Abstract
The aim of this study was to identify and assess all existing randomized studies on treatment interventions for hand fractures and joint injuries, to inform practice and plan future research. PubMed, Cochrane CENTRAL, MEDLINE and Embase were searched. We identified 78 randomized controlled trials published over 35 years, covering seven anatomical areas of the hand. We report on sources of bias, sample size, follow-up length and retention, outcome measures and reporting. In terms of interventions studied, the trials were extremely heterogeneous, so it is difficult to draw conclusions on individual treatments. The published randomized controlled clinical trial evidence for hand fractures and joint injuries is narrow in scope and of generally low methodological quality. Mapping provides a useful resource and stepping-stone for planning further research. There is a need for high-quality, collaborative research to guide management of a wider range of common hand injuries.
Keywords: Hand fractures, joint injuries, scoping review, randomized controlled trial
Introduction
The wide variety of hand injuries treated by different methods and the lack of consistency in outcome reporting and research methodological standards make existing evidence difficult to interpret and apply to clinical decision making.
The importance of studying hand injuries and the gap in the evidence base was recently highlighted by the James Lind Alliance Priority Setting Partnership on common hand and wrist conditions, a priority-setting national consensus exercise involving patients and those providing hand surgery care (James Lind Alliance, 2017). Two of the top ten research priorities highlighted the treatment of bony or ligamentous injuries of the hand. Further work is needed to inform clinical practice and help plan future high-quality clinical trials (James Lind Alliance, 2017).
A scoping review is a type of systematic review that identifies the nature and the extent of research evidence on a topic. It is the assessment of available published research with the aim of identifying the breadth of relevant evidence, as opposed to trying to answer a specific question (Grant and Booth, 2009).
The aim of this scoping review was to identify and assess existing randomized controlled trial (RCT) evidence on treatment interventions for hand fracture and joint injuries in order to inform practice and help plan future trials. The objectives were to collate and map existing RCT evidence to the anatomical sites of hand fractures or joint injuries, appraise the quality of studies using a recognized risk of bias assessment tool, summarize outcomes used and assess the length of follow-up and retention rates in published RCTs.
Methods
The study was prospectively registered on PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=102845). Preferred Reporting Items for Systematic Reviews and Meta-Analyses, Extension for Scoping Reviews (PRISMA-ScR) were followed (Tricco et al., 2018).
Scope and eligibility criteria
For the purposes of this review, hand fractures and joint injuries were defined as carpal fractures of the scaphoid, hamate, lunate and pisiform and others; metacarpal fractures; phalangeal fractures; fractures at the base of the thumb; any joint injuries, such as dislocations or fracture-dislocations; tendon avulsion and joint ligament injuries that can be associated with a small fracture; and other ligament injuries to the hand, carpus or wrist. Distal radial fractures were not included in this review, which was focused on the hand and carpus.
Inclusion and exclusion criteria are outlined in Table 1. Interventions included primary treatment (e.g. plaster or surgery for a scaphoid fracture), secondary treatment (e.g. treatment for scaphoid non-union or deformity secondary to the injury) and/or associated therapy interventions (e.g. physiotherapy). Trials were included without restrictions on publication time or language.
Table 1.
Inclusion and exclusion criteria for the scoping review.
| Criteria |
|---|
| Inclusion criteria |
| Study design |
| • Randomized controlled trials • Studies stated to be ‘randomized’, but for which there is inadequate information about sequence generation and/or allocation concealment • Quasi-randomized studies |
| Population |
| • Adults with acute hand fracture(s) and/or joint injury(ies) of the hand • In studies of mixed populations (e.g. adults and children) a randomized controlled trial is included if 90% or more of the population meet the eligibility criteria |
| Intervention |
| • Any intervention for the treatment of hand fractures and joint injuries. This includes primary, secondary treatment and/or associated therapy interventions |
| Comparator |
| • Any other intervention for the treatment of hand fractures and joint injuries as described above • Placebo or no intervention |
| Study report characteristics |
| • Full study reports published in peer-reviewed journals • Abstracts of completed studies, if the full study report is not yet available • No timeframe restrictions for trial report publication • Studies in any language |
| Exclusion criteria |
| • Separate publications of economic evaluation of the primary trial • Studies of treatment for distal radial fractures • Studies where the primary injury was trauma of nerve, vessel, tendon and/or soft tissue deficits • Review articles, unpublished and ongoing trials |
Search strategy
The search strategies were compiled with guidance from an information specialist with hand surgery expertise. The search strategy was constructed in four parts.
Names of bones, joints and ligaments of the hand (e.g. phalanx, scaphoid, collateral).
General terms for fractures and joint injuries (e.g. fracture, dislocation).
Specific terms about hand fractures and joint injuries (e.g. boxer’s, Stener, gamekeeper’s thumb).
The sensitivity-maximizing version of the Cochrane RCT filter.
(1) and (2) were combined using the Boolean ‘AND’, which was then combined with (3) using the Boolean ‘OR’. The findings were then combined with the RCT filer (4) using the Boolean ‘AND’. The search terms are detailed in Appendix S1 (available online).
The databases searched were PubMed, Cochrane CENTRAL, Ovid MEDLINE and Ovid Embase. The details of coverage and the interfaces used are shown in Table 2. The search was carried out on 27 December 2017.
Table 2.
Databases searched.
| Database | Interface | Coverage |
|---|---|---|
| PubMed | PubMed | 1946–present |
| Cochrane Central Register of Controlled Trials (Cochrane CENTRAL) | Wiley | 1999–present |
| Embase | OVID | 1980–present |
| MEDLINE | OVID (In process and non-indexed) | 1946–present |
Data management, quality assessment and data extraction
Records identified via the searches were imported into EndNote X7 (Thompson Reuters, New York, NY) and duplicates removed. Two review authors (CM and DG) independently screened all titles and abstracts for potentially eligible studies, for which full-text reports were obtained where appropriate. The quality (risk of bias) of included studies was assessed independently by two assessors (CM and SD) using the Cochrane Risk of Bias tool for RCTs and quasi-random studies (Higgins et al., 2017). Disagreements were resolved by consulting a third review author (AK) and discussion. One review author (CM) extracted the data, using a pre-piloted standard data collection form. Data extraction included details of the population, intervention, comparator and outcomes for all included trials, external funding source, registration with a trial repository, sample size, sample size calculation, method of randomization, RCT study design (single or multi-centre) and whether intention-to-treat analysis was performed. Length of follow-up, losses to follow-up and the outcomes (primary, secondary) were also extracted.
Mapping and data synthesis
The studies were mapped according to the anatomical site of the fracture or joint injury. A narrative descriptive synthesis of the findings is presented, structured around the anatomical site of the hand fracture or joint injury. Descriptive statistics (proportions, median with range, mean with standard deviation) were used to report study characteristics. Linear regression was used to test the association between length of study follow-up and study retention rates.
Results
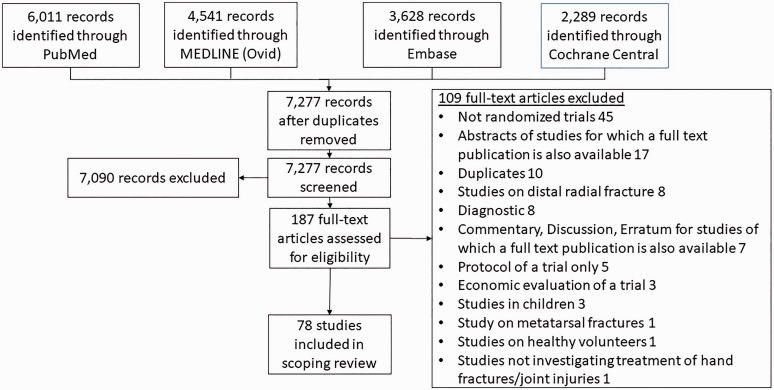
The study selection process is demonstrated via a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Moher et al., 2009) (Figure 1). Seventy-eight RCTs fulfilled the eligibility criteria and were included. The authors of three studies were contacted as it was unclear whether they were randomized. One confirmed that the study was an RCT (Sourmelis et al., 1995), one that the study was a cohort study (Gabler et al., 2001) and no reply was received from the third (Toker et al., 2015), so the study was excluded. Details of the included studies, mapped by anatomical region, are presented in Appendix S2 (Tables A–H) (available online), including report identifiers and the population, intervention, comparator and outcomes outline for each trial.
Figure 1.
Review PRISMA flow diagram.
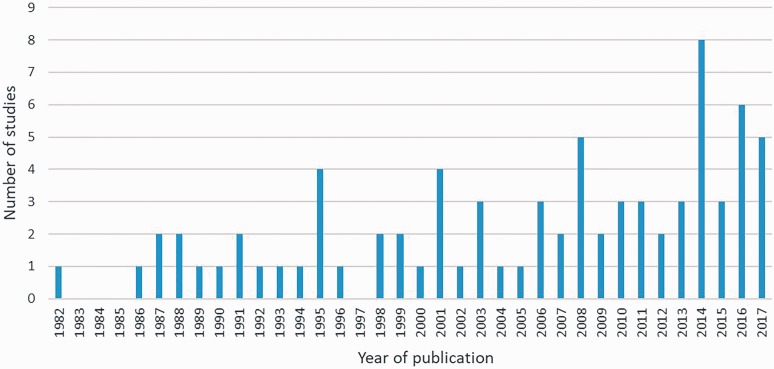
Trial publication dates ranged from 1982 to 2017 (Figure 2). Most trials were from European institutions (51/78, 65%), with fewer studies from North America (USA and Canada) (9/78 (12%)) and the rest from other parts of the world (18/78 (23%)). The five countries that reported the highest number of trials were the United Kingdom (14/78 (18%)), the United States (8/78 (10%)), Denmark (7/78, (9%)), Netherlands (5/78 (6%)) and Sweden (6/78 (8%)). Of the included trials, 46 (59%) were published after 1 July 2005, when the registration requirement for trials was implemented by the International Committee of Medical Journal Editors (ICMJE) (De Angelis et al., 2004); of those, only 8/46 (17%) indicated compliance by reporting registration in a clinical trial repository. Few trials reported a sample size calculation or intention-to-treat analysis, studies were generally small (under 100 participants) and single-centre (Table 3); median sample size was 54 (range 8–352). Only 14 of 33 (42%) trials that looked at operative treatment interventions reported the training/experience of surgeons.
Figure 2.
Distribution of trials per year of publication.
Table 3.
Study characteristics.
| n/N (%) | |
|---|---|
| Studies published after 1 July 2005 | 46/78 (59%) |
| Study report indicating registration in a trial repository | 8/46 (17%) |
| Sample size > 100 | 12/78 (15%) |
| Studies reporting a sample size calculation | 26/78 (33%) |
| Randomized controlled trial study design | |
| • Single-centre | 49/78 (63%) |
| • Multi-centre | 11/78 (14%) |
| • Inadequate information | 18/78 (23%) |
| Randomization | |
| • Randomized controlled trials | 73/78 (94%) |
| • Quasi-randomized trials | 5/78 (6%) |
| Study report indicating intention-to-treat analysis | 14/78 (18%) |
| External funding source | 13/78 (17%) |
| Comparison type | |
| • Two different types of surgical treatment | 16/78 (21%) |
| • One surgical treatment compared to one type of conservative treatment | 16/78 (21%) |
| • Two different types of conservative treatment | 46/78 (59%) |
Mapping
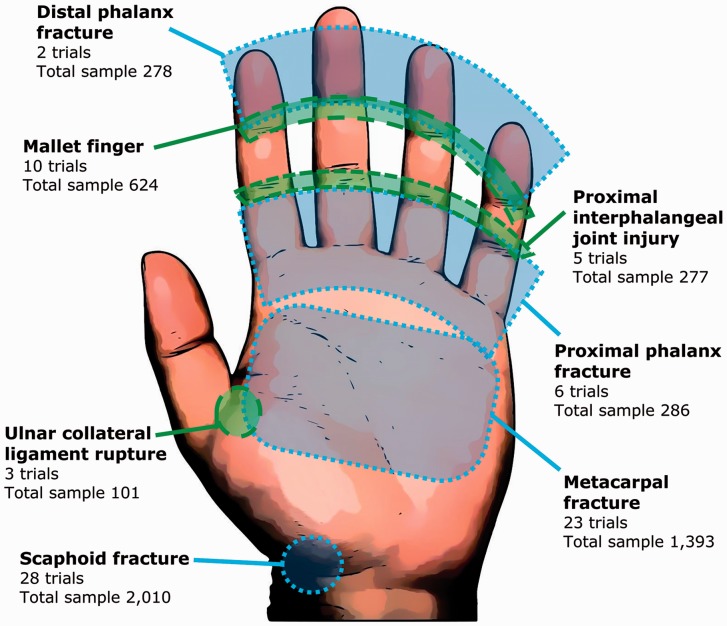
The trials were mapped according to the anatomical site of injury treated; this is presented visually in Figure 3. The four most common injuries studied were scaphoid fractures (28 trials), followed by metacarpal (23 trials), mallet fingers (ten trials) and proximal phalangeal fracture (five trials). One trial reported a mixed population of ‘closed hand bone fractures’.
Figure 3.
Mapping of the included randomized trials according to the anatomical site.
RCTs investigated the effects of a wide range of treatments, including Kirschner-wires, different types of splints, casts, or orthoses and exercise/rehabilitation programmes. An equal number of studies compared two different types of surgical treatment, and a type of surgical treatment compared with a type of conservative treatment, with the remaining comparing two conservative treatments (Table 3). Of the conservative treatments compared, 35/78 (45%) studies assessed different splints/casts/orthoses, 2/78 (3%) studies compared rehabilitation regimes, 4/78 (5%) electrical stimulation to no treatment, 2/78 (3%) ultrasound therapy to no therapy, 1/78 (1%), laser therapy to no therapy and 2/78 (3%) studies compared pharmacological interventions. Appendix S2 (available online) presents details of all included RCT intervention comparisons, mapped by anatomical site of injury.
Quality assessment
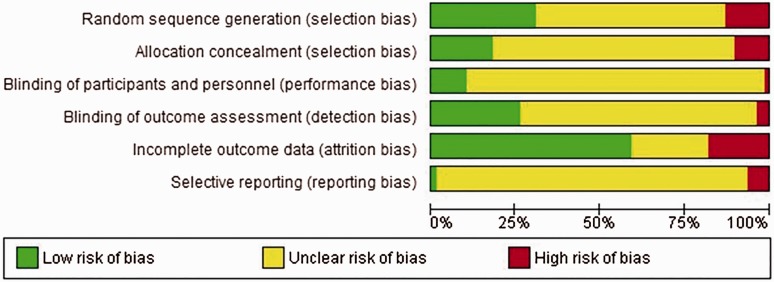
The quality assessment of included trials is visually summarized in the risk of bias graph (Figure 4). Figure S1 (Supplementary Material, available online) details the quality assessment for each individual study. A common finding was that most of the studies that claimed to be ‘randomized’ did not actually specify how the randomization was done (i.e. coin toss, sealed envelopes, computer generated sequence or other) or whether or how the allocation sequence was concealed. Only 24/78 (31%) scored ‘low risk of bias’ for random sequence generation and 14/78 (18%) for allocation concealment.
Figure 4.
Risk of bias graph: authors’ judgements about each risk of bias item presented as percentages across all included studies.
Only a small proportion of studies reported blinding, with 8/78 (10%) studies blinding the participants and/or the study personnel and 20/78 (26%) blinding the outcome assessors. Most studies did not report on ‘blinding’ status. Very few studies (5/78 (6%)) referenced a study protocol. Studies published before 2003 generally tended to score ‘unclear’ for many risks of bias domains, whereas those published after 2003 tended to report more of the information required for bias assessment, therefore scoring either ‘low’ or ‘high’ more often in the domains (Figure 4).
Outcomes and follow-up
Only 13/78 (17%) trials specified the primary outcome measure in full, including what was measured and when. The median time point for the assessment of the primary outcome out of 13 studies was 6 weeks (range 1–16). A further 4/78 (5%) trials specified the primary outcome, but this was incomplete, that is, they did not report the time point of interest. The primary outcome was ‘blinded’ in 10/78 (13%) trials. Only two trials selected a recognized standardized Patient-Reported Outcome Measure (PROM) as primary outcome measure (the QuickDASH). Table 4 shows the outcomes assessed in included trials.
Table 4.
Outcomes assessed in included trials.
| Outcomes | n/N (%) |
|---|---|
| Clinical measurements | 64/78 (82%) |
| • Range of motion | 45/78 (58%) |
| • Grip strength | 33/78 (42%) |
| Radiological | 32/78 (41%) |
| Pain | 28/78 (36%) |
| Patient-reported outcome measures (PROMs) | 19/78 (24%) |
| • Disabilities of the Arm, Shoulder and Hand (DASH) | 10/78 (13%) |
| • QuickDASH | 7/78 (9%) |
| • Patient Evaluation Measure (PEM) | 2/78 (3%) |
| • Patient-Rated Wrist Evaluation questionnaire (PRWE) | 2/78 (3%) |
| • Michigan Hand Outcomes Questionnaire (MHQ) | 1/78 (1%) |
| Return to previous occupation | 15/78 (19%) |
| Overall satisfaction with the result | 15/78 (19%) |
| Complications | 12/78 (15%) |
| Physician-reported and/or composite outcome scores | 5/78 (6%) |
| • Mayo Modified Risk score | 2/78 (3%) |
| • Green/O’Brien score | 2/78 (3%) |
| • Modified Scaphoid Outcome Scoring System | 1/78 (1%) |
| Satisfaction with cosmetic appearance | 4/78 (5%) |
| Quality of life (EQ-5D) | 1/78 (1%) |
The maximum length of individual study follow-up was highly variable (median 24 weeks; range 1 to 624 weeks). Twenty-two studies (28%) had a maximum follow-up of 1 year or more. Follow-up (retention) data were reported in 50/78 (64%) of trials; in these trials, retention was over 80% in 37/50 (74%) at the end of the study. This translates to reported follow-up over 80% for only 37/78 (47%) of trials. Retention rates of 70%–80% were reported by 6/78 (8%) and 50%–69% by 7/78 (9%) trials, with the rest providing no retention information at all. The median follow-up retention was 89% (range 55%–100%) at the end of each study. Retention did not show an association with the maximum length of study follow-up (regression coefficient 0.008; p = 0.63; 95% CI: –0.39 to 0.023).
Discussion
This comprehensive scoping review identified and assessed published RCTs on the treatment of hand fractures and joint injuries. It was guided by a search of publications developed with support from an experienced information specialist and used sound methodology informed by the PRISMA-SCR guidelines (Tricco et al., 2018). It has highlighted issues with design and reporting, informed by a recognized assessment tool (Cochrane Methods, 2018). It provides a reference for the planning of future studies as well a repository of the included trials, mapped by topic (Appendix S2). It highlights the paucity of high-level evidence to guide the clinical management of people with hand injuries.
The review identified 78 trials published over a period of 35 years, which is a surprisingly small number. To put this number into context, a systematic review on the treatment of distal radial fractures identified 90 RCTs published over 5 years from 2010 to 2015 (Lee et al., 2018). The trials identified in the present review covered only seven anatomical areas of the hand. This may be because the injuries studied are common or have potential for poor outcomes. For example, metacarpal fractures are common and scaphoid fractures can have poor outcomes if not properly treated. In terms of the interventions studied, the trials were extremely heterogeneous, and compared various types of operative and non-operative treatments, so it is difficult to draw any conclusions on individual treatments. There are further issues with the design, conduct and reporting of these trials, suggesting potential for bias.
Mandatory prospective trial registration came into effect in July 2005 (De Angelis et al., 2004). Of the RCTs published after this time, very few studies had been registered with a trial registry. Only a few studies referenced a study protocol and for the rest it is unknown whether a study protocol was available, but not reported. It was therefore not possible to assess for selective outcome reporting in studies without a protocol. In terms of quality assessment, most assessed bias domains were graded as unclear, reflecting the pressing need for greater clarity in trial reporting via the enforcement of adherence to Consolidated Standards of Reporting Trials (CONSORT) guidelines by researchers and journals (Nagendran et al., 2013).
Selection bias refers to systematic baseline differences between the two groups (Cochrane Methods, 2018). Randomization helps to control for known and unknown confounders and minimizes selection bias. Even though all studies were reported as randomized trials, the randomization and allocation concealment methods were not described in many trials.
Very few studies were blinded, which introduces performance and detection biases. Performance bias refers to the introduction of differences between the two groups other than the intervention (Cochrane Methods, 2018). Knowing which intervention a patient has received can affect the care provided by clinicians and the perception of recovery by the patients. Detection bias refers to systematic differences between groups in how outcomes are determined (Cochrane Methods, 2018). Knowing which intervention a patient has received can affect outcome assessment, especially of subjective outcomes such as pain. Though it is impossible to achieve blinding in many surgical trials, assessors should be independent and blinded whenever possible. When it is not possible to blind, this should be stated. Most of the included trials did not discuss the blinding, or explained why they did not blind.
Though most trials assessed outcomes likely to be reported directly by patients, such as pain and measures of satisfaction, only a small proportion of trials measured this in a standardized way that can be compared across studies, such as a standardized scale or patient-reported questionnaire. Only 24% used PROMs. The most frequently used PROMs were the Disabilities of the Arm Shoulder and Hand (DASH) and QuickDASH questionnaires, reflecting their prominence in orthopaedic publications. Furthermore, very few trials in this review specified their primary outcome, whereas they measured a wide range of heterogeneous secondary outcomes at differing time points (Table 4), precluding future meta-analysis in systematic reviews. Most studies also failed to report a sample size calculation and had a sample size of less than 100 participants, which is likely to be too low to draw meaningful conclusions with narrow confidence intervals (Corty and Corty, 2011).
Length of follow-up was variable and participant retention at the final follow-up point was often not reported. Only 22/78 (28%) of trials had duration of follow-up of 1 year or more. Participant retention did not show an association with follow-up length, suggesting that either most studies reporting the percentages of follow-up at the last attendance were relatively short (median follow-up 24 weeks) or possibly that those which had high percentages of losses failed to report it.
Few trials of operative treatments reported the training/experience of surgeons. Those that did said that the authors carried out the surgery. The authors were senior, likely to be enthusiasts with specialist knowledge, which would make the results less generalizable.
The low number of multi-centre studies and the lack of external funding shows that the speciality of hand surgery needs to follow other specialities in conducting larger, collaborative studies.
There needs to be consistency by better design. A core outcome set for trials relating to the treatment of hand injuries would substantially increase the transparency and consistency of reporting (Williamson et al., 2012). A core outcome set is a consensus minimum set of outcomes that should be measured and reported in all trials relating to a specific condition and is developed with the input of all relevant stakeholders, including patients, researchers, clinicians and policy makers (COMET, 2018). Furthermore, issues with poor design are important to highlight and address because a solution will require the endorsement and cooperation of researchers, funders, reviewers, journal editors and the wider clinical community.
The results of this review are compatible with other reports. Post et al. (2014) carried out an analysis of two major hand surgery journals for the level of evidence of RCTs. They found that the lack of quality may be for a number of reasons, such as economic (i.e. trials in surgery lack comparable budgets with those trials funded by pharmaceutical companies), the relatively small and heterogeneous patient populations and the inability to blind surgeons and patients. They suggested that there is a need for high-quality publications, which could be achieved by the use of the CONSORT statement as a guideline to improve the quality of RCT reporting. A systematic review of all hand surgery articles published in six journals over a 20-year period found that the number of hand surgery articles has progressively increased over the last 20 years (Sugrue et al., 2016).
This is not a systematic review of the effectiveness of specific interventions, so we cannot draw clinically relevant conclusions about the effectiveness of treatments for a particular injury. The comparatively low number of studies, heterogeneity of interventions, deficiencies in trial design and inconsistencies in outcome assessment make this very difficult, which is why so many systematic reviews in the field of hand surgery rely on lower levels of evidence by including non-randomized studies (Sugrue et al., 2016).
Supplemental Material
Supplemental material, Supplemental Material1 for Treatment interventions for hand fractures and joint injuries: a scoping review of randomized controlled trials by Christos Mousoulis, Kim Thomas, Paul Leighton, Sandeep Deshmukh, Douglas Grindlay and Alexia Karantana in Journal of Hand Surgery (European Volume)
Supplemental material, Supplemental Material2 for Treatment interventions for hand fractures and joint injuries: a scoping review of randomized controlled trials by Christos Mousoulis, Kim Thomas, Paul Leighton, Sandeep Deshmukh, Douglas Grindlay and Alexia Karantana in Journal of Hand Surgery (European Volume)
Supplemental material, Supplemental Material3 for Treatment interventions for hand fractures and joint injuries: a scoping review of randomized controlled trials by Christos Mousoulis, Kim Thomas, Paul Leighton, Sandeep Deshmukh, Douglas Grindlay and Alexia Karantana in Journal of Hand Surgery (European Volume)
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- Cochrane Methods. Assessing risk of bias in included studies 2018. http://methods.cochrane.org/bias/assessing-risk-bias-included-studies (29 June 2018).
- COMET. Core outcome set (cos). 2018. http://www.comet-initiative.org/glossary/cos/ (20 July 2018).
- Corty E, Corty R. Setting sample size to ensure narrow confidence intervals for precise estimation of population values. Nurs Res. 2011, 60: 148–53. [DOI] [PubMed] [Google Scholar]
- De Angelis C, Drazen J, Frizelle F, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004, 351: 1250–1. [DOI] [PubMed] [Google Scholar]
- Gabler C, Kukla C, Breitenseher M, Trattnig S, Vecsei V. Diagnosis of occult scaphoid fractures and other wrist injuries. Are repeated clinical examinations and plain radiographs still state of the art? Langenbecks Arch Surg. 2001, 386: 150–4. [DOI] [PubMed] [Google Scholar]
- Grant M, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009, 26: 91–108. [DOI] [PubMed] [Google Scholar]
- Higgins J, Altman D, Sterne J. Assessing risk of bias in included studies. In: Higgins J, Churchill R, Chandler J, Cumpston M. (eds) Cochrane handbook for systematic reviews of interventions version 520, Cochrane, 2017. [Google Scholar]
- James Lind Alliance. Common conditions affecting the hand and wrist, priority setting partnership. 2017. https://www.bssh.ac.uk/_userfiles/pages/files/Patients/James%20Lind/JLA%20Final%20Summary.pdf (29 November 2017).
- Lee S, Khan T, Grindlay D, Karantana A. Registration and outcome-reporting bias in randomized controlled trials of distal radial fracture treatment. JB JS Open Access. 2018, 3: e0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. BMJ. 2009, 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagendran M, Harding D, Teo W, et al. Poor adherence of randomised trials in surgery to CONSORT guidelines for non-pharmacological treatments (NPT): a cross-sectional study. BMJ Open. 2013, 3: e003898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post S, Selles R, McGrouther D et al. Levels of evidence and quality of randomized controlled trials in hand and wrist surgery: an analysis of two major hand surgery journals. J Hand Surg Eur. 2014; 39: 900–2. [DOI] [PubMed]
- Sourmelis S, Platanitis G, Korakis T, Daras A, Schinas N, Papakostas C. Static splinting vs. functional treatment in extra-articular fractures of the proximal phalanges. Orthop Trans. 1995, 19: 210. [Google Scholar]
- Sugrue C, Joyce C, Sugrue R, Carroll S. Trends in the level of evidence in clinical hand surgery research. Hand (NY). 2016, 11: 211–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker S, Turkmen F, Pekince O, Korucu I, Karalezli N. Extension block pinning versus hook plate fixation for treatment of mallet fractures. J Hand Surg Am. 2015, 40: 1591–6. [DOI] [PubMed] [Google Scholar]
- Tricco A, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018, 169: 467–73. [DOI] [PubMed] [Google Scholar]
- Williamson P, Altman D, Blazeby J, Clarke M, Gargon E. Driving up the quality and relevance of research through the use of agreed core outcomes. J Health Serv Res Policy. 2012, 17: 1–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental Material1 for Treatment interventions for hand fractures and joint injuries: a scoping review of randomized controlled trials by Christos Mousoulis, Kim Thomas, Paul Leighton, Sandeep Deshmukh, Douglas Grindlay and Alexia Karantana in Journal of Hand Surgery (European Volume)
Supplemental material, Supplemental Material2 for Treatment interventions for hand fractures and joint injuries: a scoping review of randomized controlled trials by Christos Mousoulis, Kim Thomas, Paul Leighton, Sandeep Deshmukh, Douglas Grindlay and Alexia Karantana in Journal of Hand Surgery (European Volume)
Supplemental material, Supplemental Material3 for Treatment interventions for hand fractures and joint injuries: a scoping review of randomized controlled trials by Christos Mousoulis, Kim Thomas, Paul Leighton, Sandeep Deshmukh, Douglas Grindlay and Alexia Karantana in Journal of Hand Surgery (European Volume)