Abstract
Spinach fine powder is a good source of protein, fiber, antioxidant, and minerals, making it a suitable ingredient to be used in the formulation of foods with high nutritional or biological values. In the current study, spinach nano-powder (0.50, 1.00, 1.50, and 2%) was used in the manufacturing of ultra-filtered soft chesses (UF-soft chesses). The quality of the cheeses was assessed by measuring their chemical compositions, colors, sensory, and antioxidant properties. Dynamic light scattering (DLS) showed that particles of spinach powder followed a normal distribution pattern with an average diameter of about 328 nm. By increasing the addition of spinach nano-powder with retentate its content of fiber, minerals, total phenolic content, and antioxidant activity was improved. Moreover, the total solid, protein and acidity contents increased significantly with the increased percentage of added spinach nano-powder reaching maximum values after four weeks of cold storage. Cheese containing 0.5% and 1% spinach powder demonstrated higher values for sensory parameters than other treatments. UF-cheese prepared in this work represents a novel functional dairy product that can potentially provide the human body with better nutrients.
Keywords: Food science, Food technology, Spinach powder, UF-Soft cheese, Minerals, Antioxidant activity, Fiber, Nutritional value
Food science; Food technology; Spinach powder; UF-Soft cheese; Minerals; Antioxidant activity; Fiber; Nutritional value
1. Introduction
Green vegetables consist of a large number of components such as flavonoids, tannins and other phenolic compounds that are said to have a very significant function in health management mainly in reducing the risk of chronic human diseases such as cardiovascular diseases, cancer and degenerative diseases (Suresh and Ashok, 2016; Aboulthana et al., 2019; El-Sayed et al., 2019).
Spinach (Spinacia oleracea) is a plant that grows all over the world as a cool-season annual green leafy vegetable (Vazquez et al., 2013). It is a good source of protein, fiber, and minerals, thus costituiting a functional ingredient in a new product with high nutritional and biological values. Spinach is a rich source of major micronutrients such as iron, manganese, zinc, and magnesium and also contains small quantities of vitamin E, A, C, K, folate, thiamine (B1), pyridoxine (B6) and riboflavin (B2). Moreover it is a rich source of fiber and has an added benefit of a low calorie content. It is present in food in many forms such as raw, canned, boiled, pureed, frozen, dehydrated, cooked and baked (Slavin, and Lloyd, 2012).
Spinach is well recognized for its antimicrobial, anticarcinogenic and antioxidant activity (Vazquez et al., 2013). Furthermore, supplementing the diet with spinach reduced post-ischemic stroke brain damage in rats, probably through anti-apoptosis as well as antioxidant and anti-inflammatory mechanisms (Wang et al., 2005). Spinach or spinach extracts also have been studied in cancer. It was reported that people who consumed spinach or carrots more than twice a week had a lower risk of rising breast cancer and decreased propagation of prostate cancer than those who consumed nothing (Asai et al., 2004). Moreover, an early research documented that, women who consumed spinach more than fifteen days had a smaller risk of developing breast cancer than those who didn't consume any (Longnecker et al., 1997).
Spinach encompasses a huge surface area because it contains a high quantity of moisture, may resulting in a quick deterioration. Therefore the dehydration procedure is a a significant process that is mainly applied to remove the moisture from the surface, thus enhancing the shelf life of spinach for extended usage (Szulc and Lenart, 2012). The leaves are also compressed into a powder form for easier storability, handling, and usability (Ankita and Prasad, 2015a, 2015b). In recent years, many studies have highlighted the importance of cheese as a good sources for essential nutritious for the human body. Therefore the addition of cheese to a school menu may help increase the intake of vegetables, fruits, and whole grains compared a menu without cheese (El-Sayed and Youssef, 2019). Combining the above mentioned items with cheese has received significant attention in health advertising and disease prevention in humans by delivering health-promoting factors and increasig total nutrient intake thus improveig diet quality as shown in (Scheme 1).
Scheme 1.
UF-soft cheese rich with spinach nano-powder as a beneficial nutrient health product.
On the other hand, the addition of small amounts of spinach leaves powder not only improved the nutritional values but also enhanced the color and the taste of cheese products.
Attributable to the importance mentioned about spinach leaves powder, the current study was carried out exclusively to characterize and evaluate the activity of spinach leaves nano-powder and use it as a functional material for the preparation of a beneficial healthy UF-soft cheese. The chemical analysis, nutritional composition, color measurements, and sensory quality of the cheese were assessed to determine the acceptable level of added spinach powder in the fabricated UF-cheese.
2. Materials and method
2.1. Materials
Fresh full cream UF-buffalo's milk retentate was procured from the Animal Production Research Institute, Agriculture Research Center, Dokki, Egypt. Microbial rennet powder (RENIPLUS) extracted from Mucor miehei was purchased from Gaglio Star, Spain. The spinach (spinach oleracea) leaves were purchased from the vegetable market in Giza, Egypt. Chemicals and solvents used in the study were of analytical and laboratory-grade and purchased from Sigma Chemical Company (St. Louis, U.S.A).
2.2. Methods
2.2.1. Preparation of dehydrated spinach powder
Whole spinach leaves were selected and washed with water to remove dirt and other impurities, soaked in sodium hypochlorite solution (35 mg/L) for 30 min and shade dried for one week. The dried material was ground to powder using a high-speed mixer, passed through BS 72 (120 μm) mesh and dehydrated fine spinach powder was obtained. The spinach powder was packaging in glass bottels and stored at 4 °C for chemical analysis and application studies.
2.2.2. Preparation of ultrafiltration soft cheese by using spinach powder
Ultrafiltration soft cheese was fabricated using fresh full cream UF-retentate pasteurized at 72 °C for 15 s, cooled and adjusted to 42 °C. The full cream UF-retentate was divided into five batches. The first was assisted as a control and the other four batches mixed individually with spinach powder in ratios of (0.50 g, 1.00 g, 1.50 g, and 2.00g spinach powder/100 g full cream UF-retentate) for (T1, T2, T3, and T4), respectively using the electric blender (Molinex blender). The rennet was then added and packaged in plastic cups (50mL) and all cups were incubated at 42 °C until complete coagulation (40 min). Samples of cheese from the differed treatments were kept cold (5 ± 2 °C) for 4 weeks and analyzed when fresh and at weekly intervals. Three replicates were prepared and analyzed from different treatments.
2.2.3. Sample preparation for dynamic light scattering (DLS)
About 250 mg of the fabricated spinach powder was added to 20 g of Millipore water (containing 0.1 wt% SDS and 0.1 wt% HQ relative to Millipore water) and the mixture was sonicated for 10 min at room temperature.
2.2.3.1. Particle size analyzer
NICOMP 380 ZLS, Dynamic light scattering (DLS) instrument (PSS, Santa Barbara, CA, USA), using the 632 nm line of a HeNe laser as the incident light with angel 90o and Zeta potential with external angel 18.9o. Samples were diluted in 0.1M phosphate buffer pH 7.0 and filtered through 0.45 μm membrane (Mellipore, USA) to obtain a count rate in the appropriate range 100–450 nm, to avoid multiple scattering phenomena due to inter-particle interaction (El-Shibiny et al., 2018). Immediately, the diluted sample was transferred into a polystyrene cuvette for size determination.
2.3. Antioxidant activity analysis
2.3.1. Cheese extracts preparation
100 ml quick-fit conical flask was used for the extraction of cheese, 10 grm of cheese was put into the flask then 20 ml of methanol/water mixture (80:20) were added. After shaking in the ultrasonic water bath for 30 min, in 25 ml measuring flask the remaining solution was filtered and completed to 25 ml with the extraction solvent. Then the extracts were separately collected in glass sealed vessels in addition to used for diverse analysis (Mohamed et al., 2018).
2.3.2. Total phenol content (TPC)
The total phenol content was measured calorimetrically at 625 nm with the Folin–Ciocalteau reagent along with the procedure related to Rashidinejad et al. (2013). The cheese extract solution (0.5 ml), deionized water (20 ml) and the Folin–Ciocalteau reagent (0.625 ml) were supplementary in a volumetric flask (25 ml). After 3 min, adding 2.5 ml of saturated solution of sodium carbonate (Na2CO3, 35%). Then the content was diluted using deionized water. the absorbance of the extract cheese sample was evaluated at 625 nm contrary to a blank sample via a double-beam ultraviolet–visible spectrophotometer Hitachi U-3210 (Hitachi, Ltd., Tokyo, Japan). The calibration curve was prepared using gallic acid as a standard, the data were expressed as mg of gallic acid equivalents (GAE) per g of extract.
2.3.3. Antioxidant assay
2,2-diphenyl-1-picryl-hydrazyl radical (DPPH) was used to determine the antioxidant activity of the phenol extracts in relation to a modification technique of Bandoniene et al. (2002). The addation of 0.1 ml of methanolic solutions of phenol extracts as well as 3.9 ml of methanolic solution of DPPH (0.0025 g/100 ml methanol) in a cuvette and the absorbance was 515 nm (till stabilization) the solution was measured in contrast to methanol via a double-beam ultraviolet–visible spectrophotometer Hitachi U-3210 (Hitachi, Ltd., Tokyo, Japan). Concurrently, the blank sample was measeured at 515 nm of (0.1 ml methanol + 3.9 ml methanolic solution of DPPH) contrary to CH3OH. The radical scavenging activities stated as ratio inhibition of DPPH, were designed according to the following eqution:
| % Inhibition = 100 X (A – A0) / A0, |
where A0 is the absorbance at 515 nm of the blank sample at time t=0 min and A is the final absorbance of the test sample at 515 nm.
2.4. Chemical analysis
The pH values were measured using a digital laboratory Jenway 3510 pH meter, UK. Total solids (TSs), fat, fat/dry matter (F/DM), titratable acidity (T.A), as well as ash content of cheese samples were examined according to AOAC (2007). Total nitrogen (T.N) contents were determined using the semi-micro Kjeldal method the factor N. 6.38 was used to convert nitrogen into total protein (T.P) as mentioned by Ling (1963). Crude Fiber was determined using AOAC (2005). The values of carbohydrate were achieved by calculation. % Carbohydrates = [100–(Moisture + Total ash + Protein + Fiber + Fat)]
The total fat content of spinach powder was gained by the Soxhelt extraction method. The protein of spinach powder was determined by Kjeldal procedure; the factor N. 6.25 was used to convert nitrogen into crude protein. Results of spinach powder were specified as a percent of dry weight (DW). All of the analyses were run in triplicate.
2.4.1. Mineral elements analysis
Mineral contents (Mn, K, Ca, Mg, Fe, Cu, and Zn) were evaluated using atomic absorption spectrometer (Chemtech CTA-2000, England). Spinach powder and cheese samples were digested via dry ashing and dissolved in 1M HCl. as described by Saadatu and Mshelia (2013). The minerals were stated as mg/100 g of dry weight. All determinations were done in triplicate.
2.4.2. Color measurements of cream cheese
The color of cheese samples was determined by a Hunter colorimeter model D2s A-2 (Hunter Assoc. Lab Inc., VA, USA). The color values were measured to the absolute values of a perfect white diffuser (white tile) as measured under the same geometric conditions. The values of the color namely (l, a, and b) were evaluated using the corresponding button on the colorimeter.
2.5. Sensory evaluation
Sensory evaluation was carried out according to the scheme of Meyer (1973). The evaluation was done when fresh and during a 4 week storage time at a refrigerator temperature by specific expert judges have good general health (no allergies/food intolerances and no known allergy to milk) were selected from a staff member of the Dairy Science Department, National Research Centre, Giza, Egypt, using scale of (20) points for Outer appearance, (40) points for body & texture such as (body, mouth feel, hardness and softness), (40) points for flavor and overall acceptability (sum of scores for different attributes). UF-soft Cheese was held for at least 1 h at 22 °C to equilibrate. Every one of judge was given three cubes of cheese per samples. Non-salted crackers and water were provided to clean their sense of taste between tasting.
2.6. Statistical analysis
The Vassar states (http://www.faculty.vassar.edu/lowery/anova2corr.html) computing site was used to analyze the obtained data statistically by Anova of two independent variables according to Lowry (2009). Nutritional and chemical compositions were carried out in triplicate and mean values with standard deviation (SD) were computed by using Microsoft Excel, 2007.
3. Results and discussion
3.1. Particle size analyzer
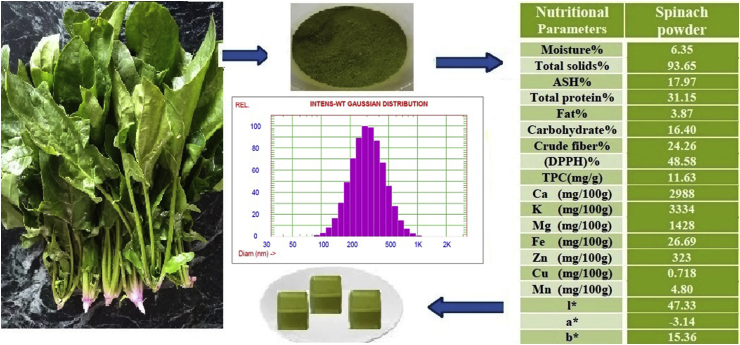
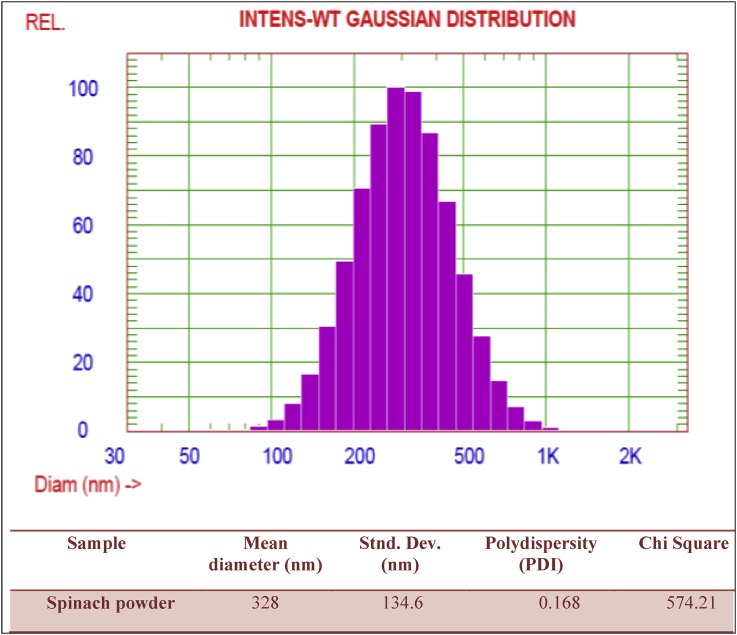
Dynamic light scattering technique was used to determine the particle size and relative measurement indices, as well as the zeta potential of dispersed spinach powder. Figure 1 illustrates the particle size distribution of dispersed spinach powder by intensity, volume, and the number of distribution. The mean particle size and polydispersity variation index are presented in (Figure 1).
Figure 1.
Mean diameter and relative particle size indices for dispersed spinach powder.
The average diameter of spinach powder is about 328 nm, which specifies that the particle size of spinach powder is in a nano-form. Furthermore, the polydispersity of spinach powder nanoparticles has led to excellent particle distribution and homogenous particle mean diameters (Danaei et al., 2018). This indicates that fine powder fraction can be exposed a significantly higher water-binding characteristic which enhances the quality of cheese product. Moreover, fine spinach powder as a nutritional fraction can be used for therapeutic purposes and therefore the fine powder can be considered to be high food functional (Ankita and Prasad, 2015b).
3.2. Chemical, nutritional composition and color measurements of spinach powder, control cheese, and spinach powder supplemented UF-soft cheeses
Chemical and nutritional composition of spinach powder and spinach powder supplemented cheeses between levels 0.5–2% are shown in Table 1. The moisture content of spinach powder was 6.35 %, while ash and fat contents were 17.97% and 3.87 % respectively (Khan et al., 2015). The carbohydrates' content of the UF-soft cheeses increased from 6.20 to 7.28% by increasing the addition of spinach powder between treatments from (T1 to T4). This increase is due to the high content of carbohydrate in spinach powder which agrees with (Kavitha and Ramadas, 2013).
Table 1.
Chemical, nutritional composition and colour measurements of spinach powder, control cheese and spinach powder supplemented UF-soft cheeses∗.
| Parameters | Spinach powder | Control | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| Moisture% | 6.35 | 68.84 | 68.65 | 68.32 | 68.02 | 67.75 |
| Total solids% | 93.65 | 31.16 | 31.35 | 31.68 | 31.98 | 32.25 |
| ASH% | 17.97 | 2.39 | 2.50 | 2.61 | 2.75 | 2.93 |
| Total protein% | 31.15 | 9.29 | 9.50 | 9.83 | 9.91 | 10.03 |
| Fat% | 3.87 | 13.00 | 13.00 | 12.50 | 12.50 | 11.50 |
| Carbohydrate% | 16.40 | 6.48 | 6.20 | 6.47 | 6.82 | 7.28 |
| Crude fiber% | 24.26 | - | 0.15 | 0.27 | 0.38 | 0.51 |
| (DPPH)% | 48.58 | 3.40 | 19.53 | 24.65 | 30.55 | 35.84 |
| TPC(mg/g) | 11.63 | 29.78 | 60.55 | 81.15 | 96.53 | 110.56 |
| Ca (mg/100g) | 2988 | 35475 | 39973 | 4034 | 50552 | 74891 |
| K (mg/100g) | 3334 | 1373 | 14678 | 15296 | 15487 | 19206 |
| Mg (mg/100g) | 1428 | 2639 | 3805.6 | 3952 | 4662 | 4732 |
| Fe (mg/100g) | 26.69 | 42.76 | 60.72 | 73.38 | 73.59 | 84.90 |
| Zn (mg/100g) | 323 | 23.25 | 25.06 | 26.23 | 31.88 | 64.40 |
| Cu (mg/100g) | 0.718 | 3.86 | 6.19 | 7.23 | 8.57 | 11.24 |
| Mn (mg/100g) | 4.80 | 4.35 | 10.92 | 13.30 | 19.40 | 19.60 |
| l* | 47.33 | 70.05 | 68.33 | 65.16 | 57.67 | 51.85 |
| a* | - 3.14 | - 1.17 | - 1.89 | - 2.11 | - 2.56 | - 2.68 |
| b* | 15.36 | 21.49 | 19.45 | 17.58 | 16.94 | 14.09 |
l* value represents darkness from black (0) to white (100). a* value represents color ranging from red (+) to green (−). b* value represents yellow (+) to blue.
Values are average of triplicate analysis with ± SD (0.001–3.5).
Spinach powder dislayed excellent quantities of protein 31.15 % and crude fiber 24.26% along with a DPPH scaving activity 48.58% and a total phenol content of 11.63 mg/g dry weight (Fernández-Segovia et al., 2018). The obtaanied results also showed that potassium, calcium and magnesium were the most abundant elements in spinach powder, while the other elements, in descending order by quantity were (Fe, Zn, Mn, and Cu). The values for spinach powder were (calcium 2988 mg/100 g, potassium 3334 mg/100 g, magnesium 1428 mg/100 g, iron 26.69 mg/100 g, zinc 3.23 mg/100 g, copper 0.718 mg/100 g, and manganese 4.8 mg/100 g), which gave a good value to the spinach powder. Increasing the supplementation of spinach powder from 0.5 to 2% has demonstated excellent improvement in protein, fiber, DPPH scaving activity, total phenol and mineral content in UF-cheese when compared to control samples (Ankita, 2015).
The combination between spinach powder and cheese is able to create new dairy products with great biological value for balanced nutrition, these results are in the same line with Roberts and Moreaua (2016) which reported that spinach is widely observed as a functional food owing to its various nutritional composition of vitamins and minerals, and to its photochemical and bioactive ingredients that support healthy nutrition. The changes in color l*, a* and b* of spinach powder were 47.33 for l* value, a* value -3.14, and b* value 15.36 (Table 1) the color values of spinach powder showed the highest green value and the green value of cheeses supplemented with spinach powder gradually increased with addition of spinach powder. Similar findings were reported for the color changes in biscuits supplemented with spinach powder (Narsing Rao et al., 2017). The other color parameters of white and yellow regularly decreased on increasing amout of spinach powder for cheese comparing with control samples. These changes in the color of cheese products may be due to the green color of spinach powder and coincide with the results of (Mohamed et al., 2018).
3.3. Changes in the chemical composition of spinach powder supplemented UF-soft cheese
Table 2 displays the changes in the total solids, fat, rotein, fat/dry matter content, acidity and pH of cheese respectively, as affected by the added percentage of spinach powder and storage period. In cheese, the total solid (TS) and total protein (TP) contents were increased significantly (P < 0.05) with the increased percentage of added spinach powder and during storage period reaching maximum values after four weeks of storage which may be mainly attributed to the moisture losses.
Table 2.
Chemical composition of spinach powder supplemented UF-soft cheese fresh and during cold storage for 4 weeks.
| Treatments | Storage (Weeks) | T.S | Fat | pH | T.A | Fat/DM | T.P |
|---|---|---|---|---|---|---|---|
| Control | Fresh | 31.16Ee | 13.00Ad | 6.36Aa | 0.14De | 41.72Ad | 9.29Ee |
| T1 | 31.35De | 13.00Ad | 6.30Ba | 0.14De | 41.46Bd | 9.50De | |
| T2 | 31.68Ce | 12.50Bd | 6.28Ca | 0.16Ce | 39.46Cd | 9.83Ce | |
| T3 | 31.98Be | 12.50Bc | 6.25Da | 0.18Be | 39.09Dd | 9.91Be | |
| T4 | 32.25Ae | 11.50Ce | 6.20Ea | 0.20Ae | 35.66Ee | 10.03Ae | |
| Control | 1 week | 31.29Ed | 13.00Ad | 6.29Ab | 0.16Ed | 41.54Ae | 9.35Ed |
| T1 | 31.46Dd | 13.00Ad | 6.23Bb | 0.18Dd | 41.32Be | 9.67Dd | |
| T2 | 31. 71Cd | 12.50Bd | 6.19Cb | 0.20Cd | 39.42Cd | 10.06Cd | |
| T3 | 32.48Bd | 12.50Bc | 6.15Db | 0.24Bd | 38.49De | 10.15Bd | |
| T4 | 32.92Ad | 12.00Cd | 6.09Eb | 0.28Ad | 36.45Ed | 10.21Ad | |
| Control | 2 weeks | 31.40Ec | 13.50Ac | 6.21Ac | 0.20Ec | 42.99Ac | 9.52Ec |
| T1 | 31.73Dc | 13.50Ac | 6.16Bc | 0.24Dc | 42.55Bc | 9. 85Dc | |
| T2 | 32.48Cc | 13.00Bc | 6.11Cc | 0.26Cc | 40.02Cc | 10.12Cc | |
| T3 | 32.88Bc | 13.00Bb | 6.06Dc | 0.28Bc | 39.54Db | 10.22Bc | |
| T4 | 33.05Ac | 12.50Cc | 6.00Ec | 0.30Ac | 37.82Ec | 10.34Ac | |
| Control | 3 weeks | 31. 65Eb | 14.00Ab | 6.16Ad | 0.22Eb | 44.23Ab | 9.68Eb |
| T1 | 31.91Db | 14.00Ab | 6.13Bd | 0.26Db | 43.87Bb | 9.93Db | |
| T2 | 32.56Cb | 14.00Ab | 6.10Cd | 0.28Cb | 42.99Cb | 10.21Cb | |
| T3 | 32.96Bb | 13.00Bb | 6.03Dd | 0.30Bb | 39.44Dc | 10.36Bb | |
| T4 | 33.18Ab | 13.00Bb | 6.00Ec | 0.32Ab | 39.18Eb | 10.72Ab | |
| Control | 4 weeks | 31.83Ea | 15.00Aa | 6.16Ad | 0.25Ea | 47.13Aa | 9.87Ea |
| T1 | 32.02Da | 15.00Aa | 6.14Bd | 0.28Da | 46.85Ba | 10 .17Da | |
| T2 | 32.81Ca | 14.50Ba | 6.10Cd | 0.30Ca | 44.19Ca | 10.43Ca | |
| T3 | 33.12Ba | 14.00Ca | 6.00De | 0.32Ba | 42.27Da | 10.56Ba | |
| T4 | 33.26Aa | 14.00Ca | 5.97Ed | 0.35Aa | 42.09Ea | 10.78Aa |
Means with the same small letter superscripts indicate insignificant difference between rows (effect of storage), and means with the same capital letter superscripts indicate insignificant difference between rows (effect of treatments).
The fat content of cheese was decreased significantly (P < 0.05) with the increased percentage of added spinach powder and with the progress of storage period, Also, fat/dry matter decreased significantly (P < 0.05) with the increased percentage of added spinach powder but it increased after one week of storage until the end of storage. Similiarly, the increase in the fat, protein, fat/dry matter percentage resulted in essence from the increase in the TSs for the duration of storage. The acidity of cheese improved gradually, the pH decreased with increased percentage of added spinach powder. The development of acidity was slightly quicker during storage. In cheese, the addition of spinach powder enhanced acid development significantly (P < 0.05). Similar results were reported for the chemical changes in soft white cheeses supplemented with Moringa oleifera Leaves Powder during storage (Hassan et al., 2017).
3.4. Sensory properties of spinach powder supplemented UF-soft cheese
Table 3 displays the scores for the appearance, flavor, body and texture of cheese made with different levels of added spinach powder during 4 weeks of cold storage. In case of all sensory attributes of cheese with 0.5% scored approximately nearer to with the control samples, when compared to other treatments supplemented with 1, 1.5 and 2% spinach powder. Both time of storage and ratio of spinach powder of all treatments had significant (P < 0.05) effects on all sensory scores of cheese, which gradually decreased during storage in control and all treatments.
Table 3.
Sensory properties of spinach powder supplemented UF-soft cheese fresh and during cold storage for 4 weeks.
| Storage (weeks) | Character assessed | Control | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|---|
| Fresh | O.A(20) | 19.16Aa | 19.15Aa | 19.13Aa | 18.34Ba | 18.00Ca |
| B&T(40) | 38.22Aa | 38.19Ba | 37.43Ca | 37.25Da | 36.33Ea | |
| A&F(40) | 39.10Aa | 39.00Ba | 39.00Ba | 38.22Ca | 38.18Da | |
| Total(100) | 96.48Aa | 96.34Ba | 95.56Ca | 94.81Da | 92.51Ea | |
| 1 Week | O.A(20) | 19.10Ab | 19.00Bb | 18.53Cb | 18.22Db | 17.78Eb |
| B&T(40) | 38.12Ab | 38.00Bb | 37.44Ca | 36.43Db | 35.24Eb | |
| A&F(40) | 38.16Ab | 38.10Bb | 37.38Cc | 37.12Db | 36.54Eb | |
| Total(100) | 95.28Ab | 95.10Bb | 93.35Cb | 91.77Db | 89.56Eb | |
| 2 weeks | O.A(20) | 18.74Ac | 18.65Bc | 18.36Cc | 17.43Dc | 16.62Ec |
| B&T(40) | 37.33Ac | 36.93Bc | 36.57Cb | 35.77Dc | 34.76Ec | |
| A&F(40) | 38.00Ac | 37.64Bc | 37.54Cb | 36.65Dc | 35.85Ec | |
| Total(100) | 94.07Ac | 93.22Bc | 92.40Cc | 89.85Dc | 87.07Ec | |
| 3 weeks | O.A(20) | 18.12Ad | 18.00Bd | 17.65Cd | 16.82Dd | 15.70Ed |
| B&T(40) | 36.86Ad | 36.61Bd | 35.50Cc | 34.23Dd | 33.93Ed | |
| A&F(40) | 37.14Ad | 36.82Be | 35.14Cd | 34.52Dd | 34.00Ed | |
| Total(100) | 92.12Ad | 91.43Bd | 88.29Cd | 85.57Dd | 83.63Ed | |
| 4 weeks | O.A(20) | 17.88Ae | 17.68Be | 16.81Ce | 15.20De | 14.34Ee |
| B&T(40) | 35.91Ae | 35.82Be | 34.70Cd | 34.12De | 33.63Ee | |
| A&F(40) | 37.00Ae | 36.72Bd | 34.85Ce | 34.33De | 33.12Ee | |
| Total(100) | 90.79Ae | 90.22Be | 86.36Ce | 83.65De | 81.09Ee |
Means in the same column and upper case superscript (effect of treatment). Means in the same row and lower case (effect of storage) are not significantly different (P > 0.05).
O.A: Outer appearance (20), B&T: Body& Texture (40), A&F: Aroma &Flavor (40).
The appearance, body and texture of cheese were affected by the storage period particularly in treatments containing high levels of spinach powder (P < 0.05). Smiliarly, flavor decreased as spinach powder increased and the unacceptable flavor was noticed as after taste in cheese with 2% spinach powder at the end of storage. The overall acceptability of cheese was affected by both spinach powder level and storage period. Cheese containing 0.5% and 1% spinach powder demonstrated higher scores for sensory parameters than cheese containing 1.5 and 2% spinach powder respectively compared to control. The lowest total scores were given to cheese with high levels of spinach powder (2%) and stored for 4weeks.
Similar observations were informed for the changes of flavor and overall scores which indicate that the flavor declined as spinach powder content increased and bitterness was observed as after taste in high ratio of spinach powders. Furthermore, overall scores were highly acceptable in low concentration of spinach powder supplemented biscuits (Narsing Rao et al., 2017).
4. Conclusions
It has been successfully established that spinach powder (spinach oleracea) in its nanoform in our daily life has enormous nutritional potentials which may guard against many human diseases. Spinach powder in the nanoform could be condensed to obtain functional UF-soft cheese extremely rich in antioxidants and containing a high concentration of minerals, protein and vegetable fiber. The addition of nano-spinach powder in the range of 0.5% and 1 % gives cheese an acceptable composition and taste. This study highlights an innovative product of cheese containing a powder form of spinach, that offers a healthy source of nutrients for normal body functions.
Declarations
Author contribution statement
Samah M. El-Sayed: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Aboulthana W.M., Youssef A.M., El-Feky A.M., Ibrahim N.E., Seif M.M., Hassan A.K. Evaluation of antioxidant efficiency of Croton tiglium L. Seeds extracts after incorporating silver nanoparticles. Egypt. J. Chem. 2019;62(2):181–200. [Google Scholar]
- Ankita, Prasad K. Characterization of dehydrated functional fractional radish leaf powder. Der Pharm. Lett. 2015;7(1):269–279. [Google Scholar]
- Ankita, Prasad K. Chemical and physical characterization of dehydrated functional fenugreek leaf powder. Asian J. Chem. 2015;27(10):3697–3703. [Google Scholar]
- Ankita K.P. Characterization of dehydrated functional fractional spinach powder. Biotechnol. Indian J. 2015;11(11):426–435. [Google Scholar]
- AOAC . Official Methods of Analysis. eighteenth ed. 2005. Association of Official Analytical Chemists. Gaithersburg, MD.,USA. [Google Scholar]
- AOAC . eighteenth ed. Official Methods of Analysis; Gaithersburg, MD., USA: 2007. Association of Official Analytical Chemists. [Google Scholar]
- Asai A., Terasaki M., Nagao A. An epoxide-furanoid rearrangement of spinach neoxanthin occurs in the gastrointestinal tract of mice and in vitro:formation and cytostatic activity of neochrome stereoisomers. J. Nutr. 2004;134(9):2237–2243. doi: 10.1093/jn/134.9.2237. [DOI] [PubMed] [Google Scholar]
- Bandoniene D., Murkovic M., Pfannhauser W., Venskutonis P.R., Gruzdiene D. Detection and activity evaluation of radical scavenging compounds by using DPPH free radical and on-line HPLC–DPPH methods. Eur. Food Res. Technol. 2002;214:143–147. [Google Scholar]
- Danaei M., Dehghankhold M., Ataei S., Hasanzadeh Davarani F., Javanmard R., Dokhani A., Mozafari M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10(2):57. doi: 10.3390/pharmaceutics10020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed S.M., Youssef A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon. 2019;5:e01989. doi: 10.1016/j.heliyon.2019.e01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed S.M., El-Naggar M.E., Hussein J., Medhat D., El-Banna M. Effect of Ficus Carica L. leaves extract loaded gold nanoparticles against cisplatin-induced acute kidney injury. Colloids Surfaces B Biointerfaces. 2019:110465. doi: 10.1016/j.colsurfb.2019.110465. [DOI] [PubMed] [Google Scholar]
- El-Shibiny S., Abd El-Gawad M.A., Assem F.M., El-Sayed S.M. The use of nanosized eggshell powder for calcium fortification of cow’s and buffalo’s milk yogurts. Acta Sci. Pol. Technol. Aliment. 2018;17(1):37–49. doi: 10.17306/J.AFS.0541. [DOI] [PubMed] [Google Scholar]
- Fernández-Segovia I., Lerma-García M.J., Fuentes A., Barat J.M. Characterization of Spanish powdered seaweeds: composition, antioxidant capacity and technological properties. Food Res. Int. 2018;111:212–219. doi: 10.1016/j.foodres.2018.05.037. [DOI] [PubMed] [Google Scholar]
- Hassan F.A., Enab A.K., Abdel-Gawad M.A., Bayoumi H.M., Youssef Y.B. Utilization of Moringa oleifera leaves powder in production of soft white cheese. Int. J. Dairy Sci. 2017;12:137–142. [Google Scholar]
- Kavitha V., Ramadas V.S. Nutritional composition of raw fresh and shade dried form of spinach leaf (Spinach oleracea) JPR BioMedRx. 2013;1:767–770. [Google Scholar]
- Khan M.A., Mahesh C., Semwal A.D., Sharma G.K. Effect of spinach powder on physico-chemical, rheological, nutritional and sensory characteristics of chapati premixes. J. Food Sci. Technol. 2015;52(4):2359–2365. doi: 10.1007/s13197-013-1198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling E.R. third ed. Vol. 2. Chapman and Hall Ltd.; London, UK: 1963. pp. 76–98. (A Text Book of Dairy Chemistry). [Google Scholar]
- Longnecker M.P., Newcomb P.A., Mittendorf R., Greenberg R., Willet W. Intake of carrots, spinach, and supplements containing vitamin A in relation to risk of breast cancer. Cancer Epidemiol. Biomark. Prev. 1997;6:887–892. [PubMed] [Google Scholar]
- Lowry R. Vassar states. 2009. http://www.faculty.vassar.edu/lowry/vassarstats.html Available from:
- Meyer A. first ed. Food Trade Press Ltd.; London, UK.: 1973. Processed Cheese Manufacture; p. 329. [Google Scholar]
- Mohamed F.A., Salama H.H., El-Sayed S.M., El-Sayed H.S., Zahran H.A. Utilization of natural antimicrobial and antioxidant of Moringa oleifera leaves extract in manufacture of cream cheese. J. Biol. Sci. 2018;18(2):92–106. [Google Scholar]
- Narsing Rao G., Prabhakarara Rao P., Balaswamy K., Math Rudrayya G., Satyanarayana A. Nutritional, textural and sensory quality of biscuits supplemented with spinach (Spinacia oleracea L.) Int. J. Gastronomy Food Sci. 2017;(7):20–26. [Google Scholar]
- Rashidinejad A., Birch E.J., Sun-Waterhouse D., Everett D.W. Effects of catechin on the phenolic content and antioxidant properties of low-fat cheese. Int. J. Food Sci. Technol. 2013;48:2448–2455. [Google Scholar]
- Roberts J.L., Moreaua R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct. 2016;7:3337–3353. doi: 10.1039/c6fo00051g. [DOI] [PubMed] [Google Scholar]
- Saadatu M.E., Mshelia M.S. Comparative study on concentration of some minerals found in garlic (Allium sativum Linn) species grown in some African countries. J. Biol. Life Sci. 2013;4:63–67. [Google Scholar]
- Slavin J.L., Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3(4):506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suresh L., Ashok K. Nutritional activity, antioxidant and anti arthritic activity of selected green leafy vegetables. Int. J. Home Sci. 2016;2(3):85–88. [Google Scholar]
- Szulc K., Lenart A. Water vapour adsorption properties of agglomerated baby food powders. J. Food Eng. 2012;109:135–141. [Google Scholar]
- Vazquez E., Garcia-Risco M., Jaime L., Reglero G., Fornari T. Simultaneous extraction of rosemay and spinach leaves and its effect on the antioxidant activity of products. J. Supercrit. Fluids. 2013;82:138–145. [Google Scholar]
- Wang Y., Chang C.F., Chou J., Chen H.L., Deng X., Harvey B.K., Cadet J.L., Bickford P.C. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Exp. Neurol. 2005;193(1):75–84. doi: 10.1016/j.expneurol.2004.12.014. [DOI] [PubMed] [Google Scholar]