Highlights
-
•
Effects of lithium treatment duration on deep grey matter shape in BD are investigated.
-
•
Treatment duration impacts nucleus accumbens shape.
-
•
Extroflection effects, are related to longer duration in accumbens core.
-
•
Left accumbens core shape is correlated with increased depression severity.
Keywords: Bipolar disorder, Nucleus accumbens, Shape analysis, Lithium, Reward system, Neuroimaging, Grey matter
Abbreviations: BD, Bipolar disorder; HC, Healthy controls; DGM, Deep grey matter structures; BHStot, Total score from beck hopelessness scale; HAM-Dsom, Somatic subscale from Hamilton depression rating scale; HAM-Dtot, Total score from Hamilton depression rating scale; L-Accu, left nucleus accumbens; R-Accu, right nucleus accumbens; LiTD, Lithium Treatment Duration; LiTD+, bipolar patients with lithium treatment duration lasting for more than 50% of illness duration; LiTD-, bipolar patients with lithium treatment duration lasting for less than 50% of illness duration; NoLi, bipolar patients never treated with lithium drugs
Abstract
The effects of lithium treatment duration on deep grey matter structures in bipolar disorder are not well known. In this cross-sectional neuroimaging case-control study, we tested the hypothesis that shape characteristics of deep grey matter structures in bipolar disorder are associated with the duration of lithium treatment and with clinical phenomenology.
In a setting of neuropsychiatry outpatient clinic, we included 74 patients with bipolar disorder (BD) and 74 matched healthy control subjects (HC). Both groups underwent a Magnetic Resonance Imaging acquisition and an exhaustive assessment of clinical and psychiatrics dimensions. Shape measures of seven deep grey matter structures (hippocampus, amygdala, caudate, nucleus accumbens, putamen, globus pallidus and thalamus) were obtained from T1 weighted images in both groups, using FSL FIRST segmentation tool. The segmented structures were then analysed vertex-by-vertex with FSL Randomise tool. First, we investigated the presence of significant associations between the duration of lithium treatment and shape measures in BD sample. Then, for structures that resulted significantly associated with the duration of lithium treatment, comparisons between BD and HC were performed either considering the BD group as a whole or dividing it in three groups based on the duration of treatment (lithium drug-naïve, short and long treated). Any deformation uncovered by group comparisons was subsequently associated with depressive and hypomanic/manic symptoms.
The relationship between structures shape and the duration of lithium treatment in BD sample was significant for bilateral nucleus accumbens. Specifically, significant bilateral extroflection effects, related to longer duration of lithium treatment, were found bilaterally over the surface shape of core accumbens nuclei (r2R-Accu-Core = 0.12, p = 0.016, r2L-Accu-Core = 0.1, p = 0.031). Moreover, introflection effect related to longer duration of treatment resulted over the shell of right accumbens (r2R-Accu-Shell = 0.17, p = 0.002). Nucleus accumbens shape did not differ between BD and HC considering BD group as a whole. By contrast, categorizing BD in subgroups as a function of the duration of lithium treatment revealed significant inward deformation on the core of left accumbens nucleus and outward deformation on the shell of the right accumbens nucleus in lithium-naive patients, compared to both patients with long duration of lithium treatment (pL-Accu-Core = 0.016, pR-Accu-Shell = 0.005) and HC (pL-Accu-Core = 0.002; pR-Accu-Shell = 0.005). Moreover, compared to HC, inward deformation on the core of the left accumbens surface was found for patients with short duration of treatment (pLAccu-Core = 0.027). Finally, measures of surface deformation on the core of left accumbens observed in the group comparison showed significant positive correlations with depressive symptoms severity, as assessed by the Hamilton Depression Rating Scale (total score: r2L-AccuCore = 0.07, p = 0.02, somatic score: r2L-Accu-Core = 0.1, p = 0.005) and Beck Hopelessness Scale (r2LAccu-Core = 0.05, p = 0.03).
Findings demonstrate that lithium untreated BD patients are characterised by localized shape abnormalities in the nucleus accumbens. Lithium treatment could act modulating these morphometric features as part of its mechanism of action in mood stabilizing.
1. Introduction
Brain changes in deep grey matter (DGM) structures have been described with some inconsistences (McDonald et al., 2004) in patients with diagnosis of Bipolar Disorder (BD) (Hibar et al., 2016). While the sources of heterogeneity are multifactorial, one much debated source of bias is the effect of mood-stabilizing medications, primarily lithium (the archetypal mood stabilizer), that is already thought to have a modulatory effect in terms of volumetric increase induced on brain structures, particularly hippocampus and amygdala (Manji et al., 2000; Shaltiel et al., 2007; De-Paula et al., 2016; Simonetti et al., 2016).
In recent years, developments in brain morphometric methodologies have led to different structural measures that can integrate data derived from volumetric analysis. Indeed, investigation of the three-dimensional surfaces of DGM structures have been shown to be more sensitive than gross morphometric techniques, identifying group abnormalities where volumetric analyses did not (Mamah et al., 2009). Since shape analysis enables the uncovering of localized changes on the surface of brain structures it can be a very specific neuroimaging measure, especially in the case of structures with explicit regional differentiation in functions, such as DGM (Herrero et al., 2002). Previous studies investigating DGM shape in BD reported that drug-naive and non-psychotic BD patients, compared to healthy controls (HC), show widespread shape alteration of the caudate (Hwang et al., 2006; Ong et al., 2012; Mamah et al., 2016; Womer et al., 2014) and putamen (Mamah et al., 2016; Womer et al., 2014), both as local inward and outward deformations. Significant shape contraction on the dorsal surface of the right accumbens and widespread contractions of the globus pallidus in non-psychotic BD patients have also been reported (Mamah et al., 2016). However, results from these studies did not consider neither the specific effect of lithium treatment nor the effect of its duration relative to illness duration. Indeed, a staging model for bipolar disorder has recently been proposed claiming that a better response to treatment generally occurs when drugs are introduced early in the course of the illness (Vieta et al., 2011). Coherently, this model posits that response to treatment is function of the duration of illness and that the longer untreated duration of illness the longer duration of treatment is necessary in order to be effective. In this perspective, treatment duration should be considered as the balance between the exposure to neuropathological (Van Gestel et al., 2019) (driven by illness duration) and neuroprotective (driven by lithium treatment) processes.
Finally, the relationship between lithium-driven DGM shape variations and clinical correlates of BD patients, such as the degree of depressive and manic symptoms, has not been investigated.
In the present work, our first aim was to analyse the potential effect of the duration of lithium treatment on the morphometric measures of DGM surfaces. As a secondary goal, we aimed at identifying the relationship between the severity of patients’ depressive and/or manic symptomatology and the degree of any localized DGM abnormalities. To these aims we analysed the shape of seven bilateral DGM structures (i.e. Accumbens, Amygdala, Caudate, Hippocampus, Pallidus, Putamen, and Thalamus) in HC and BD samples, both considering BD as a whole group and stratifying it in subsamples, according to lithium treatment duration.
We hypothesize that shape of DGM structures of BD is associated to lithium treatment duration, given the well-established neurotrophic effect of lithium (Manji et al., 2000). Further, based on previous literature focusing on volume data (Simonetti et al., 2016), we predict that differences between BD and HC subjects on DGM surface will be evident only categorizing patients as a function of duration of lithium treatment, such that shape abnormalities would emerge particularly in never treated patients. Finally, we expect that such abnormalities will be associated to the severity of depressive and hypomanic/manic patients’ symptomatology, given lithium efficacy in mood stabilization (Geddes et al., 2004).
2. Methods
This cross-sectional case-control study was approved by the local ethic committee of the Santa Lucia Foundation. All participants provided written informed consent to be included in the study.
Given the lack of prior studies about the relationship between DGM shape morphometry and lithium treatment in bipolar disorder, an a priori power calculation in G*power was used to determine the minimum sample size. Specifically, we used data from our previous study on hippocampal volumetry in bipolar disorder (Simonetti et al., 2016), which showed an effect sizes of 0.85–0.99 for the lithium-dependent modulatory effects, to inform the power calculation. To be conservative we used the lower estimate, which determined that a total sample size of at least 24 subjects would have greater than 95% power to detect a difference between groups, with an alpha value of <0.05 (two tailed).
Subjects were recruited between May 2013 and March 2017.
2.1. Participants
Eighty patients with a diagnosis of BD (49 BD type I – 31 BD type II) according to the Diagnostic and Statistical Manual of Mental Disorders IV-Edition, Text Revised (DSM-IVTR) (American Psychiatric Association 2000) were initially assessed at the Santa Lucia Foundation in Rome. The clinical psychiatrist who had been treating the patients and knew their clinical history, and who was blind to the aims of the study, made the preliminary diagnosis using the DSM- IV-TR criteria. Then, a research psychiatrist using the Structured Clinical Interview for DSM-IV-TR-Patient Edition (SCID-I/P) (First et al., 2002) confirmed the clinical diagnoses. If the diagnoses made at the two different steps were not consistent, more data were gathered and the diagnostic process continued until a final diagnostic consensus was reached. If an agreement could not be reached the patient was not included in the sample. From the original BD group (n = 80), two refused to undergo the MRI exam and four were excluded given the low quality of T1-weighted images (see exclusion criteria). The remaining sample of 74 patients included 45 with BD-I and 29 with BD-II.
We also recruited 74 healthy controls (HC) in the same geographical area, paired-matched with the patients for age, gender and educational level. All HC were screened for a current or lifetime history of DSM-IV-TR Axis I and II disorders using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition (SCID-I-NP) (First et al., 1997a) and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (First et al., 1997b); they were also assessed to confirm that no first-degree relative had a history of bipolar, mood- or schizophrenia-related disorders.
Apart from these groups-specific inclusion processes, inclusion criteria for all participants were: (i) age between 18 and 75 years, (ii) at least five years of education, and (iii) suitability for magnetic resonance imaging (MRI) scanning. Exclusion criteria were: (i) history of alcohol or drug abuse during the two years before the assessment, (ii) lifetime drug dependence, (iii) traumatic head injury with loss of consciousness, (iv) past or present major medical illness or neurological disorders, (v) any (for HC) or additional (for BD) psychiatric disorder or mental retardation, (vi) dementia or cognitive deterioration according to DSM-IV-TR criteria, and Mini-Mental State Examination (MMSE) (Folstein et al., 1975) score <25, consistent with normative data in the italian population (Measso et al., 1993), (vii) low quality of T1-weighted images (i.e. presents of severe motion or scanner-generated artefacts), (viii) any potential brain abnormality or microvascular lesion as apparent on conventional fluid attenuated inversion recovery (FLAIR) scans, potentially explaining critical phenomenology; in particular, the presence, severity, and location of vascular lesions were computed according to the semi-automated method developed by our group (Iorio et al., 2013).
2.3. Clinical assessment
Clinical characteristics were collected during a semi-structured clinical interview and all patients were under stable pharmacologic treatment for at least six months. Age at onset was defined as ‘age at onset of first affective symptoms’, which were investigated in an interview with patients and first-degree relatives. We also collected specific information about lithium treatment duration (LiTD), reported in months. We categorized patients with LiTD longer than 50% of illness duration (hereafter called LiTD+, N = 13), those with LiTD shorter than 50% of illness duration (LiTD-, N = 31), and those not taking lithium at all (NoLi, N = 30), in order to weight up LiTD as a function of patients’ illness duration. This was done in order to obtain better homogeneity (in terms of lifetime drug exposure) of samples to compare with HC group, according to the staging model of BD (Vieta et al., 2011). At the time of MRI scanning, patients in both LiTD+ and LiTD- groups all were still taking lithium.
The severity of affective depressive and hypomanic/manic symptoms was assessed using the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960), the Beck Hopelessness Scale (BHS) (Beck, 1998) and the Young Mania Rating Scale (YMRS) (Young et al., 1978). The amount of depressive symptoms were scored, rating also the psychological (HAM-Dpsy) and somatic components (HAM-Dsom) of depression, apart from the total score (HAM-D tot).
2.4. Image acquisition e processing
All 148 participants underwent the same imaging protocol, which included 3D T1-weighted, T2-weighted and FLAIR sequences using a 3T Achieva MR scanner (Philips Medical Systems, Best, The Netherlands) with a 32-channel receiving-only head coil. Whole-brain T1-weighted images were obtained using a fast-field echo sequence (echo time/repetition: time = 5.3/11 ms, flip angleec = 9°, voxel sizeec = 1 × 1 × 1 mm3). T2-weighted and FLAIR sequences were acquired to screen for brain pathology.
Segmentations of seven bilateral subcortical grey matter nuclei (Accumbens, Amygdala, Caudate, Hippocampus, Pallidus, Putamen, and Thalamus) were generated using the FIRST algorithms included in the FMRIB Software Library (FSL, version 5.0.0). Specifically, FIRST is a fully automated model-based segmentation/registration software, providing analysing tools both for volume estimations and localized differences in shape (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST). The segmentation process is based on the shape and appearance models, constructed from 336 manually segmented images using Gaussian assumptions combined with a Bayesian probabilistic approach (Patenaude et al., 2011). FIRST algorithms ascertain the most probable shape by means of linear combinations of shape variations employing the learned manually segmented models. Moreover, the segmentation process includes boundary correction and registration to a MNI152 template using 12 degrees of freedom as well as a subcortical mask to detect and eliminate voxels outside the subcortical structure. Shape was then expressed as a mean with modes of variation (principal components).
After the automated segmentation performed by the software, the outputs were manually checked and confirmed for the proper nuclei extraction of all the subcortical structures. FSLView toolbox was used for neuroimaging results visualizations (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FslView).
In case of significant results located in the nucleus accumbens, we further refined their localization in terms of specific accumbens subregions (i.e. core and shell), by using the Allan Human Brain Atlas (Hawrylycz et al., 2012) (available from: http://atlas.brain-map.org) and referring to previous evidence on accumbens anatomy (Baliki et al., 2013) (see Results section).
2.5. Statistical analyses
Comparisons between HC and BD on sociodemographic variables (i.e. age, gender, and educational level) and between BD subgroups on sociodemographic and clinical (i.e. duration of illness, number of episodes, time of lithium exposure, severity of (hypo)manic/depressive symptoms and pharmacological treatment) characteristics were performed using t-test, ANOVA or chi-square tests on StatView statistical software, considering p < 0.05 as statistical threshold for significance.
All neuroimaging statistics were performed by vertex-wise shape analysis along the surface of the segmented DGM structures, using FSL Randomise tool with a nonparametric permutation testing (Nichols and Holmes, 2002). We used n = 5000 permutations and a Threshold-Free Cluster Enhancement (TFCE, –T2) to correct for multiple comparisons (p < 0.05, after correction for multiple comparisons) (Smith and Nichols, 2009).
We preliminary checked for the presence of differences in demographical, clinical (Table 1) and DGM morphological features between BD-I and BD-II performing t-tests between patients’ subgroups. Since no difference was found between BD subtypes, subsequent analyses were performed considering BD patient groups as a whole.
Table 1.
Sociodemographic and clinical characteristics of 45 BD-I and 29 BD-II patients.
| Characteristics | BD-I | BD-II | t | df | p |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 43.51 (12.9) | 44.65 (11.7) | −0.39 | 72 | 0.70 |
| Educational level (years), mean (SD) | 13.76 (3.3) | 14.83 (2.9) | −1.37 | 72 | 0.174 |
| Duration of illness (years), mean (SD) | 14.7 (11.6) | 15.38 (9.2) | −0.27 | 72 | 0.788 |
| Duration of lithium treatment (months) | 63.84 (54.5) | 38.38 (30.2) | 1.577 | 42 | 0.122 |
df, degrees of freedom; SD, standard deviation.
The effect of lithium treatment duration on BD DGM was investigated using FSL Randomise as follows: first, linear regressions were performed between each DGM shape measure of BD (continuous dependent variable) and LiTD (continuous independent variable), including the duration of illness as covariate of no interest. The inclusion of the covariate was justified by the significant association with the independent variable (r2 = 0.125, p = 0.0186). Second, in case of significant linear regressions results, we compared BD and HC either considering patient group as a whole, either dividing patients in three groups based on LiTD. In the first case, DGM of BD and HC were compared using t-test while in the second case, ANOVA analyses were conducted, using shape as dependant variable and group (i.e. LiTD+, LiTD-, NoLi, HC groups) as four-level independent variable. We performed the last two analyses in order to assess whether the impact of the duration of lithium treatment is crucial to bring out differences between patients and controls and to investigate in which time point of medication they could be evident. Moreover, categorizing patient according to treatment duration, we investigated if lithium-associated DGM morphological characteristics found in BD were similar to these observed in physiological states. T-tests were performed for post-hoc comparisons. The relationship between values of inward/outward deformations in BD patients (independent variable) and clinical symptoms severity (i.e. HAM-Dtot, HAM-Dpsy, HAM-Dsom, BHStot) (dependent variable) was subsequently assessed performing linear regressions. We focused subsequent ANOVA post-hoc (pairwise comparisons) and linear regressions (surface deformations vs. symptom severity) analyses in those areas where significant surface outward or inward deformations emerged from results of the main ANOVA analysis.
3. Results
3.1. Sociodemographic and clinical characteristics
Sociodemographic characteristics of BD (as a whole) and HC groups are shown in Table 2. As expected, in view of the matching procedure, the groups did not differ in terms of age, gender or educational level (Table 2). No differences were also found when HC were compared to BD subgroups (NoLi, LiTD- and LiTD+) (Table 3). Furthermore, the NoLi, LiTD- and LiTD+ subgroups did not differ in terms of duration of illness, number of past episodes, HAM-D scores, YMRS scores, or – with the exception of lithium exposure – current and past pharmacotherapies (Table 3).
Table 2.
Sociodemographic and clinical characteristics of 74 BD patient and, 74 healthy subjects.
| Characteristics | BD | HC | t or χ2 | df | p |
|---|---|---|---|---|---|
| Age (years), mean (SD) | 43.96 (12.4) | 43.96 (17.2) | 0.00 | 146 | – |
| Males, n (%) | 42 (56.8) | 42 (56.8) | 0.00 | 1 | – |
| Educational level (years), mean (SD) | 14.2 (3.3) | 14.8 (2.9) | −1.22 | 146 | 0.22 |
| Duration of illness (years), mean (SD) | 14.9 (10.7) | – | – | – | – |
| Number of past manic/hypomanic episodes, mean (SD) | 5 (6.2) | – | – | – | – |
| Number of past depressive episodes, mean (SD) | 6.2 (6.4) | – | – | – | – |
| HAM-D score, mean (SD) | 7.7 (5.6) | – | – | – | – |
| YMRS score, mean (SD) | 5 (6) | – | – | – | – |
| Current medication, n (%) | 74 (100) | – | – | – | – |
| Antidepressant, n (%) | 29 (39.2) | – | – | – | – |
| Antipsychotics, n (%) | 47 (63.5) | – | – | – | – |
| Lithium, n (%) | 44 (59.5) | – | – | – | – |
| Benzodiazepines, n (%) | 29 (39.2) | – | – | – | – |
| Other mood-stabiliser, n (%) | 48 (65.8) | – | – | – | – |
df, degrees of freedom; SD, standard deviation; HAM-D, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale.
Table 3.
Sociodemographic and clinical characteristics of 74 healthy subjects and 74 BD patients stratified according to lithium treatment duration: 31 BD_LiTD-; 13 BD_LiTD+; 30 BD_NoLi.
| Characteristics | BD _LiTD- | BD _LiTD+ | BD _NoLi | HC | t, F or χ2 | df | p |
|---|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 44.7 (13) | 45 (11) | 42.7 (12) | 43.9 (17) | 0.11 | 3 | 0.952 |
| Males n (%) | 22 (71) | 6 (46.2) | 14 (46.7) | 42 (57) | 4.39 | 3 | 0.111 |
| Educational level (years), mean (SD) | 14.7 (3.5) | 14.3 (3) | 13.6 (3.1) | 14.8 (2.8) | 1.2 | 3 | 0.312 |
| Duration of illness (years), mean (SD) | 17.2 (10.5) | 11.5 (9.1) | 14.3 (11) | – | 0.21 | 71 | 0.319 |
| Duration of lithium treatment (months), mean (SD) | 41.4 (34) | 91.8 (63) | – | – | −3.4 | 42 | 0.0013* |
| Number of past manic/hypomanic episodes, mean (SD) | 4.1 (3) | 4.2 (3.1) | 6.31 (9) | – | 1.12 | 2 | 0.336 |
| Number of past depressive episodes, mean (SD) | 5.4 (4) | 5.9 (4) | 7 (8.9) | – | 0.46 | 2 | 0.633 |
| HAM-D score, mean (SD) | 7.2 (6.6) | 6.8 (5.6) | 8.5 (4.4) | – | 0.59 | 2 | 0.559 |
| YMRS score, mean (SD) | 3.2 (4.3) | 6.7 (5.5) | 6.2 (7.3) | – | 2.69 | 2 | 0.075 |
| Antidepressant, n (%) | 10 (32.3) | 3 (23.1) | 16 (53.3) | – | 4.56 | 2 | 0.102 |
| Antipsychotics, n (%) | 20 (64.5) | 8 (61.5) | 19 (63,3) | – | 0.04 | 2 | 0.982 |
| Benzodiazepines, n (%) | 13 (41.9) | 4 (30.8) | 12 (40) | – | 0.49 | 2 | 0.781 |
| Other mood-stabiliser, n (%) | 22 (45.8) | 9 (18.8) | 17 (35.4) | 1.935 | 2 | 0.381 |
df, degrees of freedom; SD, standard deviation; HAM-D, Hamilton Depression Rating Scale; YMRS, Young Mania Rating Scale. *Statistically significant differences at p < 0.05.
3.2. Shape analysis
Either comparisons between BD-I and BD-II patients or between HC and BD (considered as a single diagnostic group) did not show significant shape differences in any of the analysed DGM structures.
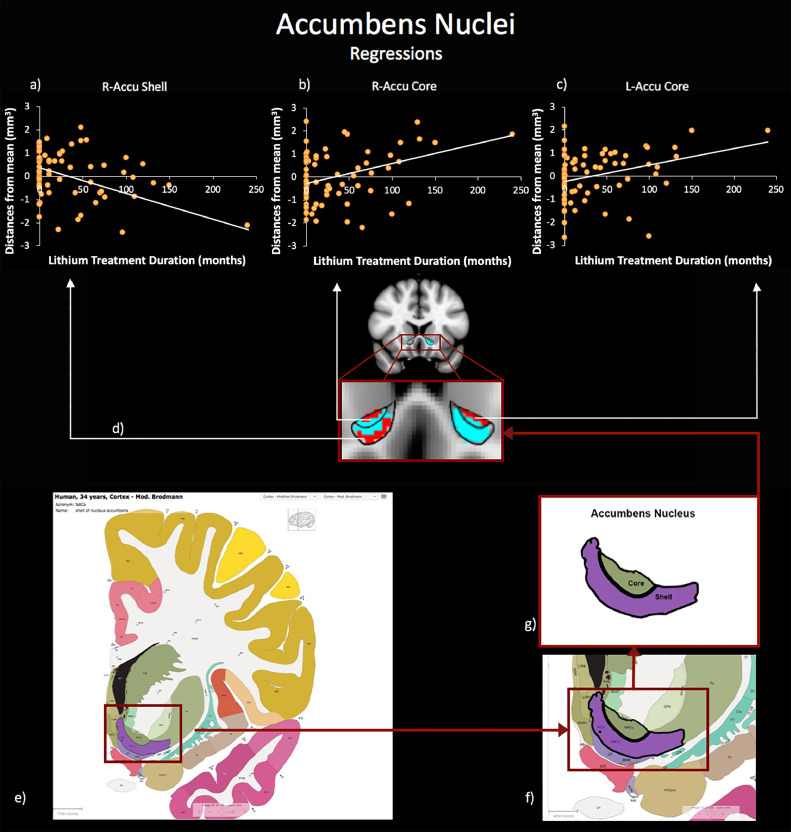
The linear relationship between LiTD and DGM shape in BD indicated both positive and negative significant associations between the duration of lithium treatment and the morphometric characteristics of clusters located on the bilateral nucleus accumbens (R-Accu and L-Accu). In order to qualify the localization of significant changes in terms of specific accumbens subregions (i.e. core or shell), we both referred results to the standard Human Brain Atlas (Hawrylycz et al., 2012) (available from: http://human.brain-map.org) and to previous evidence for human nucleus accumbens anatomy (Baliki et al., 2013) described by the MNI coordinates, which anatomically defined the core and the shell of the nucleus accumbens. According to y-axis coordinate and to boundaries obtained from Human Brain Atlas (Hawrylycz et al., 2012), our results could be selectively located on the surfaces of the accumbens nuclei core or shell. Specifically, we found positive bilateral correlations in clusters on the accumbens core sub-field (statistical peaks: corrected pR-AccuCore = 0.016; corrected pL-Accu-Core = 0.031) indicating that the longer lithium treatment duration, the more outward is the bending of the cluster on the core (i.e. core extroflection). Moreover, we found a negative correlation between shape and lithium treatment duration in a cluster on the boundary of the R-Accu shell sub-field (statistical peak: corrected pR-Accu-Shell = 0.002), indicating that lithium treatment duration is associated with inward bending in this sub-region (i.e. shell introflection) (Fig. 1).
Fig. 1.
Relationship between patients’ lithium treatment duration and nucleus accumbens shape morphometry. Linear regression results using lithium treatment duration (months) and accumbens nuclei shape deformations (distance from mean template, mm3) as variables of interest are shown. (a) right accumbens shell; (b) right accumbens core and (c) left accumbens core; (d) Statistical maps for significant (TFCE-corrected) right/left accumbens core inward and right accumbens shell outward deformations (in red) superimposed onto representative nucleus accumbens masks (light blue). Borders of shell and core nuclei are drawn using solid black lines and taken from the Human Brain Atlas. Image credit: Allen Institute. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
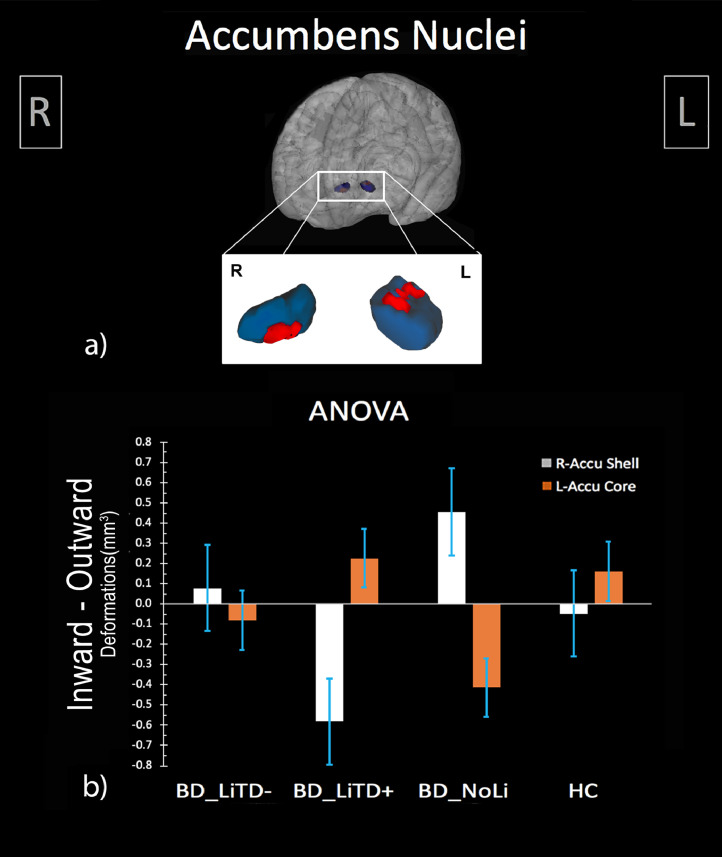
When splitting the BD group in terms of LiTD (LiTD+, LiTD-, NoLi and HC), ANOVA performed on R-Accu and L-Accu shape showed significant bilateral results (statistical peaks: corrected pL-Accu-ANOVA = 0.025; corrected pR-Accu-ANOVA = 0.025) (Fig. 2). Post-hoc t-tests revealed R-Accu shell outward deformation in the NoLi group compared to LiTD+ (statistical peak: corrected pR-Accu-Shell = 0.016) and HC (statistical peak: corrected pR-Accu-Shell = 0.002). Moreover, the LiTD- group showed L-Accu core inward deformation compared to HC (statistical peak: corrected pL-Accu-Core = 0.027). Details on results and clusters coordinates are shown in Table 4.
Fig. 2.
Comparison between healthy subjects and patients with bipolar disorder stratified according to lithium treatment duration. (a) 3D visualization of ANOVA results: right accumbens shell outward and left accumbens core inward deformations (in red) superimposed onto a representative nucleus accumbens mask (in blue); (b) Mean total deformation (distance from mean template, mm3) of right accumbens shell (light grey) and left accumbens core (orange) in patients with lithium treatment duration longer/shorter then 50% of illness duration (respectively, BD_LiTD+, BD_LiTD-), patients never treated with lithium (BD_NoLi) and healthy subjects (HC) groups. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 4.
Neuroimaging Results. Topography and statistical details of significant relationships between BD LiTD and boundary deformation on the left/right accumbens nuclei shape morphometric features. Effects have been assessed through linear regression analyses and ANOVA comparisons between BD groups stratified according to LiTD (i.e. BD_LiTD-, BD_LiTD+, BD_NoLi) and HC and related post-hoc analyses. Clinical correlates (depressive/hopefulness BD symptoms scores) of L-Accu core inward deformation are also reported.
| Anatomical region | Results | Cluster Extent (mm3) | R2/F/t | Statistical peak p (TFCE-corrected) | MNI Coordinates of the statistical peak | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Regression Analyses | |||||||
| Left Nucleus Accumbens core | Positive Correlation | 68 | 0.1 | 0.031* | −15 | 19 | −6 |
| Right Nucleus Accumbens core | Positive Correlation | 56 | 0.12 | 0.016* | 10 | 14 | −4 |
| Right Nucleus Accumbens shell | Negative Correlation | 136 | 0.17 | 0.002* | 8 | 13 | −10 |
| ANOVA | |||||||
| Left Nucleus Accumbens core | 47 | F(3;144) = 4.6 | 0.01* | −11 | 16 | −4 | |
| Right Nucleus Accumbens shell | 55 | F(3;144) = 3.5 | 0.025* | 8 | 13 | −9 | |
| Post-Hoc | |||||||
| Left Nucleus Accumbens core | BP_LiTD- < HC | 17 | 2.72 | 0.027* | −10 | 16 | −9 |
| Left Nucleus Accumbens core | BP_NoLi < HC | 47 | 3.5 | 0.002* | −10 | 16 | −9 |
| Left Nucleus Accumbens core | BP_NoLi < BP_LiTD+ | 47 | 2.32 | 0.016* | −10 | 9 | −6 |
| Right Nucleus Accumbens shell | BP_NoLi > BP_LiTD+ | 55 | 3.11 | 0.005* | 8 | 14 | −10 |
| Right Nucleus Accumbens shell | BP_NoLi > HC | 55 | 2.58 | 0.005* | 10 | 18 | −9 |
| Clinical Correlates of Left Nucleus Accumbens core | |||||||
| HAM-Dsom | Positive Correlation | 43 | 0.1 | 0.005* | −12 | 10 | −6 |
| HAM-Dtot | Positive Correlation | 41 | 0.07 | 0.02* | −12 | 9 | −6 |
| BHStot | Positive Correlation | 18 | 0.05 | 0.03* | −9 | 16 | −3 |
TFCE, Threshold-Free Cluster Enhancement; Coordinates are in Montreal Neurological Institute (MNI) Space. BD_LiTD-, bipolar disorder patients with lithium duration treatment shorter then 50% of illness duration; BD_LiTD+, bipolar disorder patients with lithium duration treatment longer then 50% of illness duration; BD_NoLi, bipolar disorder patients never treated with lithium; HC, healthy control subjects. HAM-Dsom, somatic subscale from Hamilton Depression Rating Scale; HAM-Dtot, total score from Hamilton Depression Rating Scale; BHStot, total score from Beck Hopefulness Scale.
Statistically significant differences at p < 0.05.
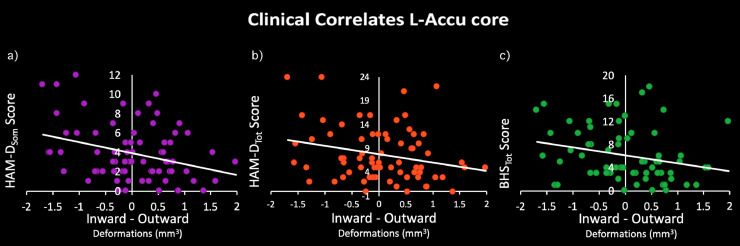
The shape of BD L-Accu-Core was positively correlated with HAM-Dtot (statistical peaks: corrected pL-Accu-core = 0.02), HAM-Dsom (statistical peaks: corrected pL-Accu-core = 0.005) and BHStot (statistical peaks: corrected pL-Accu-core = 0.03) scores (Table 4, Fig. 3), indicating the more inward deformation the higher depression symptom severity. No significant associations were found for YMRStot scores.
Fig. 3.
Clinical correlates of left accumbens inward deformation. Relationship between deformations resulted from ROI ANOVA and patients depressive/hopelessness symptom scores, assessed through (a) Somatic subscale of Hamilton Depression Rating Scale (HAMDsom); (b) Total score of Hamilton Depression Rating Scale (HAM-Dtot); (c) Total score of Beck Hopelessness Scale (BHStot).
4. Discussion
Despite a substantial amount of research over the past decades has been focused on neurotrophic and neuroprotective effects of lithium, this is the first report describing an association between lithium treatment duration and Accu shape in patients diagnosed with BD. Here we show that patients without or even with short-term personal history of lithium treatment have outward R-Accu shell and inward L-Accu core surface changes, when compared to both healthy individuals and patients under long-term lithium treatment. Moreover, in patients we showed that the increased duration of lithium treatment was associated with both an extroflection effect on the core of bilateral Accu and a concomitant introflection effect on the shell of R-Accu. Both these effects showed that the longer duration of treatment the strongest shape modulations, bringing patient whit longer treatment to have an Accu morphology similar to that of healthy controls. We also found that inward deformation of L-Accu core shape is significantly correlated with increased depression severity.
A small number of previous studies (Hwang et al., 2006; Ong et al., 2012; Mamah et al., 2016; Womer et al., 2014; Sun et al., 2017) investigated the shape of brain regions in patients with BD and half of them were restricted to the caudate (Hwang et al., 2006; Ong et al., 2012) and putamen nuclei alone (Ong et al., 2012) or did not consider DGM structures at all (Sun et al., 2017). Among the remaining studies, findings led to inconsistent results (Mamah et al., 2016; Womer et al., 2014) especially for the nucleus accumbens. Indeed, Mamah et al. (2016) found a dorsal contraction of the right nucleus accumbens that partially overlaps with our results, while Womer et al. (2014) did not. Moreover, no studies to date considered lithium treatment as a potential mediator of brain shape despite a modulatory effect of lithium treatment was clearly described on other morphometric brain measures, such as volume (Hibar et al., 2016; Simonetti et al., 2016; Sani et al., 2018) and cortical thickness (Giakoumatos et al., 2015). Indeed, lithium treatment was associated with volume preservation of deep grey matter assemblies (e.g. hippocampus, amygdala, thalamus and basal ganglia) and cortical thickening in frontal, parietal and occipital regions as well as in the precuneus and precentral areas (Abramovic et al., 2016). This was also confirmed by post-mortem studies demonstrating lower numbers of neurons among individuals with BD who are lithium-treatment naïve (Benes et al., 1998; Bowley et al., 2002).
The Accu has dopamine as a principal neurotransmitter and animal studies described that lithium modulates neuronal activity and dopamine neurotransmission in models of BD. In particular, Clock-Δ19 mice have higher dopamine levels in the Accu area (Coque et al., 2011) and display behaviours that are very similar to those found in human BD (i.e. increased exploratory drive in novel environments, significant reduction in sleep, decreased anxiety related behaviours, and increase in cocaine preference). Chronic administration of lithium decreases Clock-Δ19 mice dopamine tissue levels in the Accu and reverses many of the phenotypes of Clock-Δ19 mice. Consistently with our morphological results, it has been demonstrated that dopamine neurotransmission modulates different responses of core and shell Accu neurons and depletion of dopamine release from the ascending pathway differently affect the morphology of medium spiny neurons of the two Accu districts (Meredith et al., 1995). Specifically, in animal models dopamine depletion is associated with a significant reduction of the dendritic length of neurons in the core but not in the shell, which show a more tortuous morphology instead. It could be the case that an initial pathogenetic condition in BD lithium naïve patients is characterized by increased level of tonic dopamine, inducing oxidative neuronal loss with a concomitant reduction of dopaminergic connections. Then, dopamine deflection consequent to oxidative neuronal loss can lead to shorter dendrites in the core resulting in an inward surface deformation. Since the same input (dopamine deflection) causes an opposite effect on the neuronal dendrites of the shell, here the pathological oxidative process could lead to a more tortuous internal organization, ending in an outward surface deformation.
A number of mechanisms have been recognized as associated to the neuroprotective/neurotrophic effects of lithium that could be useful in interpreting our results. The inhibition of a protein kinase having a pro-apoptotic action (i.e. glycogen synthase kinase-3, GSK-3), the induction of intracellular molecular survival pathway, and the stimulation of GABAergic neurotransmission, are those having the most direct evidence (Malhi et al., 2013). By stimulating inhibitory GABAergic neurotransmission while reducing the excitatory dopaminergic one (Malhi et al., 2013; Chiu and Chuang, 2010), lithium reduces the neuronal loss due to oxidative stress and promotes neuronal plasticity (Gray and Mcewen, 2013). Some of these mechanisms, or probably all these coupled together, could be responsible for grey matter shape remodelling, even if the precise underling biochemical mechanism of this drugs remain poorly defined. Based on the hypothesis suggested by Van Essen (1997), the shapes of specific brain structures may be determined by the physical properties of neural tissue combined to the patterns of neural connectivity and biochemical mechanism mediating the neural signalling cascades. Being a dynamic process, it drives normal conditions, neurodegenerative and neurorestorative processes. In this context, lithium treatment could act as a trigger that elicits an extensive reprogramming of BD pathological baseline condition, finally resulting in shape remodelling.
The Accu is connected to the limbic and extrapyramidal motor systems. Recent evidence (Li et al., 2018) suggests that Accu D1 and D2 dopamine receptors in Accu core and shell receive similar inputs from diverse sources. Inputs to the core are broadly scattered whereas inputs to the shell are relatively concentrated around DGM. Specifically, the anterior cortex preferentially innervates the core for both D1 and D2, whereas the lateral hypothalamic area preferentially targets D1 in the shell. Acting as a limbic-motor interface, the Accu regulates motivation and emotional processes and is involved in a number of the most disabling neuropsychiatric disorders (Mavridis et al., 2011; De Rossi et al., 2016; Nestler and Carlezon, 2006; Francis and Lobo, 2017). Moreover, it is part of a circuit including the hippocampus, the amygdala, the prefrontal cortex and other regions mediating emotional memory and anxiety (Francis and Lobo, 2017). As such, the Accu has a central role in the cerebral reward system. It integrates excitatory inputs from these regions triggering emotionally motivated behaviours through cortico-striatal-thalamic loops within the basal ganglia, eventually driving the dorsolateral striatum to facilitate action related behaviours (Floresco, 2015). These local and global Accu circuit interactions mediate motivated and emotional behaviour, making them an attractive focus to study affective disorders such as BD and depression. Evidence for reward and dopamine neural systems alterations in mood disorders, like mania, major depression and BD, have been widely reported both in humans and animal models (Francis and Lobo, 2017; Abler et al., 2008, Abramson et al., 1989; Satterthwaite et al., 2015; Naranjo et al., 2001; Berk et al., 2007). Furthermore, it has been proposed a “dopamine dysregulation syndrome” model for the BD pathophysiology (Berk et al., 2007), where a cyclical dysregulation in quantitative dopaminergic transmission is the main construct. Despite our work does not directly asses dopamine transmission, it supports this model showing a complex pattern of lithium-dependant surface modulatory effects on such central node for dopamine and rewarding systems.
The second important result of the present study is the association between L-Accu core area inward deformation and both hopelessness and depressive symptom severity. Recently, a more accurate way for characterizing Accu functions has been proposed overcoming the pedantic view that it merely acts as a reward centre relay (Floresco, 2015). Specifically, the Accu is involved in the modulation of cerebral processing of motivationally relevant goals by promoting likelihood, efficiency, and vigour of behaviours intended to obtain those goals, driving exploration of novel stimuli and procurement of things worth having (rewards) or the avoidance of aversive consequences. Moreover, the core and shell areas modulate different functions in this motivational process: the core has a more prominent role in instigating approach behaviour toward motivationally relevant stimuli (“go to it” function) while the shell drives behaviour to stay on tasks until the rich of a goal (“stay to it” function), suppressing irrelevant or non-rewarded actions.
Our results of increased L-Accu core inward deformation associated with higher depressive symptom severity support the hypothesis of a decreasing “go to it” brain signalling as pathophysiological correlate of depressive phenomenology in BD. A recent theory was proposed to integrate the hopelessness theory of depression (Abramson et al., 1989) and Davidson's (1994) approach/withdrawal motivational theory of depression, based on the evidence that individual with high vulnerability to cognitive distress resulted in higher hopelessness and depression scores and fewer motivated goal-directed behaviour (Haeffel et al., 2008). Amotivational/anhedonic features are crucial components of depression and, interestingly, the left lateralized finding of Accu core deformation in BD may be explained by the crucial role of the dominant hemisphere in motivational behaviours (Pizzagalli et al., 2005). Studies conducted over several decades support the use of long-term lithium treatment to prevent depression relapse in patients with bipolar disorder (Geddes et al., 2004). In this context, our findings may reflect one of the neurobiological mechanisms underpinning the therapeutic effect of lithium, as part of its efficacy on preventing depression that may unfold through a long-term remodelling of cortical-subcortical connections related to the motivational/hedonic dimension.
5. Limitations
Several issues have to be considered before concluding. First, the sample size of LiTD+ group is relatively small. However, it is adequate to detect shape differences because of the moderate to large effect size we observed. Second, our results come from a cross-sectional study design and should be replicated also with a longitudinal approach for definitively demonstrating a causal effect between lithium exposure and Accu neuroprotection. Third, our patients were treated also with different psychotropic drugs that might potentially affect Accu shape. However, it could be noted that BD subgroups showed no differences in terms of non-lithium pharmacological drugs, making unlikely the probability that they may have influenced our results.
Finally, the criterium adopted here for categorising BD patients according to the 50% ratio between illness and treatment duration could be considered as arbitrary. However, as posited by the staging model of BD (Vieta et al., 2011), the impact of pharmacological treatment should be considered relatively to illness duration rather than in its absolute value, assuming the construct that the earlier intervention the better efficacy. From the other side, it should be thought that the longer time of brain exposure to pathological conditions the stronger intervention is needed to counteract the illness. Therefore, we chose the 50% threshold since it distinguishes patients having more exposure to potential neuroprotective effects (driven by lithium treatment) than to neuropathological processes (driven by the illness) from patients showing the opposite pattern. In practical terms, the 50% ratio represent the “zero” point as reference to the balance between neuropathological and neuroprotective effects.
6. Conclusions
Lithium treatment affects Accu surface morphology in BD, with different impacts on core and shell. Differences between BD and HC on Accu shape morphometry are significant in patients never treated with lithium but they progressively decrease and disappear in patients under long-term treatment. Lithium treatment dependent surface deformations of L-Accu core showed significant correlations with the severity of depressive phenomenology, being the less inward deformation associated to the lower depression symptoms, especially in terms of somatic and hopefulness components. Taken together our results suggest that the duration of lithium treatment is associated with the modulation of Accu shape as part of the mechanism of action possibly linked with its mood stabilizing effects.
CRediT authorship contribution statement
Daniela Vecchio: Conceptualization, Validation, Writing - original draft. Fabrizio Piras: Conceptualization, Validation, Writing - original draft. Federica Piras: Conceptualization, Validation. Nerisa Banaj: Conceptualization, Validation. Delfina Janiri: Conceptualization, Validation. Alessio Simonetti: Conceptualization, Validation. Gabriele Sani: Conceptualization, Validation, Supervision. Gianfranco Spalletta: Conceptualization, Validation, Supervision, Writing - review & editing, Funding acquisition, Resources.
Declarations of Competing Interest
None.
Acknowledgement
This study was partially funded by the Italian Ministry of Health, Grant/Award Number: RC‐12‐13 ‐14 ‐15 ‐16 ‐17‐18/A. Progetto finanziato grazie ai fondi 5xmille Ministero Salute Anno 2017 (the study was partly funded by the 5xmille grant of the Italian Ministry of Health, year 2017).
Contributor Information
Fabrizio Piras, Email: f.piras@hsantalucia.it.
Gianfranco Spalletta, Email: g.spalletta@hsantalucia.it.
References
- Abler B., Greenhouse I., Ongur D., Walter H., Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33(9):2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovic L., Boks M.P.M., Vreeker A. The association of antipsychotic medication and lithium with brain measures in patients with bipolar disorder. Eur. Neuropsychopharmacol. 2016;26(11):1741–1751. doi: 10.1016/j.euroneuro.2016.09.371. [DOI] [PubMed] [Google Scholar]
- Abramson L.Y., Metalsky G.I., Alloy L.B. Hopelessness depression: a theory-based subtype of depression. Psychol. Rev. 1989 [Google Scholar]
- American Psychiatric Association . 2000. Diagnostic and statistical manual of mental disorders 4th revised edition. DSM-IV-TR. [Google Scholar]
- Baliki M.N., Mansour A., Baria A.T. Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain. J. Neurosci. 2013;33(41):16383–16393. doi: 10.1523/JNEUROSCI.1731-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T. Beck hopelessness scale. J. Clin. Psychol. 1998;54:1063–1078. [Google Scholar]
- Benes F.M., Kwok E.W., Vincent S.L., Todtenkopf M.S. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol. Psychiatry. 1998;44(2):88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Berk M., Dodd S., Kauer-Sant’Anna M. Dopamine dysregulation syndrome: implications for a dopamine hypothesis of bipolar disorder. Acta Psychiatr. Scand. 2007;116(SUPPL. 434):41–49. doi: 10.1111/j.1600-0447.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- Bowley M.P., Drevets W.C., Öngür D., Price J.L. Low glial numbers in the amygdala in major depressive disorder. Biol. Psychiatry. 2002;52(5):404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- Chiu C.-.T., Chuang D.-.M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol. Ther. 2010;128:281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coque L., Mukherjee S., Cao J.L. Specific role of vta dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the clockδ19 mouse model of mania. Neuropsychopharmacology. 2011;36(7):1478–1488. doi: 10.1038/npp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J.Asymmetric brain function, affective style and psychopathology: the role of early experience and plasticity. 1994;(6):741–758.
- De Rossi P., Dacquino C., Piras F., Caltagirone C., Spalletta G. Left nucleus accumbens atrophy in deficit schizophrenia: a preliminary study. Psychiatry Res. 2016;254:48–55. doi: 10.1016/j.pscychresns.2016.06.004. [DOI] [PubMed] [Google Scholar]
- De-Paula V.J., Gattaz W.F., Forlenza O V. Long-term lithium treatment increases intracellular and extracellular brain-derived neurotrophic factor (BDNF) in cortical and hippocampal neurons at subtherapeutic concentrations. Bipolar Disord. 2016;18(8):692–695. doi: 10.1111/bdi.12449. [DOI] [PubMed] [Google Scholar]
- First M., Gibbon M., Spitzer R. Am Psychiatr Press Inc; 1997. Structured Clinical Interview For DSM-IV Axis II Personality Disorders (SCID-II) [Google Scholar]
- First M.B., Gibbon M., Spitzer R.L., Williams J.B., Benjamin L. American Psychiatric Press, Inc.; Washington DC: 1997. Structured Clinical Interview For DSM-IV Axis II Personality Disorders, (SCID-II) [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Patient Edition (SCID-I/P, 11/2002 Revision) [Google Scholar]
- Floresco S.B. The nucleus accumbens: an interface between cognition, emotion, and action. Annu. Rev. Psychol. 2015;66(1):25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. Mini-mental state. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Francis T.C., Lobo M.K. Emerging role for nucleus accumbens medium spiny neuron subtypes in depression. Biol. Psychiatry. 2017;81(8):645–653. doi: 10.1016/j.biopsych.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geddes J.R., Burgess S., Hawton K., Jamison K., Goodwin G.M. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am. J. Psychiatry. 2004;161(2):217–222. doi: 10.1176/appi.ajp.161.2.217. [DOI] [PubMed] [Google Scholar]
- Giakoumatos C.I., Nanda P., Mathew I.T. Effects of lithium on cortical thickness and hippocampal subfield volumes in psychotic bipolar disorder. J. Psychiatr. Res. 2015;61:180–187. doi: 10.1016/j.jpsychires.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.D., Mcewen B.S. Lithium's role in neural plasticity and its implications for mood disorders. Acta Psychiatr. Scand. 2013;128(5):347–361. doi: 10.1111/acps.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeffel G.J., Abramson L.Y., Brazy P.C., Shah J.Y. Hopelessness theory and the approach system: cognitive vulnerability predicts decreases in goal-directed behavior. Cognit. Ther. Res. 2008;32(2):281–290. [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylycz M.J., Lein E.S., Guillozet-Bongaarts A.L. An anatomically comprehensive atlas of the adult human brain transcriptome. Nature. 2012 doi: 10.1038/nature11405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero M.T., Barcia C., Navarro J.M. Functional anatomy of thalamus and basal ganglia. Child's Nerv. Syst. 2002;18(8):386–404. doi: 10.1007/s00381-002-0604-1. [DOI] [PubMed] [Google Scholar]
- Hibar D.P., Westlye L.T., van Erp T.G.M. Subcortical volumetric abnormalities in bipolar disorder. Mol. Psychiatry. 2016:1–7. doi: 10.1038/mp.2015.227. (October 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., In K.L., Dager S.R. Basal ganglia shape alterations in bipolar disorder. Am. J. Psychiatry. 2006;163(2):276–285. doi: 10.1176/appi.ajp.163.2.276. [DOI] [PubMed] [Google Scholar]
- Iorio M., Spalletta G., Chiapponi C. White matter hyperintensities segmentation: a new semi-automated method. Front. Aging Neurosci. 2013;5(DEC) doi: 10.3389/fnagi.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Chen Z., Fan G., Li A., Yuan J., Xu T. Cell-Type-Specific afferent innervation of the nucleus accumbens core and shell. Front. Neuroanat. 2018;12 doi: 10.3389/fnana.2018.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi G.S., Tanious M., Das P., Coulston C.M., Berk M. Potential mechanisms of action of lithium in bipolar disorder: current understanding. CNS Drugs. 2013;27(2):135–153. doi: 10.1007/s40263-013-0039-0. [DOI] [PubMed] [Google Scholar]
- Mamah D., Alpert K.I., Barch D.M., Csernansky J.G., Wang L. Subcortical neuromorphometry in schizophrenia spectrum and bipolar disorders. NeuroImage Clin. 2016;11:276–286. doi: 10.1016/j.nicl.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D., Barch D.M., Csernansky J.G. Handbook Neuropsychiatr Biomarkers, Endophenotypes Genes. 2009. Neuromorphometric measures as endophenotypes of schizophrenia spectrum disorders; pp. 87–122. [Google Scholar]
- Manji H.K., Moore G.J., Chen G. Clinical and preclinical evidence for the neurotrophic effects of mood stabilizers: implications for the pathophysiology and treatment of manic-depressive illness. Biol. Psychiatry. 2000;48(8):740–754. doi: 10.1016/s0006-3223(00)00979-3. [DOI] [PubMed] [Google Scholar]
- Mavridis I., Boviatsis E., Anagnostopoulou S. The human nucleus accumbens suffers parkinsonism-related shrinkage: a novel finding. Surg. Radiol. Anat. 2011;33(7):595–599. doi: 10.1007/s00276-011-0802-1. [DOI] [PubMed] [Google Scholar]
- McDonald C., Zanelli J., Rabe-Hesketh S. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol. Psychiatry. 2004;56(6):411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Measso G., Cavarzeran F., Zappalà G., Lebowitz B.D., Crook T.H., Pirozzolo F.J., ..., Grigoletto F. The mini‐mental state examination: normative study of an Italian random sample. Dev. Neuropsychol. 1993;9(2):77–85. [Google Scholar]
- Meredith G.E., Ypma P., Zahm D.S. Effects of dopamine depletion on the morphology of medium spiny neurons in the shell and core of the rat nucleus accumbens. J. Neurosci. 1995;15(5):3808–3820. doi: 10.1523/JNEUROSCI.15-05-03808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo C.A., Tremblay L.K., Busto U.E. The role of the brain reward system in depression. Prog Neuro-Psychopharmacol. Biol. Psychiatry. 2001;25(4):781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Nestler E.J., Carlezon W.A. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong D., Walterfang M., Malhi G.S., Styner M., Velakoulis D., Pantelis C. Size and shape of the caudate nucleus in individuals with bipolar affective disorder. Aust. N. Z. J. Psychiatry. 2012;46(4):340–351. doi: 10.1177/0004867412440191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B., Smith S.M., Kennedy D.N., Jenkinson M. A bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A., Sherwood R.J., Henriques J.B., Davidson R.J. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol. Sci. 2005;16(10):805–813. doi: 10.1111/j.1467-9280.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- Sani G., Simonetti A., Janiri D. Association between duration of lithium exposure and hippocampus/amygdala volumes in type i bipolar disorder. J. Affect. Disord. 2018:232. doi: 10.1016/j.jad.2018.02.042. [DOI] [PubMed] [Google Scholar]
- Satterthwaite T.D., Kable J.W., Vandekar L. Common and dissociable dysfunction of the reward system in bipolar and unipolar depression. Neuropsychopharmacology. 2015;40(9):2258–2268. doi: 10.1038/npp.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaltiel G., Chen G., Manji H.K. Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr. Opin. Pharmacol. 2007;7(1):22–26. doi: 10.1016/j.coph.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Simonetti A., Sani G., Dacquino C. Hippocampal subfield volumes in short- and long-term lithium-treated patients with bipolar i disorder. Bipolar Disord. 2016;18(4):352–362. doi: 10.1111/bdi.12394. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Sun Z.Y., Houenou J., Duclap D. Shape analysis of the cingulum, uncinate and arcuate fasciculi in patients with bipolar disorder. J. Psychiatry Neurosci. 2017;42(1):27–36. doi: 10.1503/jpn.150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C. A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature. 1997;385(6614):313–318. doi: 10.1038/385313a0. [DOI] [PubMed] [Google Scholar]
- Van Gestel H., Franke K., Petite J. Brain age in bipolar disorders: effects of lithium treatment. Aust. N. Z. J. Psychiatry. 2019;0(0) doi: 10.1177/0004867419857814. [DOI] [PubMed] [Google Scholar]
- Vieta E., Reinares M., Rosa A.R. Staging bipolar disorder. Neurotox. Res. 2011 doi: 10.1007/s12640-010-9197-8. [DOI] [PubMed] [Google Scholar]
- Womer F.Y., Wang L., Alpert K.I., Smith M.J., Csernansky J.G., Barch D.M. Basal ganglia and thalamic morphology in schizophrenia and bipolar disorder. Psychiatry Res. 2014;223:75–83. doi: 10.1016/j.pscychresns.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133(11):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]