Abstract
The gut microbiota (GM) is able to modulate the human immune system. The development of novel investigation methods has provided better characterization of the GM, increasing our knowledge of the role of GM in the context of hematopoietic stem-cell transplantation (HSCT). In particular, the GM influences the development of the major complications seen after HSCT, having an impact on overall survival.
In fact, this evidence highlights the possible therapeutic implications of modulation of the GM during HSCT. Insights into the complex mechanisms and functions of the GM are essential for the rational design of these therapeutics. To date, preemptive and curative approaches have been tested. The current state of understanding of the impact of the GM on HSCT, and therapies targeting the GM balance is reviewed herein.
Keywords: fecal microbiota transplantation, graft-vs-host-disease, gut microbiota, hematopoietic stem-cell transplantation, nutrition, postbiotic, prebiotic
Introduction
The human body is colonized in different sites by thousands of different bacterial species that have a symbiotic relationship with the human host and play a key role in maintaining our health or promoting disease.1 In the last 15 years, comprehension of the human microbiome has increased exponentially. In fact, identification of microbes, primarily from the gut, was achieved initially by means of cultures and characterization of microbial properties. Newer methods, involving the use of next generation DNA sequencing, both considering the whole metagenome and targeting a single marker gene, that is, the 16S rRNA gene, have allowed a more comprehensive evaluation of the composition and functionality of the human microbiome.2 The study of the gut microbiota (GM) has identified numerous microbial–host dynamics, which demonstrate the close link between human wellness and intestinal commensals, most notably involving their substantial influence on adaptive immunity.3,4 In contrast, dysbiosis, defined as a disruption of the natural balance of the microbiome composition, that is, changes in the healthy microbiota profile, may prolong, exacerbate, or induce detrimental health effects.5–8 Patients receiving allogeneic hematopoietic stem-cell transplantation (HSCT) are at high risk of infective, inflammatory, and immune-related complications, due to their underlying malignancies or immune-dysregulation, and to the extensive exposure to systemic antibiotics and chemotherapeutic agents, possibly causing major shifts in the GM. In particular, numerous laboratory and clinical observations have described the complex and multidirectional interplay between inflammation, the GM, and immune reactivity in the gut following HSCT.9,10 There is mounting evidence for the considerable effect of the human intestinal microbiome on clinical course following HSCT.
An understanding of these microbial shifts, and how they can influence complications following HSCT is crucial in understanding the deep relationship and interplay between the GM, immunity, and the intestinal barrier, and, subsequently, in developing strategies to prevent and treat transplant-related complications in this unique population. The following discussion will first provide a summary of present knowledge regarding alterations in the GM during HSCT, and the impact of these modifications on the main transplant-related complications. Second, given the impact of dysbiosis of GM on the clinical outcomes of HSCT, all microbiota-based strategies found in the literature to prevent or treat this condition will be discussed.
Gut microbiota during HSCT
HSCT and related procedures (conditioning regimen, antibiotic exposure, diet, anti-acid prophylaxis) represent a combination of upsetting events that profoundly modify the GM structure, leading to disruption of this mutualistic asset.9,11 In fact, the high biodiversity that typically characterizes the GM of a healthy subject is usually decreased after HSCT, and a significant increase in Enterococcus or Streptococcus is frequently observed.12 These changes in GM structure may have a profound impact on HSCT outcomes, affecting both transplant-related complications after HSCT, that is, infections and graft-versus-host-disease (GvHD) and the risk of relapse. In particular, it has been demonstrated that lower GM diversity at the engraftment is strictly related to transplant-related mortality.13 These observations have led to increased research interest in characterizing the role of the GM in the genesis of the principle HSCT complications.12
A first potential means of monitoring microbiome alterations was suggested by Weber and colleagues, who hypothesized that the decreased urinary excretion of 3-indoxyl sulfate, the major conjugate of indole produced from l-tryptophan by commensal bacteria, may be a marker of compromised GM.14 In their study, lower levels of this metabolite were correlated to a condition of intestinal dysbiosis characterized by a shift towards Enterococcus domination, defined as occupation of at least 30% of the microbiota by a single predominating bacterial taxon. The reduction of plasma levels of 3-indoxyl sulfate was also linked to reduced overall survival in the same cohort of patients.
Gut microbiota and transplant-related complications
Infections
Studies on gut microbiota and transplant-related complications are listed in Table 1. Preparative chemotherapy prior to transplant ablates circulating granulocytes and monocytes, while damage to gut epithelial cells mediated by radiation and chemotherapy enables commensal microbes to invade the underlying submucosa and eventually reach the bloodstream. As a result, systemic bacterial infections are frequent during the early post-transplant period, and may compromise the outcome of HSCT.15 Interestingly, patients with Escherichia coli and Klebsiella pneumoniae bloodstream infections have concomitant gut colonization with these organisms, suggesting that the damaged gut may possibly be the primary source of these infections.16
Table 1.
Review of the studies on GM in HSCT patients.
| Study | Patients | Aim | Main results | |
|---|---|---|---|---|
| Infections | Taur12 | 94 patients undergoing allo-HSCT | Characterizing the fecal microbiota of patients undergoing HSCT | Shift in intestinal diversity with domination by Enterococcus, Streptococcus, and various Proteobacteria |
| Harris17 | 94 patients undergoing allo-HSCT | To investigate whether changes in the GM are associated with pulmonary complications after HSCT | 112 PCs occurred in 66 (70.2%) patients and proteobacteria domination of fecal microbiota (HR, 2.64; 95% CI,1.10–5.65; p = 0.031) predicted PCs | |
| Ford18 | 161 patients with acute leukemia undergoing allo-HSCT | Association between VRE bacteremia and HSCT mortality | Mortality associated with postengraftment VRE bacteremia was higher than pre-engraftment bacteremia | |
| Tamburini16 | 30 patients who had bloodestram infection during HSCT | To define the gut microbiome as a potential reservoir of bloodstream pathogens in a cohort of HSCT recipients | Escherichia coli and Klebsiella pneumoniae bloodstream infections have concomitant gut colonization with these organisms. Typically nonenteric pathogens, such as Pseudomonas aeruginosa and Staphylococcus epidermidis were also found in the GM | |
| GvHD | Jenq10 | 18 Patients undergoing HSCT and GvHD murine models | To define variations in gut microbiome during GvHD | Shifts in both the human and the mouse microbiomes: loss of overall diversity and expansion of Lactobacillales and loss of Clostridiales. Eliminating Lactobacillales from the flora of mice before BMT aGvHD, whereas reintroducing the predominant species of Lactobacillus mediated significant protection against GvHD |
| Levine19 | 116 patients with gut GvHD | Evaluation of Paneth cells in duodenal biopsies | Lower numbers of Paneth cells at diagnosis correlated with clinically more severe GI GvHD (p < 0.0001) and less likelihood of response to GvHD treatment (p < 0.0001) | |
| Holler9 | 31 patients receiving HSCT | To analyze variations in the composition of the intestinal microbiome in the course of HSCT with next generation sequencing | Profound switch to Enterococcal domination in patients who subsequently developed or suffered from active gastrointestinal GvHD | |
| Jenq20 | 64 patients undergoing HSCT | To evaluate the role of intestinal bacteria in GvHD pathophysiology | Bacteria belonging to the genus Blautia were associated with reduced GvHD lethality while loss of Blautia was associated with treatment with antibiotics and receiving total parenteral nutrition | |
| Biagi11 | 10 pediatric patients undergoing HSCT | To define microbiome reconstruction in patients who suffered from gut GvHD | Non-GvHD and GvHD patients had different microbiome recovery after HSCT, the latter having a decrease in Faecalibacterium and an increase in Enterococcus | |
| D’Amico21 | 8 pediatric patients undergoing HSCT | To define the pattern of antibiotic resistance genes provided by the gut microbiome in patients undergoing HSCT | Patients developing aGvHD were characterised by post-HSCT expansion of their gut resistome, with the acquisition of new resistances as well as the consolidation of those already present before HSCT | |
| Biagi22 | 36 pediatric patients undergoing HSCT. | To explore the GM trajectory in 36 pediatric HSCT recipients in relation to aGvHD onset. | Children developing gut aGvHD had a dysbiotic GM layout before undergoing HSCT. This state was characterised by reduced diversity, lower Blautia content and an increase in Fusobacterium abundance. At the time of engraftment, the GM structure underwent a deep rearrangement in all patients. Regardless of the occurrence of aGvHD, it reacquired an eubiotic configuration from day 30. | |
| Relapse | Peled23 | 541 patients undergoing HSCT | To examine the relationship between the abundance of microbiota species and relapse/progression of disease during 2 years of follow-up after allo-HSCT | Higher abundance of a bacterial group, for the most part composed of Eubacterium limosum in the validation set was associated with a decreased risk of relapse/progression of disease |
aGvHD, acute graft versus host disease; BMT, bone marrow transplant; CI, confidence interval; GI, gastrointestinal; GM, gut microbiota; GvHD, graft versus host disease; HR, hazard rate; HSCT, hematopoietic stem-cells transplantation; PCs, pulmonary complications; VRE, Vancomycin-resistant enterccoccal.
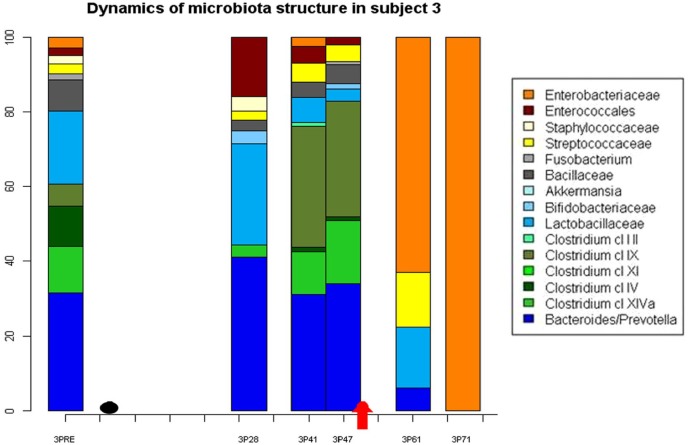
In 2012, Taur and colleagues observed extreme shifts in the intestinal microbiota composition in two-thirds of 94 patients after HSCT, with a domination of either vancomycin-resistant Enterococcus, Streptococcus, or aerobic gram-negative bacteria belonging to the phylum Proteobacteria. These perturbations correlated with the development of a corresponding bloodstream infection caused either by vancomycin-resistant Enterococcus or gram-negative bacteria, suggesting that intestinal domination in a subset of HSCT patients may precede a bloodstream infection (Figure 1).12 Regarding the importance of vancomycin-resistant Enterococcus/gram-negative infections, Ford and colleagues obtained some interesting results, observing that pre-HSCT colonization was not associated with increases in HSCT mortality, but with a higher risk of vancomycin-resistant Enterococcus bacteremia, and, possibly, bacteremia from other organisms. On the other hand, postengraftment vancomycin-resistant Enterococcus colonization was associated with increased mortality, explained largely by associated severe GvHD and relapsed leukemia.18
Figure 1.
Example of the extreme shift in GM composition characterized by intestinal domination of Enterobacteriaceae 71 days after HSCT, resulting in a bloodstream infection in a patient with gut GvHD (Black plot: day of HSCT, arrow: day of onset of GvHD).
GM, gut microbiota; GvHD, graft-versus-host disease; HSCT, hematopoietic stem-cell transplantation.
Pulmonary complications
Intestinal permeability and microbiome modifications may also be implicated in the translocation of bacteria or bacterial-derived molecules, such as lipopolysaccharides, to the lung, causing acute lung injury and sepsis-associated acute respiratory distress syndrome, events that are frequent after engraftment, and may compromise patient outcome. Harris and colleagues investigated the role of the microbiome in gut–lung crosstalk after HSCT, aiming to evaluate its role in the onset of pulmonary complications.17 They found 112 pulmonary complications (defined by radiographic parenchymal abnormalities in the setting of respiratory signs/symptoms and associated mortality) in 66 allotransplanted patients: high comorbidity index, exposure to fluoroquinolones, low-baseline diversity, and, interestingly, Gammaproteobacteria domination of GM were predictors of pulmonary complications. Gammaproteobacteria is a class of bacteria that includes respiratory pathogens, such as K. pneumoniae. These findings may indicate direct translocation of bacteria to the lungs during the early post-transplant phase, or indirect lung injury caused by microbiota stimulation of an inflammatory response as a possible mechanism; alternatively, this association might reflect overall microbiota status or antimicrobial use.
Graft versus host disease
Gut GvHD is the result of conditioning toxicity and immune activation associated with injury of the stem-cell compartments along with Paneth and goblet cells in the intestinal mucosa.24 This leads to increased intestinal permeability, inflammation, and reduction of the mucous membrane.25 In addition, the concurrent intensive antibiotic exposure in these patients can cause additional gastrointestinal damage.
A possible impact of the GM on GvHD was proposed in the 1970s, when experiments on germ-free mice demonstrated that, in this condition of being germ free, the tendency to develop gut GvHD was decreased.26,27
More recent studies on mice have demonstrated that GvHD is associated with specific dysbiosis, characterized by an increase in Enterobacteriales and a reduction in obligate anaerobic bacteria from the order Clostridiales.10,28,29 Data found in adult, and recently also in pediatric patients undergoing HSCT confirmed the results seen in murine models. In particular, with respect to non-GvHD patients, GvHD onset in humans has been associated with a drop in the abundance of the known health-promoting Faecalibacterium belonging to the order Clostridiales and higher percentages of Enterococcus. These latter microorganisms are potential contributors to increasing intestinal inflammation in both mice and humans, by means of the induction of interleukin (IL)-1 and IL-6 production, resulting in subsequent Th1 and Th17 activation.9 Another confirmation that bacteria of the order Clostridiales seems to protect from lethal GvHD was provided by Jenq and colleagues, who discovered that a higher relative abundance of bacteria belonging to the Clostridiales genus Blautia, a key contributor in maintaining an anti-inflammatory milieu in the gut, was associated with reduced GVHD lethality.20
Two different studies have found another interesting connection between dysbiosis and Paneth cells; these latter cells have been demonstrated to regulate microbial communities thanks to the secretion of alfa-defensis, peptides that kill noncommensal bacteria. The Paneth cells are ‘targeted’ by GvHD, and their destruction causes a drop in alfa-secretins, favoring the growth of E. coli, a bacteria that causes septicemia and stimulates gut GvHD, creating a negative loop for HSCT patients.19,28
Interestingly, patients with GvHD and inflammatory bowel disease share a similar pattern of dysbiosis, indicating that there might be a link between the microbiome and autoimmune disease.6,30 As seen in Crohn’s disease, clinical and experimental evidence suggests that TLR4 mutations (receptor for lipopolysaccharides) provide a lower risk of acute GvHD (aGvHD), and host mutations in the gene encoding intracellular peptidoglycan receptor nucleotide-binding oligomerization domain-containing 2 (NOD2/CARD15) have been associated with aGvHD. This association was reduced in patients with gram-positive decontamination, suggesting the important role of the interaction between immunity and the GM in GvHD.31–33
It should also be considered that the distinctive trajectory of post-HSCT GM reconstruction in GvHD and non-GvHD subjects is influenced by the pre-HSCT GM signature.11,22 Non-GvHD subjects have a higher abundance of bacterial groups known to produce short chain fatty acids (SCFAs), that is, propionate-producing Bacteroidetes and acetate-producing Blautia. SCFAs are well-studied end products of microbial fermentation of complex plant polysaccharides that cannot be digested by humans because of the insufficient repertoire of glycoside hydrolases in the human genome. SCFAs have shown a diverse array of immune-modulatory functions, both locally and systemically,34 and are one of the clearest examples of how diet and nutrient processing by the microbiota combine to shape immune responses.35
Finally, D’Amico and colleagues explored the gut resistome, that is, the total load of antibiotic resistance genes provided by the GM, in eight pediatric HSCT recipients, half of whom developed aGvHD. According to their findings, patients developing aGvHD showed post-HSCT expansion of their gut resistome, involving the acquisition of resistance not already present at the time of transplant, such as to macrolides, aminoglycosides, tetracyclines, and beta-lactams, stressing that alterations of the microbiome during GvHD might play a significant role in the clinical management of patients.21
Relapse
Since the close interaction between the GM and the immune system has been thoroughly explored and described during the last 15 years, the possible influence of the GM on the Graft versus Leukemia effect, and, thus, on the risk of relapse of the underlying malignancy following HSCT, should be considered. In a retrospective study, Peled and colleagues analyzed the abundance of microbiota species or groups of related species in 541 patients undergoing HSCT. They examined the relationship between the relapse/progression rate of disease in the 2-year period after HSCT and GM composition. Higher abundance of a bacterial group composed for the most part of Eubacterium limosum was associated with a decreased risk of relapse/progression of disease. These data suggested that the abundance or presence of some bacterial groups in the intestinal ecosystem might serve as potential biomarkers or therapeutic targets to prevent relapse and improve survival after HSCT.23
Modulation of gut microbiota in HSCT
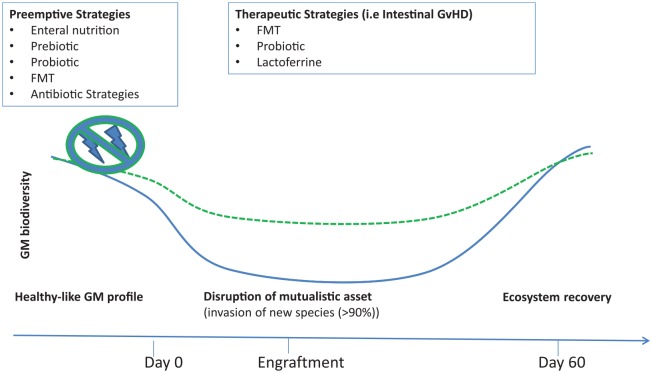
The confirmation of the strong impact of the GM on all aspects of HSCT led to the idea of modulating GM composition in order to improve clinical outcomes. The main interventions described in the literature are reviewed herein (Figure 2).
Figure 2.
Potential strategies, preemptive and therapeutic, used for preventing or treating GM dysbiosis during HSCT are summarized.
GM, gut microbiota; HSCT, hematopoietic stem-cell transplantation.
Antibiotics
As already mentioned,26,27 the observation that germ-free mice were less likely to develop GvHD introduced the idea of gastrointestinal decontamination in HSCT recipients using nonabsorbable antibiotics as well as isolation in a protective environment; however, this showed mixed results.36–38 GM dynamics were analyzed in patients undergoing gut decontamination, comparing results in children receiving total or selective decontamination. In both groups, GM richness and diversity decreased markedly, but were restored gradually after cessation of antibiotics. In the selective group, GM composition was relatively more stable and dominated by Bacteroides, while, in some of the patients receiving total decontamination, a shift towards Enterococcus or Streptococcus domination was observed.39 Weber and colleagues compared gut decontamination using ciprolfloxacin and metronidazole, or rifaxim only, which is a poorly absorbed oral antibiotic already used in inflammatory bowel disease.40–42 This latter antibiotic has some activity against Enterobacteriaceae,43 and may induce eubiotic changes in the intestinal ecosystem.44 These authors found a significant reduction in gut GvHD and 1-year transplant related mortality, and a significant increase in overall survival, with less enterococcal load and higher urinary 3-indoxyl sulfate concentrations in the rifaximin group. Furthermore, treatment of infectious complications with systemic antibiotics did not abrogate the beneficial effects of rifaximin on GM composition and on HSCT outcomes.45
All this evidence suggests that different classes of antibiotics have significantly diverse impacts on GM structure, leading to different respective impacts on clinical outcomes. First, extreme shifts in the GM composition and development of intestinal domination were found in a significant number of transplanted patients. In fact, vancomycin and metronidazole, both active against anaerobes, have been associated with Enterococcus domination, while the use of fluoroquinolonics decreased the risk of proteobacteria domination.12,46 A potential role for anaerobic bacteria, in particular Clostridiales, was supported by the observation that Blautia concentration was correlated inversely with the risk of developing gut GvHD.20 Clindamycin, an antianaerobic agent, increased the risk of GvHD by depleting anti-inflammatory clostridia.47 Moreover, cumulative antibiotic exposure with penicillin derivatives and carbapenems, both antibiotics active against anaerobic bacteria, was associated with a higher incidence of intestinal aGvHD.48 In particular, piperacillin-tazobactam and imipenem-cilastatin were associated with increased incidence, severity, and mortality in gut GvHD. Piperacillin-tazobactam also reduced Bacteroidetes and Lactobacillus.49 Furthermore, the cumulative incidence of aGvHD was significantly higher in patients receiving fourth-generation cephalosporins.50 On the other hand, the use of antibiotics that preserve anaerobic commensal bacteria, such as aztreonam or cefepime, reduced GvHD-related mortality. All these findings have suggested that the use of antibiotics sparing anaerobic bacteria may potentially reduce the risk of developing gut GvHD. This was obtained by different strategies involving narrow-spectrum antibiotics and modulating the timing and duration of treatment.
New specific molecules preventing dysbiosis
Lactoferrin is an iron-binding protein with pleiotropic functions, such as antimicrobial, anti-inflammatory, immunoregulatory, and anticancer activity, and is also involved in intestinal epithelial regeneration and iron homeostasis. Lactoferrin and N-terminal peptide-derivatives have been studied in preclinical models, and seem to reduce bacterial translocation, improving GM eubiosis.51,52 Rota and colleagues tested the administration of lactoferrin in an HSCT patient, and showed that symptoms of gut GvHD disappeared soon after lactoferrin therapy was started.53,54
Nutrition and prebiotics
Type of nutritional supplementation
The connection between nutrition and the human microbiome in maintaining human health has been reported in great detail.55–59 On the contrary, the effect of parenteral nutrition and starvation on the intestinal ecosystem during HSCT has been poorly evaluated. The role of fasting on GM is difficult to assess in healthy subjects, but it has been shown to decrease microbial richness and diversity.60,61 Parenteral nutrition for more than 10 days has been associated with the loss of commensal bacteria belonging to the genus Blautia. Moreover, parenteral nutrition induces gut mucosal atrophy, promoting bacterial translocation and altering SCFA production.20,62–69 Although there are no studies comparing microbiome composition between patients receiving enteral nutrition and parenteral nutrition, clinical data show that enteral nutrition is associated with better outcomes in terms of survival, infection, and aGvHD.70–75 Certainly, the role of the type of nutritional support in preserving GM during HSCT is worth exploring in future studies.
Prebiotics
Prebiotics are defined as ‘a substrate which is selectively utilized by host microorganisms conferring a health benefit’.76 This term usually refers to indigestible carbohydrates that are fermented in the colon by commensal bacteria, resulting in the production of SCFAs, metabolites with a potential immunomodulatory role.43 Several nutritional strategies have been studied in settings other than HSCT in order to modify the GM, such as supplementation of inulin and fructooligosaccharides, xylo-oligosaccharides, galacto-oligosaccharides, and potato starch. Tavil and colleagues utilized a diet richer in fibre in a patient in the pre-HSCT period, which correlated with earlier neutrophil engraftment and a shorter duration of febrile neutropenia.77 The retrospective trial of Iyama and colleagues evaluated enteral supplementation with prebiotics in HSCT. They found that preemptive enteral supplementation with a combination of glutamine, fiber, and oligosaccharides reduced days of moderate and severe intestinal involvement and was associated with better survival at day +100. In particular, a reduction in the severity of mucositis and the need for intravenous alimentation, as well as a reduction in weight loss and the gut bacterial translocation of Enterococcus species, thus lowering the gastrointestinal mucosal damage, was described.78
Probiotic and fecal microbiota transplantation
Single probiotics
Probiotics are defined as ‘live microorganisms, which, when administered in adequate amounts, confer a health benefit on the host’.79 Their use has been studied in a variety of gastrointestinal diseases with mixed results.80–84
Probiotics consist of traditional and commonly eaten foods; a probiotic-rich diet prior to HSCT is associated with earlier neutrophil engraftment and a shorter duration of febrile neutropenia.77 There are two studies in the literature regarding HSCT recipients that focus on the use of single strains of microorganisms. Gorshein and colleagues started a randomized probiotic enteric regimen trial in which allogeneic HSCT patients were supplemented with Lactobacillus rhamnosus GG, and found no appreciable alteration in the GM or protection against GvHD.85 Sharma and colleagues conducted a phase II trial, lacking a control group, in which the preventive use of Lactobacillus brevis CD2 lozenges seemed to reduce the incidence, duration, and severity of oral mucositis.86
On the other hand, there are some concerns regarding the safety of administering living microorganisms to immunocompromised patients presenting with, in addition to other symptoms, altered gut permeability. For instance, some authors have reported bacteremia and sepsis sustained by pathogens normally considered probiotics,87–90 and, in one report of a child undergoing HSCT, the infection resulted in meningitis.91 However, the analysis by Cohen and colleagues regarding HSCT patients supports the safety of probiotics, showing that organisms frequently incorporated in available over-the-counter probiotics are infrequent causes of bacteremia after HSCT.92 The study also evaluated the safety and feasibility of the probiotic Lactobacillus plantarum in 30 children and adolescents undergoing HSCT, and found no related bacteremia or adverse event.93
Fecal microbiota transplantation
Fecal microbiota transplantation (FMT) consists of the infusion of fecal matter from a healthy donor into the gastrointestinal tract of a recipient patient harboring dysbiotic GM. It is considered by some authors to be the ‘ultimate probiotic’, because it directly modifies the host’s intestinal microbiome composition in order to restore eubiosis and gut homeostasis.94–96
Fecal microbiota transplantation was first used for the treatment of recurrent Clostridium difficile infections (rCDI), and was found to be effective; it is being evaluated in other fields of medicine, including HSCT.97,98 Table 2 summarizes the main studies regarding FMT in HSCT. Some practical issues are addressed in order to better contextualize the topic. The source of fecal material can be either the patient themselves or a healthy donor. For patients receiving autologous FMT, stool samples should be collected in a period of clinical history with relatively good health, ensuring enough time to collect, screen, and process the patient’s stool for the moment of administration. For patients receiving FMT from a donor, the choice is between a related or an unrelated donor. Because of the genetic similarity and shared environment, a related FMT donor may have a GM composition closer to the recipient’s before the HSCT-induced dysbiosis. However, time to screen, collect, and process is needed for related donors, whereas unrelated healthy FMT donors fecal material can be collected and stored frozen in a stool bank, ready to use when needed.95,96 Donor screening is a key factor in the safety of the procedure in order to prevent iatrogenic infectious diseases potentially transmittable to the recipient.94 There are different ways of administering FMT, such as colonoscopy, esophago-gastro-duodenoscopy, nasogastric or nasoduodenal tube, enema, and oral capsule; however, none of them have shown clear superiority.99 The choice must be based on the balance of risk and benefits for each method, considering the clinical setting in which FMT is performed. It is worth mentioning that an oral capsule seems to maintain the efficacy and safety of other routes, and is less invasive for the patient,100 even if a substantial number of capsules is required to achieve the necessary microbial load.101 Optimization of all these practical aspects still needs to be addressed in the future.
Table 2.
Review of the studies about FMT in HSCT patients.
| Study | Patients | Route of administration | Main results | ||
|---|---|---|---|---|---|
| PREEMPTIVE | ARB | Bilinski102 | 20 with blood disorders colonized with ARB | Nasoduodenal tube | 60% of patients achieved complete ARB decolonization at 1 month after FMT |
| Innes103 | 1 in preparation for HSCT for Ph+ ALL | Nasogastric tube | Repeat rectal screening 7 days after FMT showed no evidence of GES-5 K. oxytoca CPE or C. difficile. By day +16 after FMT, neither CPE nor ESBL was detected on rectal screening swabs | ||
| Battipaglia104 | 10 colonized by multidrug-resistant bacteria in preparation for HSCT | Enema or nasogastric tube | Decolonization was achieved in 7 out of 10 patients. No serious adverse events were reported; 1 case of grade III gut aGvHD occurred after FMT performed before HSCT | ||
| Dysbiosis | Taur105 | 25 HSCTs with high pre-HSCT microbial diversity and without rCDI | Enema | 14 patients revealed boosted microbial diversity after FMT and reestablished the intestinal microbiota composition they had before antibiotic treatment and allo-HSCT | |
| DeFelilpp106 | 13 HSCTs for AML, MDS, NHL, CML | Oral capsules | Improving intestinal microbiome diversity associated with expansion of stool-donor taxa; 1 treatment-related abdominal pain. Two patients subsequently developed gut aGvHD, one patient presented concurrent bacteremia | ||
| Therapeutic | rCDI | Neemann107 | 1 HSCT for ALL | Nasojejunal tube | Patient symptoms resolved within 48 h and did not show signs of recurrence in 2 months of follow up |
| De Castro108 | 1 HSCT for ALL | Push enteroscopy | Patient symptoms resolved within 48 h without any adverse effects, and no recurrence of symptoms in 10 months after the FMT | ||
| Mittal109 | 1 auto-HSCT for B-cell lymphoma | Enema | Resolution of symptoms. After 6 months, the rCDI recurred and was treated successfully with another FMT | ||
| Webb110 | 7 HSCTs | Nasojejunal tube | Six patients (85.7%) had no recurrence after the first FMT. One patient needed two FMTs to reach the absence of recurrence | ||
| Bluestone111 | 3 HSCTs for AML, DiGeorge Syndrome and Hurler Syndrome | Nasogastric tube and colonoscopy | In three patients, the infection resolved but, in two patients, the rCDI recurred and required additional FMT | ||
| Moss112 | 8 HSCTs for AML, ALL, NHL, DLBCL | Oral capsules | All the patients treated achieved resolution in 8 weeks, and only 1 had a recurrence | ||
| Steroid R-D Gut aGvHD | Kakihana113 | 4 HSCTs for AML. Grade II-IV GvHD | Nasoduodenal tube | All patients responded to FMT, 3 had a complete response and 1 a partial response within 7–14 days; in 3 cases, a second FMT was needed | |
| Spindelboeck114 | 3 HCSTs for AML and MDS. Grade IV GvHD | Instillation into the terminal ileum and cecum via colonoscopy | Two patients achieved complete response with multiple FMTs while the third obtained a partial response still presenting a grade I GvHD after one course of FMT | ||
| Qi115 | 8 HSCTs for AML, ALL, CML, MDS. Grade IV gut GvHD | Nasoduodenal tube | All patient symptoms were relieved; 5 of them achieved complete response and had no recurrence | ||
| Von Lier116 | 15 HSCTs | Nasoduodenal tube | 11 patients showed a complete remission; however, 5 relapsed during cortisone therapy tapering | ||
| Kaito117 | 1 HSCT for Ph+ ALL | Oral capsules | Digestive symptoms improved soon after the initiation of FMT; GvHD improved to stage 1 after the second cycle of FMT with improvement in the endoscopic findings | ||
| Zhang118 | 1 HSCT for AML | Nasoduodenal tube | Improvement of symptoms with 3 recurrences and the need for additional FMT |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ARB, antibiotic resistant bacteria; CML, chronic myelogenous leukemia; DLBCL, diffuse large B-cell lymphoma; FMT, fecal microbiotic transplantation; HSCT, hematopoietic stem-cells transplantation; MDS, myelodysplastic syndromes; NHL, non-Hodgkin lymphoma; Ph, Philadelphia; rCDI, recurrent Clostridium difficile infections; Steroid r-d gut aGvHD, steroid refractory or resistant gut acute GvHD.
In the literature, FMT during HSCT has been used as either a preventive or a therapeutic strategy. The former has the aim of reducing dysbiosis and the growth of antibiotic-resistant bacterial strains. Bilinski and colleagues treated patients with blood disorders (40% neutropenic, 8% with chronic GvHD) colonized with antibiotic-resistant bacteria who underwent FMT via a nasoduodenal tube from unrelated donors; 60% of patients achieved complete decolonization of resistant species at 1 month after FMT.102 Innes and colleagues showed the possibility of performing FMT before HSCT to extensively eradicate drug-resistant organisms.103 After these two encouraging reports, 10 adult patients colonized by multidrug-resistant bacteria underwent FMT after (n = 6) or before (n = 4) HSCT, with stools either from related or unrelated donors, delivered via enema or nasogastric tube. Three patients needed a second transplant from the same donor due to initial failure of the procedure. Decolonization was achieved in 7 out of 10 patients. No serious adverse events were reported. Interestingly, one case of grade III gut aGvHD occurred after FMT performed before HSCT.104 FMT to prevent and decrease dysbiosis is more difficult to assess because there is no clear endpoint as in other indications. Autologous FMT after HSCT was performed in a randomized, controlled clinical trial. Compared with the control group without treatment, 16S rRNA gene sequencing of 14 patients after FMT revealed boosted microbial diversity and reestablishment of the GM composition they had before antibiotic treatment and allo-HSCT.105 A similar result was obtained using FMT from unrelated donors, administered orally in 13 patients, resulting in improved GM diversity associated with expansion of the stool-donor taxa. One treatment-related significant adverse event (abdominal pain) occurred, two patients subsequently developed acute gut GvHD, and one patient presented concurrent bacteremia.106
As mentioned before, FMT can be used with a therapeutic aim both in the context of rCDI and as a second line agent for aGvHD of the gut. Regarding rCDI, six studies have addressed this topic in the HSCT setting. Neeman and colleagues and De Castro and colleagues published two case reports regarding the successful treatment of rCDI after HSCT by injecting FMT via a nasojejunal tube, one from a related donor and the other from two different unrelated donors. In both cases, remission was obtained without adverse effects.107,108 Mittal and colleagues described the case of a patient who had undergone HSCT 1 year previously, receiving FMT from an unrelated donor via enema, which provided resolution of symptoms. At 6 months after the first FMT, a second FMT was performed using a different donor due to a second episode of rCDI, again achieving complete resolution.109 Since then, three small series have been published. Webb and colleagues published a study in which seven HSCT recipients underwent FMT from an unrelated donor after a median time of 635 days from HSCT; five of these patients were still under immunosuppressive therapy. Fecal microbiota transplantation was administered via nasojejunal tube or colonoscopy with only minor post-FMT adverse effects and no serious events. Six patients had no relapse, while one needed another FMT to obtain remission.110 Another series by Bluestone and colleagues reported FMT administration in three pediatric HSCT recipients from related and unrelated donors via a gastric tube or colonoscopy. No adverse events were reported, but only one achieved rCDI remission.111 Finally, Moss and colleagues published a study in which FMT was delivered as oral, encapsulated therapy using stools from unrelated donors in eight patients. All the patients treated achieved resolution in 8 weeks, and only one had a recurrence at a later time. Interestingly, metagenomic analysis of the stools in two patients suggested limited durability of the specific bacterial composition introduced with FMT, with short-term similarity and long-term dissimilarity between donor and recipient GM composition.112
Due to the link between GM and GvHD, FMT has been investigated as a potential therapeutic strategy for steroid-resistant or steroid-dependent gut GvHD. At present, four case series exist in the literature. Kakihana and colleagues performed FMT via a nasoduodenal tube from a related donor a median of 92 days after HSCT in four patients affected by steroid-resistant GvHD. Complete response was observed in three patients and partial response in one case. Improvement of gastrointestinal symptoms was observed within several days, and, in three cases, a second FMT was needed. An increase in peripheral effector regulatory T cells during response to FMT was also observed.113 Subsequently, Spindelboeck and colleagues reported the use of FMT from related and unrelated donors, delivered by colonoscopy, in three patients with refractory grade IV gut GvHD. Two patients achieved complete response with multiple FMT, while the other obtained a partial response, still presenting grade I GvHD after one course of FMT. A 16S rRNA gene analysis seemed to correlate FMT response to increased microbial diversity and richness.114 Another study by Qi and colleagues involved eight patients with refractory grade IV gut GvHD receiving one or two courses of FMT from unrelated donors via a nasoduodenal tube. All symptoms were relieved, and, in particular, five patients experienced complete response and no relapse. These eight patients achieved higher progression-free survival as compared with the control group. After FMT, the diversity of microbiota of the patients showed similarities with that of the donors, particularly in the composition of beneficial bacteria.115 A last case series by Von Lier and colleagues reported 15 patients who received a single FMT via nasoduodenal infusion from an unrelated donor. The FMT was well tolerated, and there were no serious adverse events. A total of 11 patients showed complete remission; however, 5 of them relapsed during cortisone therapy tapering. One week after FMT, the fecal microbial composition of patients with complete response, defined as resolution of all GvHD symptoms 4 weeks after FMT without other interventions to alleviate symptoms, resembled that of the donors.116 Two more case reports deserve mention: Kaito and colleagues reported a case of refractory gut GvHD treated effectively and safely using FMT with oral capsules, representing the only case to date in the literature.117 Zhang and colleagues treated a case of post-HSCT diarrhoea in an adult patient with repeated FMTs from two unrelated infants, one with mixed feeding (formula and complementary food) and one exclusively breastfed, leading to an improvement in symptoms. After FMT, both bacterial richness and diversity improved, but, in the case of the donor with mixed feeding, a greater proportion of Lactobacillus and Bifidobacterium among others was observed.118
FMT is an up-and-coming strategy that can be used in different situations and with different purposes in HSCT recipients. Despite being deemed safe by all the case reports or case series revised in this study, there are still some concerns regarding the administration of living microorganisms to immunocompromised patients with altered intestinal permeability.101,119 For example, infectious complications after FMT have been reported in other settings,120,121 and they may be of key relevance regarding the risk and benefit balance of this procedure in HSCT recipients.
Conclusions and future strategies
The ever-growing field of research regarding the role of the microbiome during HSCT has shed light on the complex interaction between the GM, the intestinal mucosa, and the immune system, allowing a better understanding of the influence of the GM on HSCT outcomes, Some topics that the authors believe could represent important strategies of research in the near future are discussed herein.
The major effort until now has been concentrated on characterization of the GM; however, new evidence shows the important correlation between the immune system and the symbiont microbial communities living in the human body, which are found in district-specific microbiomes. As suggested by the Human Microbiome Project, in the future, it will be necessary to study oral, skin, and airway microbiota in HSCT to improve the understanding of the correlation between the different microbiotas and their role in the development of the main complications of HSCT.122–124,137
In addition, thanks to next-generation DNA sequencing, metabolomics and the utilization of gnotobiotic models, it has been possible to dissect compositional and functional microbiome structures, as well as infer the mechanisms underlying the role of the microbiome in human biology. The study of microbial genes and metabolic pathways with newer methods, such as shotgun metagenomic sequencing, metabolomics, and metaproteomics, will further elucidate the mechanism of interaction between the GM and the host, expanding it beyond bacterial taxonomy. An interesting field of research which the authors believe will be a center of focus in the next few years is the characterization of the gut resistome,21 the virome,125 and the microbial interaction and metabolism of bile acids.126
The clinical translation of all the data coming from a better comprehension of microbiota could potentially help in developing biomarkers, aimed at precociously identifying HSCT complications and guiding clinical intervention. Some proposed methods of monitoring microbiome alterations that need to be additionally validated are the already mentioned measurement of urinary excretion of 3-indoxyl sulfate,14 serum concentrations of Reg3α,127 and fecal levels of alpha defensin.128
New studies are needed to assess the clinical and microbial impact of specific antibiotic molecules and new microbiome-sparing antibiotic strategies. A novel approach to protect the microbiome from antibiotic-mediated dysbiosis could be the use of beta-lactamase enzymes to degrade residual antibiotics in the gastrointestinal tract before the flora is harmed,129 or preventing the local action of antibiotic residuals at the colonic level by sequestering them, while leaving their small intestine absorption intact.130,131 Nutritional strategies, either by changing the route of nutritional supplementation or by the administration of specific molecules, are also promising cost- and risk-effective methods of modulating the gut microbiome. Supplementation using prebiotics, such as inulin, fructooligosaccharides, xylo-oligosaccharides, galacto-oligosaccharides, and potato starch132–134 or SCFAs, are strategies worth exploring. Another interesting area of research is the interaction between nutritional intervention and the GM, such as how enteral nutrition affects gut homeostasis. Finally, the authors believe that there is a gap in the comprehension of the exact mechanism of FMT action, and additional biological studies may provide insights for better implementing this procedure in clinical practice. Another challenge that needs to be addressed is its safety in immune-compromised patients. An interesting approach that may be of interest in reducing the risks is sterile fecal filtrate. Sterile fecal filtrate is obtained from the stool with a procedure of sterile-filtering to remove small particles and bacteria. The result contains bacterial debris, proteins, antimicrobial compounds, metabolic products, and oligonucleotides/DNA rather than intact microorganisms, and was shown to be effective in the treatment of rCDI.135 Other possible additional research strategies are a better understanding of donor microbial composition and its relationship with the outcome of FMT, and the implementation of genetic engineering techniques to manipulate the graft towards a more protective configuration.136
There is still much work to be done in order to better comprehend the precise biological mechanism and the overall clinical impact of a specific dysbiosis pattern. Regarding the possible microbiota-altering preventive and therapeutic strategies, the potential of modulating the microbiome to improve outcome is starting to be understood.
Acknowledgments
Authors Andrea Pession and Riccardo Masetti contributed equally.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: this study was supported by the Italian Ministero della Salute (Bando Ricerca Finalizzata 2013, Giovani Ricercatori section, code GR-2013-02357136 to R.M.).
Conflict of interest statement: The author(s) declare that there is no conflict of interest.
ORCID iD: Daniele Zama  https://orcid.org/0000-0002-9895-5942
https://orcid.org/0000-0002-9895-5942
Contributor Information
Daniele Zama, Pediatric Oncology and Hematology Unit ‘Lalla Seràgnoli,’ Sant’Orsola-Malpighi Hospital, University of Bologna, Via Massarenti 11, Bologna, 40137, Italy.
Gianluca Bossù, Department of Pediatrics, ‘Lalla Seràgnoli,’ Hematology-Oncology Unit, University of Bologna, Italy.
Davide Leardini, Department of Pediatrics, ‘Lalla Seràgnoli,’ Hematology-Oncology Unit, University of Bologna, Italy.
Edoardo Muratore, Department of Pediatrics, ‘Lalla Seràgnoli,’ Hematology-Oncology Unit, University of Bologna, Italy.
Elena Biagi, Department of Pharmacy and Biotechnology, University of Bologna, Bologna, Italy.
Arcangelo Prete, Department of Pediatrics, ‘Lalla Seràgnoli,’ Hematology-Oncology Unit, University of Bologna, Italy.
Andrea Pession, Department of Pediatrics, ‘Lalla Seràgnoli,’ Hematology-Oncology Unit, University of Bologna, Italy.
Riccardo Masetti, Department of Pediatrics, ‘Lalla Seràgnoli,’ Hematology-Oncology Unit, University of Bologna, Italy.
References
- 1. Integrative HMP (iHMP) Research Network Consortium. The integrative human microbiome project. Nature 2019; 569: 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Franks AH, Harmsen HJM, Raangs GC, et al. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol 1998; 64: 3336–3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoshi H, Aijima H, Horie K, et al. Lymph follicles and germinal centers in popliteal lymph nodes and other lymphoid tissues of germ-free and conventional rats. Tohoku J Exp Med 1992; 166: 297–307. [DOI] [PubMed] [Google Scholar]
- 4. Mazmanian SK, Liu CH, Tzianabos AO, et al. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005; 122: 107–118. [DOI] [PubMed] [Google Scholar]
- 5. Abrahamsson TR, Jakobsson HE, Andersson AF, et al. Low gut microbiota diversity in early infancy precedes asthma at school age. Clin Exp Allergy 2014; 44: 842–850. [DOI] [PubMed] [Google Scholar]
- 6. Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007; 104: 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karlsson FH, Tremaroli V, Nookaew I, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013; 498: 99–103. [DOI] [PubMed] [Google Scholar]
- 8. Parracho HM, Bingham MO, Gibson GR, et al. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol 2005; 54: 987–991. [DOI] [PubMed] [Google Scholar]
- 9. Holler E, Butzhammer P, Schmid K, et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem-cell transplantation: loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol Blood Marrow Transplant 2014; 20: 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jenq RR, Ubeda C, Taur Y, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med 2012; 209: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biagi E, Zama D, Nastasi C, et al. Gut microbiota trajectory in pediatric patients undergoing hematopoietic SCT. Bone Marrow Transplant 2015; 50: 992–998. [DOI] [PubMed] [Google Scholar]
- 12. Taur Y, Xavier JB, Lipuma L, et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem-cell transplantation. Clin Infect Dis 2012; 55: 905–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem-cell transplantation. Blood 2014; 124: 1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weber D, Oefner PJ, Hiergeist A, et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood 2015; 126: 1723–1728. [DOI] [PubMed] [Google Scholar]
- 15. Junghanss C, Marr KA, Carter RA, et al. Incidence and outcome of bacterial and fungal infections following nonmyeloablative compared with myeloablative allogeneic hematopoietic stem-cell transplantation: a matched control study. Biol Blood Marrow Transplant 2002; 8: 512–520. [DOI] [PubMed] [Google Scholar]
- 16. Tamburini FB, Andermann TM, Tkachenko E, et al. Precision identification of diverse bloodstream pathogens in the gut microbiome. Nat Med 2018; 24: 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris B, Morjaria SM, Littmann ER, et al. Gut microbiota predict pulmonary infiltrates after allogeneic hematopoietic cell transplantation. Am J Respir Crit Care Med 2016; 194: 450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ford CD, Gazdik MA, Lopansri BK, et al. Vancomycin-resistant enterococcus colonization and bacteremia and hematopoietic stem-cell transplantation outcomes. Biol Blood Marrow Transplant 2017; 23: 340–346. [DOI] [PubMed] [Google Scholar]
- 19. Levine JE, Huber E, Hammer STG, et al. Low Paneth cell numbers at onset of gastrointestinal graft-versus-host disease identify patients at high risk for nonrelapse mortality. Blood 2013; 122: 1505–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jenq RR, Taur Y, Devlin SM, et al. Intestinal blautia is associated with reduced death from graft-versus-host disease. Biol Blood Marrow Transplant 2015; 21: 1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D’Amico F, Soverini M, Zama D, et al. Gut resistome plasticity in pediatric patients undergoing hematopoietic stem-cell transplantation. Sci Rep 2019; 9: 5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Biagi E, Zama D, Rampelli S, et al. Early gut microbiota signature of aGvHD in children given allogeneic hematopoietic cell transplantation for hematological disorders. BMC Med Genomics 2019; 12: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peled JU, Devlin SM, Staffas A, et al. Intestinal microbiota and relapse after hematopoietic-cell transplantation. J Clin Oncol 2017; 35: 1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Magenau J, Runaas L, Reddy P. Advances in understanding the pathogenesis of graft-versus-host disease. Br J Haematol 2016; 173: 190–205. [DOI] [PubMed] [Google Scholar]
- 25. Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol 2009; 40: 909–917. [DOI] [PubMed] [Google Scholar]
- 26. Jones JM, Wilson R, Bealmear PM. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat Res 1971; 45: 577–588. [PubMed] [Google Scholar]
- 27. van Bekkum DW, Roodenburg J, Heidt PJ, et al. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst 1974; 52: 401–404. [DOI] [PubMed] [Google Scholar]
- 28. Eriguchi Y, Takashima S, Oka H, et al. Graft-versus-host disease disrupts intestinal microbial ecology by inhibiting Paneth cell production of -defensins. Blood 2012; 120: 223–231. [DOI] [PubMed] [Google Scholar]
- 29. Heimesaat MM, Nogai A, Bereswill S, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut 2010; 59: 1079–1087. [DOI] [PubMed] [Google Scholar]
- 30. Sokol H, Leducq V, Aschard H, et al. Fungal microbiota dysbiosis in IBD. Gut 2017; 66: 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holler E, Rogler G, Brenmoehl J, et al. Prognostic significance of NOD2/CARD15 variants in HLA-identical sibling hematopoietic stem-cell transplantation: effect on long-term outcome is confirmed in 2 independent cohorts and may be modulated by the type of gastrointestinal decontamination. Blood 2006; 107: 4189–4193. [DOI] [PubMed] [Google Scholar]
- 32. Penack O, Smith OM, Cunningham-Bussel A, et al. NOD2 regulates hematopoietic cell function during graft-versus-host disease. J Exp Med 2009; 206: 2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao Y, Liu Q, Yang L, et al. TLR4 inactivation protects from graft-versus-host disease after allogeneic hematopoietic stem-cell transplantation. Cell Mol Immunol 2013; 10: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koh A, De Vadder F, Kovatcheva-Datchary P, et al. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 2016; 165: 1332–1345. [DOI] [PubMed] [Google Scholar]
- 35. Smith PM, Howitt MR, Panikov N, et al. The microbial metabolites, short-chain fatty acids, regulate colonic treg cell homeostasis. Science 2013; 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Navari RM, Buckner CD, Clift RA, et al. Prophylaxis of infection in patients with aplastic anemia receiving allogeneic marrow transplants. Am J Med 1984; 76: 564–572. [DOI] [PubMed] [Google Scholar]
- 37. Storb R. Graft-versus-host disease and survival in patients with aplastic anemia treated bt marrow grafts from HLA-identical siblings. New Engl J Med 1987; 317: 141–145. [DOI] [PubMed] [Google Scholar]
- 38. Vossen JM, Heidt PJ, van den Berg H, et al. Prevention of infection and graft-versus-host disease by suppression of intestinal microflora in children treated with allogeneic bone marrow transplantation. Eur J Clin Microbiol Infect Dis 1990; 9: 14–23. [DOI] [PubMed] [Google Scholar]
- 39. Bekker V, Zwittink RD, Knetsch CW, et al. Dynamics of the gut microbiota in children receiving selective or total gut decontamination treatment during hematopoietic stem-cell transplantation. Biol Blood Marrow Transplant 2019; 25: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 40. Lopetuso LR, Petito V, Scaldaferri F, et al. Gut microbiota modulation and mucosal immunity: focus on rifaximin. Mini Rev Med Chem 2015; 16: 179–185. [DOI] [PubMed] [Google Scholar]
- 41. Maccaferri S, Vitali B, Klinder A, et al. Rifaximin modulates the colonic microbiota of patients with Crohn’s disease: an in vitro approach using a continuous culture colonic model system. J Antimicrob Chemother 2010; 65: 2556–2565. [DOI] [PubMed] [Google Scholar]
- 42. Townsend CM, Parker CE, MacDonald JK, et al. Antibiotics for induction and maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev 2019; 2: CD012730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shono Y, van den Brink Marcel RM. Gut microbiota injury in allogeneic haematopoietic stem-cell transplantation. Nat Rev Cancer 2018; 18: 283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ponziani FR, Zocco MA, D’Aversa F, et al. Eubiotic properties of rifaximin: disruption of the traditional concepts in gut microbiota modulation. World J Gastroenterol 2017; 23: 4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weber D, Oefner PJ, Dettmer K, et al. Rifaximin preserves intestinal microbiota balance in patients undergoing allogeneic stem-cell transplantation. Bone Marrow Transplant 2016; 51: 1087–1092. [DOI] [PubMed] [Google Scholar]
- 46. Ubeda C, Taur Y, Jenq RR, et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. Conflict 2010; 120: 4332–4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simms-Waldrip TR, Sunkersett G, Coughlin LA, et al. Antibiotic-induced depletion of anti-inflammatory clostridia is associated with the development of graft-versus-host disease in pediatric stem-cell transplantation patients. Biol Blood Marrow Transplant 2017; 23: 820–829. [DOI] [PubMed] [Google Scholar]
- 48. Farowski F, Bücker V, Vehreschild JJ, et al. Impact of choice, timing, sequence and combination of broad-spectrum antibiotics on the outcome of allogeneic haematopoietic stem-cell transplantation. Bone Marrow Transplant 2018; 53: 52–57. [DOI] [PubMed] [Google Scholar]
- 49. Liu C, Lieberman SR, Bhatt AS, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem-cell transplantation in human patients and mice. Sci Transl Med 2016; 8: 339ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nishi K, Kanda J, Hishizawa M, et al. Impact of the use and type of antibiotics on acute graft-versus-host disease. Biol Blood Marrow Transplant 2018; 24: 2178–2183. [DOI] [PubMed] [Google Scholar]
- 51. Drago-Serrano ME, Campos-Rodriguez R, Carrero JC, et al. Lactoferrin and peptide-derivatives: antimicrobial agents with potential use in nonspecific immunity modulation. Curr Pharm Des 2018; 24: 1067–1078. [DOI] [PubMed] [Google Scholar]
- 52. Haiwen Z, Rui H, Bingxi Z, et al. Oral administration of bovine lactoferrin derived lactoferricin (Lfcin) B could attenuate enterohemorrhagic Escherichia coli O157: H7 induced intestinal disease through improving intestinal barrier function and microbiota. J Agric Food Chem 2019; 67: 3932–3945. [DOI] [PubMed] [Google Scholar]
- 53. Inoue M, Okamura T, Yasui M, et al. Lactoferrin for gut GVHD. Bone Marrow Transplant 2001; 28: 1091–1092. [DOI] [PubMed] [Google Scholar]
- 54. van der Velden WJ, Blijlevens NM, Donnelly JP. The potential role of lactoferrin and derivatives in the management of infectious and inflammatory complications of hematology patients receiving a hematopoietic stem-cell transplantation. Transpl Infect Dis 2008; 10: 80–89. [DOI] [PubMed] [Google Scholar]
- 55. Cotillard A, Kennedy SP, Kong LC, et al. Dietary intervention impact on gut microbial gene richness. Nature 2013; 500: 585–588. [DOI] [PubMed] [Google Scholar]
- 56. Dao MC, Everard A, Aron-Wisnewsky J, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016; 65: 426–436. [DOI] [PubMed] [Google Scholar]
- 57. David LA, Maurice CF, Carmody RN, et al. Diet and reproducibly alters the humen gut micobiome. Nature 2014; 505: 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krezalek MA, Yeh A, Alverdy JC, et al. Influence of nutrition therapy on the intestinal microbiome. Curr Opin Clin Nutr Metab Care 2016; 20: 131–137. [DOI] [PubMed] [Google Scholar]
- 59. Liou AP, Paziuk M, Luevano J, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013; 5: 178ra41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mack I, Penders J, Cook J, et al. Is the impact of starvation on the gut microbiota specific or unspecific to anorexia nervosa? A narrative review based on a systematic literature search. Curr Neuropharmacol 2018; 16: 1131–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seitz J, Belheouane M, Schulz N, et al. The impact of starvation on the microbiome and gut-brain interaction in anorexia nervosa. Front Endocrinol (Lausanne) 2019; 10: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Alpers DH. Enteral feeding and gut atrophy. Curr Opin Clin Nutr Metab Care 2002; 5: 679–683. [DOI] [PubMed] [Google Scholar]
- 63. Groos S, Hunefeld G, Luciano L. Parenteral versus enteral nutrition: morphological changes in human adult intestinal mucosa. J Submicrosc Cytol Pathol 1996; 28: 61–74. [PubMed] [Google Scholar]
- 64. Kudsk KA. Gut mucosal nutritional support–enteral nutrition as primary therapy after multiple system trauma. Gut 1994; 35(Suppl. 1): S52–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. MacFie J, Reddy BS, Gatt M, et al. Bacterial translocation studied in 927 patients over 13 years. Br J Surg 2006; 93: 87–93. [DOI] [PubMed] [Google Scholar]
- 66. Pierre JF. Gastrointestinal immune and microbiome changes during parenteral nutrition. Am J Physiol Gastrointest Liver Physiol 2017; 312: G246–G256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ríos-Covián D, Ruas-Madiedo P, Margolles A, et al. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016; 7: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sax HC, Illig KA, Ryan CK, et al. Low-dose enteral feeding is beneficial during total parenteral nutrition. Am J Surg 1996; 171: 587–590. [DOI] [PubMed] [Google Scholar]
- 69. Weissman C. Nutrition in the intensive care unit. Crit Care 1999; 3: R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Azarnoush S, Bruno B, Beghin L, et al. Enteral nutrition: a first option for nutritional support of children following allo-SCT? Bone Marrow Transplant 2012; 47: 1191–1195. [DOI] [PubMed] [Google Scholar]
- 71. Gonzales F, Bruno B, Alarcón Fuentes M, et al. Better early outcome with enteral rather than parenteral nutrition in children undergoing MAC allo-SCT. Clin Nutr 2018; 37: 2113–2121. [DOI] [PubMed] [Google Scholar]
- 72. Guièze R, Lemal R, Cabrespine A, et al. Enteral versus parenteral nutritional support in allogeneic haematopoietic stem-cell transplantation. Clin Nutr 2014; 33: 533–538. [DOI] [PubMed] [Google Scholar]
- 73. Hopman GD, Peña EG, Le Cessie S, et al. Tube feeding and bone marrow transplantation. Med Pediatr Oncol 2003; 40: 375–379. [DOI] [PubMed] [Google Scholar]
- 74. Seguy D, Berthon C, Micol JB, et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem-cell transplantation following myeloablative conditioning. Transplantation 2006; 82: 835–839. [DOI] [PubMed] [Google Scholar]
- 75. Seguy D, Duhamel A, Rejeb M Ben, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem-cell transplantation. Transplantation 2012; 94: 287–294. [DOI] [PubMed] [Google Scholar]
- 76. Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: the international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 2017; 14: 491–502. [DOI] [PubMed] [Google Scholar]
- 77. Tavil B, Koksal E, Songul Yalcin S, et al. Pretransplant nutritional habits and clinical outcome in children undergoing hematopoietic stem-cell transplant. Exp Clin Transplant 2012; 10: 55–61. [DOI] [PubMed] [Google Scholar]
- 78. Iyama S, Sato T, Tatsumi H, et al. Efficacy of enteral supplementation enriched with glutamine, fiber, and oligosaccharide on mucosal injury following hematopoietic stem-cell transplantation. Case Rep Oncol 2014; 7: 692–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hill C, Guarner F, Reid G, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11: 506–514. [DOI] [PubMed] [Google Scholar]
- 80. Bjarnason I, Sission G, Hayee B. A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology 2019; 27: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Freedman SB, Williamson-Urquhart S, Farion KJ, et al. Multicenter trial of a combination probiotic for children with gastroenteritis. N Engl J Med 2018; 379: 2015–2026. [DOI] [PubMed] [Google Scholar]
- 82. Kruis W, Frič P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004; 53: 1617–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schnadower D, Tarr PI, Casper TC, et al. Lactobacillus rhamnosus GG versus placebo for acute gastroenteritis in children. N Engl J Med 2018; 379: 2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vemuri R, Gundamaraju R, Eri R. Role of lactic acid probiotic bacteria in IBD. Curr Pharm Des 2017; 23: 2352–2355. [DOI] [PubMed] [Google Scholar]
- 85. Gorshein E, Wei C, Ambrosy S, et al. Lactobacillus rhamnosus GG probiotic enteric regimen does not appreciably alter the gut microbiome or provide protection against GVHD after allogeneic hematopoietic stem-cell transplantation. Clin Transplant 2017; 31: e12947. [DOI] [PubMed] [Google Scholar]
- 86. Sharma A, Tilak T, Bakhshi S, et al. Lactobacillus brevis CD2 lozenges prevent oral mucositis in patients undergoing high dose chemotherapy followed by haematopoietic stem-cell transplantation. ESMO Open 2017; 1: e000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ambesh P, Stroud S, Franzova E, et al. Recurrent lactobacillus bacteremia in a patient with leukemia. J Investig Med High Impact Case Rep 2017; 5: 232470961774423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mehta A, Rangarajan S, Borate U. A cautionary tale for probiotic use in hematopoietic SCT patients-Lactobacillus acidophilus sepsis in a patient with mantle cell lymphoma undergoing hematopoietic SCT. Bone Marrow Transplant 2013; 48: 461–462. [DOI] [PubMed] [Google Scholar]
- 89. Salminen MK, Rautelin H, Tynkkynen S, et al. Lactobacillus bacteremia, clinical significance, and patient outcome, with special focus on probiotic L. rhamnosus GG. Clin Infect Dis 2004; 38: 62–69. [DOI] [PubMed] [Google Scholar]
- 90. Vahabnezhad E, Mochon AB, Wozniak LJ, et al. Lactobacillus bacteremia associated with probiotic use in a pediatric patient with ulcerative colitis. J Clin Gastroenterol 2013; 47: 437–439. [DOI] [PubMed] [Google Scholar]
- 91. Robin F, Paillard C, Marchandin H, et al. Lactobacillus rhamnosus meningitis following recurrent episodes of bacteremia in a child undergoing allogeneic hematopoietic stem-cell transplantation. J Clin Microbiol 2010; 48: 4317–4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cohen SA, Woodfield MC, Boyle N, et al. Incidence and outcomes of bloodstream infections among hematopoietic cell transplant recipients from species commonly reported to be in over-the-counter probiotic formulations. Transpl Infect Dis 2016; 18: 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ladas EJ, Bhatia M, Chen L, et al. The safety and feasibility of probiotics in children and adolescents undergoing hematopoietic cell transplantation. Bone Marrow Transplant 2016; 51: 262–266. [DOI] [PubMed] [Google Scholar]
- 94. Cammarota G, Ianiro G, Tilg H, et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017; 66: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. DeFilipp Z, Hohmann E, Jenq RR, et al. Fecal microbiota transplantation: restoring the injured microbiome after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2019; 25: e17–e22. [DOI] [PubMed] [Google Scholar]
- 96. Shouval R, Geva M, Nagler A, et al. Fecal microbiota transplantation for treatment of acute graft-versus-host disease. Clin Haematol Int 2019; 1: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bakken JS, Borody T, Brandt LJ, et al. Treating clostridium difficile infection with fecal microbiota transplantation. Clin Gastroenterol Hepatol 2011; 9: 1044–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rao K, Safdar N. Fecal microbiota transplantation for the treatment of Clostridium difficile infection. J Hosp Med 2016; 11: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ramai D, Zakhia K, Ofosu A, et al. Fecal microbiota transplantation: donor relation, fresh or frozen, delivery methods, cost-effectiveness. Ann Gastroenterol 2019; 32: 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Jiang ZD, Jenq RR, Ajami NJ, et al. Safety and preliminary efficacy of orally administered lyophilized fecal microbiota product compared with frozen product given by enema for recurrent Clostridium difficile infection: a randomized clinical trial. PLoS One 2018; 13: e0205064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wardill HR, Secombe KR, Bryant RV, et al. Adjunctive fecal microbiota transplantation in supportive oncology: emerging indications and considerations in immunocompromised patients. EBioMedicine 2019; 44: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bilinski J, Grzesiowski P, Sorensen N, et al. Fecal microbiota transplantation in patients with blood disorders inhibits gut colonizationwith antibiotic-resistant bacteria: results of a prospective, single-center study. Clin Infect Dis 2017; 65: 364–370. [DOI] [PubMed] [Google Scholar]
- 103. Innes AJ, Mullish BH, Fernando F, et al. Faecal microbiota transplant: a novel biological approach to extensively drug-resistant organism-related non-relapse mortality. Bone Marrow Transplant 2017; 52: 1452–1454. [DOI] [PubMed] [Google Scholar]
- 104. Battipaglia G, Malard F, Rubio MT, et al. Fecal microbiota transplantation before or after allogeneic hematopoietic transplantation in patients with hematological malignancies carrying multidrug-resistance bacteria. Haematologica 2019; 104: 1682–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Taur Y, Coyte K, Schluter J, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med 2018; 10. pii: eaap9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. DeFilipp Z, Peled JU, Li S, et al. Third-party fecal microbiota transplantation following allo-HCT reconstitutes microbiome diversity. Blood Adv 2018; 2: 745–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Neemann K, Eichele DDD, Smith PPW, et al. Fecal microbiota transplantation for fulminant Clostridium difficile infection in an allogeneic stem-cell transplant patient. Transpl Infect Dis 2012; 14: 161–165. [DOI] [PubMed] [Google Scholar]
- 108. de Castro CG, Ganc AJ, Ganc RL, et al. Fecal microbiota transplant after hematopoietic SCT: report of a successful case. Bone Marrow Transplant 2015; 50: 145. [DOI] [PubMed] [Google Scholar]
- 109. Mittal C, Miller N, Meighani A, et al. Fecal microbiota transplant for recurrent Clostridium difficile infection after peripheral autologous stem-cell transplant for diffuse large B-cell lymphoma. Bone Marrow Transplant 2015; 50: 1010. [DOI] [PubMed] [Google Scholar]
- 110. Webb BJ, Brunner A, Ford CD, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection in hematopoietic stem-cell transplant recipients. Transpl Infect Dis 2016; 18: 628–633. [DOI] [PubMed] [Google Scholar]
- 111. Bluestone H, Kronman MP, Suskind DL. Fecal microbiota transplantation for recurrent Clostridium difficile infections in pediatric hematopoietic stem-cell transplant recipients. J Pediatric Infect Dis Soc 2018; 7: e6–e8. [DOI] [PubMed] [Google Scholar]
- 112. Moss EL, Falconer SB, Tkachenko E, et al. Long-term taxonomic and functional divergence from donor bacterial strains following fecal microbiota transplantation in immunocompromised patients. PLoS One 2017; 12: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kakihana K, Fujioka Y, Suda W, et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016; 128: 2083–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Spindelboeck W, Schulz E, Uhl B, et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft- versus -host-disease. Haematologica 2017; 102: e210–e213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Qi X, Li X, Zhao Y, et al. Treating steroid refractory intestinal acute graft-vs.-host disease with fecalmicrobiota transplantation: a pilot study. Front Immunol 2018; 9: 2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. van Lier YF, Davids M, Haverkate NJE, et al. Fecal microbiota transplantation can cure steroid-refractory intestinal graft-versus-host disease. Biol Blood Marrow Transplant 2019; 25: S241. [Google Scholar]
- 117. Kaito S, Toya T, Yoshifuji K, et al. Fecal microbiota transplantation with frozen capsules for a patient with refractory acute gut graft-versus-host disease. Blood Adv 2018; 2: 3097–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang J, Ren G, Li M, et al. The effects of fecal donors with different feeding patterns on diarrhea in a patient undergoing hematopoietic stem-cell transplantation. Case Rep Hematol 2019; 2019: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Dailey FE, Turse EP, Daglilar E, et al. The dirty aspects of fecal microbiota transplantation: a review of its adverse effects and complications. Curr Opin Pharmacol 2019; 49: 29–33. [DOI] [PubMed] [Google Scholar]
- 120. Quera R, Espinoza R, Estay C, et al. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohns Colitis 2014; 8: 252–253. [DOI] [PubMed] [Google Scholar]
- 121. Schwartz M, Gluck M, Koon S. Norovirus gastroenteritis after fecal microbiota transplantation for treatment of clostridium difficile infection despite asymptomatic donors and lack of sick contacts. Am J Gastroenterol 2013; 108: 1367. [DOI] [PubMed] [Google Scholar]
- 122. Gonçalves SM, Lagrou K, Duarte-Oliveira C, et al. The microbiome-metabolome crosstalk in the pathogenesis of respiratory fungal diseases. Virulence 2017; 8: 673–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Muro M, Soga Y, Higuchi T, et al. Unusual oral mucosal microbiota after hematopoietic cell transplantation with glycopeptide antibiotics: potential association with pathophysiology of oral mucositis. Folia Microbiol (Praha) 2018; 63: 587–597. [DOI] [PubMed] [Google Scholar]
- 124. Santos E, Sousa P, Bennett CL, Chakraverty R. Unraveling the mechanisms of cutaneous graft-versus-host disease. Front Immunol 2018; 9: 963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Legoff J, Resche-Rigon M, Bouquet J, et al. The eukaryotic gut virome in hematopoietic stem-cell transplantation: new clues in enteric graft-versus-host disease. Nat Med 2017; 23: 1080–1085. [DOI] [PubMed] [Google Scholar]
- 126. McKenney PT, Yan J, Vaubourgeix J, et al. Intestinal bile acids induce a morphotype switch in vancomycin-resistant enterococcus that facilitates intestinal colonization. Cell Host Microbe 2019; 25: 695–705.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Weber D, Frauenschläger K, Ghimire S, et al. The association between acute graft-versus-host disease and antimicrobial peptide expression in the gastrointestinal tract after allogeneic stem-cell transplantation. PLoS One 2017; 12: e0185265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Eriguchi Y, Nakamura K, Hashimoto D, et al. Decreased secretion of Paneth cell α-defensins in graft-versus-host disease. Transpl Infect Dis 2015; 17: 702–706. [DOI] [PubMed] [Google Scholar]
- 129. Kokai-Kun JF, Roberts T, Coughlin O, et al. Use of ribaxamase (SYN-004), a β-lactamase, to prevent Clostridium difficile infection in β-lactam-treated patients: a double-blind, phase 2b, randomised placebo-controlled trial. Lancet Infect Dis 2019; 19: 487–496. [DOI] [PubMed] [Google Scholar]
- 130. De Gunzburg J, Ducher A, Modess C, et al. Targeted adsorption of molecules in the colon with the novel adsorbent-based medicinal product, DAV132: a proof of concept study in healthy subjects. J Clin Pharmacol 2014; 55: 10–16. [DOI] [PubMed] [Google Scholar]
- 131. de Gunzburg J, Ghozlane A, Ducher A, et al. Protection of the human gut microbiome from antibiotics. J Infect Dis 2018; 217: 628–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr 2004; 80: 1658–1664. [DOI] [PubMed] [Google Scholar]
- 133. Lecerf JM, Dépeint F, Clerc E, et al. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 2012; 108: 1847–1858. [DOI] [PubMed] [Google Scholar]
- 134. Vulevic J, Juric A, Walton GE, et al. Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. Br J Nutr 2015; 114: 586–595. [DOI] [PubMed] [Google Scholar]
- 135. Ott SJ, Waetzig GH, Rehman A, et al. Efficacy of sterile fecal filtrate transfer for treating patients with clostridium difficile infection. Gastroenterology 2017; 152: 799–811.e7. [DOI] [PubMed] [Google Scholar]
- 136. Woloszynek S, Pastor S, Mell JC, et al. Engineering human microbiota: influencing cellular and community dynamics for therapeutic applications. Int Rev Cell Mol Biol 2016, 324: 67–124. [DOI] [PubMed] [Google Scholar]
- 137. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486: 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]