Abstract
Brugada syndrome (BrS) is an inherited ion channel channelopathy predisposing to ventricular arrhythmias and sudden cardiac death. Originally believed to be predominantly associated with mutations in SCN5A encoding for the cardiac sodium channel, mutations of 18 genes other than SCN5A have been implicated in the pathogenesis of BrS to date. Diagnosis is based on the presence of a spontaneous or drug-induced coved-type ST segment elevation. The predominant electrophysiological mechanism underlying BrS remains disputed, commonly revolving around the three main hypotheses based on abnormal repolarization, depolarization or current-load match. Evidence from computational modelling, pre-clinical and clinical studies illustrates that molecular abnormalities found in BrS lead to alterations in excitation wavelength (λ), which ultimately elevates arrhythmic risk. A major challenge for clinicians in managing this condition is the difficulty in predicting the subset of patients who will suffer from life-threatening ventricular arrhythmic events. Several repolarization risk markers have been used thus far, but these neglect the contributions of conduction abnormalities in the form of slowing and dispersion. Indices incorporating both repolarization and conduction based on the concept of λ have recently been proposed. These may have better predictive values than the existing markers. Current treatment options include pharmacological therapy to reduce the occurrence of arrhythmic events or to abort these episodes, and interventions such as implantable cardioverter-defibrillator insertion or radiofrequency ablation of abnormal arrhythmic substrate.
Keywords: Brugada syndrome, Ion channel, Repolarization, Depolarization, Risk stratification
1. Introduction
Brugada syndrome (BrS) is an inherited cardiac disorder first described by Pedro and Josep Brugada in 1992. The term “Brugada syndrome” was coined later in recognition of their identification of this important disease [1]. Four years after Yan and Antzelevitch [2] approached the cellular basis underlying the ECG abnormalities displayed by patients affected by Brugada syndrome ECG, BrS is frequently associated with mutations in the SCN5A gene, which encodes for the pore-forming alpha subunit of the cardiac Na+ channels. To date, multiple pathogenic variants of genes have been shown to alter the normal function of Na+, K+, Ca2+ and hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which mediate the ionic currents responsible for the cardiac action potentials [3]. Both depolarization and repolarization abnormalities have been described in BrS [4], [5]. Patients with this syndrome can present with aborted sudden cardiac death, agonal breathing syncope, or palpitations. Precipitating factors include fever, increased vagal tone and other drugs such as tricyclic antidepressants and alcohol [6], [7]. These in turn predispose to malignant ventricular tachycardia and fibrillation (VT/VF) and sudden cardiac death (SCD) [8]. BrS was also found to be associated with sick sinus syndrome (SSS) [9], atrial flutter, atrial fibrillation [10], AV nodal reentrant tachycardia and Wolff-Parkinson-White syndrome [11].
2. Types of Brugada ECG patterns
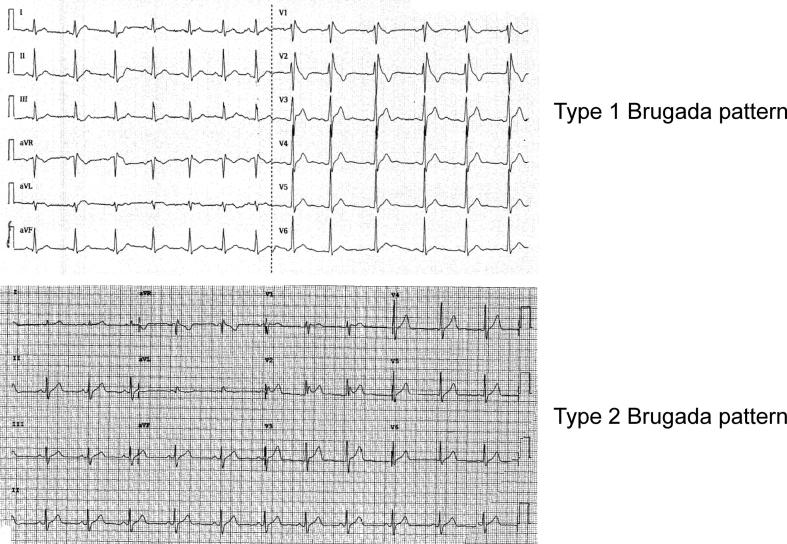
Brugada patterns can be divided into two types (Fig. 1) [12]. Type 1 pattern has a characteristic coved-shaped ST segment elevation (STE) 2 mm, J-point elevation, a gradually descending ST segment which terminates with a negative T-wave in the right precordial leads (V1, V2 and V3) with or without a class I anti-arrhythmic drug challenge, such as flecainide [13]. Type 2 pattern is characterized by a saddleback morphology with a minimum 2 mm J-point elevation along with ST segment elevation of at least 1 mm. A type 2 pattern can be converted to a type 1 pattern upon pharmacological challenge or other stressors such as fever.
Fig. 1.
Type 1 (top) and type 2 (bottom) Brugada ECG patterns.
3. Epidemiology
In 1992, the Brugada investigators initially estimated that BrS was responsible for 12% of SCD cases in the general population [14], but recent epidemiological studies suggested the prevalence to be much lower, at least 0.05% with marked regional variability [15], [16]. It was also found that Southeast Asians are at an increased risk of BrS as compared to other ethnicities, with only 0.1% showing BrS-type ECG pattern [17]. This variance is supported by comparing epidemiological studies in Denmark against Chinese subjects. In Denmark, a low prevalence of 0.001% was found as compared to the 3.3% found in Chinese subjects (although a Type 1 pattern was only observed in 0.08% of these subjects) [18], [19]. In terms of gender distribution, BrS has a strong male correlation, affecting men four times more frequently than women and also affecting younger adults than infants or children [20]. Recent insights from SABRUS a multi-center survey, which reported important ethnic differences [21]. They found that Asians present almost exclusively as male adults, with a higher frequency of aborted SCD and spontaneous type 1 ECG pattern but showed lower frequency of family history of SCD and SCN5A mutations compared to Caucasians.
4. Genetic basis and heterogeneity underlying BrS
There is significant genetic heterogeneity underlying BrS. The most common mutation is loss-of-function mutations in SCN5A, the gene responsible for the α-subunit of the Na+ channel, are frequently associated with a type 1 pattern. Since 2001 there have been more than 80 mutations in SCN5A gene that have been associated with Brugada syndrome [22]. These lead to reduced expression or function of Na+ channels, leading to conduction or repolarization abnormalities that produce the characteristic ECG patterns of right bundle branch block and ST segment elevation primarily observed in the right precordial leads [23]. Type 2 pattern has also been associated with mutations in SCN5A, glycerol-3-phosphate dehydrogenase 1-like (GPD1L), which is the domain responsible for a site homologous to SCN5A [24], and CACNA1C, the gene responsible for the α-subunit of cardiac L-type calcium channels (LTCC) [25].
BrS was believed to be a Mendelian disease with an autosomal dominant inheritance pattern with incomplete penetrance [26]. However recent evidence suggests that this may not be completely true [27]. There is a poor genotype-phenotype correlation. A recent study investigated co-segregation of SCN5A mutations amongst large genotyped families, demonstrating that some affected family members did not carry the familial mutation [28]. This could mean that mutations in other genes are responsible for BrS [29], [30]. Another possibility is incomplete penetrance despite the presence of the mutated gene or variable expressivity [31]. This has been observed in a frameshift mutation in SCN5A found in a proband from Spain with recurrent episodes of ventricular fibrillation and presenting bradycardia and paroxysmal atrial fibrillation without a spontaneous or drug-induced Brugada pattern [32]. By contrast, two family members of the proband showed type 1 BrS following flecainide challenge, and another suffered from only permanent atrial fibrillation. Some putatively pathogenic genetic mutations do not produce an abnormal clinical phenotype [33]. Recently, a genome-wide association study successfully identified two common genetic variants in SCN5A-SCN10A and HEY2, a translational repressor [34]. This approach will continue to elucidate the role of proteins that may serve as genetic modifiers to influence the disease phenotype [35].
5. Differential diagnosis: J-wave syndromes and other causes of Brugada pattern
BrS has been classified as part of the J-wave syndromes that include early repolarization (ER) variants. Antzelevitch’s group suggested dividing ER syndrome into three types [36]. Type 1 ER pattern in lateral precordial leads is prevalent in healthy male athletes and rarely observed in VF survivors. Type 2 refers to ER pattern in inferior or inferolateral leads and is associated with idiopathic VF and is also prevalent in healthy young males. Type 3 refers to ERS pattern observed globally in the inferior, lateral and right precordial leads. This subtype is thought to be high risk of VT/VF [37]. Another classification scheme divides ERS into benign and malignant forms [38]. A J-wave followed by an ascending ST segment is considered benign, whereas J-wave followed by horizontal or descending ST segment is considered malignant. A recent consensus conference report addresses the similarities and differences between BrS and ERS [39]. ERS continue to be associated with higher risk of SCD [40]. The estimated prevalence of ERS spans between 1 and 13% of the general population and is thought to contribute to 15 to 70% of idiopathic VF cases [41], [42].
The cellular basis of J-point elevation has been intensively studied in pre-clinical models using coronary-perfused wedge preparations. Thus, the ventricular epicardium expresses the transient outward current (Ito) in high levels, resulting in an AP notch, whereas the ventricular endocardium expresses Ito at low levels and therefore does not have this notch [43], [44]. These differences therefore create a transmural repolarization gradient that is responsible for J-point elevation seen in ERS. However, it has been pointed out that a wedge is not a heart, and the electrophysiological mechanisms may be different in the intact heart [45].
The term Brugada phenocopy (BrP) has been coined to describe a group of heterogeneous conditions that induce Brugada ECG patterns [46], such as hyperkalaemia [47], hypokalaemia [48], left ventricular aneurysm [49], pericarditis [50], pulmonary embolism [51], and many other causes. These conditions must be distinguished from true BrS as these are potential reversible causes and do not necessitate invasive treatments such as implantable cardioverter-defibrillator (ICD) insertion. The diagnosis of BrP is established with a negative drug challenge [52].
6. Electrophysiological mechanisms underlying arrhythmogenesis in Brugada syndrome
To understand the electrophysiological basis of BrS, the ionic determinants of the normal cardiac action potential (AP) need to be discussed. AP depolarization (phase 0) is mediated by voltage-gated Na+ channels (INa) [53]. This is followed by early repolarization (phase 1) due to activation of the fast and slow transient outward potassium currents, Ito,f and Ito,s. The AP plateau (phase 2) is determined by a balance between inward currents mediated by the voltage-gated L-type Ca2+ channel (LTCC, ICa,L) and Na+-Ca2+ exchanger (INCX), and outward currents mediated by the voltage-gated delayed rectifier K+ channels (IK: IKr and IKs) [54]. During delayed repolarization (phase 3), relatively greater outward K+ currents compared to inward currents are due to LTCC inactivation.
-
(i)
Sodium channels and BrS
The voltage-gated Na+ channels are made of α subunits associated with other proteins, such as β subunits (SCN1B, SCN2B and SCN3B). The SCN5A gene encodes for the α subunit of the cardiac sodium channel. Loss-of-function mutations in SCN5A have been associated with BrS [55], [56], SSS [57], progressive cardiac conduction defect (PCCD, or Lenegre disease) [58] and overlap disorders between these conditions [59]. Loss-of-function mutations are observed in approximately 25% of BrS cases [60]. These lead to reduced sodium current availability during the phases 0 (upstroke) of the cardiac action potential, which is associated with impaired expression of non-functional proteins and reduced ionic exchange across the cell membrane. Even though most mutations involved in the development of BrS are found in the SCN5A gene, mutations in the associated β subunit proteins have also been observed [61], [62], [63], [64]. Interestingly, a study by Hu et al. also discussed the involvement of the SCN10A gene, mainly involved in expressing the sodium channel specific to neurons, in causing a large proportion of BrS cases. However, there is increasing conjecture about the genotype-phenotype correlation between Brugada syndrome and previously reported “pathogenic variants” in genes other than SCN5A” [65]. The reduction in sodium current availability is not limited to genes encoding for the sodium channels. Genes expressing the glycerol-3-phosphate dehydrogenase 1-like (GPD1-L) protein [24], cardiac sodium channel regulator MOG1 [66], sarcolemmal membrane-associated protein (SLMAP) [67], desmosomal component plakophilin-2 [68], fibroblast growth factor homologous factor-1 (FGF-2) [69] and the transcriptional factor HEY2 [34] have been suggested to give rise to BrS.
-
(ii)
Calcium channels and BrS
The calcium current is mediated by L-type calcium channels (LTCC). Each LTCC consists of 4 protein subunits α1 (CACNA1C), β2 (CACNB2), α2 (CACNA2D), and δ (CACNA2D). Similar to SCN5A mutations, Antzelevitch et al. suggested that loss-of-function mutations in these genes precipitate abnormal trafficking, reduced expression or function of LTCC, leading to reduced calcium influx current during phase 2 [25], [70]. As a result, BrS secondary to the reduced functionality of LTCCs are associated with shorter QT intervals compared to classical SCN5A mutation BrS where QT interval remains unaltered.
-
(iii)
Potassium channels and BrS
Gain-of-function mutations in genes encoding for potassium channels have also been implicated in BrS. Genes influencing Ito include KCNE3, KCND3 and SEMA3A (semaphoring, an endogenous K+ channel inhibitor) [71], [72], [73], [74], [75], [76] while KCNJ8 and ABCC9 (encoding for SUR2A, the ATP-binding cassette transporter for the KATP channel) mutations affected the IK,ATP [77], [78]. KCNH2, which encodes for IKr was also proposed by Wang et al. to be involved in BrS development [79]. Most recently, dysfunction in the KCNAB2, which encodes the voltage-gated K+ channel β2-subunit, was associated with increased Ito activity and identified as a putative gene involved in BrS [80].
-
(iv)
Other proteins
The potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 4 is a protein mainly found in the pacemaker region of mammalian hearts, encoded by the HCN4 gene, controlling the heart rate [81]. Mutation in the HCN4 gene was associated with bradycardia and idiopathic VT [82]. Lastly, the transient receptor potential melastatin protein protein 4 gene (TRPM4), which encode for a calcium-activated nonselective ion channel that mediates transport of monovalent cations across the plasmolemma, was also found to be associated with BrS [83].
7. Brugada phenotypes
There are three leading theories on the electrophysiological mechanisms underlying BrS, which based on abnormal depolarization, abnormal repolarization and current-load-fmismatch [4] (Fig. 2).
-
(i)
Depolarization hypothesis
Fig. 2.
Summary of arrhythmogenic mechanisms underlying Brugada syndrome. Adapted from [189] with permission.
Conduction velocity (CV) of the propagating cardiac action potentials involves both sodium channel activation leading to cellular depolarization, followed by gap junction conduction across successive cardiomyocytes. Any form of disruption to the normal AP generation or propagation can lead to conduction defects and arrhythmogenesis [84]. Approximately a quarter of BrS cases have been attributed to loss-of-function mutations in the SCN5A gene, leading to a decreased inward current during phase 0 [85]. The resulting slower upstroke during phase 0 and the consequent delay in AP generation has been shown to play an important role in mediating ventricular arrhythmogenesis in BrS. Martini and colleagues in 1989 observed fibrotic changes in the right ventricles, which may produce the RBBB and ST segment elevation on the ECG [86]. This theory has been supported by multiple studies investigating the disruption of SCN5A in mice models, which found targeted disruption of Scn5a (Scn5a+/−), Scn5a1798insD/+ and SCN5aG1408R mice to be associated with reduced CV in interstitial fibrosis [87], [88], [89]. Scn5a+/− mice also show progressive conduction defects that are suggestive of Lenègre disease [90]. In an explanted heart and in right ventricular biopsies it was found that structural changes such as fibrosis, apoptosis and myocarditis were present [91], [92]. These findings are in concordance with the observed late potentials and fragmented electrograms, which reflect discontinuous conduction through a diseased myocardium [93], [94].
Under physiological conditions, the specific subcellular distribution of gap junctions together with the tight packaging of the rod-shaped cardiomyocytes underlies anisotropic conduction, which is continuous at the macroscopic scale. Due to its nature, it was initially assumed that gap junctions were predominantly found at the ends of the cardiomyocytes to facilitate AP conduction, away from sodium channel sites. However, it was shown by Cohen et al. in 1996 that both sodium channels and gap junctions co-exist at the intercalated disks [95]. This phenomenon was later confirmed by numerous studies by the macrostructure known as the connexome. The concept of the connexome revolves around the notion that the cardiac intercalated disc is the host of a protein interacting network including desmosomes, gap junctions and sodium channel complexes [96]. Autopsy findings support the idea that components of the connexome are not independent of each other, by demonstrating increased myocardial fibrosis from collagen deposition and reduced gap junction expression in the RVOT of hearts from BrS patients [97].
There is a close relationship between BrS phenotype on the ECG and RVOT abnormalities. An abnormal delayed potential was recorded from the epicardium of the RVOT in patients with BrS through the electrode inserted into the conus branch of the right coronary artery (RCA) [98]. Also, individuals with a normal ECG at baseline occasionally display BrS-type ECG patterns during an AMI of the RVOT [99]. Most recently, a panoramic ventricular mapping study in humans showed electrogram prolongation and fractionation, reflecting reduced CV and increased CV dispersion [100]. Defective depolarization may contribute to the pathology to different extents depending on the subtype. For example, in cases of BrS where abnormal function in calcium or potassium is observed, repolarization abnormalities may play a dominant role since the currents mediated by these channels contribute to the plateau rather than the depolarizing phase of the cardiac action potential.
-
(ii)
Repolarization hypothesis
The repolarization theory states that differential action potential duration (APD) shortening across the myocardial wall is primarily responsible for the BrS phenotype. Loss-of-function SCN5A mutations can have opposing effects on the fast and slow inactivation of Na+ channels with distinct effects on repolarization [101]. Disruptions in fast inactivation leads to a sustained Na+ current, which prolongs repolarization at slow heart rates. However, the intermediate kinetic component of slow inactivation is augmented, delaying Na+ channel recovery, reducing the Na+ current and shorten APD at fast heart rates. This biphasic behaviour of APD of prolongation followed by shortening has subsequently been observed in a panoramic mapping study in BrS patients [100].
Experiments conducted on transmural ventricular wedges of canines have provided important information on the mechanism underlying the heterogeneities in repolarization and re-entry due to reduced inward currents [8], [102], [103]. Comparing APD values obtained from the epicardium (specifically the RVOT epicardium) with those obtained from the endocardium, shortening of the APD was seen at greater degree in the epicardium due to a relatively greater transient outward current (Ito). This is reflected in the more prominent loss of dome-shaped AP morphology seen in the epicardium. This is thought to underlie reentry by a phase 2 reentrant mechanism, initially hypothesized by Yan and Antzelevitch in 1999. Phase 2 reentry requires electrotonic interactions and propagation of epicardial sites with an AP dome to sites where this dome is abolished [104]. It could well underlie the R-on-T phenomenon leading to an extrasystolic action potential that can initiating ventricular arrhythmias in the presence of favourable reentrant substrates [8]. Steep and reversal of repolarization gradients lead to the ST segment elevation and T-wave inversion, respectively, in the ECG. Increased Tpeak – Tend, an ECG marker of repolarization, is observed in BrS patients and associated with higher incidence of arrhythmic events or sudden cardiac death [105]. Indeed, studies of electrogram recordings have found a combination of steep repolarization gradient and delayed repolarization at the right ventricular outflow tract (RVOT) [100]. In patients with BrS due to defective calcium or potassium currents, a defective repolarization is the likely cause of VT/VF. Indeed, a reentrant mechanism as a result of loss-of-function Ca2+ channel mutation has been proposed [25].
Lastly, the restitution hypothesis states that a slope of the APD restitution curve >1 has been associated with the generation of repolarization alternans [106]. APD alternans can produce steep gradients in repolarization and refractoriness, unidirectional conduction block, wavebreak, and reentry. Both abnormal restitution and T-wave alternans have been observed in BrS [107], [108], [109], [110].
-
(iii)
Current-load-mismatch, depolarization-repolarization balance and excitation wavelength (λ)
In 2010, Hoogendijk and colleagues introduced the current-to-load mismatch phenomenon in the subepicardium to underlie ventricular arrhythmias in patients with BrS signs. This was performed using computer simulations of right ventricular structural discontinuities. Reduction in sodium current due to channel dysfunction or size of the pores was found to cause subepicardial excitation failure or delayed activation by current-to-load mismatch. Computational modeling work also showed that disruption to the inward-outward current equilibrium could affect excitation and therefore causing ST segment elevation subsequently [111], [112]. It was confirmed in an explanted human heart model that only the failure of local excitation correlated with ST-elevation, not delayed activation or early repolarization [113]. Therefore, by altering the Ito or ICa accordingly to compensate for reduced sodium current, the extent of ST-elevation will decrease [111]. In order to simulate similar conditions of ST-elevation in pseudo-ECG recordings, conduction block and excitatory failure via sodium channel blocking was induced using ajmaline [112]. At sites of local ST-segment elevation, the subepicardium was interspersed with adipose tissue and contained more fibrous tissue than either the left ventricle or control hearts [114].
Cardiac structural abnormalities are observed in BrS patients, specifically in the right ventricle (RV) and RVOT, predisposes to current-load-mismatch and excitation failure. This was confirmed recently by Ten Sande and colleagues using cardiac activation mapping, illustrating that structural abnormalities in the subepicardial sites in the RV and RVOT are the likely cause of conduction changes and ST segment elevation [114]. This structural-electrophysiological relationship is in keeping with ventricular arrhythmias found in BrS patients in their 30 s, when cardiac interstitial fibrosis is more evident [91]. Another study suggested that current-to-load mismatches at discontinuities were capable of causing a degree of conduction block, which explains the RBBB morphology found in BrS patients. It should also be recognized that discontinuities are usually associated with depolarization abnormalities when producing arrhythmia in BrS [115]. It also interacts with action potential repolarization and recovery to determine the excitation (λ) given by CV × ERP. Decreased λ has been associated with increased likelihood of reentrant arrhythmias not only in pre-clinical animal models, but also in BrS patients [116], [117].
8. Therapy and arrhythmic risk stratification
Since the underlying cause of BrS is reduced magnitude of inward currents, pharmacological agents that act to increase the inward currents or decrease the outward currents can restore the balance. Currently available drugs which are effective in preventing arrhythmic episodes in BrS are quinidine (a Class Ia Na+ channel and Ito inhibitor), bepridil (Ito inhibitor and INa enhancer) and cilostazol (phosphodiesterase III inhibitor) [118], [119], [120], [121]. Beta agonists and phosphodiesterase III inhibitors can be used to treat VF storms [122], [123]. Future therapy can aim to restore the inward-outward balance, by enhancing Ca2+ currents (e.g. with cilostazol or milrinone [124]) or suppressing Ito (e.g. 4-aminopyridine [125]) to reduce the AP notch, TDR and the likelihood of phase 2 reentry. Interventional options include ICD insertion and radiofrequency ablation [126], [127]. ICD insertion appears to be safe in the long term and reduces cardiovascular mortality in BrS patients [128], [129]. However, its use is not without significant morbidity, as complications such as lead failure and infections can occur [130]. Moreover, the quality of life is affected from inappropriate shocks, most often due to the presence of supraventricular arrhythmias [127]. In some cases, radiofrequency ablation can be used to successfully prevent VT/VF occurrence [131]. The electrophysiological substrates have frequently been localized to the RVOT in BrS. Once the location of the substrates are confirmed by epicardial and endocardial mapping, they can be eliminated using radiofrequency ablation [132]. A multicenter randomized study, Ablation in Brugada Syndrome for the Prevention of VF Episodes (BRAVE study), will provide exciting findings on the utility of ablation without the need of ICD insertion [132].
There are many risk factors that have been associated with higher likelihood of developing VT/VF [133], [134], [135]. These include male gender [136], [137], occurrence of syncope [138], genetic mutations in SCN5A [139], [140], the presence of a spontaneous type 1 Brugada pattern [141], [142], early repolarization pattern in inferolateral leads [143], S-wave in lead I [144], T-wave alternans [145], fragmented QRS morphology [94], burden of Brugada ECG pattern on Holter monitoring [146], augmented ST elevation during exercise recovery [147], an abbreviated ventricular refractory period of <200 ms [148], activation-recovery interval prolongations [149], and inducible arrhythmias observed during programmed electrical stimulation [150]. Several ECG repolarization markers have been proposed to stratify arrhythmic risk in the BrS population (Table 1) [151]. First, Tpeak – Tend, the interval between the peak and the end of the T wave, have been associated with increased arrhythmic risk [152]. Experiments conducted in arterially-perfused canine wedge preparations showed that the end of epicardial repolarization coincided with the Tpeak and at the M-cell coincided with Tend suggesting that Tpeak – Tend reflected increased TDR [153]. Subsequent experiments in swines later reported that Tpeak – Tend was a marker of global rather than transmural dispersion of repolarization [152], [154], [155]. Tpeak – Tend changes with heart rate and demonstrate significant inter-individual variability [156]. Dividing it by the QT interval produces relatively constant values of 0.17 to 0.23 and has been proposed to be a better indicator for arrhythmia prediction [156]. Increases in Tpeak – Tend and (Tpeak – Tend) / QT have been associated with arrhythmia inducibility. Thus, Letsas and colleagues reported that patients with spontaneous or drug-induced Type 1 Brugada pattern with inducible VT/VF displayed an increased Tpeak – Tend interval in leads V2 (88.82 vs. 78.33 ms) and V6 (76.33 vs. 66.66) and a greater (Tpeak – Tend)/QT ratio in lead V6 (0.214 vs. 0.180) compared with those without arrhythmias [157]. Recent meta-analyses conducted by our group confirmed the value of both indices for risk stratification in BrS [158], [159]. Moreover, markers based on conduction have also demonstrated utility for risk stratification. Fragmented QRS complex, represents an increased dispersion of conduction [160], [161], [162], can create unidirectional block, whereas wide QRS reflecting reduced conduction velocity, will shorten the excitation wavelength [163]. Both will predispose to reentrant arrhythmias.
Table 1.
Electrocardiographic indices for risk stratification in Brugada syndrome.
| Depolarization | Repolarization | Depolarization-repolarization |
|---|---|---|
| Prolonged QRS | QT and QTc intervals | iCEB (QRS/QT), iCEBc |
| Increased QRS dispersion | QT and QTc dispersion | |
| fQRS | Tpeak-Tend, Tpeak-Tend/QT ratio, Tpeak-Tend dispersion | |
| Epsilon-like waves | JTpeak, JTpeak dispersion | |
| Concomitant RBBB | Early repolarization pattern (in >=2 contiguous inferior/lateral leads | |
| First degree AV block | ||
| RVOT delay signs: positive R-wave in aVR, S-wave in lead I, SII > SIII | ||
| Positive Tzou criteria: V1 R-wave > 0.15 mV V6 S-wave > 0.15 mV V6 S-wave: R-wave ratio > 0.2 |
Given the insights from pre-clinical studies, it was recognized that conduction abnormalities need to be incorporated into the risk markers to increase their accuracy of risk prediction [164], [165], [166], [167], [168], [169]. For example, the index of Cardiac Electrophysiological Balance (iCEB), given by QT / QRS, is a surrogate marker of λ and its use has led to improved risk stratification [170], [171]. Furthermore, abnormal action potential restitution appears to contribute to the arrhythmic substrate [172], [173], and given recent work has developed restitution indices in clinical cohorts [174], [175], [176], whether they will incremental value in risk stratification in patients with Brugada syndrome remains to be elucidated. Finally, given the dynamicity in both the Brugada pattern [177], [178], [179] and arrhythmic risk [180], it would follow that temporal variability in ECG indices could offer additional value for risk stratification. Indeed, a high temporal burden of type 1 ST-segment elevation assessed using 24-hour Holter monitoring has been associated with an increased arrhythmic risk in BrS [146], [178].
Other techniques such as electroanatomical mapping are also crucial for aiding risk stratification. For example, endocardial unipolar voltage mapping of the RVOT can detect low voltage areas that possibly reflect epicardial structural lesions in BrS [181]. We have recently shown that BrS patients with broad endocardial unipolar voltage abnormalities are more vulnerable to VF induction during programmed ventricular stimulation. On the contrary, subjects with normal electroanatomical maps were non-inducible [182]. Detection of magnetic signals has traditionally been used to characterize structural properties [183], [184], [185], but recent work shown that magnetocardiography can provide incremental value for arrhythmic risk prediction [186], [187], [188].
9. Conclusion
The Brugada syndrome is an inherited primary arrhythmia syndromeoriginally thought to involve structurally normal hearts. Recent evidence implicates structural alterations of fibrotic change in the right ventricle. Risk stratification is based on a combination of genetic studies, symptoms, the presence of spontaneous or induced Brugada pattern on the ECG, ECG conduction and repolarization parameters as well as programmed electrical stimulation procedures to test for VT inducibility. High risk patients require ICD implantation. New developments such as subcutaneous ICDs might reduce the complication rates of transvenous ICDs, but its use is limited by the considerable rate of sensing screening failure. If electrophysiological substrates arising from the RVOT are confirmed by mapping, they can be eliminated using catheter ablation.
Acknowledgements
GT thanks the Croucher Foundation of Hong Kong for the support of his Clinical Assistant Professorship.
References
- 1.Kobayashi T., Shintani U., Yamamoto T., Shida S., Isshiki N., Tanaka T. Familial occurrence of electrocardiographic abnormalities of the Brugada-type. Int. Med. 1996;35:637–640. doi: 10.2169/internalmedicine.35.637. [DOI] [PubMed] [Google Scholar]
- 2.Yan G.X., Antzelevitch C. Cellular basis for the electrocardiographic J wave. Circulation. 1996;93:372–379. doi: 10.1161/01.cir.93.2.372. [DOI] [PubMed] [Google Scholar]
- 3.Grant A.O. Cardiac Ion Channels. Circulation: Arrhythmia Electrophysiol. 2009;2:185–194. doi: 10.1161/CIRCEP.108.789081. [DOI] [PubMed] [Google Scholar]
- 4.Wilde A.A., Postema P.G., Di Diego J.M., Viskin S., Morita H., Fish J.M. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J. Mol. Cell Cardiol. 2010;49:543–553. doi: 10.1016/j.yjmcc.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tse G., Wong S.T., Tse V., Yeo J.M. Depolarization vs. repolarization: what is the mechanism of ventricular arrhythmogenesis underlying sodium channel haploinsufficiency in mouse hearts? Acta Physiol. (Oxf) 2016 doi: 10.1111/apha.12694. [DOI] [PubMed] [Google Scholar]
- 6.Madeira M., Caetano F., Providencia R., Almeida I., Trigo J., Nascimento J., Costa M., Leitao Marques A. Fever-induced type 1 Brugada pattern. Rev. Port. Cardiol. 2015;34(287):e1–e7. doi: 10.1016/j.repc.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Achaiah A.A.N. Intoxication with alcohol: An underestimated trigger of Brugada syndrome? JRSM Open. 2016;7 doi: 10.1177/2054270416640153. 2054270416640153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan G.X., Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 9.Letsas K.P., Korantzopoulos P., Efremidis M., Weber R., Lioni L., Bakosis G. Sinus node disease in subjects with type 1 ECG pattern of Brugada syndrome. J. Cardiol. 2013;61:227–231. doi: 10.1016/j.jjcc.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Bordachar P., Reuter S., Garrigue S., Cai X., Hocini M., Jais P., Haissaguerre M., Clementy J. Incidence, clinical implications and prognosis of atrial arrhythmias in Brugada syndrome. Eur. Heart J. 2004;25:879–884. doi: 10.1016/j.ehj.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Eckardt L., Kirchhof P., Johna R., Haverkamp W., Breithardt G., Borggrefe M. Wolff-Parkinson-White syndrome associated with Brugada syndrome. Pacing Clin. Electrophysiol.: PACE. 2001;24:1423–1424. doi: 10.1046/j.1460-9592.2001.01423.x. [DOI] [PubMed] [Google Scholar]
- 12.Bayes de Luna A., Brugada J., Baranchuk A., Borggrefe M., Breithardt G., Goldwasser D. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report. J. Electrocardiol. 2012;45:433–442. doi: 10.1016/j.jelectrocard.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Priori S.G., Wilde A.A., Horie M., Cho Y., Behr E.R., Berul C. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes. Europace. 2013;15:1389–1406. doi: 10.1093/europace/eut272. [DOI] [PubMed] [Google Scholar]
- 14.Brugada P., Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J. Am. Coll. Cardiol. 1992;20:1391–1396. doi: 10.1016/0735-1097(92)90253-j. [DOI] [PubMed] [Google Scholar]
- 15.Postema P.G. About Brugada syndrome and its prevalence. EP Europace. 2012;14:925–928. doi: 10.1093/europace/eus042. [DOI] [PubMed] [Google Scholar]
- 16.Vutthikraivit W., Rattanawong P., Putthapiban P., Sukhumthammarat W., Vathesatogkit P., Ngarmukos T. Worldwide prevalence of brugada syndrome: a systematic review and meta-analysis. Acta Cardiologica Sinica. 2018;34:267–277. doi: 10.6515/ACS.201805_34(3).20180302B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng C.T., Ong H.Y., Cheok C., Chua T.S., Ching C.K. Prevalence of electrocardiographic abnormalities in an unselected young male multi-ethnic South-East Asian population undergoing pre-participation cardiovascular screening: results of the Singapore Armed Forces Electrocardiogram and Echocardiogram screening protocol. Europace. 2012;14:1018–1024. doi: 10.1093/europace/eur424. [DOI] [PubMed] [Google Scholar]
- 18.Juang J.M., Chen C.Y., Chen Y.H., Wu I.C., Hsu C.C., Chen L.N. Prevalence and prognosis of Brugada electrocardiogram patterns in an elderly Han Chinese population: a nation-wide community-based study (HALST cohort) Europace. 2015;17(Suppl 2):ii54-62. doi: 10.1093/europace/euv141. [DOI] [PubMed] [Google Scholar]
- 19.Holst A.G., Jensen H.K., Eschen O., Henriksen F.L., Kanters J., Bundgaard H., Svendsen J.H., Haunso S., Tfelt-Hansen J. Low disease prevalence and inappropriate implantable cardioverter defibrillator shock rate in Brugada syndrome: a nationwide study. Europace. 2012;14:1025–1029. doi: 10.1093/europace/eus002. [DOI] [PubMed] [Google Scholar]
- 20.Nademanee K., Veerakul G., Nimmannit S., Chaowakul V., Bhuripanyo K., Likittanasombat K., Tunsanga K., Kuasirikul S., Malasit P., Tansupasawadikul S., Tatsanavivat P. Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation. 1997;96:2595–2600. doi: 10.1161/01.cir.96.8.2595. [DOI] [PubMed] [Google Scholar]
- 21.Milman A., Andorin A., Postema P.G., Gourraud J.B., Sacher F., Mabo P. Ethnic differences in patients with brugada syndrome and arrhythmic events: new insights from SABRUS. Heart Rhythm. 2019 doi: 10.1016/j.hrthm.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Antzelevitch C., Brugada P., Borggrefe M., Brugada J., Brugada R., Corrado D. Brugada syndrome: report of the second consensus conference. Heart Rhythm. 2005;2:429–440. doi: 10.1016/j.hrthm.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Dumaine R., Towbin J.A., Brugada P., Vatta M., Nesterenko D.V., Nesterenko V.V. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ. Res. 1999;85:803–809. doi: 10.1161/01.res.85.9.803. [DOI] [PubMed] [Google Scholar]
- 24.London B., Michalec M., Mehdi H., Zhu X., Kerchner L., Sanyal S. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007;116:2260–2268. doi: 10.1161/CIRCULATIONAHA.107.703330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antzelevitch C., Pollevick G.D., Cordeiro J.M., Casis O., Sanguinetti M.C., Aizawa Y. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–449. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sicouri S., Blazek J., Belardinelli L., Antzelevitch C. Electrophysiological characteristics of canine superior vena cava sleeve preparations: effect of ranolazine. Circ. Arrhythmia Electrophysiol. 2012;5:371–379. doi: 10.1161/CIRCEP.111.969493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gourraud J.B., Barc J., Thollet A., Le Scouarnec S., Le Marec H., Schott J.J., Redon R., Probst V. The Brugada Syndrome: A Rare Arrhythmia Disorder with Complex Inheritance. Front. Cardiovasc. Med. 2016;3:9. doi: 10.3389/fcvm.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Probst V., Wilde A.A., Barc J., Sacher F., Babuty D., Mabo P., Mansourati J., Le Scouarnec S., Kyndt F., Le Caignec C., Guicheney P., Gouas L., Albuisson J., Meregalli P.G., Le Marec H., Tan H.L., Schott J.J. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ. Cardiovasc. Genet. 2009;2:552–557. doi: 10.1161/CIRCGENETICS.109.853374. [DOI] [PubMed] [Google Scholar]
- 29.Marian A.J. Nature's genetic gradients and the clinical phenotype. Circ. Cardiovasc. Genet. 2009;2:537–539. doi: 10.1161/CIRCGENETICS.109.921940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden D.M. Brugada syndrome: Lots of questions, some answers. Heart Rhythm. 2010;7:47–49. doi: 10.1016/j.hrthm.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Giudicessi J.R., Ackerman M.J. Determinants of incomplete penetrance and variable expressivity in heritable cardiac arrhythmia syndromes. Transl. Res.: J. Lab. Clin. Med. 2013;161:1–14. doi: 10.1016/j.trsl.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolz-Gaitón P., Núñez M., Núñez L., Barana A., Amorós I., Matamoros M. Functional Characterization of a Novel Frameshift Mutation in the C-terminus of the Nav1.5 Channel Underlying a Brugada Syndrome with Variable Expression in a Spanish Family. PLoS ONE. 2013;8:e81493. doi: 10.1371/journal.pone.0081493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Driest Sara L., Wells Quinn S., Stallings Sarah. Association of Arrhythmia-Related Genetic Variants With Phenotypes Documented in Electronic Medical Records. JAMA. 2016;315(1):47. doi: 10.1001/jama.2015.17701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bezzina C.R., Barc J., Mizusawa Y., Remme C.A., Gourraud J.B., Simonet F. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 2013;45:1044–1049. doi: 10.1038/ng.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abriel H. Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J. Mol. Cell. Cardiol. 2010;48:2–11. doi: 10.1016/j.yjmcc.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Templin C., Ghadri J.R., Rougier J.S., Baumer A., Kaplan V., Albesa M. Identification of a novel loss-of-function calcium channel gene mutation in short QT syndrome (SQTS6) Eur. Heart J. 2011;32:1077–1088. doi: 10.1093/eurheartj/ehr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nam G.B., Kim Y.H., Antzelevitch C. Augmentation of J waves and electrical storms in patients with early repolarization. N. Engl. J. Med. 2008;358:2078–2079. doi: 10.1056/NEJMc0708182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junttila M.J., Sager S.J., Tikkanen J.T., Anttonen O., Huikuri H.V., Myerburg R.J. Clinical significance of variants of J-points and J-waves: early repolarization patterns and risk. Eur. Heart J. 2012;33:2639–2643. doi: 10.1093/eurheartj/ehs110. [DOI] [PubMed] [Google Scholar]
- 39.Antzelevitch C.Y., Yan G.X., Ackerman M.J., Borggrefe M., Corrado D., Guo J., Gussak I., Hasdemir C., Horie M., Huikuri H., Ma C., Morita H., Nam G.B., Sacher F., Shimizu W., Viskin S., Wilde A.A. J-Wave syndromes expert consensus conference report: Emerging concepts and gaps in knowledge: Endorsed by the Asia Pacific Heart Rhythm Society (APHRS), the European Heart Rhythm Association (EHRA), the Heart Rhythm Society (HRS), and the Latin American Society of Cardiac Pacing and Electrophysiology (Sociedad Latinoamericana de Estimulacifin Cardiaca y Electro fi siologia [SOLAECE]) Europace. 2016 [Google Scholar]
- 40.Haïssaguerre M., Derval N., Sacher F., Jesel L., Deisenhofer I., de Roy L. Sudden cardiac arrest associated with early repolarization. N. Engl. J. Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 41.Derval N., Simpson C.S., Birnie D.H., Healey J.S., Chauhan V., Champagne J., Gardner M., Sanatani S., Yee R., Skanes A.C., Gula L.J., Leong-Sit P., Ahmad K., Gollob M.H., Haissaguerre M., Klein G.J., Krahn A.D. Prevalence and characteristics of early repolarization in the CASPER registry: cardiac arrest survivors with preserved ejection fraction registry. J. Am. Coll. Cardiol. 2011;58:722–728. doi: 10.1016/j.jacc.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 42.Haruta D., Matsuo K., Tsuneto A., Ichimaru S., Hida A., Sera N., Imaizumi M., Nakashima E., Maemura K., Akahoshi M. Incidence and prognostic value of early repolarization pattern in the 12-lead electrocardiogram. Circulation. 2011;123:2931–2937. doi: 10.1161/CIRCULATIONAHA.110.006460. [DOI] [PubMed] [Google Scholar]
- 43.Antzelevitch C., Sicouri S., Litovsky S.H., Lukas A., Krishnan S.C., Di Diego J.M. Heterogeneity within the ventricular wall. Electrophysiology and pharmacology of epicardial, endocardial, and M cells. Circ. Res. 1991;69:1427–1449. doi: 10.1161/01.res.69.6.1427. [DOI] [PubMed] [Google Scholar]
- 44.Litovsky S.H., Antzelevitch C. Transient outward current prominent in canine ventricular epicardium but not endocardium. Circ. Res. 1988;62:116–126. doi: 10.1161/01.res.62.1.116. [DOI] [PubMed] [Google Scholar]
- 45.Opthof T., Coronel R., Janse M.J., Rosen M.R. A wedge is not a heart. Heart Rhythm. 2007;4:1116–1119. [Google Scholar]
- 46.Baranchuk A., Nguyen T., Ryu M.H., Femenia F., Zareba W., Wilde A.A. Brugada phenocopy: new terminology and proposed classification. Ann. Noninvasive Electrocardiol. 2012;17:299–314. doi: 10.1111/j.1542-474X.2012.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuo M., Li J., Lecker S.H. Brugada phenocopy induced by hyperkalemia. Kidney Int. 2019;95:471. doi: 10.1016/j.kint.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 48.Baranchuk A., Antiperovitch P., Gottschalk B.H. Brugada phenocopy due to hypokalemia. J. Family Med. Prim. Care. 2018;7:1141–1142. doi: 10.4103/jfmpc.jfmpc_64_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gul E.E., Haseeb S., Al Amoudi O., Baranchuk A. Brugada phenocopy associated with left ventricular aneurysm. J. Electrocardiol. 2018;51:963–965. doi: 10.1016/j.jelectrocard.2018.08.032. [DOI] [PubMed] [Google Scholar]
- 50.Yu M., Zhang Q., Huang X., Zhao X. Type 1 Brugada phenocopy in a patient with acute pericarditis. J. Electrocardiol. 2018;51:1121–1123. doi: 10.1016/j.jelectrocard.2018.10.087. [DOI] [PubMed] [Google Scholar]
- 51.Zhang N., Liu T., Tse G., Yu S., Fu H., Xu G. Brugada phenocopy in a patient with acute pulmonary embolism presenting with recurrent syncope. Oxf. Med. Case Rep. 2017;2017:omx014. doi: 10.1093/omcr/omx014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dendramis G. Brugada syndrome and Brugada phenocopy. The importance of a differential diagnosis. Int. J. Cardiol. 2016;210:25–27. doi: 10.1016/j.ijcard.2016.02.097. [DOI] [PubMed] [Google Scholar]
- 53.Kunze D.L., Lacerda A.E., Wilson D.L., Brown A.M. Cardiac Na currents and the inactivating, reopening, and waiting properties of single cardiac Na channels. J. Gen. Physiol. 1985;86:691–719. doi: 10.1085/jgp.86.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carmeliet E. Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol. Rev. 1999;79:917–1017. doi: 10.1152/physrev.1999.79.3.917. [DOI] [PubMed] [Google Scholar]
- 55.Chen Q., Kirsch G.E., Zhang D., Brugada R., Brugada J., Brugada P. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998;392:293–296. doi: 10.1038/32675. [DOI] [PubMed] [Google Scholar]
- 56.Christien Li K.H., Liu T., Ling To O.T., Sun Chan Y., Tse G., Yan B.P. A1427S Missense Mutation in SCN5A Causes Type 1 Brugada Pattern, Recurrent Ventricular Tachyarrhythmias and Right Ventricular Structural Abnormalities. Res. Cardiovasc. Med. 2016;inpress:e42085. [Google Scholar]
- 57.Benson D.W., Wang D.W., Dyment M., Knilans T.K., Fish F.A., Strieper M.J., Rhodes T.H., George A.L., Jr. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A) J. Clin. Invest. 2003;112:1019–1028. doi: 10.1172/JCI18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tan H.L., Bink-Boelkens M.T., Bezzina C.R., Viswanathan P.C., Beaufort-Krol G.C., van Tintelen P.J. A sodium-channel mutation causes isolated cardiac conduction disease. Nature. 2001;409:1043–1047. doi: 10.1038/35059090. [DOI] [PubMed] [Google Scholar]
- 59.Remme C.A., Wilde A.A., Bezzina C.R. Cardiac sodium channel overlap syndromes: different faces of SCN5A mutations. Trends Cardiovasc. Med. 2008;18:78–87. doi: 10.1016/j.tcm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 60.Probst V., Veltmann C., Eckardt L., Meregalli P.G., Gaita F., Tan H.L., Babuty D., Sacher F., Giustetto C., Schulze-Bahr E., Borggrefe M., Haissaguerre M., Mabo P., Le Marec H., Wolpert C., Wilde A.A. Long-term prognosis of patients diagnosed with Brugada syndrome: Results from the FINGER Brugada Syndrome Registry. Circulation. 2010;121:635–643. doi: 10.1161/CIRCULATIONAHA.109.887026. [DOI] [PubMed] [Google Scholar]
- 61.Watanabe H., Koopmann T.T., Le Scouarnec S., Yang T., Ingram C.R., Schott J.J., Demolombe S., Probst V., Anselme F., Escande D., Wiesfeld A.C., Pfeufer A., Kaab S., Wichmann H.E., Hasdemir C., Aizawa Y., Wilde A.A., Roden D.M., Bezzina C.R. Sodium channel beta1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J. Clin. Invest. 2008;118:2260–2268. doi: 10.1172/JCI33891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hu D., Barajas-Martinez H., Burashnikov E., Springer M., Wu Y., Varro A., Pfeiffer R., Koopmann T.T., Cordeiro J.M. A mutation in the beta 3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ. Cardiovasc. Genet. 2009;270:8. doi: 10.1161/CIRCGENETICS.108.829192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riuró H., Beltran‐Alvarez P., Tarradas A., Selga E., Campuzano O., Verges M., Pagans S., Iglesias A., Brugada J., Brugada P., Vazquez F.M., Perez G.J., Scornik F.S., Brugada R. A missense mutation in the sodium channel beta2 subunit reveals SCN2B as a new candidate gene for Brugada syndrome. Hum. Mutat. 2013;34:961–966. doi: 10.1002/humu.22328. [DOI] [PubMed] [Google Scholar]
- 64.Ricci M.T., Menegon S., Vatrano S., Mandrile G., Cerrato N., Carvalho P., De Marchi M., Gaita F., Giustetto C., Giachino D.F. SCN1B gene variants in Brugada Syndrome: a study of 145 SCN5A-negative patients. Sci. Rep. 2014;4:6470. doi: 10.1038/srep06470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gray M.P., Saba S., Zhang Y., Hernandez I. Outcomes of Patients With Atrial Fibrillation Newly Recommended for Oral Anticoagulation Under the, American Heart Association/American College of Cardiology/Heart Rhythm Society Guideline. J. Am. Heart Assoc. 2014;2018:7. doi: 10.1161/JAHA.117.007881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kattygnarath D., Maugenre S., Neyroud N., Balse E., Ichai C., Denjoy I., Dilanian G., Martins R.P., Fressart V., Berthet M., Schott J.J., Leenhardt A., Probst V., Le Marec H., Hainque B., Coulombe A., Hatem S.N., Guicheney P. MOG1: a new susceptibility gene for Brugada syndrome. Circ. Cardiovasc. Genet. 2011;4:261–268. doi: 10.1161/CIRCGENETICS.110.959130. [DOI] [PubMed] [Google Scholar]
- 67.Ishikawa T., Sato A., Marcou C.A., Tester D.J., Ackerman M.J., Crotti L., Schwartz P.J., On Y.K., Park J.E., Nakamura K., Hiraoka M., Nakazawa K., Sakurada H., Arimura T., Makita N., Kimura A. A novel disease gene for Brugada syndrome: sarcolemmal membrane-associated protein gene mutations impair intracellular trafficking of hNav1.5. Circ. Arrhythmia Electrophysiol. 2012;5:1098–1107. doi: 10.1161/CIRCEP.111.969972. [DOI] [PubMed] [Google Scholar]
- 68.Cerrone M., Lin X., Zhang M., Agullo-Pascual E., Pfenniger A., Chkourko Gusky H. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation. 2014;129:1092–1103. doi: 10.1161/CIRCULATIONAHA.113.003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hennessey J.A., Marcou C.A., Wang C., Wei E.Q., Wang C., Tester D.J., Torchio M., Dagradi F., Crotti L., Schwartz P.J., Ackerman M.J., Pitt G.S. FGF12 is a candidate Brugada syndrome locus. HeartRhythm: Off. J. Heart Rhythm Soc. 2013;10:1886–1894. doi: 10.1016/j.hrthm.2013.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burashnikov E., Pfeiffer R., Barajas-Martinez H., Delpon E., Hu D., Desai M. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm: Off. J. Heart Rhythm Soc. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delpón E., Cordeiro J.M., Nunez L., Thomsen P.E., Guerchicoff A., Pollevick G.D., Wu Y., Kanters J.K., Larsen C.T., Hofman-Bang J., Burashnikov E., Christiansen M., Antzelevitch C. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ. Arrhythmia Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giudicessi J.R., Ye D., Tester D.J., Crotti L., Mugione A., Nesterenko V.V., Albertson R.M., Antzelevitch C., Schwartz P.J., Ackerman M.J. Transient outward current (I(to)) gain-of-function mutations in the KCND3-encoded Kv4.3 potassium channel and Brugada syndrome. Heart Rhythm: Off. J. Heart Rhythm Soc. 2011;8:1024–1032. doi: 10.1016/j.hrthm.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohno S., Zankov D.P., Ding W.G., Itoh H., Makiyama T., Doi T., Shizuta S., Hattori T., Miyamoto A., Naiki N., Hancox J.C., Matsuura H., Horie M. KCNE5 (KCNE1L) variants are novel modulators of Brugada syndrome and idiopathic ventricular fibrillation. Circ. Arrhythmia Electrophysiol. 2011;4:352–361. doi: 10.1161/CIRCEP.110.959619. [DOI] [PubMed] [Google Scholar]
- 74.Giudicessi J.R., Ye D., Kritzberger C.J., Nesterenko V.V., Tester D.J., Antzelevitch C., Ackerman M.J. Novel mutations in the KCND3-encoded Kv4.3 K+ channel associated with autopsy-negative sudden unexplained death. Hum. Mutat. 2012;33:989–997. doi: 10.1002/humu.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakajima T., Wu J., Kaneko Y., Ashihara T., Ohno S., Irie T., Ding W.G., Matsuura H., Kurabayashi M., Horie KCNE3 T4A as the genetic basis of Brugada-pattern electrocardiogram. Circ. J.: Off. J. Japanese Circ. Soc. 2012;76:2763–2772. doi: 10.1253/circj.cj-12-0551. [DOI] [PubMed] [Google Scholar]
- 76.Boczek N.J., Ye D., Johnson E.K., Wang W., Crotti L., Tester D.J., Dagradi F., Mizusawa Y., Torchio M., Alders M., Giudicessi J.R., Wilde A.A., Schwartz P.J., Nerbonne J.M., Ackerman M.J. Characterization of SEMA3A-encoded semaphorin as a naturally occurring Kv4.3 protein inhibitor and its contribution to Brugada syndrome. Circ. Res. 2014;115:460–469. doi: 10.1161/CIRCRESAHA.115.303657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medeiros-Domingo B.H., Crotti L., Tester D.J., Eckhardt L., Cuoretti A., Kroboth S.L., Song C., Zhou Q., Kopp D., Schwartz P.J., Makielski J.C., Ackerman M.J. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm: Off. J. Heart Rhythm Soc. 2010;7:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu D., Barajas-Martínez H., Terzic A., Park S., Pfeiffer R., Burashnikov E., Wu Y., Borggrefe M., Veltmann C., Schimpf R., Cai J.J., Nam G.B., Deshmukh P., Scheinman M., Preminger M., Steinberg J., Lopez-Izquierdo A., Ponce-Balbuena D., Wolpert C., Haissaguerre M., Sanchez-Chapula J.A., Antzelevitch C. ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene. Int. J. Cardiol. 2014;171:431–442. doi: 10.1016/j.ijcard.2013.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Q.I., Ohno S., Ding W.G., Fukuyama M., Miyamoto A., Itoh H., Makiyama T., Wu J., Bai J., Hasegawa K., Shinohara T., Takahashi N., Shimizu A., Matsuura H., Horie M. Gain-of-function KCNH2 mutations in patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2014;25:522–530. doi: 10.1111/jce.12361. [DOI] [PubMed] [Google Scholar]
- 80.Portero V., Le Scouarnec S., Es-Salah-Lamoureux Z., Burel S., Gourraud J.B., Bonnaud S., Lindenbaum P., Simonet F., Violleau J., Baron E., Moreau E., Scott C., Chatel S., Loussouarn G., O'Hara T., Mabo P., Dina C., Le Marec H., Schott J.J., Probst V., Baro I., Marionneau C., Charpentier F., Redon R. Dysfunction of the Voltage-Gated K+ Channel beta2 Subunit in a Familial Case of Brugada Syndrome. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.115.003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baruscotti M., Bucchi A., Viscomi C., Mandelli G., Consalez G., Gnecchi-Rusconi T. Deep bradycardia and heart block caused by inducible cardiac-specific knockout of the pacemaker channel gene Hcn4. Proc Natl Acad Sci U S A. 2011;108:1705–1710. doi: 10.1073/pnas.1010122108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ueda K., Hirano Y., Higashiuesato Y., Aizawa Y., Hayashi T., Inagaki N., Tana T., Ohya Y., Takishita S., Muratani H., Hiraoka M., Kimura A. Role of HCN4 channel in preventing ventricular arrhythmia. J. Hum. Genet. 2009;54:115–121. doi: 10.1038/jhg.2008.16. [DOI] [PubMed] [Google Scholar]
- 83.Liu S., Simard C., Syam N., Salle L., Probst V., Morel J., Millat G., Lopez M., Abriel H., Schott J.J., Guinamard R., Bouvagnet P. Molecular genetics and functional anomalies in a series of 248 Brugada cases with 11 mutations in the TRPM4 channel. PLoS ONE. 2013;8:e54131. doi: 10.1371/journal.pone.0054131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tse G., Yeo J.M. Conduction abnormalities and ventricular arrhythmogenesis: The roles of sodium channels and gap junctions. Int. J. Cardiol. Heart Vasc. 2015;9:75–82. doi: 10.1016/j.ijcha.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kyndt F., Probst V., Potet F., Demolombe S., Chevallier J.-C., Baro I. Novel SCN5A mutation leading either to isolated cardiac conduction defect or brugada syndrome in a large french family. Circulation. 2001;104:3081–3086. doi: 10.1161/hc5001.100834. [DOI] [PubMed] [Google Scholar]
- 86.Martini B., Nava A., Thiene G., Buja G.F., Canciani B., Scognamiglio R. Ventricular fibrillation without apparent heart disease: description of six cases. Am. Heart J. 1989;118:1203–1209. doi: 10.1016/0002-8703(89)90011-2. [DOI] [PubMed] [Google Scholar]
- 87.Leoni A.L., Gavillet B., Rougier J.S., Marionneau C., Probst V., Le Scouarnec S., Schott J.J., Demolombe S., Bruneval P., Huang C.L., Colledge W.H., Grace A.A., Le Marec H., Wilde A.A., Mohler P.J., Escande D., Abriel H., Charpentier F. Variable Na(v)1.5 protein expression from the wild-type allele correlates with the penetrance of cardiac conduction disease in the Scn5a(+/-) mouse model. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boukens B.J., Sylva M., de Gier-de Vries C., Remme C.A., Bezzina C.R., Christoffels V.M., Coronel R. Reduced sodium channel function unmasks residual embryonic slow conduction in the adult right ventricular outflow tract. Circ. Res. 2013;113:137–141. doi: 10.1161/CIRCRESAHA.113.301565. [DOI] [PubMed] [Google Scholar]
- 89.Schweizer P.A., Fink T., Yampolsky P., Seyler C., Fabritz L., Kirchhof P., Becker R., Koenen M., Katus H.A., Thomas D. Generation and characterization of SCN5A loss-of-function mutant mice modeling human brugada syndrome. Eur. Heart J. 2014;34 [Google Scholar]
- 90.Royer A., van Veen T.A., Le Bouter S., Marionneau C., Griol-Charhbili V., Leoni A.L. Mouse model of SCN5A-linked hereditary Lenegre's disease: age-related conduction slowing and myocardial fibrosis. Circulation. 2005;111:1738–1746. doi: 10.1161/01.CIR.0000160853.19867.61. [DOI] [PubMed] [Google Scholar]
- 91.Coronel R., Casini S., Koopmann T.T., Wilms-Schopman F.J., Verkerk A.O., de Groot J.R. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005;112:2769–2777. doi: 10.1161/CIRCULATIONAHA.105.532614. [DOI] [PubMed] [Google Scholar]
- 92.Frustaci A., Priori S.G., Pieroni M., Chimenti C., Napolitano C., Rivolta I. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation. 2005;112:3680–3687. doi: 10.1161/CIRCULATIONAHA.105.520999. [DOI] [PubMed] [Google Scholar]
- 93.Fujiki A., Usui M., Nagasawa H., Mizumaki K., Hayashi H., Inoue H. ST segment elevation in the right precordial leads induced with class IC antiarrhythmic drugs: insight into the mechanism of Brugada syndrome. J. Cardiovasc. Electrophysiol. 1999;10:214–218. doi: 10.1111/j.1540-8167.1999.tb00662.x. [DOI] [PubMed] [Google Scholar]
- 94.Morita H., Kusano K.F., Miura D., Nagase S., Nakamura K., Morita S.T. Fragmented QRS as a marker of conduction abnormality and a predictor of prognosis of Brugada syndrome. Circulation. 2008;118:1697–1704. doi: 10.1161/CIRCULATIONAHA.108.770917. [DOI] [PubMed] [Google Scholar]
- 95.Cohen S.A. Immunocytochemical localization of rH1 sodium channel in adult rat heart atria and ventricle. Presence in terminal intercalated disks. Circulation. 1996;94:3083–3086. doi: 10.1161/01.cir.94.12.3083. [DOI] [PubMed] [Google Scholar]
- 96.Rhett J.M., Veeraraghavan R., Poelzing S., Gourdie R.G. The perinexus: sign-post on the path to a new model of cardiac conduction? Trends Cardiovasc. Med. 2013;23:222–228. doi: 10.1016/j.tcm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nademanee K., Raju H., De Noronha S.V., Papadakis M., Robinson L., Rothery S., Makita N., Kowase S., Boonmee N., Vitayakritsirikul V., Ratanarapee S. Fibrosis, connexin-43, and conduction abnormalities in the brugada syndrome. J. Am. Coll. Cardiol. 2015;66(18):1976–1986. doi: 10.1016/j.jacc.2015.08.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nagase S., Kusano K.F., Morita H., Fujimoto Y., Kakishita M., Nakamura K., Emori T., Matsubara H., Ohe T. Epicardial electrogram of the right ventricular outflow tract in patients with the brugada syndromeUsing the epicardial lead. J. Am. College Cardiol. 2002;39:1992–1995. doi: 10.1016/s0735-1097(02)01888-0. [DOI] [PubMed] [Google Scholar]
- 99.Kataoka H. Electrocardiographic patterns of the Brugada syndrome in right ventricular infarction/ischemia. Am. J. Cardiol. 2000;86:1056. doi: 10.1016/s0002-9149(00)01351-5. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J., Sacher F., Hoffmayer K., O’Hara T., Strom M., Cuculich P., Silva J., Cooper D., Faddis M., Hocini M., Haïssaguerre M. Cardiac Electrophysiological Substrate Underlying the ECG Phenotype and Electrogram Abnormalities in Brugada Syndrome Patients. Circulation. 2015;131:1950–1959. doi: 10.1161/CIRCULATIONAHA.114.013698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Veldkamp M.W., Viswanathan P.C., Bezzina C., Baartscheer A., Wilde A.A., Balser J.R. Two distinct congenital arrhythmias evoked by a multidysfunctional Na(+) channel. Circ. Res. 2000;86:E91–E97. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- 102.Antzelevitch C. Transmural dispersion of repolarization and the T wave. Cardiovasc. Res. 2001;50:426–431. doi: 10.1016/s0008-6363(01)00285-1. [DOI] [PubMed] [Google Scholar]
- 103.Fish J.M.A.C. Role of sodium and calcium channel block in unmasking the Brugada syndrome. Heart Rhythm: Off. J. Heart Rhythm Soc. 2004;1:210–217. doi: 10.1016/j.hrthm.2004.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maoz A.C., David J., Krogh-Madsen Trine. Dependence of phase-2 reentry and repolarization dispersion on epicardial and transmural ionic heterogeneity: a simulation study. Europace. 2014;16:458–465. doi: 10.1093/europace/eut379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maury P., Sacher F., Gourraud J.B., Pasquie J.L., Raczka F., Bongard V. Increased Tpeak-Tend interval is highly and independently related to arrhythmic events in Brugada syndrome. Heart Rhythm. 2015;12:2469–2476. doi: 10.1016/j.hrthm.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 106.Pastore J.M.G., Laurita S.D., Akar K.R., Rosenbaum F.G.D.S. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 107.Nishii N., Nagase S., Morita H., Kusano K.F., Namba T., Miura D., Miyaji K., Hiramatsu S., Tada T., Murakami M., Watanabe A. Abnormal restitution property of action potential duration and conduction delay in Brugada syndrome: both repolarization and depolarization abnormalities. Europace. 2010;12:544–552. doi: 10.1093/europace/eup432. [DOI] [PubMed] [Google Scholar]
- 108.Nishizaki M.F., Sakurada H., Kimura H., Hiraoka A.M. Spontaneous T wave alternans in a patient with Brugada syndrome–responses to intravenous administration of class I antiarrhythmic drug, glucose tolerance test, and atrial pacing. J. Cardiovasc. Electrophysiol. 2005;16:217–220. doi: 10.1046/j.1540-8167.2004.40411.x. [DOI] [PubMed] [Google Scholar]
- 109.Uchimura‐Makita Y.U., Nakano Y., Tokuyama T., Fujiwara M., Watanabe Y., Sairaku A., Kawazoe H., Matsumura H., Oda N., Ikanaga H., Motoda C., Kajihara K., Oda N., Verrier R.L., Kihara Y. Time-domain T-wave alternans is strongly associated with a history of ventricular fibrillation in patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2014;25:1021–1027. doi: 10.1111/jce.12441. [DOI] [PubMed] [Google Scholar]
- 110.Sakamoto S., Takagi M., Tatsumi H., Doi A., Sugioka K., Hanatani A., Yoshiyama M. Utility of T-wave alternans during night time as a predictor for ventricular fibrillation in patients with Brugada syndrome. Heart Vessels. 2016;31:947–956. doi: 10.1007/s00380-015-0692-y. [DOI] [PubMed] [Google Scholar]
- 111.Hoogendijk M.G., Potse M., Linnenbank A.C., Verkerk A.O., den Ruijter H.M., van Amersfoorth S.C. Mechanism of right precordial ST-segment elevation in structural heart disease: excitation failure by current-to-load mismatch. Heart Rhythm. 2010;7:238–248. doi: 10.1016/j.hrthm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 112.Hoogendijk M.G.P., Vinet M., de Bakker A., Coronel J.M.R. ST segment elevation by current-to-load mismatch: an experimental and computational study. Heart Rhythm: Off. J. Heart Rhythm Soc. 2011;8:111–118. doi: 10.1016/j.hrthm.2010.09.066. [DOI] [PubMed] [Google Scholar]
- 113.Hoogendijk M.G., Opthof T., Postema P.G., Wilde A.A., de Bakker J.M., Coronel R. The Brugada ECG pattern: a marker of channelopathy, structural heart disease, or neither? Toward a unifying mechanism of the Brugada syndrome. Circ. Arrhythmia Electrophysiol. 2010;3:283–290. doi: 10.1161/CIRCEP.110.937029. [DOI] [PubMed] [Google Scholar]
- 114.ten Sande J.N., Coronel R., Conrath C.E., Driessen A.H., de Groot J.R., Tan H.L., Nademanee K., Wilde A.A., de Bakker J.M., van Dessel P.F. ST-Segment Elevation and Fractionated Electrograms in Brugada Syndrome Patients Arise From the Same Structurally Abnormal Subepicardial RVOT Area but Have a Different Mechanism. Circ. Arrhythmia Electrophysiol. 2015;8:1382–1392. doi: 10.1161/CIRCEP.115.003366. [DOI] [PubMed] [Google Scholar]
- 115.Postema P.G., van Dessel P.F., de Bakker J.M., Dekker L.R., Linnenbank A.C., Hoogendijk M.G., Coronel R., Tijssen J.G., Wilde A.A., Tan H.L. Slow and discontinuous conduction conspire in Brugada syndrome: a right ventricular mapping and stimulation study. Circ. Arrhythmia Electrophysiol. 2008;1:379–386. doi: 10.1161/CIRCEP.108.790543. [DOI] [PubMed] [Google Scholar]
- 116.Robyns T., Lu H.R., Gallacher D.J., Garweg C., Ector J., Willems R., Janssens S., Nuyens D. Evaluation of Index of Cardio-Electrophysiological Balance (iCEB) as a New Biomarker for the Identification of Patients at Increased Arrhythmic Risk. Ann. Noninvasive Electrocardiol. 2016 doi: 10.1111/anec.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tse G., Yan B.P. Traditional and novel electrocardiographic conduction and repolarization markers of sudden cardiac death. Europace. 2016 doi: 10.1093/europace/euw280. [DOI] [PubMed] [Google Scholar]
- 118.Aizawa Y., Yamakawa H., Takatsuki S., Katsumata Y., Nishiyama T., Kimura T. Efficacy and safety of bepridil for prevention of ICD shocks in patients with Brugada syndrome and idiopathic ventricular fibrillation. Int. J. Cardiol. 2013;168:5083–5085. doi: 10.1016/j.ijcard.2013.07.187. [DOI] [PubMed] [Google Scholar]
- 119.Shinohara T., Ebata Y., Ayabe R., Fukui A., Okada N., Yufu K. Combination therapy of cilostazol and bepridil suppresses recurrent ventricular fibrillation related to J-wave syndromes. Heart Rhythm. 2014;11:1441–1445. doi: 10.1016/j.hrthm.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 120.Viskin S., Wilde A.A., Tan H.L., Antzelevitch C., Shimizu W., Belhassen B. Empiric quinidine therapy for asymptomatic Brugada syndrome: time for a prospective registry. Heart Rhythm. 2009;6:401–404. doi: 10.1016/j.hrthm.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Belhassen B., Viskin S., Antzelevitch C. The Brugada syndrome: is an implantable cardioverter defibrillator the only therapeutic option? Pacing Clin. Electrophysiol. 2002;25:1634–1640. doi: 10.1046/j.1460-9592.2002.01634.x. [DOI] [PubMed] [Google Scholar]
- 122.Ohgo T., Okamura H., Noda T., Satomi K., Suyama K., Kurita T. Acute and chronic management in patients with Brugada syndrome associated with electrical storm of ventricular fibrillation. Heart Rhythm. 2007;4:695–700. doi: 10.1016/j.hrthm.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 123.Tsuchiya T., Ashikaga K., Honda T., Arita M. Prevention of ventricular fibrillation by cilostazol, an oral phosphodiesterase inhibitor, in a patient with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2002;13:698–701. doi: 10.1046/j.1540-8167.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 124.Szel T., Koncz I., Antzelevitch C. Cellular mechanisms underlying the effects of milrinone and cilostazol to suppress arrhythmogenesis associated with Brugada syndrome. Heart Rhythm. 2013;10:1720–1727. doi: 10.1016/j.hrthm.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gussak I. Ito Blockade by 4-Aminopyridine: A New Approach to Stabilise the Electrical Activity of the Heart. Emerging Therapeutic Targets. 1997;1:133–135. [Google Scholar]
- 126.Nademanee K., Veerakul G., Chandanamattha P., Chaothawee L., Ariyachaipanich A., Jirasirirojanakorn K. Prevention of ventricular fibrillation episodes in brugada syndrome by catheter ablation over the anterior right ventricular outflow tract epicardium. Circulation. 2011;123:1270–1279. doi: 10.1161/CIRCULATIONAHA.110.972612. [DOI] [PubMed] [Google Scholar]
- 127.McNamara D.A., Goldberger J.J., Berendsen M.A., Huffman M.D. Implantable defibrillators versus medical therapy for cardiac channelopathies. Cochrane Database Syst. Rev. 2015:CD011168. doi: 10.1002/14651858.CD011168.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Binbin Y., Jingping L., Bing Y., Minglong C., Jiangang Z., Kejiang C. Long-term outcome after cardioverter-defibrillator implantation in patients with Brugada syndrome. Zhonghua Xin Xue Guan Bing Za Zhi. 2015;43:690–694. [PubMed] [Google Scholar]
- 129.Nademanee K., Veerakul G., Mower M., Likittanasombat K., Krittayapong R., Bhuripanyo K. Defibrillator Versus β-Blockers for Unexplained Death in Thailand (DEBUT) Circulation. 2003;107:2221–2226. doi: 10.1161/01.CIR.0000066319.56234.C8. [DOI] [PubMed] [Google Scholar]
- 130.Irfan G., Czapla J., Saitoh Y., Ciconte G., Mugnai G., Conte G. Implantable cardioverter defibrillator therapy in young individuals: comparison of conventional and subcostal approaches-a single-centre experience. Europace. 2016 doi: 10.1093/europace/euv455. [DOI] [PubMed] [Google Scholar]
- 131.Rodriguez-Manero M., Sacher F., de Asmundis C., Maury P., Lambiase P.D., Sarkozy A. Monomorphic ventricular tachycardia in patients with Brugada syndrome: A multicenter retrospective study. Heart Rhythm. 2016;13:669–682. doi: 10.1016/j.hrthm.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 132.Nademanee K., Hocini M., Haïssaguerre M. Epicardial substrate ablation for Brugada syndrome. Heart Rhythm. 2017;14:457–461. doi: 10.1016/j.hrthm.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 133.Shi S., Barajas-Martinez H., Liu T., Sun Y., Yang B., Huang C. Prevalence of spontaneous Brugada ECG pattern recorded at standard intercostal leads: A meta-analysis. Int. J. Cardiol. 2017 doi: 10.1016/j.ijcard.2017.11.113. [DOI] [PubMed] [Google Scholar]
- 134.Letsas K.P., Asvestas D., Baranchuk A., Liu T., Georgopoulos S., Efremidis M. Prognosis, risk stratification and management of asymptomatic individuals with Brugada syndrome: a systematic review. Pacing Clin. Electrophysiol. 2017 doi: 10.1111/pace.13214. [DOI] [PubMed] [Google Scholar]
- 135.Adler A., Rosso R., Chorin E., Havakuk O., Antzelevitch C., Viskin S. Risk stratification in Brugada syndrome: clinical characteristics, electrocardiographic parameters, and auxiliary testing. Heart Rhythm. 2016;13:299–310. doi: 10.1016/j.hrthm.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 136.Milman A., Gourraud J.B., Andorin A., Postema P.G., Sacher F., Mabo P. Gender differences in patients with Brugada syndrome and arrhythmic events: data from a survey on arrhythmic events in 678 patients. Heart Rhythm. 2018;15:1457–1465. doi: 10.1016/j.hrthm.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 137.Li X., Sacher F., Kusano K.F., Barajas-Martinez H., Liu N., Li Y. Pooled Analysis of Risk Stratification of Spontaneous Type 1 Brugada ECG: Focus on the Influence of Gender and EPS. Front. Physiol. 2018;9:1951. doi: 10.3389/fphys.2018.01951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okamura H., Kamakura T., Morita H., Tokioka K., Nakajima I., Wada M. Risk stratification in patients with Brugada syndrome without previous cardiac arrest – prognostic value of combined risk factors. Circ. J. 2015;79:310–317. doi: 10.1253/circj.CJ-14-1059. [DOI] [PubMed] [Google Scholar]
- 139.Makarawate P., Chaosuwannakit N., Vannaprasaht S., Sahasthas D., Koo S.H., Lee E.J.D. SCN5A Genetic Polymorphisms Associated With Increased Defibrillator Shocks in Brugada Syndrome. J. Am. Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yang Y., Hu D., Sacher F., Kusano K.F., Li X., Barajas-Martinez H. Meta-Analysis of Risk Stratification of SCN5A With Brugada Syndrome: Is SCN5A Always a Marker of Low Risk? Front. Physiol. 2019;10:103. doi: 10.3389/fphys.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bayoumy A., Gong M.Q., Christien Li K.H., Wong S.H., Wu W.K., Li G.P. Spontaneous type 1 pattern, ventricular arrhythmias and sudden cardiac death in Brugada Syndrome: an updated systematic review and meta-analysis. J. Geriatr. Cardiol. 2017;14:639–643. doi: 10.11909/j.issn.1671-5411.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tse G., Li K.H.C., Li G., Liu T., Bazoukis G., Wong W.T. Higher Dispersion Measures of Conduction and Repolarization in Type 1 Compared to Non-type 1 Brugada Syndrome Patients: An Electrocardiographic Study From a Single Center. Front. Cardiovasc. Med. 2018;5:132. doi: 10.3389/fcvm.2018.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Georgopoulos S., Letsas K.P., Liu T., Kalafateli M., Korantzopoulos P., Burkle G. A meta-analysis on the prognostic significance of inferolateral early repolarization pattern in Brugada syndrome. Europace. 2017 doi: 10.1093/europace/euw394. [DOI] [PubMed] [Google Scholar]
- 144.Calo L., Giustetto C., Martino A., Sciarra L., Cerrato N., Marziali M. A New Electrocardiographic Marker of Sudden Death in Brugada Syndrome: The S-Wave in Lead I. J. Am. Coll. Cardiol. 2016;67:1427–1440. doi: 10.1016/j.jacc.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 145.Uchimura-Makita Y., Nakano Y., Tokuyama T., Fujiwara M., Watanabe Y., Sairaku A. Time-domain T-wave alternans is strongly associated with a history of ventricular fibrillation in patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2014;25:1021–1027. doi: 10.1111/jce.12441. [DOI] [PubMed] [Google Scholar]
- 146.Gray B., Kirby A., Kabunga P., Freedman S.B., Yeates L., Kanthan A. Twelve-lead ambulatory electrocardiographic monitoring in Brugada syndrome: Potential diagnostic and prognostic implications. Heart Rhythm. 2017;14:866–874. doi: 10.1016/j.hrthm.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 147.Makimoto H., Nakagawa E., Takaki H., Yamada Y., Okamura H., Noda T. Augmented ST-segment elevation during recovery from exercise predicts cardiac events in patients with Brugada syndrome. J. Am. Coll. Cardiol. 2010;56:1576–1584. doi: 10.1016/j.jacc.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 148.Priori S.G., Gasparini M., Napolitano C., Della Bella P., Ottonelli A.G., Sassone B. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J. Am. Coll. Cardiol. 2012;59:37–45. doi: 10.1016/j.jacc.2011.08.064. [DOI] [PubMed] [Google Scholar]
- 149.Bhar-Amato J., Finlay M., Santos D., Orini M., Chaubey S., Vyas V. Pharmacological Modulation of Right Ventricular Endocardial-Epicardial Gradients in Brugada Syndrome. Circ. Arrhythm Electrophysiol. 2018;11:e006330. doi: 10.1161/CIRCEP.118.006330. [DOI] [PubMed] [Google Scholar]
- 150.Letsas K.P., Liu T., Shao Q., Korantzopoulos P., Giannopoulos G., Vlachos K. Meta-analysis on risk stratification of asymptomatic individuals with the brugada phenotype. Am. J. Cardiol. 2015;116:98–103. doi: 10.1016/j.amjcard.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 151.Asvestas D., Tse G., Baranchuk A., Bazoukis G., Liu T., Saplaouras A. High risk electrocardiographic markers in Brugada syndrome. Int. J. Cardiol. Heart Vasc. 2018;18:58–64. doi: 10.1016/j.ijcha.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xia Y., Liang Y., Kongstad O., Holm M., Olsson B., Yuan S. Tpeak-Tend interval as an index of global dispersion of ventricular repolarization: evaluations using monophasic action potential mapping of the epi- and endocardium in swine. J. Interv. Card Electrophysiol. 2005;14:79–87. doi: 10.1007/s10840-005-4592-4. [DOI] [PubMed] [Google Scholar]
- 153.Antzelevitch C., Shimizu W., Yan G.X., Sicouri S., Weissenburger J., Nesterenko V.V. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J. Cardiovasc. Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 154.Xia Y., Liang Y., Kongstad O., Liao Q., Holm M., Olsson B. In vivo validation of the coincidence of the peak and end of the T wave with full repolarization of the epicardium and endocardium in swine. Heart Rhythm. 2005;2:162–169. doi: 10.1016/j.hrthm.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 155.Opthof T., Coronel R., Wilms-Schopman F.J., Plotnikov A.N., Shlapakova I.N., Danilo P., Jr Dispersion of repolarization in canine ventricle and the electrocardiographic T wave: Tp-e interval does not reflect transmural dispersion. Heart Rhythm. 2007;4:341–348. doi: 10.1016/j.hrthm.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 156.Gupta P., Patel C., Patel H., Narayanaswamy S., Malhotra B., Green J.T. T(p-e)/QT ratio as an index of arrhythmogenesis. J. Electrocardiol. 2008;41:567–574. doi: 10.1016/j.jelectrocard.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 157.Letsas K.P., Weber R., Astheimer K., Kalusche D., Arentz T. Tpeak-Tend interval and Tpeak-Tend/QT ratio as markers of ventricular tachycardia inducibility in subjects with Brugada ECG phenotype. Europace. 2010;12:271–274. doi: 10.1093/europace/eup357. [DOI] [PubMed] [Google Scholar]
- 158.Tse G., Gong M., Wong W.T., Georgopoulos S., Letsas K.P., Vassiliou V.S. The Tpeak – Tend interval as an electrocardiographic risk marker of arrhythmic and mortality outcomes: a systematic review and meta-analysis. Heart Rhythm. 2017 doi: 10.1016/j.hrthm.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 159.Tse G., Gong M., Li C.K.H., Leung K.S.K., Georgopoulos S., Bazoukis G. Tpeak-Tend, Tpeak-Tend/QT ratio and Tpeak-Tend dispersion for risk stratification in Brugada Syndrome: A systematic review and meta-analysis. J. Arrhythm. 2018;34:587–597. doi: 10.1002/joa3.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meng L., Letsas K.P., Baranchuk A., Shao Q., Tse G., Zhang N. Meta-analysis of Fragmented QRS as an Electrocardiographic Predictor for Arrhythmic Events in Patients with Brugada Syndrome. Front. Physiol. 2017;8:678. doi: 10.3389/fphys.2017.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rattanawong P., Riangwiwat T., Prasitlumkum N., Limpruttidham N., Kanjanahattakij N., Chongsathidkiet P. Baseline fragmented QRS increases the risk of major arrhythmic events in Brugada syndrome: Systematic review and meta-analysis. Ann. Noninvasive Electrocardiol. 2017 doi: 10.1111/anec.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morita H., Watanabe A., Morimoto Y., Kawada S., Tachibana M., Nakagawa K. Distribution and Prognostic Significance of Fragmented QRS in Patients With Brugada Syndrome. Circ. Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004765. [DOI] [PubMed] [Google Scholar]
- 163.Morita H., Watanabe A., Kawada S., Miyamoto M., Morimoto Y., Nakagawa K. Identification of electrocardiographic risk markers for the initial and recurrent episodes of ventricular fibrillation in patients with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2017 doi: 10.1111/jce.13349. [DOI] [PubMed] [Google Scholar]
- 164.Ohkubo K., Watanabe I., Okumura Y., Ashino S., Kofune M., Nagashima K. Prolonged QRS duration in lead V2 and risk of life-threatening ventricular Arrhythmia in patients with Brugada syndrome. Int. Heart J. 2011;52:98–102. doi: 10.1536/ihj.52.98. [DOI] [PubMed] [Google Scholar]
- 165.Turrini P., Corrado D., Basso C., Nava A., Bauce B., Thiene G. Dispersion of Ventricular Depolarization-Repolarization: A Noninvasive Marker for Risk Stratification in Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation. 2001;103:3075–3080. doi: 10.1161/01.cir.103.25.3075. [DOI] [PubMed] [Google Scholar]
- 166.Tse G. Novel conduction-repolarization indices for the stratification of arrhythmic risk. J. Geriatric. Cardiol.: JGC. 2016;13:811–812. doi: 10.11909/j.issn.1671-5411.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tse G. (Tpeak - Tend)/QRS and (Tpeak - Tend)/(QT x QRS): novel markers for predicting arrhythmic risk in the Brugada syndrome. Europace. 2017;19:696. doi: 10.1093/europace/euw194. [DOI] [PubMed] [Google Scholar]
- 168.Zumhagen S., Stallmeyer B., Eckardt L., Schulze-Bahr E. (Tpeak - Tend)/QRS and (Tpeak - Tend)/(QT x QRS) as risk markers in Brugada syndrome: authors' reply. Europace. 2017;19:696–697. doi: 10.1093/europace/euw210. [DOI] [PubMed] [Google Scholar]
- 169.Tse G., Yan B.P. Novel arrhythmic risk markers incorporating QRS dispersion: QRSd x (Tpeak - Tend)/QRS and QRSd x (Tpeak - Tend)/(QT x QRS) Ann. Noninvasive Electrocardiol. 2017;22 doi: 10.1111/anec.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lu H.R., Yan G.-X., Gallacher D.J. A new biomarker – index of Cardiac Electrophysiological Balance (iCEB) – plays an important role in drug-induced cardiac arrhythmias: beyond QT-prolongation and Torsades de Pointes (TdPs) J. Pharmacol. Toxicol. Methods. 2013;68:250–259. doi: 10.1016/j.vascn.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 171.Tse G. Both transmural dispersion of repolarization and of refractoriness are poor predictors of arrhythmogenicity: a role for iCEB (QT/QRS)? J. Geriatr. Cardiol. 2016;13:813–814. doi: 10.11909/j.issn.1671-5411.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tse G., Wong S.T., Tse V., Yeo J.M. Determination of action potential wavelength restitution in Scn5a(+/-) mouse hearts modelling human Brugada syndrome. J. Geriatr. Cardiol. 2017;14:595–596. doi: 10.11909/j.issn.1671-5411.2017.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tse G., Wong S.T., Tse V., Yeo J.M. Variability in local action potential durations, dispersion of repolarization and wavelength restitution in aged wild-type and Scn5a(+/-) mouse hearts modeling human Brugada syndrome. J. Geriatr. Cardiol. 2016;13:930–931. doi: 10.11909/j.issn.1671-5411.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Trethewey S.P., Nicolson W.B., Ng G.A. Investigation of the relationship between two novel electrocardiogram-based sudden cardiac death risk markers and autonomic function. J. Electrocardiol. 2018;51:889–894. doi: 10.1016/j.jelectrocard.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 175.Nicolson W.B., McCann G.P., Smith M.I., Sandilands A.J., Stafford P.J., Schlindwein F.S. Prospective evaluation of two novel ECG-based restitution biomarkers for prediction of sudden cardiac death risk in ischaemic cardiomyopathy. Heart. 2014;100:1878–1885. doi: 10.1136/heartjnl-2014-305672. [DOI] [PubMed] [Google Scholar]
- 176.Nicolson W.B., McCann G.P., Brown P.D., Sandilands A.J., Stafford P.J., Schlindwein F.S. A novel surface electrocardiogram-based marker of ventricular arrhythmia risk in patients with ischemic cardiomyopathy. J. Am. Heart Assoc. 2012;1:e001552. doi: 10.1161/JAHA.112.001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Richter S., Sarkozy A., Veltmann C., Chierchia G.B., Boussy T., Wolpert C. Variability of the diagnostic ECG pattern in an ICD patient population with Brugada syndrome. J. Cardiovasc. Electrophysiol. 2009;20:69–75. doi: 10.1111/j.1540-8167.2008.01282.x. [DOI] [PubMed] [Google Scholar]
- 178.Castro Hevia J., Dorantes Sanchez M., Martinez Lopez F., Castaneda Chirino O., Falcon Rodriguez R., Puga Bravo M. Multiple serial ECGs aid with the diagnosis and prognosis of Brugada syndrome. Int. J. Cardiol. 2019;277:130–135. doi: 10.1016/j.ijcard.2018.08.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Abe A., Kobayashi K., Yuzawa H., Sato H., Fukunaga S., Fujino T. Comparison of late potentials for 24 hours between Brugada syndrome and arrhythmogenic right ventricular cardiomyopathy using a novel signal-averaging system based on Holter ECG. Circ. Arrhythm Electrophysiol. 2012;5:789–795. doi: 10.1161/CIRCEP.111.969865. [DOI] [PubMed] [Google Scholar]
- 180.Aizawa Y., Takatsuki S., Kaneko Y., Noda T., Katsumata Y., Nishiyama T. Comparison of circadian, weekly, and seasonal variations of electrical storms and single events of ventricular fibrillation in patients with Brugada syndrome. IJC Heart Vasc. 2016;11:104–110. doi: 10.1016/j.ijcha.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Letsas K.P., Efremidis M., Vlachos K., Georgopoulos S., Karamichalakis N., Asvestas D. Right ventricular outflow tract high-density endocardial unipolar voltage mapping in patients with Brugada syndrome: evidence for electroanatomical abnormalities. Europace. 2017 doi: 10.1093/europace/eux079. [DOI] [PubMed] [Google Scholar]
- 182.Letsas K.P., Efremidis M., Asvestas D., Vlachos K., Georgopoulos S., Tse G. Right ventricular outflow tract electroanatomical abnormalities predict ventricular fibrillation inducibility in brugada syndrome. Circ Arrhythm Electrophysiol. 2018;11:e005928. doi: 10.1161/CIRCEP.117.005928. [DOI] [PubMed] [Google Scholar]
- 183.Tse G., Ali A., Alpendurada F., Prasad S., Raphael C.E., Vassiliou V. Tuberculous Constrictive Pericarditis. Res. Cardiovasc. Med. 2015;4:e29614. doi: 10.5812/cardiovascmed.29614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tse G., Ali A., Prasad S.K., Vassiliou V., Raphael C.E. Atypical case of post-partum cardiomyopathy: an overlap syndrome with arrhythmogenic right ventricular cardiomyopathy? BJR|Case Rep. 2015;1:20150182. doi: 10.1259/bjrcr.20150182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Vassiliou V., Chin C., Perperoglou A., Tse G., Ali A., Raphael C. 93 ejection fraction by cardiovascular magnetic resonance predicts adverse outcomes post aortic valve replacement. Heart. 2014;100:A53–A54. [Google Scholar]
- 186.Ito Y., Shiga K., Yoshida K., Ogata K., Kandori A., Inaba T. Development of a magnetocardiography-based algorithm for discrimination between ventricular arrhythmias originating from the right ventricular outflow tract and those originating from the aortic sinus cusp: a pilot study. Heart Rhythm. 2014;11:1605–1612. doi: 10.1016/j.hrthm.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 187.Kandori A., Miyashita T., Ogata K., Shimizu W., Yokokawa M., Kamakura S. Electrical space-time abnormalities of ventricular depolarization in patients with Brugada syndrome and patients with complete right-bundle branch blocks studied by magnetocardiography. Pacing Clin. Electrophysiol. 2006;29:15–20. doi: 10.1111/j.1540-8159.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 188.Kandori A., Miyashita T., Ogata K., Shimizu W., Yokokawa M., Kamakura S. Magnetocardiography study on ventricular depolarization-current pattern in patients with brugada syndrome and complete right-bundle branch blocks. Pacing Clin. Electrophysiol. 2006;29:1359–1367. doi: 10.1111/j.1540-8159.2006.00548.x. [DOI] [PubMed] [Google Scholar]
- 189.Tse G., Liu T., Li K.H., Laxton V., Chan Y.W., Keung W. Electrophysiological mechanisms of Brugada syndrome: insights from pre-clinical and clinical studies. Front. Physiol. 2016;7:467. doi: 10.3389/fphys.2016.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]