Abstract
Background and Purpose
Previous studies have explored the association between retinal vascular changes and cognitive impairment. The retinal vasculature shares some characteristics with the cerebral vasculature, and quantitative changes in it could indicate cognitive impairment. Hence, a comprehensive meta-analysis was performed to clarify the potential relationship between retinal vascular geometric changes and cognitive impairment.
Methods
Relevant databases were scrupulously and systematically searched for retinal vascular geometric changes including caliber, tortuosity, and fractal dimension (FD), and for cognitive impairment. The Newcastle-Ottawa Scale was used to evaluate the methodological quality of included studies. RevMan was used to perform the meta-analysis and detect publication bias. Sensitivity analyses were also performed.
Results
Five studies that involved 2,343 subjects were finally included in the meta-analysis. The results showed that there was no significant association between central retinal artery equivalents (Z=1.17) or central retinal venular equivalents (Z=1.74) and cognitive impairment (both p>0.05). Similarly, no significant difference was detected in retinal arteriolar tortuosity (Z=0.91) and venular tortuosity (Z=1.31) (both p>0.05). However, the retinal arteriolar FD (mean difference: −0.03, 95% CI: −0.05, −0.01) and venular FD (mean difference: −0.03, 95% CI: −0.05, −0.02) were associated with cognitive impairment.
Conclusions
A smaller retinal microvascular FD might be associated with cognitive impairment. Further large-sample and well-controlled original studies are required to confirm the present findings.
Keywords: retina, cognitive impairment, meta-analysis, retinal vessels
INTRODUCTION
The aging of the world's population has resulted in a tremendous increase in the incidence of aging-related brain diseases. Cognitive impairment is one such disease that has become very common among the elderly population. All kinds of aging-related brain diseases adversely impact the health and quality of life of elderly people, and they are expensive to diagnose, treat, and manage, hence representing a huge economic burden on the affected families as well as society as a whole.1 Previous studies have investigated different types of aging populations comprising people older than 70 years, and have found that 14% of this population has mild cognitive impairment (MCI), which involves a cognitive dysfunction severity between normal aging and dementia.2,3 When this condition worsens, the patient will suffer from Alzheimer's disease (AD).1 According to Alzheimer's Association International, 44 million people currently suffer from dementia worldwide, and this number will increase to 76 million in 2030 and 135 million in 2050.4 Such growth will inevitably lead to enormous increases in the economic and health burdens on societies worldwide.
Cognitive function can be quantified using several psychological questionnaires into different levels based on the cognitive decline5 and mental disability.6 In clinical situations, several imaging protocols such as CT and MRI are sometimes used to detect structural changes in the brain or cerebral vasculature. Such imaging modalities are valuable diagnosing the severity of cognitive impairment,7,8,9,10 but they are also expensive and can only be performed in large hospitals. Due to the similarity between the retinal and cerebral vasculatures, imaging biomarkers on high-resolution retinal images are promising features comparable with low-resolution brain images obtained by CT or MRI. Moreover, retinal imaging is less expensive and can be applied in community hospitals, thus providing the potential for the large-scale screening of aging populations.
The advances of computer programs developed for medical imaging have facilitated several computerized measurements on captured retinal images for discovering the relationship between retinal changes and clinical diseases.11,12 There are increasing data on the retinal changes and some diseases, including those affecting the brain. Several studies have revealed a relationship between retinal vascular changes and the incidence of dementia.13 Several clinical investigations have attempted to determine whether the retinal vasculature could be an imaging biomarker for cognitive impairment, and the results suggest that there is an association between retinal changes and cognitive impairment. However, some reported findings have been found to be inconsistent, and so in this study we performed a comprehensive meta-analysis to determine the relationship between retinal vascular geometric changes and cognitive impairment.
METHODS
Search strategy
We performed a comprehensive search of the following electronic databases up to July 2018: China National Knowledge Infrastructure (CNKI), Chinese VIP Information, Wanfang, PubMed, and Embase. Our main aim was to include all published studies that investigated the relationship between retinal vascular geometric changes and cognitive impairment. We used the following search terms to identify these published studies: retina*, retinopathy, fundus, vascular, microvascular, blood vessel, microvascula*, microvasculature, fractal dimension (FD), Df, FD, tortuosity, curvature, width, caliber, central retinal arteriolar equivalents (CRAE), CRAE, central retinal venular equivalents (CRVE), CRVE, branching angle, geometric changes, cogniti*, Alzheimer's disease, AD, MCI, dementia, aphrenia, loss of memory, dysfunction, impairment, abnormalities, and decline. The above terms were combined in various ways to form a complete retrieval system. The reference lists of identified studies were also examined rigorously to determine whether they met our inclusion criteria. We also performed a manual search of the gray literature, including theses, dissertations, and conference proceedings. We obtained raw data by contacting the original authors by email.
The study protocol was conducted in accordance with the ethical guidelines of the 1995 Declaration of Helsinki and was approved by Ethics Committee of Affiliated Hospital Nantong University (IRB No. 2018-L084).
Selection criteria and exclusion criteria
The inclusion criteria for the clinical trials were as follows:
1) Involving patients with different levels of cognitive impairment, from memory loss to MCI and AD. These patients were diagnosed using various instruments, including the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria (NINDS-ADRDA criteria)14 and the Mini Mental State Examination.15
2) Reporting on measures and quantitative data on retinal microvascular changes, including CRAE, CRVE, FD, and tortuosity.
3) A case–control study design.
4) Published in the medical literature written in English or Chinese.
-
5) Published between December 2012 and August 2018.
The exclusion criteria for the clinical trials were as follows:
- 1) Inadequate data on retinal vascular parameters.
- 2) Reported in a language other than English or Chinese.
- 3) Inclusion of cases or controls with other confounding diseases that might affect mental health.
Quality assessment and data extraction
We appraised the quality of all included studies using the Newcastle-Ottawa Scale16 and RevMan (version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). A high-quality case–control study had the following characteristics: adequate definition and representativeness of the case, clear definition and selection of controls, reasonable comparability of cases and controls based on the study design or analysis, ascertainment of exposure, using the same method to as certain cases and controls, and a low no-response rate. Two reviewers (W.C. & C.C.) evaluated and cross-checked the quality of the studies, and if there were any discrepancies, another author (W.H.) was consulted to reach a consensus. Fig. 1 illustrates the risk of bias among the five included studies.
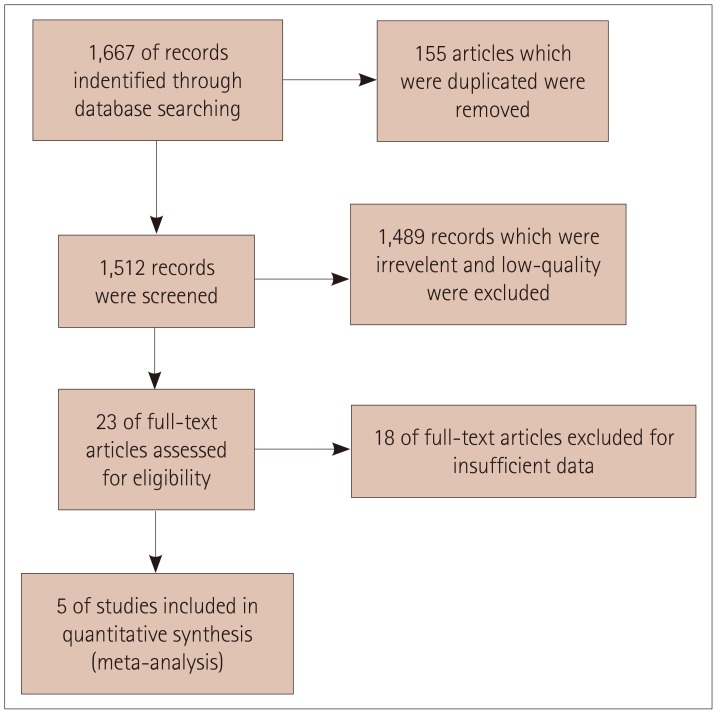
Fig. 1. Flow chart of included and excluded studies.
Two reviewers (W.C. & C.C.) independently extracted data using a data-extraction form. To identify all of the published literature studies, they considered the name of the first author and the year of publication. The following data were extracted for the reported studies: year of publication, inclusion and exclusion criteria, sample size, age, racial group, sex, the number or percentage of diabetes mellitus patients, pre-existing eye diseases, ophthalmic camera and imaging conditions, retinal microvascular abnormalities, cognitive measures and outcomes, and other confounding risk factors such as the history of smoking and hypertension. All duplicated studies were excluded in this systematic review.
Statistical analysis
The meta-analysis was performed using RevMan software (version 5.3). In the statistical analysis we calculated the mean difference (MD) and its 95% confidence interval (CI) as effect factors. Heterogeneity was determined with the chi-square test, and quantified by calculating the inconsistency index I2. We determined the impact of the combined effects by synthesizing all studies using different statistical models according to the heterogeneity. The results were considered to be statistically significant when the two-sided p value was less than 0.05. In addition, funnel plots were constructed to detect the presence of publication bias and other types of bias. Finally, sensitivity analyses were performed to improve the accuracy of the tests.
RESULTS
Search results
After retrieving 1,667 references from the PubMed, Embase, CNKI, and Wanfang databases, we finally included 5 studies3,17,18,19,20 that were consistent with the inclusion and exclusion criteria of this meta-analysis. Fig. 1 shows a flow chart of the study selection process.
Summary characteristics of included studies
While the 5 included studies involved 2,343 subjects, only 671 of them had cognitive impairment or AD. Frost et al.,17 Cheung et al.,18 and Williams et al.19 all used NINDS-ADRDA criteria to diagnose AD. Most of the participants included in the present meta-analysis were aged between 60 to 80 years. Both males and females were included in the case and control groups. The participants in the included studies were Asian or Caucasian. Each study had a cross-sectional design. Most of the included participants in both the case and control groups had hypertension. Nearly one-third of the participants in the study of Jiang3 were smokers. All of the studies except for that of Frost et al.17 captured retinal images after performing pupil dilation. In three studies,17,18,20 a digital ophthalmic camera was positioned at 45° to acquire images. In all of these studies, retinal microvascular abnormalities were determined by measuring the FD, tortuosity, and caliber. The retinal vascular caliber was measured using the Singapore I Vessel Assessment method,21 and caliber data were summarized into CRAE and CRVE by using Knudtson et al.22 revised formulas. The vascular tortuosity of retinal arterioles and venules was calculated as a ratio of the integral of the curvature squared along the path of the vessel relative to the total path length.23 FD was calculated using the box-counting method to represent the complexity of a skeletonized retinal arteriole and venule network, which represents a ‘global’ measure summarizing the entire branching pattern of the retinal vascular tree.24 The risk factors (additional adjustments) were vascular diseases, reflecting that some participants have vascular problems of different intensities. Table 1 provides a detailed description of all of the features included in these studies.
Table 1. Characteristics of the included studies.
| Study (year) | Sample size (number) | Age (years)* | Ethnic group | Male (%)* | Smoking (%)* | Hypertension (%)* | DM (%)* | Pre-existing eye diseases | Study design | Photograph | Measures | Cognitive diagnosis and outcome | Risk factors (additional adjustments) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Devices | Method | |||||||||||||
| Cheung et al.18 (2014) | 426 | 74.77±5.69 (73.87±4.61) | Asian | 47 (53) | 8.8 (11.4) | 76.5 (60.6) | 42.6 (24.8) | No cataract | Crosssectional | 45° Canon CR-Dgi 10D/CR-1 40D | SIVA3.0 after dilation | CRAE, CRVE, FD, tortuosity | DSM-IV criteria AD NINDS-ADRDA criteria, AMT | Vascular risk factors, hypercholesterolemia, ethnicity, history of myocardial infarction, history of cerebrovascular disease |
| Outcome: NS | ||||||||||||||
| Jiang et al.3 (2015) | 60 | 62.22±5.00 (67.22±6.73) | Asian | 60.87 (27.03) | 34.78 (35.14) | 56.52 (81.08) | 30.43 (29.73) | NS | Crosssectional | Color fundus camera | After dilation | CRAE, CRVE, AT, VT, FD | MMSE MoCA CDR ADL RAVLT TMT-B DSST VF | Vascular risk factors |
| Outcome: MMSE: 25.7± 1.88 vs. 28.87±2.07 (cases vs. controls), MoCA: 22.57±1.91 vs. 27.70±1.02* | ||||||||||||||
| CDR: 0.5 vs. 0* | ||||||||||||||
| Cheung et al.20 (2014) | 1202 | 70.57±5.42 (67.89±5.27) | Asian | 21.4 (58.9) | 9.7 (16.3) | 90.1 (85.7) | 30.2 (32.6) | NS | Crosssectional | 45° Canon CR-Dgi with a 10D SLR digital camera back | SIVA1.0 after dilation | FD, tortuosity, branching angle, caliber | AM | Cardiovascular risk factors |
| Outcome: | ||||||||||||||
| Cases: AMT score≤6/10 (with 0-6 yrs of formal education) or ≤8/10 (those with more than 6 yrs formal education) | ||||||||||||||
| Williams et al.19 (2016) | 507 | 79.6±7.8 (76.3±6.6) | Caucasian | 36 (40) | 7 (5) | 38 (42) | 9 (11) | No ophthalmic history | Crosssectional | 500 Canon CR-Dgi | OD centered, SIVA 3.0 after dilation | CRAE, CRVE, FD, tortuosity, branching angle | NINDS-ADRDA criteria, MMSE | Vascular risk factors medications with a cohort frequency greater than 5% |
| Outcome: | ||||||||||||||
| Controls: MMSE score more than 26 of 30 | ||||||||||||||
| Frost et al.17 (2013) | 148 | 72.4±7.5 (71.6±5.6) | Caucasian | 48 (45) | 8 (4) | 44 (36) | 8 (5) | No history of glaucoma, cataract or DR | Crosssectional | 45° Canon CR-1 nonmydriatic | OD centered, SIVA | CRAE, CRVE, FD, tortuosity | NINDS-ADRDA criteria, PET | Vascular risk factors, ApoE |
| Outcome: NS | ||||||||||||||
*Data are cases vs. controls values.
AMT: Abbreviated Mental Test, AT: arteriolar tortuosity, CDR: clinical dementia rating, DR: diabetic retinopathy, DSM: Diagnostic and Statistical Manual of Mental Disorders, FD: fractal dimension, MMSE: Mini-Mental State Examination, MoCA: Montreal Cognitive assessment, NINDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association, NS: no specified, OD: optic disc, VT: venular tortuosity.
Quality of included studies
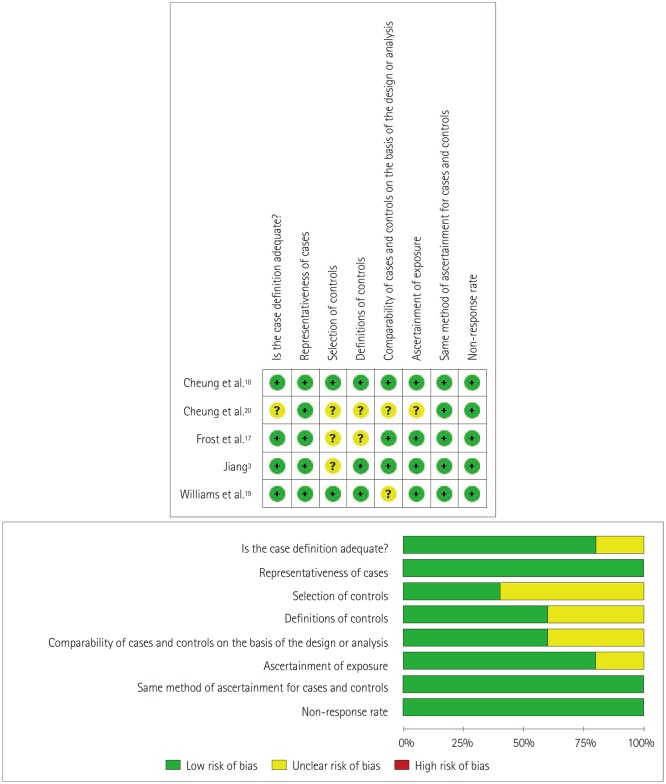
The methodological quality of the included studies is graphically demonstrated in Fig. 2. The representativeness of cases as well as the method of ascertainment for cases and controls were quality controlled in the included studies. The definition and selection of the controls were only vaguely described for some of the included studies, and the ascertainment of exposure for cases and controls might have been opaque in some of the studies. In general, the overall quality of the included studies indicated a low risk of bias, although for some the risk of bias was unclear.
Fig. 2. Details of the quality assessment of studies using the Newcastle-Ottawa Scale.
Association between retinal vascular caliber and cognitive impairment
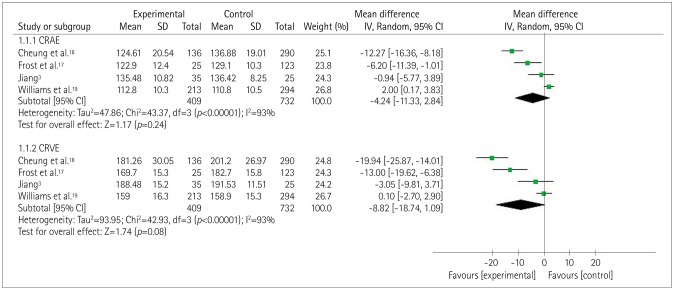
The forest plot in Fig. 3 depicts the association between cognitive impairment and CRAE or CRVE. In four studies3,17,18,19 there was a considerable heterogeneity between the controls and the patients with cognitive impairment (p<0.0001, I2=93%), with MD total-effect values of −4.24 (95% CI: −11.33, 2.84) and −8.82 (95% CI: −18.74, 1.09), respectively. The Z values for CRAE and CRVE were 1.17 (p=0.24) and 1.74 (p=0.08), respectively. These data indicate that there was no significant association between CRAE or CRVE and cognitive impairment. The sensitivity analysis showed that the heterogeneity decreased slightly when excluding one study19 while analyzing the results for CRAE and CRVE. A particularly interesting finding was that the previous reported association between CRAE and cognitive impairment remained unchanged, while a decreased CRVE was found to be related to dementia.
Fig. 3. Forest plot of the association between the retinal vascular caliber and cognitive impairment. CRAE: central retinal arteriolar equivalents, CRVE: central retinal venular equivalents.
Association between retinal vascular tortuosity and cognitive impairment
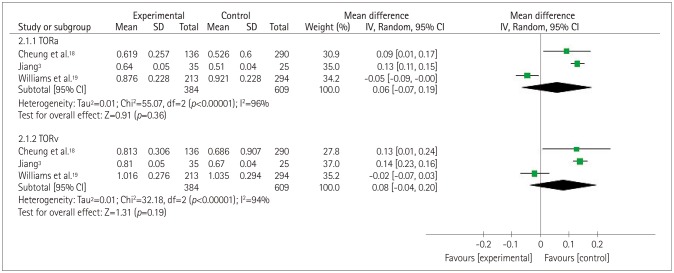
Fig. 4 shows that there was severe heterogeneity (p<0.00001, I2=96% or 94%) across three of the included studies.3,18,19 The MD total-effect estimates were 0.06 (95% CI: −0.07, 0.19) and 0.08 (95% CI: −0.04, 0.20), respectively. The Z values for arteriolar tortuosity (TORa) and venular tortuosity (TORv) were 0.91 (p=0.36) and 1.31 (p=0.19), respectively. These results indicated that retinal vascular tortuosity was not significantly correlated with cognitive impairment. The sensitivity analysis showed that when the study of Williams et al.19 was excluded from the meta-analysis, the heterogeneity of TORa and TORv decreased from 96% and 94%, respectively, to 0%. Furthermore, it was found that the tortuosity was greater in the patients with cognitive impairment.
Fig. 4. Forest plot of the association between the retinal vascular tortuosity and cognitive impairment. TORa: arteriolar tortuosity, TORv: venular tortuosity.
Association between retinal vascular FD and cognitive impairment
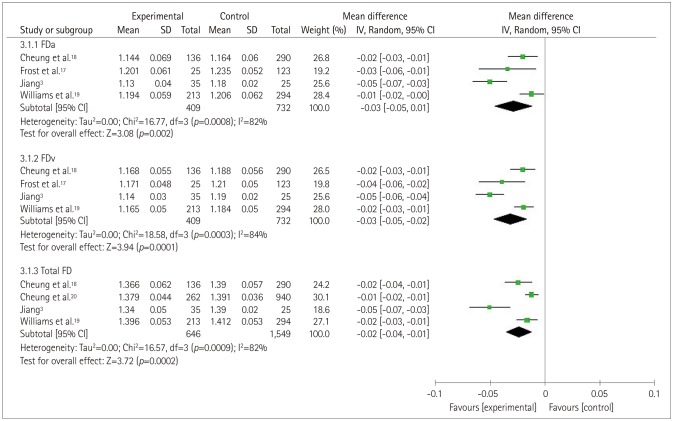
The forest plot in Fig. 5 depictsthe association between retinal FD and cognitive impairment. In four of the original studies,3,17,18,19 the authors measured the arteriolar FD (FDa) and the venular FD (FDv),for which the MD total-effect values were −0.03 (95% CI: −0.05, −0.01) and −0.03 (95% CI: −0.05, −0.02), respectively, and the Z values were 3.08 (p=0.002) and 3.72 (p<0.0001), respectively. In these four selected studies,3,17,18,19 heterogeneity was also observed in the total FD (TFD) group (p=0.0009, I2=82%). The effect value of MD was −0.02 (95% CI: −0.04, −0.01). The test for the overall TFD effect produced a Z value of 3.72 (p=0.0002). These results suggest that retinal FD is related to cognitive impairment. The sensitivity analysis showed that when one study3 was eliminated, the heterogeneity in the FDa and TFD groups decreased to 27% and 35%, respectively, from 82%, while in the FDv group it decreased from 84% to 35%.
Fig. 5. Forest plot of the association between the retinal vascular FD and cognitive impairment. FD: fractal dimension, FDa: arteriolar FD, FDv: venular FD.
Publication bias
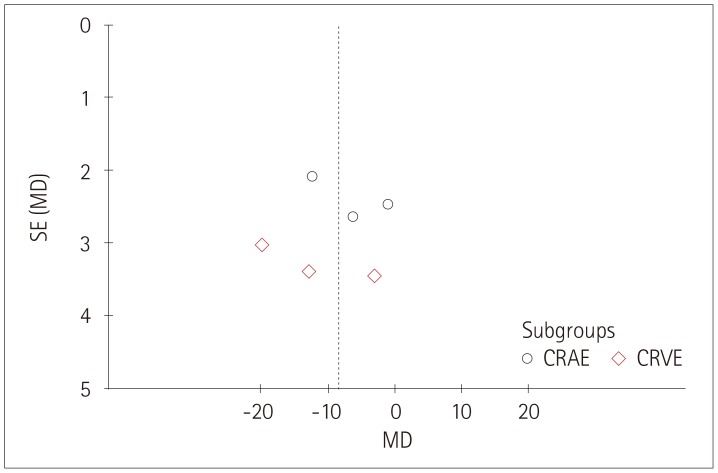
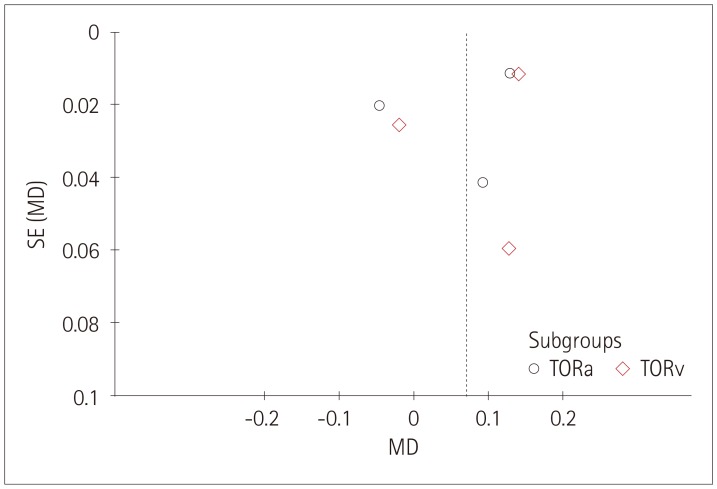
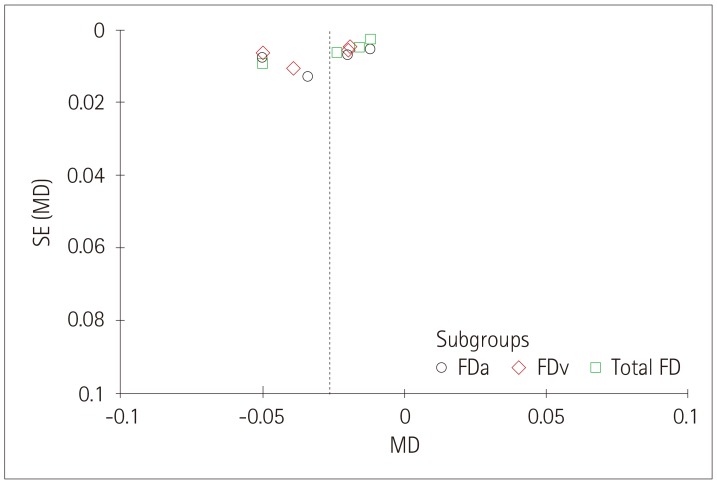
The funnel plots produced in this study for each group showed associations between retinal vessel caliber, tortuosity, FD and cognitive impairments, and those funnel plots without outlines were due to the use of a random-effects model (Figs. 6, 7, 8).
Fig. 6. Funnel plot of publication bias for the association between the retinal vascular caliber and cognitive impairment. CRAE: central retinal arteriolar equivalents, CRVE: central retinal venular equivalents, MD: mean difference, SE: standard error.
Fig. 7. Funnel plot of publication bias for the association between the retinal vascular tortuosity and cognitive impairment. MD: mean difference, SE: standard error, TORa: arteriolar tortuosity, TORv: venular tortuosity.
Fig. 8. Funnel plot of the association between the retinal vascular FD and cognitive impairment. FD: fractal dimension, FDa: arteriolar FD, FDv: venular FD, MD: mean difference, SE: standard error.
DISCUSSION
This study performed a meta-analysis to determine the relationship between retinal vascular geometric changes and cognitive impairment or dementia. Our investigation of 2,343 individuals indicated that retinal vascular FD rather than caliber and tortuosity was associated with cognitive dysfunction. The findings of our meta-analysis were similar to those of previous qualitative systematic reviews performed by Heringa et al.25 and Ding et al.26 However, some of our findings are inconsistent with previously reported investigations. The Los Angeles Latino Eye Study27 found that variables related to the retinal microvasculature caliber such as CRAE and CRVE can provide surprising insights into cognitive impairment in Latino populations. However, their reported data were qualitative rather than quantitative, and so could not been synthesized in our meta-analysis. A similar situation was found in the Circulatory Risk in Communities Study study,28 whose authors reported that arteriolar narrowing and overall retinal abnormalities may be used as markers to identify persons who are more likely to be affected by dementia. In contrast, our meta-analysis did not confirm the presence of such a relationship between arteriolar narrowing and cognitive impairment. Our study mainly disclosed that patients with cognitive impairment might have a smaller vascular FD or a lower complexity of fundus microvascular networks. This is consistent with the finding of Jiang et al.29 that AD patients had sparser retinal microvascular networks than controls, indicating the presence of retinal microvascular dysfunction in AD individuals. Ong et al.30 also indicated that decreased FDa and FDv values were associated with cognitive impairment, which is in complete agreement with the results of our meta-analysis. It is also interesting that Mroczkowska et al.31 found that mild-AD patients with relatively minor cognitive impairment exhibited signs of microvascular dysfunction even in the absence of a history of significant systemic vascular disease. This suggests that retinal changes are potentially useful as an early indicator of cognitive impairment.
The exact biological mechanism underling the association between retinal FD changes and cognitive impairment remains unclear. We consider that there are several possible reasons. The branching complexity is less when FD is smaller, and so rarefaction of retinal vessels is associated with decreased FD, representing corresponding geometric changes in the cerebral microvasculature, further indicating hypoperfusion that may switch on hypoxia-induced pathways, which would increase the amyloid-beta load19,32 or tau pathology.33 All of these undesirable events would lead to the development of AD in patients. Moreover, amyloid plaques in those AD patients may result in vascular damage that undermines the microvasculature. The risk of cerebrovascular disease increases in patients with impaired microcirculation and decreased blood flow.34,35 which may further lead to decreased retinal FD since the retinal vasculature is an observable microcirculation tissue.
Our study was subject to several limitations. At the study and outcome levels, publication bias and the risk of bias may interfere with the detected outcomes. At the review level, our findings were also influenced by eligible research studies that produced incomplete retrievable results. The reliability of the results is impaired by the huge heterogeneity of data present in the selected reports. We therefore performed a sensitivity analysis in addition to applying a random-effects model to tackle the considerable heterogeneity. We speculated that sex and the smoking ratio did impact the results of the retinal FD sensitive analysis. The cerebrovascular perfusion is better in females than in males,36 and so the vascular density of males may be smaller than that of females. Smoking is a risk factor that damages vascular health and increases vascular stiffness,37 and thus might change the branching pattern of the retinal vasculature. For the sensitive analysis of TORa and TORv, we believed that the disparity could be attributed to the participants of this study being Caucasians, while participants of other studies were Asians; in other words, that race can influence retinal measurements. The small number of included studies made it difficult to perform subgroup analysis and a meta-regression analysis. Moreover, the conclusions cannot be generalized since only small populations were analyzed in the included studies. The correlation between the severity of cognitive impairments and the extent of retina microvascular abnormalities therefore needs further investigations to confirm the significance of a retinal imaging biomarker as a predictor for cognitive impairments.
In conclusion, a smaller retinal microvascular FD might be associated with cognitive impairment. Such a relationship could be used to establish a novel retinal image biomarker that might allow the early detection of cognitive impairment in an affordable and convenient way. Further large-sample and well-controlled original studies are required to confirm the present findings.
Acknowledgements
This work was supported by the grant from National Key R&D Program of China (2018YFC1314900, 2018YFC1314902), Nantong “226 Project” and Excellent Key Teachers in the “Qing Lan Project” of Jiangsu Colleges and Universities.
Footnotes
- Conceptualization: Huiqun Wu.
- Data curation: Chendong Wang.
- Formal analysis: Cong Chen, Xiaotao Xu, Yi Zhu.
- Funding acquisition: Huiqun Wu.
- Investigation: Huiqun Wu, Chendong Wang.
- Methodology: Chendong Wang.
- Project administration: Kui Jiang, Jiancheng Dong.
- Supervision: Aimin Sang.
- Validation: Cong Chen.
- Visualization: Chendong Wang.
- Writing—original draft: Chendong Wang.
- Writing—review & editing: Huiqun Wu.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011;7:137–152. doi: 10.1038/nrneurol.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men. The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang YS. Retinal microvascular pathology and vascular risk factors in cognitive impairment: a cross-sectional study [dissertation] Wuhan: Wuhan University; 2015. pp. 1–83. [Google Scholar]
- 4.James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82:1045–1050. doi: 10.1212/WNL.0000000000000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesage SR, Mosley TH, Wong TY, Szklo M, Knopman D, Catellier DJ, et al. Retinal microvascular abnormalities and cognitive decline: the ARIC 14-year follow-up study. Neurology. 2009;73:862–868. doi: 10.1212/WNL.0b013e3181b78436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DH, Chaves PH, Newman AB, Klein R, Sarnak MJ, Newton E, et al. Retinal microvascular signs and disability in the Cardiovascular Health Study. Arch Ophthalmol. 2012;130:350–356. doi: 10.1001/archophthalmol.2011.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikram MK, De Jong FJ, Van Dijk EJ, Prins ND, Hofman A, Breteler MM, et al. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006;129:182–188. doi: 10.1093/brain/awh688. [DOI] [PubMed] [Google Scholar]
- 8.Schrijvers EM, Buitendijk GH, Ikram MK, Koudstaal PJ, Hofman A, Vingerling JR, et al. Retinopathy and risk of dementia: the Rotterdam Study. Neurology. 2012;79:365–370. doi: 10.1212/WNL.0b013e318260cd7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawasaki R, Cheung N, Mosley T, Islam AF, Sharrett AR, Klein R, et al. Retinal microvascular signs and 10-year risk of cerebral atrophy: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2010;41:1826–1828. doi: 10.1161/STROKEAHA.110.585042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R, et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain. 2010;133:1987–1993. doi: 10.1093/brain/awq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dashtbozorg B, Mendonça AM, Penas S, Campilho A. RetinaCAD, a system for the assessment of retinal vascular changes. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6328–6331. doi: 10.1109/EMBC.2014.6945076. [DOI] [PubMed] [Google Scholar]
- 12.Ponto KA, Werner DJ, Wiedemer L, Laubert-Reh D, Schuster AK, Nickels S, et al. Retinal vessel metrics: normative data and their use in systemic hypertension: results from the Gutenberg Health Study. J Hypertens. 2017;35:1635–1645. doi: 10.1097/HJH.0000000000001380. [DOI] [PubMed] [Google Scholar]
- 13.De Jong FJ, Schrijvers EM, Ikram MK, Koudstaal PJ, De Jong PT, Hofman A, et al. Retinal vascular caliber and risk of dementia: the Rotterdam study. Neurology. 2011;76:816–821. doi: 10.1212/WNL.0b013e31820e7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 15.Ridha B, Rossor M. The mini mental state examination. Pract Neurol. 2005;5:298–303. [Google Scholar]
- 16.Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Appl Eng Agric. 2000;18:727–734. [Google Scholar]
- 17.Frost S, Kanagasingam Y, Sohrabi H, Vignarajan J, Bourgeat P, Salvado O, et al. Retinal vascular biomarkers for early detection and monitoring of Alzheimer's disease. Transl Psychiatry. 2013;3:e233. doi: 10.1038/tp.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, et al. Microvascular network alterations in the retina of patients with Alzheimer's disease. Alzheimers Dement. 2014;10:135–142. doi: 10.1016/j.jalz.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 19.Williams MA, McGowan AJ, Cardwell CR, Cheung CY, Craig D, Passmore P, et al. Retinal microvascular network attenuation in Alzheimer's disease. Alzheimers Dement (Amst) 2015;1:229–235. doi: 10.1016/j.dadm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung CY, Ong S, Ikram MK, Ong YT, Chen CP, Venketasubramanian N, et al. Retinal vascular fractal dimension is associated with cognitive dysfunction. J Stroke Cerebrovasc Dis. 2014;23:43–50. doi: 10.1016/j.jstrokecerebrovasdis.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Cheung CY, Hsu W, Lee ML, Wang JJ, Mitchell P, Lau QP, et al. A new method to measure peripheral retinal vascular caliber over an extended area. Microcirculation. 2010;17:495–503. doi: 10.1111/j.1549-8719.2010.00048.x. [DOI] [PubMed] [Google Scholar]
- 22.Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. doi: 10.1076/ceyr.27.3.143.16049. [DOI] [PubMed] [Google Scholar]
- 23.Hart WE, Goldbaum M, Côté B, Kube P, Nelson MR. Measurement and classification of retinal vascular tortuosity. Int J Med Inform. 1999;53:239–252. doi: 10.1016/s1386-5056(98)00163-4. [DOI] [PubMed] [Google Scholar]
- 24.Liew G, Wang JJ, Cheung N, Zhang YP, Hsu W, Lee ML, et al. The retinal vasculature as a fractal: methodology, reliability, and relationship to blood pressure. Ophthalmology. 2008;115:1951–1956. doi: 10.1016/j.ophtha.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Heringa SM, Bouvy WH, Van den Berg E, Moll AC, Kappelle LJ, Biessels GJ. Associations between retinal microvascular changes and dementia, cognitive functioning, and brain imaging abnormalities: a systematic review. J Cereb Blood Flow Metab. 2013;33:983–995. doi: 10.1038/jcbfm.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding J, Patton N, Deary IJ, Strachan MW, Fowkes FG, Mitchell RJ, et al. Retinal microvascular abnormalities and cognitive dysfunction: a systematic review. Br J Ophthalmol. 2008;92:1017–1025. doi: 10.1136/bjo.2008.141994. [DOI] [PubMed] [Google Scholar]
- 27.Gatto NM, Varma R, Torres M, Wong TY, Johnson PL, Segal-Gidan F, et al. Retinal microvascular abnormalities and cognitive function in Latino adults in Los Angeles. Ophthalmic Epidemiol. 2012;19:127–136. doi: 10.3109/09286586.2011.615452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jinnouchi H, Kitamura A, Yamagishi K, Kiyama M, Imano H, Okada T, et al. Retinal vascular changes and prospective risk of disabling dementia: the circulatory risk in communities study (CIRCS) J Atheroscler Thromb. 2017;24:687–695. doi: 10.5551/jat.37291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H, Wei Y, Shi Y, Wright CB, Sun X, Gregori G, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol. 2018;38:292–298. doi: 10.1097/WNO.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong YT, Hilal S, Cheung CY, Xu X, Chen C, Venketasubramanian N, et al. Retinal vascular fractals and cognitive impairment. Dement Geriatr Cogn Dis Extra. 2014;4:305–313. doi: 10.1159/000363286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mroczkowska S, Benavente-Pérez A, Patel S, Qin L, Bentham P, Gherghel D. Retinal vascular dysfunction relates to cognitive impairment in Alzheimer disease. Alzheimer Dis Assoc Disord. 2014;28:366–367. doi: 10.1097/WAD.0b013e3182a2e221. [DOI] [PubMed] [Google Scholar]
- 32.Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, et al. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koike MA, Garcia FG, Kitazawa M, Green KN, Laferla FM. Long term changes in phospho-APP and tau aggregation in the 3xTg-AD mice following cerebral ischemia. Neurosci Lett. 2011;495:55–59. doi: 10.1016/j.neulet.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patton N, Aslam T, Macgillivray T, Pattie A, Deary IJ, Dhillon B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: a rationale based on homology between cerebral and retinal microvasculatures. J Anat. 2005;206:319–348. doi: 10.1111/j.1469-7580.2005.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray CD. The physiological principle of minimum work I. The vascular system and the cost of blood volume. Proc Natl Acad Sci U S A. 1926;12:207–214. doi: 10.1073/pnas.12.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Jerskey BA, Cote DM, Walsh EG, Hassenstab JJ, Ladino ME, et al. Cerebrovascular perfusion among older adults is moderated by strength training and gender. Neurosci Lett. 2014;560:26–30. doi: 10.1016/j.neulet.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YX, Fitch RM. Vascular stiffness: measurements, mechanisms and implications. Curr Vasc Pharmacol. 2004;2:379–384. doi: 10.2174/1570161043385448. [DOI] [PubMed] [Google Scholar]