Summary
Using high-resolution diffusion magnetic resonance imaging (dMRI) and a suite of old and new staining techniques, the beginnings of a multi-scale connectome map of the squid brain is erected. The first of its kind for a cephalopod, this includes the confirmation of 281 known connections with the addition of 145 previously undescribed pathways. These and other features suggest a suite of functional attributes, including (1) retinotopic organization through the optic lobes and into other brain areas well beyond that previously recognized, (2) a level of complexity and sub-division in the basal lobe supporting ideas of convergence with the vertebrate basal ganglia, and (3) differential lobe-dependent growth rates that mirror complexity and transitions in ontogeny.
Subject Areas: Biological Sciences, Neuroscience, Systems Neuroscience
Graphical Abstract
Highlights
-
•
The first MRI-based connectome in a cephalopod
-
•
Retinotopic organization through the optic lobes and into other brain areas
-
•
Subdivided basal lobe system defines topographic information from optic lobes
-
•
A new chiasm is proposed to coordinate vision and countershading camouflage
Biological Sciences; Neuroscience; Systems Neuroscience
Introduction
Cephalopods have the most complicated central nervous system (CNS) of all invertebrates at both anatomical and functional levels as demonstrated by the pioneering neuroanatomical work of Cajal and Young decades or indeed a century ago (Cajal, 1917, Young, 1961, Young, 1971, Young, 1974, Young, 1976, Young, 1977, Young, 1979). The brains of all coleoid cephalopod groups (octopus, cuttlefish, and squid but not nautilus) are built around a circum-esophageal set of ganglia or lobes that have expanded dramatically (Figures 1 and S1 and Videos S1 and S2). In particular, their complex visual system and limb-based tactile capability set the cephalopods apart from other molluscs (Figure 1) (Wells and Wells, 1957, Bullock and Horridge, 1965, Nixon and Young, 2003, Chung and Marshall, 2014, Liu and Chiao, 2017, Shigeno et al., 2018). Early work on the organization of the cephalopod sensory and motor control systems combined with comparative studies in behavioral changes before and after brain-region ablation suggested a useful model for studying sensory function, learning, and memory (Boycott and Young, 1955, Boycott and Young, 1957, Wells and Wells, 1957, Boycott, 1961, Boycott, 1965, Young, 1971, Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979, Abbott et al., 1995). Based on distinct morphological and functional features, the coleoid cephalopod brain can be divided into four major divisions: (1) the vertical lobe complex (learning and memory); (2) a pair of optic lobes (vision-related tasks); (3) supraoesophageal mass (higher motor control centers coordinating sensory inputs and behavioral responses); (4) suboesophageal mass (lower motor control centers executing simple movement of fins and arms, and mantle activities for respiration) (Video S2). A detailed list of categories of sub-regions within the lobe systems can be found in Table 1. However, more recent model systems in neuroscience have moved away from the cephalopods and, with few exceptions, their functional neuroanatomy has languished (Boycott, 1961, Miyan and Messenger, 1995, Brown et al., 2006, Zullo et al., 2009, Liu and Chiao, 2017, Shomrat et al., 2008).
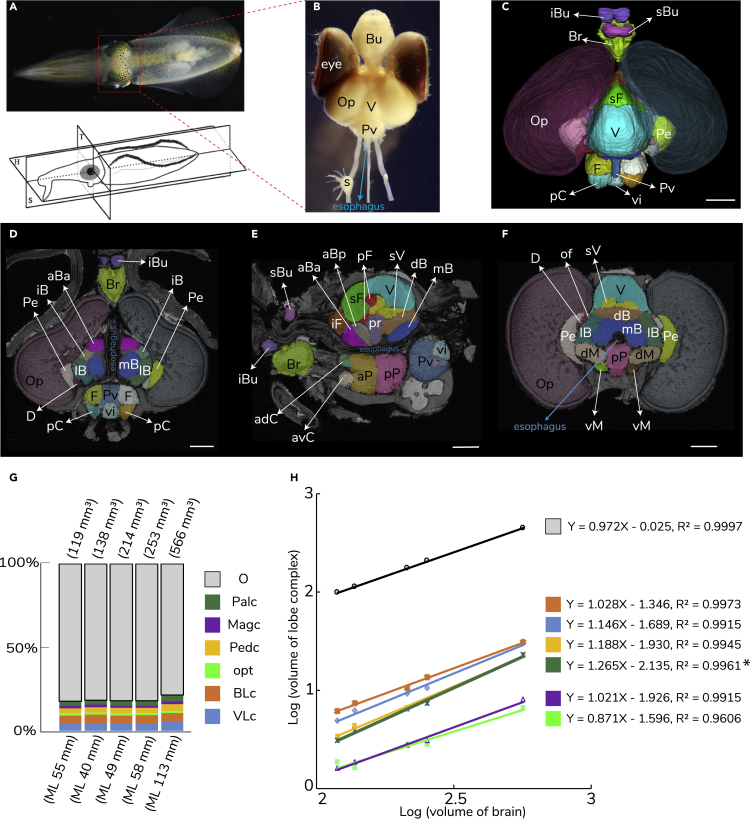
Figure 1.
Squid (Sepioteuthis lessoniana) Multi-lobed Brain
A juvenile reef squid brain.
(A) Close-up of squid head. Live specimen (dorsal view) and a schematic drawing represents three anatomical planes.
(B) Isolated squid brain and eyes. Bu, buccal mass; Op, optic lobe; Pv, palliovisceral lobe; s, stellate ganglion; V, vertical lobe.
(C) MRI-based 3D reconstruction of squid multi-lobed brain. Br, brachial lobe; F, fin; iBu, inferior buccal; pC, posterior chromatophore; Pe, peduncle; sBu, superior buccal; sF, superior frontal; vi, visceral.
(D–F) Parcellation of the squid brain derived by anatomical landmarks. (D) Sagittal section. (E) Transverse section. (F) Horizontal section. aBa, Anterior basal lobe; adC, anterior dorsal chromatophore; aBp, anterior posterior basal; aP, anterior pedal; avC, anterior ventral chromatophore; dB, dorsal basal; dM, dorsal magnocellular; D, dorsolateral; iB, interbasal; iF, inferior frontal; lB, lateral basal; mB, median basal; pP, posterior pedal; pF, posterior frontal; pr, precommisural; sV, subvertical; vM, ventral magnocellular. Scale bar: 1 mm.
(G) Brain volume and percentage of each lobe complex volume in five squid individuals. Owing to partial damage of brachial and buccal lobes in two specimens, the brain volume excludes the brachial lobe complex. ML, mantle length. Color-coded symbols represent the lobe complex as: BLc, basal lobe complex; Magc, magnocellular lobe complex; O, optic lobes; opt, optic track complex; Palc, palliovisceral lobe complex; Pedc, pedal lobe complex; VLc, vertical lobe complex. Detailed volumetric data can be found in Table S1. All abbreviations also in Table 1.
(H) Allometric analysis of the lobe complex. Color-coded regression line for each lobe complex showing significant variance of the slopes among seven regression lines (ANOVA, df = 6, p < 0.0001). In particular, the palliovisceral lobe complex volume scales with positive allometry (slope 1.2645) and with a 95% confidence interval around the slope (1.1167–1.4124) excludes 1 (isometry), indicating that this lobe complex grows disproportionately and its disproportionality increases with increased volume of the brain.
Table 1.
List of Squid Brain Lobes with Abbreviations Used through the Text
| Lobe System and Function | Lobe | Abbreviation |
|---|---|---|
| Vertical lobe complex (VLc)—memory and learning | Inferior frontal | iF |
| Superior frontal | sF | |
| Posterior frontal | pF | |
| Subvertical | sV | |
| Vertical | V | |
| Basal lobe complex (BLc)—higher motor control | Anterior anterior basal | aBa |
| Anterior posterior basal | aBp | |
| Precommisural | pr | |
| Dorsal basal* | dB | |
| Interbasal* | iB | |
| Median basal | mB | |
| Lateral basal* | lB | |
| Optic track complex (opt)—intermediate visual-motor center and olfaction | Peduncle* | Pe |
| Olfactory* | of | |
| Dorsolateral* | D | |
| Brachial lobe complex (Brc)—arm and feeding control | Inferior buccal | iBu |
| Superior buccal | sBu | |
| Brachial | Br | |
| Pedal lobe complex (Pedc)—intermediate and lower motor center for locomotion control | Anterior dorsal chromatophore* Ψ | adC |
| Anterior ventral chromatophore* Ψ | avC | |
| Anterior pedal | aP | |
| Lateral pedal* | lP | |
| Posterior pedal | pP | |
| Magnocellular lobe complex (Magc)—intermediate motor center | Dorsal magnocellular* | dM |
| Ventral magnocellular* | vM | |
| Posterior magnocellular* | pM | |
| Palliovisceral lobe complex (Palc)—lower motor center for locomotion and mantle activities | Palliovisceral | Pv |
| Lateral ventral palliovisceral* | lvP | |
| Fin* | F | |
| Posterior chromatophore* | pC | |
| Visceral | vi | |
| Optic lobes (O)—vision | Optic* | Op |
The main functions of the lobe systems based on work by Young and his colleagues (Messenger, 1979, Young, 1961, Young, 1971, Young, 1974, Young, 1976, Young, 1977, Young, 1979, Boycott and Young, 1955, Boycott and Young, 1957, Wells and Wells, 1957, Nixon and Young, 2003). Supraoesophageal mass includes basal lobe and optic track complexes. Suboesophageal mass consists of the brachial lobe, pedal lobe, magnocellular lobe, and palliovisceral lobe complexes. * indicates that the lobe is further divided into the left and right lobe. Ψ indicates a further sub-division of the anterior chromatophore lobes into dorsal and ventral halves.
Two series of transverse sections show the anatomical features (top) and the corresponding direction-encoded color map of fiber orientation distribution (FOD) (bottom) along with the long body axis (from anterior end [the inferior buccal lobe] to posterior end [visceral lobe]). The color-coded tensors show the direction of water diffusion along with neural bundles related to the body axes. A, anterior; D, dorsal; L, lateral.
The color-coded neural tracts show the orientation of neural bundles related to the body axes. A, anterior; D, dorsal; L, lateral.
By contrast, several studies have focused on the behavioral neurobiology of the cephalopods and their remarkably rapid and apparently smart reactions to novel challenges. Such behaviors suggest that the coleoids at least have developed alternative ways of problem solving compared with standard model species such as mice, zebrafish, and fruit flies (Darmaillacq et al., 2014, Bublitz et al., 2017, Liscovitch-Brauer et al., 2017, Hanlon and Messenger, 2018, Schnell and Clayton, 2019). Most famous cases in this respect are the remarkable color-blind camouflage, mimicry, and other communication abilities that they are capable of (Marshall and Messenger, 1996, Chung and Marshall, 2016, Lin et al., 2017, How et al., 2017, Hanlon and Messenger, 2018). Other studies reveal the richness of cephalopod behavioral repertoires (e.g., pattern recognition, bipedal walking, mate guarding, social cognition, and observational learning) and suggest that some instances are comparable with the abilities of higher vertebrates (Sutherland et al., 1963, Fiorito and Scotto, 1992, Darmaillacq et al., 2014, Bublitz et al., 2017, Hanlon and Messenger, 2018, Schnell and Clayton, 2019). Interestingly, some of these studies suggest that a somatotopic relationship from brain to the outside world is lacking (Zullo et al., 2009, Liu and Chiao, 2017), but results we present here as well as recent suggestions from others (Grasso, 2014, Shigeno et al., 2018) suggest that this visual world to motor output does exist.
Despite intense interest and research progress concerning cephalopod complex behavioral and cognitive abilities, even for the common octopus, Octopus vulgaris, which is the most well-studied species (Boycott and Young, 1955, Boycott and Young, 1957, Young, 1961, Young, 1971, Sutherland et al., 1963, Fiorito and Scotto, 1992, Nixon and Young, 2003, Shomrat et al., 2008, Zullo et al., 2009, Wollesen et al., 2012, Darmaillacq et al., 2014), understanding its brain-wide neural network and the associated functional circuits remains incomplete. Our current knowledge of cephalopod nervous systems is derived from multiple levels and may be summarized in the following categories: (1) decoding the genomic sequence (Albertin et al., 2015, Liscovitch-Brauer et al., 2017, Edsinger and Dölen, 2018); (2) molecular and synaptic activities (Shomrat et al., 2008, Wollesen et al., 2012, Lee et al., 2013, Shigeno and Ragsdale, 2015, Edsinger and Dölen, 2018); (3) electrophysiological studies (Miyan and Messenger, 1995, Chrachri and Williamson, 1998, Shomrat et al., 2008, Hu et al., 2009, Zullo et al., 2009, Liu and Chiao, 2017); (4) gross neural anatomy and connectivity using classical histology (Cajal, 1917, Young, 1971, Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979, Maddock and Young, 1987, Shigeno et al., 2001, Nixon and Young, 2003, Wollesen et al., 2009, Kobayashi et al., 2013, Wild et al., 2015, Koizumi et al., 2016); (5) magnetic resonance imaging (MRI) of the brain (Chung and Marshall, 2014, Chung and Marshall, 2017, Liu et al., 2018). It is in this latter category, using modern neuroanatomical techniques, that we aim to contribute toward describing new neural pathways and the behaviors they mediate, and we start with the reef squid, Sepioteuthis lessoniana. This loliginid species has been studied extensively in terms of its sensory ecology and behavior making it a suitable first subject (Hu et al., 2009, Sugimoto and Ikeda, 2012, Chung and Marshall, 2014, Chung and Marshall, 2016, Lin et al., 2017, Lin and Chiao, 2017, Liu and Chiao, 2017, Lu and Chung, 2017). It is also closely related to previously studied loliginid squid species (Anderson, 2000, Messenger, 1979, Strugnell et al., 2005, Vecchione et al., 1998, Young, 1974, Young, 1976, Young, 1977, Young, 1979), allowing confidence in the cross-species comparisons made here between old and new methods. Descriptions of cuttlefish, coastal benthic octopuses, and vampire squid brain connectomes using similar techniques are ongoing.
One major challenge in mapping any brain is in the untangling of inter-woven neural fibers into the discrete bundles related to specific functional circuits rather than those that are simply anatomically concomitant. With this in mind, three long-standing problems have produced variable results in cephalopods: the inconsistent stochastic nature of silver staining methods, practical difficulties in examining a large-sized brain, and the methodological constraint of classical histology to a single section angle per specimen (Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979, Valverde, 1998).
The large-scale, unfamiliar mollusc plan and neuronal diversity of cephalopods make a synapse-level wiring diagram unlikely in the near future. For other invertebrates with smaller brains, such as Drosophila and nematode worms, these detailed connectomes are becoming reality (Cook et al., 2019, Meinertzhagen, 2018, Shih et al., 2015, Takemura et al., 2017, White et al., 1986) and are an important first step in understanding function and cognition in any animal. The brain of a mouse, which is around the same size as the squid used in this study, now has what is described as a complete connectome at mesoscale (Oh et al., 2014, Liu et al., 2016). The mouse Allen Brain Atlas (ABA) is based on a huge quantity of histological and neuronal tracing data and is now beginning to be supported by cross comparisons with high-resolution (9.4 and 16.4 Tesla) MRI (Calamante et al., 2012, Kurniawan et al., 2014, Calabrese et al., 2015, Liu et al., 2016) as well as serial block face electron microscopy (Deweerdt, 2019). The same basic cross-correlative ideas are applied here to map and model the elements and potential interactions of the squid brain (Bullmore and Sporns, 2009, Bassett and Sporns, 2017, Assaf et al., 2019). As noted in mouse and other vertebrate work (Jbabdi and Johansen-Berg, 2011, Donahue et al., 2016, Maier-Hein et al., 2017, Aydogan et al., 2018, Suarez et al., 2018, Sotiropoulos and Zalesky, 2019), several caveats should be applied to probabilistic MRI-based tractography, and it is often best viewed as supportive and predictive rather than a fully validated functional connectome.
Several morphological and methodological factors detailed below allow a closer correlation and greater confidence of anatomical accuracy from pathways predicted from MRI projections alone in squid compared with those in mouse. First, the invertebrate nervous systems share a more similar layout between individuals than the vertebrates (Bullock and Horridge, 1965, Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979, Nixon and Young, 2003, Strausfeld, 2005, Strausfeld et al., 2016, Suarez et al., 2018). Also, organization of brain lobes and the known underlying connections share a high degree of similarity across close phylogenetic groups (e.g., cuttlefish and squid) (Cajal, 1917, Boycott, 1961, Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979, Nixon and Young, 2003, Wild et al., 2015). Therefore, structural features, including individual cells and wiring patterns, may be reliably identified and re-visited between individuals or indeed between species and used to predict function. In addition, the higher signal-to-noise ratio and long scan time in the 16.4 T MRI (compared with 9.4 T) and highly conservative acceptance threshold (detail in Methods) that we use bring the scale of MRI and direct anatomical methods closer than in previous studies (Calamante et al., 2012, Kurniawan et al., 2014, Alomair et al., 2015, Ziegler et al., 2018). Combined with congruence of both previous (Cajal, 1917, Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979) and the current study and anatomical tract data with MRI-predicted projections, we may be more confident that the new regional interconnections proposed here are real.
Connectomes and brain maps confirm known functional interactions and where detailed enough may also predict function (Jbabdi and Johansen-Berg, 2011, Jbabdi et al., 2013, Shih et al., 2015, Meinertzhagen, 2018). Our findings in squid and possible new neuronal functions that they suggest may be summarized as follows:
-
(1)
A similar number of lobes were found using MRI as by the traditional serial sectioning techniques of, e.g., Young (Young, 1974, Young, 1976, Young, 1977, Young, 1979, Nixon and Young, 2003), but MRI delineated these much more clearly, allowing more accurate volumetric analysis to define differentials of lobe growth rate (Figures 1, S1, Tables 1, and S1).
-
(2)
Tractography from five individuals combined with over 1,000 silver-stained sections and fluorescent neural tracers defined 145 new major pathways in the brain along with 99.65% of the previously known ones (Figures 2, 3, 4, and 5).
-
(3)
Differentials of lobe growth and increasing complexity of the underlying neural network during growth mirror the sophisticated behavior repertoires that emerge during ontogeny (Figures 1G and 1H, 5D, S1, and Table S1).
-
(4)
New aspects of spatial organization were uncovered in the optic lobes, including a preservation of retinotopicity to deeper layers than previously known. This topographic information could also project through to the basal lobe system (Figures 6 and 7 and Video S5).
-
(5)
Within the basal lobe system, a new network of sub-divisions and interconnections was elucidated, probably coordinating visual and motor activity such as color change and body orientation (Figures 5, 6, 7, and 8). This provides another example of convergent evolution between cephalopods and vertebrates and supports recent hypotheses around a common brain-to-body bauplan convergence in many animals (Shigeno et al., 2018).
-
(6)
A previously undescribed second chiasm between the lateral basal lobe and the anterior chromatophore lobes reorients the visual scene to the chromatophore display (Figures 8C–8E). This may enable coordination of camouflage and communication in body patterning.
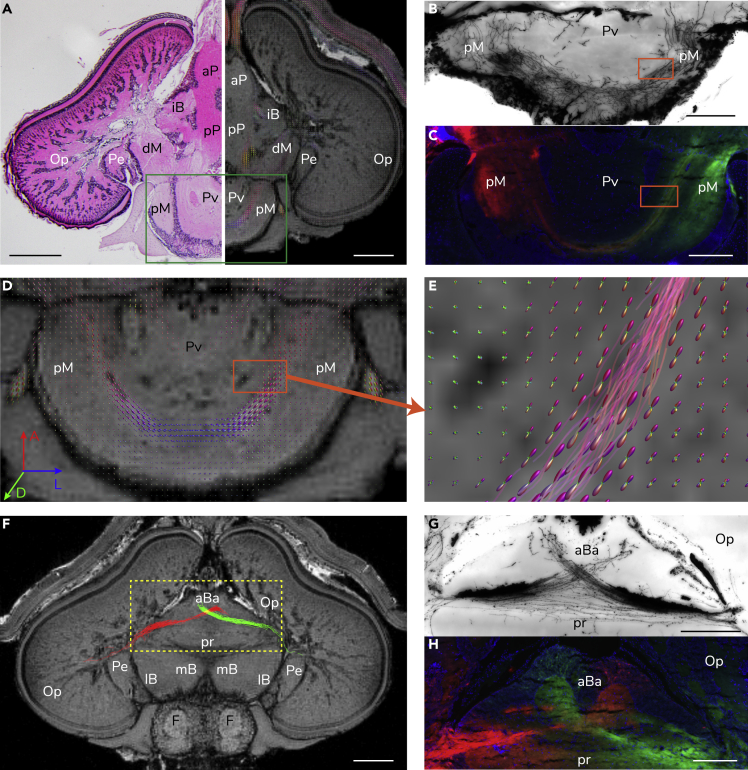
Figure 2.
Cross-validation of the Squid Neural Tracts
(A) A comparison between conventional histology (left) (10-μm slice stained with hematoxylin and eosin) and magnetic resonance histology (right) (isotropic resolution 30 μm). The green rectangle indicates the posterior suboesophageal mass.
(B–E) An example of well-matched fiber orientation distribution (FOD) of neural tracts (orange rectangles) of the posterior magnocellular lobe (pM), which were validated among (B) Cajal-Golgi impregnation (40-μm slice), (C) fluorescent lipophilic dye tracers (25-μm slice), and (D) FOD. (E) A close-up of FOD and the corresponding streamlines (probabilistic tractography of the right pM), which were reconstructed using the optimized algorithm and acceptance criteria. The direction-encoded colors indicate orientation of body axis. A, anterior; D, dorsal; L, lateral.
(F–H) A sample of the matched neural tracts derived from two small regions of interest (each ROI voxel = 120 μm × 120 μm × 120 μm) in the anterior-median lobule of the anterior basal lobe (aBa) among (F) probabilistic tractography, (G) Cajal-Golgi impregnation (40-μm slice), and (H) fluorescent stained contralateral tracts (25-μm slice). Scale bar: 1 mm.
See also Figures S2 and S3.
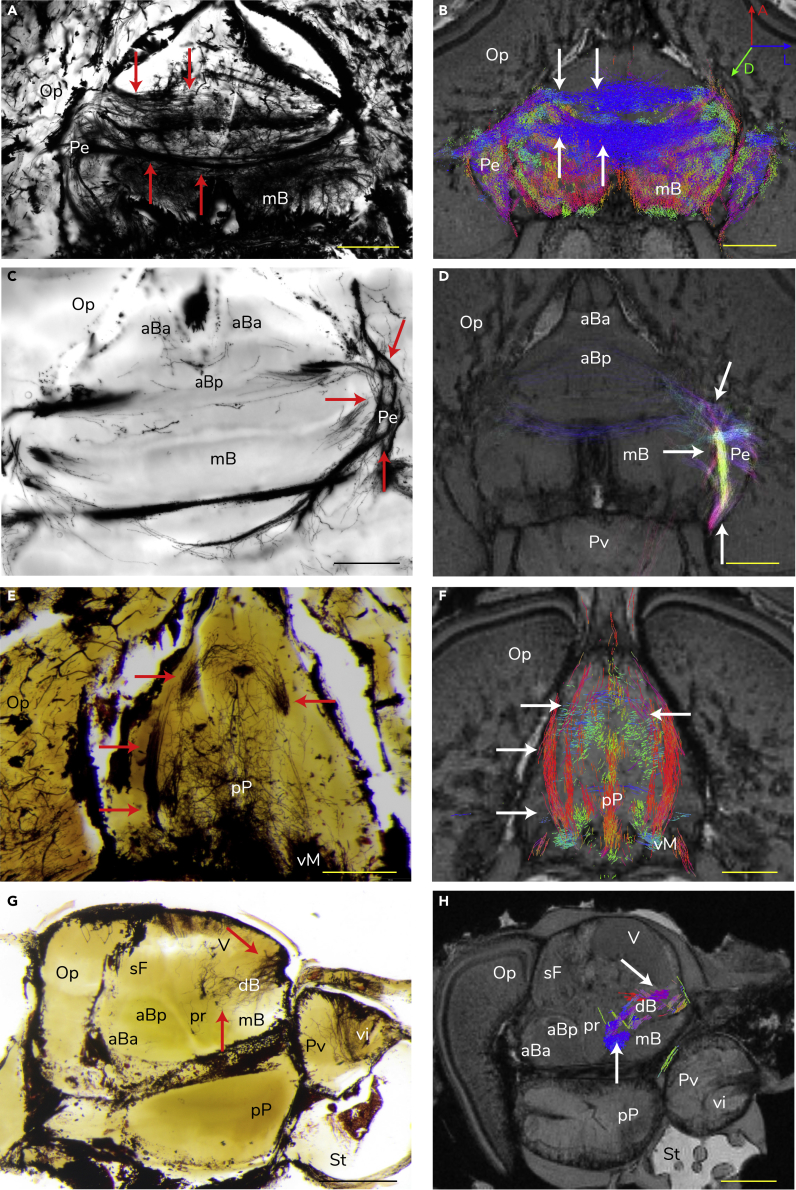
Figure 3.
Comparisons of the Squid Neural Tract Counterparts between the Cajal-Golgi Impregnation and Tractography
Four pairs of examples showed the well-matched major tracts between two methods. Red arrows indicate silver stained neural bundles and white arrows indicate the tractographic counterparts. The color-coded neural tracts of tractography show the orientation of neural bundles equivalent to the body axes. A, anterior; D, dorsal; L, lateral. Scale bar: 1 mm. (A and B) Major neural bundles derived from the median basal lobe (mB) (horizontal section).
(C and D) Neural bundles associated within the peduncle lobe (Pe) (horizontal section).
(E and F) Neural bundles linked to the posterior pedal lobe (pP) and the ventral magnocellular lobe (vM) (horizontal section).
(G and H) Neural bundles in the dorsal basal lobe (dB) (sagittal section).
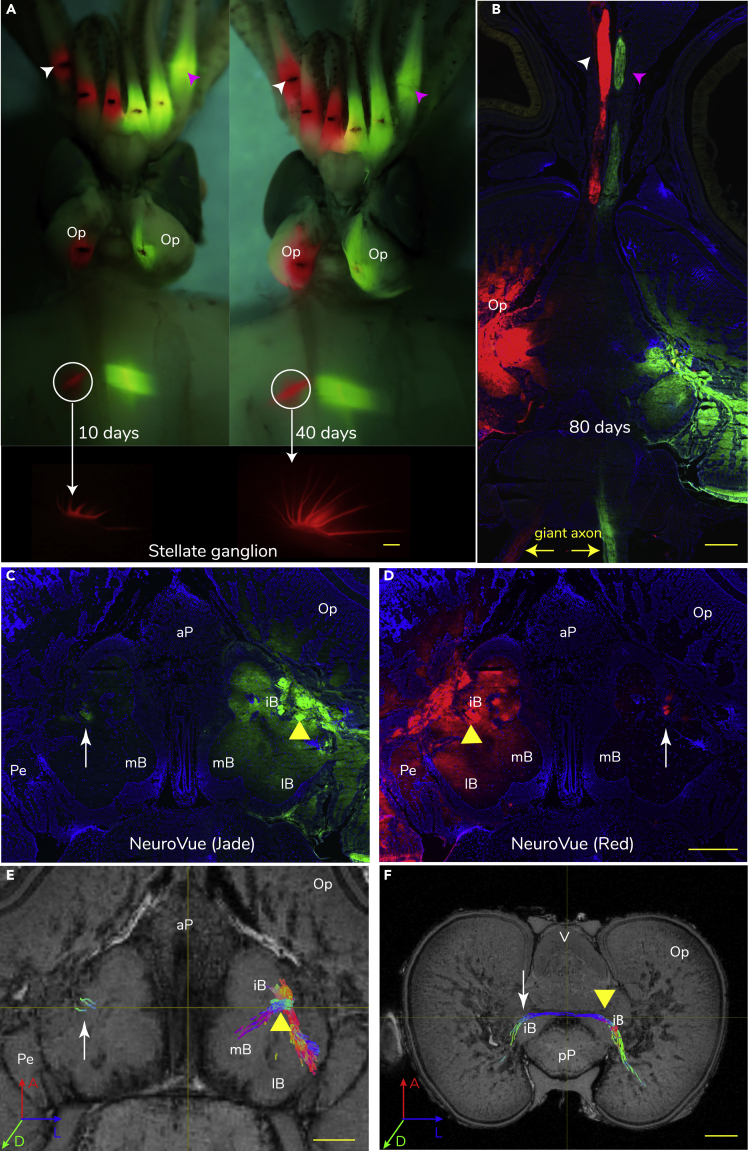
Figure 4.
Lipophilic Dye Tracing of the Squid Neural Tracts
(A and B) An example of fluorescent dyes diffusion down squid neural tissue in a time series. (A) The lipophilic dye tracers, NeuroVue red (left) and jade (right), were applied symmetrically to parts of the squid neural tissue, including arms, optic lobes, and stellate ganglia. Arrows indicate the stellate ganglion; arrow heads indicate the dye diffusing along the brachial nerve bundles. (B) A sample of slice showing that both dyes diffused along the brachial nerves of the third pair of arms with similar speed (arrow heads). Two yellow arrows indicate the stained giant axon, which the dyes were loaded into the stellate ganglion.
(C–E) Comparisons of the contralateral connection of the interbasal lobe (iB) tracts between the fluorescent dye tracing and tractography (horizontal section). (C and D) Triangles indicate that two dyes were loaded separately in the left and right iB, and arrows show the dye diffusion toward the contralateral side of iB. (E) Tractography projection of iB showing a good match to the dye tracing shown above.
(F) The same iB tractography was viewed in the transverse section. The color-coded neural tracts show the orientation of neural bundles equivalent to the body axes. A, anterior; D, dorsal; L, lateral. Scale bar: 1 mm.
Figure 5.
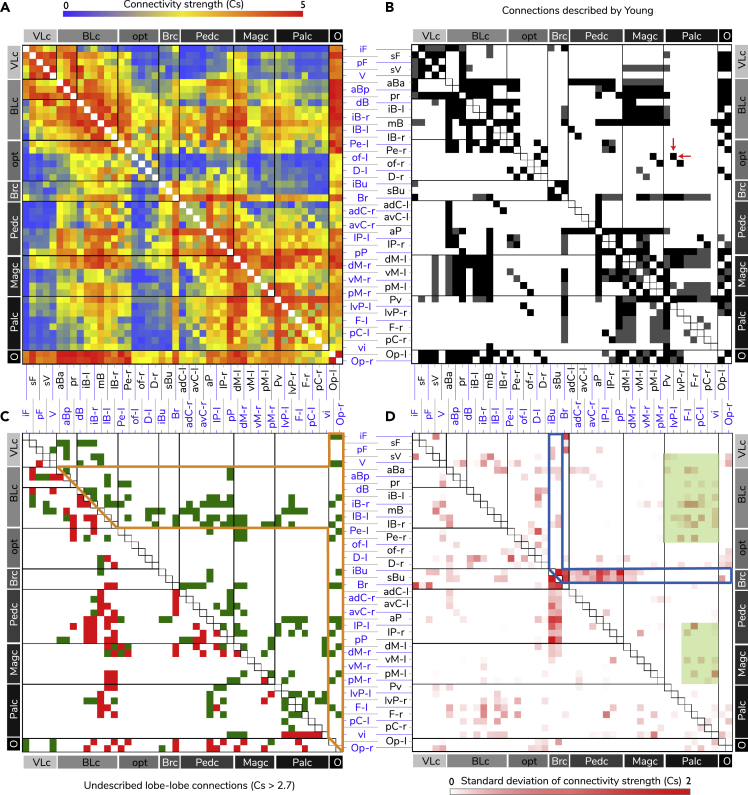
Connectivity Matrices of the Squid Brain
(A) An averaged probabilistic tractography connectivity matrix of five individuals. The heatmap indicates log10-transfered connection strength (Cs).
(B) This matrix summarized all described lobe-lobe neural connections of the loliginid squid, including 282 neural connections based on the Cajal-Golgi impregnation results by J.Z. Young and his colleagues (Young, 1974, Young, 1976; 1977, 1979; Messenger, 1979). Black squares indicate the well-defined tracts; gray squares are the partially stained ones that were defined as possible tracts by Young. Red arrows indicate the only tract (between olfactory lobe and the lateral-ventral palliovisceral lobe) that was not recovered from the averaged tractography matrix.
(C) The new lobe-to-lobe tracts and their distribution pattern. This matrix is visualized against the known 282 tracts and is subtracted from all tracts recovered by the selected conservative Cs of 2.7, suggesting 145 new tracts (green squares) where 93 have been confirmed by histology (red squares). The highlighted region (within the orange line) contains over 62% of these new tracts for which the tractographic connection patterns are likely to response to the visual-motor control.
(D) Matrix summarizing the pairwise connections (red squares) with a high degree of variation of Cs among the five individuals. The region within the blue line shows high variation of Cs due to two damaged buccal lobes in two samples (see Figure S1B and Table S1) during brain extraction and a resulting low connection strength. The green shaded areas contain regions showing rapid lobe enlargement (lower motor control-related lobes) inducing increased values of Cs.
See also Figures S1, S3, and S4; Table S1; Videos S2, S3, S4, and S5.
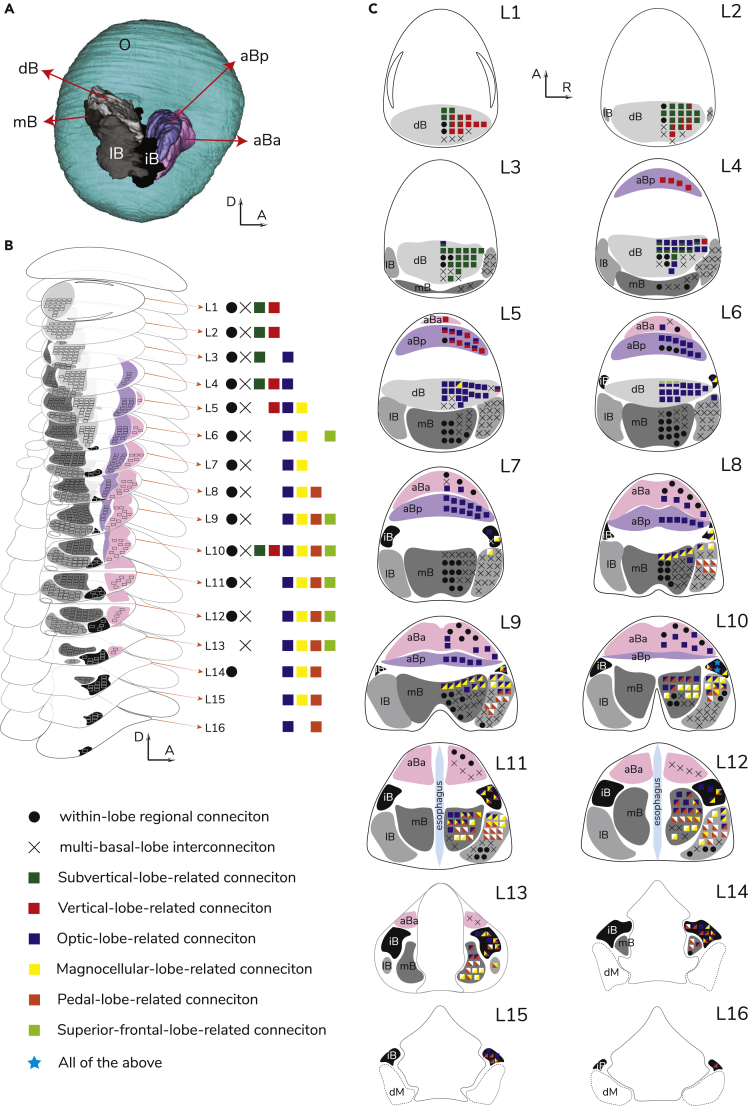
Figure 6.
The Neural Network of the Multi-layered Basal Lobe System
(A) 3D reconstruction of the basal-optic lobe system. D, dorsal; A, anterior; R, right side of animal. Other abbreviations as in Table 1.
(B) Schematic drawings represented the subdivided small regions of interest (ROIs color and symbol coded as in Key) (n = 604) in the right half of the basal lobe system throughout the dorsoventral axis (16 levels L1–L16, each level thickness is 120 μm).
(C) Schematic drawings represented the dominant connectivity patterns between the ROIs and the projection lobe(s) throughout this system. Along with tractography derived from these small ROIs, the dominant connectivity of each ROI is identified by the color-coded symbols, revealing the detailed tractographic network of this high-order visual-motor control center. Two blue stars in the interbasal lobe at level 10 indicate that all eight types of connections can be found in these two ROIs.
See also Video S5.
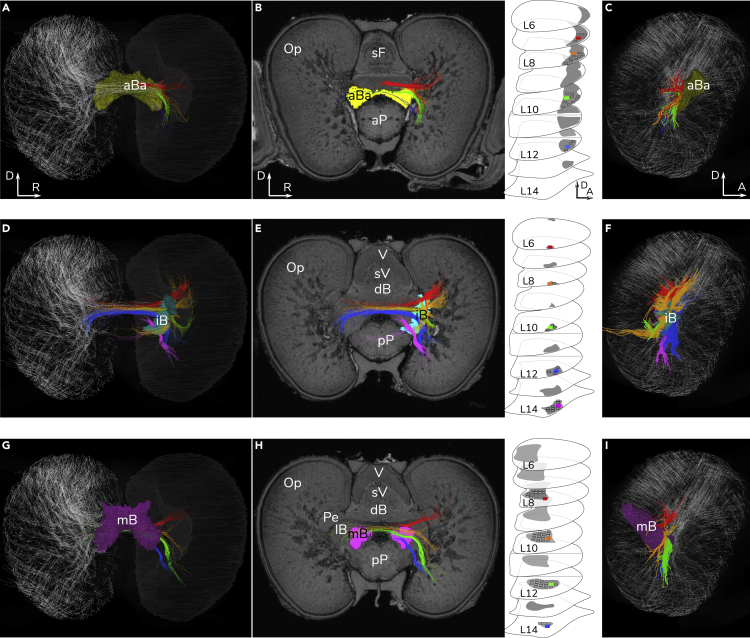
Figure 7.
Topographic Representation in the Optic Lobe and Basal Lobe Connections
(A–C) Anterior basal lobe (aBa). D, dorsal; A, anterior; R, right side of animal. (A) 3D reconstruction of this optic-basal system. To visualize the grid-like network in the medulla of the left optic lobe, 0.5% of the streamlines extracted from the probabilistic tractography of the optic lobe were used here. This structural network may function to keep the peripheral retinotopicity deeper within the medulla of optic lobe and directly couple to the tracts of aBa. (B) Transverse section showing the color-coded aBa tracts of level 7 (red), 8 (orange), 10 (green), and 12 (blue) and the levels of the basal lobe system (the same as Figure 4) shown to the right. The corresponding projections ended in different layers of the medulla (dorsoventral plane). (C) The distribution of the aBa projections inside the medulla viewed from the side. Detailed 3D geometric information of projections can be found in Video S5.
(D–F) Interbasal lobe (iB). (D) 3D reconstruction of the optic-interbasal system and the associating tracts. (E) Transverse section showing the color-coded tracts of level 6 (red), 8 (orange), 10 (green), 12 (blue), and 14 (pink). (F) The distribution of the iB projections inside the medulla.
(G–I) Median basal lobe (mB). (G) 3D reconstruction of the optic-median basal system and the associating tracts. (H) Transverse section showing the color-coded tracts of level 8 (red), 10 (orange), 12 (green), and 14 (blue). (I) The distribution of the mB projections inside the medulla.
See also Video S5.
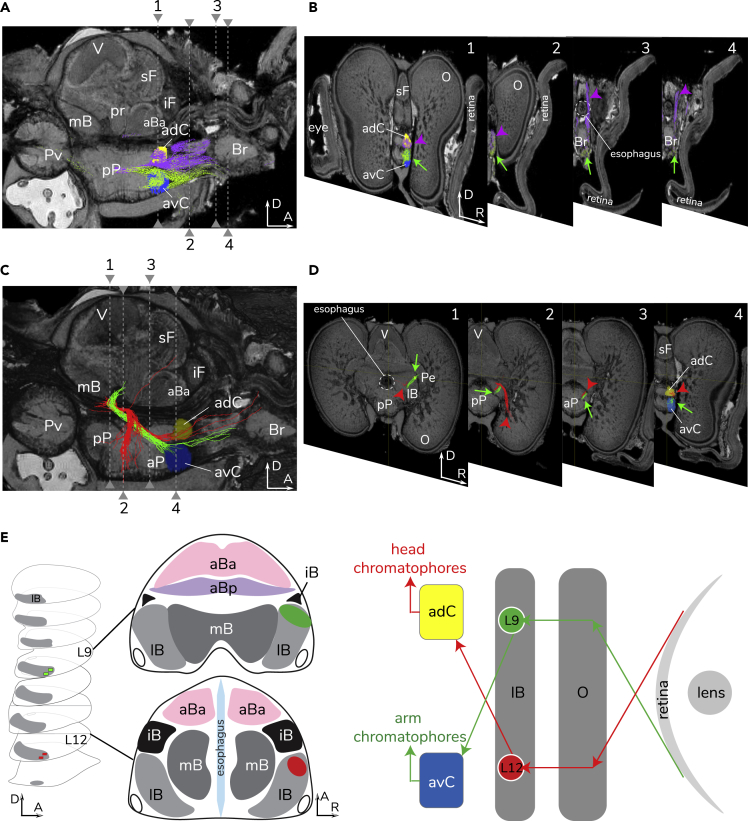
Figure 8.
A Second Chiasm in the Coloration Control Circuit
(A) Two color-coded tracts derived from the two anterior chromatophore lobes, including the purple tracts of the anterior dorsal chromatophore lobe (adC) and the green tracts of the anterior ventral chromatophore lobe (avC). D, dorsal; A, anterior; R, right side of animal.
(B) Four transverse sections (from posterior to anterior) showing the progression of the purple tracts of adC toward the head (arrow heads) and the green tracts of avC toward arms (green arrows).
(C) Two additional circuits proposed here from Levels 9 and 12 of the basal lobe system (see Figure 4, and color-coded green and red, respectively, throughout the figure) linking adC and avC to the lateral basal (lB) and optic (O) lobes (sagittal section), forming a second chiasm.
(D) Four transverse sections (from posterior to anterior) showing the progression of green and red tracts toward the avC (green arrows) and the adC (red arrow heads).
(E) A schematic drawing of this second chiasm and the levels of the lateral basal lobe shown to the left.
It is worth underlining that, although MRI-based tractography has previously attracted a great deal of criticism (Thomas et al., 2014, Maier-Hein et al., 2017, Aydogan et al., 2018, Sotiropoulos and Zalesky, 2019), the methods and cross-checks applied here enable us to suggest that all new tracts proposed are valid (and see Calabrese et al. [2015]). Specifically, in a comparison with the previously known 282 squid neural tracts described by Young and his colleague (Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979), 281 were re-found using the high angular resolution diffusion-weighted imaging (HARDI) method combined with an ultra-conservative level for tractography acceptance employed here. In addition, only 36% of the new tracts proposed remain prediction-only with 64% confirmed through numerous Golgi impregnations or NeuroVue dye. Finally, long-scan-time 16.4 T resolution moves beyond general tract tracing and is closer to the scale of the individual neuron bundle at around 80 μm, reducing false positives and loss of tracing route.
Based largely on the size and neuronal number within the squid brain (Young, 1974, Maddock and Young, 1987), we do not yet claim that what is presented here is a “brain atlas” or a “map” of the squid brain but just the beginning of one. As with vertebrate examples, using the complementary techniques of MRI, traditional histology, and dye-based tract tracing, this putative “circuit diagram” for squid neural architecture will lead to a better understanding of their complex behaviors. It also supports emerging hypotheses around anatomical and functional convergence with parts of the vertebrate CNS, including projections of overall body axes. Notably, it provides a firm base upon which to place the currently fashionable but contentious cephalopod cognitive capabilities. We hope that results here will encourage focal regions to be examined both for the new connections suggested and the actual functions they likely coordinate.
Results
The Multi-lobed Squid Brain and Differentials of Lobe Growth Rate
Contrast-enhanced MR images (isotropic resolution 30 μm) of the brain of S. lessoniana allowed a re-examination of the known lobes of the squid brain and its three-dimensional (3D) detailed structure. Identification of brain lobes was based on the published anatomical studies as an initial aid in determining the boundaries between tissue types (Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979, Nixon and Young, 2003, Chung and Marshall, 2017) (Figures 1 and 2A). The 31 lobes (15 of which are bilateral) defined by Young's work (Young, 1974, Young, 1976, Young, 1977, Young, 1979) on five similar loliginid species (Tables 1, S1, and S2) were identified here, but we suggest a further sub-division of the anterior chromatophore lobe into dorsal and ventral halves based on a clear within-lobe anatomical sub-division (Figures 1E, 8A, 8B, S1A–S1E, Tables 1, and S1). A series of MR image stacks (e.g., 11 slices for an olfactory lobe and 186 slices for an optic lobe of the smallest squid brain) allowed accurate volumetric estimates of the 47 lobes defined here, revealing distinct brain enlargement and corresponding morphological changes of lobes in the rapidly growing juvenile squid (n = 5) (Figures 1G, 1H, S1, and Table S1). Allometric analysis of the lobe complex volume showed significant variance of the slopes among 7 regression lines (ANOVA, df = 6, p < 0.0001). In particular, the palliovisceral lobe complex volume scales with positive allometry (slope 1.2645) and with a 95% confidence interval around the slope (1.1167–1.4124) excludes 1 (isometry). That is, this lobe complex grows disproportionately and its disproportionality increases with increased volume of the brain (Figure 1H).
dMRI and Tractography of the Squid Brain
Using HARDI, 30 diffusion-weighted orientations at a b-value of 3,000 s/mm2 and two b0 images were acquired without diffusion weighting at the isotropic resolution 80 μm (n = 5) using a 16.4-T (700-MHz) vertical wide-bore micro-imaging system (Bruker Biospin, Karlsruhe, Germany) (detail in Methods). Validation of the squid tractography process was applied at multiple levels to quantify the match between the experimental estimated orientation and the true physical orientation of the fiber.
First, an initial comparison between full k-space (scan time approximately 39 h) and partial Fourier acceleration acquisitions (approximately 24 h) confirmed a high degree of similarity in defined fiber orientation distribution (FOD) in two squid specimens (Figures S3A and S3B). This includes, for example, the well-known optic nerves between optic lobes, four pairs of brachial nerves and funnel nerves (Figures S3A and S3B). As a result, with a realistic time frame in terms of scan and computation time in mind, another three specimens, so five in total, were scanned using this acceleration protocol (approximately 24 h for each individual) (Table S1). The following analyses were thus mainly based on data obtained using the accelerated protocol unless specifically noted.
Reconstructions of probabilistic tractography of the squid brain, where regions of interest (ROIs) and FOD are used, vary both in number and orientation with the selected algorithms and parameters chosen (see detail in Methods and Figures 2, 3, and 4). Cross-validation by spatially registering histological results and tractography was conducted, largely following protocols and caveats from previous HARDI brain studies (Valverde, 1998, Calamante et al., 2012, Kurniawan et al., 2014, Calabrese et al., 2015, Suarez et al., 2018). Two histological methods were used, the well-established method of Cajal-Golgi silver impregnation developed by Strausfeld (Strausfeld, 1980) with further modification (Figures 2B and 3) and a new lipophilic dye tracer NeuroVue (Shigeno et al., 2014) (Figures 2C, 2H, and 4) (see detail in Methods). After examining over 1,000 silver stains from 40 individuals, lipophilic dye tracing was then applied specifically to 42 targeted potential tracts to further clarify the silver staining results, particularly those with long contralateral connections that were often stained incompletely by silver impregnation, rendering 30 long tracts confirmed in five individual brains using multiple dye colors (Figures 2C, 2H, and 4B–4D).
Based on fiber geometries obtained from histology in this study and previous work by Young and Messenger (Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979, Nixon and Young, 2003), the selected lobes (e.g., basal, peduncle, and pedal lobes) were then used to test in detecting the choice of local fiber orientation and to optimize matches between tractography and histology (Figures 2, 3, 4, and S2). Imposing neuro-anatomical knowledge (e.g., trajectory of tracts of interest) to eliminate false positives led to increasingly accurate reconstruction of the local fiber architecture and lobe-to-lobe connectivity (Figures 2, 3, 4, 5, and S2). We then used the probabilistic algorithm and the optimized parameters defined by the 375 instances of exact anatomical match (silver-stained neurons or fluorescent tracing) to establish the lobe-dependent probabilistic tractography (Figures 5 and S2).
Squid whole-brain tractography results in an orientationally encoded color tract map, revealing an overall bilateral symmetry along any stereotaxis plane examined (Figures 3B, 3F, 4C–4F, 5A, S3, and S4 and Videos S2, S3, and S4). Using this color-coded map, the specific tracts of interest can be identified at a given slice level or three-dimensionally (Figures 2, 3, 4, 6, 7, 8, and S2). Specific tracts derived from lobes that are paired (Table 1 and Figures 2C, 2F–2H, 3A, 3B, 3E, 3F, 5, and S2) show network patterns precisely mirroring each other again providing confidence in their description as functional interconnections. Comparison between full k-space (long scan) and partial Fourier acceleration acquisitions confirmed a high degree of similarity for the squid whole-brain tractography (98.98% and 96.48% match in two individuals, respectively) (Figures S3C and S3D), suggesting no significant improvement for the mesoscale brain-wide tractography visualization and connectivity matrix under long scan and over-restrictive thresholding for tract acceptance (Figure S3). Probabilistic tractography data, pairwise connections, and the corresponding connectivity strength index (Cs: the logarithm of numbers of streamlines intersecting a pair of regions) were then used to generate a brain-wide neural connectivity matrix for each individual and the averaged connectivity matrix, respectively (Figures 5A, S3, and S4).
The color-coded neural tracts show the orientation of neural bundles related to the body axes. A, anterior; D, dorsal; L, lateral.
The color-coded neural tracts show the orientation of neural bundles related to the body axes. A, anterior; D, dorsal; L, lateral.
Among the 282 already known neural network pathways of squid brain described by Young and his colleague (Figure 5B) (Messenger, 1979, Young, 1974, Young, 1976, Young, 1977, Young, 1979), the averaged connectivity matrix identified 281, that is, a 99.65% true-positive rate between previous Cajal-Golgi silver-stained results and tractography (range of Cs value: 0.477–5.084) (Figure 5A). This included 76 suggestions that were defined as possible tracts by Young (Young, 1974, Young, 1976, Young, 1977, Young, 1979) (Figure 5B). The only one mismatch was the track between olfactory lobe and the lateral-ventral palliovisceral lobe (Figure 5B). In addition, 45 blank spots (Cs = 0) (Figure 5A) in the averaged connectivity matrix from tractography are well matched with the blanks from previous histology (Figure 5B).
Topographic Representation and Estimates of 145 New Interconnections
A mesoscale brain-wide tractography-based connectivity matrix for squid with an additional 145 (Cs > 2.7) previously unknown lobe-lobe tracts is proposed here (Figure 5C). Ninety-three new tracts (64.14%) were also confirmed with Cajal-Golgi or dye tracing (Figure 5C). We are confident in the remaining 36%, currently without direct backup from Cajal-Golgi or dye tracing, owing to both the highly conservative methods just described and the great match rate between previous connections identified and our results.
Over 62% of the 145 new structural connections are linked to the vision (optic lobes) and motor control (basal lobes) systems (Figure 5C). Sixty-seven of these are found within the basal lobe system, a brain area controlling higher motor activity, and another 23 tracts connecting the optic lobes to other brain areas (Figures 5C, 6, 7, and 8). The already well-known chiasma of optic nerves and the associated projections toward the outer granule layer of the optic lobe can be clearly identified (Videos S3 and S5). As the secondary visual nerves enter the medulla of the optic lobe, tractography presents a previously unseen grid-like network of ongoing connections (Figure 7 and Video S5). The peripheral retinotopicity that optic chiasma allows (Figure 7 and Video S5) apparently travels deeper within the optic lobe than previously noted (beyond the zone of radial columns described by Young [1974]) and this most likely impacts processing of visual information. Interestingly, at this point in the optic lobe medullary region, there are many motor control circuits that directly couple to this network and project to the basal lobe (Figures 6 and 7 and Video S5). This would allow a topographical overlay of the outside world as viewed by the eye on the motor command units controlling locomotion (fins and funnel) and use of arms as discussed later (Figure 6). This provides another example of convergence with the vertebrate brain as noted by Shigeno et al. (2018) before the strong supporting evidence that we provide here.
The tracts of the basal lobe system divide into two major orientations, one ipsilateral and one contralateral as shown in the neural connectivity matrix (Figures 2F–2H, 3A, 3B, 4C–4F, 5, and 7). The contralateral tracts include those going from the left anterior-median lobule of the anterior basal lobe (aBa) to the right optic lobe and some that travel from the right anterior-median lobule of the aBa to the left optic lobe (Figures 2F–2H). There are also two separate contralateral connections between the left and right interbasal lobes joining the left and right lateral basal lobes, for example (Figure 5A). Aside from examining the lobe-dependent tractography, with the distinct anatomical features of the neighbor basal lobes, examination of the basal lobe system of the smallest brain by dividing its right half into 604 small ROIs (each ROI voxel = 120 μm × 120 μm × 120 μm) evenly distributed across this system throughout the dorsoventral axis revealed that the corresponding tractography of ROIs represents specified neural connectivity patterns distributing in 16 levels (L1–L16) (Figure 6). The spatial arrangement here is complex but highly organized containing elements of the visual and lower motor control areas (Figures 6 and 7). Overall, the basal lobes construct a well-defined relay station. For example, in the upper region of the basal lobe system, there are interconnections between the vision (optic lobes) and the memory formation and learning areas (vertical and subvertical lobes). There is also a complex set of serially interconnected networks at different depth levels allowing signal-relay wiring patterns between vision (optic lobes) and lower motor centers (e.g., pedal and magnocellular lobes), mediating with eye movement, locomotion by funnel and fins. More specifically these networks identified include:
-
(1)
The upper levels of the dorsal basal lobe (L1 and L2) and anterior basal lobes (L4 and L5) show direct connection with the learning and memory center, including vertical and subvertical lobes (Figures 5 and 6). The power of HARDI is evident here as previous work missed the connections between the basal lobe and the vertical lobe entirely (Young, 1977).
-
(2)
There are a significant number of short tracts forming a complex network within adjacent basal lobe regions (e.g., dorsal, median, and lateral basal lobes throughout the basal lobe system, Figure 6).
-
(3)
A multilayered structure found in all basal lobes could retain the spatial information projecting from the optic lobe. Projections from the upper layers of the basal lobes connect only with the upper level of the optic lobe, whereas the projections from the lower levels of the basal lobes shift toward lower levels of the optic lobe accordingly (Figure 7 and Video S5). This forms an orderly topographical representation from the basal lobe system toward the medulla of the optic lobe at different depth levels (L3–L16) (Figures 6 and 7).
-
(4)
The median-basal layers (L5–L16) mediate lower motor control including connections to arm movements (pedal lobes) and funnel and fin movements (magnocellular lobes). Most of these motor control tracts are congruent with optic relay pathways, suggesting a role in visual-motor coordination (Figures 5, 6, and 7).
-
(5)
New interconnections in response to coloration control are described where the dorsal area of the anterior chromatophore lobe projects mainly to the head and the ventral area to the arms (Figures 8A and 8B). Furthermore, two additional circuits linked to the anterior chromatophore lobes and the resulting second chiasm are identified: (i) the lateral basal lobe (L9) to the anterior ventral chromatophore lobe and (ii) the lateral basal lobe (L12) to the anterior dorsal chromatophore lobe control arm and head chromatophores, respectively (Figures 8C–8E). This second chiasm forms a previously unknown dorsoventral cross-over between the optic lobe and the chromatophore lobes via the basal lobe system (Figures 8C–8E).
-
(6)
A unique connection has been found between the dorsal basal and superior frontal lobe at L6, derived from the interbasal lobe at different depth levels (L9–13). The potential function of this connection is unknown in squid (Figure 6). In octopus the superior frontal lobe is involved in chemosensory learning and memory (Boycott, 1965, Wells, 1963).
Discussion
Using the modern imaging techniques of high-resolution dMRI that we adapted from established vertebrate methods combined with both old (Golgi) and new (NeuroVue) histological results, we have started the first mesoscale MRI-based cephalopod brain atlas. New insights reveal inter-individual variability of squid brain in both volumetric and tractographic network aspects. This dataset provides a foundation for proposing new functional morphology and interactions between brain regions. It also supports emerging hypotheses around anatomical and functional convergence with parts of the vertebrate central nervous system (Packard, 1972, Shigeno et al., 2018).
New Pathways and Functional Importance
Squid are voracious predators using their “simple eyes” to conduct a vision-dominated lifestyle. Their eye is famously convergent to the vertebrate eye (Packard, 1972, Chung and Marshall, 2017, Hanlon and Messenger, 2018), but although much is known about the optical and retinal elements (Sweeney et al., 2007, Chung and Marshall, 2014, Chung and Marshall, 2016, Chung and Marshall, 2017, Gagnon et al., 2016), the optic lobe remains one of the most mysterious neural integration centers in visual neuroscience (Cajal, 1917, Boycott, 1961, Young, 1974, Liu and Chiao, 2017, Liu et al., 2018). The pioneering neuroanatomical work of Cajal (cuttlefish and squid) and Young (octopus and squid) demonstrated a retinal topography preserved through to the retina profunda, the outer layers of the optic lobe (Cajal, 1917, Young, 1971, Young, 1974). Also known as deep retina, this is where neurons of the outer and inner granular layers are assumed to process visual input and retinotopicity was known to remain (Young, 1974). That is, a general plan of the outside world remained fixed to the two-dimensional layout of the layers present. Before our work here, deeper into the optic lobe, the amorphous arrangement of the millions of neurons within the medulla made anatomical or functional correlations difficult. The tractography presented here now demonstrates a clear grid-like and retinotopic network that is maintained deep within the medulla of the optic lobes and indeed well beyond it to other brain areas (Figure 7 and Video S5). This network of cells was described by Young as centrifugal axons, multipolar cells, large horizontal cells, and efferent neurons connected with other lobes, and with a possible organization that we now confirm (Young, 1974) (Figure 7 and Video S5). Based on tractography of the optic lobe, this grid structure initially appears below the inner granular layer and is continuous within the medulla, forming a layered organization. Although the projections of the basal lobes into the medulla apparently reach different locations and depths (Figure 7 and Video S5), neural coding via spatial and temporal coherence via this grid network could integrate visual signals and coordinate complex vision-related activities. If that is the case, there are parallels to similar mechanism in vertebrate's visual cortex (Hubel and Wiesel, 1968, Shigeno et al., 2018). This structure-function link now makes it possible to support the idea of a retinal topographic representation connected through to two previously known somatotopic maps, including the anterior-median lobule of the aBa (control of posture and movement of head and eyes) (Young, 1977) and the peduncle lobe (coordinating motor activities) (Messenger, 1979). In addition, our new tractography adds two further topographic representations in the basal lobe system, specifically in the median and inter basal lobes (Figures 6 and 7 and Video S5). This again underlines the orderly topographic congruence of visual scene and motor control system that Young originally proposed but lacked evidence for (Young, 1974, Young, 1977). Counter suggestions since Young in fact suggested that a somatosensory congruence in cephalopods was unlikely (Zullo et al., 2009, Liu and Chiao, 2017).
Shigeno et al. (2018) recently highlighted similarities between cephalopod and vertebrate brains in terms of lobe organization, functional analogies, and development. They equated, for example, the optic lobe to the vertebrate tectum, dorsal basal lobe to thalamus, and anterior basal lobe to basal ganglia. This again follows the original suggestions of convergence of the cephalopod and vertebrate brain from Young (1971) and adds to a growing body of literature finding parallels between vertebrate and invertebrate brains and their functional sub-units (Packard, 1972, Shigeno et al., 2018). There are well-known and orderly topographic maps of a variety of sorts with both sensory and somatic origins in vertebrate cortex (Penfield and Boldrey, 1937, Kaas, 1997, Jbabdi et al., 2013). The squid tractography we present here suggests another example of convergence in visual systems, adding to that of the more famous optical arrangement of the eye and the initial suggestions from Boycott (1961) and Young (1974). We hope that the detail and new features of this outside map to spatio-motor control will guide research in function, behavior, and cognition.
Another new feature of squid brain that our combined MRI/dye-trace/histology approach can map is the highly subdivided network of the basal lobe system (Figure 6). As noted in the vertebrate cortex (Hubel and Wiesel, 1962, Alexander et al., 1986), within-lobe and inter-lobe connections at different levels may group neurons that frequently interact and provide a processing platform with short interconnections. These sorts of functional groupings overcome some of the distributed network problems in brain design (Kaas, 1997, Betzel and Bassett, 2017, Lynn and Bassett, 2019), a solution noted in Drosophila and Caenorhabditis connectomics also (White et al., 1986, Shih et al., 2015, Cook et al., 2019). We suggest that the multi-layered basal lobe system, which receives visual scene input from the optic lobe (Figures 6 and 7) may translate and distribute the control commands in a spatiotemporal manner to different motor units. These include direction of tentacular strike, schooling, arm coordination over the outside visual field, and courtship display through body posture and color changes (Jantzen and Havenhand, 2003, Mather et al., 2010, Sugimoto and Ikeda, 2012, Lin et al., 2017, Hanlon and Messenger, 2018). This latter possibility deserves further elaboration using electrical recording, stimulation, and biochemical studies.
Multiple Levels of Control in Coloration and Patterns
The rapidity and versatility of body color and pattern change in cephalopods for both communication and camouflage is almost legendary (Jantzen and Havenhand, 2003, Mather et al., 2010; How et al., 2017; Lin et al., 2017, Hanlon and Messenger, 2018, Reiter et al., 2018). The skin elements, chromatophores, irridophores, and leucophores, are under direct neural control in response to visual perception of environment as well as behavioral mood changes (Darmaillacq et al., 2014; How et al., 2017; Hanlon and Messenger, 2018). Using computational analysis to quantify the states of chromatophores of live cuttlefish revealed that a hierarchical motor control mechanism governs development of skin patterns and dynamic coloration (Laan et al., 2014, Reiter et al., 2018). This view is also supported by both neuroanatomical, electrophysiological, and now our tractography approaches, suggesting that this skin-display system is hierarchically organized via the optic, lateral basal, peduncle, and chromatophore lobes (Boycott, 1961, Young, 1974, Dubas et al., 1986, Novicki et al., 1990) (Figures 6 and 8).
Our tractography and mapping also provides new evidence for a sub-division of the anterior chromatophore lobe into dorsal and ventral units in squid, the dorsal lobe controlling dorsal head color and ventral, arm color (Figures 8A and 8B). The two paired anterior chromatophore lobes may link body form and color display while squid hover in water column such as countershading camouflage that a task is distinct to the benthic octopus, which retains only one pair of anterior chromatophore lobes (Young, 1971). The reason for this is explored next.
A new chiasm is clear from the tractography presented here, between the lateral basal lobe and the anterior chromatophore lobes (Figures 8C–8E). This second dorsoventral crossing of tracts within the coloration control circuit we hypothesize may control open water countershading camouflage. The second chiasm ensures the seafloor image falling on the dorsal retina is sent to the anterior dorsal chromatophore lobe, which controls dark coloration on the head to match with the seafloor background. The other tract would guide the ventral side of head and arms to show light color patterns to match with the visual scene above the squid. Aside from this proposed coloration circuit, a similar chiasm-like network for the coordination of head and eye movement was previously noted in the anterior-median lobule of the aBa by Young (1977) (confirmed here also by our tractography and dye tracing [Figures 2F–2H]). This kind of crossing arrangement could be a common feature of the basal lobe system (Young, 1977). Chiasmata in any nervous system are of interest owing to mapping inversions and other non-linear relationships as well as re-configuration of evolutionary trajectory of vision and visual behaviors (Sinakevitch et al., 2003, Strausfeld, 2005).
A Comparison between Cephalopod Species
Table S2 lists the loliginid squid species including the five species from the North Atlantic Ocean (Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979; Wild et al., 2015) and S. lessoniana from the Indo-Pacific Ocean (Shigeno et al., 2001, Kobayashi et al., 2013), our work here, upon which much of our knowledge of the squid brain is based. Despite different geographic distribution and phylogenetic differences (six species from three genera where the genus Sepioteuthis is the stable basal in-group taxa in family Loliginidae) (Anderson, 2000, Strugnell et al., 2005, Vecchione et al., 1998), organization of the CNS, number of lobes in the circum-esophageal ring, and the interregional connections are very similar between species. Perhaps more surprising, a comparison between cuttlefish and squid also indicates these coleoid groups (decapodiform) share a basically similar layout (Cajal, 1917, Boycott, 1961, Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979, Nixon and Young, 2003). The decapodiform cephalopods may mirror brain organization bauplans from the life needs of a rapid visual predator and also conduct courtship display in the water column.
Considering the evolutionary divergence time of major cephalopod groups (Strugnell et al., 2006), the brain maps of cuttlefish, octopus, and vampire squid using the techniques developed here are underway. In contrast to the elongated CNS arrangement in decapodiforms, the compact octopus brain (octopodiform) has evolved distinct changes, particularly the noticeable five gyri of the vertical lobe (learning and memory) and sub-divisions of the frontal lobe system (tactile and chemosensory) (Wells, 1963, Boycott, 1965, Young, 1971, Shigeno and Ragsdale, 2015). Development of these octopus-specialized lobes is likely linked to their benthic life such as searching invisible food from crevices using nimble arms. Furthermore, the vampire squid (the basal clade of coleoids), which inhabits in midwater (ca. 600–1,000 m depth), possesses features of both octopodiform and decapodiform in body form (e.g., eight arms with two tentacle-like filaments) and brain organization (squid-like suboesophageal and octopus-like supraoesophageal mass) (Young, 1964, Nixon and Young, 2003). A comparison between coleoid brains could further extend our understanding of the evolutionary history between cephalopod brain design and life styles.
We erect the start of a connectome for the squid brain, the first of its kind for the cephalopods with input from high-resolution dMRI, previous histology, new histology with old (Cajal-Golgi silver impregnation), and new fluorescent neuronal tracers. This study does not claim to build a complete connectivity atlas but to erect an initial predictive mesoscale connectome that reveals hitherto unsuspected pathways. As is the result of any connectome, several new functional circuits are suggested by this map, as well bolstering the previously known functional sub-units that underlie behaviors. The apparently complex cognitive tasks cephalopods perform need this kind of solid background evidence before anthropomorphic speculations lead to misconceptions around these unique and wonderful creatures.
Limitations of the Study
This study reveals the first mesoscale MRI-based neural connectivity in the squid brain; however, it is possible that some limitations could affect the results presented here.
Indirect Quantitative Measurement of Tractography: Visualization of 3D direction-coded color tractography of the squid brain reveals rich information that is not possible to see in conventional histology. However, the MRI-based tractography is based on a mapping from water diffusion to fiber orientation. In other words, an estimate of the confidence on the route of least hindrance to diffusion can thus indirectly reflect the connecting neural bundles between pairs of regions, making analysis of tractography and the resulting connectivity matrix less quantitative. Detailed advantages and potential pitfalls about the tractography can be found in the review by Sotiropoulos and Zalesky (2019).
Species Selection: Our current knowledge of the neural network of the squid brain is majorly based on a series of studies in the nervous system of Loligo (Young, 1974, Young, 1976, Young, 1977, Young, 1979, Messenger, 1979). It is worth noting that the five species (see Table S2) used in Young's studies are phylogenetically close to the species used in this study. Some new findings in this study (i.e., subdivisions of lobes and new tracts) might be arguable owing to species selection. Although all five species in Young's study are not available in Australian waters, selecting S. lessoniana is a good start and a reliable bridge to the classical studies of loliginid squid.
No Information of Synaptic Connection: Our current macroscale tractography and histological results are unable to reveal synaptic connections yet. Further efforts to label neural tracts and comparative studies across different cephalopod groups are ongoing, and hopefully will lead to further defined neural connectivity in cellular level.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work is supported by the Australian Research Council (ARC) (FL140100197) (Australian Laureate Fellowship to N.J.M.). The 16.4 T is supported by the Queensland State Government through the Queensland NMR Network and the Australian Government through National Collaborative Research Infrastructure Strategy (NCRIS) and the National Imaging Facility. Histological imaging was performed at the Queensland Brain Institute's Advanced Microscopy Facility using Zeiss Axio Imager.
Author Contributions
W.-S.C designed and performed most experiments and analysis and wrote the first version of the manuscript. N.D.K. and W.-S.C. performed MRI and tractography. N.J.M. supervised the project and co-wrote the manuscript with input from all authors. All authors contributed to data analysis, interpretation, and revision of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: January 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.100816.
Contributor Information
Wen-Sung Chung, Email: w.chung1@uq.edu.au.
N. Justin Marshall, Email: justin.marshall@uq.edu.au.
Data and Code Availability
The authors confirm that the data supporting the findings of this study are available within the article, its Supplemental Information, and Mendeley Data (https://doi.org/10.17632/pwkh3s2t33.1).
Supplemental Information
References
- Abbott N.J., Williamson R., Maddock L. Oxford University Press; 1995. Cephalopod Neurobiology. [Google Scholar]
- Albertin C.B., Simakov O., Mitros T., Wang Z.Y., Pungor J.R., Edsinger-Gonzales E., Brenner S., Ragsdale C.W., Rokhsar D.S. The octopus genome and the evolution of cephalopod neural and morphological novelties. Nature. 2015;524:220–224. doi: 10.1038/nature14668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.E., Delong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alomair O.I., Brereton I.M., Smith M.T., Galloway G.J., Kurniawan N.D. In vivo high angular resolution diffusion-weighted imaging of mouse brain at 16.4 Tesla. PLoS One. 2015;10:e0130133. doi: 10.1371/journal.pone.0130133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson F.E. Phylogeny and historical biogeography of the loliginid squids (Mollusca: cephalopoda) based on mitochondrial DNA sequence data. Mol. Phylogenet. Evol. 2000;15:191–214. doi: 10.1006/mpev.1999.0753. [DOI] [PubMed] [Google Scholar]
- Assaf Y., Johansen-Berg H., Thiebaut de Schotten M. The role of diffusion MRI in neuroscience. NMR Biomed. 2019;32:e3762. doi: 10.1002/nbm.3762. [DOI] [PubMed] [Google Scholar]
- Aydogan D.B., Jacobs R., Dulawa S., Thompson S.L., Francois M.C., Toga A.W., Dong H.-W., Knowles J.A., Shi Y.-G. When tractography meets tracer injections: a systematic study of trends and variation sources of diffusion-based connectivity. Brain Struct. Funct. 2018;223:2841–2858. doi: 10.1007/s00429-018-1663-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett D.S., Sporns O. Network neuroscience. Nat. Neurosci. 2017;20:353–364. doi: 10.1038/nn.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel R.F., Bassett D.S. Multi-scale brain networks. Neuroimage. 2017;160:73–83. doi: 10.1016/j.neuroimage.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott B.B. The functional organization of the brain of the cuttlefish Sepia officinalis. Proc. Biol. Sci. 1961;153:503–534. [Google Scholar]
- Boycott B.B. Learning in the Octopus. Sci. Am. 1965;212:42–50. doi: 10.1038/scientificamerican0365-42. [DOI] [PubMed] [Google Scholar]
- Boycott B.B., Young J.Z. A memory system in Octopus vulgaris Lamarck. Proc. Biol. Sci. 1955;143:449–480. doi: 10.1098/rspb.1955.0024. [DOI] [PubMed] [Google Scholar]
- Boycott B.B., Young J.Z. Effects of interference with the vertical lobe on visual discriminations in Octopus vulgaris Lamarck. Proc. Biol. Sci. 1957;146:439–459. doi: 10.1098/rspb.1957.0023. [DOI] [PubMed] [Google Scholar]
- Brown E.R., Piscopo S., de Stefano R., Giuditta A. Brain and behavioural evidence for rest-activity cycles in Octopus vulgaris. Behav. Brain Res. 2006;172:355–359. doi: 10.1016/j.bbr.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Bublitz A., Weinhold S.R., Strobel S., Dehnhardt G., Hanke F.D. Reconsideration of serial visual reversal learning in octopus (Octopus vulgaris) from a methodological perspective. Front. Physiol. 2017;8:54. doi: 10.3389/fphys.2017.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E., Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullock T.H., Horridge G.A. Freeman; 1965. Structure and Function in the Nervous Systems of Invertebrates. [Google Scholar]
- Cajal S.R. Contribucion al conocimiento de la retina y centros opticos de los cefalopodos. Tarbajos del laboratorio de investigacious biologicas de la universidad de Madrid. 1917;15:1–82. https://bipadi.ub.edu/digital/collection/atlesmed/id/103317 [Google Scholar]
- Calabrese E., Badea A., Cofer G., Qi Y., Johnson G.A. A diffusion MRI tractography connectome of the mouse brain and comparison with neuronal tracer data. Cereb. Cortex. 2015;25:4628–4637. doi: 10.1093/cercor/bhv121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamante F., Tournier J.D., Kurniawan N.D., Yang Z., Gyengesi E., Galloway G.J., Reutens D.C., Connelly A. Super-resolution track-density imaging studies of mouse brain: comparison to histology. Neuroimage. 2012;59:286–296. doi: 10.1016/j.neuroimage.2011.07.014. [DOI] [PubMed] [Google Scholar]
- Chrachri A., Williamson R. Synaptic interactions between crista hair cells in the statocyst of the squid Alloteuthis subulata. J. Neurophysiol. 1998;80:656–666. doi: 10.1152/jn.1998.80.2.656. [DOI] [PubMed] [Google Scholar]
- Chung W.-S., Marshall N.J. Range-finding in squid using retinal deformation and image blur. Curr. Biol. 2014;24:R64–R65. doi: 10.1016/j.cub.2013.11.058. [DOI] [PubMed] [Google Scholar]
- Chung W.-S., Marshall N.J. Comparative visual ecology of cephalopods from different habitats. Proc. Biol. Sci. 2016;283:20161346. doi: 10.1098/rspb.2016.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung W.-S., Marshall N.J. Complex visual adaptations in squid for specific tasks in different environments. Front. Physiol. 2017;8:105. doi: 10.3389/fphys.2017.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S.J., Jarrell T.A., Brittin C.A., Wang Y., Bloniarz A.E., Yakovlev M.A., Nguyen K.C.Q., Tang L.T., Bayer E.A., Duerr J.S. Whole-animal connectomes of both Caenorhabditis elegans sexes. Nature. 2019;571:63–71. doi: 10.1038/s41586-019-1352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmaillacq A.-S., Dickel L., Mather J.A. Cambridge University Press; 2014. Cephalopod Cognition. [Google Scholar]
- Deweerdt S. Deep connections. Nature. 2019;571:S6–S8. doi: 10.1038/d41586-019-02208-0. [DOI] [PubMed] [Google Scholar]
- Donahue C.J., Sotiropoulos S.N., Jbabdi S., Hernandez-Fernandez M., Behrens T.E., Dyrby T.B., Coalson T., Kennedy H., Knoblauch K., van Essen Using diffusion tractography to predict cortical connection strength and distance: a quantitative comparison with tracers in the monkey. J. Neurosci. 2016;36:6758–6770. doi: 10.1523/JNEUROSCI.0493-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubas F., Hanlon R.T., Ferguson G.P., Pinsker H.M. Localization and stimulation of chromatophore motoneurons in the brain of the squid, Lolliguncula brevis. J. Exp. Biol. 1986;121:1–25. doi: 10.1242/jeb.121.1.1. [DOI] [PubMed] [Google Scholar]
- Edsinger E., Dölen G. A conserved role for serotonergic neurotransmission in mediating social behavior in octopus. Curr. Biol. 2018;28:3136–3142. doi: 10.1016/j.cub.2018.07.061. [DOI] [PubMed] [Google Scholar]
- Fiorito G., Scotto P. Observational learning in Octopus vulgaris. Science. 1992;256:545–547. doi: 10.1126/science.256.5056.545. [DOI] [PubMed] [Google Scholar]
- Gagnon Y.L., Osorio D.C., Wardill T.J., Marshall N.J., Chung W.-S., Temple S.E. Can chromatic aberration enable color vision in natural environments? Proc. Natl. Acad. Sci. U S A. 2016;113:E6908–E6909. doi: 10.1073/pnas.1612239113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso F.W. The octopus with two brains: how are distributed and central representations integrated in the octopus central nervous system? In: Darmaillacq A.-S., Mather J., Dickel L., editors. Cephalopod Cognition. Cambridge University Press; 2014. pp. 94–122. [Google Scholar]
- Hanlon R.T., Messenger J.B. Cambridge University Press; 2018. Cephalopod Behaviour. [Google Scholar]
- How M.J., Norman M.D., Finn J., Chung W.-S., Marshall N.J. Dynamic skin patterns in cephalopods. Front. Physiol. 2017;8:393. doi: 10.3389/fphys.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M.Y., Yan H.Y., Chung W.-S., Shiao J.C., Hwang P.P. Acoustically evoked potentials in two cephalopods inferred using the auditory brainstem response (ABR) approach. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009;153:278–283. doi: 10.1016/j.cbpa.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H., Wiesel T.N. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen T.M., Havenhand J.N. Reproductive behavior in the squid Sepioteuthis australis from south Australia: ethogram of reproductive body patterns. Biol. Bull. 2003;204:290–304. doi: 10.2307/1543600. [DOI] [PubMed] [Google Scholar]
- Jbabdi S., Johansen-Berg H. Tractography: where do we go from here? Brain Connect. 2011;1:169–183. doi: 10.1089/brain.2011.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jbabdi S., Sotiropoulos S.N., Behrens T.E. The topographic connectome. Curr. Opin. Neurobiol. 2013;23:207–215. doi: 10.1016/j.conb.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J.H. Topographic maps are fundamental to sensory processing. Brain Res. Bull. 1997;44:107–112. doi: 10.1016/s0361-9230(97)00094-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Takayama C., Ikeda Y. Ontogeny of the brain in oval squid Sepioteuthis lessoniana (Cephalopoda: Loliginidae) during the post-hatching phase. J. Mar. Biol. Assoc. U K. 2013;93:1663–1671. [Google Scholar]
- Koizumi M., Shigeno S., Mizunami M., Tanaka N.K. Three-dimensional brain atlas of pygmy squid, Idiosepius paradoxus, revealing the largest relative vertical lobe system volume among the cephalopods. J. Comp. Neurol. 2016;524:2142–2157. doi: 10.1002/cne.23939. [DOI] [PubMed] [Google Scholar]
- Kurniawan N.D., Richards K.L., Yang Z.Y., She D., Ullmann J.F.P., Moldrich R.X., Liu S., Yaksic J.U., Leanage G., Kharatishvili I. Visualization of mouse barrel cortex using ex-vivo track density imaging. Neuroimage. 2014;87:465–475. doi: 10.1016/j.neuroimage.2013.09.030. [DOI] [PubMed] [Google Scholar]
- Laan A., Gutnick T., Kuba M.J., Laurent G. Behavioral analysis of cuttlefish traveling waves and its implications for neural control. Curr. Biol. 2014;24:1737–1742. doi: 10.1016/j.cub.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Lee Y.H., Chang Y.C., Yan H.Y., Chiao C.C. Early visual experience of background contrast affects the expression of NMDA-like glutamate receptors in the optic lobe of cuttlefish, Sepia pharaonis. J. Exp. Mar. Biol. Ecol. 2013;447:86–92. [Google Scholar]
- Lin C.-Y., Chiao C.C. Female choice leads to a switch in oval squid male mating tactics. Biol. Bull. 2017;233:219–226. doi: 10.1086/695718. [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Tsai Y.-C., Chiao C.-C. Quantitative analysis of dynamic body patterning reveals the grammar of visual signals during the reproductive behavior of the oval squid Sepioteuthis lessoniana. Front. Ecol. Evol. 2017;5:30. [Google Scholar]
- Liscovitch-Brauer N., Alon S., Porath H.T., Elstein B., Unger R., Ziv T., Admon A., Levanon E.Y., Rosenthal J.J., Eisenberg E. Trade-off between transcriptome plasticity and genome evolution in cephalopods. Cell. 2017;169:191–202. doi: 10.1016/j.cell.2017.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.R., Li Y.H., Edwards T.J., Kurniawan N.D., Richards L.J., Jiang T.Z. Altered structural connectome in adolescent socially isolated mice. Neuroimage. 2016;139:259–270. doi: 10.1016/j.neuroimage.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Liu T.-H., Chiao C.-C. Mosaic organization of body pattern control in the optic lobe of squids. J. Neurosci. 2017;37:768–780. doi: 10.1523/JNEUROSCI.0768-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-C., Chung W.-S., Yu C.-C., Hsu S.-T., Chan F.-L., Liu T.-H., Su C.-H., Hwu Y., Marshall N.J., Chiao C.C. Morphological changes of the optic lobe from late embryonic to adult stages in oval squids Sepioteuthis lessoniana. J. Morphol. 2018;279:75–85. doi: 10.1002/jmor.20755. [DOI] [PubMed] [Google Scholar]
- Lu C.-C., Chung W.-S. Huayu Nature Book Trade; 2017. Guide of the Cephalopods of Taiwan. [Google Scholar]
- Lynn C.W., Bassett D.S. The physics of brain network structure, function and control. Nat. Rev. Phys. 2019;1:318–332. [Google Scholar]
- Maddock L., Young J.Z. Quantitative differences among the brains of cephalopods. J. Zool. 1987;212:739–767. [Google Scholar]
- Maier-Hein K.H., Neher P.F., Houde J.C., Cote M.A., Garyfallidis E., Zhong J., Chamberland M., Yeh F.C., Lin Y.C., Ji Q. The challenge of mapping the human connectome based on diffusion tractography. Nat. Commun. 2017;8:1349. doi: 10.1038/s41467-017-01285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall N.J., Messenger J.B. Colour-blind camouflage. Nature. 1996;382:408–409. [Google Scholar]
- Mather J.A., Griebel U., Byrne R.A. Squid dances: an ethogram of postures and actions of Sepioteuthis sepioidea squid with a muscular hydrostatic system. Mar. Freshw. Behav. Phys. 2010;43:45–61. [Google Scholar]
- Meinertzhagen I.A. Of what use is connectomics? A personal perspective on the Drosophila connectome. J. Exp. Biol. 2018;221:jeb164954. doi: 10.1242/jeb.164954. [DOI] [PubMed] [Google Scholar]
- Messenger J.B. The nervous system of Loligo. IV. Peduncle and olfactory lobes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1979;285:275–309. [Google Scholar]
- Miyan J.A., Messenger J.B. Intracellular recordings from the chromatophore lobes of Octopus. In: Abbott N.J., Williamson R., Maddock L., editors. Cephalopod Neurobiology Neuroscience Studies in Squid, octopus, and Cuttlefish. Oxford University Press; 1995. pp. 415–429. [Google Scholar]
- Nixon M., Young J.Z. Oxford University Press; 2003. The Brains and Lives of Cephalopods. [Google Scholar]
- Novicki A., Budelmann B.U., Hanlon R.T. Brain pathways of the chromatophore system in the squid Lolliguncula brevis. Brain Res. 1990;519:315–323. doi: 10.1016/0006-8993(90)90094-r. [DOI] [PubMed] [Google Scholar]
- Oh S.W., Harris J.A., Ng L., Winslow B., Cain N., Mihalas S., Wang Q., Lau C., Kuan L., Henry A.M. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A. Cephalopods and fish - the limits of convergence. Biol. Rev. Camb. Philos. Soc. 1972;47:241–307. [Google Scholar]
- Penfield W., Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Reiter S., Hulsdunk P., Woo T., Lauterbach M.A., Eberle J.S., Akay L.A., Longo A., Meier-Credo J., Kretschmer F., Langer J.D. Elucidating the control and development of skin patterning in cuttlefish. Nature. 2018;562:361–366. doi: 10.1038/s41586-018-0591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell A.K., Clayton N.S. Cephalopod cognition. Curr. Biol. 2019;29:R726–R732. doi: 10.1016/j.cub.2019.06.049. [DOI] [PubMed] [Google Scholar]
- Shigeno S., Andrews P.L.R., Ponte G., Fiorito G. Cephalopod brains: an overview of current knowledge to facilitate comparison with vertebrates. Front. Physiol. 2018;9:952. doi: 10.3389/fphys.2018.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeno S., Ogura A., Mori T., Toyohara H., Yoshida T., Tsuchida S., Fujikura K. Sensing deep extreme environments: the receptor cell types, brain centers, and multi-layer neural packaging of hydrothermal vent endemic worms. Front. Zool. 2014;11:82. doi: 10.1186/s12983-014-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeno S., Ragsdale C.W. The gyri of the octopus vertical lobe have distinct neurochemical identities. J. Comp. Neurol. 2015;523:1297–1317. doi: 10.1002/cne.23755. [DOI] [PubMed] [Google Scholar]
- Shigeno S., Tsuchiya K., Segawa S. Embryonic and paralarval development of the central nervous system of the loliginid squid Sepioteuthis lessoniana. J. Comp. Neurol. 2001;437:449–475. doi: 10.1002/cne.1295. [DOI] [PubMed] [Google Scholar]
- Shih C.T., Sporns O., Yuan S.L., Su T.S., Lin Y.J., Chuang C.C., Wang T.Y., Lo C.C., Greenspan R.J., Chiang A.S. Connectomics-based analysis of information flow in the Drosophila brain. Curr. Biol. 2015;25:1249–1258. doi: 10.1016/j.cub.2015.03.021. [DOI] [PubMed] [Google Scholar]
- Shomrat T., Zarrella I., Fiorito G., Hochner B. The octopus vertical lobe modulates short-term learning rate and uses LTP to acquire long-term memory. Curr. Biol. 2008;18:337–342. doi: 10.1016/j.cub.2008.01.056. [DOI] [PubMed] [Google Scholar]
- Sinakevitch I., Douglass J.K., Scholtz G., Loesel R., Strausfeld N.J. Conserved and convergent organization in the optic lobes of insects and isopods, with reference to other crustacean taxa. J. Comp. Neurol. 2003;467:150–172. doi: 10.1002/cne.10925. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos S.N., Zalesky A. Building connectomes using diffusion MRI: why, how and but. NMR Biomed. 2019;32:e3752. doi: 10.1002/nbm.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausfeld N.J. The Golgi method: Its application to the insect nervous system and the phenomenon of stochastic impregnation. In: Strausfeld N.J., Miller T.A., editors. Neuroanatomical Techniques. Springer; 1980. pp. 131–203. [Google Scholar]
- Strausfeld N.J. The evolution of crustacean and insect optic lobes and the origins of chiasmata. Arthropod Struct. Dev. 2005;34:235–256. [Google Scholar]
- Strausfeld N.J., Ma X., Edgecombe G.D. Fossils and the evolution of the arthropod brain. Curr. Biol. 2016;26:R989–R1000. doi: 10.1016/j.cub.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Strugnell J., Jackson J., Drummond A.J., Cooper A. Divergence time estimates for major cephalopod groups: evidence from multiple genes. Cladistics. 2006;22:89–96. doi: 10.1111/j.1096-0031.2006.00086.x. [DOI] [PubMed] [Google Scholar]
- Strugnell J., Norman M., Jackson J., Drummond A.J., Cooper A. Molecular phylogeny of coleoid cephalopods (Mollusca: cephalopoda) using a multigene approach; the effect of data partitioning on resolving phylogenies in a Bayesian framework. Mol. Phylogenet. Evol. 2005;37:426–441. doi: 10.1016/j.ympev.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Suarez R., Paolino A., Fenlon L.R., Morcom L.R., Kozulin P., Kurniawan N.D., Richards L.J. A pan-mammalian map of interhemispheric brain connections predates the evolution of the corpus callosum. Proc. Natl. Acad. Sci. U S A. 2018;115:9622–9627. doi: 10.1073/pnas.1808262115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto C., Ikeda Y. Ontogeny of schooling behavior in the oval squid Sepioteuthis lessoniana. Fish. Sci. 2012;78:287–294. [Google Scholar]
- Sutherland N.S., Mackintosh J., Mackintosh N.J. The visual discrimination of reduplicated patterns by octopus. Anim. Behav. 1963;11:106–110. [Google Scholar]
- Sweeney A.M., Haddock S.H., Johnsen S. Comparative visual acuity of coleoid cephalopods. Integr. Comp. Biol. 2007;47:808–814. doi: 10.1093/icb/icm092. [DOI] [PubMed] [Google Scholar]
- Takemura S., Aso Y., Hige T., Wong A., Lu Z.Y., Xu C.S., Rivlin P.K., Hess H., Zhao T., Parag T. A connectome of a learning and memory center in the adult Drosophila brain. Elife. 2017;6:e26975. doi: 10.7554/eLife.26975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C., Ye F.Q., Irfanoglu M.O., Modi P., Saleem K.S., Leopold D.A., Pierpaoli C. Anatomical accuracy of brain connections derived from diffusion MRI tractography is inherently limited. Proc. Natl. Acad. Sci. U S A. 2014;111:16574–16579. doi: 10.1073/pnas.1405672111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F. Springer-Verlag; 1998. Golgi Atlas of the Postnatal Mouse Brain. [Google Scholar]
- Vecchione M., Brakoniechki T.F., Natsukari Y., Hanlon R.T. A provisional generic classification of the family Loliginidae. In: Voss, editor. Cephalopod International Advisory Council. Smithsonian Institution Press; 1998. pp. 215–222. [Google Scholar]
- Wells M.J. Taste by touch: some experiments with octopus. J. Exp. Biol. 1963;40:187–193. [Google Scholar]
- Wells M.J., Wells J. The effect of lesions to the vertical and optic lobes on tactile discrimination in Octopus. J. Exp. Biol. 1957;34:378–393. [Google Scholar]
- White J.G., Southgate E., Thomson J.N., Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Wild E., Wollesen T., Haszprunar G., Hess M. Comparative 3D microanatomy and histology of the eyes and central nervous systems in coleoid cephalopod hatchlings. Org. Divers. Evol. 2015;15:37–64. [Google Scholar]
- Wollesen T., Loesel R., Wanninger A. Pygmy squids and giant brains: mapping the complex cephalopod CNS by phalloidin staining of vibratome sections and whole-mount preparations. J. Neurosci. Methods. 2009;179:63–67. doi: 10.1016/j.jneumeth.2009.01.021. [DOI] [PubMed] [Google Scholar]
- Wollesen T., Sukhsangchan C., Seixas P., Nabhitabhata J., Wanninger A. Analysis of neurotransmitter distribution in brain development of benthic and pelagic octopod cephalopods. J. Morphol. 2012;273:776–790. doi: 10.1002/jmor.20023. [DOI] [PubMed] [Google Scholar]
- Young J.Z. Learning and discrimination in the octopus. Biol. Rev. Camb. Philos. Soc. 1961;36:32–96. doi: 10.1111/j.1469-185x.1961.tb01432.x. [DOI] [PubMed] [Google Scholar]
- Young J.Z. Clarendon Press; 1971. The Anatomy of the Nervous System of Octopus vulgaris. [Google Scholar]
- Young J.Z. The central nervous system of Loligo. I. The optic lobe. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1974;267:263–302. doi: 10.1098/rstb.1974.0002. [DOI] [PubMed] [Google Scholar]
- Young J.Z. The nervous system of Loligo. II. Subesophageal centers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1976;274:101–167. doi: 10.1098/rstb.1976.0041. [DOI] [PubMed] [Google Scholar]
- Young J.Z. The nervous system of Loligo. III. Higher motor centers - the basal supraesophageal lobes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1977;276:351–398. [Google Scholar]
- Young J.Z. The nervous system of Loligo. V. The vertical lobe complex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1979;285:311–354. [Google Scholar]
- Young R.E. University of Sothern California; 1964. The Anatomy of the Vampire Squid. Master. [Google Scholar]
- Ziegler A., Bock C., Ketten D.R., Mair R.W., Mueller S., Nagelmann N., Pracht E.D., Schröder L. Digital three-dimensional imaging techniques provide new analytical pathways for malacological research. Am. Malacol. Bull. 2018;36:248–273. [Google Scholar]
- Zullo L., Sumbre G., Agnisola C., Flash T., Hochner B. Nonsomatotopic organization of the higher motor centers in octopus. Curr. Biol. 2009;19:1632–1636. doi: 10.1016/j.cub.2009.07.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two series of transverse sections show the anatomical features (top) and the corresponding direction-encoded color map of fiber orientation distribution (FOD) (bottom) along with the long body axis (from anterior end [the inferior buccal lobe] to posterior end [visceral lobe]). The color-coded tensors show the direction of water diffusion along with neural bundles related to the body axes. A, anterior; D, dorsal; L, lateral.
The color-coded neural tracts show the orientation of neural bundles related to the body axes. A, anterior; D, dorsal; L, lateral.
The color-coded neural tracts show the orientation of neural bundles related to the body axes. A, anterior; D, dorsal; L, lateral.
The color-coded neural tracts show the orientation of neural bundles related to the body axes. A, anterior; D, dorsal; L, lateral.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article, its Supplemental Information, and Mendeley Data (https://doi.org/10.17632/pwkh3s2t33.1).