT3 resulted in upregulation of osteogenesis-related proteins. Therefore, pharmaceutical interventions that specifically target miR-21 and the JAK2/STAT3 pathway may be a promising avenue in the innovation of future AS therapies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This article was funded by the National Natural Science Foundation of Guangdong Province of China (No.2017A030310293), National Natural Science Foundation of China (No.81703883), Southern Medical University Research Start-up Project (No.PY2017N031), China Postdoctoral Science Foundation Funded Project (No.2018M630971), 12th Special Foundation of China Postdoctoral Science (No,2019T120744), and the Project of Administration of Traditional Chinese Medicine Guangdong Provincial of China (No.20171176).
ORCID iD: Gang Liu  https://orcid.org/0000-0002-6426-3155
https://orcid.org/0000-0002-6426-3155
References
- 1. Vanaki N, Aslani S, Jamshidi A, Mahmoudi M. Role of innate immune system in the pathogenesis of ankylosing spondylitis. Biomed Pharmacother. 2018;105:130–143. [DOI] [PubMed] [Google Scholar]
- 2. Smith JA. Update on ankylosing spondylitis: current concepts in pathogenesis. Curr Allergy Asthma Rep. 2015;15(1):489. [DOI] [PubMed] [Google Scholar]
- 3. Watad A, Bridgewood C, Russell T, Marzo-Ortega H, Cuthbert R, McGonagle D. The early phases of ankylosing spondylitis: emerging insights from clinical and basic science. Front Immunol. 2018;9:2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sari I, Haroon N. Radiographic progression in ankylosing spondylitis: from prognostication to disease modification. Curr Rheumatol Rep. 2018;20(4):82. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- 6. Denli AM, Tops BB, Plasterk RH, et al. Processing of primary microRNAs by the microprocessor complex. Nature. 2004;432(5):231–235. [DOI] [PubMed] [Google Scholar]
- 7. Pauley KM, Cha S, Chan EK. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmun. 2009;32(4):189–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li Z, Wong SH, Shen J, et al. The role of MicroRNAS in ankylosing spondylitis. Medicine (Baltimore). 2016;95(1):e3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krichevsky AM, Gabriely G. MiR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13(1):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sugatani T, Vacher J, Hruska KA. A microRNA expression signature of osteoclastogenesis. Blood. 2011;117(13):3648–3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mei Y, Bian C, Li J, et al. MiR-21 modulates the ERK-MAPK signaling pathway by regulating SPRY2 expression during human mesenchymal stem cell differentiation. J Cell Biochem. 2013;114(6):1374–1384. [DOI] [PubMed] [Google Scholar]
- 12. Wei F, Yang S, Guo Q, et al. MicroRNA-21 regulates osteogenic differentiation of periodontal ligament stem cells by targeting smad5. Sci Rep. 2017;7(2):16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang N, Wang G, Hu C, et al. Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res. 2013;28(3):559–573. [DOI] [PubMed] [Google Scholar]
- 14. Xu K, Xiao J, Zheng K, et al. MiR-21/STAT3 signal is involved in odontoblast differentiation of human dental pulp stem cells mediated by TNF-α. Cell Reprogram. 2018;20(3):107–116. [DOI] [PubMed] [Google Scholar]
- 15. Sheedy FJ. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol. 2015;6(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murugaiyan G, Da Cunha AP, Ajay AK, et al. MicroRNA-21 promotes Th17 differentiation and mediates experimental autoimmune encephalomyelitis. J Clin Invest. 2015;125(3):1069–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sherlock JP, Joyce-Shaikh B, Turner SP, et al. IL-23 induces spondyloarthropathy by acting on ROR- ROR-γt+ CD3+CD4-CD8- entheseal resident T cells. Nat Med. 2012;18(7):1069–1076. [DOI] [PubMed] [Google Scholar]
- 18. He T, Huang Y, Zhang C, et al. Interleukin-17A-promoted MSC2 polarization related with new bone formation of ankylosing spondylitis. Oncotarget. 2017;8(57):96993–97008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang CH, Wei JC, Chang WC, et al. Higher expression of whole blood microRNA-21 in patients with ankylosing spondylitis associated with programmed cell death 4 mRNA expression and collagen cross-linked C-telopeptide concentration. J Rheumatol. 2014;41(6):1104–1111. [DOI] [PubMed] [Google Scholar]
- 20. Zou YC, Gao YP, Yin HD, Liu G. Serum miR-21 expression correlates with radiographic progression but also low bone mineral density in patients with ankylosing spondylitis: a cross-sectional study. Innate Immun. 2019;25(5):314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zou YC, Yang XW, Yuan SG, Zhang P, Ye YL, Li YK. Downregulation of dickkopf-1 enhances the proliferation and osteogenic potential of fibroblasts isolated from ankylosing spondylitis patients via the Wnt/β-catenin signaling pathway in vitro. Connect Tissue Res. 2016;57(3):200–211. [DOI] [PubMed] [Google Scholar]
- 22. Lin S, Qiu M, Chen J. Il-4 modulates macrophage polarization in ankylosing spondylitis. Cell Physiol Biochem. 2015;35(2):2213–2222. [DOI] [PubMed] [Google Scholar]
- 23. Van der Heijde D, Sieper J, Maksymowych WP, et al. 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis. 2011;70(6):905–908. [DOI] [PubMed] [Google Scholar]
- 24. Osman MS, Maksymowych WP. An update on the use of tumor necrosis factor alpha inhibitors in the treatment of ankylosing spondylitis. Expert Rev Clin Immunol. 2017;13(2):125–131. [DOI] [PubMed] [Google Scholar]
- 25. Zong HX, Xu SQ, Tong H, et al. Effect of Anti-tumor necrosis factor α treatment on radiographic progression in patient with ankylosing spondylitis: a systematic review and meta-analysis. Mod Rheumatol. 2018;15(2):1–19. [DOI] [PubMed] [Google Scholar]
- 26. Zhang JR, Liu XJ, Xu WD, et al. Effects of tumor necrosis factor-α inhibitors on new bone formation in ankylosing spondylitis. Joint Bone Spine. 2016;83(3):257–264. [DOI] [PubMed] [Google Scholar]
- 27. Molnar C, Scherer A, Baraliakos X, et al. TNF blockers inhibit spinal radiographic progression in ankylosing spondylitis by reducing disease activity: results from the Swiss Clinical Quality Management cohort. Ann Rheum Dis. 2018;77(1):63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jeong H, Eun YH, Kim IY, et al. Effect of tumor necrosis factor α inhibitors on spinal radiographic progression in patients with ankylosing spondylitis. Int J Rheum Dis. 2018;21(5):1098–1105. [DOI] [PubMed] [Google Scholar]
- 29. Maas F, Arends S, Brouwer E, et al. Reduction in spinal radiographic progression in ankylosing spondylitis patients receiving prolonged treatment with tumor necrosis factor inhibitors. Arthritis Care Res (Hoboken). 2017;69(7):1011–1019. [DOI] [PubMed] [Google Scholar]
- 30. van der Heijde D, Landewé R, Baraliakos X, et al. Radiographic findings following two years of infliximab therapy in patients with ankylosing spondylitis. Arthritis Rheum. 2008;58(10):3063–3070. [DOI] [PubMed] [Google Scholar]
- 31. Van der Heijde D, Landewé R, Einstein S, et al. Radiographic progression of ankylosing spondylitis after up to two years of treatment with etanercept. Arthritis Rheum. 2008;58(5):1324–1331. doi:10.1002/art.23471. [DOI] [PubMed] [Google Scholar]
- 32. van der Heijde D, Salonen D, Weissman BN, et al. Assessment of radiographic progression in the spines of patients with ankylosing spondylitis treated with adalimumab for up 2 years. Arthrtis Res Ther. 2009;11(4):R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maksymowych WP, Chiowchanwisawakit P, Clare T, Pedersen SJ, Østergaard M, Lambert RG. Inflammatory lesions of the spine on magnetic resonance imaging predict the development of new syndesmophytes in ankylosing spondylitis: evidence of a relationship between inflammation and new bone formation. Arthritis Rheum. 2009;60(1):93–102. [DOI] [PubMed] [Google Scholar]
- 34. Pedersen SJ, Sørensen I, Lambert RG, et al. Radiographic progression is associated with resolution of systemic inflammation in patients with axial spondylarthritis treated with tumor necrosis factor α inhibitors: a study of radiographic progression, inflammation on magnetic resonance imaging, and circulating biomarkers of inflammation, angiogenesis, and cartilage and bone turnover. Arthritis Rheum. 2011;63(12):3789–3800. [DOI] [PubMed] [Google Scholar]
- 35. Wang L, Zhang J, Wang C, et al. Low concentrations of TNF-α promote osteogenic differentiation via activation of the ephrinB2-EphB4 signalling pathway. Cell Prolif. 2017;50(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daniele S, Natali L, Giacomelli C, et al. Osteogenesis is improved by low tumor necrosis factor alpha concentration through the modulation of Gs-coupled receptor signals. Mol Cell Biol. 2017;37(8):e00442–004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lu Z, Wang G, Dunstan CR, Zreiqat H. Short-term exposure to tumor necrosis factor-alpha enables human osteoblasts to direct adipose tissue-derived mesenchymal stem cells into osteogenic differentiation. Stem Cells Dev. 2012;21(13):2420–2429. [DOI] [PubMed] [Google Scholar]
- 38. Huang H, Zhao N, Xu X, et al. Dose-specific effects of tumor necrosis factor alpha on osteogenic differentiation of mesenchymal stem cells. Cell Prolif. 2011;44(5):420–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Glass GE, Chan JK, Freidin A, Feldmann M, Horwood NJ, Nanchahal J. TNF-alpha promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc Natl Acad Sci U S A. 2011;108(4):1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chiba T, Yamada M, Aiso S. Targeting the JAK2/STAT3 axis in Alzheimer’s disease. Expert Opin Ther Targets. 2009;13(10):1155–1167. [DOI] [PubMed] [Google Scholar]
- 41. Xiu W, Ma J, Lei T, et al. Immunosuppressive effect of bladder cancer on function of dendritic cells involving of Jak2/STAT3 pathway. Oncotarget. 2016;7(1):63204–63214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu X, Li Z, Wan Q, et al. Inhibition of JAK2/STAT3 signaling suppresses bone marrow stromal cells proliferation and osteogenic differentiation, and impairs bone defect healing. Biol Chem. 2018;399(11):1313–1323. [DOI] [PubMed] [Google Scholar]
- 43. Zhang Y, Wang D, Xu J, et al. Stat3 activation is critical for pluripotency maintenance. J Cell Physiol. 2019;234(2):1044–1051. [DOI] [PubMed] [Google Scholar]
- 44. Parham C, Chirica M, Timans J, et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23 R. J Immunol. 2002;168(11):5699–5708. [DOI] [PubMed] [Google Scholar]
- 45. Yu X, Wan Q, Cheng G, et al. CoCl2, a mimic of hypoxia, enhances bone marrow mesenchymal stem cells migration and osteogenic differentiation via STAT3 signaling pathway. Cell Biol Int. 2018;42(10):1321–1329. [DOI] [PubMed] [Google Scholar]
- 46. Davidson SI, Liu Y, Danoy PA, et al. Association of STAT3 and TNFRSF1A with ankylosing spondylitis in Han Chinese. Ann Rheum Dis. 2011;70(2):289–292. [DOI] [PubMed] [Google Scholar]
- 47. Danoy P, Pryce K, Hadler J, et al. Association of variants at 1q32 and STAT3 with ankylosing spondylitis suggests genetic overlap with Crohn’s disease. PLoS Genet. 2010;6(12):e1001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saadi A, Dang J, Shan S, et al. Ankylosing spondylitis: analysis of gene-gene interactions between IL-12β, JAK2, and STAT3 in Han Chinese and Algerian cohorts. Cent Eur J Immunol. 2019;44(1):65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. van der Fits L, van Kester MS, Qin Y, et al. MicroRNA-21 expression in CD4+ T cells is regulated by STAT3 and is pathologically involved in Sézary syndrome. J Invest Dermatol. 2011;131(3):762–768. [DOI] [PubMed] [Google Scholar]
- 50. Chen LY, Wang X, Qu XL, et al. Activation of the STAT3/microRNA-21 pathway participates in angiotensin II-induced angiogenesis. J Cell Physiol. 2019;234(11):19640–19654. doi:10.1002/jcp.28564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cao W, Shi P, Ge JJ. MiR-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via CADM1/STAT3 pathway. BMC Cardiovasc Disord. 2017;17(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Torgutalp M, Poddubnyy D. IL-17 inhibition in axial spondyloarthritis: current and future perspectives. Expert Opin Biol Ther. 2019;19(7):1–11. [DOI] [PubMed] [Google Scholar]