Abstract
Introduction
Shear wave elastography ultrasound is a relatively new technique that evaluates the tissue elasticity by applying an acoustic radiation force impulse. It is undetermined how reliable this modality is in assessing rotator cuff tendons. The aim of this study, therefore, was to evaluate the reliability of shear wave elastography ultrasound to assess the stiffness of normal and tendinopathic supraspinatus tendons.
Methods
An inter- and intra-rater reliability trial was carried out using shear wave elastography to assess the supraspinatus tendon at its distal insertion, by measuring shear wave velocity and elasticity. Twenty participants with a mean age of 37 (21–69) years old were evaluated. Ten subjects with normal supraspinatus tendon and 10 subjects with tendinopathic tendon were selected. The Virtual Touch Imaging Quantification program was used to generate the acoustic radiation force impulse and to obtain the elastography data. Three raters with different experience in conventional ultrasound were used for the inter-rater trial in normal tendons and the most experienced rater examined all subjects for the intra-rater reliability evaluation. Each rater obtained three readings in three different examinations per subject over a one-week period.
Results
The mean (±SEM) shear wave velocity for the normal supraspinatus tendon was 9.96 ± 0.02 m/s (=297 kPa), while in the tendinopathic supraspinatus tendon was 8.3 ± 0.2 m/s (=207 kPa) (p < 0.001). The intra-rater trial agreement was excellent, with an intraclass correlation coefficient = 0.96. In the inter-rater testing, the mean shear wave velocity in normal tendons was 9.90 ± 0.07 m/s (=294 kPa), with intraclass correlation coefficient = 0.45.
Conclusion
Shear wave elastography ultrasound was able to show that tendinopathic tendons were less stiff than normal tendons. It was a reliable imaging technique to assess the supraspinatus tendon, especially when used by a single experienced musculoskeletal sonographer.
Keywords: musculoskeletal ultrasound, shear wave elasotography, shoulder elastography, shoulder ultrasound, supraspinatus tendon
Background
Ultrasound of the shoulder is commonly used to assess the integrity of the supraspinatus tendon.1 Ultrasound elastography (EUS) was developed by Sarvazyan2,3 in 1998 to measure the degree of tissue distortion in response to an internal or external force. This imagining tool has been used to qualify the difference of tissue stiffness in the evaluation of benign and malignant changes in soft tissues such as the breast, liver, and thyroid lesions.4–6 There are few published studies in which EUS has been used for musculoskeletal applications.7,8 Most of these were focused on the thigh and gastrocnemius muscles,9 inflammatory diseases,10 the Achilles tendon,11–13 and soft tissue tumors.14
Several elastography ultrasound methods have been developed for stress application to tissue. The two main described techniques are strain elastography and shear wave elastography ultrasound (SWEUS). The first application evaluates the elasticity of the underlying tissue by measuring the degree of deformation while applying mechanical compression, either manually or by physiologic movements; but operator-dependence is potentially high. In contrast, shear wave elastography is a unique application based on acoustic radiation force impulse (ARFI),15 whereby, tissue is compressed using an acoustic push beam, that compares tissue stiffness to surrounding structures without relying on the operator compressing the tissue.16
Shear waves are generated after the application of the ARFI and propagate perpendicular to the push pulse. Shear waves travel through tissues and are rapidly attenuated, with an attenuation coefficient significantly higher than longitudinal ultrasound waves. Shear wave velocity (Vs) is dependent and has close correlation to tissue elasticity/stiffness.
In tissue, both longitudinal and shear strains are usually present when manual compression or radiation force is applied. Poisson’s ratio for a given tissue type provides a ratio of transverse strain to the normal longitudinal strain. Hooke’s law (F = kx) gives us a relationship between stress and strain. This is called Young’s modulus (modulus of elasticity) in the elastic portion of the stress/strain curve. The shear modulus G (modulus of rigidity) is calculated from a corresponding shear stress versus strain curve. In homogeneous tissue, there are two types of waves: pressure (longitudinal compression) and transverse (shear) waves. In elastic materials, the relationship between the velocity of a shear wave and shear modulus is = , where G is the tissue shear modulus and ρ is the solid tissue density. Therefore, tissues with a higher shear modulus, less compliant to shear forces, will have a higher vs than tissues with a lower shear modulus.
When assessing the stiffness of a structure using Vs, the virtual touch imaging quantification (VTIQ) of the ACUSON S2000™ ultrasound system (HELIX Evolution ultrasound system; Siemens Medical Solutions®, Mountain View, California. USA) creates a color-coded Vs image that can be both qualitatively and quantitatively assessed. Therefore, Vs can be measured and used to evaluate tissue stiffness/elasticity by calculating Young’s elastic modulus according to the formula: E ≈ 3 × Vs2, where E = Young’s modulus in kPa units and Vs in m/s.17
Velocity is slower in soft tissue and faster in stiff tissue. The color bar shown in the VTIQ system displays the minimum and maximum range of the shear wave velocity from blue (0.5 m/s) to red (10 m/s), respectively. A shear wave sampling box can be positioned within the supraspinatus tendon to record the depth (in cm), from the skin to the targeted region of interest, and the movement of tissue within the sampled area.
Displacement of tissue is higher in soft tissue (fat) and lower in stiff tissue (tendon). The property of a stiff structure identified using elastography would be of high Vs and low displacement compared to the surrounding tissue, whilst low Vs and high tissue displacement would be appreciated in soft structures. In this way, an increased Vs correlates closely with increasing tissue stiffness.
Currently, two studies, one in vivo-type study18 and another in vitro-type study,19 have been published that evaluates the reliability of the shear wave elastography in the quantitative measurement of the supraspinatus muscle elasticity. Both studies showed good reliability of this imaging technique; however, the in-vivo study assessed the supraspinatus tendon in its medial portion close to the muscle–tendon junction, which is not the common location for pathology. In addition, the reliability in the assessment of a specific targeted area within the supraspinatus tendon had not been reported in vivo-type studies.
The aim of this study was to evaluate the intra- and inter-rater reliability of SWEUS to assess the stiffness of the supraspinatus tendon in individuals with healthy tendons and with sonographic tendinopathic tendon changes.
Methods
Ten subjects with normal supraspinatus tendon and 10 subjects with tendinopathic tendon changes were identified by conventional B-mode gray scale ultrasound imaging. An inter- and intra-rater reliability trial was undertaken using shear wave elastography to assess the supraspinatus tendon at the level of the footprint of the greater tuberosity of the humerus.
The study was conducted in accordance with ethics approval from the local ethics committee of the Orthopedic Research Institute, St George Hospital, Sydney, NSW, Australia.
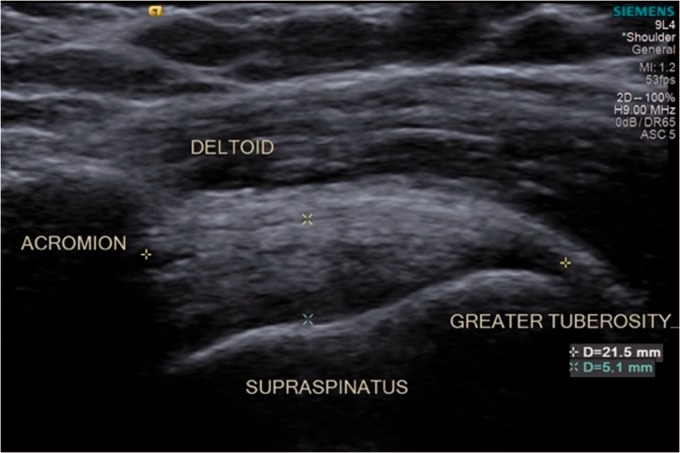
The inclusion criteria for the normal tendon group included subjects with conventional sonographic evidence of a normal tendon, showing continual bright echogenic lines inserting into the greater tuberosity footprint without disruption. The supraspinatus tendon resembles a “bird’s beak” configuration with the tendon fibers horizontal to the beam and probe orientation (Figure 1).
Figure 1.
Normal anatomy of the supraspinatus tendon visualized by conventional ultrasound.
The tendinopathic supraspinatus tendon group included subjects who had ultrasound signs of abnormal tendon where the tendon matrix was disorganized, and there was a decrease in echogenicity within the tendon complex, to no more than 50% of the tendon. The conventional ultrasound image of a tendinopathic tendon shows a circumscribed focal area of decreased echogenicity compared to the surrounding echogenic material with disorganization of fibers.
The exclusion criteria for all groups included: < 18 years old of age, gleno-humeral arthritis, calcific tendonitis, avulsed fracture, previous rotator cuff repair or supraspinatus tear evidenced by conventional ultrasound imagining.
Equipment
An ACUSON S2000™ ultrasound system with a Linear 9L4 transducer was used for all examinations. A B-mode gray scale image of the supraspinatus tendon was identified. The VTIQ application was activated for shear wave elastography to record velocity (Vs) in m/s, tissue elasticity in kPa, and the depth of the sampling box position in cm, within the supraspinatus at each examination.
Raters
Three raters were used for this study. Rater A (LH, a musculo-skeletal sonographer with over 20 years’ experience), rater B (KS, a research assistant with no experience in ultrasound imaging), and rater C (MS, a medical practitioner with limited ultrasound experience).
All examiners were briefed on the study protocol. A training session was conducted to ensure familiarization and standardization of examination, which included: patient positioning, imaging, application of SWEUS, and sample box placement within the supraspinatus tendon.
Inter-rater trial
For the inter-rater reliability evaluation, the subjects of the normal tendon group were examined by the three raters during three different examinations. The readings were recorded three times during each examination, which was conducted in a 30-min session, with a 5-min rest interval between each scan on all subjects.
The examiners were blinded to the results obtained by the other examiner performing the study to ensure reliability of probe position, patient position, activation of SWEUS, and sample box position.
Intra-rater trial
For the intra-rater reliability evaluation, Rater A examined all subjects obtaining three readings per examination. All subjects were examined and results were recorded in one session and repeated on day 3 and day 5. The data were recorded on the hard drive of the ultrasound machine; then transferred to a picture archiving and communication system.
Patient positioning
The subjects were positioned in seated position with the examiner in front of the patient and the ultrasound machine was to the left of the examiner.
The subject’s arm being examined was placed in a neutral position, with the elbow flexed at 90°, and the hand in supination resting on the patients’ lap. The arm was positioned in extension to 35° to visualize the supraspinatus tendon in the longitudinal view.
Scanning protocol
To ensure standardization of the ultrasound probe placement, specific bony landmarks were incorporated into the scanning protocol. The acromion (medial) and greater tuberosity footprint (lateral) of the shoulder were the reference points used to identify the supraspinatus tendon in the longitudinal view. The bicipital groove was the reference point in the antero-posterior plane.
The supraspinatus tendon of all subjects was identified and evaluated using B-mode gray scale conventional ultrasound.
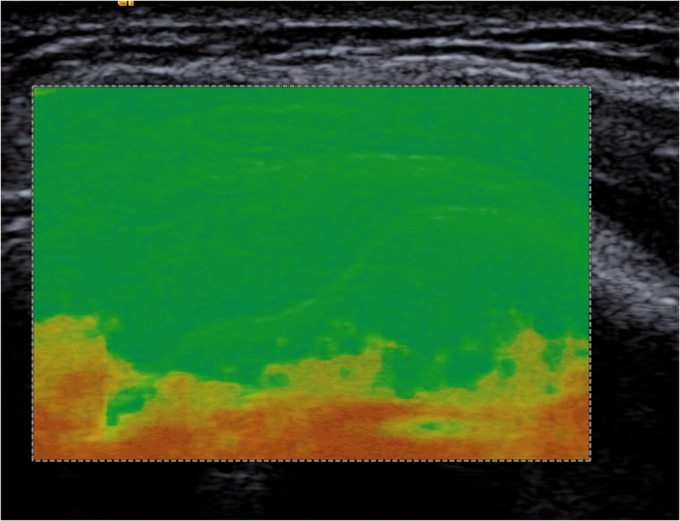
A “quality control” color map is applied to the gray scale B-mode image for interpreting whether the shear wave was of sufficient magnitude with adequate signal to noise (SN) to accurately estimate shear wave velocity (Figure 2).
Figure 2.
Quality color map (green) indicating optimal image quality to proceed with shear wave elastography.
The examiners were asked to position the ultrasound probe in the longitudinal axis of the supraspinatus tendon, 5 mm posterior to the posterior wall of the bicipital groove. The VTIQ application was activated and the color-coded shear wave image is applied to the gray scale image.
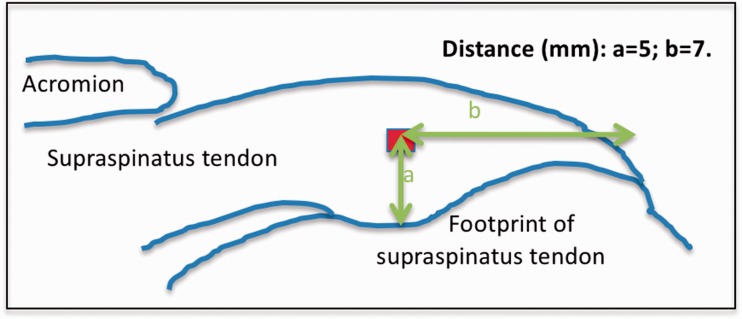
Then, the examiners placed a region of interest (ROI) sample box in the color-code image, which has a manufactures’ dimension of 0.7 × 0.7 mm. The raters were asked to place the sample box within the supraspinatus tendon at 5 mm superior to the footprint (targeting the mid-tendon thickness) of the greater tuberosity and 7 mm medial to the lateral end of the footprint (Figure 3).
Figure 3.
Illustration that represents the location of the sample box placement within the supraspinatus tendon.
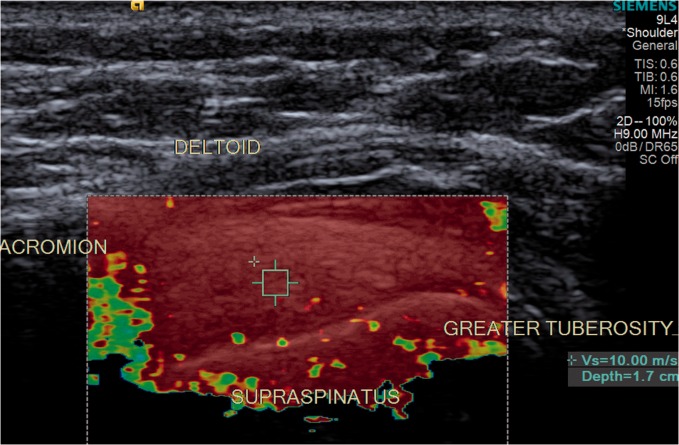
The sample box was then used to register the Vs and elasticity algorithm of the tendon, and to measure the depth of the sample box position within the tendon (Figure 4).
Figure 4.
Color-coded shear wave velocity image. Sample box positioning within the supraspinatus tendon.
Statistical analysis
To assess the reliability of sonoelastography of the supraspinatus tendon stiffness, two-way random effects intraclass correlation coefficients (ICCs), with 95% confidence intervals, were calculated using SPSS version 24 (SPSS, Inc., Chicago, IL, USA). In accordance with Fleiss, an ICC value < 0.4 was considered to represent poor reliability, with values between 0.4 and 0.75 representing fair to good reliability, whereas values > 0.75 were deemed to represent excellent reliability.
Results
The 20 selected subjects consisted of 14 females and 6 males. The mean age in the normal and tendinopathic groups was 25 (21–53) and 49 (27–69) years old, respectively.
Inter-rater testing
The mean depth of the ROI sampling box placement was 1.2 cm ± 0.09 cm (mean ± SEM). The inter-rater reliability for the depth of sample box positioning within the supraspinatus tendon was excellent (ICC = 0.95). All values recorded by all examiners in the normal tendon group showed a minimum shear wave velocity (Vs) of 9.33 m/s (261 kPa), with a mean of 9.9 ± 0.07 m/s (=294 kPa) (p < 0.001). The ICC values of the Vs/stiffness recorded by the different raters varied during the three different readings were: 0.31, 0.62, and 0.32 for readings 1, 2, and 3, respectively. The overall inter-rater ICC for elastography values was moderate (ICC = 0.45).
Intra-rater testing
The average depth of sample box placement within the supraspinatus tendon was 1.2 ± 0.08 cm (mean ± SEM) with excellent reliability (ICC = 0.94). The average shear wave velocity (Vs) recorded during the three scanning sessions in the normal tendon group was 9.96 ± 0.02 m/s (297 kPa) (p < 0.001). On the other hand, the tendinopathic tendon group resulted in an average shear wave velocity of 8.3 ± 0.2 m/s (207 kPa) (p < 0.001), with a minimum value recorded of 7.3 m/s (159 kPa) and a maximum of 9.39 m/s (264 kPa) between all tendinopathic tendons. The reliability of the intra-rater assessment was excellent (ICC = 0.96).
Discussion
This study showed lower shear wave velocity values; therefore, decreased tissue stiffness in tendinopathic supraspinatus tendons was compared to normal tendons. The reliability of this imaging tool was excellent when was used by a musculoskeletal trained sonographer, and fair to good when the technique is used by raters with different levels of experience in ultrasound imaging. The reliability of sampling box positioning was excellent in all groups.
Our data regarding elastography values of supraspinatus tendon were consistent with the data reported by Chen et al.13 in other tendons. They found that in the Achilles tendon shear wave elastography values ranged from 9.86 m/s (291.91 kPa) for the normal tendon to 4.3–4.8 ms (56.48 kPa–68.59 kPa) for the ruptured tendon.
The reliability and reproducibility of elastography have been investigated in some studies. Golatta et al.20 reported good intra- and inter-examiner reliability of shear wave elastography in breast lesions (r = 0.93). Rosskopf et al.21 reported excellent inter examiner reliability (ICC = 0.89) of shear wave elastography in the assessment of supraspinatus muscle and fatty infiltration; however, they did not report values regarding the supraspinatus tendon. Cortez et al.9 also examined its reliability in normal skeletal muscles and found good results (ICC: 0.73–0.96).
To our knowledge, our study is the first study to examine the supraspinatus tendon at its insertion. Baumer et al. from Detroit, USA, also reported good intra- and inter-user repeatability (ICC = 0.87 and 0.73, respectively) of shear wave elastography in the longitudinal plane of supraspinatus tendon from 10 human subjects, assessed by two users.22 However, this study evaluated the tendon at the musculo-tendinous junction, whereas in our study, the supraspinatus tendon was assessed at its distal insertion. In addition, the values of Vs/kPa reported were lower than our study, which may be explained by the fact that the stiffness property of muscle is lower compared to tendon. The consequence of lower tendon stiffness for patients would mean that they have a pathologic tendon. Like several musculoskeletal diseases, the abnormalities shown by complementary tests and imaging such as magnetic resonance imaging and conventional ultrasound do not always correlate with symptoms. Shear wave elastography ultrasound is a relatively new technology that provides objective data (numbers) of the tissue of the supraspinatus tendon, rather than only an image to be interpreted. Therefore, treatment is decided by taking into consideration all available data, including the clinician’s physical assessment and severity of symptoms.
The strengths of this study were the significant number of cases used for the reliability evaluation of shear wave elastography, the assessment of this technique in two different types of tendons (normal and tendinopathic), and it is the first study that assessed the supraspinatus tendon in its clinical relevant region of pathology using this imaging tool. The limitations included that the elastography application of the ACUSON S2000™ ultrasound machine had a maximum elastography values threshold of 10 m/s and 300 kPa, and we did not include the tendinopathic tendon group in the inter-rater reliability evaluation that could explain the resulted lower reliability in this testing.
This study was able to show that tendinopathic tendons were less stiff than normal tendons using SWEUS. This imaging technique was reliable to assess the supraspinatus tendon, especially when used by a single experienced musculoskeletal sonographer.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The paper has not been presented at any society or meeting.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Review and Patient Consent
All patients provided written consent for inclusion in this study.
References
- 1.Briggs L, Murrell GA. Diagnostic ultrasound examination of the shoulder. Tech Should Surg 2011; 12: 101–107. [Google Scholar]
- 2.Sarvazyan A. Mechanical imaging: a new technology for medical diagnostics. Int J Med Inform 1998; 49: 195–216. [DOI] [PubMed] [Google Scholar]
- 3.Sarvazyan AP, et al. Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics. Ultrasound Med Biol 1998; 24: 1419–1435. [DOI] [PubMed] [Google Scholar]
- 4.Ciledag N, et al. The utility of ultrasound elastography and micropure imaging in the differentiation of benign and malignant thyroid nodules. Am J Roentgenol 2012; 198: W244–W249. [DOI] [PubMed] [Google Scholar]
- 5.Bohte AE, et al. Non-invasive evaluation of liver fibrosis: a comparison of ultrasound based transient elastography and MR elastography in patients with viral hepatitis B and C. Eur Radiol 2014; 24: 638–648. [DOI] [PubMed] [Google Scholar]
- 6.Ghajarzadeh M, Sodagari F, Shakiba M. Diagnostic accuracy of sonoelastography in detecting malignant thyroid nodules: a systematic review and meta-analysis. Am J Roentgenol 2014; 202: W379–W389. [DOI] [PubMed] [Google Scholar]
- 7.Arda K, et al. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am J Roentgenol 2011; 197: 532–536. [DOI] [PubMed] [Google Scholar]
- 8.Chino K, Akagi R, Dohi M, et al. Reliability and validity of quantifying absolute muscle hardness using ultrasound elastography. PLoS One 2012; 7: e45764–e45764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortez CD, Hermitte L, Ramain A, et al. Ultrasound shear wave velocity in skeletal muscle: a reproducibility study. Diagn Interv Imaging 2016; 97: 71–79. [DOI] [PubMed] [Google Scholar]
- 10.Botar-Jid C, Damian L, Dudea SM, et al. The contribution of ultrasonography and sono-elastography in assessment of myositis. Med Ultrasound 2010; 12: 120–126. [PubMed] [Google Scholar]
- 11.De Zordo T, et al. Real-time sonoelastography: findings in patients with symptomatic Achilles tendons and comparison to healthy volunteers. Ultraschall Med 2010; 31: 394–400. [DOI] [PubMed] [Google Scholar]
- 12.Drakonaki EE, Allen GM, Wilson DJ. Real-time ultrasound elastography of the normal Achilles tendon: reproducibility and pattern description. Clin Radiol 2009; 64: 1196–1202. [DOI] [PubMed] [Google Scholar]
- 13.Chen XM, Cui LG, He P, et al. Shear wave elastographic characterisation of normal and torn Achilles tendons: a pilot study. J Ultrasound Med 2013; 32: 449–455. [DOI] [PubMed] [Google Scholar]
- 14.Magarelli N, Carducci C, Bucalo C. Sono-elastography for qualitative and quantitative evaluation of superficial soft tissue lesions: a feasibility study. Eur J Radiol 2014; 24: 566–573. [DOI] [PubMed] [Google Scholar]
- 15.Wojcinski S, et al. Acoustic radiation force impulse imaging with virtual touch tissue quantification: measurements of normal breast tissue and dependence on the degree of pre-compression. Ultrasound Med Biol 2013; 39: 2226–2232. [DOI] [PubMed] [Google Scholar]
- 16.Lupsor M, Badea R, Stefanescu H, et al. A performance of a new elastogrpahic method (ARFI) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. J Gastrointestin Liver Dis 2009; 18: 303–310. [PubMed] [Google Scholar]
- 17.Rosen J, Brown DJ, De S, et al. Biomechanical properties of abdominal organs in vivo and postmortem under compression loads. J Biomech Eng 2008; 130: 021020–021021. [DOI] [PubMed] [Google Scholar]
- 18.Rosskopf A, Ehrmann C, Buck F, et al. Quantitative shear-wave us elastography of the supraspinatus muscle: reliability of the method and relation to tendon integrity and muscle quality. Radiology 2016; 278: 465–475. [DOI] [PubMed] [Google Scholar]
- 19.Hatta T, Giambini H, Uehara K, et al. Quantitative assessment of rotator cuff muscle elasticity: reliability and feasibility of shear wave elastography. J Biomech Eng 2015; 48: 3853–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golatta M, Schweitzer-Martin M, Harcos A, et al. Evaluation of virtual touch tissue imaging quantification, a new shear wave velocity imaging method, for breast lesion assessment by ultrasound. Biomed Res Int. 2014; 2014:960262. doi: 10.1155/2014/960262. [DOI] [PMC free article] [PubMed]
- 21.Rosskopf AB, Ehrmann C, Buck F, et al. Quantitative shear-wave US elastography of the supraspinatus muscle: reliability of the method and relation to tendon integrity and muscle quality. J Biomech 2016; 48: 3853–3858. [DOI] [PubMed] [Google Scholar]
- 22.Baumer TG, Davis L, Dischler J, et al. Shear wave elastography of the supraspinatus muscle and tendon: repeatability and preliminary findings. J Biomech 2017; 53: 201–204. [DOI] [PubMed] [Google Scholar]