Abstract
Background
Severe glenohumeral arthritis in the young/active patient remains challenging. Historically, glenohumeral arthrodesis was recommended with limited return of function. Total shoulder arthroplasty has shown increasing survivorship at 15 years; however it is still not ideal for young patients. Biologic resurfacing of the glenoid with humeral head replacement has shown promising results.
Methods
The PubMed and Embase databases were queried for studies evaluating outcomes of glenoid biologic resurfacing with autograft or allograft. Two independent reviewers performed a systematic review according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines.
Results
Eleven studies (268 shoulders, 264 patients) were included. Minimum follow-up was 24 months in all but one study; patient age ranged from 14 to 75 years. Glenoid grafts used included 44.3% lateral meniscus allografts, 25.4% human acellular dermal matrix, 14.2% Achilles tendon allografts, 11.6% shoulder joint capsules, and 4.5% fascia lata autografts. Studies reported significantly improved American Shoulder and Elbow Surgeons, Visual Analog Scale, and Simple Shoulder Test scores postoperatively; 43.3% were failures (Neer’s evaluation of unsatisfactory or requiring revision). Infection occurred in 12/235.
Conclusions
Biologic resurfacing of the glenoid with a metallic humeral component can provide a significant improvement in pain, motion, and standardized outcomes scores in the well-indicated situation. Appropriate counseling is required with an appreciated complication rate of over 36% and a revision rate of 34%.
Keywords: glenohumeral arthritis, soft tissue resurfacing, allograft resurfacing, total shoulder arthroplasty, biologic resurfacing, shoulder resurfacing arthroplasty
Introduction
Glenohumeral arthritis in young, active patients remains a challenge. Treatment options are limited in this cohort, and many young, active patients with glenohumeral arthritis have been traditionally treated with glenohumeral arthrodesis. However, the morbidity of a fusion procedure is considerable.1,2 Total shoulder arthroplasty (TSA) has also been utilized as a first-line option, but the high risk of revision is correlated with glenoid component loosening and polyethylene wear; the 10-year survival of TSA in young patients has been demonstrated to be as low as 62.5%.3–9 Finally, as the results of glenoid revision in TSA have been poor, alternative therapeutic options are required.1,2,10,11 One solution to address the shortcomings of TSA was hemiarthroplasty (HA). However, humeral head implant erosion of glenoid cartilage and bone is a common occurrence and frequent cause of loss of joint space and poor outcomes.8,9,12–18 In addition, pain improvement and function are of concern, as several studies have found HA to be inferior to TSA in several parameters.4,6,12,13,15,19–22
As another option for the treatment of glenohumeral arthritis in the young patient, biologic resurfacing of the glenoid has been utilized. During biologic resurfacing, the proximal humerus undergoes an HA and the glenoid is resurfaced with a soft covering. The concept behind this dates back to 1860, when Verneriel positioned muscle and fascia into the temporomandibular joint. This topic was reviewed in 2011 by Namdari et al., looking at seven different studies and a total of 180 patients treated with biologic glenoid surface replacement.23 This excellent study showed an improvement in American Shoulder and Elbow Surgeons (ASES) scores to close to 74.1, significantly improved range of motion (ROM), and an overall complication rate of 13.3% and a reoperation rate of 26.6%. Considerable research has resulted since the publication of those findings. As additional difference, our current review of 11 studies and more than 250 patients will focus only on biologic glenoid resurfacing performed with humeral head replacement; in the prior review by Namdari et al., only 5 of these studies were available. The purpose of this systematic review is to evaluate the functional and radiographic outcomes, complications, and failures of biologic glenoid surfacing and humeral head replacement.
Methods
A systematic review was performed in June 2017 using Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines. PubMed and Embase were queried for studies subsequent to 1985 using the following terms: “shoulder,” “glenoid,” and “glenohumeral” in combination with “biologic,” “resurfacing,” “interposition,” “autograft,” or “allograft.” After removing duplicates, the abstracts were reviewed for eligible studies. Inclusion criteria were studies in English examining humeral head replacement in combination with glenoid resurfacing without a glenoid arthroplasty component for the treatment of glenohumeral arthritis. Exclusion criteria included non-English studies, non-arthroplasty studies, arthroscopic studies, studies lacking a biologic implant, biomechanical studies, review articles, technique papers, cadaveric studies, animal studies, case reports, and osteochondral or bone grafts studies. After relevant studies had been identified, the references section of each paper was reviewed for additional relevant studies that may not have been identified by the initial search. Each paper was then carefully reviewed. To be included, the paper had to include clinical outcomes for patients who had undergone a metallic humeral component and any type of non-prosthetic soft covering to the glenoid (allograft meniscus, anterior capsule, allograft Achilles tendon, etc). From each of the final papers chosen, the following data were extracted: publication year, sample size, minimum follow-up, population variables, preoperative diagnosis, graft type, postoperative standardized outcomes measures, postoperative ROM, infection rate, postoperative radiographic characteristics, and revision rate.
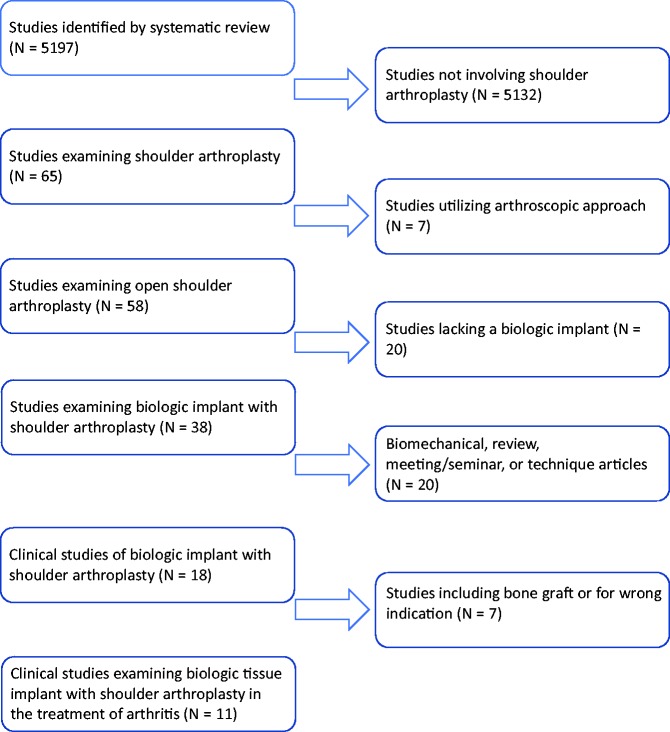
The systematic review yielded 5197 relevant publications. The progression is illustrated in Figure 1. After screening study titles for the exclusion and inclusion criteria, 65 studies were identified. Selecting for studies utilizing an open approach resulted in 58 remaining studies. Another 20 studies were eliminated due to a lack of utilization of a biologic implant. After selecting out biomechanical studies, review articles, meeting/seminar abstracts, and technique articles, 18 remained. An additional seven were excluded for the use of bone graft or incorrect indication. Following abstract review, 11 studies were deemed appropriate to be included in this review (Table 1).24–34
Figure 1.
Diagram demonstrating progression of systematic review.
Table 1.
Study population and methods of analysis.
| References | N = number of shoulders evaluated | Graft type (%) | Minimum Follow-up (months) | Outcome measures | Mean age (years) |
|---|---|---|---|---|---|
| Johnson et al.27 | 16 | 16 LMA (100) | 24 | VAS pain, ROM | NR |
| Krishnan et al.28 | 36 | 7 ASJC (19.4), 11 AFL (30.6), 18 ATA (50) | 24 | Neer, ASES, VAS pain, ROM | 51 |
| Elhassan et al.25 | 13 | 11 ATA (84.6), 1 ASJC (7.7), 1 AFL (7.7) | 24 | VAS pain, subjective shoulder value, Constant score | 34 |
| Lee et al.29 | 17 | 17 ASJC (100) | 24 | VAS pain, Constant, ASES, ROM | 54.8 |
| Hammond et al.26 | 20 | 8 HADM (40), 12 LMA (60) | 24 | ROM, SANE, SST, ASES, VAS pain | 37.7 |
| Lee et al.30 | 19 | 19 LMA (100) | 24 | SST, DASH, VAS pain | 57 |
| Muh et al.32 | 16 | 7 HADM (43.8), 9 ATA (56.2) | 24 | ASES, VAS pain | 36.1 |
| Straus et al.34 | 41 | 41 LMA (100) | 8 | ASES, SST, VAS pain | 42.2 |
| Bois et al.24 | 26 | 26 LMA (100) | 24 | ASES, SST, VAS pain | 46 |
| Puskas et al.33 | 17 | 6 HADM (35.3), 6 ASJC (35.3), 5 LMA (29.54) | 9 | Constant | 47 |
| Lo et al.31 | 47 | 47 HADM (100) | 24 | WOOS, ASES, VAS pain, SANE | 50 |
| Total | 268 | 12 AFL (4.5), 38 ATA (14.2), 31 ASJC (11.5), 119 LMA (44.4), 68 HADM (25.4) | N/A | N/A | N/A |
AFL: autogenous fascia lata; ATA: Achilles tendon allograft; ASJC: anterior shoulder joint capsule; LMA: lateral meniscus allograft; HADM: human acellular dermal matrix; NR: not reported; N/A: not applicable; ASES: American Shoulder and Elbow Surgeon; VAS: Visual Analog Scale; SST: Simple Shoulder Test; ROM: range of motion; SANE: Single Assessment Numerical Evaluation score; DASH: Disabilities of the Arm, Shoulder and Hand score; WOOS: Western Ontario Osteoarthritis of the Shoulder index.
Results
The 11 studies included 268 shoulders from 264 patients. Minimum follow-up was 24 months in all but one study in which 9 months was reported as the minimum follow-up. Mean follow-up ranged from 17 to 99 months. Patient age ranged from 14 to 75 years, reported by 10 studies. Eight studies reported the sex of the patient, resulting in 136 (76.0%) males and 43 (24.0%) females. Seven studies reported that 67.6% of shoulders operated on were on the side of the patient’s dominant upper extremity. Specific demographics of each study can be found in Table 2. Preoperative diagnoses included primary glenohumeral osteoarthritis, posttraumatic arthritis, postreconstruction osteoarthritis, and avascular necrosis. Exclusion criteria utilized by most studies were major glenoid osseous deficiency, active infection, rotator cuff tear, and a history of prior glenoid implants.
Table 2.
Study demographics.
| References | Average age (range) | M/F | Dominant/non-D | Preoperative diagnosis |
|---|---|---|---|---|
| Johnson et al.27 | NR | NR | NR | Glenohumeral arthritis (16) |
| Krishnan et al.28 | 51 (30–75) | 30/4 | 33/3 | Primary osteoarthritis (18), postreconstruction arthritis (12), and posttraumatic arthritis(5), osteonecrosis (1) |
| Elhassan et al.25 | 34 (18–49) | 9/4 | 10/3 | Primary osteoarthritis (5), posttraumatic arthritis (4), and postreconstruction arthritis (4) |
| Lee et al.29 | 54.8 (36–68) | 15/6 | 8/12 | Primary osteoarthritis (19), postinstability arthritis (2), and rheumatoid arthritis (1) |
| Hammond et al.26 | 37.7 (19–53.7) | 12/8 | 10/10 | Postreconstructive arthritis (10), glenohumeral osteoarthritis (6), and vascular necrosis (1) |
| Lee et al.30 | 57 (30–73) | 14/3 | NR | Primary osteoarthritis (14), postreconstruction arthritis (4), and posttraumatic arthritis (1) |
| Muh et al.32 | 36.1 (14–45) | 12/4 | NR | Glenohumeral arthritis (11), postreconstructive arthritis (3), chondrolysis (1), and instability arthropathy (1) |
| Strauss et al.34 | 42.2 (18.1–60.2) | 30/11 | 24/17 | Primary glenohumeral osteoarthritis (29), posttraumatic arthritis (7), capsulorarrhaphy arthropathy (7), chondrolysis (1), and avascular necrosis (1) |
| Bois et al.24 | 46 (27–55) | NR | NR | Glenohumeral arthritis (26) |
| Puskas et al.33 | 47 (34–57) | 14/3 | 13/4 | Dislocation arthropathy (13), primary osteoarthritis (3), and posttraumatic avascular necrosis (1) |
| Lo et al.31 | 50 (23–65) | NR | 30/17 | Primary osteoarthritis (38), postreconstruction osteoarthritis (9), dislocation osteoarthritis (6), and septic arthritis (2) |
NR: not reported.
All 264 patients underwent humeral head replacement in addition to biologic glenoid surface replacement. Various humeral head replacement techniques were encountered including stemmed vs. non-stemmed, cemented vs. cementless, and resurfacing vs. arthroplasty. Five different types of biologic glenoid resurfacing techniques were utilized in the analyzed studies: 119 (44.3%) lateral meniscus allografts, 68 (25.4%) human acellular dermal matrix, 38 (14.2%) Achilles tendon allografts, 31 (11.6%) shoulder joint capsules, and 12 (4.5%) fascia lata autografts (Table 1).
The standardized outcome tools utilized varied between studies. The most commonly encountered included ASES score, Visual Analog Scale (VAS), the Constant score, and the Simple Shoulder Test (SST). Most studies also documented forward elevation, external rotation, and internal rotation. Forward elevation was reported both preoperatively and postoperatively by six studies, and this showed a statistical improvement in five of the six studies.24,26,28,31,33,34 Mean preoperative values ranged from 70° to 119°, and mean postoperative values ranged from 83° to 140°. External rotation was reported both preoperatively and postoperatively by five studies, and this illustrated a statistical improvement in all five studies.24,28,31,33,34 Internal rotation was reported both preoperatively and postoperatively by two studies, showing a statistical improvement in both.24,28
Postoperative pain measures were reported by three studies. Of studies using pain outcome measures of “excellent, satisfactory, and unsatisfactory,” 22/36 reported excellent and 9/36 reported “satisfactory.”28 Elhassan et al. found 11/13 of patients to be in persistent severe pain postoperatively.25
Six studies documented on both preoperative and postoperative ASES values, with five reporting significant improvements and one reporting significant worsening. An additional two studies either commented on preoperative or postoperative values but not both. Five studies commented on both preoperative and postoperative VAS pain scale values, with all reporting significant improvements. Three studies commented on both preoperative and postoperative SST values, with all three reporting significant improvements. Two studies commented on both preoperative and postoperative Constant scores, with one reporting significant improvements and one reporting insignificant improvement. The available clinical outcomes are collected in Table 3.
Table 3.
Available outcomes scores.
| References | ASES | Constant | VAS Pain | SST |
|---|---|---|---|---|
| Johnson et al.27 | ||||
| Krishnan et al.28 | Pre-op: 39 Post-op: 91 | Pre-op: 7.7 Post-op: 2.1 | ||
| Elhassan et al.25 | Pre-op: 24 Post-op: 43 | Pre-op: 8 Post-op: 6 | ||
| Lee et al.29 | Pre-op: ukn Post-op: 74.4 | Pre-op: unk Post-op: 71.4 | ||
| Hammond et al.26 | Pre-op: unk Post-op: 59.5 | Pre-op: unk Post-op: 1.8 | Pre-op: unk Post-op: 6.9 | |
| Lee et al.30 | Pre-op: unk Post-op: 3.5 | Pre-op: unk Post-op: 8 | ||
| Muh et al.32 | Pre-op: 23.2 Post-op: 57.7 | Pre-op: 8.0 Post-op: 5.8 | ||
| Straus et al.34 | Pre-op: 36.8 Post-op: 62 | Pre-op: 6.3 Post-op: 3.0 | Pre-op: 4.0 Post-op: 7.0 | |
| Bois et al.24 | Pre-op: 31.6 Post-op: 59.6 | Pre-op: 2.8 Post-op: 6.3 | ||
| Puskas et al.33 | Graftjacket: 32→29 Meniscus allo: 40→51 Capsule: 43→58 | |||
| Lo et al.31 | Pre-op: 22 Post-op: 76 | Pre-op: unk Post-op: 2.4 |
ASES: American shoulder and elbow surgeon; VAS: visual analog scale; SST: simple shoulder test.
Notable radiologic features commented on in the literature include glenohumeral joint space (5/11 studies), glenoid erosion (6/11 studies), and humeral head subluxation (3/11 studies). Two studies compared preoperative glenohumeral joint space to immediate postoperative values, all showing improvement with a range of 0.94 mm to 2.9 mm.28,30 Two studies compared preoperative glenohumeral joint space to most recent follow-up values, with improvements ranging from 0.45 to 1.55 mm.28,34 Two studies compared immediate postoperative glenohumeral joint space to most recent follow-up values, all showing reduction ranging from 1.6 to 2.2 mm.24,28 One studies reported “no joint space narrowing” at most recent follow-up.31
Various methods of tracking glenoid erosion were utilized in 6 of the 11 studies. Two studies reported zero glenoid erosion at most recent follow-up in 46 patients.24,25 Krishnan et al. reported average glenoid erosion of 7.2 mm at final follow-up in 36 shoulders,28 while Puskas et al. reported a value of 1.9 mm at 13 months.33 Lee et al. classified glenoid erosion as none in 1/17 subjects, mild in 6/17, moderate in 6/17, and severe in 3/17.29 A lack of posterior subluxation at most recent follow-up was reported by Bois et al. for a total of 26 patients.24 Lee et al., in 2009, having radiographic data on 16 of 17 patients, found two subjects to have mild superior subluxation and two others to have moderate superior subluxation, but no incidence of posterior subluxation.29
Failure rates, revision rates, and total complication rates were provided by all 11 included studies and infection rates by 9 of 11 studies (Table 4). Global complication rate was found to be 36.2% (97/268 shoulders) and included infection, instability, persistent pain and stiffness (some resolving with medical treatment, many requiring further surgery), brachial plexitis, synovitis, and upper extremity deep vein thrombosis. Nine studies commented on infection in 235 shoulders, finding an infection rate of 5.1% (12/235). Failure was separately defined as a procedure that resulted in either a Neer’s evaluation of unsatisfactory outcomes and/or required a subsequent revision procedure. Of the 268 shoulders evaluated in our 11 studies, 116 (43.3%) were classified as a failure by these criteria. Revisions were reported by all 11 studies, with 91 of 268 (34.0%) shoulders requiring revision surgeries. Common indications for revision included persistent pain and loss of function, infection, instability, and graft displacement (Table 5). Revision procedures included diagnostic arthroscopy with lysis of adhesion, graft removal and glenoid reaming, debridement and placement of antibiotic spacers in infections, and conversion to revision HA or TSA. Overall, the rate of conversion to total anatomic or reverse shoulder arthroplasty was 22.7% (61/268). In those studies that reported it, the time to conversion to arthroplasty ranged from 15 months to greater than 9 years but predominantly within 2.5 years from the index resurfacing.
Table 4.
Complications and revisions.
| References | Infections (%) | Revisions (%) | Complications (%) | Failures (%)a |
|---|---|---|---|---|
| Johnson et al.27 | NR | 12 (75) | 12 (75) | 12 (75) |
| Krishnan et al.28 | 3 (15.7) | 5 (13.9) | 7 (19.4) | 5 (13.9) |
| Elhassan et al.25 | 2 (15.4) | 12 (92.3) | 12 (92.3) | 12 (92.3) |
| Lee et al.29 | 1 (5.9) | 5 (29.4) | 5 (29.4) | 5 (29.4) |
| Hammond et al.26 | 1 (5) | 6 (30) | 6 (30) | 12 (60) |
| Lee et al.30 | 0 (0) | 6 (31.6) | 6 (31.6) | 6 (31.5) |
| Muh et al.32 | 0 (0) | 7 (43.8) | 7 (43.8) | 7 (44) |
| Straus et al.34 | 1 (2.4) | 11 (26.8) | 9 (22) | 21 (51.2) |
| Bois et al.24 | 3 (11.5) | 9 (34.6) | 9 (34.6) | 11 (44) |
| Puskas et al.33 | NR | 12 (70.6) | 13 (76.5) | 13 (76.5) |
| Lo et al.31 | 1 (2.1) | 6 (12.8) | 11 (23.4) | 12 (25.5) |
| Total | 12/235 (5.1) | 91/268 (34.0) | 97/268 (36.2) | 116/268 (43.3) |
Note: NR: not reported.
aFailure defined as a procedure that resulted in either a Neer’s evaluation of unsatisfactory and/or required a subsequent revision procedure.
Table 5.
Reoperations.
| References | Post-op complications | Revision surgeries |
|---|---|---|
| Johnson et al.27 | Not detailed | 8 convert to TSA (before 2 years), 1 convert to hemiarthroplasty, 2 capsular release, and 1 rotator cuff repair |
| Krishnan et al.28 | 3 instability after anterior capsule reconstruction, 2 infections, 1 brachial plexitis, and 1 upper extremity DVT | 3 conversion to TSA (before 5 years), 1 infection I&D, and 1 biceps tenodesis |
| Elhassan et al.25 | 6 persistent pain, 4 persistent pain and stiffness, and 2 infections | 10 convert to TSA (16 months), 1 I&D, and 1 resection arthropalsty |
| Lee et al.29 | 1 subacromial impingement, 1 brachial neuritis, 2 persistent loss of motion, and 1 infection | 1 convert to TSA (15 months), 1 subacromial decompression, 2 MUA, and 1 I&D and graft removal for infection |
| Hammond et al.26 | 5 persistent pain and loss of motion and 1 infection | 4 convert to TSA (28.5 months), 1 capsular release, and 1 graft removal |
| Lee et al.30 | 6 with progressive pain and loss of motion | 3 convert to TSA (25 months), 1 to revision hemiarthroplasty, 1 synovectomy, and 1 LOA |
| Muh et al.32 | 6 persistent pain and stiffness and 1 MVA w/ pain after | 7 convert to TSA (36 months) |
| Strauss et al.34 | 5 low ASES score, 2 persistent loss of motion, and 1 deep infection | 7 conversion to TSA, 1 conversion to RSA (∼3 years), 2 arthroscopic capsular release, and 1 infection I&D |
| Bois et al.24 | 3 infections and 6 with persistent loss of motion | 3 convert to TSA (9.3 years), 2 arthroscopic debridement, 1 revision meniscal allograft resurfacing, 1 open I&D for infection, 1 resection arthroplasty, and 1 antibiotic spacer as definitive treatment |
| Puskas et al.33 | 13 persistent pain and loss of motion | Graftjacket: 4 convert to TSA and 1 to RSA (16 months) Meniscal allograft: 1 convert to TSA, 1 arthrodesis, and 1 debridement (22 months) Capsule interposition: 4 convert to TSA (34 months) |
| Lo et al.31 | 9 persistent pain and loss of motion, 1 infection, and 1 intraoperative fracture | 5 convert to TSA |
ASES: American shoulder and elbow surgeon; TSA: total shoulder arthroplasty; RSA: reverse total shoulder arthroplasty; LOA: lysis of adhesions; MUA: manipulation under anesthesia; I&D: irrigation and debridement; DVT: deep vein thrombosis; MVA: motor vehicle accident.
Discussion
Glenohumeral arthritis in the young, active adult represents a difficult clinical situation for the treating surgeon. With data demonstrating poor results from HA and early failure from TSA, soft tissue resurfacing procedures can offer an intermediate option that allows the arthritic glenoid to be addressed but removes the risk of glenoid component loosening or failure.3–9,12–18 This systematic review was able to identify 11 studies with 268 soft tissue resurfacing procedures. A mix of grafts were chosen with lateral meniscus, human acellular dermal matrix, and Achilles tendon allografts being the most common. All the procedures were combined with a metallic replacement of the humeral head. Overall, the data demonstrated improved outcomes for patient undergoing a soft tissue resurfacing.
To assess clinical improvement, several studies used similar standardized clinical outcome tools. The ASES score, Constant score, SST, and VAS score were commonly utilized for examining progression. The results of this review demonstrated a trend for significant improvement for all standardized outcome scores for all the types of procedures performed. A similar trend was appreciated for pre- and postoperative ROM. Forward elevation, external rotation, and internal rotation were noted to significantly improve in follow-up. The results are encouraging, with overall trends illustrating improved function, improved pain, and improved ROM. The results can be used to support the continued use of this procedure as a viable treatment option for young patients with glenohumeral arthritis.
Additional outcomes also supported the continued use of soft tissue resurfacing. Two of the 11 studies were able to demonstrate that soft tissue resurfacing was able to initially improve the postoperative joint space,28,30 and three of the studies followed the postoperative joint space chronically.24,28,31 In one study that followed the postoperative joint space chronically, the results were also encouraging, demonstrating stabilization of joint space narrowing at final follow-up.31 A similar encouraging pattern was demonstrated for glenoid erosion and subluxation. Six of the 11 studies reported on glenoid erosion, with all showing zero erosion at final follow-up, suggesting that soft tissue resurfacing is a reliable way to maintain glenoid bone stock while still addressing the degenerative surface.24,26,28,29,33,34 To further strengthen the data, two studies demonstrated subluxation to be an uncommon postoperative event.24,29 Thus, soft tissue resurfacing has demonstrated the ability to prevent volumetric glenoid bone loss while not having an association with an increased risk of subluxation.
Despite the trend towards improvement in pain and function, these clinical improvements did not come without risk. Overall, a complication rate of 36.2% was appreciated. This rate occurred with a 5.1% rate of infection and a 43.3% rate of clinical failure. The risk of failure was often correlated with a need for revision surgery, and many of these reported revisions were a conversion to a TSA. Thus, despite the substantial clinical improvement, patients must be appropriately counseled in regard to the risk of postoperative complications and failure. In particular, the rate of infection requires further investigation to understand what the driving cause is.
There are several limitations to this review. First of all, the data were obtained from non-randomized trials, but to date, no high level prospective randomized trials have been published. Each of the studies reviewed had a relatively low sample size, but as this is a less common clinical presentation, this seems reflective of clinical practice. A heterogeneous mix of outcomes were reported. Despite the variance in clinical and standardized outcome tools chosen, the vast majority utilized common, well accepted, and validated shoulder specific measures. As there is no data suggesting that one or more of these measures are superior for this treatment, we believe conclusions can still be drawn from the comparisons. A variety of techniques and graft choices were also used in the literature. Although a difference in the type of soft tissue covering is a confounding variable, we believe including a comparison of all is needed as one has not been shown to be definitively clinically superior. Finally, some of the papers included did include patients who were elderly. As the data were not presented for each patient in the papers, an accurate subgroup analysis of younger patients was not possible.
Overall, glenohumeral arthritis in the young, active individual remains a challenging presentation. This review, with two-year minimum follow-up, demonstrates that a soft tissue resurfacing procedure used in conjunction with a metallic humeral component can serve as an intermediate alternative to commonly used procedures of arthrodesis, TSA, and HA to provide significant improvement in pain, standardized outcome scores, and motion while preserving the glenoid. This does come with significant risk, as the overall complication rate was over 33%, the revision to TSA/arthrodesis rate was nearly 25%, and the overall infection rate was over 5%. Thus, the procedure may be used in well indicated situations with caution and appropriate counseling to the patient, taking into account patient specific factors such as age, desire to remain active and for how long, risk factors for infection, glenoid bone stock, etc.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent
Not required for this study.
References
- 1.Neyton L, Walch G, Nove-Josserand L, et al. Glenoid corticocancellous bone grafting after glenoid component removal in the treatment of glenoid loosening. J Shoulder Elbow Surg 2006; 15: 173–179. [DOI] [PubMed] [Google Scholar]
- 2.Richards R. Shoulder arthrodesis. In: Ninth combined meeting of Orthopaedic Associations of the English-Speaking World, Toronto, Canada, 21–26 June 1992.
- 3.Bohsali KI, Wirth MA, Rockwood CA., Jr Complications of total shoulder arthroplasty. J Bone Joint Surg Am 2006; 88: 2279–2292. [DOI] [PubMed] [Google Scholar]
- 4.Colonna PC. Arthroplastic operation for congenital dislocation of the hip. A two-stage procedure. Surg Gynecol Obstel 1936; 63: 777–777. [Google Scholar]
- 5.Denard PJ, Ladermann A, Burkhart SS. Long-term outcome after arthroscopic repair of type II SLAP lesions: results according to age and workers’ compensation status. Arthroscopy 2012; 28: 451–457. [DOI] [PubMed] [Google Scholar]
- 6.Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg Br 1987; 69: 45–55. [DOI] [PubMed] [Google Scholar]
- 7.Kalandiak S, Wirth MA and Rockwood CA Jr. Complications of shoulder arthroplasty. In: Williams GR (ed). Shoulder and elbow arthroplasty. Philadelphia, PA: Lippincott Williams & Wilkins, 2005, pp. 229–249.
- 8.Sperling JW, Cofield RH, Rowland CM. Minimum fifteen-year follow-up of Neer hemiarthroplasty and total shoulder arthroplasty in patients fifty years or younger. J Shoulder Elbow Surg 2004; 13: 604–613. [DOI] [PubMed] [Google Scholar]
- 9.Sperling JW, Cofield RH, Rowland CM. Neer hemiarthroplasty and Neer total shoulder arthroplasty in patients fifty years old or less: long term results. J Bone Joint Surg Am 1998; 80: 464–473. [DOI] [PubMed] [Google Scholar]
- 10.Cofield RH. Hemiarthroplasty of the shoulder. In: Fifth international conference on surgery of the shoulder, Paris, France, 12–15 July 1992.
- 11.Ernstbrunner L, Werthel JD, Wagner E, et al. Glenoid bone grafting in primary reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017; 26: 1441–1447. [DOI] [PubMed] [Google Scholar]
- 12.Bryant D, Litchfield R, Sandow M, et al. A comparison of pain, strength, range of motion, and functional outcome after hemiarthroplasty and total shoulder arthroplasty in patients with osteoarthritis of the shoulder. J Bone Joint Surg Am 2005; 87: 1947–1956. [DOI] [PubMed] [Google Scholar]
- 13.Gartsman GM, Roddey TS, Hammerman SM. Shoulder arthroplasty with or without resurfacing of the glenoid in patients who have osteoarthritis. J Bone Joint Surg Am 2000; 82: 26–34. [DOI] [PubMed] [Google Scholar]
- 14.Levine WN, Djurasovic M, Glasson JM, et al. Hemiarthroplasty for glenohumeral osteoarthritis: results correlated to degree of glenoid wear. J Shoulder Elbow Surg 1997; 6: 449–454. [DOI] [PubMed] [Google Scholar]
- 15.Lo IK, Litchfield RB, Griffin S, et al. Quality-of-life outcome following hemiarthroplasty or total shoulder arthroplasty in patients with osteoarthritis. A prospective, randomized trial. J Bone Joint Surg Am 2005; 87: 2178–2185. [DOI] [PubMed] [Google Scholar]
- 16.Parsons IM, Millett PJ, Warner JJP. Glenoid wear after shoulder hemiarthroplasty: quantitative radiographic analysis. Clin Orthop Relat Res 2004; 421: 120–125. [DOI] [PubMed] [Google Scholar]
- 17.Rispoli DM, Sperling JW, Athwal GS, et al. Humeral head replacement for the treatment of osteoarthritis. J Bone Joint Surg Am 2006; 88: 2637–2644. [DOI] [PubMed] [Google Scholar]
- 18.Sperling JW, Antuna SA, Sanchez-Sotelo J, et al. Shoulder arthroplasty for arthritis after instability surgery. J Bone Joint Surg Am 2002; 84-A: 1775–1781. [DOI] [PubMed] [Google Scholar]
- 19.Averill RM, Sledge CB, Thomas WH. Neer total shoulder arthroplasty. In: Bennett JC (ed). Arthritis and rheumatism, Philadelphia, PA: Lippincott-Raven Publisher, 1980, pp. 650–650. [Google Scholar]
- 20.Cofield RH, Daly PJ. Total shoulder arthroplasty with a tissue-ingrowth glenoid component. J Shoulder Elbow Surg 1992; 1: 77–85. [DOI] [PubMed] [Google Scholar]
- 21.Neer CS. Follow-up notes on articles previously published in the journal: articular replacement for the humeral head. J Bone Joint Surg Am 1964; 46: 1607–1610. [PubMed] [Google Scholar]
- 22.Pfahler M, Jena F, Neyton L, et al. Hemiarthroplasty versus total shoulder prosthesis: results of cemented glenoid components. J Shoulder Elbow Surg 2006; 15: 154–163. [DOI] [PubMed] [Google Scholar]
- 23.Namdari S, Alosh H, Baldwin K, et al. Biological glenoid resurfacing for glenohumeral osteoarthritis: a systematic review. J Shoulder Elbow Surg 2011; 20: 1184–1190. [DOI] [PubMed] [Google Scholar]
- 24.Bois AJ, Whitney IJ, Somerson JS, et al. Humeral head arthroplasty and meniscal allograft resurfacing of the glenoid: a concise follow-up of a previous report and survivorship analysis. J Bone Joint Surg Am 2015; 97: 1571–1577. [DOI] [PubMed] [Google Scholar]
- 25.Elhassan B, Ozbaydar M, Diller D, et al. Soft-tissue resurfacing of the glenoid in the treatment of glenohumeral arthritis in active patients less than fifty years old. J Bone Joint Surg Am 2009; 91: 419–424. [DOI] [PubMed] [Google Scholar]
- 26.Hammond LC, Lin EC, Harwood DP, et al. Clinical outcomes of hemiarthroplasty and biological resurfacing in patients aged younger than 50 years. J Shoulder Elbow Surg 2013; 22: 1345–1351. [DOI] [PubMed] [Google Scholar]
- 27.Johnson D, Humphrey S, Norris T. Glenoid resurfacing with use of a lateral meniscal allograft. Tech Orthop 2007; 22: 55–61. [Google Scholar]
- 28.Krishnan SG, Nowinski RJ, Harrison D, et al. Humeral hemiarthroplasty with biologic resurfacing of the glenoid for glenohumeral arthritis. Two to fifteen-year outcomes. J Bone Joint Surg Am 2007; 89: 727–734. [DOI] [PubMed] [Google Scholar]
- 29.Lee KT, Bell S, Salmon J. Cementless surface replacement arthroplasty of the shoulder with biologic resurfacing of the glenoid. J Shoulder Elbow Surg 2009; 18: 915–919. [DOI] [PubMed] [Google Scholar]
- 30.Lee BK, Vaishnav S, Rick Hatch GF, 3rd, et al. Biologic resurfacing of the glenoid with menisal allograft: long-term results with minimum 2-year follow-up. J Shoulder Elbow Surg 2013; 22: 253–260. [DOI] [PubMed] [Google Scholar]
- 31.Lo EY, Flanagin BA, Burkhead WZ. Biologic resurfacing arthroplasty with acellular human dermal allograft and platelet-rich plasma (PRP) in young patients with glenohumeral arthritis-average of 60 months of at mid-term follow-up. J Shoulder Elbow Surg 2016; 25: e199–e207. [DOI] [PubMed] [Google Scholar]
- 32.Muh SJ, Streit JJ, Shishani Y, et al. Biologic resurfacing of the glenoid with humeral head resurfacing for glenohumeral arthritis in the young patient. J Shoulder Elbow Surg 2014; 23: e185–e190. [DOI] [PubMed] [Google Scholar]
- 33.Puskas GJ, Meyer DC, Lebschi JA, et al. Unacceptable failure of hemiarthroplasty combined with biological glenoid resurfacing in the treatment of glenohumeral arthritis in the young. J Shoulder Elbow Surg 2015; 24: 1900–1907. [DOI] [PubMed] [Google Scholar]
- 34.Strauss EJ, Verma NN, Salata MJ, et al. The high failure rate of biologic resurfacing of the glenoid in young patients with glenohumeral arthritis. J Shoulder Elbow Surg 2014; 23: 4409–4419. [DOI] [PubMed] [Google Scholar]