Short abstract
Gut bacterial translocation following impaired gut barrier is a critical determinant of initiating and aggravating acute pancreatitis (AP). Antibiotic combination (ABX; vancomycin, neomycin and polymyxin b) is capable of reducing gut bacteria, but its efficacy in AP prevention and the underlying mechanism have not been investigated yet. AP was induced in BALB/c mice by caerulein (CAE) hyperstimulation. We found that ABX supplementation attenuated the severity of AP as evidenced by reduced pancreatic oedema and myeloperoxidase activity. The protective effect was also confirmed by improved histological morphology of the pancreas and decreased pro-inflammatory markers (IL-1β, TNF-α, MCP-1) in pancreas. ABX administration inhibits the activation of colonic TLR4/NLRP3 inflammasome pathway. Subsequently, down-regulated NLRP3 resulted in decreased colonic pro-inflammation (IL-1β, IL-6, MCP-1) and enhanced gut physical barrier as evidenced by up-regulation of tight junction proteins including occludin, claudin-1 and ZO-1, as well as improved histological morphology of the colon. Together, combinatory ABX therapy inhibited the translocation of gut bacteria to pancreas and its amplification effects on pancreatic inflammation by inhibiting the pancreatic NLRP3 pathway, and inhibiting intestinal-pancreatic inflammatory responses. The current study provides the basis for potential clinical application of ABX in AP.
Keywords: Gut bacterial translocation, antibiotic, gut barrier, acute pancreatitis, Toll-like receptor 4
Introduction
Acute pancreatitis (AP) is a pancreatic inflammatory disorder caused by gallstones, alcohol misuse, and other risk factors (such as genetic factors and drugs).1 During the past decades, the worldwide incidence of AP has grown.1 The direct cause of AP is abnormal activation of proenzymes2 and uncontrolled diffuse inflammation in the pancreas and adjacent organs,3 but the mechanism of AP remains unclear. Complications of severe AP, such as multiple organ failure and sepsis, enormously increase the mortality,4 so it is critically important to elucidate the aggravated pathogenesis of mild AP turning to severe AP.
Gut bacteria have been increasingly recognized for their role in the regulation of local and distant inflammatory responses.5 Severe illness enables gut bacteria to enter the extraintestinal site (such as blood, pancreas and other organs);6 this phenomenon is referred to as ‘bacteria translocation’. Delayed intestinal transit time induced by AP changes the composition of the intestinal microflora, promotes intestinal bacterial overgrowth, and subsequently promotes intestinal bacterial translocation.7,8 In addition, severe AP is characterized by impairment of the intestinal barrier, intestinal dysmotility, ischemia, hyperpermeability, bacterial translocation and increased endotoxin. These studies suggest that dysfunctional intestinal homeostasis in the early stage of AP may enhance intestinal bacterial translocation, subsequently exacerbating AP. Gut decontamination by prophylactic antibiotic treatment may protect against severe AP by inhibiting intestinal bacterial translocation.
Bacteria can activate the host innate immune system via PRR Toll-like receptor 4 (TLR4) and its downstream leucine-rich repeat containing molecules (NLRs).9 TLR4 triggers the inflammatory responses by mediating organ dysfunction and bacterial translocation in severe AP.10 Thus, reduction of intestinal bacterial translocation may alleviate the activation of TLR4 and NLRP3, thereby inhibiting the uncontrolled diffuse inflammation in pancreas and adjacent organs during AP.
During AP, gut bacteria translocation is usually caused by an impaired gut barrier, gut bacterial overgrowth, and increased inflammation.11–13 In the later stage of AP, bacteria in the blood and other organs account for multiple organ failure, sepsis and other serious complications. However, how the escaped gut bacteria participate in amplifying inflammation in the blood, pancreas and adjacent organs remains unknown.
The current study aimed to elucidate the role of escaped gut bacteria in AP. To elucidate the effects and mechanisms, first, a broad-spectrum antibiotic combination (vancomycin, neomycin and polymyxin b – ABX) was used to reduce gut bacteria. Eight days later, caerulein was used to induce AP in mice. Pathology and inflammation-associated indexes were then examined. The effect of ABX treatment on gut bacteria translocation in relation to the progression of AP was investigated. This study will facilitate the understanding on the gut–pancreas immune environment and provide a novel strategy for intervening in AP.
Materials and methods
Animals
For this study, 8-week-old female BALB/c mice (Su Pu Si Biotechnology Co., Ltd. Suzhou, Jiangsu, China) were maintained in specific pathogen-free environment at the Animal Housing Unit of Jiangnan University (Wuxi, Jiangsu, China) under 23–25°C and 12-h light/dark cycle with unlimited access to food and water. All mice were allowed to acclimatize to laboratory conditions over the course of 1 week prior to the experiments. All experimental procedures in this study were approved by the Institutional Animal Ethics Committee of Jiangnan University (JN.No20170711-20171020-84) and carried out in compliance with national and international guidelines for the Care and Use of Laboratory Animals.
Antibiotic treatment and AP induction
Vancomycin is one of the last antibiotics used to treat life-threatening infections caused by Gram-positive bacteria.14–17 Polymyxin b is often used in combination with neomycin to deplete intestinal Gram-negative bacteria, and the combination has been used to treat Gram-negative infections approved by the FDA.18–20 In this study, the purpose of the triple antibiotic regimen is to decontaminate intestinal Gram-positive and Gram-negative bacteria.
Female BALB/c mice (20 ± 2g) were randomly assigned to three groups (n = 7): (1) the control (CON) mice were fed with autoclaved water; (2) the caerulein-treated (CAE) mice were fed with autoclaved water; (3) the antibiotic-treated mice were fed with autoclaved water with antibiotic combination (ABX, 0.5 .mg·ml−1 vancomycin; 1 mg·ml−1 neomycin; 0.3 mg·ml−1 polymyxin b, all in Sangon, China) for 8 d. Then the CON-mice received hourly intraperitoneal injections with saline for 10 h, and the CAE-mice and ABX + CAE-mice received hourly intraperitoneal injections with caerulein for 10 h.
Tissues sampling
Mice were euthanized and sacrificed with pentobarbitone sodium (100 mg/kg) 1 h after the last caerulein injection. For serum analysis, blood samples were centrifuged (3000 g, 15 min), and supernatant was collected and stored at –80°C. Tissues (pancreas and colon) were excised, fixed in 4% paraformaldehyde or snap frozen in liquid nitrogen and stored at –80°C for later analysis. The colon was collected, cut along the axis of the intestine and washed three times with phosphate-buffered saline, and then stored at –80°C until used for subsequent western blotting/PCR analysis. For pancreatic and colonic cytokine assays, tissues (pancreas and colon) were homogenized with phosphate-buffered saline, and centrifuged (3000 g, 15 min). Supernatant was then collected and stored at –80°C.
Pancreatic oedema measurement
A portion of freshly harvested pancreatic tissue was trimmed of fat and weighed. Pancreatic water content was evaluated by the ratio of initial mass (wet mass) of the pancreas to its mass after incubation at 80°C for 48 h (dry weight).21
Pancreatic myeloperoxidase (MPO) measurement
The MPO activity was measured in homogenized pancreas using MPO assay kit according to the manufacturer’s protocol (Jian Cheng Bioengineering Institute, China) to determine the neutrophil infiltration in the pancreas.
Bacterial culture
On TSA plates, we cultured bacterial colonies following a standard method for pancreatic bacteria.22 After euthanasia, mouse skin was sterilized with 70% ethanol before opening the abdomen. Freshly harvested pancreatic and pancreatic lymph node (PLNs) samples (0.1 g) were obtained aseptically in a safety vertical laminar flow hood, homogenized in with phosphate-buffered saline (1:9). The dilution (0.2 ml) was plated on tryptone soy agar plates, incubated at 37°C for 48–72 h (until CFUs were evident). Tryptone soy agar plate counts (CFUs) were examined to determine whether bacterial translocation had occurred as previously described.22–25
Gut bacterial translocation
To evaluate the effect of ABX on the gut bacterial translocation during AP, after 8 d of antibiotic treatment, mice were given Lactobacillus plantarum with GFP-labelled plasmids suspended in saline (1 × 1010 CFU/kg) by gavage 18 h before the mice were euthanized. The L. planterum-GFP were detected in PLNs and mesenteric lymph nodes (MLNs) by flow cytometry.
The L. planterum-GFP (http://www.paper.edu.cn/releasepaper/content/201902-90) were kindly provided by Dr Bingyong Mao (State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, China).
Preparation of single cell suspensions
PLNs and MLNs were harvested and ground with gentle MACS™ Dissociators (Miltenyi Biotec, Bergisch Gladbach, Germany) and filtered with 70 μm filter screen.26–28 GFP+ L. plantarum were analysed on an Invitrogen™ Attune™ NxT Flow Cytometer (Thermo Fisher Scientific, Massachusetts, USA).
Short chain fatty acid (SCFAs) analysis
Faecal acetate, propionate and butyrate were detected by gas chromatography coupled mass spectrometry (GC-MS) as previously described29.
Histological examination
Freshly harvested pancreatic samples were fixed with 4% paraformaldehyde overnight, washed with ddH2O, dehydrated with gradient ethanol solutions and embedded with paraffin. Prepared sections (5 μm) were stained with hematoxylin and eosin (H&E) using standardized protocols. Morphological changes of pancreas were examined under a DM2000 light microscope (Leica, Germany).
Pancreatic injury was evaluated based on oedema, inflammatory cell infiltration, haemorrhage and necrosis.30 The histopathologic scores were in accordance with the pathological scoring system of pancreas regulated by Schmidt et (Table 1), and Schmidt scores of normal pancreas were 0–3.
Table 1.
Schmidt score of pancreatic pathology.
| Scores | Interstitial edema | Inflammatory infiltration | Parenchymal necrosis | Parenchymal haemorrhage |
|---|---|---|---|---|
| 0 | – | – | – | – |
| 1 | Mild | < 20 | < 5% | 1–2 |
| 2 | Moderate | 20–50 | 5–20% | 3–5 |
| 3 | Serious | > 50 | > 20% | > 20% |
DNA extraction
The stool samples were stored at –80°C until analysis. Stool sample DNA was extracted using FastDNA® SPIN Kit for Soil (MP Biomedicals, 6560-200, California, USA), following the manufacturer’s instructions. In detail, 50 mg frozen stools were added to Lysing Matrix A tube, then 1.0 ml CLS-TC were added to Sample Tube. The mixture was homogenized in the FastPrep Instrument for 40 s at a speed setting of 6.0, then centrifuged at 14,000 g for 5–10 min to pellet debris. Next, the supernatant was transfer to a 2.0 ml microcentrifuge tube, an equal volume of Binding Matrix was added, and the samples were mixed and incubated with gentle agitation for 5 min at room temperature on a rotator. Then, the suspension was transferred to a SPIN™, filtered and centrifuged (14,000 g, 1 min) twice. Subsequently, the pellet was re-suspended gently with 500 µl prepared SEWS-M, and centrifuged (14,000 g, 1 min). The contents of Catch Tube were discarded and the Catch Tube was replaced, centrifuged (14,000 g, 1 min) without any addition of liquid. Catch Tubes were replaced with new, clean Catch Tubes, the Binding Matrix above the SPIN filter was resuspended with 100 µl DNase/Pyrogen-Free Water to elute DNA, centrifuged at 14,000 g for 1 min to bring eluted DNA into the clean Catch Tube after incubating the tubes at 55°C for 5 min. DNA was now ready for downstream applications and was stored at –80°C until use.
RNA isolation, reverse transcription, and real-time quantitative polymerase chain reaction (RT-qPCR)
We detected the pancreatic and colonic cytokines by RT-qPCR in our study. Mice were euthanized and sacrificed with pentobarbitone sodium (100 mg/kg) 1 h after the last caerulein injection. Pancreas and colon tissues were collected and stored at –80°C until they were used for RNA extraction. Total RNA of pancreas and colon was homogenized in TRIzol (Life Technologies, MA, USA), quantitated by spectrophotometry (Thermo, USA) and subjected to reverse transcription using the Prime-Script RT reagent kit (TaKaRa Bio, Japan) following the manufacturer’s instructions. SYBR® Green RT-PCR reagents (Yeasen, China) were used with a real-time PCR system (BIO RAD CFX Connect, CA, USA). Calculations were made based on the comparative cycle threshold method (2-DDCt). Relative mRNA expression was normalized to the mRNA levels of β-actin (housekeeping control).21,31–36 Detailed primer sequences are shown in Table 2.
Table 2.
Specific primers for qPCR.
| Target gene | Forward primer | Reverse primer |
|---|---|---|
| MCP-1 | 5′- GTGCTGACCCCAATAAGGAA -3′ | 5′- TGAGGTGGTTGTGGAAAAGA -3′ |
| TNF-α | 5′-AGGGTCTGGGCCATAGAACT-3′ | 5′-CCACCACGCTCTTCTGTCTAC-3′ |
| IL-1β | 5′-CTGAACTCAACTGTGAAATGC-3′ | 5′-TGATGTGCTGCTGCGAGA-3′ |
| IL-6 | 5′-CTCTGCAAGAGACTTCCATCCAGT-3′ | 5′-GAAGTAGGGAAGGCCGTGG-3′ |
| Oldn | 5′-TTGAAAGTCCACCTCCTTACAGA-3′ | 5′-CCGGATAAAAAGAGTACGCTGG-3′ |
| Cldn1 | 5′-GGGGACAACATCGTGACCG-3′ | 5′-AGGAGTCGAAGACTTTGCACT-3′ |
| Tjp1 | 5′-GCCGCTAAGAGCACAGCAA-3′ | 5′-TCCCCACTCTGAAAATGAGGA-3′ |
| Muc2 | 5′-CAAGGGCTCGGAACTCCAG-3′ | 5′-ATGCCCACCTCCTCAAAGAC-3′ |
| Reg3g | 5′-ATGCTTCCCCGTATAACCATCA-3′ | 5′-GGCCATATCTGCATCATACCAG-3′ |
| Reg3b | 5′-TACTGCCTTAGACCGTGCTTTCTG-3′ | 5′-GACATAGGGCAACTTCACCTCACA-3′ |
| Defb1 | 5′-GCACAAGAAGGTCACACGGA-3′ | 5′-CTAAGGTTGCAGATGGGGTGT-3′ |
| 16SrDNA | 5′-TCCTACGGGAGGCAGCAGT-3′ | 5′-GACTACCAGGGTATCTAATCCTGTT-3′ |
| β-Actin | 5′-GGCTGTATTCCCCTCCATCG-3′ | 5′-CCAGTTGGTAACAATGCCATGT-3′ |
Western blot analysis
Mice were euthanized and sacrificed with pentobarbitone sodium (100 mg/kg) 1 h after the last caerulein injection. Pancreas and colon tissues were collected and stored at –80°C until used for Western blot analysis. The tissues were homogenized in ice-cold RIPA lysis buffer (Beyotime, China) containing cocktail protease inhibitors (Beyotime, Shanghai, China), and centrifuged at 10,000 g for 15 min at 4°C; the supernatant was used for Western blot analysis at an equaled amount of protein (30 µg), and protein concentration was quantified using a BCA protein assay Kit (Beyotime, Shanghai, China). Equal amounts of total proteins were separated via SDS-PAGE, transferred onto polyvinylidene difluoride membranes. Membranes were blocked with blocking buffer for 1 h at room temperature, washed with TBST, finally incubated overnight at 4°C with anti-cleaved-caspase-1 p20, anti-cleaved-IL-1β, anti-NLRP3, anti-TLR4 (CST, Beverly, MA, USA) and anti-GAPDH (Biogot, Nanjing, China). Incubation with fluorescently labelled secondary HRP-conjugated secondary antibodies (1:5000) was performed for 2 h at room temperature. Immunoreactivity was analysed using Western Lightening Plus enhanced chemiluminescence (PerkinElmer, MA, USA) according to the manufacturer’s instructions. GAPDH was adopted as internal standard to control for unwanted sources of variation, and relative protein expression values were expressed as ‘fold mean of the controls’ by comparing with the corresponding control value,32,37–40 and the control value was normalized to 1.0.
Statistic analysis
All data were expressed as mean ± SD. ANOVA was performed to determine the significance among three groups followed by the indicated post hoc test. Independent t-test was used for two independent groups. A p-value less than 0.05 was considered as a significant difference. All data were analysed using GraphPad Prism 5 software (version 5; GraphPad Software Inc, San Francisco, CA, USA).
Results
ABX supplementation alleviates the severity of caerulein-induced AP
As shown in Figure 1a, ABX + CAE-mice exhibited significantly lower body mass than the CAE-mice. ABX pretreated mice exhibited reduced pancreatic edema (Figure 1b) and MPO levels (Figure 1c) induced by caerulein injection. Morphological examination confirmed the protective effect of ABX on caerulein-induced AP mice evidenced by improved cellular morphology, pancreatic oedema, reduced inflammatory cell infiltration and acinar necrosis (Figure 1d). The pancreas pathology score of the CON group was 0, and there was no hyperemia, oedema or inflammatory cells infiltration, and there was less hyperemia, oedema and inflammatory in the CAE-ABX group than that in the CAE group (Figure 1d).
Figure 1.
ABX supplementation alleviates the severity of caerulein-induced AP. Female BALB/c mice received normal water (CON and CAE group) or antibiotic-containing water (ABX + CAE group) for 8 days before AP induction by caerulein. Body mass (a), pancreatic oedema (b) and pancreatic MPO activity (c) were determined. (d) Representative photographs showed histomorphology of pancreatic tissues by H&E staining for the indicated groups (bar = 50 µm), and the pancreas pathology score. The results are shown as mean ± SD, n ≥ 3. *P < 0.05, **P < 0.01 and ***P < 0.001 by one-way ANOVA followed by Dunnett’s test.
These findings demonstrate that the ABX may reduce the translocation of gut bacteria to the pancreas during caerulein-induced AP.
ABX supplementation inhibits pancreatic inflammation during caerulein-induced AP
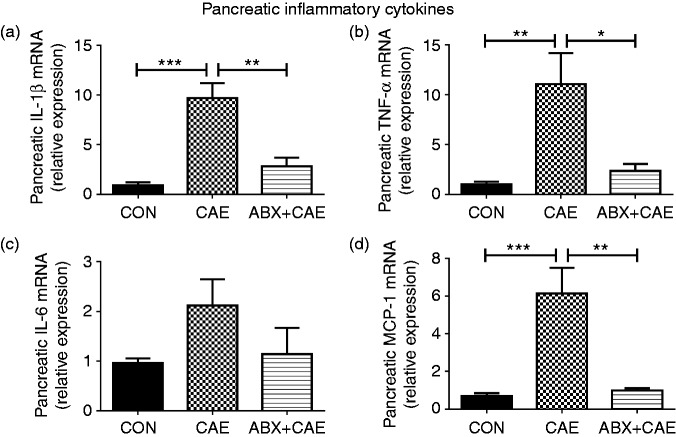
Progression of AP is accompanied by increased production of pancreatic pro-inflammatory cytokine, which amplifies the condition and promotes systemic inflammatory responses.41 To detect the effects of ABX on inflammatory responses during AP, we determined the mRNA levels of pro-inflammatory cytokines in the pancreas. The results showed that ABX significantly down-regulated the pancreatic pro-inflammation markers (IL-1β, TNF-α, MCP-1) compared with the caerulein-treated mice (Figure 2), suggesting that ABX administration effectively reduces pancreatic inflammatory tone.
Figure 2.
ABX supplementation inhibits pancreatic inflammations during caerulein-induced AP. Levels of pancreatic IL-1β (a), TNF-α (b), IL-6 (c) and MCP-1 (d) were determined by qPCR. The results are shown as mean ± SD, n ≥ 3. *P < 0.05, **P < 0.01 and ***P < 0.001 by one-way ANOVA followed by Dunnett’s test.
ABX supplementation enhances the gut barrier during caerulein-induced AP
Impaired gut physical barrier with down-regulated tight junction protein expression is an early hallmark of severe AP.42,43 To investigate the effects of ABX on the gut barrier, we determined the expression of physical barrier-associated markers as well as chemical barrier-associated markers in colon.44,45 As shown in Figure 3 and Supplementary Figure 1, both physical barrier-associated markers and chemical barrier-associated markers were greatly impaired by caerulein injection, while ABX protected against caerulein-induced down-regulation of physical barrier-associated markers (occludin, claudin-1, ZO-1) (Figure 3a–e) but not the mucus and the chemical barrier-associated markers (Supplementary Figure 1). Moreover, we found that ABX prevented the reduction of goblet cells and crypt length in the colon (Figure 3f), which was a sign of improved gut barrier dysfunction. These data demonstrate that ABX administration effectively restores the impaired gut physical barrier induced by AP.
Figure 3.
ABX supplementation enhances gut barrier function during caerulein-induced AP. Levels of colonic physical barrier markers occludin (a), claudin-1 (b), ZO-1 (c), Muc2 (d) were determined by qPCR. (e) Levels of colonic occludin were determined by Western blot. (f) Representative photographs showed histomorphology of colon by H&E staining for the indicated groups (bar = 100 µm). The results are shown as mean ± SD, n ≥ 3. *P < 0.05, **P < 0.01 and ***P < 0.001 by one-way ANOVA followed by Dunnett’s test.
ABX supplementation inhibits colonic inflammation during caerulein-induced AP
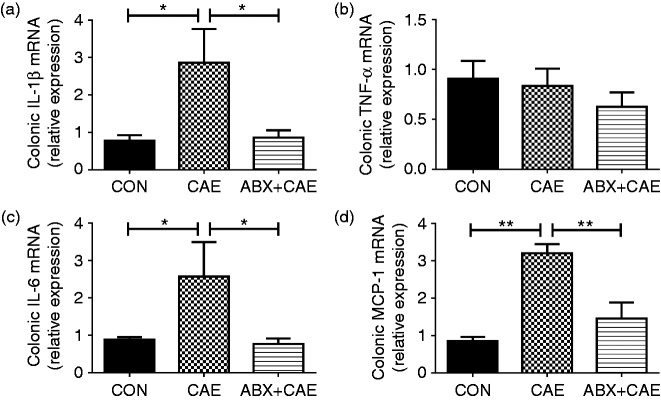
The integrity of the gut barrier is tightly coupled to the degree of severity in AP, and the intestine is the origin of systemic inflammation.46 Having found clear effects on the gut integrity (Figure 3), we subsequently measured the colonic pro-inflammatory markers by qPCR to elucidate whether ABX lowered inflammatory tone in the colon. The ABX + CAE-treated mice had significant lower IL-1β, IL-6 and MCP-1 levels compared with the CAE-mice (Figure 4), suggesting the protective effect of ABX on AP could be potently induced by decreased inflammatory tone in colon.
Figure 4.
ABX supplementation inhibits colonic inflammation during caerulein-induced AP. Levels of colonic cytokines IL-1β (a), TNF-α (b), IL-6 (c) and chemokine MCP-1 (d) were determined by qPCR. The results are shown as mean ± SD, n ≥ 3. *P < 0.05 and **P < 0.01 by one-way ANOVA followed by Dunnett’s test.
ABX supplementation suppresses the translocation of gut bacteria to pancreas
Previous studies have suggested a possible interaction between altered intestinal bacteria and pancreas.22,23 We next detected the bacteria inside the faeces and pancreas by nanodrop and 16S PCR analysis, and found that the ABX + CAE-mice exhibited significantly lower faecal (Figure 5a) and pancreatic bacterial DNA (Figure 5b) compared with the CAE-mice.
Figure 5.
ABX supplementation suppresses the translocation of gut bacteria to pancreas. (a) Faecal bacterial DNA concentration. (b) Pancreatic bacterial DNA content. CFUs were counted on culture plates of pancreas (c) and PLNs (d). The GFP+ events were detected in PLNs (e) and MLNs (f) by flow cytometry. (g) Faecal SCFAs concentration measured by GC-MS. The results are shown as mean ± SD, n ≥ 3. *P < 0.05, **P < 0.01 and ***P < 0.001 by one-way ANOVA followed by Dunnett’s test.
In addition, we detected the bacteria inside the pancreas and PLNs by CFU analysis. Interestingly, we found a significant increase of CFU in the pancreas and PLNs of CAE-mice compared with CON-mice (Figure 5c, 5d), suggesting the translocation of gut bacteria to pancreas during AP. ABX treatment effectively lowered AP-induced increase in CFU (Figure 5c, 5d).
To evaluate the effect of ABX on the gut bacterial translocation during AP, mice were given L. planterum-GFP suspended in saline (1 × 1010 CFU/kg) by gavage 18 h before the mice were euthanized. After the mice were sacrificed, the GFP+ events were detected in PLNs and MLNs. We found that CAE-mice harboured more GFP+ events in both PLNs (Figure 5e) and MLNs (Figure 5f) compared with the CON-mice, which was significantly inhibited by ABX treatment (Figure 5e, 5f).
In addition, GC-MS analyses revealed that ABX significantly reduced SCFA production (Figure 5g).
These data suggest that ABX administration attenuates intestinal bacterial content and inhibits their translocation to pancreas during caerulein-induced AP.
ABX supplementation down-regulates colonic TLR4 expression during caerulein-induced AP
We next examined pancreatic and colonic TLR4 levels by Western blotting. Interestingly, colonic TLR4 was significantly activated in the CAE-mice compared with the CON-mice, while ABX treatment significantly reduced colonic TLR4 levels (Figure 6). However, there was no significant difference observed in pancreatic TLR4 levels among the groups. These findings demonstrate that the anti-AP mechanism of ABX may be associated with the inhibition of colonic TLR4.
Figure 6.
ABX supplementation down-regulates colonic TLR4 expression during caerulein-induced AP. Protein expression and quantitative analysis of TLR4 in pancreas (a) and colon (b) were examined by Western blot and analysed by Alphaview SA. The results are shown as mean ± SD, n ≥ 3. *P < 0.05 and **P < 0.01 by one-way ANOVA followed by Dunnett’s test.
ABX supplementation inhibits the activation of pancreatic and colonic NLRP3 inflammasome during caerulein-induced AP
Next, we investigated the signalling molecular pathways underlying the protective effects by ABX supplementation. Recent reports have suggested that NLRP3 inflammasome can be activated by bacteria during AP.47,48 Thus we investigated whether the protective effect of ABX on AP was associated with the NLRP3 inflammasome pathway. We found that both pancreatic and colonic NLRP3 inflammasome pathways were significantly activated in caerulein-induced AP mice, while ABX supplementation effectively suppressed the activation of NLRP3 and its downstream signalling molecules (caspase-1 and cleaved 1β) (Figure 7). These data suggest that ABX supplementation inhibits the activation of pancreatic and colonic NLRP3 inflammasome pathway.
Figure 7.
ABX supplementation inhibits pancreatic and colonic NLRP3 inflammasome activation during caerulein-induced AP. Protein expression and quantitative analysis of NLRP3, caspase-1 (p20), cleaved-IL-1β in pancreas (a) and colons (b) were examined by Western blot and analysed by Alphaview SA. The results are shown as mean ± SD, n ≥ 3. *P < 0.05, **P < 0.01 and ***P < 0.001 by one-way ANOVA followed by Dunnett’s test.
Discussion
The present study demonstrates that prophylactic ABX supplementation delays AP development by three graded actions: (1) reducing pancreatic inflammation and damage; (2) preventing intestinal barrier dysfunction and inflammation, and reducing gut bacterial translocation to the pancreas; (3) delaying the progression of AP into a systemic inflammatory response as the consequence. To our knowledge, this is the first study to reveal the positive effect of prophylactic ABX treatment on AP development.
Developmentally controlled lymphogenesis establishes a preferential trafficking route from the gut to the PLNs, where T cells can be activated by antigen drained from the gastrointestinal tract. Furthermore, intestinal stress also modifies the presentation of pancreatic self-antigens in PLNs.11,49 Prophylactic broad-spectrum treatment prevents gut bacterial translocation to the PLNs in both streptozotocin-induced type 1 diabetes (T1D) and non-obese diabetic mice, subsequently protecting the mice from T1D.23 With the progression of AP, pro-inflammatory cytokines including TNF-α and IL-1β are produced in the pancreas and reach the colon through the microcirculation.21,43 These cytokines recruit more leukocytes and inflammatory mediators, ultimately contributing to intestinal barrier dysfunction and increased intestine permeability.43 This promotes intestinal bacterial overgrowth and its translocation to the pancreas, subsequently resulting in a second round of inflammatory events in the pancreas and initiating excessive inflammatory responses and multi-organ dysfunctions.50 In the present study, combinatory ABX therapy significantly inhibited gut bacterial translocation to the pancreas, finally alleviating intestinal-pancreatic inflammatory responses to prevent secondary excessive inflammatory responses.
The innate immune system recognizes specific bacterial antigens through an extensive family of PRRs. The inflammasome complex is typically composed of three components: NLR, ASC and caspase-1, of which the NLRP3 inflammasome is the most investigated.51 The combination of TLR4 (one of the PRRs, the receptor of LPS, which is a Gram-negative bacterial component) and LPS activates the downstream NLRP3. Once activated, NLRP3/ASC adaptor promotes the recruitment of pro-caspase-1 to generate enzymatically active caspase-1, which then converts pro-IL-1β into IL-1β (its mature active form).52,53 IL-1β is an important mediator in systemic inflammation reaction syndrome and plays an important role in the early stages of AP.12,54 However, there were no differences in pancreatic TLR4 levels among these three groups, probably due to relatively low abundance of bacteria in pancreas compared with that in colon. Consistent with previous studies, in colon, combinatory ABX therapy inhibits the activation of TLR4/NLRP3 pathway, subsequently resulting in decreased colonic inflammation and enhanced gut physical barrier. Enhanced gut physical barrier inhibited the translocation of gut bacteria to pancreas and pancreatic inflammation by inhibiting the pancreatic NLRP3 pathway, ultimately inhibiting the inflammatory response in pancreas.
The translocation of gut bacteria to pancreas is the underlying cause of exacerbating AP. Inhibition of gut bacterial translocation to pancreas improves the outcome of AP.42,55 Antibiotics with high pancreatic tissue penetration significantly reduce pancreatic infection and mortality.56,57 In addition, in human studies, both systemic antibiotic use and selective intestinal antibiotic intervention are beneficial for AP.58,59 Consistently, in our results, 8 d of ABX pre-treatment significantly down-regulated total gut bacteria and their migration to pancreas, subsequently improving the outcome of AP. However, pre-treatment with broad-spectrum antibiotic meropenem for 2 d increases the mortality of SAP mice60, which is probably because 2 d of meropenem treatment is insufficient to reduce gut bacteria and their translocation to pancreas. Instead it may lead to excessive proliferation of Enterococcus faecium.60 These data suggest that different antibiotics or different intervention time may account for discrepant effects.
Our results demonstrate that ABX administration significantly improves the impaired gut barrier by enhancing the gut physical barrier-associated markers but not chemical barriers-associated markers. Gut microbiota modulates mucus composition and mucus thickness, and the mucus layer in germ-free mice, is relatively thinner than control mice.61 In addition, the metabolites of gut microbiota (SCFAs, especially butyrate) also stimulate epithelial cells to produce chemical barrier-associated markers after sensing the luminal environment.61,62 Moreover, previous findings show that butyrate up-regulates Muc 2 expression in a dose-dependent manner.63–66 In our current study, ABX treatment contributed to drastically reduced gut bacteria and decreased SCFAs in the caerulein-induced AP mice, which might lead to a reduced stimulus signal and unraised chemical barrier-associated markers, including Muc 2.
In this study, we observed a significantly increase of TNF-α in pancreas but not colon in response to caerulein injection or ABX administration. Moreover, in colon, the other pro-inflammatory cytokines (IL-1β, IL-6 and MCP-1) of ABX-treated mice were significantly lower than in CAE-mice, probably because TNF-α can be rapidly cleared by liver2,67 and disrupted by inactivating enzymes in the bloodstream (neutrophil elastase).2,68,69 Therefore, serum and colonic TNF-α are difficult to detect in patients with AP, and TNF-α is not a good indicator of AP.67 In addition, differential cytokine regulation in different tissues is also common in experimental AP.70 Moreover, cytokine production differs based on their tissue-dependent cellular sources and the time point examined. In the current study, we measured a variety of cytokines to demonstrate their differential production in tissues and regulation by ABX treatment.
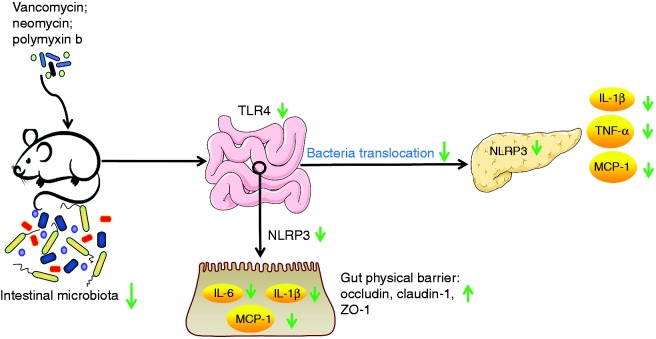
In summary, the study demonstrates that combinatory ABX administration protects against AP and delineates the underlying mechanism. The protective effects of ABX administration were achieved by attenuating pancreas inflammation, which was the result of decreased gut bacterial translocation to pancreas. Thus, our results suggest that ABX treatment targeting gut bacteria translocation may act as a novel strategy for prevention and treatment of AP and associated complication in clinical practice (Figure 8).
Figure 8.
The ABX administration inhibits the activation of colonic TLR4/NLRP3 inflammasome pathway. Down-regulated NLRP3 resulted in decreased colonic inflammation (IL-1β, IL-6, MCP-1) and enhanced gut physical barrier, subsequently inhibiting the translocation of gut bacteria to pancreas and its amplification effects on pancreatic inflammation by inhibiting pancreatic NLRP3 pathway.
Supplemental Material
Supplemental material, INI881502 Supplemental Material for Combinatory antibiotic treatment protects against experimental acute pancreatitis by suppressing gut bacterial translocation to pancreas and inhibiting NLRP3 inflammasome pathway by Lingling Jia, Hao Chen, Jun Yang, Xin Fang, Wenying Niu, Ming Zhang, Jiahong Li, Xiaohua Pan, Zhengnan Ren, Jia Sun and Li-Long Pan in Innate Immunity
Acknowledgements
The authors thank Dr. Bingyong Mao (State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, China) for providing the Lactobacillus plantarum with green fluorescent protein-labelled plasmid.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. All authors participated in the discussion and commented on the paper.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by funds from the National Natural Science Foundation of China (Grant Nos. 91642114, 81870439, 31570915 and 81573420), Jiangsu Province Recruitment Plan for High-level, Innovative and Entrepreneurial Talents, the Fundamental Research Funds for the Central Universities (Grant No. JUSRP11866), Jiangsu Province “Six Summit Talents” program (2019-YY-038), National first-class discipline program of Food Science and Technology (Grant No. JUFSTR20180103), Wuxi Social Development Funds for International Science & Technology Cooperation (Grant No. WX0303B010518180007PB), Chinese Postdoctoral Science Foundation (Grant No. 2018M642169), Jiangsu Postdoctoral Fund (Grant No. 2018K237C), Wuxi Medical Youth Talents Project of “Science and Technology Strengthening Health Project” (QNRC001) and Project of Public Health Research Center of Jiangnan University (JUPH201825).
Supplemental material
Supplemental material for this article is available online.
References
- 1.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet 2015; 386(9988): 85–96. [DOI] [PubMed] [Google Scholar]
- 2.Manohar M, Verma AK, Venkateshaiah SU, et al. Pathogenic mechanisms of pancreatitis. World J Gastrointest Pharmacol Ther 2017; 8(1): 10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao K, Yu L, Wang X, et al. Clostridium butyricum regulates visceral hypersensitivity of irritable bowel syndrome by inhibiting colonic mucous low grade inflammation through its action on NLRP6. Acta Biochim Biophys Sin (Shanghai) 2018; 50(2): 216–223. [DOI] [PubMed] [Google Scholar]
- 4.Liu T, Huang W, Szatmary P, et al. Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br J Surg 2017; 104(9): 1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soares FS, Amaral FC, Silva NLC, et al. Antibiotic-induced pathobiont dissemination accelerates mortality in severe experimental pancreatitis. Front Immunol 2017; 8: 1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaworek J, Tudek B, Kowalczyk P, et al. Effect of endotoxemia in suckling rats on pancreatic integrity and exocrine function in adults: A review report. Gastroenterol Res Pract 2018; 2018: 6915059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papoff P, Ceccarelli G, d'Ettorre G, et al. Gut microbial translocation in critically ill children and effects of supplementation with pre- and pro biotics. Int J Microbiol 2012. ;2012: 151393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Gong Z, Wu K, et al. Gastrointestinal dysmotility in patients with acute pancreatitis. J Gastroenterol Hepatol 2003; 18(1): 57–62. [DOI] [PubMed] [Google Scholar]
- 9.Lin TH, Su HH, Kang HY, et al. The interactive roles of lipopolysaccharides and dsRNA/viruses on respiratory epithelial cells and dendritic cells in allergic respiratory disorders: The hygiene hypothesis. Int J Mol Sci 2017; 18(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawa H, Ueda T, Takeyama Y, et al. Role of toll-like receptor 4 in the pathophysiology of severe acute pancreatitis in mice. Surg Today 2007; 37(10): 867–873. [DOI] [PubMed] [Google Scholar]
- 11.Vaishnavi C. Translocation of gut flora and its role in sepsis. Indian J Med Microbiol 2013; 31(4): 334–342. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Zhao HX, Bai C, et al. Blockade of high-mobility group box 1 attenuates intestinal mucosal barrier dysfunction in experimental acute pancreatitis. Sci Rep 2017; 7(1): 6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen W, Zheng H, Jiang Y, et al. Effect of intestinal epithelial autophagy on bacterial translocation in severe acute pancreatitis. Clin Res Hepatol Gastroenterol 2017; 41(6): 703–710. [DOI] [PubMed] [Google Scholar]
- 14.Russell SL, Gold MJ, Reynolds LA, et al. Perinatal antibiotic-induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol 2015; 135(1): 100–109. [DOI] [PubMed] [Google Scholar]
- 15.Hansen CH, Krych L, Nielsen DS, et al. Early life treatment with vancomycin propagates Akkermansia muciniphila and reduces diabetes incidence in the NOD mouse. Diabetologia 2012; 55(8): 2285–2294. [DOI] [PubMed] [Google Scholar]
- 16.Jeffres MN. The whole price of vancomycin: Toxicities, troughs, and time. Drugs 2017; 77(11): 1143–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin M, Hathorn JW, Marshall D, et al. Gram-positive infections and the use of vancomycin in 550 episodes of fever and neutropenia. Ann Intern Med 1988; 108(1): 30–35. [DOI] [PubMed] [Google Scholar]
- 18.Hubbard BK, Walsh CT. Vancomycin assembly: Nature's way. Angew Chem Int Ed Engl 2003; 42(7): 730–765. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Peng J, Tai N, et al. Maternal antibiotic treatment protects offspring from diabetes development in nonobese diabetic mice by generation of tolerogenic APCs. J Immunol 2015; 195(9): 4176–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011; 141(2): 599–609, e1–3. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, He Y, Wang F, et al. Low-methoxyl lemon pectin attenuates inflammatory responses and improves intestinal barrier integrity in caerulein-induced experimental acute pancreatitis. Mol Nutr Food Res 2017; 61(4): 1600885. [DOI] [PubMed] [Google Scholar]
- 22.Ahuja M, Schwartz DM, Tandon M, et al. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab 2017; 25(3): 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa FR, Francozo MC, de Oliveira GG, et al. Gut microbiota translocation to the pancreatic lymph nodes triggers NOD2 activation and contributes to T1D onset. J Exp Med 2016; 213(7): 1223–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Fouts DE, Starkel P, et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe 2016; 19(2): 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wan X, Bi J, Gao X, et al. Partial enteral nutrition preserves elements of gut barrier function, including innate immunity, intestinal alkaline phosphatase (IAP) level, and intestinal microbiota in mice. Nutrients 2015; 7(8): 6294–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Tian J, Tang X, et al. Exosomes released by granulocytic myeloid-derived suppressor cells attenuate DSS-induced colitis in mice. Oncotarget 2016; 7(13): 15356–15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Lang M, Zhao T, et al. Cancer-FOXP3 directly activated CCL5 to recruit FOXP3 + Treg cells in pancreatic ductal adenocarcinoma. Oncogene 2017; 36(21): 3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Q, Adams JY, Penaranda C, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity 2008; 28(5): 687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun J, Furio L, Mecheri R, et al. Pancreatic beta-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 2015; 43(2): 304–317. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt J, Lewandrowsi K, Warshaw AL, et al. Morphometric characteristics and homogeneity of a new model of acute pancreatitis in the rat. Int J Pancreatol 1992; 12(1): 41–51. [DOI] [PubMed] [Google Scholar]
- 31.He Y, Wu C, Li J, et al. Corrigendum: Inulin-type fructans modulates pancreatic-gut innate immune responses and gut barrier integrity during experimental acute pancreatitis in a chain length-dependent manner. Front Immunol 2018; 9: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao L, Lin X, Tan S, et al. beta-Arrestin1 alleviates acute pancreatitis via repression of NF-kappaBp65 activation. J Gastroenterol Hepatol 2019; 34(1): 284–292. [DOI] [PubMed] [Google Scholar]
- 33.Miao B, Qi WJ, Zhang SW, et al. miR-148a suppresses autophagy by down-regulation of IL-6/STAT3 signaling in cerulein-induced acute pancreatitis. Pancreatology 2019; 19(4): 557–565. [DOI] [PubMed] [Google Scholar]
- 34.Seo JY, Pandey RP, Lee J, et al. Quercetin 3-O-xyloside ameliorates acute pancreatitis in vitro via the reduction of ER stress and enhancement of apoptosis. Phytomedicine 2019; 55: 40–49. [DOI] [PubMed] [Google Scholar]
- 35.Xie H, Yang M, Zhang B, et al. Protective role of TNIP2 in myocardial injury induced by acute pancreatitis and its mechanism. Med Sci Monit 2017; 23: 5650–5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng J, Wu J, Chen J, et al. Therapeutic effects of quercetin on early inflammation in hypertriglyceridemia-related acute pancreatitis and its mechanism. Pancreatology 2016; 16(2): 200–210. [DOI] [PubMed] [Google Scholar]
- 37.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology 2011; 141(1): 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie Q, Fei M, Fa Z, et al. Methane-rich saline alleviates cerulein-induced acute pancreatitis by inhibiting inflammatory response, oxidative stress and pancreatic apoptosis in mice. Int Immunopharmacol 2017; 51: 17–24. [DOI] [PubMed] [Google Scholar]
- 39.Huang HL, Tang GD, Liang ZH, et al. Role of Wnt/beta-catenin pathway agonist SKL2001 in caerulein-induced acute pancreatitis. Can J Physiol Pharmacol 2019; 97(1): 15–22. [DOI] [PubMed] [Google Scholar]
- 40.Terao K, Wake H, Adachi N, et al. Histidine-rich glycoprotein suppresses hyperinflammatory responses of lung in a severe acute pancreatitis mouse model. Pancreas 2018; 47(9): 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh VK, Wu BU, Bollen TL, et al. Early systemic inflammatory response syndrome is associated with severe acute pancreatitis. Clin Gastroenterol Hepatol 2009; 7(11): 1247–1251. [DOI] [PubMed] [Google Scholar]
- 42.Signoretti M, Roggiolani R, Stornello C, et al. Gut microbiota and pancreatic diseases. Minerva Gastroenterol Dietol 2017; 63(4): 399–410. [DOI] [PubMed] [Google Scholar]
- 43.He Y, Wu C, Li J, et al. Inulin-type fructans modulates pancreatic-gut innate immune responses and gut barrier integrity during experimental acute pancreatitis in a chain length-dependent manner. Front Immunol 2017; 8: 1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groschwitz KR, Hogan SP. Intestinal barrier function: Molecular regulation and disease pathogenesis. J Allerg Clin Immunol 2009; 124(1): 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ayabe T, Satchell DP, Pesendorfer P, et al. Activation of Paneth cell alpha-defensins in mouse small intestine. J Biol Chem 2002; 277(7): 5219–5228. [DOI] [PubMed] [Google Scholar]
- 46.de Jong PR, Gonzalez-Navajas JM, Jansen NJ. The digestive tract as the origin of systemic inflammation. Crit Care 2016; 20(1): 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, Pan Y, Gao L, et al. Dexmedetomidine attenuates pancreatic injury and inflammatory response in mice with pancreatitis by possible reduction of NLRP3 activation and up-regulation of NET expression. Biochem Biophys Res Commun 2018; 495(4): 2439–2447. [DOI] [PubMed] [Google Scholar]
- 48.Sendler M, Weiss F-U, Golchert J, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology 2018; 154(3): 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turley SJ, Lee JW, Dutton-Swain N, et al. Endocrine self and gut non-self intersect in the pancreatic lymph nodes. Proc Natl Acad Sci U S A 2005; 102(49): 17729–17733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leal-Lopes C, Velloso FJ, Campopiano JC, et al. Roles of commensal microbiota in pancreas homeostasis and pancreatic pathologies. J Diabetes Res 2015; 2015: 284680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bi J, Song S, Fang L, et al. Porcine reproductive and respiratory syndrome virus induces IL-1beta production depending on TLR4/MyD88 pathway and NLRP3 inflammasome in primary porcine alveolar macrophages. Mediat Inflamm 2014. ;2014: 403515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo W, Hu S, Elgehama A, et al. Fumigaclavine C ameliorates dextran sulfate sodium-induced murine experimental colitis via NLRP3 inflammasome inhibition. J Pharmacol Scie 2015; 129(2): 101–106. [DOI] [PubMed] [Google Scholar]
- 53.Otani K, Watanabe T, Shimada S, et al. Colchicine prevents NSAID-induced small intestinal injury by inhibiting activation of the NLRP3 inflammasome. Sci Rep 2016; 6: 32587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Flint RS, Windsor JA. The role of the intestine in the pathophysiology and management of severe acute pancreatitis. HPB 2003; 5(2): 69–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen X, Valente JF, Alexander JW. The effect of sennosides on bacterial translocation and survival in a model of acute hemorrhagic pancreatitis. Pancreas 1999; 18(1): 39–46. [DOI] [PubMed] [Google Scholar]
- 56.Mourad MM, Evans R, Kalidindi V, et al. Prophylactic antibiotics in acute pancreatitis: Endless debate. Ann R Coll Surg Engl 2017; 99(2): 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arlt A, Erhart W, Schafmayer C, et al. Antibiosis of necrotizing pancreatitis. Viszeralmedizin 2014; 30(5): 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Brunschot S, van Santvoort H, Besselink M, et al. Infected necrotising pancreatitis: Antibiotic administration remains the first step - Authors' reply. Lancet 2018; 391(10139): 2502. [DOI] [PubMed] [Google Scholar]
- 59.Powell JJ, Miles R, Siriwardena AK. Antibiotic prophylaxis in the initial management of severe acute pancreatitis. Br J Surg 1998; 85(5): 582–587. [DOI] [PubMed] [Google Scholar]
- 60.Soares FS, Amaral FC, Silva NLC, et al. Antibiotic-induced pathobiont dissemination accelerates mortality in severe experimental pancreatitis. Front Immunol 2017; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cornick S, Tawiah A, Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers 2015; 3(1-2): e982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaji I, Karaki S, Kuwahara A. Taste sensing in the colon. Curr Pharm Des 2014; 20(16): 2766–2774. [DOI] [PubMed] [Google Scholar]
- 63.Burger-van Paassen N, Vincent A, Puiman PJ, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem J 2009; 420(2): 211–219. [DOI] [PubMed] [Google Scholar]
- 64.Hatayama H, Iwashita J, Kuwajima A, Abe T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem Biophys Res Commun 2007; 356(3): 599–603. [DOI] [PubMed] [Google Scholar]
- 65.Finnie IA, Dwarakanath AD, Taylor BA, et al. Colonic mucin synthesis is increased by sodium butyrate. Gut 1995; 36(1): 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shimotoyodome A, Meguro S, Hase T, et al. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp Biochem Physiol A Mol Integr Physiol 2000; 125(4): 525–531. [DOI] [PubMed] [Google Scholar]
- 67.Grewal HP, Kotb M, el Din AM, et al. Induction of tumor necrosis factor in severe acute pancreatitis and its subsequent reduction after hepatic passage. Surgery 1994; 115(2): 213–221. [PubMed] [Google Scholar]
- 68.Pooran N, Indaram A, Singh P, et al. Cytokines (IL-6, IL-8, TNF): Early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol 2003; 37(3): 263–266. [DOI] [PubMed] [Google Scholar]
- 69.Alsfasser G, Antoniu B, Thayer SP, et al. Degradation and inactivation of plasma tumor necrosis factor-alpha by pancreatic proteases in experimental acute pancreatitis. Pancreatology 2005; 5(1): 37–43; discussion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun J, Bhatia M. Blockade of neurokinin-1 receptor attenuates CC and CXC chemokine production in experimental acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol 2007; 292(1): G143–G153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, INI881502 Supplemental Material for Combinatory antibiotic treatment protects against experimental acute pancreatitis by suppressing gut bacterial translocation to pancreas and inhibiting NLRP3 inflammasome pathway by Lingling Jia, Hao Chen, Jun Yang, Xin Fang, Wenying Niu, Ming Zhang, Jiahong Li, Xiaohua Pan, Zhengnan Ren, Jia Sun and Li-Long Pan in Innate Immunity