Abstract
Background and Purpose
The high prevalence of antiphospholipid antibodies (aPL) and antinuclear antibodies (ANA) in patients with epilepsy may be associated with either the disease itself or the antiepileptic treatment. The purpose of this prospective study was to determine the prevalence of aPL and ANA in children with idiopathic epilepsy before and during treatment with antiepileptic drugs.
Methods
aPL, including both anticardiolipin and anti-β2-glycoprotein I antibodies, and ANA statuses were determined in 40 healthy children, 30 children treated with sodium valproate (VPA) monotherapy, and 20 children treated with carbamazepine (CBZ) monotherapy before and at 6, 12, and 24 months after treatment initiation.
Results
Fifteen children (50%) in the VPA-treated group and 7 (35%) in the CBZ-treated group showed positivity for aPL before treatment initiation, compared with only 4 of the 40 controls. Nine children (30%) in the VPA-treated group and 4 (20%) in the CBZ-treated group showed positivity for ANA before treatment initiation, compared with only 2 of the 40 controls. The subgroup analysis found nonsignificant associations at the different time points regarding the positivity of all of the autoantibodies. Only patients treated with VPA had a significantly decreased risk of aPL positivity after 6 months of treatment.
Conclusions
The increased prevalence of autoantibodies in children with idiopathic epilepsy is strongly associated with the disease itself.
Keywords: antibodies, anticardiolipin, antinuclear antibodies, beta 2-Glycoprotein I, children, epilepsy
INTRODUCTION
Antiphospholipid antibodies (aPL) comprise a large heterogeneous group of immunoglobulins directed against negatively charged phospholipids of the cell cytoplasmic membrane, including endothelial cells, platelets, and plasma proteins that bind negatively charged phospholipids.1 Antinuclear antibodies (ANA) are also a heterogeneous group of autoantibodies directed against various nuclear antigens.2 It has been observed that children and adults with epilepsy have increased numbers of aPL and ANA, which implicates immune mechanisms in the pathogenesis of epilepsy.3 In addition, it is known that various antiepileptic drugs may induce autoantibody formation.4 However, long-term prospective studies evaluating the prevalence of aPL and ANA in patients with epilepsy before and after the initiation of antiepileptic drug treatment are lacking.
This situation prompted us to seek evidence of aPL and ANA positivity in children with new-onset idiopathic epilepsy before and during treatment with antiepileptic drugs. To the best of our knowledge, this is the first study to have prospectively evaluated these autoantibodies in children with newly diagnosed idiopathic epilepsy. This analysis was performed over a period of up to 2 years.
METHODS
The study was approved by the Institutional Review Board of “Attikon” University Hospital (IRB No. 6358/2009). Informed written consent was obtained from the families of all participants. Patients diagnosed with new-onset idiopathic epilepsy between January 2010 and December 2016 were included in the study. Epilepsy was classified according to the International League Against Epilepsy 1989 criteria, and patients diagnosed with idiopathic generalized and focal epilepsy were included. Idiopathic epilepsy was diagnosed based on clinical and EEG characteristics. None of the patients had an underlying cause. All patients underwent brain MRI except from those diagnosed with any of the following epileptic syndromes: childhood absence, juvenile myoclonic, or epilepsy with centrotemporal spikes. Patients receiving chronic treatment for other medical conditions or diagnosed with diseases associated with aPL positivity (thromboembolic episodes, rheumatologic diseases, or recent infections) were excluded from the study.
Patients were divided into 2 groups: 30 children [15 females; age 9.0±3.6 years (mean±SD), age range 4–14 years] with tonic-clonic seizure episodes and absence seizure episodes who were prescribed sodium valproate (VPA) (Group A), and 20 children (8 females; age 9.2±3.2 years, age range 4–14 years) with focal seizures who were prescribed carbamazepine (CBZ) (Group B). The children were prospectively followed up for 24 months, with serum obtained before and at 6, 12, and 24 months after treatment initiation. Additionally, 40 control healthy children (19 females; age 8.9±3.0 years, age range 3.5–14 years) who were free of chronic diseases that might affect the autoantibody status were randomly selected among those who presented to the outpatient department for routine pediatric care and were age matched to the cases. Serum was also obtained from the controls.
In-house ELISAs were used to determine the concentrations of circulating anticardiolipin antibodies (ACL) immunoglobulin G (IgG) and IgM and β2-glycoprotein I (β2-GPI) IgG antibodies (anti-β2-GPI IgG). Normal values were determined by measuring healthy serum samples, whose concentrations were normalized to 0–100%. Any test serum samples with concentrations exceeding this range were considered positive. Briefly, cardiolipin or β2-GPI was coated onto ELISA plates, and after blocking with 10% bovine serum or Tween/gelatin in phosphate buffered saline, respectively, diluted serum (1:50) was added. Then, an alkaline-phosphatase-conjugated secondary antibody (anti-IgG or anti-IgM) was added at 1:2,000 dilution (Jackson Immunoresearch; West Grove, PA, USA). The reaction was visualized with the substrate, with the intensity measured at 410 nm. All values were expressed as percentages relative to reference negative samples. ANA were assessed by indirect immunofluorescence using a commercially available ANA kit (INOVA; Inova Diagnostics, San Diego, CA, USA). The VPA and CBZ levels were also measured in each sample.
Fisher's exact test was used to assess differences between the two groups of patients and the age-matched healthy controls regarding aPL and ANA positivity. In addition, crude odds ratios (ORs) and 95% CIs were calculated in order to explore the potential association of the different time periods [before treatment (reference category) and 6, 12, and 24 months after treatment initiation] with the risk of autoantibody positivity in Groups A and B separately. The proportions of patients with autoantibody positivity before treatment were also compared among the two study groups. A p-value of <0.05 was considered statistically significant. Data were analyzed using the SPSS (version 23.0, IBM Corp., Armonk, NY, USA).
RESULTS
Twenty-two children treated with VPA were diagnosed with generalized tonic—clonic seizures, and eight had absence seizures (Group A). Twenty children had focal seizures and were prescribed CBZ (Group B). Three patients (one in Group A and two in Group B) presented with one seizure episode during the first few months (at 2, 3, and 5 months of treatment), and their dose of antiepileptic medication had to be increased. All of the other patients in both groups remained seizure free throughout the study period.
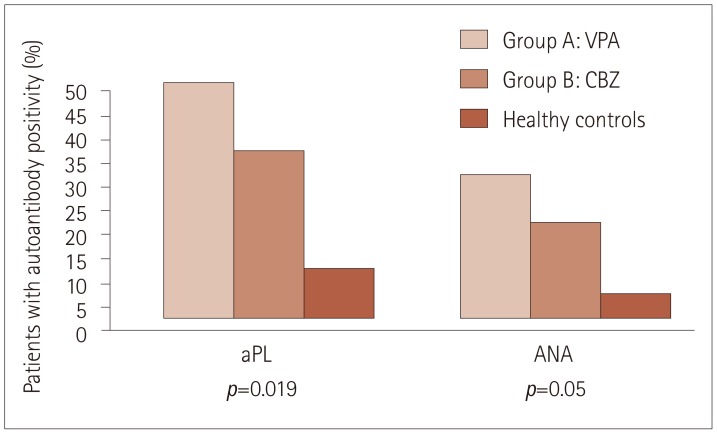
We detected significant differences between patients and controls at the diagnosis and before treatment initiation, since 22 (44%) patients were positive for aPL (either ACL or anti-β2-GPI IgG) compared to 4 (10%) healthy controls (p=0.019). Similarly, there were 13 (26%) patients positive for ANA (>1:160) compared to 2 (5%) positive cases in the healthy control group (p=0.05) (Fig. 1).
Fig. 1. Positivity for aPL (including both ACL and anti-β2-GPI) and ANA among patients treated with sodium VPA (Group A) and CBZ (Group B) before treatment initiation, as well as in age-matched healthy controls. Fisher's exact test was used to compare groups (Groups A and B vs. controls). ACL: anticardiolipin antibodies, ANA: antinuclear antibodies, anti-β2-GPI: anti-β2-glycoprotein I antibodies, aPL: antiphospholipid antibodies, CBZ: carbamazepine, VPA: valproate.
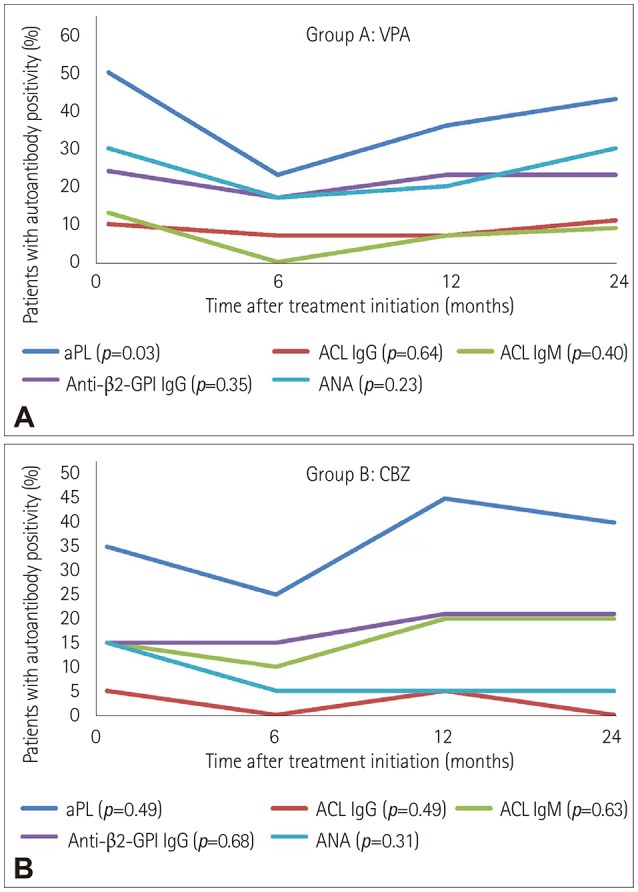
The comparison of the two groups of patients with epilepsy (Groups A and B) before treatment initiation yielded nonsignificant associations regarding the positive titers of aPL (p=0.39), ACL IgG (p=0.64), ACL IgM (p=0.59), anti-β2-GPI IgG (p=0.49), and ANA (p=0.32). In the analysis of each group of patients before treatment initiation (reference category) and 6, 12, and 24 months after treatment initiation, nonsignificant associations were also found at the different time points regarding positivity for all of the autoantibodies (Table 1). The only exception was found in Group A (patients treated with VPA), in which the risk of aPL positivity was significantly lower after 6 months of treatment compared to the pretreatment period (OR=0.30, 95% CI=0.10–0.92). Trends in the autoantibody positivity among patients treated with VPA (Group A) and CBZ (Group B) before and at 6, 12, and 24 months after treatment initiation are shown in Fig. 2.
Table 1. Crude OR and 95% CIs of the positive autoantibodies before and after treatment initiation among the studied groups of patients (A and B).
| Follow-up | Patients with positive autoantibodies | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aPL (ACL or anti-β2-GPI) | ACL IgG | ACL IgM | Anti-β2-GPI IgG | ANA | |||||||||||
| n (%) | OR | 95% CIs | n (%) | OR | 95% CIs | n (%) | OR | 95% CIs | n (%) | OR | 95% CIs | n (%) | OR | 95% CIs | |
| Group A: sodium valproate (n=30) | |||||||||||||||
| Before treatment | 15 (50) | Baseline | 3 (10) | Baseline | 4 (13) | Baseline | 8 (24) | Baseline | 9 (30) | Baseline | |||||
| 6 m | 7 (23)* | 0.30* | 0.10–0.92* | 2 (7) | 0.64 | 0.10–4.15 | 0 (0) | - | - | 5 (17) | 0.55 | 0.16–1.93 | 5 (17) | 0.47 | 0.14–1.61 |
| 12 m | 11 (36) | 0.58 | 0.21–1.62 | 2 (7) | 0.64 | 0.10–4.15 | 2 (7) | 0.46 | 0.08–2.75 | 7 (23) | 0.84 | 0.26–2.70 | 6 (20) | 0.58 | 0.18–1.91 |
| 24 m | 13 (43) | 0.76 | 0.28–2.11 | 3 (10) | 1.00 | 0.19–5.40 | 3 (10) | 0.72 | 0.15–3.54 | 7 (23) | 0.84 | 0.26–2.70 | 9 (30) | 1.00 | 0.33–3.02 |
| Group B: carbamazepine (n=20) | |||||||||||||||
| Before treatment | 7 (35) | Baseline | 1 (5) | Baseline | 3 (15) | Baseline | 3 (15) | Baseline | 3 (15) | Baseline | |||||
| 6 m | 5 (25) | 0.62 | 0.16–2.43 | 0 (0) | - | - | 2 (10) | 0.63 | 0.09–4.24 | 3 (15) | 1.00 | 0.18–5.67 | 1 (5) | 0.30 | 0.03–3.15 |
| 12 m | 9 (45) | 1.52 | 0.43–5.43 | 1 (5) | 1.00 | 0.06–17.18 | 4 (20) | 1.42 | 0.27–7.34 | 4 (20) | 1.42 | 0.27–7.34 | 1 (5) | 0.30 | 0.03–3.15 |
| 24 m | 8 (40) | 1.24 | 0.34–4.46 | 0 (0) | - | - | 4 (20) | 1.42 | 0.27–7.34 | 4 (20) | 1.42 | 0.27–7.34 | 1 (5) | 0.30 | 0.03–3.15 |
*Statistically significant associations.
ACL: anticardiolipin antibodies, ANA: antinuclear antibodies, anti-β2-GPI: anti-β2-glycoprotein I antibodies, aPL: antiphospholipid antibodies, CI: confidence interval, Ig: immunoglobulin, m: months, OR: odds ratios.
Fig. 2. Trends of positivity for aPL, ACL IgG and IgM, anti-β2-GPI IgG, and ANA among patients treated with VPA (A) and CBZ (B) before and at 6, 12, and 24 months after treatment initiation. ACL: anticardiolipin antibodies, ANA: antinuclear antibodies, anti-β2-GPI: anti-β2-glycoprotein I antibodies, aPL: antiphospholipid antibodies, CBZ: carbamazepine, Ig: immunoglobulin, OR: odds ratios, VPA: valproate.
CBZ and VPA were prescribed at therapeutic dosages of 16–20 and 17–38 mg/kg/day, respectively. Serum CBZ concentrations remained within the therapeutic range (5–10 mg/L) during the study period (6.05±1.44, 6.3±2.12, and 7.22±1.48 mg/L at 6, 12, and 24 months after treatment initiation, respectively), as did the VPA concentrations (therapeutic range=50–100 mg/L): 68.14±15.92, 69.15±16.90, and 71.74±13.88 mg/L, respectively.
DISCUSSION
Several previous clinical studies have found an association between epilepsy and immune dysregulation, with epilepsy being more common in patients with autoimmune disorders such as systemic lupus erythematosus or celiac disease.5,6 The current prospective study found that children with idiopathic epilepsy had increased levels of ANA (26%) and aPL (44%) compared to healthy controls (5% and 10%, respectively).
This long-term (2-year) prospective study showed that the use of VPA or CBZ in children with well-controlled idiopathic epilepsy does not induce further aPL or ANA formation. Antibody titers were not influenced from baseline to 12 and 24 months later in either group. Those results are consistent with other cross-sectional studies also not showing a difference,7,8,9 despite it being possible that medications, including antiepileptics, induce autoantibody production.4 Only the VPA-treated group presented a significantly decreased risk of aPL positivity at 6 months of treatment compared to the pretreatment period. This decrease may be associated with a short-term effect of VPA or with a short-term downregulation of these autoantibodies after the onset of epilepsy.
aPL positivity has been reported in 19–26% of adults7,10,11 and in 13–44% of children with epilepsy,9,12,13 although this is suggested to simply be an epiphenomenon. The underlying mechanism remains to be elucidated, and could result from ischemic damage to the brain, the binding of the autoantibodies to brain tissue, or the effect of neurosynapse depolarization, as has been shown in both human and animal models.14
It is particularly interesting that we found no significant changes in the prevalence of aPL or ANA between before and during long-term treatment (after 12 and 24 months) with VPA and CBZ in children with idiopathic epilepsy. The presence of aPL has been associated with recent and refractory seizure episodes.15 In contrast, despite the presence of well-controlled epilepsy in our study, the prevalence of autoantibodies did not change significantly during a 24-month follow-up period. This is the first study to show increased titers of aPL and ANA (compared to healthy controls) at the time of newly diagnosed idiopathic epilepsy, but with no change in these autoantibodies during a 24-month follow-up, despite excellent control of the seizures. Further studies are therefore needed to clarify whether this finding represents an antiepileptic drug controlling seizure-related immune activation or suggests the involvement of these antibodies mainly in the onset rather than in the progression or prognosis of epilepsy.
One weakness of this study is the lack of a population with refractory epilepsy, which would have allowed the assessment of the evolution of aPL and ANA titers during treatment. Another limitation is the small number of children investigated in the VPA- and CBZ-treated groups. However, the strengths of the present study are its prospective design lasting for 24 months, the well-controlled disease status, and the homogeneity of the included groups.
In conclusion, this prospective study has demonstrated that children with well-controlled idiopathic epilepsy have persistently increased titers of aPL and ANA, and that the administration of CBZ and VPA does not induce further antibody production. These observations increase the likelihood that immune mechanisms are involved in the pathogenesis of epilepsy, although further studies in children are needed to elucidate the roles of aPL and ANA in the pathogenesis, clinical manifestations, and prognosis of epilepsy.
Footnotes
- Conceptualization: Achilleas Attilakos, Lambros Fotis, Argirios Dinopoulos, Anastasia Garoufi.
- Data curation: Harris Alexopoulos, Aikaterini Vasileiou Theofilopoulou, Maria Karalexi, Athanasios George Tzioufas, Sotiria Mastroyianni.
- Formal analysis: Harris Alexopoulos, Aikaterini Vasileiou Theofilopoulou, Maria Karalexi.
- Writing—original draft: Achilleas Attilakos, Lambros Fotis, Argirios Dinopoulos, Anastasia Garoufi.
- Writing—review & editing: Achilleas Attilakos, Lambros Fotis, Argirios Dinopoulos, Anastasia Garoufi, Harris Alexopoulos, Aikaterini Vasileiou Theofilopoulou, Maria Karalexi, Athanasios George Tzioufas, Sotiria Mastroyianni.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Rumsey DG, Myones B, Massicotte P. Diagnosis and treatment of antiphospholipid syndrome in childhood: a review. Blood Cells Mol Dis. 2017;67:34–40. doi: 10.1016/j.bcmd.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Agmon-Levin N, Damoiseaux J, Kallenberg C, Sack U, Witte T, Herold M, et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis. 2014;73:17–23. doi: 10.1136/annrheumdis-2013-203863. [DOI] [PubMed] [Google Scholar]
- 3.Constantin T, Kálovics T, Ponyi A, Nagy E, Sallai K, Szabó L, et al. Prevalence of antiphospholipid and antinuclear antibodies in children with epilepsy. Med Sci Monit. 2009;15:CR164–CR169. [PubMed] [Google Scholar]
- 4.Dlott JS, Roubey RA. Drug-induced lupus anticoagulants and antiphospholipid antibodies. Curr Rheumatol Rep. 2012;14:71–78. doi: 10.1007/s11926-011-0227-1. [DOI] [PubMed] [Google Scholar]
- 5.Brey RL, Muscal E, Chapman J. Antiphospholipid antibodies and the brain: a consensus report. Lupus. 2011;20:153–157. doi: 10.1177/0961203310396748. [DOI] [PubMed] [Google Scholar]
- 6.Julian T, Hadjivassiliou M, Zis P. Gluten sensitivity and epilepsy: a systematic review. J Neurol. 2019;266:1557–1565. doi: 10.1007/s00415-018-9025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verrot D, San-Marco M, Dravet C, Genton P, Disdier P, Bolla G, et al. Prevalence and signification of antinuclear and anticardiolipin antibodies in patients with epilepsy. Am J Med. 1997;103:33–37. doi: 10.1016/s0002-9343(97)90046-2. [DOI] [PubMed] [Google Scholar]
- 8.Peltola JT, Haapala A, Isojärvi JI, Auvinen A, Palmio J, Latvala K, et al. Antiphospholipid and antinuclear antibodies in patients with epilepsy or new-onset seizure disorders. Am J Med. 2000;109:712–717. doi: 10.1016/s0002-9343(00)00617-3. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson K, Peltola J, Keränen T, Haapala AM, Koivikko M. High prevalence of antiphospholipid antibodies in children with epilepsy: a controlled study of 50 cases. Epilepsy Res. 2001;46:129–137. doi: 10.1016/s0920-1211(01)00273-x. [DOI] [PubMed] [Google Scholar]
- 10.Specchio N, Fusco L, Claps D, Vigevano F. Epileptic encephalopathy in children possibly related to immune-mediated pathogenesis. Brain Dev. 2010;32:51–56. doi: 10.1016/j.braindev.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Arnson Y, Shoenfeld Y, Alon E, Amital H. The antiphospholipid syndrome as a neurological disease. Semin Arthritis Rheum. 2010;40:97–108. doi: 10.1016/j.semarthrit.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Angelini L, Granata T, Zibordi F, Binelli S, Zorzi G, Besana C. Partial seizures associated with antiphospholipid antibodies in childhood. Neuropediatrics. 1998;29:249–253. doi: 10.1055/s-2007-973570. [DOI] [PubMed] [Google Scholar]
- 13.Cimaz R, Romeo A, Scarano A, Avcin T, Viri M, Veggiotti P, et al. Prevalence of anti-cardiolipin, anti-β2 glycoprotein I, and anti-prothrombin antibodies in young patients with epilepsy. Epilepsia. 2002;43:52–59. doi: 10.1046/j.1528-1157.2002.00701.x. [DOI] [PubMed] [Google Scholar]
- 14.Graf J. Central nervous system manifestations of antiphospholipid syndrome. Rheum Dis Clin North Am. 2017;43:547–560. doi: 10.1016/j.rdc.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Liimatainen S, Peltola M, Fallah M, Kharazmi E, Haapala AM, Peltola J. The high prevalence of antiphospholipid antibodies in refractory focal epilepsy is related to recurrent seizures. Eur J Neurol. 2009;16:134–141. doi: 10.1111/j.1468-1331.2008.02373.x. [DOI] [PubMed] [Google Scholar]