Highlights
-
•
Progressive resistance exercise leads to long-term cognitive benefits in MCI.
-
•
Resistance exercise slows post-training CA1, subiculum and dentate atrophy.
-
•
Within-training preservation of PCC predicts long-term hippocampal preservation.
-
•
Neuroprotection of AD-vulnerable hippocampal subfields mediate long-term cognition.
-
•
Long-term neuroprotection is not mediated by post-training fitness adaptations.
Keywords: Resistance exercise, Mild cognitive impairment, Plasticity, Hippocampus, Subfields, Randomised controlled trial
Abstract
Dementia affects 47 million individuals worldwide, and assuming the status quo is projected to rise to 150 million by 2050. Prevention of age-related cognitive impairment in older persons with lifestyle interventions continues to garner evidence but whether this can combat underlying neurodegeneration is unknown. The Study of Mental Activity and Resistance Training (SMART) trial has previously reported within-training findings; the aim of this study was to investigate the long-term neurostructural and cognitive impact of resistance exercise in Mild Cognitive Impairment (MCI). For the first time we show that hippocampal subareas particularly susceptible to volume loss in Alzheimer's disease (AD) are protected by resistance exercise for up to one year after training.
One hundred MCI participants were randomised to one of four training groups: (1) Combined high intensity progressive resistance and computerised cognitive training (PRT+CCT), (2) PRT+Sham CCT, (3) CCT+Sham PRT, (4) Sham physical+sham cognitive training (SHAM+SHAM). Physical, neuropsychological and MRI assessments were carried out at baseline, 6 months (directly after training) and 18 months from baseline (12 months after intervention cessation). Here we report neuro-structural and functional changes over the 18-month trial period and the association with global cognitive and executive function measures.
PRT but not CCT or PRT+CCT led to global long-term cognitive improvements above SHAM intervention at 18-month follow-up. Furthermore, hippocampal subfields susceptible to atrophy in AD were protected by PRT revealing an elimination of long-term atrophy in the left subiculum, and attenuation of atrophy in left CA1 and dentate gyrus when compared to SHAM+SHAM (p = 0.023, p = 0.020 and p = 0.027). These neuroprotective effects mediated a significant portion of long-term cognitive benefits. By contrast, within-training posterior cingulate plasticity decayed after training cessation and was unrelated to long term cognitive benefits. Neither general physical activity levels nor fitness change over the 18-month period mediated hippocampal trajectory, demonstrating that enduring hippocampal subfield plasticity is not a simple reflection of post-training changes in fitness or physical activity participation. Notably, resting-state fMRI analysis revealed that both the hippocampus and posterior cingulate participate in a functional network that continued to be upregulated following intervention cessation.
Multiple structural mechanisms may contribute to the long-term global cognitive benefit of resistance exercise, developing along different time courses but functionally linked. For the first time we show that 6 months of high intensity resistance exercise is capable of not only promoting better cognition in those with MCI, but also protecting AD-vulnerable hippocampal subfields from degeneration for at least 12 months post-intervention. These findings emphasise the therapeutic potential of resistance exercise; however, future work will need to establish just how long-lived these outcomes are and whether they are sufficient to delay dementia.
1. Introduction
Dementia affects 47 million individuals worldwide and assuming the status quo is projected to rise to 150 million by 2050 (Alzheimer's Disease International, 2016). However, there are good reasons to predict otherwise, as 30–40% of dementia is estimated to be attributable to modifiable risk factors such as physical and cognitive inactivity (Livingston et al., 2017; Norton et al., 2014). Consequently, there is now intense international interest in large-scale clinical trials targeted for the purpose of slowing cognitive-functional decline and potentially delaying dementia onset (Hill et al., 2017; Ngandu et al., 2015; Richard et al., 2009; Scarmeas, 2017; Vellas et al., 2014; Yassine and Schneider, 2017). In this regard, delay is equivalent to population-level prevention because of death from other causes. Modelling suggests that an intervention that delays onset by just one year, implemented in 2010, could avert 1.3 million dementia cases in the US by 2050 (Zissimopoulos et al., 2014).
Key to such prevention strategies is when to begin, how long to intervene, and to understand if it is possible to modify the course of underlying neurodegeneration. Cognitive symptoms emerge many years before dementia diagnosis, and there is generally a transition through the preclinical state, Mild Cognitive Impairment (MCI). MCI increases the risk of progression to dementia by five to ten-fold (Petersen et al., 1999), with post mortem examinations indicating substantial neuronal loss in the hippocampus (Scheff et al., 2006). In fact, MRI studies have shown that hippocampal atrophy can precede cognitive impairment by several years (Jack et al., 2000) and correlates with the degree of cognitive dysfunction upon MCI diagnosis (Jack et al., 2000; Morra et al., 2009). Both imaging and histological studies suggest that hippocampal atrophy in MCI is not homogeneous, with the most severe volume loss being in CA1 and subiculum subfields, a pattern associated with progression from MCI to Alzheimer's dementia (AD) (Apostolova et al., 2006, 2010; Costafreda et al., 2011; Rossler et al., 2002; West et al., 1994). Yet no intervention, behavioural or pharmacologic, has been found to influence the trajectory of AD-vulnerable subfield degeneration.
Aerobic exercise has been suggested as a possible promoter of hippocampal plasticity, but trials in healthy older adults have produced mixed results. For example, ten Brinke et al., 2015 found that aerobic training significantly increased hippocampal volume in older women with probable MCI (ten Brinke et al., 2015). Furthermore, Erickson et al., 2011 found that one year of moderate-intensity walking not only reversed whole hippocampal atrophy in cognitively healthy older adults but triggered volumetric expansion; conversely, memory and cognitive outcomes were not differentially impacted compared to a control group engaged in progressive multi-modal exercise sessions which included moderate intensity resistance training, yoga, balance and stretching exercises (Erickson et al., 2011), reflecting conflicting clinical trial outcomes more generally (Baker et al., 2010; Gates et al., 2013; Wang et al., 2014; Young et al., 2015). Other studies have failed to replicate a positive trophic effect and rather shown attenuated atrophy over the duration of the aerobic intervention (i.e., within-training effect) (Smith et al., 2010). Whether any physical exercise can modify degenerative trajectories in MCI at either the whole-hippocampal level or within AD-vulnerable subfields is unknown (Firth et al., 2018).
Previously, in the Study of Mental Activity and Resistance Training (SMART) Trial (Gates et al., 2011), we found that 6 months of high intensity progressive resistance training (PRT) in MCI was efficacious for the trial's primary outcome, general cognition (Fiatarone Singh et al., 2014; Mavros et al., 2017), and furthermore, this was therapeutically-mediated by structural plasticity in the posterior cingulate (PC) (Suo et al., 2016). We examined PRT because it is feasible in frail and sarcopenic older individuals, is particularly effective for general health outcomes such as cardiovascular disease, mobility impairment/falls, osteoporosis, and depression (Cooney et al., 2014; Irvine and Taylor, 2009; Peterson and Gordon, 2011), and continues to be under-investigated in relation to cognition (Liu-Ambrose and Donaldson, 2009). Within-training effects on the PC are promising because it is another target of degeneration and dysfunction in early AD (Choo et al., 2010), changes that coincide with hippocampal and entorhinal cortical degeneration (Huang et al., 2002).
Given that research on neuroplasticity has so far been limited to within-training effects, investigating what happens to the brain after training cessation is of particular importance. The SMART Trial cohort is unique in this regard because we continue to see positive cognitive effects up to 12 months post-training (Fiatarone Singh et al., 2014). Understanding the mechanistic basis for this was therefore our primary aim. Here, we report for the first time the long-term impact of PRT on hippocampal subfields and PC structural and functional trajectories. Finally, we model aerobic capacity, muscle strength and exercise participation after the cessation of formal training, to determine any physical activity (PA) behaviour or physical fitness mediation of neuroplasticity.
2. Materials and methods
The SMART trial was approved by the Human Research Ethics Committee of the University of Sydney. Australian and New Zealand Clinical Trials Registry Protocol No: X08-0064. Registry No: ACTRN12608000489392.
2.1. SMART trial design
Community-dwelling, non-depressed, non-demented participants aged ≥55, diagnosed with MCI (Petersen original criteria) (Petersen et al., 1999), with a Mini-mental State Examination score of 24–28, and a Clinical Dementia Rating Score ≤0.5, were recruited. Participants were randomised into one of four training groups in which they completed 90-min supervised, centre-based training, two or three times a week, for 26 weeks. (1) Combined high intensity progressive resistance and computerized cognitive training (PRT+CCT), (2) PRT+Sham computerized cognitive training, (3) CCT+Sham stretching and toning training and finally, (4) Sham physical+sham cognitive training (SHAM+SHAM). Physical, metabolic, and a battery of neuropsychological tests as well as MRI assessments were carried out at baseline, 6 months and 18 months from baseline (Fig. 1). In addition, genotyping for the apolipoprotein E polymorphism ε4 allele (APOE-e4) was carried out at baseline. Full details describing the SMART trial have been previously reported (Gates et al., 2011).
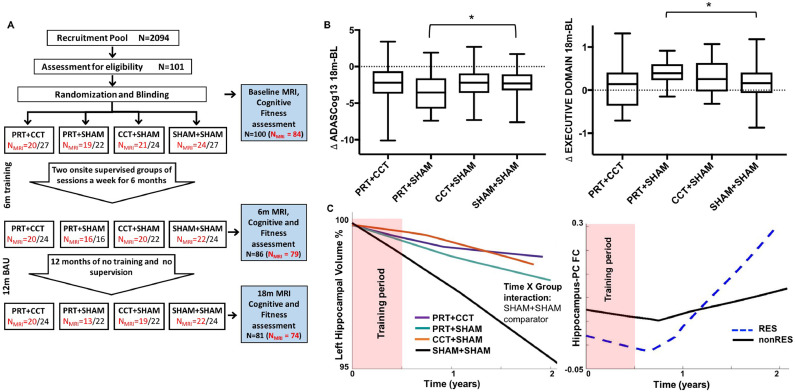
Fig. 1.
SMART Trial design and statistical models. (A) Randomization was into one of four training groups – combined progressive resistance training and computerised cognitive training (PRT+CCT), PRT and cognitive sham (PRT+SHAM), CCT and sham exercise (CCT+SHAM), sham exercise and cognitive sham, (SHAM+SHAM). MRI, cognitive and fitness assessments were carried out at baseline, directly after 6 months of training (6M) and at 18 months (18M), following a one-year usual care (UC) period with weekly telephone contact by research staff to administer health/adverse event checks in all participants. In addition, cognitive, physical, social, recreational, volunteer and religious activity participation was recorded daily for the entire 18 months in a logbook by each participant. (B) Raw change in the trial's primary outcome (ADASCog error scores) over the complete 18-month period, as well as neuropsychologically-defined Executive domain scores were compared between SHAM+SHAM and the three training groups. Box-and-Whisker plots show the median (horizontal line), interquartile range (box) and upper and lower quartiles (whiskers) of change in outcome scores for each training group. Dotted line indicates no change in outcome over the 18-month period. LME analysis found significantly (*Time x Group p < 0.05 superimposed on the Box-and-Whisker plots) improved cognition in the PRT+SHAM group compared to SHAM+SHAM on both outcomes (model including covariates age, sex and education, as well as group factor and time as continuous variable). (C) Basic Linear Mixed Effects (LME) model including baseline covariates (age, sex, education), time (as continuous variable), group (SHAM+SHAM comparator) and Time x Group interaction. Unadjusted locally-weighted mean trajectories were plotted over a sliding temporal window (i.e., lowess plot) for hippocampal volume as percentage of baseline and functional connectivity z-scores for all four groups over the 18-month trial period. Temporal relationship determined the subsequent modelled time interaction function in the LME.
2.2. Training interventions
Physical exercise training. Pneumatic resistance machines (Keiser Sports Health Equipment, Ltd, Fresno, CA, USA) were used for training at high intensity (80% of peak capacity, continuously progressed), three sets of eight repetitions of each of five–six exercises/session for most major muscle groups (chest press, leg press, seated row, standing hip abduction, knee extension). PRT was supervised by experienced research assistants (exercise physiologists and physiotherapists) in a physician-supervised clinic at the University of Sydney Faculty of Health Sciences campus in a ratio of one trainer to four–five participants. Sham physical exercise included stretching and seated calisthenics, designed not to notably increase heart rate or aerobic capacity or improve balance or strength.
Cognitive training. All cognitive training was conducted at the university campus under supervision. COGPACK program (www.cogpack.com/USA/frames.htm), a multidomain, computer-based software package developed for neurorehabilitation was used for CCT. Sham CCT was also computer based: participants watched video clips of general interest documentary topics, followed by a set of simple questions regarding the presented material (Gates et al., 2011). CCT and sham cognitive training were matched for duration, setting and sensorimotor stimulation.
2.3. Cognitive assessments
Cognitive status was evaluated via the Alzheimer's Disease Assessment Scale–cognitive subscale (ADASCog) and Executive domain, assessed using the Matrices and Similarities subtests of the Wechsler Adult Intelligence Scale, Third Edition (WAIS-III) and verbal fluency (Controlled Oral Words Association Test (COWAT), Animal Naming). The MCI diagnosis was retrospectively sub-categorised to amnestic and non-amnestic (aMCI, naMCI) to characterise the cohort at baseline. Specifically, aMCI was defined by performance falling ≥1 SD below normative values or equivalent on two or more of three memory domain tests: ADASCog word list, BVRT-revised or Logical Memory.
2.4. Exercise mediation measures
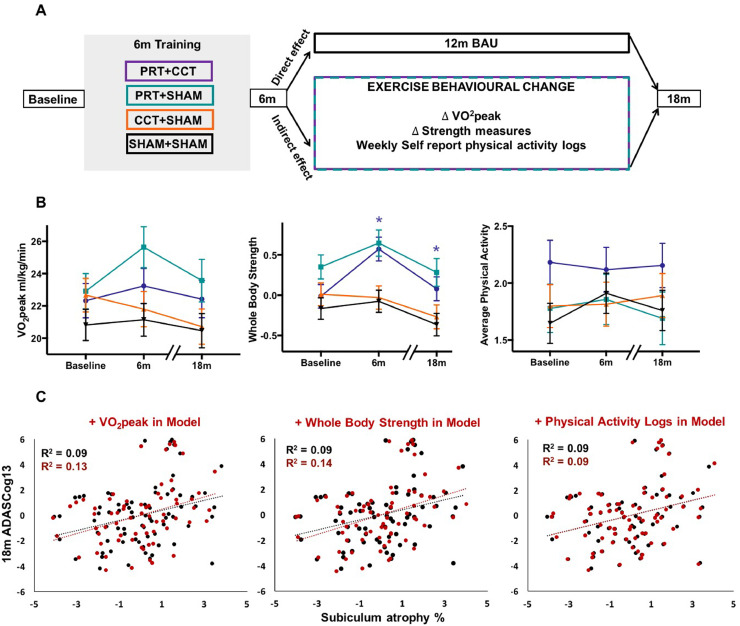
To determine whether long-term benefits of resistance exercise are a direct consequence of training dose, or rather, mediated by habitual physical activity behaviour and/or physical fitness adaptations three different variables (Fiatarone Singh et al., 2014) were tested: peak oxygen consumption measurements (VO2peak) via treadmill stress test; z-score whole-body strength via one-repetition maximum testing on Keiser machines; and self-reported, daily written physical activity (PA) logs. PA logs were defined as activities over and above activities of daily living (excluding intervention) (Mavros et al., 2017). Individual weekly averages were calculated. Baseline assessments was carried out over 2 weeks prior to intervention commencement. The 6- and 18-month PA scores were calculated as an average of the weekly average scores between baseline-6 month and 7–18-month assessments and then square root transformed to achieve normal distribution. Baseline, 6-month and 18-month scores for all three variables were entered into the statistical linear mixed effects model for each individual so that change in behaviour/fitness was modelled over the trial period. Full details of fitness testing are provided in Mavros et al. (2017) and described in Supplementary materials and model details are given in Supplementary Fig. 2E.
2.5. MRI acquisition
MRI data were acquired on a 3-Tesla Philips Achieva Scanner (Amsterdam, Netherlands) with 8-channel receive head coil, full details are reported in Suo et al. (2016). Structural measures were derived from 3 D-T1TFE sequences (1 mm isotropic resolution, TR/TE/FA = 5.39 ms/2.43 ms/8°). Functional connectivity (FC) was analysed from resting-state fMRI (rsfMRI), T2* echo-planar BOLD sequences acquired with eyes closed (FOV = 250 × 250 mm, matrix size = 64 × 64, 29 slices, slice thickness = 4.5 mm, TR/TE = 2000/30 ms, 200 volumes).
2.6. MRI post-processing
3 D-T1TFE datasets were processed with the longitudinal analysis stream in FreeSurfer (v6.0) to extract reliable volume and thickness estimates (Reuter et al., 2012; Iglesias et al., 2015). All datasets were visually assessed both for data quality prior to FreeSurfer processing and post-processing segmentation quality. Manual edits were then carried out if necessary following the FreeSurfer guidelines. Hippocampal subfield segmentation was then carried out on the longitudinal processed output data using the longitudinal hippocampal subfield segmentation tool in FreeSurfer (Iglesias et al., 2016). All subsequent reported whole-hippocampal measures are derived from the whole hippocampal volumes generated by the final hippocampal-specific subfield segmentation pipeline and not the output from the preceding longitudinal whole-brain pipeline. rsfMRI datasets were again visually inspected for data quality and then processed with the SPM8-based Data Processing Assistant for Resting-State fMRI Advanced (DPARSFA) as part of the DPABI V2.1 toolbox (www.restfmri.net/) based on published protocols (Chao-Gan and Yu-Feng, 2010). This involved: excluding the first ten volumes, motion correction, co-registration, normalization to standard MNI space (DARTEL stream), resampling, smoothing and removing global signal trends, bypass filtering of 0.01–0.08 Hz and finally regressing out nuisance signals related to white matter, whole-brain and cerebrospinal fluid signal. Co-registration outputs between structural and functional scans were visually inspected to ensure accurate registration and between-slice motion correction. Poor quality, motion artefacted data was excluded from further analysis. Individual 116 × 166 FC matrices were then generated using the Anatomical Automatic Labelling (AAL) template. The Fisher z-score correlation between PC and hippocampal regions for each individual at each time point were then extracted. All MRI pre-processing and analysis was carried out by a single observer.
2.7. Statistical analysis
Contrast: The SMART trial was originally designed as a fully-factorial trial and previous statistical analyses utilized this flexible design to investigate the effect of cognitive and physical training (Suo et al., 2016). However, we found no therapeutic benefit from the combined training group (Fiatarone Singh 2014) compared to PRT alone, and postulated that the combined intervention could have caused possible mental and/or physical stress rather than the hypothesized augmented benefits (Suo et al., 2016). As this secondary analysis aims to understand the long-term impact of cognitive and physical training on neuroplasticity, with prior knowledge of the ineffectiveness of the combined training group, we investigated the 2 original RES (PRT+CCT and PRT+SHAM vs CCT+SHAM and SHAM+SHAM) and COG (PRT+CCT and CCT+SHAM vs PRT+SHAM and SHAM+SHAM) factors as well as the 3 individual intervention arms vs SHAM+SHAM control contrasts. Statistical contrasts are shown in Supplementary Fig. 1 and false discovery rate (FDR) was used to correct for multiple comparisons.
Subfield validation: Currently, the “gold standard” hippocampal subfield segmentation approach requires a T2-weighted, high spatial resolution acquisition (Iglesias et al., 2015; Yushkevich et al., 2015). We have previously cross-validated TI and T2 subfield segmentations in an independent clinical MCI cohort (Broadhouse et al., 2019), defining valid T1-weighted subfield segmentations as those with a Pearson's correlation of >0.9 with T2-weighted segmentations. Presubiculum, Subiculum, CA1, CA4 and dentate gyrus met our criteria and were consequently included in this analysis.
Longitudinal modelling: Longitudinal changes in hippocampal volume (as a percentage of baseline volume), PC thickness and functional connectivity between these two regions were investigated using a freely available univariate linear mixed effects (LME) Matlab tool within the FreeSufer framework (Bernal-Rusiel et al., 2013). Full details regarding model designs and analyses are given in the Supplementary materials and Supplementary Fig. 2. Left and right hemispheres were analysed separately, and subsequent subfield analysis was carried out in the left hippocampus only. Time was modelled as a continuous variable, with Time x Group as the main contrast of interest. The marginal means (±SEM) of idealised/collapsed time point plots (baseline, 6M, 18M) shown in subsequent figures were generated for visualisation purposes and to provide a measure of effect size. Full details regarding effect size calculations are given in Supplementary materials. When modelling functional connectivity changes, lowess plots revealed that an exponential (not linear) time component was appropriate (Fig. 1C). Age, years of education and sex were used as covariates in all models. Finally, the three previously defined PA and fitness variables (baseline, 6-month and 18-month VO2peak, whole-body strength and PA values) were added into the hippocampal model as covariates to investigate mediation effect.
Correlation analysis: The relationship between left hippocampal subfield atrophy rate and cognitive outcome scores was investigated by carrying out a backward wise multiple linear regression analysis with age at baseline scan, years of education, sex and significant subfields volumes as independent variables. Partial correlation analysis was carried out to investigate the relationship between change in left hippocampal and PC functional connectivity and change in ADASCog between baseline and 18-month follow up correcting for covariates. All multiple linear regression and correlation analyses was carried out in SPSS (IBM, Armonk, NY, USA. Release 24).
3. Results
Eighty-four (58 female) of the 100 participants had a baseline MRI assessment (mean age = 69.5 (SD = 6.6)). Participant dropout or poor-quality 3 D-T1TFE datasets led to 79 (53 female) participants at 6-month and 74 (50 female) participants at 18-month follow-up (Fig. 1). In addition, due to poor quality and motion artefact, seven baseline and four 18-month rsfMRI datasets were excluded from functional connectivity analysis. All subsequent analysis was carried out on this MRI subset (there was no significant difference in demographics (age or sex) between this subset and the whole cohort). Fisher exact tests identified no significant differences in amnestic vs. non-amnestic MCI subtype diagnosis at baseline (aMCI = 11% of total cohort, p = 0.27) or in number of APOE-e4 carriers (APOE-e4 carriers = 29% of total cohort, p = 0.63) between groups.
3.1. Resistance exercise leads to long-term cognitive benefits
Previously, we found that resistance exercise led to better global cognitive and executive function at 18 months compared to no resistance training in factorial analysis (RES contrast) and individual group investigation revealed that only PRT+SHAM was significantly different from SHAM+SHAM (Fiatarone Singh et al., 2014). For continuity we investigated these effects at the individual group level within the MRI subset and again found that PRT+SHAM selectively benefited global ADASCog performance, as well as executive function, compared to SHAM+SHAM group (Time x Group p = 0.028; Cohen's d = −0.36; p = 0.046, Cohen's d = 0.32) (Fig. 1B).
3.2. Within-training exercise-induced PC plasticity is not sustained long-term
To investigate whether the within-training resistance exercise-induced PC structural plasticity previously reported in Suo et al. (2016) was sustained long-term we first replicated the within-training results using our updated LME within the FreeSurfer framework. As anticipated, the FreeSurfer-based LME mirrored the previous findings (Suo et al., 2016) and indicated that resistance exercise increases cortical thickness during training, although the FreeSurfer hemispheric differentiation of cortical regions revealed that this was localised to the left PC. However, building on this, a second analysis modelling long-term thickness trajectory over the 18-month period revealed that this PC structural benefit was not evident 12 months after training cessation (Fig. 2A). Full results are given in Supplementary Table 1. Resistance exercise therefore seems to stimulate an important cortico-structural mechanism during training, which is not sustained long-term.
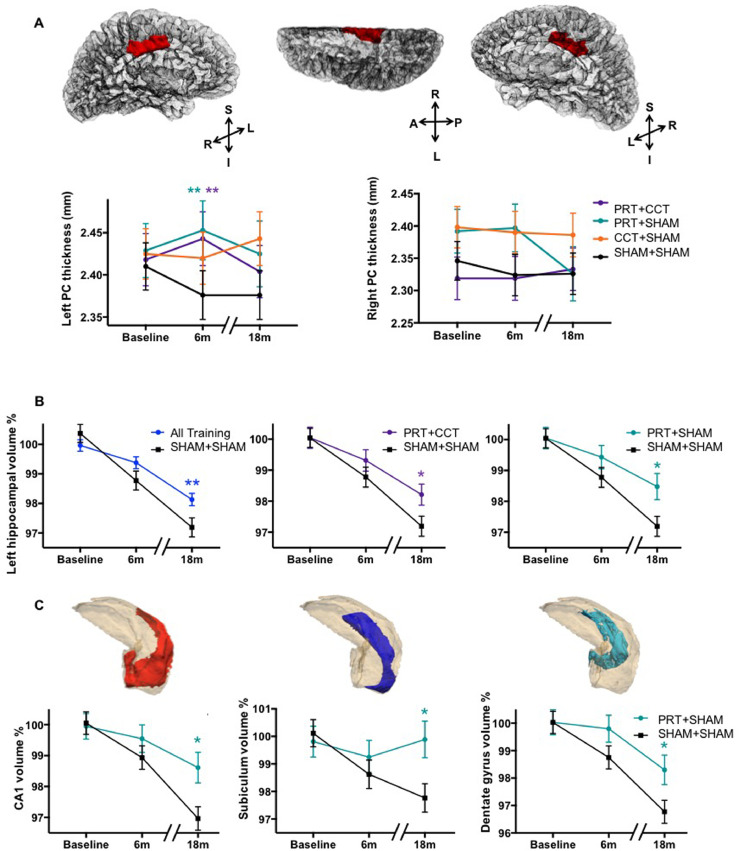
Fig. 2.
Resistance exercise preserves and protects Alzheimer-vulnerable hippocampal subfields for up to one year after training. (A) Left but not right PC cortical thickness was enhanced by any resistance exercise training as opposed to double-sham; however, this advantage was lost one year after training cessation. (**) refers to significant within-training effect of PRT+CCT vs SHAM+SHAM (F = 11.1, p = 0.005 FDR corrected, DF = 39.2) and PRT+SHAM vs SHAM+SHAM (F = 15.2, p = 0.005 FDR corrected, DF = 36.4), using an identical LME model restricted to baseline and 6 months. No Time x Group effects were observed when testing the complete 18-month model. (B) Left hippocampal volume trajectory is protected from atrophy by any training compared to double-sham (left; Time x Group F = 7.27, p = 0.008, DF = 157.9), the effect specific to groups that included resistance training (PRT+CCT: middle, F = 5.10, p = 0.041 FDR corrected, DF = 84.0; and PRT+SHAM: F = 7.22, p = 0.027 FDR corrected, DF = 79.6). (C) Resistance exercise alone protects Alzheimer-vulnerable hippocampal subfields, including: left CA1 (F = 8.81, p = 0.020 FDR corrected, DF = 81.0); Subiculum (F = 7.14, p = 0.023 FDR corrected, DF = 80.2); and dentate gyrus (F = 5.53, p = 0.035 FDR corrected, DF = 79.1). For each of these subfields, 6 months of resistance exercise protected participants from 2% to 3% volumetric loss over the complete 18-month follow-up period. Means (±SEM) adjusted for baseline age, years of education, sex, and baseline volume normalized by ICV. (*) refers to significant Time x Group interaction at p < 0.05. NB: Idealised time points (baseline, 6M, 18M) are displayed for visualisation purposes only; statistics are solely from LME models where real-time is treated as a continuous variable.
3.3. Resistance exercise slows post-training CA1, subiculum and dentate atrophy
Next, LME analysis was used to investigate the long-term impact of the interventions, over the 18-month trial period, on whole hippocampal volume. We found significant effects for left, but not right hippocampal atrophy rates. Specifically, both the PRT+CCT and PRT+SHAM groups exhibited significantly slower atrophy rates in the left hippocampus over the 18-month period compared to SHAM+SHAM (Fig. 2B; FDR corrected p = 0.042 and 0.027). PRT+CCT did not show this effect; concordant with our previous findings on cognition and fitness adaptations (Fiatarone Singh et al., 2014; Mavros et al., 2017), the neuroplastic benefits of PRT were attenuated when combined with CTT. Full results for whole hippocampal LME analysis are given in Table 1A. Focusing on the left hippocampus, subfield LME analysis revealed an elimination of long-term atrophy in the left subiculum, and attenuation of atrophy in left CA1 and dentate gyrus for the PRT+SHAM group compared to SHAM+SHAM (Fig 2C; FDR corrected p = 0.023, Cohen's d = 0.99; p = 0.020 Cohen's d = 1.02; and p = 0.027, Cohen's d = 0.82, respectively). Full results for subfield hippocampal LME analyses are given in Tables 1B and C.
Table. 1.
(A)Whole hippocampal and (B) Subfield LME analysis. Significant p-values are shown in bold, individual intervention analysis shows FDR corrected p-values.
| A | Left | Right | ||||
|---|---|---|---|---|---|---|
| Long-term effect | Fvalue | p value | DF | F value | p value | DF |
| RES | 3.60 | 0.060 | 159.2 | 0.19 | 0.663 | 159.9 |
| COG | 1.53 | 0.219 | 158.8 | 0.71 | 0.401 | 159.5 |
| PRT+CCT vs SHAM+SHAM | 5.10 | 0.041 | 83.8 | 0.77 | 0.650 | 84.0 |
| PRT+SHAM vs SHAM+SHAM | 7.22 | 0.027 | 79.6 | 0.62 | 0.650 | 79.9 |
| CCT+SHAM vs SHAM+SHAM | 2.70 | 0.104 | 84.7 | 0.18 | 0.650 | 85.0 |
| B | PRT+CCT vs SHAM+SHAM | PRT+SHAM vs SHAM+SHAM | ||||
| F value | p value | DF | F value | p value | DF | |
| Subiculum | 0.74 | 0.392 | 83.6 | 7.14 | 0.023 | 80.2 |
| CA1 | 2.85 | 0.119 | 84.6 | 8.81 | 0.020 | 81.0 |
| Dentate gyrus | 6.05 | 0.075 | 83.5 | 5.53 | 0.035 | 79.1 |
| CA4 | 4.67 | 0.083 | 83.7 | 3.39 | 0.086 | 79.6 |
| Presubiculum | 2.87 | 0.119 | 83.7 | 0.59 | 0.442 | 80.9 |
RES = PRT+CCT and PRT+SHAM vs CCT+SHAM and SHAM+SHAM.
COG = PRT+CCT and CCT+SHAM vs PRT+SHAM and SHAM+SHAM.
PRT = progressive resistance training.
CCT = computerised cognitive training.
3.4. Within-training preservation of PC volume predicts long-term hippocampal preservation
Next, the relationship between within-training PC and long-term hippocampal structural plasticity was investigated. Whole group correlation analysis established a weak yet significant negative relationship between the within-training structural PC plasticity and the long-term hippocampal subfield neuroprotection described above. Specifically, individuals who underwent the most within-training increment in PC cortical thickness had less subiculum and dentate gyrus atrophy one year post-training (Pearson's correlation = −0.24, p = 0.04 and Pearson's correlation = −0.26, p = 0.03, respectively).
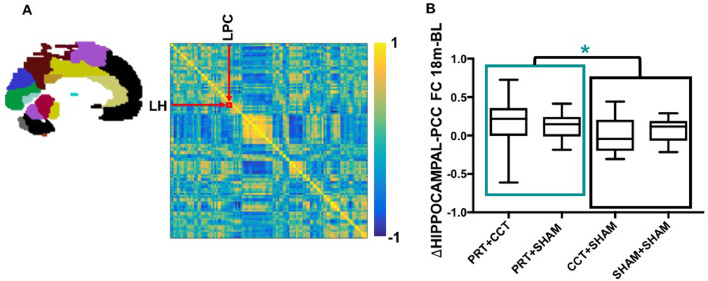
3.5. PRT increases functional connectivity between PC and hippocampus
To further investigate this link between the spatiotemporally distinct PC and hippocampal plasticity we examined the change in functional connectivity between left PC and hippocampus. A significant RES x Time interaction over the 18-month period was found, such that long-term functional connectivity between PC and hippocampus was significantly strengthened in PRT+CCT and PRT+SHAM compared to CCT+SHAM and SHAM+SHAM (p = 0.018) (Fig. 3). However, significant p-values did not survive FRD correction for the individual group analysis. Full results for the functional connectivity LME analysis are given in Table 2. Partial correlation analysis across the whole group revealed a non-significant relationship between change in left hippocampal and PC functional connectivity and change in ADASCog between baseline and 18-month follow (p = 0.097).
Fig. 3.
Resistance exercise leads to long term increase in functional connectivity between left hippocampus and posterior cingulate. (A) An individual functional connectome from one SMART participant at baseline showing the correlation between all 116 AAL-atlas brain regions (left) in the form of a correlation heat map (right). Individual connectomes were generated at each time point, and correlation values between the left hippocampus and PC regions extracted to investigate the change in functional connectivity using our LME model as outlined in Fig 1E. (B) Raw change in the functional connectivity between left hippocampus and PC over the complete 18-month period is depicted at the individual level. Box-and-Whisker plots show the median (horizontal line), interquartile range (box) and upper and lower quartiles (whiskers) of change in functional connectivity between left PCC and hippocampus for each training group. LME analysis found significantly increased connectivity for those two groups that underwent resistance exercise training (PRT+CCT and PRT+SHAM) compared to those that did not (CCT+SHAM and SHAM+SHAM). *Time x Group p < 0.05; model included covariates age, sex and education, as well as group factor and time as continuous variable.
Table. 2.
LME analyses of PC-hippocampal functional connectivity z-scores. Significant p-values are shown in bold, individual intervention analysis shows FDR corrected p-values.
| Within-training | Long-term | |||||
|---|---|---|---|---|---|---|
| F value | p value | DF | F value | p value | DF | |
| RES | 0.07 | 0.789 | 77.3 | 5.72 | 0.018 | 148.0 |
| COG | 0.38 | 0.538 | 76.5 | 0.02 | 0.887 | 147.1 |
| PRT+CCT vs SHAM+SHAM | 0.02 | 0.903 | 39.8 | 1.82 | 0.180 | 77.9 |
| PRT+SHAM vs SHAM+SHAM | 0.00 | 0.985 | 38.2 | 1.68 | 0.199 | 70.4 |
| CCT+SHAM vs SHAM+SHAM | 0.81 | 0.374 | 39.6 | 0.22 | 0.637 | 77.8 |
RES = PRT+CCT and PRT+SHAM vs CCT+SHAM and SHAM+SHAM.
COG = PRT+CCT and CCT+SHAM vs PRT+SHAM and SHAM+SHAM.
PRT = progressive resistance training.
CCT = computerised cognitive training.
3.6. Neuroprotection of AD-vulnerable areas mediate long-term cognition
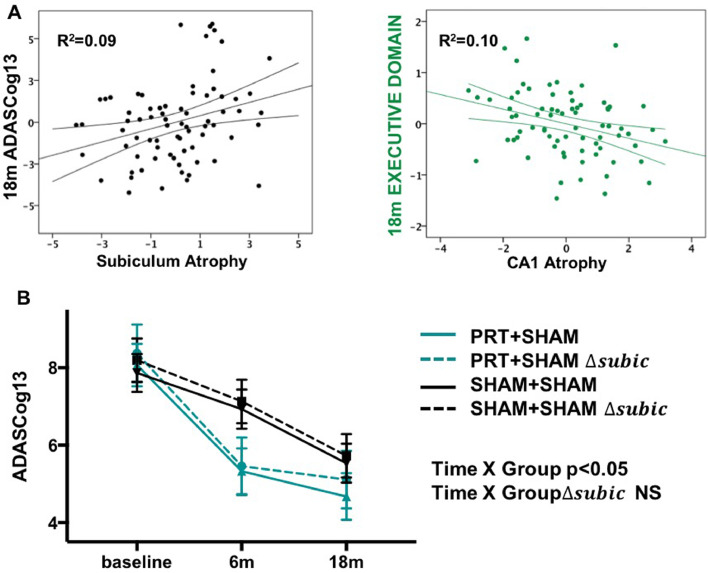
Next, backward multiple linear regression was used to investigate the association between left hippocampal subfield atrophy trajectory over the 18-month period and previously observed, long-term cognitive gains (Fiatarone Singh et al., 2014) (Fig. 1B). For ADASCog, sex (β = −0.32, p = 0.002), subiculum atrophy rate (β = 0.28, p = 0.011), education (β = −0.24, p = 0.022), and baseline age (β = 0.22, p = 0.04) were significant independent predictors (overall model fit adjusted-R2 = 0.28; Fig. 4A), where higher ADASCog scores indicate poorer global cognitive function. Interestingly, for the trial's primary outcome (ADASCog), rate of subicular atrophy accounted for more unique variance than age or education (subiculum: adjusted R2 = 9%; age: R2 = 6% and education: R2 = 7%). For executive function, education (β = 0.53, p < 0.0001), baseline age (β = −0.30, p = 0.001) and CA1 atrophy (β = −0.25, p = 0.007) were significant and independent predictors (overall model fit aR2 = 0.45, Fig. 4A). To further investigate this association between subiculum atrophy on our primary outcome measure ADASCog, we re-ran the cognitive LME model in Fig 1B with 18-month change in subiculum volume as an additional covariate and found no significant PRT+SHAM x Time interaction when compared to SHAM+SHAM (Fig 4B). Since resistance exercise protects and preserves subiculum volume (Fig 2), is therapeutically effective for cognition in the long term, and the former predicts the latter, it follows that the addition of subiculum atrophy abolished the therapeutic effects of resistance exercise on final ADASCog, indicating that resistance exercise-dependent plasticity of the subiculum contributes to mediation of long-term therapeutic benefits on cognition.
Fig. 4.
Therapeutic benefits of resistance exercise on cognition one year after training are mediated by preservation of AD-vulnerable hippocampal subfields. (A) Multiple regression analysis tested the unique variance accounted in the primary outcome (ADASCog) and Executive function by 18-month subiculum or CA1 atrophy (as percent change from baseline; age, sex and education were also in the model). Partial plots here show that subiculum atrophy was an independent predictor of ADAGCog13, whilst CA1 atrophy was an independent predictor of Executive function. The beta values for each of these (β = 0.28 and −0.25, respectively) were of similar magnitude to clinically meaningful predictors age (β = 0.22 and −0.30) and education (β = −0.24 and 0.53). (B) Since resistance exercise protects and preserves subiculum and CA1 volume (Fig 2C), is therapeutically effective on cognition in the long term (A), and the former predict the latter (B), we re-ran the cognitive LME model in (A) with 18-month change in subiculum volume (Δsubic; expressed as percentage change from baseline) as an additional covariate. This abolished the therapeutic effect of resistance exercise on ADASCog. Accordingly, resistance exercise-dependent plasticity of the subiculum mediates long-term therapeutic benefits on global cognition.
3.7. Long-term neuroprotection is not mediated by post-training physical activity or fitness adaptations
Finally, the challenging question as to whether the long-term brain benefits of resistance exercise are a direct and extended consequence of a time-limited training dose, or rather, mediated by habitual PA engagement outside of the intervention or fitness adaptations was investigated. There are many indirect pathways by which an exercise intervention could continue to impact the brain and cognition. However, in this context, changes in either physical fitness or post-training “exercise behaviour”, are most relevant, and are conceptualized in Fig. 5A. LME analysis revealed a significant increase in whole body strength in the PRT+CCT group compared to SHAM+SHAM over the 18-month period (Fig. 5B). This is potentially important therapeutically, since we have previously shown that increases in muscle strength during training mediate clinical improvements in general cognition (Mavros et al., 2017). However, when entering the three PA and fitness variables (VO2peak, whole-body strength and PA) as covariates into our subfield LME model, CA1, subiculum and dentate gyrus atrophy trajectories were still significantly attenuated in PRT+SHAM compared to SHAM+SHAM (Fig. 5C). Suggesting that long-term protective effects of PRT on atrophic subfield trajectories are not mediated by exercise behavioural change outside the intervention. Rather, our data indicate that this discrete 6-month PRT intervention extends a direct and lasting neuroprotective effect on cognitively-relevant and AD-vulnerable hippocampal subfields. Full LME results with the addition of the three exercise and fitness variables are given in Supplementary Table 2.
Fig. 5.
Physical fitness and physical activity behaviour after training does not mediate long-term protection of hippocampal subfields. (A) A conceptual schema showing how the long-term effects of resistance exercise on the brain could arise directly following training in the context of reverting to business-as-usual (BAU) or could be mediated by changes in physical fitness or physical activity behaviour unrelated to the interventions. We modelled exercise-related behaviour and physical fitness in three different ways, using long term changes in physiologic VO2peak and whole-body muscle strength measurements, as well as daily physical activity logs over 18 months. (B) Change in fitness and exercise behaviour is charted over time (mean (±SEM)) using a LME model, revealing a significant Time x Group (*) effect of PRT+CCT on whole body strength during training and over the complete 18-month period compared to SHAM+SHAM (F = 15.22, p = 0.001 FDR corrected, DF = 38.52 and F = 5.47, p = 0.018 FDR corrected, DF = 76.03, respectively). We therefore confirm that 6 months of resistance exercise training continues to modify whole body muscle strength for up to a year after the cessation of formal supervised study-related resistance training. (C) However, after inclusion of these fitness measures and non-study physical activities in our LME model of hippocampal subfield change, the neuroprotective effect of resistance exercise was not diminished. This is further visualised by partial plots of subiculum atrophy against the trial's primary cognitive outcome (ADASCog error scores), showing no moderation of relationship before (black) or after (red) inclusion of whole-body muscle strength, aerobic capacity (VO2 peak) and total number of physical activity sessions outside of the intervention in the model. Long-term protective effects of resistance exercise on AD-vulnerable hippocampal subfields are hence direct, delayed and protracted, independent of PA behaviour after formal training ends.
4. Discussion
Cognitive benefit directly following lifestyle intervention is now well established in older persons (Mavros et al., 2017; Ngandu et al., 2015; Rebok et al., 2014; Richard et al., 2009), however the underlying mechanisms are poorly understood and how these evolve long-term, essentially unknown. For the first time with any intervention, we show that resistance exercise can not only lead to cognitive benefits but also protects AD-vulnerable hippocampal subfields long-term. Moreover, this form of neuroprotection helps to explain such lasting cognitive benefits, spatiotemporally distinct from PC plasticity that mediates cognitive improvement only during exercise. These two temporally distinct targets of structural protection are linked by a long-term upregulation of functional inter-connectivity following resistance exercise. Finally, we demonstrate that enduring hippocampal subfield plasticity is not a simple reflection of post-training changes in fitness or physical activity participation. Rather, resistance exercise can effectively support ongoing general cognition in at-risk elders by directly protecting hippocampal subfields most at risk for degeneration in AD.
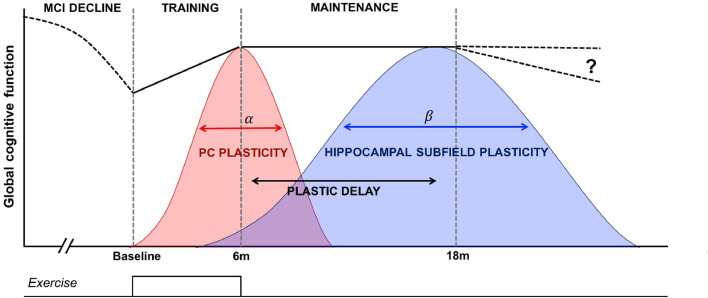
It is widely assumed that the same mechanisms elicited during exercise will either persist or diminish after training cessation, which in turn determines long-term therapeutic efficacy. Our data contradict this and suggest that distinct mechanisms emerge during- and post-training, in effect a cascade of therapeutic mechanisms that combine to support cognition (Fig. 6). Our past work indicates that within-training cognitive gains are coupled with short-to-medium term structural plasticity in the PC (Suo et al., 2016). Here we show this decays after the offset of training. However, plasticity in left CA1, subiculum and dentate gyrus, areas highly sensitive to AD degeneration, only becomes evident well after training has finished. Interestingly, whilst such structural benefits can be distinguished in time, we reveal a possible underlying explanation in the form of upregulated hippocampal-PC functional connectivity, a change unique to resistance exercise (RES contrast) that persisted across the entire 18-month period.
Fig. 6.
Model in which different mechanisms are responsible for therapeutic cognitive effects during and after training. Global cognition presumably declines before observations begin at baseline because of the MCI status of participants (dashed line). A discrete dose of resistance exercise (bottom trace) produces a significant improvement to cognition, coupled with contemporaneous structural plasticity in the posterior cingulate (PC) that is time-limited and wanes after training offset. Resistance exercise also leads to a time-delayed structural response within select hippocampal subvolumes, evident up to one year after training offset. This delayed hippocampal subfield plasticity helps explains ongoing maintenance of cognition. In this conceptual schema several parameters are unknown, including: the shape, symmetry, peak and width of α and β, how they interact, or how long therapeutic cognitive effects last.
The PC has strong reciprocal connections with several cortical and subcortical regions, most pertinently with the hippocampus (Leech and Sharp, 2014). In MCI and AD, functional and diffusion tensor imaging studies show a decrease in connectivity between the PC and hippocampus (Zhou et al., 2008), part of a well characterized reduction in functional connectivity in the encompassing default mode network (DMN) (Greicius et al., 2004; Zhou et al., 2008). Previous studies suggest that disrupted connectivity between these two regions may account for PC hypometabolism commonly detected in early AD (Greicius et al., 2004). The DMN may also be plastic to exercise, as witnessed in a recent trial in healthy older adults that found that stretching led to an increase DMN functional connectivity after 6 months, while aerobic showed similar results at 12 months (Voss et al., 2010). It is thereby possible that resistance exercise triggers increased functional connectivity between these two key hubs of the DMN, regions that then undergo structural plasticity along different time courses. More generally, recent studies have indicated that resting state functional connectivity maybe be a promising biomarker for AD as functional brain changes are thought to precede structural changes. Decreased functional connectivity has been observed in healthy aging, mild cognitive impairment, a prodromal stage of AD, and AD. However, as the exact role of aberrant functional connectivity within the cascade of pathophysiological processes of AD remains poorly understood, it is unclear how the observed upregulated hippocampal-PC functional connectivity will impact or delay the onset of dementia. Further research is therefore required to determine the temporal sequence and inter-dependency of functional and structural brain changes triggered by PRT.
4.1. Neuroprotection of AD-vulnerable hippocampal subfields
We found strong evidence for left asymmetric plasticity in the cortex and within the hippocampus. Longitudinal human studies consistently show asymmetrical volume loss in MCI and AD, with higher atrophy rates in the left hippocampus (Shi et al., 2009). More specifically, this asymmetry is reserved to the anterior part of the hippocampus and asymmetry increases with disease progression (Maruszak and Thuret, 2014). Since CA1 and subiculum subfields lie within the anterior hippocampus, our findings of left-lateralised effects after resistance exercise gain added relevance. Furthermore, atrophy progresses nonlinearly, with periods of acceleration predicting transition from MCI to dementia (Apostolova et al., 2010; Maruszak and Thuret, 2014; Rossler et al., 2002). An intervention that effectively protects against volume loss in AD-vulnerable hippocampal subfields could potentially delay transition to dementia.
Accordingly, the positive outcome seen here using resistance exercise suggests this non-pharmacological intervention may not only be clinically effective, but capable of targeting a key aspect of AD pathogenesis. Our results are also of interest because the cohort was population-based and trial entry non-specific for subtype of MCI. In fact, our sample was generally non-amnestic MCI (89%) and only about one-third held the AD-risk gene APOE4. Yet hippocampal AD pathology and neurodegeneration are as common in amnestic MCI as in non-amnestic MCI (Dugger et al., 2015), primarily because pathology in MCI and AD is highly heterogenous (Schneider et al., 2009). Furthermore, functional imaging studies have revealed that the hippocampus is implicated in far more than just memory consolidation and retrieval, forming an import hub in several networks involved in higher order executive functioning, impulse control, cognitive flexibility and decision making (Mars et al., 2012; Vincent et al., 2008). It has been suggested that the functional disruptions within these neurocircuitries lead to maladaptive responses during cognitive and emotional processing, which may underpin cognitive decline in this cohort (Liu et al., 2014). For these reasons, AD-related mechanisms are as relevant to non-amnestic MCI as amnestic MCI, and for community-orientated preventative intervention such as resistance exercise there is no compelling justification to make the distinction.
At the same time, caution against over interpretation is required. Annualized rates of hippocampal atrophy in those with stable versus declinate MCI are 2.6% and 3.7% (Jack et al., 2000) respectively – a ~1% difference per year. Mapping cognitive decline showed that on average non-converter and converter MCI subjects declined by 0.75 and 2.93 ADASCog points over a 12-month period (Evans et al., 2010). We observed that 6 months of resistance exercise produced a 12-month- advantage to general cognition of 1–2 ADASCog points indicating that the training-dependent effects observed in SMART are comparable to ~0.7–2years of naturalistic decline but in the opposite direction. Furthermore, we see a preservation of CA1 and subiculum volume by ~3%. If such linear trends are extrapolated, our intervention could preserve key hippocampal substructures by ~3 years compared to usual care. This would be a significant improvement to the individual, but MCI is a long-term condition of indeterminate prognosis, and so a circumscribed dose of resistance exercise would be unlikely to provide indefinite protection against cognitive decline or neurodegeneration, as would be the case if it were used for sarcopenia or osteoporosis, for example. This therefore poses an implementation challenge as prescription of PRT may need to be lifelong, similar to the prescription of pharmacologic agents or exercise for diabetes or cardiovascular disease.
4.2. CA1 and subiculum function
CA1 and subiculum volumetric plasticity is also interesting given their putative role in higher-order cognitive function. Classically, they participate in the trisynaptic circuit, a mnemonic loop where afferents from CA3 synapse onto CA1 and information is passed onto the subiculum before delivery to cortical and contralateral targets. In rodents, CA1 is strongly implicated in episodic memory (Kyle et al., 2015; Reitz et al., 2009) and spatial navigation (O'Keefe, 1991). Similarly, the subiculum has been heavily implicated in mnemonic consolidation processes (O'Mara et al., 2000). It may therefore appear counterintuitive that selective plasticity in these areas by PRT seemed to primarily benefit executive function. However, this is less surprising considering the presence of dense monosynaptic CA1 to medial-orbital prefrontal connections in primate studies (Ongur and Price, 2000), suggesting a more complex role for the hippocampus (O'Mara et al., 2000), not just in memory but also in executive processes and affect regulation (Godsil et al., 2013; Ongur and Price, 2000). Resistance exercise may potentially alter the connections between CA1 and prefrontal structures, providing for more nuanced and effective integration of mnemonic and affective information. Because a precise role for these subfields in humans is still emerging, resistance exercise may prove useful in disambiguating hippocampal subfield function.
The primary outstanding question remains why and how any exercise regime should benefit cognition. The relative impact of peripheral- versus centrally-induced molecular changes remains poorly understood, with some evidence that exercise-induced myokines may cross the blood-brain barrier and possibly mediate some forms of brain plasticity (Bostrom et al., 2012; Kobilo et al., 2011; Wrann et al., 2013). Whilst some mechanisms may be shared with aerobic exercise, there is abundant evidence that anabolic training produces its own metabolic signature. Resistance exercise, for example, is particularly effective at counteracting insulin resistance, sarcopenia and stimulating osteogenic mechanisms (Cooney et al., 2014; Irvine and Taylor, 2009; Peterson and Gordon, 2011). Notably, in the EXCEL RCT, the only head-to-head comparison of resistance and aerobic training in older women with probable MCI published to date, 6 months of resistance training significantly improved selective attention/conflict resolution, and associative memory compared to the sham-exercise control, along with functional changes in three regions of cortex the right lingual and occipital-fusiform gyri, and the right frontal pole during the encoding and recall of associations (ten Brinke et al., 2015). Additionally, there was a significant positive correlation between change in hemodynamic activity in the right lingual gyrus and change in behavioural associative memory performance in the resistance training group. None of these changes were seen in the aerobic exercise group (Nagamatsu et al., 2012). However, compared with the control group, aerobic training significantly increased left, right, and total hippocampal volumes, but the increased left hippocampal volume was independently associated with reduced verbal memory and learning performance (ten Brinke et al., 2015). Consequently, whether resistance exercise has unique plastic effects on the older brain is still unclear and warrants further investigation.
4.3. Limitations
Although the long-term, resistance exercise induced, structural and functional plasticity reported here is encouraging in the MCI population, caution is needed when interpreting such findings. Firstly, our results derive from a single community-based cohort in Sydney, Australia, and our main analyses centred around the SHAM+SHAM and PRT+SHAM groups, a total sample of 43 participants for MRI and 49 for cognition. For replication purposes, assuming 80% power, and the observed Cohen's d effect sizes for cognition outcomes (ADASCog and executive domain scores), group sample sizes of 150 and 190 are recommended, respectively. By contrast, subfield analysis revealed large effect sizes translating to recommended sample sizes of 21–31 participants in each group depending on the subfield of interest. We also recognise that power calculations are complex for longitudinal designs due to missing data and autocorrelation between measures over time. Consequently, we calculated the realized power (Bernal-Rusiel et al., 2013a, 2013b) associated with the CA1, subiculum and dentate gyrus linear mixed-effects model which provided an estimate of power of 0.83, 0.78 and 0.63 respectively (see Supplementary materials for details). For some outcomes our study was therefore underpowered. Furthermore, the relatively small numbers and trial design did not allow dichotomization of the groups into the MCI subtypes, amnestic and non-amnestic MCI. It is thought that amnestic MCI increases the risk of conversion to AD further however, the wider generalizability of our findings will need to be tested in large and more diverse MCI populations along with determining links to other AD biomarkers.
Secondly, only 3 D-T1TFE datasets were acquired for segmentation analysis, although datasets were visually inspected for data quality, the lower resolution and lower within structure contrast may lead to inaccuracies in subfield segmentations. However, it is important to note that although a dedicated high resolution T2-weighted sequence is considered by many as the “gold standard” for subfield segmentation, as with all MR sequences there are tradeoffs. The improved grey/white matter contrast is achieved with a spin echo sequence – a gradient readout that is inherently longer and resultant contrast that provides less signal or “entropy” than the T1-weighted counterpart. Furthermore, to achieve reasonable scan times the T2 sequence is not 3 D and as a result has anisotropic resolution (typically 0.4 × 0.4 × 2 mm). These factors need to be considered when carrying out accurate subfield segmentation. In a cohort where hippocampal atrophy occurs along the anterior-posterior axis and speed to reduce motion artefacts is priority, the faster, 3 D T1-weighted, isotropic resolution sequence may often be preferable and as robust.
Finally, we found strong evidence for long-term left asymmetric plasticity in the hippocampus. Longitudinal human studies consistently show asymmetrical volume loss in MCI and AD, with higher atrophy rates in the left hippocampus (Shi et al., 2009). Therefore, our findings of left-lateralised plastic effects after resistance exercise gain clinical relevance but are essentially unexplained. Furthermore, we do not understand the dependency, if any, between the previously reported within-training PC cortical mechanisms (Suo et al., 2016) and the delayed and protracted plasticity observed after training in AD-vulnerable subfields. The two phenomena are correlated, and these regions participate within a functional network stimulated by resistance exercise, but their causal relationship is unclear. Further work is needed to investigate the long-term structural and functional, resistance exercise-induced plasticity in MCI and whether these plastic effects are diverse between subtypes.
4.4. Conclusion
Given the great challenge of dementia to modern society it is promising to show for the first time that 6 months of high intensity resistance exercise is capable of not only promoting cognition in those with MCI, but also protecting AD-vulnerable hippocampal subfields from degeneration for at least 12 months post-intervention. Future work will need to establish just how long-lived these outcomes are and whether they are sufficient enough to delay cognitive decline. Despite this, given the strength of our findings we recommend that resistance exercise be considered an integral part of lifestyle-based prevention programs in older persons.
Funding
This study was funded by the National Health and Medical Research Council (NHMRC) of Australia Dementia Research Grant, Project Grant ID No. 512672 from 2008 to 2011. Additional funding for a research assistant position was sourced from the NHMRC Program Grant ID No. 568969.
CRediT authorship contribution statement
Kathryn M. Broadhouse: Formal analysis, Investigation, Methodology, Writing - original draft, Writing - review & editing. Maria Fiatarone Singh: Supervision, Project administration, Conceptualization, Funding acquisition, Data curation, Methodology, Writing - review & editing. Chao Suo: Data curation, Methodology, Writing - review & editing. Nicola Gates: Formal analysis, Data curation, Methodology, Writing - review & editing. Wei Wen: Formal analysis, Methodology, Writing - review & editing. Henry Brodaty: Conceptualization, Funding acquisition, Methodology, Writing - review & editing. Nidhi Jain: Formal analysis, Data curation, Methodology, Writing - review & editing. Guy C. Wilson: Data curation, Methodology, Writing - review & editing. Jacinda Meiklejohn: Data curation, Methodology, Writing - review & editing. Nalin Singh: Data curation, Methodology, Writing - review & editing. Bernhard T. Baune: Data curation, Methodology, Writing - review & editing. Michael Baker: Data curation, Methodology, Writing - review & editing. Nasim Foroughi: Data curation, Methodology, Writing - review & editing. Yi Wang: Data curation, Methodology, Writing - review & editing. Nicole Kochan: Data curation, Methodology, Writing - review & editing. Kevin Ashton: Data curation, Methodology, Writing - review & editing. Matt Brown: Data curation, Methodology, Writing - review & editing. Zhixiu Li: Data curation, Methodology, Writing - review & editing. Yorgi Mavros: Funding acquisition, Formal analysis, Data curation, Methodology, Writing - review & editing. Perminder S. Sachdev: Supervision, Conceptualization, Funding acquisition, Data curation, Methodology, Writing - review & editing. Michael J.Valenzuela: Supervision, Conceptualization, Funding acquisition, Data curation, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors have no conflicts of interest to declare.
Acknowledgements
We would like to acknowledge the generous support and contribution of all our SMART Trial participants. MV was supported by a National Health and Medical Research Council of Australia career development fellowship APP1112813 and The University of Sydney.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.nicl.2020.102182.
Contributor Information
Kathryn M. Broadhouse, Email: kbroadhouse@usc.edu.au.
Michael J.Valenzuela, Email: michael.valenzuela@sydney.edu.au.
Appendix. Supplementary materials
Data for reference
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
- Alzheimer's Disease International . 2016. World Alzheimer Report 2016. Retrieved from London. [Google Scholar]
- Apostolova L.G., Dutton R.A., Dinov I.D., Hayashi K.M., Toga A.W., Cummings J.L., Thompson P.M. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch. Neurol. 2006;63(5):693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- Apostolova L.G., Mosconi L., Thompson P.M., Green A.E., Hwang K.S., Ramirez A.…de Leon M.J. Subregional hippocampal atrophy predicts alzheimer's dementia in the cognitively normal. Neurobiol. Aging. 2010;31(7):1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L.D., Frank L.L., Foster-Schubert K., Green P.S., Wilkinson C.W., McTiernan A.…Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch. Neurol. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Rusiel J.L., Greve D.N., Reuter M., Fischl B., Sabuncu M.R. Statistical analysis of longitudinal neuroimage data with Linear Mixed Effects models. Neuroimage. 2013;66:249–260. doi: 10.1016/j.neuroimage.2012.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Rusiel J.L., Reuter M., Greve D.N., Fischl B., Sabuncu M.R. Spatiotemporal linear mixed effects modeling for the mass-univariate analysis of longitudinal neuroimage data. Neuroimage. 2013;81:358–370. doi: 10.1016/j.neuroimage.2013.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostrom P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C.…Spiegelman B.M. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhouse K.M., Mowszowski L., Duffy S., Leung I., Cross N., Valenzuela M.J., Naismith S.L. Memory performance correlates of hippocampal subfield volume in mild cognitive impairment subtype. Front. Behav. Neurosci. 2019;13:259. doi: 10.3389/fnbeh.2019.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. DPARSF: a MATLAB toolbox for “Pipeline” data analysis of resting-state fMRI. Front. Syst. Neurosci. 2010;4(13) doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo I.H., Lee D.Y., Oh J.S., Lee J.S., Lee D.S., Song I.C.…Woo J.I. Posterior cingulate cortex atrophy and regional cingulum disruption in mild cognitive impairment and Alzheimer's disease. Neurobiol. Aging. 2010;31(5):772–779. doi: 10.1016/j.neurobiolaging.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Cooney G., Dwan K., Mead G. Exercise for depression. JAMA. 2014;311(23):2432–2433. doi: 10.1001/jama.2014.4930. [DOI] [PubMed] [Google Scholar]
- Costafreda S.G., Dinov I.D., Tu Z., Shi Y., Liu C.Y., Kloszewska I.…Simmons A. Automated hippocampal shape analysis predicts the onset of dementia in mild cognitive impairment. Neuroimage. 2011;56(1):212–219. doi: 10.1016/j.neuroimage.2011.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugger B.N., Davis K., Malek-Ahmadi M., Hentz J.G., Sandhu S., Beach T.G.…Sabbagh M.N. Neuropathological comparisons of amnestic and nonamnestic mild cognitive impairment. BMC Neurol. 2015;15(146) doi: 10.1186/s12883-015-0403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson K.I., Voss M.W., Prakash R.S., Basak C., Szabo A., Chaddock L.…Kramer A.F. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M.C., Barnes J., Nielsen C., Kim L.G., Clegg S.L., Blair M.… Volume changes in Alzheimer's disease and mild cognitive impairment: cognitive associations. Eur. Radiol. 2010;20(3):674–682. doi: 10.1007/s00330-009-1581-5. [DOI] [PubMed] [Google Scholar]
- Singh Fiatarone, A. M., Gates N., Saigal N., Wilson G.C., Meiklejohn J., Brodaty H.…Valenzuela M. The Study of Mental and Resistance Training (SMART) study-resistance training and/or cognitive training in mild cognitive impairment: a randomized, double-blind, double-sham controlled trial. J. Am. Med. Dir. Assoc. 2014;15(12):873–880. doi: 10.1016/j.jamda.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Firth J., Stubbs B., Vancampfort D., Schuch F., Lagopoulos J., Rosenbaum S., Ward P.B. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage. 2018;166:230–238. doi: 10.1016/j.neuroimage.2017.11.007. [DOI] [PubMed] [Google Scholar]
- Gates N., Fiatarone Singh M.A., Sachdev P.S., Valenzuela M. The effect of exercise training on cognitive function in older adults with mild cognitive impairment: a meta-analysis of randomized controlled trials. Am. J. Geriatr. Psychiatry. 2013;21(11):1086–1097. doi: 10.1016/j.jagp.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Gates N.J., Valenzuela M., Sachdev P.S., Singh N.A., Baune B.T., Brodaty H.…Fiatarone Singh M.A. Study of Mental Activity and Regular Training (SMART) in at risk individuals: a randomised double blind, sham controlled, longitudinal trial. BMC Geriatr. 2011;11(19) doi: 10.1186/1471-2318-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsil B.P., Kiss J.P., Spedding M., Jay T.M. The hippocampal-prefrontal pathway: the weak link in psychiatric disorders. Eur. Neuropsychopharmacol. 2013;23(10):1165–1181. doi: 10.1016/j.euroneuro.2012.10.018. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Srivastava G., Reiss A.L., Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. USA. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill N.T., Mowszowski L., Naismith S.L., Chadwick V.L., Valenzuela M., Lampit A. Computerized cognitive training in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Am. J. Psychiatry. 2017;174(4):329–340. doi: 10.1176/appi.ajp.2016.16030360. [DOI] [PubMed] [Google Scholar]
- Huang C., Wahlund L.O., Svensson L., Winblad B., Julin P. Cingulate cortex hypoperfusion predicts Alzheimer's disease in mild cognitive impairment. BMC Neurol. 2002;2:9. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Augustinack J.C., Nguyen K., Player C.M., Player A., Wright M.… A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. Neuroimage. 2015;115:117–137. doi: 10.1016/j.neuroimage.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias J.E., Van Leemput K., Augustinack J., Insausti R., Fischl B., Reuter M. Bayesian longitudinal segmentation of hippocampal substructures in brain MRI using subject-specific atlases. Neuroimage. 2016;141:542–555. doi: 10.1016/j.neuroimage.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine C., Taylor N.F. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: a systematic review. Aust. J. Physiother. 2009;55(4):237–246. doi: 10.1016/s0004-9514(09)70003-0. [DOI] [PubMed] [Google Scholar]
- Jack C.R., Jr., Petersen R.C., Xu Y., O'Brien P.C., Smith G.E., Ivnik R.J.…Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T., Yuan C., van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn. Mem. 2011;18(2):103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle C.T., Stokes J.D., Lieberman J.S., Hassan A.S., Ekstrom A.D. Successful retrieval of competing spatial environments in humans involves hippocampal pattern separation mechanisms. Elife. 2015;4 doi: 10.7554/eLife.10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R., Sharp D.J. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Yu C., Zhang X., Liu J., Duan Y., Alexander-Bloch A.F.…Bullmore E. Impaired long distance functional connectivity and weighted network architecture in Alzheimer's disease. Cereb. Cortex. 2014;24(6):1422–1435. doi: 10.1093/cercor/bhs410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T., Donaldson M.G. Exercise and cognition in older adults: is there a role for resistance training programmes. Br. J. Sports Med. 2009;43(1):25–27. doi: 10.1136/bjsm.2008.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D.…Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017 doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Mars R.B., Neubert F.X., Noonan M.P., Sallet J., Toni I., Rushworth M.F. On the relationship between the “default mode network” and the “social brain. Front. Hum. Neurosci. 2012;6:189. doi: 10.3389/fnhum.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruszak A., Thuret S. Why looking at the whole hippocampus is not enough-a critical role for anteroposterior axis, subfield and activation analyses to enhance predictive value of hippocampal changes for Alzheimer's disease diagnosis. Front. Cell. Neurosci. 2014;8:95. doi: 10.3389/fncel.2014.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavros Y., Gates N., Wilson G.C., Jain N., Meiklejohn J., Brodaty H.…Fiatarone Singh M.A. Mediation of cognitive function improvements by strength gains after resistance training in older adults with mild cognitive impairment: outcomes of the study of mental and resistance training. J. Am. Geriatr. Soc. 2017;65(3):550–559. doi: 10.1111/jgs.14542. [DOI] [PubMed] [Google Scholar]
- Morra J.H., Tu Z., Apostolova L.G., Green A.E., Avedissian C., Madsen S.K.… Automated 3 D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer's disease, mild cognitive impairment, and elderly controls. Hum. Brain Mapp. 2009;30(9):2766–2788. doi: 10.1002/hbm.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu L.S., Handy T.C., Hsu C.L., Voss M., Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch. Intern. Med. 2012;172(8):666–668. doi: 10.1001/archinternmed.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngandu T., Lehtisalo J., Solomon A., Levalahti E., Ahtiluoto S., Antikainen R.…Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- Norton S., Matthews F.E., Barnes D.E., Yaffe K., Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13(8):788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. An allocentric spatial model for the hippocampal cognitive map. Hippocampus. 1991;1(3):230–235. doi: 10.1002/hipo.450010303. [DOI] [PubMed] [Google Scholar]
- O'Mara S.M., Commins S., Anderson M. Synaptic plasticity in the hippocampal area CA1-subiculum projection: implications for theories of memory. Hippocampus. 2000;10(4):447–456. doi: 10.1002/1098-1063(2000)10:4<447::AID-HIPO11>3.0.CO;2-2. 10.1002/1098-1063(2000)10:4<447::AID-HIPO11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ongur D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10(3):206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Peterson M.D., Gordon P.M. Resistance exercise for the aging adult: clinical implications and prescription guidelines. Am. J. Med. 2011;124(3):194–198. doi: 10.1016/j.amjmed.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Rebok G.W., Ball K., Guey L.T., Jones R.N., Kim H.Y., King J.W.…Group A.S. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J. Am. Geriatr. Soc. 2014;62(1):16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz C., Brickman A.M., Brown T.R., Manly J., DeCarli C., Small S.A., Mayeux R. Linking hippocampal structure and function to memory performance in an aging population. Arch. Neurol. 2009;66(11):1385–1392. doi: 10.1001/archneurol.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E., Van den Heuvel E., Moll van Charante E.P., Achthoven L., Vermeulen M., Bindels P.J., Van Gool W.A. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer Dis. Assoc. Disord. 2009;23(3):198–204. doi: 10.1097/WAD.0b013e31819783a4. [DOI] [PubMed] [Google Scholar]
- Rossler M., Zarski R., Bohl J., Ohm T.G. Stage-dependent and sector-specific neuronal loss in hippocampus during Alzheimer's disease. Acta Neuropathol. 2002;103(4):363–369. doi: 10.1007/s00401-001-0475-7. [DOI] [PubMed] [Google Scholar]
- Scarmeas N. Dementia: multimodal dementia prevention – does trial design mask efficacy. Nat. Rev. Neurol. 2017;13(6):322–323. doi: 10.1038/nrneurol.2017.73. [DOI] [PubMed] [Google Scholar]
- Scheff S.W., Price D.A., Schmitt F.A., Mufson E.J. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol. Aging. 2006;27(10):1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schneider J.A., Arvanitakis Z., Leurgans S.E., Bennett D.A. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann. Neurol. 2009;66(2):200–208. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Liu B., Zhou Y., Yu C., Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and Alzheimer's disease: meta-analyses of MRI studies. Hippocampus. 2009;19(11):1055–1064. doi: 10.1002/hipo.20573. [DOI] [PubMed] [Google Scholar]
- Smith P.J., Blumenthal J.A., Hoffman B.M., Cooper H., Strauman T.A., Welsh-Bohmer K.…Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom. Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo C., Singh M.F., Gates N., Wen W., Sachdev P., Brodaty H.…Valenzuela M.J. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry. 2016;21(11):1645. doi: 10.1038/mp.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ten Brinke L.F., Bolandzadeh N., Nagamatsu L.S., Hsu C.L., Davis J.C., Miran-Khan K., Liu-Ambrose T. Aerobic exercise increases hippocampal volume in older women with probable mild cognitive impairment: a 6-month randomised controlled trial. Br. J. Sports Med. 2015;49(4):248–254. doi: 10.1136/bjsports-2013-093184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellas B., Carrie I., Gillette-Guyonnet S., Touchon J., Dantoine T., Dartigues J.F.…Andrieu S. Mapt study: a multidomain approach for preventing Alzheimer's disease: design and baseline data. J. Prev. Alzheimers Dis. 2014;1(1):13–22. [PMC free article] [PubMed] [Google Scholar]
- Vincent J.L., Kahn I., Snyder A.Z., Raichle M.E., Buckner R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100(6):3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss M.W., Prakash R.S., Erickson K.I., Basak C., Chaddock L., Kim J.S.…Kramer A.F. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front. Aging Neurosci. 2010;2 doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C., Yu J.T., Wang H.F., Tan C.C., Meng X.F., Tan L. Non-pharmacological interventions for patients with mild cognitive impairment: a meta-analysis of randomized controlled trials of cognition-based and exercise interventions. J. Alzheimers Dis. 2014;42(2):663–678. doi: 10.3233/JAD-140660. [DOI] [PubMed] [Google Scholar]
- West M.J., Coleman P.D., Flood D.G., Troncoso J.C. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer's disease. Lancet. 1994;344(8925):769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Wrann C.D., White J.P., Salogiannnis J., Laznik-Bogoslavski D., Wu J., Ma D.…Spiegelman B.M. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab. 2013;18(5):649–659. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine H.N., Schneider L.S. Lessons from the multidomain alzheimer preventive trial. Lancet Neurol. 2017;16(8):585–586. doi: 10.1016/S1474-4422(17)30227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J., Angevaren M., Rusted J., Tabet N. Aerobic exercise to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst. Rev. 2015;4 doi: 10.1002/14651858.CD005381.pub4. CD005381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich P.A., Amaral R.S., Augustinack J.C., Bender A.R., Bernstein J.D., Boccardi M.…Hippocampal Subfields G. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage. 2015;111:526–541. doi: 10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Dougherty J.H., Hubner K.F., Bai B., Cannon R.L., Hutson R.K. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer's disease and mild cognitive impairment. Alzheimers Dement. 2008;4(4):265–270. doi: 10.1016/j.jalz.2008.04.006. Jr. [DOI] [PubMed] [Google Scholar]
- Zissimopoulos J., Crimmins E., Clair St. The value of delaying Alzheimer's disease onset. Forum Health Econ. Policy. 2014;18(1):25–39. doi: 10.1515/fhep-2014-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.