Highlights
-
•
Longitudinal data reveals continuity in the neural system supporting theory of mind as it develops from 4- to 7-year-old.
-
•
4-year ToM performance and source-localized baseline EEG predicts 7-year ToM-specific fMRI activity.
-
•
Longitudinal associations were specific to the dorsal medial prefrontal cortex.
-
•
Data suggest links between brain’s early underlying organization and its later functional recruitment.
Keywords: Theory of mind, EEG, fMRI, Longitudinal, Childhood, Neural correlates, Continuity
Abstract
Children’s explicit theory of mind (ToM) understandings change over early childhood. We examined whether there is longitudinal stability in the neurobiological bases of ToM across this time period. A previous study found that source-localized resting EEG alpha attributable to the dorsal medial prefrontal cortex (DMPFC) and right temporoparietal junction (RTPJ) was associated with children’s performance on a battery of theory of mind tasks. Here, we investigated a small subset of children (N = 12) in that original study as a preliminary investigation of whether behavioral measures of ToM performance, and/or EEG localized to the DMPFC or RTPJ predicted ToM-specific fMRI responses 3.5 years later. Results showed that preschoolers’ behavioral ToM-performance positively predicted later ToM-specific fMRI responses in the DMPFC. Preschoolers’ resting EEG attributable to the DMPFC also predicted later ToM-specific fMRI responses in the DMPFC. Given the small sample, results represent a first exploration and require replication. Intriguingly, they suggest that early maturation of the area of the DMPFC related to ToM reasoning is positively linked with its specific recruitment for ToM reasoning later in development, affording implications for characterizing conceptual ToM development, and its underlying neural supports.
1. Introduction
Children across the world come to explicitly reason about internal mental states as person-specific representations of the world that are constrained by experience (Wellman, 2014). Development of this “Theory of Mind” (ToM) is a dramatic example of developing higher cognition: Major conceptual advancements occur in ToM understandings between 3–6 years old (e.g., Wellman et al., 2001) and are connected with concomitant gains in both social and cognitive functioning (see Devine and Hughes, 2014; Milligan et al., 2007 for meta-analyses). These explicit developments set the stage for teaching (Davis-Unger and Carlson, 2008; Ziv and Frye, 2004), cooperation (Moll and Tomasello, 2007; Tomasello et al., 2005), and moral reasoning (e.g., Killen et al., 2011; Lane et al., 2010). The current study provides the first exploration of possible longitudinal continuity in the neurobiological bases of ToM as it develops over this important early period of change in children’s behavioral-cognitive performance.
Much is already known about the neural correlates of ToM. For adults, brain responses to ToM tasks are selective and reliable, consistently comprising medial prefrontal cortex (MPFC), temporoparietal junction (TPJ), precuneus, (PC), temporal lobes (TL), and inferior frontal gyri (IFG) (see Schurz et al., 2014 for meta-analysis). However, ToM is a developmental phenomenon, with clear improvements in conceptual understandings over early to middle childhood (Wellman et al., 2001). How the brain supports ToM across these critically early developments is still an open question. On one hand, the functional neural specializations for adult ToM may represent a consequence of development rather than a starting place (Karmiloff-Smith, 1997; Elbert et al., 2001). On the other hand, some aspects of the ToM neural system may exhibit early specialization and longitudinal stability such that individual differences in functional neural specialization present early in development predict individual differences in specialization of the same neural regions later on.
Continuity and change in the neural specializations for ToM have not yet been examined longitudinally, however, cross-sectional neuroscientific research provides initial insight. Evidence from functional magnetic resonance imaging (fMRI) demonstrates that brain regions implicated in adult ToM reasoning such as the TPJ, MPFC, and PC are also recruited in school-aged children (Saxe et al., 2009; Gweon et al., 2012; Kobayashi et al., 2007; Sommer et al., 2010). Further, the more that certain brain regions (e.g., RTPJ) are recruited for reasoning about mental-state content (and not other general social characteristics), the better children perform on behavioral ToM tasks, demonstrating increasing “selectivity” of these regions for mental-state processes (Saxe et al., 2009; Gweon et al., 2012).
Homologies in adults’ and school-aged children’s ToM neural network may not be all that surprising; school-aged children are past the point at which explicit ToM reasoning abilities first emerge and thus any neural discontinuity may more likely be evident in preschool children for whom explicit conceptual ToM developments are still unfolding. Neuroscience research on ToM in children younger than 6-years-old is still limited, though intriguingly there is some evidence that similarities in the brain regions supporting ToM extend from adults to 3 year-old children (Richardson et al., 2018) and potentially even infants as young as 7 months (Hyde et al., 2018).
Sabbagh et al. (2009) also provide evidence that the neural regions associated with mental-state reasoning in older children and adults similarly support explicit ToM understandings as they first emerge. Importantly, they examined brain activity and its relation to ToM in 4-year-old children—an age exhibiting crucial individual differences in children’s explicit understanding of others’ mental states as person-specific representations of the world, capturing an important window of development of ToM (see Wellman et al., 2001).Sabbagh et al. used resting/baseline source-localized electroencephalographic (EEG) alpha power to index 4-year-old children’s task-independent neural activity. Research has shown that during the preschool years, alpha gradually becomes the highest amplitude resting EEG rhythm over all regions of the scalp, and peaks at 6–9 Hz in children (Marshall et al., 2002). The regional increases in resting/baseline alpha power and coherence (e.g., Marshall et al., 2002; Thatcher, 1994; Thatcher et al., 1987) are thought to reflect more mature functional organization of the underlying neurocognitive systems (Thatcher, 1994; see Sabbagh et al., 2009 and supplemental material for more details on baseline EEG alpha in children). Sabbagh et al. found that individual differences in preschoolers’ resting/baseline EEG alpha source-localized to DMPFC and RTPJ were positively associated with performance on standard behavioral measures of ToM (e.g., Wellman and Liu, 2004; Wimmer and Perner, 1983; Flavell et al., 1986). These associations held after controlling for children’s executive functioning and vocabulary skills. The authors interpreted these findings as evidence that maturation of regions of the dorsal MPFC and right TPJ could support preschoolers’ ToM development. A recent study demonstrating that 3- and 4-year-old children’s maturation of local white matter structure in the MPFC, TPJ, and precuneus is associated with better theory of mind reasoning (Grosse Wiesmann et al., 2017) is in line with this view. Thus, cross sectional findings taken together show that – despite differences in behavioral ToM – preschoolers, school-aged children, and adults share common neural substrates for ToM reasoning, hinting at intriguing continuity in the ToM neural network.
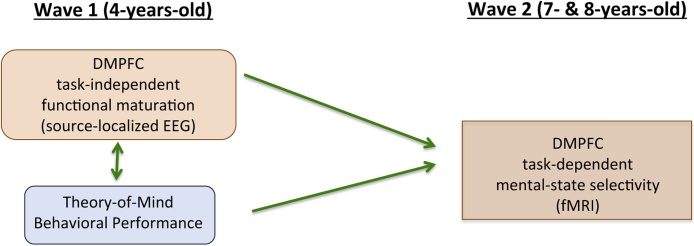
However, existing cross-sectional research still falls short of demonstrating continuity in the neural system supporting children’s developing ToM. Although findings that similar neural regions are implicated in ToM across age groups provides an important starting point, given the multidetermined and stochastic nature of development, longitudinal research is required to examine psychological and biological factors supporting developmental processes over time (see e.g., Bergman et al., 1989). Here, we aim to address this critical limitation by employing a longitudinal design that captures relations between preschoolers’ task-independent baseline EEG measures, and school-aged children’s fMRI responses during ToM tasks. Specifically, the present study represents a preliminary investigation of the relation between source-localized task-independent EEG and task-dependent fMRI in a longitudinal sample, as the first exploration of longitudinal continuity and change in the neural system supporting ToM over early to middle childhood. This approach has the added advantage of examining the relation between task-independent (baseline/resting) source-localized EEG and task-dependent fMRI, which is currently debated and unclear (e.g., Papo, 2013; Deco et al., 2011). More generally, links between source-localized EEG results and fMRI activity, which provide confidence in EEG source estimates, have implications for the broader field of developmental neuroscience given the EEG method is more child-friendly compared to MRI due to its quieter application and the ability to maintain contact between caregiver and child.
In the present study, a subsample of preschoolers from Sabbagh et al. (2009) was followed up to directly examine how the brain supports ToM across development, and to investigate the developmental relationship between indices of task-independent neural activity assessed with resting-state/baseline EEG alpha and later task-dependent functional selectivity assessed with fMRI. A small sample of children for whom we had measures of resting EEG alpha, behavioral ToM, executive functioning, and language performance at age 4 years (Sabbagh et al.) underwent fMRI while engaging in mental-state reasoning, 3.5 years later when children were 7- and 8-years-old. The fMRI task was identical to that used in Gweon et al. (2012; adapted from Saxe et al., 2009) and required children to process peoples’ mental states, general social (but non-mental) personal characteristics, and physical descriptions of scenes. This fMRI task was used to index the selectivity of brain regions for mental-state-specific reasoning beyond both non-mental and general social reasoning.
This study advances understandings of ToM and its development by exploring whether individual differences in selectivity for ToM reasoning in the dorsal MPFC and right TPJ measured at ages 7 and 8 are predicted by: (1) individual differences in ToM reasoning as assessed by standard behavioral performance measures at age 4, and (2) individual differences in task-independent neural activity in the dorsal MPFC and right TPJ indexed by source-localized resting EEG alpha at age 4. Positive associations would suggest that early maturational changes (assessed with task-independent measures) can predict later increased neurospecialization (assessed with task-dependent measures), providing preliminary evidence that early task-independent neural activity can predict later functional outcome. More broadly, positive associations would point to an underlying common factor supporting children’s ToM even from the time of preschoolers’ early conceptual changes. Demonstration of these principles in development of cognition, brain structure, and brain function have important implications for studying the development of higher cognition more generally, and set the stage for important future research into the neurodevelopmental basis of ToM.
2. Methods
2.1. Participants
Sixteen typically-developing 7- and 8-year-old children (5 males) were recruited to participate in the second wave of a longitudinal study. These children were a subset of 29 children who participated in wave 1, 3.5 years prior when they were 4-years-old (Sabbagh et al., 2009). All children were right handed, with normal or corrected-to-normal vision. Parents reported that children were born within ± 2 weeks of their due date, were developing typically, and had no history of neuropsychological disorder or trauma. The sample was predominantly middle class European-Canadian, reflecting the community from which they were recruited. At both waves, children gave assent and parents provided written informed consent. Parents received monetary compensation and children were given small toy prizes at the end of the session.
Four children were excluded from the wave 2 sample: 2 due to excessive motion artefact in their fMRI data, 1 refused to enter the MRI scanner, and 1 due to a technical issue. The final sample consisted of 12 children (4 males). At wave 1, children were 49 to 59 months old (M = 53.67, SD = 3.52). At wave 2, ages ranged from 91 to 106 months (M = 97.88 months, SD = 4.55). The time between wave 1 and wave 2 data collection was 35 to 47 months (M = 43.64 months, SD = 3.49). Across waves, children were tested by the same experimenter at the same institution.
2.2. Wave 1 (age 4 years): behavioral theory-of-mind and resting EEG
Wave 1 methods were originally published in Sabbagh et al. (2009). Brief descriptions can be found below; additional details are provided in the supplemental material.
2.2.1. Behavioral tasks
Behavioral tasks consisted of a ToM battery (tasks taken from Wellman and Liu, 2004; Gopnik and Astington, 1988; Wimmer and Perner, 1983; Flavell et al., 1986), an executive functioning battery (tasks taken from Carlson and Moses, 2001; Zelazo, 2006; Carlson et al., 2005), and a language measure (PPVT; Dunn and Dunn, 1997).
2.2.2. Behavioral variable for analysis
The wave 1 behavioral variable of interest was children’s individual ToM performance scores, residualized for executive-functioning performance, language performance, and age at time of test. Using the residuals helps assure that relations between ToM and brain measures represent the neural processes related specifically to ToM, and are not likely accounted for by these other domain general constructs.
2.2.3. EEG measures
EEG was recorded from the scalp using a 128-channel Geodesic Sensor Net (EGI, Eugene, OR), during passive, “resting” or “baseline” viewing intervals. Participants watched alternating video clips (30 s each; 6 min total) of a still picture of a rocket ship and an animation of a green line that mapped out a spiral. Only EEG recorded during the rocket ship segments was analyzed (adapted from Fox et al., 1995). All participants in the final sample contributed at least 25 artefact-free segments of EEG (i.e., 50 s of data). Cross-spectral matrices were created for the single-subject average-referenced data in the child alpha band (i.e., 6–9 Hz), and sLORETA software computed three-dimensional distributions of the standardized current density using standardized estimates of the minimum norm inverse solution (Pascual-Marqui, 2002). The sLORETA transformation results in current density values for 6239 “voxels’’ (5 mm3) located within cortical gray matter and hippocampus, as defined by the Probability Atlas from the Montreal Neurological Institute, yielding a final measure of the regional current-source density values in the alpha band at each voxel, for each child.
2.2.4. EEG variable for analysis
The final wave 1 EEG variable of interest consisted of children’s individual current density estimates, residualized for age at time of test as well as the number of useable (artefact-free) raw EEG segments contributed by each child. Using the residualized current density estimates helps ensure that relations between brain measures and other variables of interest represent relations with actual underlying EEG current density, and are not likely simply reflections of external relations with age or amount of data.
2.3. Wave 2 (age 7/8 years): fMRI
2.3.1. fMRI stimuli
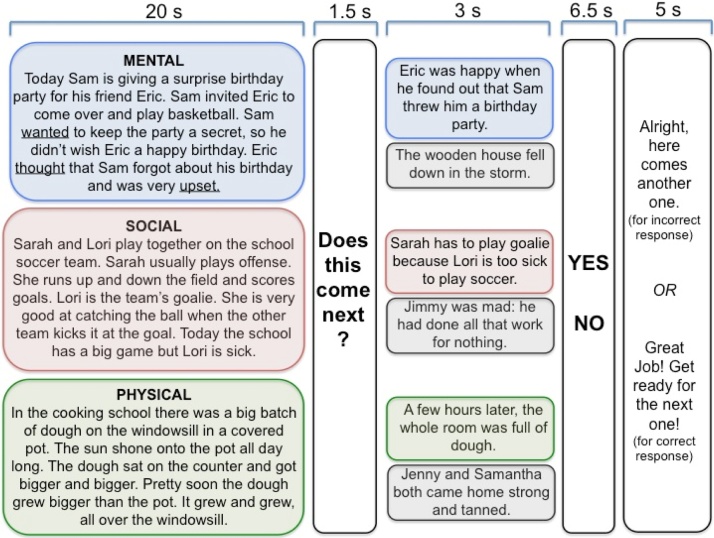
To measure task-dependent brain activation for ToM reasoning at wave 2, we used the same stimuli as those used in Gweon et al. (2012): acoustically delivered stories, read by one of three female speakers in child-directed prosody, designed to fit one of three conditions1 : 1) Mental stories which described characters’ thoughts, beliefs, desires, and emotions 2) Social stories which described characters’ social relationships and physical appearance, and 3) Physical stories which described physical states and objects in the world. Critically, though both Mental and Social stories contained social information (about peoples’ appearances, actions, and interactions), only Mental stories contained specific descriptions of peoples’ mental states (e.g., Sam wanted to keep the party a secret so he didn’t wish Eric a happy birthday. Eric thought that Sam forgot about his birthday and was very upset). Additionally, Physical stories contained neither mental-state nor social content. Stories were matched across conditions for number of words (M = 51.6 words), number of sentences (4.7), duration (20 s), and Flesch Reading Ease Level (M = 90.4). See Fig. 1 for a schematic of the task and an example story in each condition. See supplemental material for more details on stimuli presentation, counterbalancing, and analysis of behavioral performance.
Fig. 1.
Schematic of the task administered at wave 2 for functional MRI data collection (adapted from Gweon et al., 2012). The task consists of 5 components outlined in columns from left to right, with durations of each component labeled across the top. Examples of stories in mental (blue) social (red) and physical (green) conditions are shown in the boxes in the first column. The mental-state content unique to the mental condition is underlined. A given story is followed by the question “Does this come next?” (column 2), and then children either hear a sentence that continues from the previous story (in matching colors) or a sentence irrelevant to the previous story (in grey). Children judge whether the probe sentence is a match or non-match with the previous story (column 4) and then hear a post-response encouragement that changes depending on whether the participant’s response was correct or incorrect (column 5). Only data from the initial story component (column 1) was analyzed; the subsequent task components served to keep children engaged and focused on processing the story information.
2.3.2. fMRI data collection and analysis
Data were collected on a 3 T Siemens Tim Trio scanner using a 12-channel head coil. A T1-weighted MPRAGE was conducted to obtain an anatomical image (176 sagittal slices, slice thickness =1.0 mm, TE =2.2 ms, TR =1480 ms, flip angle = 9.0 degrees). Functional data were acquired in the axial plane with echo-planar images covering the whole brain at a resolution of 3.3 mm isotropic voxels (32 slices, TE =30 ms, TR =1970 ms, flip angle = 77 degrees). The first 4 volumes of each run were excluded from the analysis to ensure steady-state magnetization.
Analytic procedures were the same as those used in Gweon et al. (2012) and adapted from Saxe et al. (2009). In brief, data were analyzed using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8) and custom software written in Matlab.
Two focal regions of interest (ROIs) were defined based on results of wave 1 data: dorsal medial prefrontal cortex (DMPFC) and right temporoparietal junction (RTPJ). At wave 1, source localized (via sLORETA) EEG alpha in each of these regions positively related to children’s behavioral ToM performance; thus, examination of fMRI BOLD signal in each of these regions at age 7 and 8 addresses whether there is continuity or change in each of these regions’ relation to ToM across early childhood development. Previous research (Gweon et al., 2012; Saxe et al., 2009) has also implicated left temporoparietal junction (LTPJ), precuneus (PC), and middle, and ventral prefrontal cortex (MMPFC, VMPFC) in ToM reasoning in middle childhood; thus, these four additional ROIs were also created. Examination of fMRI BOLD signal in these regions is important to capture the larger picture of ToM neural correlates at ages 7 and 8, and allows exploration of the possibility that ToM neural regions in early childhood may relate to other regions in the ToM neural network across development.
Following Gweon et al. (2012) all six ROIs were defined using both anatomical location (MNI coordinates identified in previous literature) and functional activation: We used anatomical MNI coordinates (from Saxe and Kanwisher, 2003; Saxe et al., 2009; and Gweon et al., 2012) as spatial landmarks, and defined each ROI as the voxels in a 9 mm radius surrounding the anatomical coordinates that showed the diagnostic function of (a) clusters of at least 10 voxels (k >10), that were (b) significantly more active to the Mental condition versus the Physical condition (p < .0005, uncorrected). BOLD responses to the Mental, Social, and Physical stories were calculated in each of these 6 ROIs for each participant using percent signal change (PSC = 100*[BOLD response – Baseline]/Baseline). See supplemental material for details. The use of anatomical coordinates ensures we are investigating, at the broadest level, what has come to be identified as the “ToM” network based on much prior neuroimaging research with adults. Thus, these coordinates guide our broadest search space. The functional criteria allow for the possibility that children’s neuroanatomy may vary across individuals, and as a group could be different from the adult brains upon which the anatomical criterion were set. That is, children’s ToM neural network may not precisely match the network outlined in average adult anatomy, but may be functionally equivalent (i.e., show more activity for mental versus non-mental reasoning). Additionally, there is no purely anatomical definition that would allow precise characterization of each region in each individual brain. Further, areas within the anatomical regions of interest may exhibit selectivity for non-ToM cognitive processes. Using an anatomical ROI from a prior study leaves open the possibility that we would be interrogating different functional regions across participants. Defining ROIs individually ensures we are studying the same functional region across participants. The two ROI selection criterion taken together thus provide better assurance that the brain activity we examine in our sample can be appropriately interpreted as activity in a “ToM” neural network.
2.3.3. fMRI variable for analysis
The key wave 2 variable of interest was children’s individual mental-state selectivity scores, which were calculated as a proportion of their average PSC values in the Mental, Social, and Physical conditions (i.e., (PSCMental – PSCSocial) / (PSCMental - PSCPhysical), directly following Saxe et al. (2009) and Gweon et al.’s (2012) analytic procedures. A low selectivity score indicates that activation in an ROI for the Social stories was about as high as its activation to the Mental stories (i.e., Mental and Social activation are essentially equivalent and both differentiate from the Physical control). In contrast, a high selectivity score indicates that a given ROI’s response to the Social stories was about as low as the response to the Physical stories (i.e., Mental activation is unique and differentiates from both the Social and Physical control conditions, which themselves are essentially equivalent).
Note that use of selectivity scores importantly indexes the extent to which brain regions are selective for supporting mental-state reasoning beyond both non-mental reasoning and general social reasoning (measured as the relative differences of [mental-social]/[mental-physical]). Specifically, recall that the mental and social conditions both include general social descriptions of people. Thus, even though we selected ROIs based on ‘Mental > Physical’ activity, this is not a direct test of whether those ROIs will also exhibit ‘Mental > Social’ activity, because the mental condition includes just as much ‘social’ information as the social condition (though importantly only the mental condition includes additional information about internal mental states). ROIs that show greater activity to the mental condition relative to the social condition indicate that the ROIs were “selective” for reasoning about peoples’ internal mental states beyond social reasoning about people more generally.
Following Saxe et al., 2009 and Gweon et al., 2012, we quantified the extent to which brain regions associated with ToM reasoning are functionally selective for specifically mental state reasoning (beyond general social reasoning) using the calculation for mental state selectivity scores presented above. A high selectivity score indicates that mental activation is higher than both Physical and Social activations, suggesting specificity or specialization for mental-state reasoning. See Saxe et al. (2009); Gweon et al. (2012), and the supplemental material for more detailed descriptions of selectivity score calculations.
Selectivity scores were calculated for each child, in each of the 6 ROIs, allowing comparison of mental-state selectivity across individuals, and critically, an examination of the degree to which children’s mental-state selectivity varied as a function of (a) their performance on the battery of wave 1 behavioral ToM tasks, and (b) their wave 1 source localized (sLORETA) EEG alpha (current density).
3. Results
We first report preliminary analyses of the fMRI data alone, followed by report of focal analyses correlating wave 1 and wave 2 data. For analyses of EEG data alone, see Sabbagh et al. (2009). In line with established developmental trends over childhood (e.g., Wellman et al., 2001; Peterson et al., 2012), a subset of the wave 1 ToM behavioral battery administered at wave 2 confirmed that while only 43% of the sample passed the most complex false-belief location-change task (Wimmer and Perner, 1983) at wave 1, 100% of the sample passed the task at wave 2.
3.1. Preliminary fMRI analyses
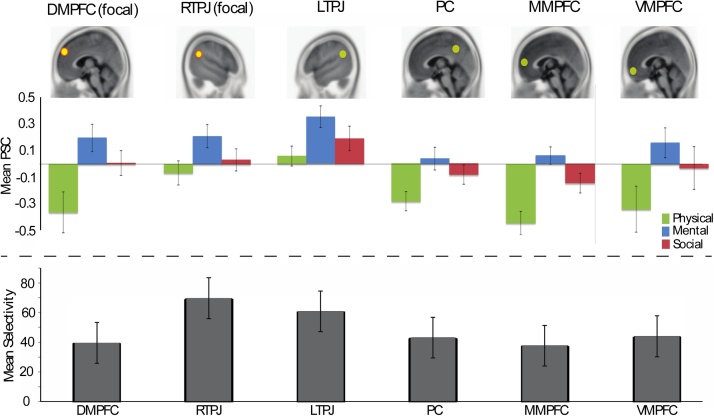
All 12 child participants showed fMRI activity that met criteria for ROI selection in the DMPFC, RTPJ, PC, and MMPFC. Eleven out of 12 children met criteria for LTPJ and VMPFC ROI selection. Thus, each region showed significantly greater activation to the Mental condition versus the Physical control condition, evincing a general response to social information across these ROIs (see top panel Fig. 2). These results replicate findings from Saxe et al. (2009) and Gweon et al. (2012), giving confidence that our sample is in line with extant findings.
Fig. 2.
Top panel shows percent signal change (from rest) averaged across participants, and averaged across the story blocks for the Physical (green), Mental (blue), and Social (red) conditions in the 6 regions of interest. All regions show significantly greater Mental versus Physical activation, as per the functional criteria for ROI selection. All regions also exhibit greater Mental versus Social activation, suggesting some degree of selectivity for mental-state reasoning across all ROIs (confirmed in bottom panel). Bottom panel shows average mental-state selectivity scores for each ROI (derived from the patterns of PSC depicted in the top panel). This panel shows similar magnitude and variation in selectivity scores across ROIs, with RTPJ exhibiting the highest average mental-state selectivity and DMPFC exhibiting the lowest (though scores did not statistically differ across ROIs).
The bottom panel of Fig. 2 depicts the average mental-state selectivity for each ROI, which was derived from the proportion of activity across all three conditions (recall that a higher selectivity score for a given ROI demonstrates that ROI is more selective for mental-state processing—beyond both physical- and social-processing). All ROIs demonstrated some degree of mental-state selectivity, also in line with previous research (Gweon et al., 2012). Visual inspection of normality plots for these fMRI selectivity scores across the six ROIs revealed varying and non-normal distributions. Thus, following a standard technique for non-parametric analyses, selectivity scores were converted to ranks (1–12) in each ROI, and Spearman’s correlation tests were used to compare wave 1 and wave 2 data.
3.2. Focal analyses: correlations between wave 1 and wave 2 data
Focal analyses tested for longitudinal relations. Specifically, we examined whether wave 2 outcome measures of ToM-specific fMRI responses in the DMPFC and the RTPJ were predicted by wave 1 measures of (a) behavioral ToM performance and (b) EEG alpha attributed to the DMPFC and RTPJ. Though not focal, we also examined correlations with mental-state selectivity in the four additional ROIs (PC, LTPJ, VMPFC, MMPFC).
3.2.1. Behavioral ToM (wave 1) and fMRI mental-state selectivity (wave 2)
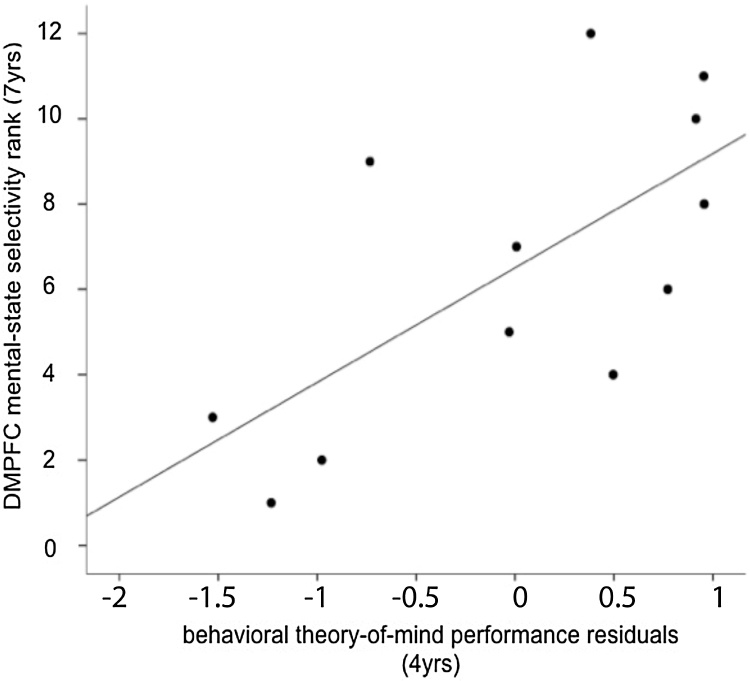
Behavioral ToM performance (controlled for age, executive functioning, and language performance) at 4 years (wave 1) showed a significant positive relation with later (wave 2) ToM-specific fMRI responses only in the DMPFC (rs = .66, p = .020); see Fig. 3. No other wave 2 ROI exhibited any significant relations with wave 1 behavioral ToM (ps < .152), including our second focal ROI, the RTPJ (rs = -.27, p = .50).
Fig. 3.
Correlations between wave 1 behavioral theory-of-mind performance (residualized for covarying effects of age, executive functioning and language performance) and wave 2 fMRI mental-state selectivity in the DMPFC. Greater DMFPC fMRI selectivity at age 7 and 8 years is associated with greater ToM performance at age 4 years.
3.2.2. EEG alpha (wave 1) and fMRI mental-state selectivity (wave 2)
Our most critical question was whether individual differences in the functional maturation of the ToM network measured at wave 1 (indexed by localized EEG alpha current density) predicted the functional specialization of that same network measured directly (with fMRI) at wave 2. Thus, we examined whether mental-state selectivity scores in the 6 ROIs at 7- and 8-years-old were predicted by EEG current density in homologous brain areas of interest (AOIs) at 4-years-old.
Following the analytic approach in Sabbagh et al. (2009), we conducted voxel-wise Spearman correlations between the EEG current density (one value per voxel within a pre-defined AOI) and children’s fMRI mental-state selectivity (one score per ROI). These AOI analyses provide the most sensitive approach to identifying continuity in neural regions over early to middle childhood because they investigate the extent to which functional maturation of a given brain area predicts neural specialization in that same area, three years later. These analyses limit the number of voxel-wise comparisons, and are similar to small volume corrections employed in fMRI analyses (Poldrack, 2007): specifically, we used the fMRI results to guide selection of a small volume of EEG voxels (constituting an AOI), and then performed voxel-wise analyses within this small volume. As described in Poldrack (2007), we controlled for multiple comparisons across voxels within an ROI, but did not control for multiple comparisons across 6 ROIs, which represented a-priori planned comparisons.
The grand average coordinates of the ROIs identified in the fMRI data (at wave 2) (fMRI ROIs) were used to define homologous group-level AOIs in the EEG data (at wave 1) (EEG AOIs). The EEG AOIs consisted of voxels in a 2 cm radius around the grand average coordinate (i.e., averaged across each individual child’s ROI) of the six fMRI ROIs (rounded to the nearest coordinate to match the spatial resolution in sLORETA) to create six brain area homologues: DMPFC and RTPJ (focal AOIs), and LTPJ, PC, MMPFC, and VMPFC (additional AOIs). The larger radius for the EEG AOIs versus the fMRI ROIs (i.e., 9 mm) was chosen to account for the lower resolution of the sLORETA data in order to achieve the most comparable brain regions across wave 1 and wave 2 data.
Six separate voxel-wise Spearman correlation analyses compared wave 1 EEG current density to wave 2 fMRI mental-state selectivity across each set of homologous ROIs. For example, DMPFC current density at wave 1 was correlated with DMPFC mental-state selectivity at wave 2, RTPJ current density at wave 1 was correlated with RTPJ mental-state selectivity at wave 2, and so on for all six ROIs. To determine significance criteria for each EEG AOI, permutation analyses were conducted. Specifically, two thousand random permutations of fMRI selectivity index ranks 1 through 12 were correlated with the current density estimates in each voxel within each EEG AOI. Permutation tests were also conducted using fMRI selectivity ranks 1 through 11 to determine cluster criterion for the VMPFC and LTPJ AOIs that only had 11 participants meet criteria for fMRI ROI selection. The cluster criterion associated with a family-wise alpha of .05 was unique for each of the six EEG AOIs (see Table 1).
Table 1.
Centroid Coordinates, Size (Number of Voxels), and Cluster Criterion for each of the 6 EEG AOIs.
| EEG ROI | Centroid MNI Coordinates | Size of ROI (# of voxels) | Cluster Criterion (# of voxels) |
|---|---|---|---|
| DMPFC | 5, 55, 30 | 109 | 12 |
| RTPJ | 55, -55, 25 | 115 | 16 |
| LTPJ | −50, -55, 30 | 125 | 15 |
| PC | −5, -50, 40 | 179 | 30 |
| MMPFC | 0, 55, 10 | 134 | 18 |
| VMPFC | 5, 55, -10 | 134 | 18 |
Note. Cluster criterion indicates the minimum number of contiguous voxels (each significant at p < .05) needed within a given ROI to reach a family-wise alpha of .05.
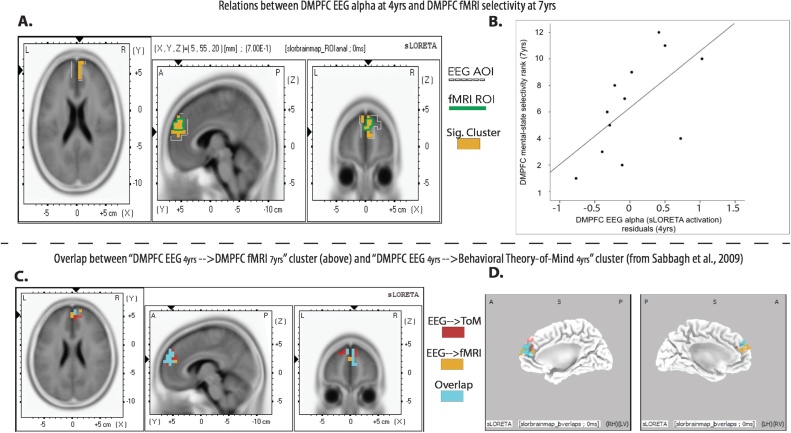
Under the established significance criteria, results showed that wave 1 EEG current density positively related to wave 2 fMRI mental-state selectivity in only one fMRI ROI: the DMPFC (rs = .59–.71, ps = .013–.048, bootstrapped CI [.091, .921], k = 25 contiguous voxels, peak voxel coordinates: 5, 55, 20; see Table S1 in supplemental material for complete list of r and p-values in the cluster); see Fig. 4a and b. All voxels in this significant cluster fell within the DMPFC EEG AOI, which by definition, consisted of the same DMPFC region that exhibited fMRI mental-state selectivity at 7-years. Given the small sample, we conducted cross-validation analyses to assess the robustness of this effect (see supplemental materials for details and Fig. S1). Results of these analyses provide confidence that our findings of links between dMPFC EEG current density and dMPFC fMRI selectivity are not an artifact of our particular sample, and not driven by outliers.
Fig. 4.
Top panel: Threshold statistical map (A) and scatterplot (B) of correlations between wave 2 DMPFC fMRI selectivity and wave 1 EEG current density. The significant cluster of EEG voxels corresponding to the DMPFC at 4yrs that positively related to DMPFC mental-state selectivity at 7 and 8yrs (at p < .05) is shown in orange in (A). The 2cm-radius for the DMPFC EEG AOI is outlined in dashed white. All voxels in the significant cluster fell within this DMPFC EEG AOI, and overlapped completely with the DMPFC fMRI ROI (outlined in solid green). Bottom panel: threshold statistical map slice view (C) and alternate cortex map view (D) demonstrating the overlap (blue) between the cluster of EEG voxels that related to DMPFC fMRI selectivity at 7 years (orange), and the cluster of EEG voxels that related to concurrent behavioral theory-of-mind (ToM) performance at 4 years (red). Images depict comprehensive overlap between the two clusters.
An additional Spearman correlation between DMPFC mental-state selectivity scores and aggregate EEG alpha current density averaged across all voxels in the AOI further confirmed the effect. Specifically, current density averaged across an AOI with a 1.3 cm radius (chosen to replicate the size of the cluster that showed positive correlations between current density and ToM behavioral performance in Sabbagh et al., 2009) surrounding the same MNI coordinates as before (5, 55, 30—the average coordinate of the DMPFC fMRI ROI) correlated positively with DMPFC fMRI mental-state selectivity scores: rs = .59, p = .045, bootstrapped CI [.075, .894].
To test whether this association between fMRI selectivity and functional maturation was specific to the DMPFC AOI, we then searched the whole brain2 for regions correlated with DMPFC fMRI selectivity. Though this analysis had the potential to reveal correlations between DMPFC fMRI mental-state selectivity indices and EEG alpha current density estimates in any part of the brain, only the cluster of voxels in the DMPFC (that matched the cluster from the prior DMPFC AOI analysis) was significant (k = 25, peak coordinate 5, 55, 20; Broadmann areas (BA) = 9/10; rs = .59–.71, ps = .013–.048).
When the significant DMPFC EEG cluster was compared to the original 4-year-old DMPFC EEG cluster that exhibited a positive correlation with ToM performance from Sabbagh et al. (2009), there was striking overlap: 19/25 voxels or 76% of the present study DMPFC cluster overlapped with the voxels included in the original DMPFC cluster (see Fig. 4c and d). Moreover, in just the subsample of children who contributed data at wave 2, we observed the previously reported association between wave 1 DMPFC EEG and wave 1 behavioral ToM performance residualized for executive functioning, vocabulary skills, and age observed by Sabbagh et al.: r = .598, p = .040. These analyses thus evince intriguing continuity in the association between DMPFC and ToM over early to middle childhood: 4-year-olds’ DMFPC resting EEG alpha (1) was associated with children’s concurrent behavioral ToM performance, and (2) longitudinally predicted selective recruitment of the DMPFC for mental-state processing as assessed 3.5 years later with fMRI (see Fig. 5).
Fig. 5.
Visual summary of correlations between wave 1 and wave 2 data for DMPFC.
No other region reached significance under our established family-wise error cluster criterion, including the RTPJ. RTPJ selectivity at wave 2 (along with selectivity in all other regions) was not significantly predicted by wave 1 EEG (only 2 non-contiguous, singular voxels yielded correlations of rs > .60, p < .05, and thus did not constitute a cluster).
4. Discussion
Over early to middle childhood, children’s ToM becomes more sophisticated, allowing it to be applied to a broader range of social circumstances (Wellman et al., 2001; Harris et al., 1989; Wellman and Liu, 2004; Peterson et al., 2012). The present study examined how the brain supports ToM across these developments, and provides preliminary evidence for longitudinal continuity in the neural system that supports ToM reasoning. Specifically, individual differences in older children’s ToM-specific DMPFC responses were predicted by (a) individual differences in children’s earlier behavioral ToM performance, and (b) individual differences in EEG alpha power attributable to the DMPFC (see Fig. 5). There was striking similarity in the region of the DMPFC that was associated with concurrent ToM at age 4 years and the region that showed ToM-specific fMRI responses 3.5 years later.
Our findings of longitudinal continuity are in line with prior cross-sectional research showing that the DMPFC is connected with ToM reasoning in preschoolers, older children, and adults. Extending these prior findings, our data demonstrate that individual differences in ToM-relevant DMPFC development at age 4 are associated with ToM-selective responses in the same brain region 3.5 years later. Of course, a number of other patterns of results could have been possible, each with different implications for understanding how the brain supports ToM development. As one example, 4-year-olds’ resting EEG could have failed to predict later ToM-specific fMRI activity in homologous brain regions, leaving 4-year-old ToM performance the sole predictor of fMRI selectivity at ages 7 and 8. This result would have raised questions about the extent to which task- independent measures of neural activity (source-localized resting EEG alpha) may be disconnected from task-dependent measures of functional neurospecialization (fMRI selectivity). Moreover, a lack of longitudinal continuity in regions implicated in ToM reasoning at both early and middle childhood (i.e., DMPFC) could have pointed to changes in how the brain supports ToM as children develop, possibly suggesting that the early neural foundations of ToM are distinct and discontinuous with later developments that ultimately promote more mature ToM performance. In contrast to these alternate possibilities, our findings provide preliminary evidence that the early developments in the region of DMPFC that is important for ToM in 4-year-olds are also associated with the extent of functional specialization of that same region for ToM reasoning 3.5 years later, suggesting early stability in the neural system for ToM reasoning, and longitudinal continuity in this neural system despite behavioral-cognitive advancements.
Two key aspects of our study design at both wave 1 and 2 provide confidence that the continuities in DMPFC relate to ToM reasoning, per se. First, we statistically controlled for skills that typically covary with ToM reasoning (e.g., language and executive functioning). Therefore, concurrent preschool relations between source localized resting EEG alpha and ToM performance are not likely attributable solely to the influence of these constructs. Second, the Mental, Social and Physical conditions – from which the mental-state selectivity index was created – all placed equal demands on executive functioning and language comprehension. Thus, it is unlikely that the present results reflect domain-general contributions to ToM reasoning that might be similar across ages; rather our data indicate domain-specific continuity in how the brain supports ToM.
It is important to note that the number of children who returned for fMRI testing at wave 2 was small; thus, the present results should be viewed as preliminary. While the positive results observed in the DMPFC were corrected to reduce type I error, more powerful designs would provide increased confidence in the results reported here and – given the higher number of girls in our sample – could explore potential moderating effects of sex (though a prior meta-analysis demonstrated a lack of sex effects; Wellman and Liu, 2004). More powerful designs may also reveal stability in other regions of the ToM neural network, and could explore additional questions of links between current density of early ToM neural networks and later activity across the whole brain. Importantly though, the possibility that other brain regions (e.g., those supporting domain-general processes) may also support ToM development across early to middle childhood would not undermine the positive results for stability associated with the DMPFC reported here. Additional caution should be taken in interpreting small sample results given possible increased danger of finding spurious correlations. Our cross-validation analyses (see supplemental material) provide important confidence in our findings, and the positive correlations between time 1 and time 2 data were found across both brain and behavioral measures. We therefore view our results as preliminary but promising, and importantly we demonstrate a novel method and approach that should be adopted for replication of our results in a larger more generalizable sample, and to address the possibility of stability and change in a broader network of brain regions across development.
4.1. Speculations on the significance of “neural stability”
The above limitations notwithstanding, these preliminary data offer opportunity for speculations that can shape interpretation of existing findings and theories, and open important avenues for future research.
4.1.1. Does stability in the brain index stability in early-developing cognition?
Our data offer preliminary evidence that the relation between DMPFC and ToM remains stable over early to middle childhood, despite behavioral evidence for advancements in conceptual ToM understanding recorded over the same time period; children tested on behavioral ToM measures at age 7 and 8 years were all at ceiling despite failing the same measures 3.5 years earlier. It is possible that if we had administered a more challenging behavioral assessment at wave 2, we would have found individual differences that may or may not have related to brain or behavior at either wave. Future longitudinal research should include more challenging behavioral ToM assessments in subsequent waves to address these open questions. Nonetheless, the neural stability demonstrated in our data over periods of change in conceptual performance points to the possibility that some aspects of ToM reasoning—those supported by the DMPFC—could be established earlier in development and exhibit stability by early and middle childhood.
While our data do not have direct implications for understanding ToM prior to age 4 years, thinking about such early-developing ToM-relevant skills informs speculations about what aspects of ToM could be established by age 4 and exhibit stability over early to middle childhood. For example, by age 2 years, older infants and toddlers demonstrate abilities that are either continuous with or are important components of later ToM reasoning such as distinguishing self and other (Legerstee et al., 1998; Rochat and Striano, 2002), identifying the goals and intentions behind others’ actions (Wellman et al., 2008; Yamaguchi et al., 2009), and discriminating faces and emotional expressions (Young-Browne et al., 1977; Righi and Nelson, 2013). FMRI research with adults and older children shows that the DMPFC (unlike other brain regions in the ToM neural network) is recruited for multiple aspects of ToM including those also present very early in development such as affective processes (Krause et al., 2012; Sebastian et al., 2011) and self-other distinctions (Schuwerk et al., 2014). Thus, an intriguing possibility is that continuity in the DMPFC may in part reflect continuity in these early-present ToM concepts. Future research should examine whether DMPFC is associated with young children’s performance on tasks assessing these early-developing ToM-relevant skills. The methods and design of the present study can be used to guide this future research.
4.1.2. DMPFC as a process-specific mechanism or ToM module?
Rather than supporting early-developing, specific, stable ToM concepts (such as affective processes, or self-other distinction), an intriguing possibility is that DMPFC may support a process-specific mechanism established early in life that is recruited across multiple ToM concepts. Indeed, beyond supporting ToM directly, DMPFC is active during tasks requiring cognitive control (Hartwright et al., 2012) and mental representation (Hartwright et al., 2014), which both support successful ToM reasoning. Moreover, evidence suggests that DMPFC, unlike other regions of the ToM neural network (e.g., TPJ), is recruited similarly for reasoning across different mental state constructs (e.g., belief- and desire-reasoning; Bowman et al., 2012; Liu et al., 2009; Bowman et al., 2015) and across individuals with different socio-cultural experiences (e.g., individuals from Japan and the United States; Kobayashi et al., 2006). This versatility is consistent with the possibility that the DMPFC supports a process-specific mechanism recruited across multiple aspects of ToM reasoning. The notion of a process-specific mechanism supporting ToM is compatible with long-standing characterizations of ToM development that emphasize a “Theory of Mind Module”—an innate neural mechanism dedicated to ToM reasoning that is in place in infancy and gradually ‘comes on-line’ over the course of development (see e.g., Fodor, 1983; Scholl and Leslie, 1999; Leslie et al., 2004; German and Hehman, 2006). Future longitudinal research that examines selective involvement of the DMPFC, in both younger and older populations, and that includes domain-general controls, will be useful in further investigating the DMPFC as a potential ToM module.
4.1.3. Endogenous and exogenous sources of DMPFC stability?
Future research is needed to address questions about the cause of DMPFC stability, which could be endogenous or exogenous. Considering endogenous factors, dopamine has been indirectly linked to ToM proficiency in 4- and 5-year-old preschool children (Lackner et al., 2010, 2012). Considering exogenous factors, a meta-analysis demonstrates family environments could influence children’s developing understanding of false-beliefs (Devine and Hughes, 2016). Future research could employ the methods and approach outlined in the present study, and include assessments of these possible endogenous/exogenous influences to investigate whether dopamine and/or continuity in the family environment relate to neural correlates of children’s ToM, and in particular to the development and function of DMPFC.
4.1.4. Links between the brain’s early underlying organization and its later functional recruitment?
The longitudinal relations between source-localized resting EEG alpha at age 4 and fMRI rank orders at ages 7 and 8 demonstrate associations between task-independent neural activity and task-dependent functional selectivity. These relations could suggest that there are direct developmental links between the brain’s early underlying organization and its later functional recruitment, and offer new insight into the decades-long debate regarding the significance of task-independent brain activity (Kubo, 1966; Papo, 2013; Deco et al., 2011; Gabard-Durnam et al., 2014). An intriguing open question is whether we might see similar associations for other aspects of core cognition across development. The methods and design of the present study can be adopted for these important future pursuits.
4.2. Conclusions
We found preliminary evidence for a stable role for DMPFC in ToM: individual differences in ToM performance at age 4 years were associated with both concurrent functional maturation DMPFC (indexed with source-localized resting EEG alpha) and with rank-ordered functional selectivity of the same region of the DMPFC 3.5 years later. Moreover, 4-year-olds’ task-independent neural activity in the DMPFC predicted task-dependent functional selectivity of the DMPFC 3.5 years later. Given the small sample, these results present a first exploration continuity in the neural system for ToM, and require replication with larger, more representative sample. Nonetheless, our approach and preliminary findings have implications for characterizing conceptual ToM development, and its underlying neural supports. Our methods and design provide a foundation for future research to investigate functional continuity and change in neural systems supporting precise social-cognitive and conceptual developments.
Declaration of Competing Interest
There are no conflicts of interest to report.
Acknowledgements
This work was funded by an NSF CAREER grant (0955818) awarded to Saxe, an NSERC PreDoctoral grant (PGSD2389020-2010) awarded to Bowman, and an NSERC Discovery Grant (RGPIN 250004-11) awarded to Sabbagh. We extend our gratitude to Anastasia Christopher who helped with data collection, and to the parents and children who participated in the study.
Footnotes
Participants also heard two additional condition types – stories in a foreign language, and music. Neither of these additional conditions was relevant for the purposes of the current study and thus neither was included in analyses.
2 Cluster criterion for the whole-brain analysis was taken to match that in the original whole-brain analyses with the sLORETA data in Sabbagh et al. that was associated with a family-wise alpha level of p < .05: correlations were considered meaningful if at least 20 contiguous sLORETA voxels correlated with fMRI mental-state selectivity at p < .05.
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2019.100705.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Bergman L.R., Edklund R., Magnusson D. Studying individual development: problems and methods. In: Magnusson D., Begman L.R., editors. Problems and Methods in Longitudinal Research: Stability and Change. Cambridge University Press; Cambridge, MA: 1989. [Google Scholar]
- Bowman L.C., Kovelman I., Hu X., Wellman H.M. Children’s belief-and desire-reasoning in the temporoparietal junction: evidence for specialization from functional near-infrared spectroscopy. Front. Hum. Neurosci. 2015;9 doi: 10.3389/fnhum.2015.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman L.C., Liu D., Meltzoff A.N., Wellman H.M. Neural correlates of belief- and desire-reasoning in 7- and 8-year-old children: an event-related potential study. Dev. Sci. 2012;15:618–632. doi: 10.1111/j.1467-7687.2012.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson S.M., Moses L.J. Individual differences in inhibitory control and children’s theory of mind. Child Dev. 2001;72:1032–1063. doi: 10.1111/1467-8624.00333. [DOI] [PubMed] [Google Scholar]
- Carlson S.M., Davis A., Leach J.G. Less is more: executive function and symbolic representation in preschool children. Psychol. Sci. 2005;16:609–616. doi: 10.1111/j.1467-9280.2005.01583.x. [DOI] [PubMed] [Google Scholar]
- Davis-Unger A.C., Carlson S.M. Development of teaching skills and relations to theory of mind in preschoolers. J. Cogn. Dev. 2008;9(1):26–45. [Google Scholar]
- Deco G., Jirsa V.K., McIntosh A.R. Nature Neuroscience Reviews. 2011;12:43–56. doi: 10.1038/nrn2961. [DOI] [PubMed] [Google Scholar]
- Devine R.T., Hughes C. Relations between false belief understanding and executive function in early childhood: a meta-analysis. Child Dev. 2014 doi: 10.1111/cdev.12237. [DOI] [PubMed] [Google Scholar]
- Devine R.T., Hughes C. Family correlates of false belief understanding in early childhood: a meta‐analysis. Child Dev. 2016 doi: 10.1111/cdev.12682. [DOI] [PubMed] [Google Scholar]
- Dunn L.M., Dunn L.M. 3rd ed. Pearson Assessments.; Bloomington, MN: 1997. Peabody Picture Vocabulary Test. [Google Scholar]
- Elbert T., Heim S., Rockstroh B. Neural plasticity and development. In: Nelson C.A., Luciana M., editors. Handbook of Developmental Cognitive Neuroscience. MIT Press; Cambridge, MA: 2001. Ch 14. [Google Scholar]
- Flavell J.H., Green F.L., Flavell E.R. Development of knowledge about the appearance-reality distinction. Monogr. Soc. Res. Child Dev. 1986;51 [PubMed] [Google Scholar]
- Fodor J. MIT Press; Cambridge, MA: 1983. Modularity of Mind. [Google Scholar]
- Fox N.A., Rubin K.H., Calkins S.D., Marshall T.R. Frontal activation asymmetry and social competence at four years of age. Child Dev. 1995;66:1770–1784. [PubMed] [Google Scholar]
- Gabard-Durnam L.J., Flannery J., Goff B., Gee D.G., Humphreys K.L., Telzer E., Hare T., Tottenham N. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage. 2014;95:193–207. doi: 10.1016/j.neuroimage.2014.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German T.P., Hehman J.A. Representational and executive selection resources in ‘theory of mind’: evidence from compromised belief-desire reasoning in old age. Cognition. 2006;101:129–152. doi: 10.1016/j.cognition.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Gopnik A., Astington J.W. Children’s understanding of representational change and its relation to the understanding of false belief and the appearance- reality distinction. Child Dev. 1988;59:26–37. doi: 10.1111/j.1467-8624.1988.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Grosse Wiesmann C., Schreiber J., Singer T., Steinbeis N., Friederici A.D. White matter maturation is associated with the emergence of Theory of Mind in early childhood. Nat. Commun. 2017;8:14692. doi: 10.1038/ncomms14692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gweon H., Dodell-Feder D., Bedny M., Saxe R. Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Dev. 2012;83:1853–1868. doi: 10.1111/j.1467-8624.2012.01829.x. [DOI] [PubMed] [Google Scholar]
- Harris P.L., Johnson C.N., Hutton D., Andrews A., Cooke T. Young children’s theory of mind and emotion. Cogn. Emot. 1989;3:379–400. [Google Scholar]
- Hartwright C.E., Apperly I.A., Hansen P.C. Multiple roles for executive control in belief-desire reasoning: distinct neural networks are recruited for self perspective inhibition and complexity of reasoning. Neuroimage. 2012;61:921–930. doi: 10.1016/j.neuroimage.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Hartwright C., Apperly I.A., Hansen P.C. Representation, control, or reasoning? Distinct functions for theory of mind within the medial prefrontal cortex. J. Cogn. Neurosci. 2014;26:683–698. doi: 10.1162/jocn_a_00520. [DOI] [PubMed] [Google Scholar]
- Hyde D.C., Simon C.E., Ting F., Nikolaeva J.I. Functional organization of the temporal–parietal junction for theory of mind in preverbal infants: a near-infrared spectroscopy study. J. Neurosci. 2018;38(18):4264–4274. doi: 10.1523/JNEUROSCI.0264-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Crucial differences between developmental cognitive neuroscience and adult neuropsychology. Dev. Neuropsychol. 1997;13:513–524. [Google Scholar]
- Killen M., Mulvey K.L., Richardson C., Jampol N., Woodward A. The accidental transgressor: morally-relevant theory of mind. Cognition. 2011;119(2):197–215. doi: 10.1016/j.cognition.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi C., Glover G.H., Temple E. Cultural and linguistic influence on neural bases of ‘Theory of mind’: an fMRI study with Japanese bilinguals. Brain Lang. 2006;98:210–220. doi: 10.1016/j.bandl.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Kobayashi C., Glover G.H., Temple E. Children’s and adults’ neural bases of verbal and nonverbal ‘theory of mind’. Neuropsychologia. 2007;45(7):1522–1532. doi: 10.1016/j.neuropsychologia.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause L., Enticott P.G., Zangen A., Fitzgerald P.B. The role of medial prefrontal cortex in theory of mind: a deep rTMS study. Behav. Brain Res. 2012;228:87–90. doi: 10.1016/j.bbr.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Kubo R. The fluctuation-dissipation theorem. Rep. Prog. Phys. 1966;29:255–284. [Google Scholar]
- Lackner C.L., Bowman L.C., Sabbagh M.A. Dopaminergic functioning and preschoolers’ theory of mind. Neuropsychologia. 2010;48(6):1767–1774. doi: 10.1016/j.neuropsychologia.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Lackner C., Sabbagh M.A., Hallinan E., Liu X., Holden J.J. Dopamine receptor D4 gene variation predicts preschoolers’ developing theory of mind. Dev. Sci. 2012;15(2):272–280. doi: 10.1111/j.1467-7687.2011.01124.x. [DOI] [PubMed] [Google Scholar]
- Lane J.D., Wellman H.M., Olson S.L., LaBounty J., Kerr D.C. Theory of mind and emotion understanding predict moral development in early childhood. Br. J. Dev. Psychol. 2010;28(4):871–889. doi: 10.1348/026151009x483056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerstee M., Anderson D., Schaffer A. Five‐and eight‐month‐Old infants recognize their faces and voices as familiar and social stimuli. Child Dev. 1998;69(1):37–50. [PubMed] [Google Scholar]
- Leslie A.M., Friedman O., German T.P. Core mechanisms in ‘theory of mind’. Trends Cogn. Sci. (Regul. Ed.) 2004;8:528–533. doi: 10.1016/j.tics.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Liu D., Meltzoff A.N., Wellman H.M. Neural correlates of belief- and desire-reasoning. Child Dev. 2009;80:1163–1171. doi: 10.1111/j.1467-8624.2009.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall P.J., Bar-Haim Y., Fox N.A. Development of the EEG from 5 months to 4 years of age. Clin. Neurophysiol. 2002;113:1199–1208. doi: 10.1016/s1388-2457(02)00163-3. [DOI] [PubMed] [Google Scholar]
- Milligan K., Astington J.W., Dack L.A. Language and theory of mind: meta-analysis of the relation between language ability and false-belief understanding. Child Dev. 2007;78:622–646. doi: 10.1111/j.1467-8624.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- Moll H., Tomasello M. Cooperation and human cognition: the Vygotskian intelligence hypothesis. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2007;362(1480):639–648. doi: 10.1098/rstb.2006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papo D. Why should cognitive neuroscientists study the brain’s resting state? Front. Hum. Neurosci. 2013;7:1–4. doi: 10.3389/fnhum.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;24:5–12. [PubMed] [Google Scholar]
- Peterson C.C., Wellman H.M., Slaughter V. The mind behind the message: advancing theory of mind scales for typically developing children, and those with deafness, autism, or Asperger Syndrome. Child Dev. 2012;83:469–485. doi: 10.1111/j.1467-8624.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R. Region of interest analysis for fMRI. SCAN. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson H., Lisandrelli G., Riobueno-Naylor A., Saxe R. Development of the social brain from age three to twelve years. Nat. Commun. 2018;9(1):1027. doi: 10.1038/s41467-018-03399-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi G., Nelson C.A., III . The neural architecture and developmental course of face processing. In: Rubenstein J., Rakic P., editors. Comprehensive Developmental Neuroscience: Neural Circuit Development and Function in the Healthy and Diseased Brain. Elsevier; Oxford: 2013. [Google Scholar]
- Rochat P., Striano T. Who’s in the mirror? Self–Other discrimination in specular images by four‐and nine‐month‐Old infants. Child Dev. 2002;73(1):35–46. doi: 10.1111/1467-8624.00390. [DOI] [PubMed] [Google Scholar]
- Sabbagh M.A., Bowman L.C., Evraire L.E., Ito J.M.B. Neurodevelopmental correlates of theory of mind in preschool children. Child Dev. 2009;80:1147–1162. doi: 10.1111/j.1467-8624.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- Saxe R.R., Whitfield‐Gabrieli S., Scholz J., Pelphrey K.A. Brain regions for perceiving and reasoning about other people in school‐aged children. Child Dev. 2009;80(4):1197–1209. doi: 10.1111/j.1467-8624.2009.01325.x. [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind.”. NeuroImage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Scholl B.J., Leslie A.M. Modularity, development and ‘theory of mind’. Mind Lang. 1999;14:131–153. [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Schuwerk T., Schecklmann M., Langguth B., Dohnel K., Sodian Beate, Sommer M. Brain structure and function inhibiting the posterior medial prefrontal cortex by rTMS decreases the discrepancy between self and other in theory of mind reasoning. Behav. Brain Res. 2014;274:312–318. doi: 10.1016/j.bbr.2014.08.031. [DOI] [PubMed] [Google Scholar]
- Sebastian C., Fontaine N., Bird G., Blakemore S., De Brito S., McCrory E., Viding E. Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Soc. Cogn. Affect. Neurosci. 2011;2011:1–11. doi: 10.1093/scan/nsr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M., Meinhardt J., Eichenmüller K., Sodian B., Döhnel K., Hajak G. Modulation of the cortical false belief network during development. Brain Res. 2010;1354:123–131. doi: 10.1016/j.brainres.2010.07.057. [DOI] [PubMed] [Google Scholar]
- Thatcher R.W. Cyclic cortical reorganization: origins of human cognitive development. In: Dawson G., Fischer K.W., editors. Human Behavior and the Developing Brain. Guil- ford; New York: 1994. pp. 232–266. [Google Scholar]
- Thatcher R.W., Walker R.A., Guidice S. Human cerebral hemispheres develop at different rates and age. Science. 1987;236:1110–1113. doi: 10.1126/science.3576224. [DOI] [PubMed] [Google Scholar]
- Tomasello M., Carpenter M., Call J., Behne T., Moll H. Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain Sci. 2005;28:675–735. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- Wellman H.M. Oxford University Press; 2014. Making Minds: How Theory of Mind Develops. [Google Scholar]
- Wellman H.M., Liu D. Scaling of theory-of-mind tasks. Child Dev. 2004;75:523–541. doi: 10.1111/j.1467-8624.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- Wellman H.M., Cross D., Watson J. Meta-analysis of theory of mind development: the truth about false belief. Child Dev. 2001;72:655–684. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Wellman H.M., Lopez-Duran S., LaBounty J., Hamilton B. Infant attention to intentional action predicts preschool theory of mind. Dev. Psychol. 2008;44(2):618. doi: 10.1037/0012-1649.44.2.618. [DOI] [PubMed] [Google Scholar]
- Wimmer H., Perner J. Beliefs about beliefs: representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition. 1983;13:103–128. doi: 10.1016/0010-0277(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Kuhlmeier V.A., Wynn K., VanMarle K. Continuity in social cognition from infancy to childhood. Dev. Sci. 2009;12(5):746–752. doi: 10.1111/j.1467-7687.2008.00813.x. [DOI] [PubMed] [Google Scholar]
- Young-Browne G., Rosenfeld H.M., Horowitz F.D. Infant discrimination of facial expressions. Child Dev. 1977:555–562. [Google Scholar]
- Zelazo P.D. The dimensional change card sort (DCCS): a method of assessing executive function in children. Nat. Protoc. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Ziv M., Frye D. Children’s understanding of teaching: the role of knowledge and belief. Cogn. Dev. 2004;19(4):457–477. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.