Abstract
Opiates are the second most prevalent abused illicit substance after cannabis in the world. The latest United Nations Office on Drugs and Crime (UNODC) report estimated 30% increment in opium cultivation worldwide. High prevalence of opium consumption in eastern countries may be due to the high availability and traditional misconceptions. Opium consumption has been linked to hypertension, diabetes mellitus, dyslipidemia, and coronary artery diseases (CAD). In this review, we will review the association between opium use, cardiovascular diseases, and clinical outcomes. The present evidence suggests that chronic opiate consumption may increase the risk of cardiovascular diseases and related mortality.
Keywords: Opium, Cardiovascular disease, Drug interactions, Metabolic effects, Mortality
Introduction
Poppy (Papaver somniferum L.) is one of the ancient plants that was cultivated for millennia and used for both medicinal and recreational purposes (1). Opium is a dark sticky or crumbly mass exuded from the ripening capsule of opium poppy and consists of several alkaloids including approximately twelve percent morphine with lesser amounts of noscapine, codeine, papaverine and thebaine (2).
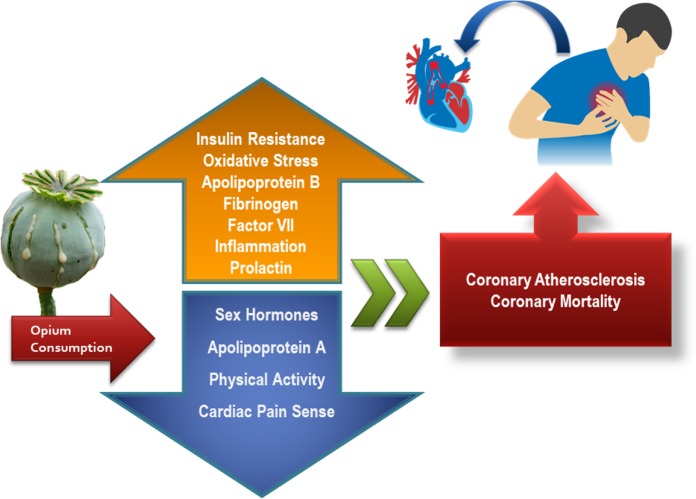
Raw opium is the second most prevalent abused substance after tobacco in most Asian countries. The 2017 report of the United Nations Office of Drugs and Crime (UNODC) estimated the overall production of opium to be 6,380 tons worldwide with 30% increase compared to the previous year and they reported the number of illicit users to be up to 17.7 million in 2015 (3). The high rate of opium consumption in Asian countries could be due to several factors such as widespread availability and long-standing misconceptions among ordinary people and even healthcare professionals regarding the purported alleviating effects of opium on coronary artery diseases (CAD), dyslipidemia, hypertension, and diabetes mellitus (DM) (4) (Fig. 1). Patients in the Middle East usually approach physicians with questions about the effectiveness of opium in controlling diabetes or cardiovascular conditions (5). The lack of sufficient evidence makes it difficult to answer those inquiries (6).
Fig. 1:
Adverse outcomes of opioids consumption
Recently opium has been proposed as a risk factor for cardiovascular diseases (CVD) (7–9). Tachycardia, bradycardia, and orthostatic hypotension are common cardiovascular problems seen in opium-addicted individuals (10). Plasma fibrinogen levels, coagulation, and atherosclerosis are adversely affected by opium abuse (11, 12). Low-density lipoprotein (LDL) cholesterol and triglyceride (TG) and blood glucose levels are other important factors changing in addicted people. They are known risk factors of CVD (13). Moreover, the deleterious effects of opium consumption on CVD risk factors were found to be proportional to the duration of consumption and the route of administration (14).
There is a paucity of consistent and reliable information on the association between opium dependency and CVD progression. Therefore, in the present article, we have reviewed the latest findings related to this issue.
Pharmacology of Opiates
In 1806, Friedrich Wilhelm Sertürner isolated morphine as the active component of the opium poppy, and there the modern opioid pharmacology was born (15). More than 40 alkaloids exist in the milky latex fluid obtained from the opium poppy. The six major alkaloids that account for almost all of the natural alkaloid composition in opium are morphine, noscapine, codeine, papaverine, thebaine, and narceine (16). Thebaine is not used therapeutically, but several drugs such as naloxone, naltrexone, oxycodone, and buprenorphine are synthesized from thebaine.
The opioid receptors are categorized according to the International Union of Pharmacology (IUPHAR) recommendation to μ-(MOP), κ-(KOP), and δ-opioid (DOP) receptors, which are G-protein-coupled receptors (17). Cyclic AMP and/or ion channels (K+) are second messenger systems of opiate receptors. Studies suggested that modifications in the levels of cyclic AMP during chronic opiate consumption are associated with the development of tolerance and physical dependence (18).
Interactions with cardiovascular medications
Opioid addicted patients may concurrently suffer from other comorbidities. Cardiovascular and pulmonary diseases are common among chronic opiate abusers but dose-dependent exact interactions are not studied well. Hence, the use of opiates (either therapeutic or on an abusive basis) along with cardiovascular medications (including anticoagulants, antiarrhythmic, cardiotonic, and antihypertensive drugs) may increase the risk of drug-drug interactions.
Concomitant administration of opiates with cardiovascular drugs may potentiate or reduce pharmacologic effects of cardiovascular medications such as warfarin or digoxin (19, 20). The interaction can affect the pharmacokinetics (absorption, distribution, metabolism or elimination) or pharmacodynamics (molecular mechanism of action) and the ultimate therapeutic status of the CV drugs (21, 22).
Opium contains alkaloids that have a direct impact on the cardiovascular or hemostatic systems. They may also have several indirect effects through interactions with the effect of other medications. Therefore, health care professionals should have a good knowledge base to identify possible opiate– drug interactions and to warn the patients about the possibility of complications. The lack of information on the interactions of opiates with concurrent medications needs to be addressed by well-designed clinical trials to assess the potential interactions and unknown side effects. Table 1 summarizes some important interactions of opiates with cardiovascular medications.
Table 1:
Interactions between opiates and other medications
| Opiate | Medication | Extent of Interference | Mechanism | Comment |
|---|---|---|---|---|
| Opium alkaloids | Isocarboxazide Linezolide Phenelazine Rasagiline Selegiline Tranylcypromine |
Serious | Unknown | Risk of hypotension, hyperpyrexia, somnolence or death. Should separate by 2 weeks.(75) |
| Procarbazine | Serious | Unknown | Potentiate CNS depression and hypotension(75) | |
| Fentanyl | Serious | Unknown | Risk of hypotension, respiratory depression and profound sedation, coma, death.(75) | |
| Amiodarone | Monitor closely | Cyp2d6 | Prevents conversion of opiates to metabolites(75) | |
| Aspirin | Monitor closely | Unknown | Increase aspirin resistance(75 ) | |
| CCBS | Monitor closely | Unknown | Potentiate effects of opiates(76) | |
| Dobutamine | Monitor closely | Unknown | Not clear | |
| Dopamine | Monitor closely | Unknown | Not clear | |
| Dronedarone | Monitor closely | Cyp2d6 | Prevents conversion of opiates to metabolites | |
| Digoxin | Monitor closely | Unknown | Increase the risk of Digoxin toxicity(77) | |
| Ephedrine | Monitor closely | Unknown | Not clear | |
| Isoproterenol | Monitor closely | Unknown | Not clear | |
| Metaproterenol | Monitor closely | Unknown | Not clear | |
| Moxonidine | Monitor closely | Unknown | Not clear(75) | |
| Phenylephrine | Monitor closely | Unknown | Not clear | |
| Quinidine | Monitor closely | Cyp2d6 | Prevents conversion of opiates to metabolites(75) | |
| Ticlopidine Ticagrelor(78) Clopidogrel(79) Prasugre(80) |
Monitor closely | Cyp2d6 | Prevents conversion of opiates to metabolites, decrease concentration and effects of ADP inhibitors | |
| Yohimbine | Monitor closely | Unknown | Not clear | |
| Warfarin and vk antagonists | Monitor closely | Unknown | Increase INR (81) |
CCB: calcium channel blockers; CNS: Central nervous system; INR: international normalized ratio; PT: prothrombin time
The effects of opium on CV risk factors
It seems that despite a few old studies, recent articles have emphasized the adverse effect of opium on cardiovascular risk factors. In the following sections, we will review the studies that explored the effect of opium consumption on cardiovascular risk factors such as hypertension, etc.
Blood pressure and hypertension
There are misconceptions in the general population or even among some medical professionals, that opium could have favorable impacts on lowering blood pressure in hypertension while many experts may disagree. Opium use had no significant ameliorative effect on hypertension in either occasional or dependent users (9).
Similarly, several other studies failed to find a correlation between opium consumption and hypertension prevention (23–26). However, not all of these studies were consistent. A cohort study of 9,264 adults showed a lower prevalence of hypertension in opium users as compared to their counterparts, probably because of the younger age of opium users in that study population (27). In the contrary, in a cohort study on 5,332 participants, high systolic and diastolic blood pressures were more prevalent in opium users than in others (3). A case-control study reported the rate of hypertension to be significantly higher in addict patients with ischemic stroke than controls (28).
Endogenous opioid systems and opioid receptor agonists are purported to modulate the arterial pressure to some extent. Stimulation of peripheral opioid receptors may reduce arterial hypertension, especially in those with pronounced sympathetic hyperactivity or stress-based high blood pressure (29). Morphine administration could decrease systolic and diastolic blood pressures, however, in several other cases, morphine exposure enhanced hypertension through its effects on brain noradrenergic mechanisms or central opiate receptors (30–32). The dosage and duration of drug abuse seem to be critical factors here. Generally, short term and low dose exposure to opium lower blood pressure, whereas long-term dependence leads to hypertension. The former effect is attributed to vasodilation and opium's effect on reducing sympathetic tone. Opium-induced high blood pressure might be secondary to its long-term deleterious impact on the structure and function of body organs, particularly in the cardiovascular system, including microvascular coronary dysfunction, elevated plasma levels of homocysteine and fibrinogen, atheroma formation and related vascular stenosis (7, 11, 12). In general, data on opium-induced blood pressure alterations suffer from inadequacy and inconsistency, and further studies are warranted to shed some light on this issue.
Effects on coronary disease and myocardial infarction
Opium users have shown to have a higher susceptibility to coronary artery disease compared to non-users with a dose-response relationship reported between these two (9, 33). Chronic opium consumers have an overall increase in their ECG abnormalities. The ECG abnormalities are more frequently observed in male opiate consumers than in females. QTc prolongation (13%), R and/or S wave abnormalities (11%), and poor R progression (10%) were the most reported ECG changes (34). Niaki et al. showed that opium consumption was a significant risk factor of MI with an adjusted odds ratio of 26.3. However, they did not find any association between opium abuse and extent of MI (35). In patients admitted with the diagnosis of MI the prevalence of opium addiction was 19%, while it was 2.8% in general population (36). Sadeghian et al. presented the opium abuse as a major risk factor for ischemic heart disease (8). In a study, they introduced diabetes mellitus in women and opium abuse in men as two major risk factors of CAD in Iran (37).
Hosseini et al. found a significant association between the dose of opium used by addicted patients and the Gensini score (β = 0.27, p = 0.04) (38). On the other hand, Dehghani et al. studied 239 opium addicts and 221 non-addicts with first MI and reported a lower rate of anterior wall MI and lower related early mortality in addicted as compared to non-addicted patients (39). Killip class and left ventricular ejection fraction (LVEF) were similar between addicted and non-addicted groups (39). None of the oral or inhaled routes of opium abuse have increased the occurrence of CAD in multivariate analysis (39).
Patients with MI should receive medical care promptly. Some addicted patients use opium to get relief from chest pain (41). Opium uses by relieving chest pain and inducing drowsiness can increase the time elapsed from symptom onset to admission to the hospital. This delay increases the mortality among opium users. Nevertheless, Bafghi et al. reported similar rates of chest pain between opium users and non-users, and hence, they attributed this delay in seeking medical care to other factors such as socioeconomic issues (36).
Effect on heart failure
Heart failure and functional class seem to be no different in opium-addicted patients after MI than in non-addicted individuals. Davoodi et al. reported no difference between addicted and non-addicted patients regarding functional class, angiographic findings, and the need for CABG (42). Similarly, post-MI LVEF was not different between addicted and non-addicted patients (p = 0.4) (43). Safaei also reported no difference between the LVEF of addicted and non-addicted patients before and 6 months after CABG (44).
The effect of opium in patients with heart failure (HF) is still unclear. Morphine can lower some of the symptoms occurring in the late stages of HF such as dyspnea (45). Similarly, morphine seems to relieve the ischemia symptoms in patients with cardiovascular risk factors (46). The underlying pharmacological mechanism of morphine has encouraged some of the researchers to conclude that morphine may be cardioprotective in HF patients (47). However, one study revealed that patients with HF who use morphine along with nitroglycerine and furosemide had higher mortality rates (48). However, there is no enough data to provide evidence-based recommendations regarding the cessation of opium abuse in cardiac patients (49).
Effects on cerebrovascular disease and stroke
A few studies assessed the effect of chronic opium consumption on the development of ischemic stroke. A case-control study showed a significant rise in ischemic stroke in opium-addicted patients in Kerman (P < 0.0001) (50). In another cross-sectional sonographic study conducted on 97 patients with ischemic stroke shown that there was no significant difference in the frequency of atherosclerosis and the type of involved vessels among opium addicts and non-addict patients (51). Rezvani and Ghandehari studied 558 opium users with a mean age of 56.2 years. They claimed that opium inhalation did not have a significant effect on occurrence cerebrovascular events and they concluded that opium may have a protective effect on ischemic stroke (40). Khademi et al in Golestan cohort study conducted on 5000 Iranian adults demonstrated that chronic abuse of opium was associated with an increased risk of ischaemic heart disease and cerebrovascular events (52). A recent case-control study presented opium addiction and hypertension as two independent predictors of stroke. After adjusting for tobacco smoking, opium abuse increased the risk of stroke by 2.3-fold (28).
Effect on other serum parameters
In the majority of studies, opium addicts had higher levels of potassium (53–56), whilst opium consumption had either no or enhancing the effect on sodium levels (53, 55). Fe2+ levels were higher and total iron-binding capacity was lower in addicted diabetics as compared to non-addict diabetics (54). Albumin was lower among opium addicts; however, no significant difference was observed in albumin/globulin ratios (54). Urea and creatinine levels were enhanced by opium consumption in some studies (55, 57), but unchanged in others (58).
Serum transaminases are shown to be associated with the severity of coronary atherosclerosis, and both cardiovascular and all-cause mortality (4). Radmard et al conducted a cohort study on 1599 participants demonstrated a significant reduction in aspartate transaminase (AST) and alanine transaminase (ALT) in normal addicted people, while a statistical notable elevation in alkaline phosphatase (ALP) and gamma-glutamyl transferase (GGT) (59). As well as Karam et al. reported lower aspartate transaminase (AST) and alanine transaminase (ALT) in diabetic addicts as compared to non-addict diabetics (54). However, several animal and human studies have shown the opium consumption to be associated with increased levels of AST, ALT, ALP, lactate dehydrogenase (LDH) and GGT (55 , 57, 60, 61).
Opium abuse could potentiate cardiometabolic risk factors such as apolipoprotein B/apolipoprotein A-I index, which is a strong predictor of cardiovascular death (62). Other cardiac biomarkers including factor VII, fibrinogen, and homocysteine could be changed by chronic opium consumption (63). These findings might explain the increased risk of MI or stroke in opium users.
Complication related to opium contamination
Opium impurities can impose a great health risk to chronic opium consumers. Chemical compounds such as lead, arsenic, and thallium are frequently presented in opium extract in different amounts. Depending on the area of opium production, product’s appearance and rout of consumption, smugglers forge opium by adding other impurities to maximize their profit (64). The motive behind adding lead to opium by drug smugglers is to increase the product’s weight (65–69). This will enhance the risk of lead poisoning among opium consumers. Signs and Symptoms include abdominal pain, constipation, anorexia, anemia and nephropathy (67–70). Acute and chronic accumulation of lead in the body causes cardiac and vascular damages with potentially life-threatening consequences (69). The blood lead level was found to be higher in opium consumers than non-addict individuals (65, 66, 70), but not across all studies (69). Opium inhalation seems to cause more lead surge in addicted subjects than oral use (65). Iron and calcium levels may affect lead absorption and their serum level may confound the lead-mortality association (71).
Opium consumption and clinical outcomes
Despite common misconceptions among opium consumers, there is a higher rate of mortality and morbidity in opium addicts than non-users. There is a paucity of data on this matter mainly due to the lack of comprehensive large-scale, long-term, controlled studies. In-hospital mortality was significantly higher in opium-addicted patients compared to non-users. Due to the pain masking properties of opium, drug users may delay one-hour longer than non-users since the onset of MI symptoms to reach the emergency department (36). Morphine therapy in acute de-compensated heart failure increased cardiac biomarker (troponin I) levels significantly and raised the rate of adverse events such as mechanical ventilation, intensive care unit (ICU) admissions, prolonged hospitalization, and death (72). Safaii et al. reported chronic opium consumption to be associated with rehospitalization with cardiac cause within 6 months after CABG surgery (73). Other studies found no association between opium use and in-hospital mortality (39, 75), but it should be noted that these studies suffered from limitations such as small sample size and short-term follow-up periods.
Conclusion
The incremental rise of opioid abuse requires us to inform and educate the patients regarding its possible detrimental effects. Several large-scale studies imply that opium is hazardous cardiovascular and metabolic disorders and even may worsen these complications.
Successive data demonstrated that there is an association between habitual opium consumption and the risk of ischemic events. While vernacular wrong believes and short-term and small-sized studies accentuate the usefulness of opium consumption. Therefore, clinicians and patients should be noticed about the deleterious effects of opium addiction on various vascular events. Besides these, further studies are warranted to elucidate the effects of opium use in cardiovascular conditions. Nevertheless, the existing data points to opium consumption as a risk factor of CAD and hence, strategies should be developed and implemented for the prevention and cessation of opioid abuse in the at-risk population.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
There was no financial source for this study.
Footnotes
Conflict of interests
The authors declare that there are no conflicts of interest.
References
- 1.Pathan H, Williams J. (2012). Basic opioid pharmacology: an update. Br J Pain, 6:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warner M, Trinidad JP, Bastian BA, Minino AM, Hedegaard H. (2016). Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2010–2014. Natl Vital Stat Rep, 65:1–15. [PubMed] [Google Scholar]
- 3.Yousefzadeh G, Shokoohi M, Najafipour H, Eslami M, Salehi F. (2015). Association between opium use and metabolic syndrome among an urban population in Southern Iran: Results of the Kerman Coronary Artery Disease Risk Factor Study (KERCADRS). ARYA Atheroscler, 11:14–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Masoomi M, Ramezani MA, Karimzadeh H. (2010). The relationship of opium addiction with coronary artery disease. Int J Prev Med, 1:182–186. [PMC free article] [PubMed] [Google Scholar]
- 5.Masoudkabir F, Sarrafzadegan N, Eisenberg MJ. (2013). Effects of opium consumption on cardiometabolic diseases. Nat Rev Cardiol, 10:733–40. [DOI] [PubMed] [Google Scholar]
- 6.Ahmadi J, Benrazavi L. (2002). Substance use among Iranian cardiovascular patients. Eur J Med Res, 7:89–92. [PubMed] [Google Scholar]
- 7.Nadimi AE, Amiri FP, Fathollahi MS, et al. (2016). Opium addiction as an independent risk factor for coronary microvascular dysfunction: A case–control study of 250 consecutive patients with slow–flow angina. Int J Cardiol, 219:301–27. [DOI] [PubMed] [Google Scholar]
- 8.Sadeghian S, Darvish S, Davoodi G, et al. (2007). The association of opium with coronary artery disease. Eur J Cardiovasc Prev Rehabil, 14:715–717. [DOI] [PubMed] [Google Scholar]
- 9.Najafipour H, Masoomi M, Shahesmaeili A, et al. (2015). Effects of opium consumption on coronary artery disease risk factors and oral health: Results of Kerman Coronary Artery Disease Risk factors Study a population-based survey on 5900 subjects aged 15–75 years. Int J Prev Med, 6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farahani MA, Ghaffari F, Fatemi NS. (2015). Opium addiction in patients with coronary artery disease: a grounded theory study. Med J Islam Repub Iran, 29:267. [PMC free article] [PubMed] [Google Scholar]
- 11.Masoomi M, Azdaki N, Shahouzehi B. (2015). Elevated Plasma Homocysteine Concentration in Opium-Addicted Individuals. Addict Health, 7:149–56. [PMC free article] [PubMed] [Google Scholar]
- 12.Najafipour H, Beik A. (2016). The Impact of Opium Consumption on Blood Glucose, Serum Lipids and Blood Pressure, and Related Mechanisms. Front physiol, 7:436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aghadavoudi O, Eizadi-Mood N, Najarzadegan MR. (2015). Comparing cardiovascular factors in opium abusers and non-users candidate for coronary artery bypass graft surgery. Adv Biomed Res, 4:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asgary S, Sarrafzadegan N, Naderi G-A, Rozbehani R. (2008). Effect of opium addiction on new and traditional cardiovascular risk factors: do duration of addiction and route of administration matter? Lipids Health Dis, 7:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atanasov AG, Waltenberger B, Pferschy-Wenzig EM, et al. (2015). Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol Adv, 33:1582–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tekelioglu O. (2016). The relationship of methadone maintenance treatment patients' age, depression, anxiety, and stress. Alliant International University. [Google Scholar]
- 17.Botz B, Bölcskei K, Helyes Z. (2017). Challenges to develop novel anti-inflammatory and analgesic drugs. Wiley Interdiscip Rev Nanomed Nanobiotechnol, 9(3). [DOI] [PubMed] [Google Scholar]
- 18.Mamiya T, Noda Y, Ren X, et al. (2001). Involvement of cyclic AMP systems in morphine physical dependence in mice: prevention of development of morphine dependence by rolipram, a phosphodiesterase 4 inhibitor. Br J Pharmacol, 132:1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pottegard A, dePont Christensen R, et al. (2014). Pharmacoepidemiological assessment of drug interactions with vitamin K antagonists. Pharmacoepidemiol Drug Saf, 23:1160–7. [DOI] [PubMed] [Google Scholar]
- 20.McCance-Katz EF, Sullivan L, Nallani S. (2010). Drug Interactions of Clinical Importance among the Opioids, Methadone and Buprenorphine, and other Frequently Prescribed Medications: A Review. Am J addict, 19:4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BJ. (2015). The influence of herbal medicine on platelet function and coagulation: a narrative review. Semin Thromb Hemost, 41(3)300–14. [DOI] [PubMed] [Google Scholar]
- 22.Ondieki G, Nyagblordzro M, Kikete S, et al. (2017). Cytochrome P450 and PGlycoprotein-Mediated Interactions Involving African Herbs Indicated for Common Noncommunicable Diseases. Evid Based Complement Alternat Med, 2017:258263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najafi M, Sheikhvatan M. (2012). Does analgesic effect of opium hamper the adverse effects of severe coronary artery disease on quality of life in addicted patients? Anesth Pain Med., 2:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayani M, Nazemi S, Niaki MK, Ramezani M, Khani A. (2014). Opium consumption and lipid and glucose parameters in diabetic patients with acute coronary syndrome: a survey in northern Iran. Tunis Med, 92:497–500. [PubMed] [Google Scholar]
- 25.Javadi HR, Allami A, Mohammadi N, Alauddin R. (2014). Opium dependency and in-hospital outcome of acute myocardial infarction. Med J Islam Repub Iran, 28:122. [PMC free article] [PubMed] [Google Scholar]
- 26.Rahimi N, Gozashti MH, Najafipour H, et al. (2014). Potential effect of opium consumption on controlling diabetes and some cardiovascular risk factors in diabetic patients. Addict Health, 6(1-2):1–6. [PMC free article] [PubMed] [Google Scholar]
- 27.Fallahzadeh MA, Salehi A, Naghshvarian M, et al. (2017). Epidemiologic Study of Opium Use in Pars Cohort Study: A Study of 9000 Adults in a Rural Southern Area of Iran. Arch Iran Med, 20:205–210. [PubMed] [Google Scholar]
- 28.Ebrahimi H, Haghjoo Javanmard S, Asgary S, et al. (2018). Opium Addiction and Ischemic Stroke in Isfahan, Iran: A Case-Control Study. Eur Neurol, 79:82–85. [DOI] [PubMed] [Google Scholar]
- 29.Badzynska B, Lipkowski AW, Olszynski KH, Sadowski J. (2016). Different blood pressure responses to opioids in 3 rat hypertension models: role of the baseline status of sympathetic and renin-angiotensin systems. Can J Physiol Pharmacol, 94(11):1159–1169. [DOI] [PubMed] [Google Scholar]
- 30.Gomes C, Svensson TH, Trolin G. (1976). Effects of morphine on central catecholamine turnover, blood pressure and heart rate in the rat. Naunyn Schmiedebergs Arch Pharmacol, 294:141–7. [DOI] [PubMed] [Google Scholar]
- 31.May CN, Ham IW, Heslop KE, Stone FA, Mathias CJ. (1988). Intravenous morphine causes hypertension, hyperglycaemia and increases sympatho-adrenal outflow in conscious rabbits. Clin Sci (Lond), 75:71–7. [DOI] [PubMed] [Google Scholar]
- 32.Shanazari AA, Aslani Z, Ramshini E, Alaei H. (2011). Acute and chronic effects of morphine on cardiovascular system and the baroreflexes sensitivity during severe increase in blood pressure in rats. ARYA Atheroscler, 7:111–7. [PMC free article] [PubMed] [Google Scholar]
- 33.Masoumi M, Shahesmaeili A, Mirzazadeh A, Tavakoli M, Ali AZ. (2010). Opium addiction and severity of coronary artery disease: a case-control study. J Res Med Sci, 15:27–32. [PMC free article] [PubMed] [Google Scholar]
- 34.Wallner C, Stöllberger C, Hlavin A, Finsterer J, et al. (2008). Electrocardiographic abnormalities in opiate addicts. Addiction, 103:1987–1993. [DOI] [PubMed] [Google Scholar]
- 35.Khosoosi Niaki MR, Hamid M, Farshidi F, et al. (2013). Evaluation of the role of opium addiction in acute myocardial infarction as a risk factor. Caspian J Intern Med, 4:585–589. [PMC free article] [PubMed] [Google Scholar]
- 36.Sadr Bafghi SM, Rafiei M, Bahadorzadeh L, et al. (2005). Is opium addiction a risk factor for acute myocardial infarction? Acta Med Iran, 43:218–222. [Google Scholar]
- 37.Sadeghian S, Graili P, Salarifar M, et al. (2010). Opium consumption in men and diabetes mellitus in women are the most important risk factors of premature coronary artery disease in Iran. Int J Cardiol, 141:116–8. [DOI] [PubMed] [Google Scholar]
- 38.Hosseini SK, Masoudkabir F, Vasheghani-Farahani A, et al. (2011). Opium consumption and coronary atherosclerosis in diabetic patients: a propensity score-matched study. Planta Med, 77:1870–5. [DOI] [PubMed] [Google Scholar]
- 39.Dehghani F, Masoomi M, Haghdoost AA. (2013). Relation of opium addiction with the severity and extension of myocardial infarction and its related mortality. Addiction & Health, 5(1–2):35–42. [PMC free article] [PubMed] [Google Scholar]
- 40.Rezvani MR, Ghandehari K. (2012). Is opium addiction a risk factor for ischemic heart disease and ischemic stroke? J Res Med Sci, 17:958–61. [PMC free article] [PubMed] [Google Scholar]
- 41.Najafipour H, Joukar S, Malekpour-Afshar R, et al. (2010). Passive opium smoking does not have beneficial effect on plasma lipids and cardiovascular indices in hypercholesterolemic rabbits with ischemic and non-ischemic hearts. J Ethnopharmacol, 127:257–263. [DOI] [PubMed] [Google Scholar]
- 42.Davoodi G, Sadeghian S, Akhondzadeh S, et al. (2006). Comparison of Specifications, Short Term Outcome and Prognosis of Acute Myocardial Infarction in Opium Dependent Patients and Nondependents. J Tehran Univ Heart Cent, 1:48–53. [Google Scholar]
- 43.Roohafza H, Talaei M, Sadeghi M, et al. (2013). Opium decreases the age at myocardial infarction and sudden cardiac death: a long- and short-term outcome evaluation. Arch Iran Med, 16:154–60. [PubMed] [Google Scholar]
- 44.Safaei N. (2008). Outcomes of coronary artery bypass grafting in patients with a history of opiate use. Pak J Biol Sci, 11:2594–8. [DOI] [PubMed] [Google Scholar]
- 45.Darrouj J, Karma L, Arora R. (2009). Cardiovascular manifestations of sedatives and analgesics in the critical care unit. Am J Ther, 16:339–353. [DOI] [PubMed] [Google Scholar]
- 46.Wang T, Hung C. (2003). Enhanced endothelin-1 degradation by intravenous morphine in patients with congestive heart failure: role of neutral endopeptidase 24.11. Heart, 89:211–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wuerz RC, Meador SA. (1992). Effects of prehospital medications on mortality and length of stay in congestive heart failure. Ann Emerg Med, 21:669–674. [DOI] [PubMed] [Google Scholar]
- 48.LoCasale R, Kern DM, Chevalier P, et al. (2014). Description of cardiovascular event rates in patients initiating chronic opioid therapy for noncancer pain in observational cohort studies in the US, UK, and Germany. Adv Ther, 31:708–723. [DOI] [PubMed] [Google Scholar]
- 49.Dispennette R, Elliott D, Nguyen L, Richmond R. (2014). Drug Burden Index score and anticholinergic risk scale as predictors of readmission to the hospital. Consult Pharm, 29:158–168. [DOI] [PubMed] [Google Scholar]
- 50.Moqaddam A, Musavi S, Khademizadeh K. (2009). Relationship of opium dependency and stroke. Addict Health, 1(1), 6–10. [PMC free article] [PubMed] [Google Scholar]
- 51.Hamzei-Moghaddam A, Shafa MA, Khanjani N, Farahat R. (2013). Frequency of Opium Addiction in Patients with Ischemic Stroke and Comparing their Cerebrovascular Doppler Ultrasound Changes to Non-Addicts. Addict Health, 5(3–4):95–101. [PMC free article] [PubMed] [Google Scholar]
- 52.Khademi H, Malekzadeh R, Pourshams A, et al. (2012). Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50 000 adults in Iran. BMJ, 344:e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karam GA, Rashidinejad HR, Aghaee MM, et al. (2008). Opium can differently alter blood glucose, sodium and potassium in male and female rats. Pak J Pharm Sci, 21:180–4. [PubMed] [Google Scholar]
- 54.Karam GA, Reisi M, Kaseb AA, et al. (2004). Effects of opium addiction on some serum factors in addicts with non-insulin-dependent diabetes mellitus. Addict Biol, 9(1):53–8. [DOI] [PubMed] [Google Scholar]
- 55.Mami S, Eghbali M, Cheraghi J, et al. (2011). Effect of opium addiction on some serum parameters in rabbit. Glob Vet, 7:310–4. [Google Scholar]
- 56.Bossone CA, Hannon JP. (1991). Metabolic actions of morphine in conscious chronically instrumented pigs. Am J Physiol, 260:R1051–7. [DOI] [PubMed] [Google Scholar]
- 57.Sumathi T, Niranjali Devaraj S. (2009). Effect of Bacopa monniera on liver and kidney toxicity in chronic use of opioids. Phytomedicine, 16:897–903. [DOI] [PubMed] [Google Scholar]
- 58.Kouros D, Tahereh H, Mohammadreza A, Minoo MZ. (2010). Opium and heroin alter biochemical parameters of human's serum. Am J Drug Alcohol Abuse, 36:135–9. [DOI] [PubMed] [Google Scholar]
- 59.Radmard AR, Khorasanizadeh F, Poustchi H, et al. (2018). Dilemma of Common Bile Duct Dilatation in Opium-addicts: A Population-based Study on Prevalence and Clinical Outcome. Am J Med Sci, 365(1). [DOI] [PubMed] [Google Scholar]
- 60.Lurie E, Soloviova A, Alyabieva T, et al. (1995). Effect of novel aromatic derivative of GABA on lipid peroxidation in chronically morphinized rats. Biochem Mol Biol Int, 36:13–9. [PubMed] [Google Scholar]
- 61.Panchenko LF, Pirozhkov SV, Nadezhdin AV, et al. (1999). [Lipid peroxidation, peroxyl radical-scavenging system of plasma and liver and heart pathology in adolescence heroin users]. Vopr Med Khim, 45:501–6. [PubMed] [Google Scholar]
- 62.Pereska ZJ, Bozinovska C, Dimitrovski C, et al. (2011). Plasma apo/lipoproteins disturbances as a precondition for metabolic syndrome in HCV seronegative heroin addicts. Am J Drug Alcohol Abuse, 37:196–202. [DOI] [PubMed] [Google Scholar]
- 63.Ghazavi A, Mosayebi G, Solhi H, Rafiei M, Moazzeni SM. (2013). Serum markers of inflammation and oxidative stress in chronic opium (Taryak) smokers. Immunol Lett, 153:22–26. [DOI] [PubMed] [Google Scholar]
- 64.Soltaninejad K, Shadnia S. (2018). Lead poisoning in opium abuser in Iran: A systematic review. Int J Prev Med, 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nemati A, Jafari S, Afshari M, Dahmardeh S, Tabrizian K. (2016). Comparing Blood Lead Level among Oral/inhaled Opium Addicts with a Non-addict Control Group in the Southeast of Iran. Addict Health, 8:235–241. [PMC free article] [PubMed] [Google Scholar]
- 66.Salehi H, Sayadi A, Tashakori M, et al. (2009). Comparison of serum lead level in oral opium addicts with healthy control group. Arch Iran Med, 12(6), 555–8. [PubMed] [Google Scholar]
- 67.Masoodi M, Zali MR, Ehsani-Ardakani M, et al. (2006). Abdominal pain due to lead-contaminated opium: a new source of inorganic lead poisoning in Iran. Arch Iran Med, 9(1):72–5. [PubMed] [Google Scholar]
- 68.Beigmohammadi MT, Aghdashi M, Najafi A, et al. (2008). Quadriplegia due to lead-contaminated opium. Middle East J Anaesthesiol, 19:1411–6. [PubMed] [Google Scholar]
- 69.Jalili M, Azizkhani R. (2009). Lead toxicity resulting from chronic ingestion of opium. West J Emerg Med, 10:244–6. [PMC free article] [PubMed] [Google Scholar]
- 70.Amiri M, Amini R. (2012). A comparison of blood-lead level (BLL) in opium-dependant addicts with healthy control group using the graphite furnace/atomic absorption spectroscopy (GF-AAS) followed by chemometric analysis. Iran Red Crescent Med J, 14:488–91. [PMC free article] [PubMed] [Google Scholar]
- 71.Aoki Y, Brody DJ, Flegal KM, et al. (2016). Blood lead and other metal biomarkers as risk factors for cardiovascular disease mortality. Medicine (Baltimore), 95(1):e2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peacock W, Hollander J, Diercks D, et al. (2008). Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J, 25:205–209. [DOI] [PubMed] [Google Scholar]
- 73.Safaii N, Kazemi B. (2010). Effect of opium use on short-term outcome in patients undergoing coronary artery bypass surgery. Gen Thorac Cardiovasc Surg, 58:62–67. [DOI] [PubMed] [Google Scholar]
- 74.Azarasa M, Azarfarin R, Changizi A, Alizadehasl A. (2009). Substance use among Iranian cardiac surgery patients and its effects on short-term outcome. Anesth Analg, 109:1553–9. [DOI] [PubMed] [Google Scholar]
- 75.Rodrigues AD. (2019). Drug-drug interactions. CRC Press. [Google Scholar]
- 76.Weizman R, Getslev V, Pankova IA, Schrieber S, Pick CG. (1999). Pharmacological interaction of the calcium channel blockers verapamil and flunarizine with the opioid system. Brain Res, 818:187–195. [DOI] [PubMed] [Google Scholar]
- 77.Fetrow CW, Avila JR. (2001). Professional's handbook of complementary & alternative medicines. ed. Springhouse Publishing Company. [Google Scholar]
- 78.Kubica J, Kubica A, Jilma B, et al. (2016). Impact of morphine on antiplatelet effects of oral P2Y12 receptor inhibitors. Int J Cardiol, 215:201–208. [DOI] [PubMed] [Google Scholar]
- 79.Hobl E-L, Stimpfl T, Ebner J, et al. (2014). Morphine decreases clopidogrel concentrations and effects: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol, 63:630–635. [DOI] [PubMed] [Google Scholar]
- 80.Hobl E-L, Reiter B, Schoergenhofer C, et al. (2016). Morphine interaction with prasugrel: a double-blind, cross-over trial in healthy volunteers. Clin Res Cardiol, 105:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hansen PW, Clemmensen L, Sehested T, Fosbøl E, et al. (2016). Identifying Drug–Drug Interactions by Data Mining: A Pilot Study of Warfarin-Associated Drug Interactions. Circ Cardiovasc Qual Outcomes, 9:621–8. [DOI] [PubMed] [Google Scholar]