Abstract
Background and Purpose
Square-wave jerks (SWJs) are the most common saccadic intrusion in progressive supranuclear palsy (PSP), but their genesis is uncertain. We aimed to determine the characteristics of SWJs in PSP (the Richardson subtype) and Parkinson's disease (PD) and to map the brain structures responsible for abnormal SWJ parameters in PSP.
Methods
Eye movements in 12 patients with PSP, 12 patients with PD, and 12 age-matched healthy controls were recorded using an infrared corneal reflection device. The rate, mean amplitude, and velocity of SWJs were analyzed offline. Voxel-based morphometry using a 3-Tesla MRI scanner was performed to relate changes in brain volume to SWJ parameters.
Results
The SWJ rate was more than threefold higher in PSP patients than in both PD patients and controls (mean rates: 33.5, 10.3, and 4.3 SWJs per minute, respectively). The volumes of neither the midbrain nor other infratentorial brain regions were correlated with the SWJ rate. Instead, highly significant associations were found for atrophy in the superior, middle, and inferior temporal gyri in the PSP group.
Conclusions
SWJs in PSP are not mediated by midbrain atrophy. Instead, supratentorial cortical structures located mainly in the temporal lobe appear to be deeply involved in the generation of abnormally high SWJ rates in these patients. Known anatomical connections of the temporal lobe to the superior colliculus and the cerebellum might play a role in SWJ genesis.
Keywords: progressive supranuclear palsy, square-wave jerks, eye movements, Parkinson's disease, voxel-based morphometry
INTRODUCTION
Apart from the impairments in classical vertical eye movements that characterize progressive supranuclear palsy (PSP), a second ocular motor dysfunction was described early on: PSP patients exhibit frequent square-wave jerks (SWJs) whose presence—at least in quantitative eye-movement recordings—is practically universal.1 Nonetheless, the genesis of SWJs is still uncertain and clear evidence of structural brain abnormalities that are causally linked to SWJs is lacking.
Clinical data favor either the “cerebellar hypothesis” or the “brainstem hypothesis” of SWJ generation. The former is based on numerous observations in neurological conditions such as Friedreich ataxia, X-linked ataxia, spinocerebellar ataxia 3, spinocerebellar ataxia 6, and oculomotor apraxia type 2.2,3,4,5,6,7,8 Frequent SWJs have also been noted in structural cerebellar disorders such as Arnold-Chiari malformation, Langerhans cell histiocytosis, anti-GAD ataxia, and multiple sclerosis.9,10,11,12 On the other hand, the brainstem hypothesis provides a morestraightforward explanation for the increased frequency of SWJs in PSP. This theory stems mainly from the elegant model of Otero-Millan et al.13 that assumes the presence of a disturbance in the brainstem ocular motor network. The latter includes the excitatory burst neurons (EBNs), inhibitory burst neurons (IBNs), omnipause neurons (OPNs), and their connections with the superior colliculus (SC). At the core of the model is the emergence of enhanced random fluctuations of neural activity within the SC that leads to increased input to burst neurons and decreased input to OPNs. When the inhibition exerted by the IBNs on the OPNs overcomes the inhibition of the OPNs on the IBNs, a short burst of activity appears in the EBNs that produces a small saccade. In turn, this produces a small retinal error that is detected in the SC network and results in a second saccade in the opposite direction, completing a SWJ. Given that the SC receives input from the basal ganglia, this model offers an explanation for the increased occurrence of SWJs and microsaccades in parkinsonian syndromes. Nonetheless, the cerebellar and brainstem hypotheses are not necessarily mutually exclusive: through its direct projections to IBN, the cerebellar fastigial nucleus directly modifies the brainstem ocular motor network.14,15 Hence, cerebellar (fastigial) damage would ultimately result in a disinhibition of EBNs and motoneurons leading to an increased SWJ occurrence.11
In the present study we aimed to determine the characteristics of SWJs in PSP and Parkinson's disease (PD) and, more importantly, to relate various SWJ features to findings in volumetric brain imaging. Our main goal was to map the brain structures responsible for the increased prevalence of SWJ in patients with PSP.
METHODS
Two patient groups were studied: 1) those with PSP (the Richardson subtype) and 2) those with PD. All participants underwent standardized clinical-neurological examinations performed by two movement disorder specialists (V.C., C.P.), and were diagnosed according to the current standardized consensus criteria for PSP and PD.16,17 Eye-movement data were also compared to recordings from matched healthy controls.
All subjects gave written informed consent for participation in the study, which was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Department of Neurology of the University of Athens (IRB No. 165-20/03/2017).
Eye movements
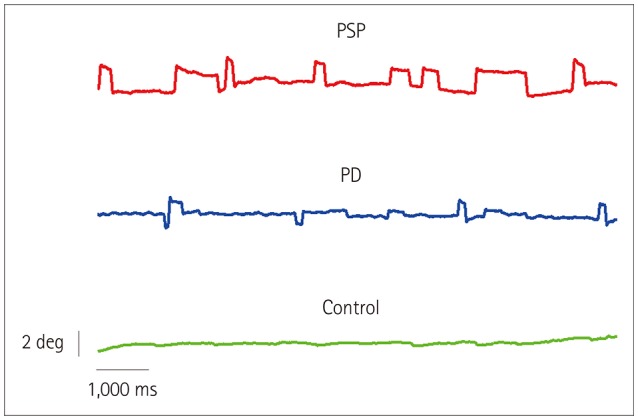
Horizontal eye movements were recorded using an infrared corneal reflection device (IRIS system, Skalar, Delft, the Netherlands) and sampled at 500 Hz with 14-bit resolution via a National Instruments external A/D card using a passband extending from direct current to 70 Hz. Eye-position records were smoothed offline using a second-degree, eight-samples Savitzky-Golay filter, and the instantaneous velocity was derived thereafter by digitally differentiating the displacement signal. Saccades were detected using a 30°/s velocity criterion and were verified visually by the examiner. SWJs were defined as a saccade with an amplitude of ≤4°, taking the gaze away from the current position, followed within 300 ms by another saccade with an amplitude similar to the first (difference in amplitude between saccades <30%), which takes the gaze back toward the initial position.18 The onset of a SWJ was the time at which the eye velocity exceeded 10°/s and continued to increase for at least three frames (6 ms). The number of SWJs during the fixation period for each participant was counted using an algorithm implementing the above rules. Eye movements smaller than 0.4° were not included in order to avoid distortion introduced by consecutive parameter calculations due to recording noise. This maximum amplitude criterion of 4° was chosen for our custom SWJ identification software based on it providing a reasonable trade-off between artifact exclusion (e.g., blinks) and SWJ identification sensitivity. Identified SWJs were also verified visually by the examiner (see the example in Fig. 1). The amplitude, duration, and peak velocity of SWJs and saccades were determined using a custom-made interactive program that extracted the peak velocity and saccade duration from the velocity trace and the saccade amplitude from the displacement trace.19
Fig. 1. Eye position while fixating an LED straight ahead for 10 s in three subjects of the same age (69 years): a PSP patient, a PD patient, and a control subject. Note the increased rate of square-wave jerks in the PSP patient. PD: Parkinson's disease, PSP: progressive supranuclear palsy.
Participants were seated on a chair in a dark room with their head restrained by a forehead-chin headrest. The visual targets were red LEDs mounted on a horizontal bar located 140 cm from the subject's head. At the beginning of the session, a calibration was performed using targets at 0°, +15°, and −15°.
Subjects were tested in two different conditions, each lasting 60 s. First, in the target-on condition, the instruction was to fixate a visual target located straight ahead (0°). Then, in the target-off condition, the LED disappeared and the subjects had to continue fixating the location of the now-absent target. Prior to the second condition, participants were asked to execute 5 leftward and 5 rightward saccades toward targets at eccentricities of 5°, 10°, and 15° (i.e., 15 leftward and 15 rightward saccades). This protocol was used for two reasons: 1) to fit the main sequence relationship between amplitude and velocity according to the formula Peak Velocity=Vmax×(1-e-Amplitude/τ), whereas Vmax is the peak velocity asymptote and τ is the exponential constant,20,21 and 2) to recalibrate the entire time series when necessary.
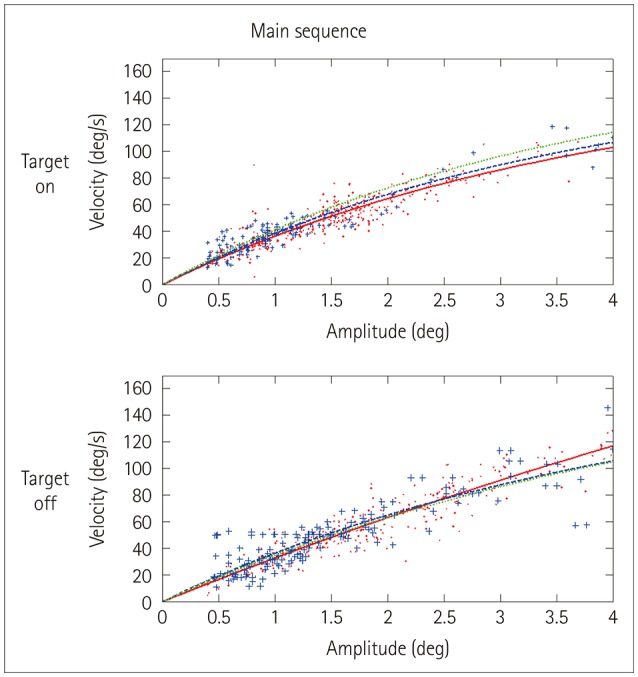
The main sequence curves were modeled only in (macro) saccades and not in SWJs, since there were too few SWJs in both the PD patients and normal controls to achieve meaningful exponential fits. Instead, the peak velocity-to-amplitude ratio22 was employed for group comparisons. Nonetheless, the main sequence curves were fitted for pooled SWJ data from the control, PD, and PSP groups, and allowed for visualization and gross comparisons of τ and Vmax.
All of the PD patients and most of the PSP patients received antiparkinsonian treatment, and recordings were performed in the medication-on state.
Imaging
All participants underwent a routine MRI examination using a 3-Tesla MRI scanner (Achieva TX, Philips, Best, the Netherlands). A clinical brain MRI protocol was employed, which included a three-dimensional, high-spatial-resolution T1-weighted gradient echo pulse sequence for the acquisition of detailed anatomical images (repetition time=9.9 ms, echo time=3.7 ms, flip angle=7°, voxel size = 1×1×1 mm3, and sagittal orientation).
Volumetric analysis was performed using the CAT12 computational anatomy toolbox, which is a toolbox of version 12 of the Statistical Parametric Mapping (SPM12) software package (Wellcome Department of Cognitive Neurology) implemented on MATLAB R2015b (The MathWorks, Natick, MA, USA). The exported registered image and preprocessing parameters were quantitatively assessed, and data with a weighted overall quality measure lower than C+ were excluded from further analyses. The remaining normalized and modulated images were smoothed with an 8-mm full-width-at-half-maximum isotropic Gaussian kernel using a standard SPM12 module.
Statistics
Oculomotor data
After testing for normality, statistical differences among the PSP, PD, and normal control groups were calculated using one-way ANOVA followed by post-hoc multiple comparisons with Scheffe's method. The analyzed parameters were the SWJ rate, amplitude, and velocity-to-amplitude ratio. Separate analyses were performed for target-on and target-off trials. (Macro) saccades were analyzed with regard to their amplitude and main-sequence parameters.
Imaging data
Preprocessed images were fed into SPM12 statistical models. Between-group whole-brain differences in gray-matter volumes were determined using the two-sample t-test with age, sex, and total intracranial volume as nuisance variables to account for any potentially contributing effects on the pattern of local changes.23,24,25 In addition, whole-brain analysis of correlations between gray-matter and white-matter volumes and eye-movement parameters (SWJ rate, amplitude, and ratio velocity/amplitude for both fixation-on and fixation-off conditions) was performed using the “multiple regression” SPM12 function. In these analyses the SWJ parameters were used as the covariate of interest, whilst age, sex, and total intracranial volume were used as confounding variables. In the same regression model we applied an additional region of interest (ROI) analysis using the SC as an inclusive mask. The criterion for statistical significance was set at p<0.001, with an extended criterion applied equal to the expected number of voxels per cluster. Anatomical ROIs covering the entire volumes of clusters were defined using the WFU PickAtlas tool of SPM1226,27 and the Automated Anatomical Labeling software package.28
RESULTS
This study included 12 PSP patients (the Richardson subtype), 12 PD patients, and 12 age-matched healthy controls. The clinical features and demographics of all subjects are summarized in Supplementary Table 1 (in the online-only Data Supplement).
Oculomotor data
During the target-on condition, PSP patients displayed a mean of 33.5 SWJs per minute, which was far higher than for the PD patients and control subjects (p<0.001). Post-hoc comparisons revealed significant differences between PSP and PD patients and between PSP patients and controls, but not between PD patients and controls. The same was true during the target-off condition, where PSP patients exhibited a mean of 22.5 SWJs per minute; that is, a far higher rate than the PSP and PD patients (p<0.001). Again, post-hoc analysis revealed differences only between PSP and PD patients and between PSP patients and controls. Although a tendency for higher SWJ amplitudes was observed in the target-off condition, there were no significant differences in the SWJ amplitude in either this or the target-on condition (Table 1). The peak SWJ-velocity-to-amplitude ratios were also similar among the three subject groups (the main sequence curves for pooled data from all subjects are depicted in Fig. 2). On the other hand, velocity-to-amplitude ratios for (macro) saccades showed markedly decreased values, reflecting slow saccades in PSP patients relative to PD patients or control subjects (p<0.001), as expected.
Table 1. Rate, amplitude, and velocity/amplitude ratios of SWJs in PSP patients, PD patients, and healthy control subjects.
| PSP | PD | Controls | Significance | |
|---|---|---|---|---|
| Fixation on | ||||
| Rate, SWJs per minute | 33.5±12.4 | 10.3±8.7 | 4.3±3.6 | † |
| Amplitude, deg | 1.3±0.5 | 1.4±1.1 | 1.3±1.1 | n.s. |
| Velocity/amplitude ratio, per s | 33.4±4.0 | 34.9±5.4 | 40.7±11.0 | n.s. |
| Fixation off | ||||
| Rate, SWJs per minute | 22.5±13.4 | 13.3±13.2 | 4.2±2.7 | * |
| Amplitude, deg | 2.0±0.7 | 1.6±0.6 | 1.5±0.7 | n.s. |
| Velocity/amplitude ratio, per s | 30.9±3.7 | 32.1±4.3 | 35.5±9.8 | n.s. |
Data are mean±SD values.
*p<0.01, †p<0.001.
n.s.: not significant, PD: Parkinson's disease, PSP: progressive supranuclear palsy, SWJs: square-wave jerks.
Fig. 2. Amplitude-vs.-velocity relationship (“main sequence”) for square-wave jerks pooled for all progressive supranuclear palsy patients (dots and solid line), all Parkinson's disease patients (crosses and dashed line), and all control subjects (dotted line). No differences are evident in either target-on or target-off condition. To improve clarity, only the fitted curve (and not the single data points) is shown for the control subjects.
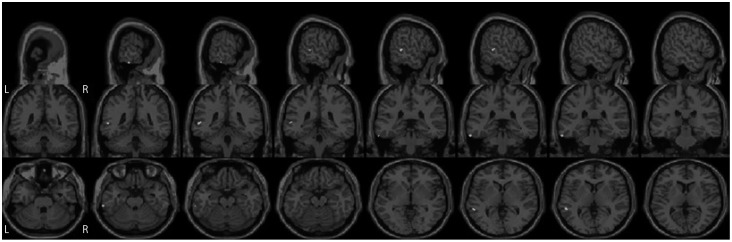
SWJ imaging correlations
Between-group morphometric comparisons revealed that the midbrain volume was lower in PSP patients than in the PD group (p<0.001). Also, PSP patients had smaller superior temporal gyrus, supramarginal gyrus, and cerebellar lobules VI and X (p<0.001). The rather trivial finding of midbrain volume loss in PSP did not yield any significant results in the analyses of correlations of brain volumes with SWJ parameters. Here, the PSP group exhibited significant associations (p<0.001) between the SWJ rate and volume reductions in the middle and inferior temporal gyri for the target-on condition (Table 2 and Fig. 3), and correlations between the SWJ rate and atrophy in the superior temporal, inferior temporal, and superior occipital gyri for the target-off condition. PD patients showed correlations between the SWJ rate and reductions in putamen and pallidum volumes for the target-on condition, but no significant correlations for the target-off condition (Table 2). Furthermore, temporal cortical areas along with other gray-matter sites such as parts of the occipital and frontal lobes were related to the SWJ amplitude and velocity/amplitude ratios in both PSP and PD patients (Tables 3, 4).
Table 2. Significant correlations between the SWJ rate and areas with reduced brain volumes in PSP and PD patients.
| SWJ recording condition | Side | Area | MNI | Cluster size | t | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| PSP | Target on | Left | Middle temporal gyrus | −56 | −42 | 3 | 47 | 13.29 |
| Left | Inferior temporal gyrus | −65 | −33 | −24 | 30 | 8.39 | ||
| Target off | Right | Inferior temporal gyrus | 59 | −30 | −21 | 20 | 10.44 | |
| Left | Superior temporal gyrus | −59 | 3 | −5 | 35 | 10.03 | ||
| Right | Superior occipital gyrus | 30 | −81 | 23 | 17 | 8.29 | ||
| PD | Target on | Left | Putamen/pallidum | −11 | 3 | −5 | 62 | 9.90 |
| Right | Pallidum | 21 | 0 | 3 | 27 | 7.93 | ||
| Target off | No significant correlations | |||||||
p<0.001.
MNI: coordinates according to the Montreal Neurological Institute brain atlas, PD: Parkinson's disease, PSP: progressive supranuclear palsy, SWJ: square-wave jerk.
Fig. 3. Cortical regions of progressive supranuclear palsy patients showing significant (p<0.001) correlations with the square-wave jerk rate in the target-on condition.
Table 3. Significant correlations between the SWJ amplitude and areas with reduced brain volumes in PSP and PD patients.
| SWJ recording condition | Side | Area | MNI | Cluster size | t | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| PSP | Target on | Left | Middle cingulate gyrus | −14 | −36 | 51 | 59 | 8.45 |
| Left | Middle frontal gyrus | −26 | 20 | 42 | 19 | 7.95 | ||
| Right | Superior temporal gyrus | 56 | −17 | −3 | 34 | 7.82 | ||
| Target off | Left | Cerebellum crus I | −17 | −81 | −20 | 56 | 18.94 | |
| Right | Superior temporal gyrus | 53 | −42 | 24 | 18 | 11.07 | ||
| Right | Postcentral gyrus | 21 | −41 | 69 | 23 | 9.29 | ||
| Left | Precuneus | −15 | −48 | 57 | 44 | 9.16 | ||
| PD | Target on | Left | Middle cingulate gyrus | −9 | −32 | 50 | 30 | 10.36 |
| Right | Inferior temporal gyrus | 48 | −65 | −3 | 99 | 10.20 | ||
| Right | Superior medial frontal gyrus | 9 | 59 | 11 | 85 | 7.44 | ||
| Left | Lingual gyrus/fusiform | −27 | −66 | −3 | 141 | 6.89 | ||
| Target off | Left | Superior temporal gyrus | −56 | −9 | 0 | 79 | 10.48 | |
| Right | Inferior temporal gyrus/fusiform | 44 | −24 | −24 | 40 | 6.13 | ||
p<0.001.
MNI: coordinates according to the Montreal Neurological Institute brain atlas, PD: Parkinson's disease, PSP: progressive supranuclear palsy, SWJ: square-wave jerk.
Table 4. Significant correlations between a decreased SWJ velocity/amplitude ratio and areas with reduced brain volumes in PSP and PD patients.
| SWJ recording condition | Side | Area | MNI | Cluster size | t | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| PSP | Target on | Left | Inferior temporal gyrus | −38 | −5 | −42 | 243 | 15.71 |
| Right | Superior parietal gyrus | 21 | −56 | 72 | 21 | 10.26 | ||
| Left | Fusiform gyrus | −42 | −54 | −8 | 16 | 9.26 | ||
| Left | Precuneus | −14 | −71 | 54 | 24 | 9.00 | ||
| Left | Middle temporal gyrus | −57 | −56 | 18 | 23 | 8.79 | ||
| Right | Cuneus | 5 | −83 | 27 | 31 | 8.56 | ||
| Left | Precentral gyrus | −50 | −5 | 23 | 25 | 7.94 | ||
| Target off | Right | Middle occipital gyrus | 39 | −74 | 2 | 17 | 8.99 | |
| Left | Middle frontal gyrus | −29 | 44 | 23 | 18 | 8.09 | ||
| Right | Superior medial frontal gyrus/superior frontal gyrus | 15 | 66 | 9 | 17 | 7.12 | ||
| PD | Target on | Right | Cuneus | 9 | −75 | 30 | 101 | 11.00 |
| Left | Rolandic operculum | −57 | 8 | 0 | 37 | 8.54 | ||
| Right | Inferior frontal operculum | 47 | 3 | 26 | 89 | 8.52 | ||
| Right | Superior medial frontal gyrus/anterior cingulate gyrus | 8 | 54 | 9 | 70 | 7.34 | ||
| Right | Inferior temporal gyrus | 48 | −62 | −12 | 29 | 6.84 | ||
| Right | Inferior operculum frontal/Rolandic operculum | 51 | 6 | 11 | 59 | 6.67 | ||
| Target off | Right | Lingual gyrus/calcarine/inferior-middle-superior occipital gyrus | 9 | −96 | −5 | 889 | 12.34 | |
| Right | Middle occipital gyrus | 45 | −77 | 9 | 38 | 7.12 | ||
| Left | Cuneus | −9 | −78 | 36 | 48 | 7.07 | ||
| Left | Inferior occipital gyrus | −18 | −92 | −9 | 26 | 6.21 | ||
p<0.001.
MNI: coordinates according to the Montreal Neurological Institute brain atlas, PD: Parkinson's disease, PSP: progressive supranuclear palsy, SWJ: square-wave jerk.
DISCUSSION
Various oculomotor abnormalities in parkinsonian syndromes have long been known and have been described in detail using quantitative eye-movement recordings.29,30,31 However, the search for the causative brain alterations in living patients has only been possible following recent advancements in structural and functional neuroimaging techniques. Indeed, combined studies of eye movements and morphometry- or connectivity-based MRI analyses have confirmed the traditional hypotheses regarding oculomotor deficits in parkinsonian syndromes: decreased peak eye velocities of saccades in PSP (the Richardson subtype) are significantly correlated with midbrain atrophy, hypometric (“staircase”) saccades to visual targets in PD are linked to functional connectivity changes in cortical areas, and saccadic smooth pursuit in multiple-system atrophy is related to cerebellar and pontine volume reductions.32,33,34 Nonetheless, common oculomotor findings such as the increased latency of reactive saccades in PD—traditionally considered a dysexecutive syndrome—did not show any significant correlation with regional brain volume changes.34
In PSP, SWJs—which are the second-most-obvious eye-movement abnormality after the slowed vertical saccadic eye movements—have not been investigated in the context of the underlying reduction in brain volume. It is possible that the only oculomotor parameter with a conceptual proximity to SWJs is the accumulated intrusion index examined by Vintonyak et al.,34 which was not correlated with volumetric changes in any of the investigated brain structures. However, the accumulated intrusion index differs fundamentally from classic saccadic intrusions such as SWJs, in that it is obtained during an active saccadic task (i.e., not during fixation) and, more importantly, it excludes all saccades smaller than 2° while keeping all macrosaccades without an upper amplitude limit. Thus, the findings of such combined oculomotor-MRI analyses are not directly comparable with those in the present study.
As expected, we found that the SWJ rate was markedly higher in the PSP group than in both the PD patients and controls. This increase was more than threefold in the fixation-on condition, whereas the effect was more moderate in the fixation-off condition, although it was still highly statistically significant. The PD patients also showed a tendency toward more SWJs compared to controls, but this difference was not significant. The mean SWJ amplitude, particularly in the fixation-off condition, showed a nonsignificant tendency toward higher values in PSP patients, while the SWJ velocity (expressed by the velocity-to-amplitude ratio) did not differ among the three patient groups. On the other hand, voluntary (macro) saccades had much slower velocities in the PSP group than in both the PD patients and controls. Thus, the present data replicated the well-known hallmark of fixational abnormality in PSP, namely the increased SWJ rate.1
Not surprisingly, voxel-based morphometry demonstrated that the midbrain volume was lower in our PSP patients than in the PD patients. A particularly interesting finding was that the midbrain volume loss was not associated with SWJ parameters.1 Focused MRI analysis of the SC using dedicated ROI calculations also did not reveal any correlation with SWJs. Thus, although reductions in the midbrain volume have been previously shown to be related to impairments in vertical eye-movement performance in PSP,34 it appears that they do not play a major role in SWJ generation.
Instead, our morphometric-oculographic correlation analysis using a stringent statistical significance threshold (p<0.001) indicated that supratentorial rather than infratentorial structures are involved in SWJ generation in PSP. In particular, atrophy in the middle and inferior temporal gyri was correlated with increased SWJ rates in the fixation-on condition, while volume losses in the inferior temporal, superior temporal, and superior occipital gyri were associated with higher SWJ rates in the fixation-off condition. Although of less importance due to the absence of significant SWJ abnormalities in PD patients, the SWJ rates in that group were correlated with volume reductions in the pallidum and putamen in the fixation-on condition, while no correlations were evident in the fixation-off condition. Also, our analyses of the MRI data set with regard to other SWJ parameters, such as the SWJ amplitude and velocity, proved to have a rather questionable heuristic value, since these oculomotor parameters did not differ significantly in PSP patients. It is perhaps worth noting that cortical rather than infratentorial sites displayed correlations with the SWJ amplitude and velocity in both PSP and PD patients, with the exception of a significant association of a volume reduction in cerebellar crus I and an increased mean SWJ amplitude in PSP.
Thus, gyri of the temporal lobe appear to play a crucial role in SWJ generation in PSP. Neuropathological studies of PSP brains have traditionally focused on changes in the brainstem, basal ganglia, and frontal lobe, providing straightforward correlations with the cardinal signs of the disease, namely supranuclear saccade slowing, parkinsonism, and frontal-type dementia.35,36 However, recent cross-sectional and longitudinal neuroimaging studies have found small but significant amounts of atrophy in the temporal lobe of PSP patients, whereas most researchers tend to associate these changes with behavioral deficits.37,38,39,40 Unfortunately, no previous study has attempted to link any parts of the temporal lobe with SWJs.
A completely different line of evidence provides indirect support for an association of the temporal cortex with SWJ generation. Although less extensively investigated in the context of ocular motility, patients with Alzheimer's disease—which perhaps is the prototype of degenerative temporal-lobe atrophy—reportedly show abnormally high SWJ rates. Jones et al.41 described the first series of Alzheimer patients with a high prevalence of SWJs, demonstrating SWJ rates ranging from 51 to 74 per minute. Additional oculomotor deficits such as saccadic smooth pursuit were subsequently shown to cooccur with SWJs in Alzheimer's disease.42 More recently, a comparison of typical Alzheimer's patients with patients with posterior cortical atrophy revealed that the former show significantly higher SWJ rates, whereas the latter exhibit more macrosaccade intrusions.43 Few studies have investigated SWJs in focal hemispheric lesions. The systematic study of Sharpe et al.44 analyzed 17 patients with pathological SWJs due to solitary brain tumors or infarctions, and measured the highest SWJ rate (65 per minute) in a patient with parietotemporal astrocytoma. There were no pure temporal or parietal lesions in this cohort to allow more precise comparisons, but patients with lesions in the frontal or occipital lobe exhibited fewer SWJs.
It is worthwhile to consider how temporal cortical areas could influence the generation of SWJs. There is only indirect neuroanatomical evidence linking the temporal neocortex to pontine neurons (EBN and OPN), which are presumably responsible for SWJ generation. With regard to the brainstem SWJ hypothesis,13 several anatomical staining and anterograde degeneration studies have demonstrated connections of neocortical areas of the temporal lobe to parts of the SC.45,46,47 It could be hypothesized that reduced control on SC neurons due to temporal cortex atrophy would lead to an enhancement of random fluctuations of neural activity within the SC, ultimately leading to increased input to EBNs and decreased input to OPNs. Alternatively, older neuroanatomical investigations indicated the presence of direct connections of the temporal neocortex to the vestibulocerebellum, presumably via cortico-ponto-cerebellar mossy fiber projections.48,49,50 These pathways could play a role in fastigial-nucleus-mediated disinhibition of EBNs that is presumably consistent with the cerebellar hypothesis of SWJ triggering (see the Introduction).
The present study was subject to some limitations. First, a larger sample might have revealed more SWJ-related brain volume changes. Second, the availability of MRI data for the control subjects, who were included only for oculomotor comparisons, might have revealed interesting correlations between voxel-based morphometry and SWJs in healthy individuals, at least in those who exhibited some SWJs. Third, it is worth noting that SWJs are linked to temporal cortex volume loss in PSP but not in PD, and the SWJ rate was correlated with reduced putamen and pallidum volumes in the latter patients. Although this association should be interpreted with caution since the PD patients did not show significantly increased SWJs compared to controls, this difference in voxel-based morphometric correlations between PSP and PD with regard to SWJs remains an open issue that requires further investigations. Nonetheless, it is not unusual for the same ocular motor phenomenon to be associated with different regional brain atrophies in different diseases. For instance, the increased latency of voluntary saccades that is observed to the same degree in PD, PSP, and multiple-system atrophy seems to be mediated by different brain structures in each syndrome.34 Another example is the disturbances of smooth-pursuit eye movements being very similar in PSP and multiple system atrophy, which shows a correlation with cerebellar and pontine atrophy in the latter but not in the former syndrome.34 With respect to the present findings, we speculate that the core neural circuit responsible for SWJ generation in the brainstem becomes hyperexcitable by damage or atrophy of multiple upstream brain regions.
In summary, this study is the first to use voxel-based morphometry and quantitative oculography to demonstrate that SWJs (a universal fixation abnormality) are not mediated by midbrain atrophy. Instead, supratentorial cortical structures located mainly in the temporal lobe appear to be deeply involved in the generation of abnormally high SWJ rates. A focused, multidisciplinary research approach that aligns precise eye-movement recordings with advanced structural and functional neuroimaging has the potential to elucidate the origin of saccadic intrusions in various movement disorders and might ultimately contribute to the understanding of the neural mechanisms underlying visual fixation.
Footnotes
- Conceptualization: Evangelos Anagnostou, Constantinos Potagas.
- Data curation: Evangelos Anagnostou, Efstratios Karavasilis, Irini Potiri, Efstathios Efstathopoulos.
- Formal analysis: Evangelos Anagnostou, Efstratios Karavasilis, Irini Potiri, Efstathios Efstathopoulos.
- Investigation: Evangelos Anagnostou, Vasileios Constantinides, Elisavet Kapaki, Constantinos Potagas.
- Writing—original draft: Evangelos Anagnostou, Efstratios Karavasilis, Irini Potiri, Vasileios Constantinides, Efstathios Efstathopoulos, Elisavet Kapaki, Constantinos Potagas.
- Writing—review & editing: Evangelos Anagnostou, Efstratios Karavasilis, Irini Potiri, Vasileios Constantinides, Efstathios Efstathopoulos, Elisavet Kapaki, Constantinos Potagas.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.1.37.
Clinical and demographic characteristics
References
- 1.Troost BT, Daroff RB. The ocular motor defects in progressive supranuclear palsy. Ann Neurol. 1977;2:397–403. doi: 10.1002/ana.410020509. [DOI] [PubMed] [Google Scholar]
- 2.Spieker S, Schulz JB, Petersen D, Fetter M, Klockgether T, Dichgans J. Fixation instability and oculomotor abnormalities in Friedreich's ataxia. J Neurol. 1995;242:517–521. doi: 10.1007/BF00867423. [DOI] [PubMed] [Google Scholar]
- 3.Ribaï P, Pousset F, Tanguy ML, Rivaud-Pechoux S, Le Ber I, Gasparini F, et al. Neurological, cardiological, and oculomotor progression in 104 patients with Friedreich ataxia during long-term follow-up. Arch Neurol. 2007;64:558–564. doi: 10.1001/archneur.64.4.558. [DOI] [PubMed] [Google Scholar]
- 4.Fahey MC, Cremer PD, Aw ST, Millist L, Todd MJ, White OB, et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain. 2008;131:1035–1045. doi: 10.1093/brain/awm323. [DOI] [PubMed] [Google Scholar]
- 5.Verhagen WI, Huygen PL, Arts WF. Multi-system signs and symptoms in X-linked ataxia carriers. J Neurol Sci. 1996;140:85–90. doi: 10.1016/0022-510x(96)00116-5. [DOI] [PubMed] [Google Scholar]
- 6.Bürk K, Fetter M, Abele M, Laccone F, Brice A, Dichgans J, et al. Autosomal dominant cerebellar ataxia type I: oculomotor abnormalities in families with SCA1, SCA2, and SCA3. J Neurol. 1999;246:789–797. doi: 10.1007/s004150050456. [DOI] [PubMed] [Google Scholar]
- 7.Christova P, Anderson JH, Gomez CM. Impaired eye movements in presymptomatic spinocerebellar ataxia type 6. Arch Neurol. 2008;65:530–536. doi: 10.1001/archneur.65.4.530. [DOI] [PubMed] [Google Scholar]
- 8.Clausi S, De Luca M, Chiricozzi FR, Tedesco AM, Casali C, Molinari M, et al. Oculomotor deficits affect neuropsychological performance in oculomotor apraxia type 2. Cortex. 2013;49:691–701. doi: 10.1016/j.cortex.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Passo M, Shults WT, Talbot T, Palmer EA. Acquired esotropia. A manifestation of Chiari I malformation. J Clin Neuroophthalmol. 1984;4:151–154. doi: 10.3109/01658108409034894. [DOI] [PubMed] [Google Scholar]
- 10.Anagnostou E, Papageorgiou SG, Potagas C, Alexakis T, Kalfakis N, Anastasopoulos D. Square-wave jerks and smooth pursuit impairment as subtle early signs of brain involvement in Langerhans' cell histiocytosis. Clin Neurol Neurosurg. 2008;110:286–290. doi: 10.1016/j.clineuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Brokalaki C, Kararizou E, Dimitrakopoulos A, Evdokimidis I, Anagnostou E. Square-wave ocular oscillation and ataxia in an anti-GAD-positive individual with hypothyroidism. J Neuroophthalmol. 2015;35:390–395. doi: 10.1097/WNO.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 12.Dell'Osso LF, Troost BT, Daroff RB. Macro square wave jerks. Neurology. 1975;25:975–979. doi: 10.1212/wnl.25.10.975. [DOI] [PubMed] [Google Scholar]
- 13.Otero-Millan J, Macknik SL, Serra A, Leigh RJ, Martinez-Conde S. Triggering mechanisms in microsaccade and saccade generation: a novel proposal. Ann N Y Acad Sci. 2011;1233:107–116. doi: 10.1111/j.1749-6632.2011.06177.x. [DOI] [PubMed] [Google Scholar]
- 14.Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–348. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- 15.Scudder CA, McGee DM, Balaban CD. Connections of monkey saccade-related fastigial nucleus neurons revealed by anatomical and physiological methods. Soc Neurosci Abstr. 2000;26:971. [Google Scholar]
- 16.Williams DR, De Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005;128:1247–1258. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- 17.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leigh RJ, Zee DS. The neurology of eye movements. 4th ed. New York: Oxford University Press; 2006. [Google Scholar]
- 19.Anagnostou E, Kemanetzoglou E, Papadimas G, Kararizou E, Evdokimidis I. Extraocular muscle function in adult-onset Pompe disease tested by saccadic eye movements. Neuromuscul Disord. 2014;24:1073–1078. doi: 10.1016/j.nmd.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci. 1975;24:191–204. [Google Scholar]
- 21.Baloh RW, Sills AW, Kumley WE, Honrubia V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology. 1975;25:1065–1070. doi: 10.1212/wnl.25.11.1065. [DOI] [PubMed] [Google Scholar]
- 22.Versino M, Rossi B, Beltrami G, Sandrini G, Cosi V. Ocular motor myotonic phenomenon in myotonic dystrophy. J Neurol Neurosurg Psychiatry. 2002;72:236–240. doi: 10.1136/jnnp.72.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stonnington CM, Tan G, Klöppel S, Chu C, Draganski B, Jack CR, Jr, et al. Interpreting scan data acquired from multiple scanners: a study with Alzheimer's disease. Neuroimage. 2008;39:1180–1185. doi: 10.1016/j.neuroimage.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostić VS, Agosta F, Petrović I, Galantucci S, Spica V, Jecmenica-Lukic M, et al. Regional patterns of brain tissue loss associated with depression in Parkinson disease. Neurology. 2010;75:857–863. doi: 10.1212/WNL.0b013e3181f11c1d. [DOI] [PubMed] [Google Scholar]
- 25.Barnes J, Ridgway GR, Bartlett J, Henley SM, Lehmann M, Hobbs N, et al. Head size, age and gender adjustment in MRI studies: a necessary nuisance? Neuroimage. 2010;53:1244–1255. doi: 10.1016/j.neuroimage.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 26.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 27.Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 29.Highstein S, Cohen B, Mones R. Changes in saccadic eye movements of patients with Parkinson's disease before and after L-dopa. Trans Am Neurol Assoc. 1969;94:277–279. [PubMed] [Google Scholar]
- 30.Dix MR, Harrison MJ, Lewis PD. Progressive supranuclear palsy (the Steele-Richardson-Olszewski syndrome). A report of 9 cases with particular reference to the mechanism of the oculomotor disorder. J Neurol Sci. 1971;13:237–256. doi: 10.1016/0022-510x(71)90029-3. [DOI] [PubMed] [Google Scholar]
- 31.Anderson T, Luxon L, Quinn N, Daniel S, David Marsden C, Bronstein A. Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Mov Disord. 2008;23:977–984. doi: 10.1002/mds.21999. [DOI] [PubMed] [Google Scholar]
- 32.Gorges M, Müller HP, Lulé D, Ludolph AC, Pinkhardt EH, Kassubek J. Functional connectivity within the default mode network is associated with saccadic accuracy in Parkinson’s disease: a resting-state FMRI and videooculographic study. Brain Connect. 2013;3:265–272. doi: 10.1089/brain.2013.0146. [DOI] [PubMed] [Google Scholar]
- 33.Rosskopf J, Gorges M, Müller HP, Lulé D, Uttner I, Ludolph AC, et al. Intrinsic functional connectivity alterations in progressive supranuclear palsy: differential effects in frontal cortex, motor, and midbrain networks. Mov Disord. 2017;32:1006–1015. doi: 10.1002/mds.27039. [DOI] [PubMed] [Google Scholar]
- 34.Vintonyak O, Gorges M, Müller HP, Pinkhardt EH, Ludolph AC, Huppertz HJ, et al. Patterns of eye movement impairment correlate with regional brain atrophy in neurodegenerative parkinsonism. Neurodegener Dis. 2017;17:117–126. doi: 10.1159/000454880. [DOI] [PubMed] [Google Scholar]
- 35.Wakabayashi K, Takahashi H. Pathological heterogeneity in progressive supranuclear palsy and corticobasal degeneration. Neuropathology. 2004;24:79–86. doi: 10.1111/j.1440-1789.2003.00543.x. [DOI] [PubMed] [Google Scholar]
- 36.Jellinger KA. Neuropathology and pathogenesis of extrapyramidal movement disorders: a critical update-I. Hypokinetic-rigid movement disorders. J Neural Transm (Vienna) 2019;126:933–995. doi: 10.1007/s00702-019-02028-6. [DOI] [PubMed] [Google Scholar]
- 37.Josephs KA, Xia R, Mandrekar J, Gunter JL, Senjem ML, Jack CR, Jr, et al. Modeling trajectories of regional volume loss in progressive supranuclear palsy. Mov Disord. 2013;28:1117–1124. doi: 10.1002/mds.25437. [DOI] [PubMed] [Google Scholar]
- 38.Caso F, Agosta F, Volonté MA, Ferraro PM, Tiraboschi P, Copetti M, et al. Cognitive impairment in progressive supranuclear palsy-Richardson's syndrome is related to white matter damage. Parkinsonism Relat Disord. 2016;31:65–71. doi: 10.1016/j.parkreldis.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Höglinger GU, Schöpe J, Stamelou M, Kassubek J, Del Ser T, Boxer AL, et al. Longitudinal magnetic resonance imaging in progressive supranuclear palsy: a new combined score for clinical trials. Mov Disord. 2017;32:842–852. doi: 10.1002/mds.26973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agosta F, Caso F, Ječmenica-Lukić M, Petrović IN, Valsasina P, Meani A, et al. Tracking brain damage in progressive supranuclear palsy: a longitudinal MRI study. J Neurol Neurosurg Psychiatry. 2018;89:696–701. doi: 10.1136/jnnp-2017-317443. [DOI] [PubMed] [Google Scholar]
- 41.Jones A, Friedland RP, Koss B, Stark L, Thompkins-Ober BA. Saccadic intrusions in Alzheimer-type dementia. J Neurol. 1983;229:189–194. doi: 10.1007/BF00313742. [DOI] [PubMed] [Google Scholar]
- 42.Parkinson J, Maxner C. Eye movement abnormalities in Alzheimer disease: case presentation and literature review. Am Orthopt J. 2005;55:90–96. doi: 10.3368/aoj.55.1.90. [DOI] [PubMed] [Google Scholar]
- 43.Shakespeare TJ, Kaski D, Yong KX, Paterson RW, Slattery CF, Ryan NS, et al. Abnormalities of fixation, saccade and pursuit in posterior cortical atrophy. Brain. 2015;138:1976–1991. doi: 10.1093/brain/awv103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharpe JA, Herishanu YO, White OB. Cerebral square wave jerks. Neurology. 1982;32:57–62. doi: 10.1212/wnl.32.1.57. [DOI] [PubMed] [Google Scholar]
- 45.Cranford JL, Ladner SJ, Campbell CB, Neff WD. Efferent projections of the insular and temporal neocortex of the cat. Brain Res. 1976;117:195–210. doi: 10.1016/0006-8993(76)90730-7. [DOI] [PubMed] [Google Scholar]
- 46.Maioli MG, Domeniconi R, Squatrito S, Riva Sanseverino E. Projections from cortical visual areas of the superior temporal sulcus to the superior colliculus, in macaque monkeys. Arch Ital Biol. 1992;130:157–166. [PubMed] [Google Scholar]
- 47.Webster MJ, Bachevalier J, Ungerleider LG. Transient subcortical connections of inferior temporal areas TE and TEO in infant macaque monkeys. J Comp Neurol. 1995;352:213–226. doi: 10.1002/cne.903520205. [DOI] [PubMed] [Google Scholar]
- 48.Ruwaldt MM, Snider RS. Projections of vestibular areas of cerebellum to the cerebrum. J Comp Neurol. 1956;104:387–401. doi: 10.1002/cne.901040304. [DOI] [PubMed] [Google Scholar]
- 49.Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978;101:251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- 50.Schmahmann JD, Pandya DN. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol. 1991;308:224–248. doi: 10.1002/cne.903080209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical and demographic characteristics