Abstract
Fathers play a crucial role in their children’s socio-emotional and cognitive development. A plausible intermediate phenotype underlying this association is father’s impact on infant brain. However, research on the association between paternal caregiving and child brain biology is scarce, particularly during infancy. Thus, we used magnetic resonance imaging (MRI) to investigate the relationship between observed father–infant interactions, specifically paternal sensitivity, and regional brain volumes in a community sample of 3-to-6-month-old infants (N = 28). We controlled for maternal sensitivity and examined the moderating role of infant communication on this relationship. T2-weighted MR images were acquired from infants during natural sleep. Higher levels of paternal sensitivity were associated with smaller cerebellar volumes in infants with high communication levels. In contrast, paternal sensitivity was not associated with subcortical grey matter volumes in the whole sample, and this was similar in infants with both high and low communication levels. This preliminary study provides the first evidence for an association between father-child interactions and variation in infant brain anatomy.
Keywords: Fathers, Father-infant interactions, Infant brain volume, MRI, Infancy, Paternal sensitivity
1. Introduction
There is compelling evidence that fathers play a critical role in their children’s development (Cabrera et al., 2014). Specifically, the quality of father-infant interactions independently contributes to a broad range of developmental outcomes in the child (for reviews, please see Barker et al., 2017; Sarkadi et al., 2007). For instance, paternal sensitivity and engagement in infancy and early childhood are linked to better cognitive and language development in their children (Sethna et al., 2017a; Tamis-LeMonda et al., 2004). In contrast, poor quality paternal caregiving (e.g., child-directed negativity) is a risk factor for maladaptive outcomes (Gauvain et al., 2002; Kok et al., 2018). Although the mediating mechanisms underpinning the impact of fathers on their children are not fully understood, a plausible intermediate phenotype underlying these associations may be differences in brain development. Consistent with this, preclinical evidence suggests that paternal care influences offspring brain (Braun and Champagne, 2014). For example, father-deprived degus pups show synaptic reductions in the cortex (Ovtscharoff et al., 2006). The human literature reports differences between the long-term effect of maternal and paternal care on offspring brain and social-cognitive-emotional development; on differences between maternal and paternal parenting behaviour in relation to their neurobiological functioning (Gordon et al., 2010), and on the brain response of mothers and fathers to their infants (Abraham et al., 2014; Abraham et al., 2016). However, despite evidence from our group and others that normal variation in maternal care influences brain biology in their children, even as early as infancy (Als et al., 2004; Luby et al., 2012; Sethna et al., 2017b), links between paternal behaviour and infant brain in humans have been less studied.
Those studies that have examined the impact of father-child relationship on brain development have only investigated older children. These include a preliminary finding that increased paternal sensitivity (observed at 1, 3 and 4 years) was associated with larger ventricular volumes at 6-weeks and total grey matter volumes in children when they were aged 8 years (Kok et al., 2015) – although the associations were not statistically significant. In this Dutch birth cohort, 6-week infant brain structure examination comprised ultrasound measurement of the ventricular system and head circumference, whilst MRI was acquired in middle childhood. In a subsequent study, grey matter volume at age 8, mediated the association between parental sensitivity (average maternal and paternal sensitivity) and prosocial behaviour in girls aged 9 years (Kok et al., 2018). However, potential links between paternal sensitivity in infancy and brain structure in infants, at that same early timepoint, were not examined. In addition, these studies did not account for potential relationships between maternal sensitivity and offspring brain (Kok et al., 2015; Rifkin-Graboi et al., 2015; Sethna et al., 2017b).
Furthermore, in these preliminary studies, the relationship between infant behaviour and infant brain was also not accounted for (Blasi et al., 2015; Sethna et al., 2017b). There is now increasing evidence to suggest that some children are more susceptible than others to a range of environmental influences, including parenting by fathers (Ramchandani et al., 2010). Moreover, links between infant communicative and attentive behaviours have also been linked with the infant brain (Blasi et al., 2015; Mundy et al., 2000; Sethna et al., 2017b). Thus, it is possible that infant behaviours will interact with the caregiving environment to which they are exposed, in moderating the influence that these environments exercise on brain development.
We therefore carried out a cross-sectional MRI study to examine, for the first time, the relationship between father-infant interactions and regional brain volumes, in a typically developing sample of infants, aged 3-to-6 months. In our previous study (Sethna et al., 2017b), we found that some regions of the developing brain are especially ‘responsive’ to the (maternal) parenting environment, specifically the subcortex and cerebellum. Therefore, although data was collected from whole-brain, we predicted that the father-infant interaction would be related to the anatomy of these regions. We controlled for maternal sensitivity and since parenting effects are often moderated by characteristics of the child (Pluess and Belsky, 2010), we also tested whether any influence of father’s behaviour on infant brain was moderated by infant behaviour.
Based on brain imaging evidence in older children that maternal and paternal sensitivity and offspring brain structure are linked in similar ways (Kok et al., 2015), we expected that the direction of relationships between father-infant behaviour and the brain would mirror those we reported previously for mother-infant behaviour (Sethna et al., 2017b). Hence, high levels of paternal sensitivity would be associated with larger subcortical volumes and/or smaller cerebellar volumes in the infant.
2. Method
2.1. Participants
A community sample of 46 families was recruited from South East London. Eligibility was based on parents having a working knowledge of the English language and being free from any current or past major psychiatric illness, or any antenatal/obstetric complications potentially altering infant development (e.g. perinatal asphyxia). Infants were included in the study if they were aged between 3 and 6 months, born at term (gestational age >36 weeks) and free from any congenital abnormalities. Exclusion criteria included contraindications for MRI scanning (e.g. metallic implants or pacemakers).
Of the 46 families assessed, 34 fathers agreed to participate – 6 families comprised single mothers and 6 fathers declined to participate. The study was approved by the UK National Research Ethics Committee (REC 07/H0807/70 and 12/LO/2017) and written informed consent for participation was obtained from both parents.
Observational face-to-face interactions were available for 31 father-infant dyads – 3 interaction assessments were excluded from analyses due to the inability to code speech in a foreign language. MRI assessments were available for 31 infants – 3 scans were excluded from the analysis due to motion artefacts (n = 2) and an incidental anatomical brain anomaly found at MRI scanning (n = 1). Therefore, the final sample comprised 28 father-infant dyads with complete data on both father-infant interactions and infant strucutral MRI collected at 3-to-6 months.
At the time of the MRI assessment, fathers had a mean age of 37 years (SD = 6 years). The majority of fathers were of a white ethnic background (82%) and held managerial or professional occupations (61%). Infants had a mean age of 144 days (SD = 30 days) and 43% were male. Infant and paternal demographic information for the total sample is displayed in Table 1.
Table 1.
Infant and paternal demographic characteristics for the total sample (N = 28).
| Demographic characteristics | Total sample |
|---|---|
| Infant demographics | |
| Age at MRI scan (days): mean (±SD) | 144 (30) |
| Gestational age (weeks): mean (±SD) | 40 (2) |
| Birth weight (grams): mean (±SD) | 3374.89 (700.17) |
| Birth order: n (%) | |
| 1st born | 16 (57%) |
| 2nd born | 11 (40% |
| 3rd born | 1 (3%) |
| Infant sex: n (%) | |
| Male | 12 (43%) |
| Female | 16 (57%) |
| Paternal demographics | |
| Age at MRI scan (years): mean (±SD) | 37 (6) |
| Ethnicity: n (%) | |
| White | 23 (82%) |
| Non-white | 5 (18%) |
| Employment status | |
| Employed | 27 (96%) |
| Employed, but on sick leave | 1 (4%) |
| Occupational level: n (%) | |
| Managerial and professional | 17 (61%) |
| Intermediate level occupation | 6 (21%) |
| Routine and manual | 5 (18%) |
SD, standard deviation.
2.2. Measures
2.2.1. Parent-infant interactions at 3-to-6 months
Observed father-infant interactions were video-recorded during a face-to-face setting – fathers were asked to talk to and play with their infant for 5 min, as they would normally, without the use of any toys. Similar assessments of father-infant face‐to‐face interactions have been used in previous studies (Sethna et al., 2017a). The Global Rating Scale (GRS, Murray et al., 1996, 1996a) was used to assess parent and infant behaviours. Specifically, we focussed on paternal sensitivity because it is a key behavioural feature of early caregiving and predictive of children’s cognitive outcomes (Lewis and Lamb, 2003; Mills-Koonce et al., 2015). Since we previously found that maternal sensitivity was related to infant brain and that the relationship was moderated by infant communication (Sethna et al., 2017b), we also controlled for maternal sensitivity and tested whether infant communication moderated the relationship between paternal sensitivity and infant brain.
2.2.2. Paternal sensitivity (exposure)
Paternal interactions were coded using the Global Rating Scales (GRS, Murray et al., 1996). Paternal behaviors were rated on a series of 5‐point scales (1–5), with lower scores indicating inadequate interactions. The sensitivity dimension was derived as per standard use in previous studies with fathers, and defined as paternal response to the infant's communication cues in a way that is appropriate to the infant's needs and experiences, including attitude and feelings toward the infant (Rajhans et al., 2019; Sethna et al., 2017a).
Interrater Intraclass Correlations (ICC, Shrout and Fleiss, 1979) for paternal sensitivity, based on 20% of the sample independently coded, was 0.96.
2.2.3. Maternal sensitivity (potential confounder)
Maternal sensitivity was assessed during observed mother-infant interactions at 3-to-6 months and coded using the GRS, as per standard use in previous studies (Gunning et al., 2018; Mäntymaa et al., 2003; Murray et al., 1996; Sethna et al., 2017a,b). ICC for maternal sensitivity based on 20% of the sample independently coded was 0.91.
2.2.4. Infant communication (potential moderator)
Infant communication during father-infant interactions was scored on a standard five-point scale, where 1 corresponds to “poor” interactive infant behaviour and 5 to most “optimal” behaviour (Murray et al., 1996, 1996a). Infant communication was defined as the infant’s level of engagement and communication (i.e., positive vocal and non-vocal activities directed towards the father). Communication included the amount of visual attention, and positive vocalizations, in addition to other forms of exchange (for example, mouthing, movement of limbs and positive affect). ICC for infant communication based on a randomly selected 20% of the interactions was 0.98.
2.2.5. Structural magnetic resonance imaging (outcome)
2.2.5.1. MRI data acquisition
MRI data were collected using a 1.5 T General Electric TwinSpeed MRI scanner (GE Medical Systems, Milwaukee, WI, USA), equipped with an 8-channel head coil. Infants were scanned in natural sleep with no sedation, wrapped in a blanket, and placed in a Med-Vac Infant Immobilization Bag (DFI Medical Solutions) to minimise infant movement. The scanner bore was insulated with sound attenuating foam (Ultra Barrier, American Micro Industries) and infants wore MiniMuff noise attenuators (Natus Medical) with MR compatible piezoelectric headphones (MR Confon) to reduce exposure to MRI scanner noise (Blasi et al., 2011; Sethna et al., 2017b). A pulse oximeter was attached to the infant’s toe to monitor heart rate and oxygen saturation.
A T2-weighted (T2w) fast spin echo (T2w) sequence was acquired with the following imaging parameters: number of slices = 20; slice thickness = 4 mm; slice gap = 2 mm; repetition time = 3000/4500 ms; echo time = 115 ms; field of view = 180 mm; flip angle 90°; matrix size = 256 × 224. All MRI scans assessed for clinical abnormalities by a radiologist.
2.2.6. Image pre-processing and volumetric segmentation
Scans were analysed blind to family characteristics using an in-house developed protocol for low resolution images as previously reported (Sethna et al., 2017b). In brief, T2w MR images were skull-stripped, and the masked images were then segmented using an atlas-based method, which adapted the Statistical Parametric Mapping software (v. SPM8) and a probabilistic neonatal brain atlas (Kuklisova-Murgasova et al., 2011) as an input to the software. The SPM segmentation model unifies tissue classification, image bias correction, and non-linear atlas registration (Ashburner and Friston, 2005). Following this, the segmented cerebrospinal fluid (CSF) was refined, and partial volume misclassifications corrected based on tissue connectivity using second order Markov random fields. Once this automated segmentation was complete, a single rater examined all images in a final manual editing process using ITK-SNAP (v. 2.2). For more details on the pre-processing and segmentation process, please see Sethna et al. (2017b).
The main analyses of this study focused on subcortical grey matter (including the caudate, putamen, globus pallidus and thalamus) and cerebellar volumes. To account for differences in total brain volume, these regional brain volumes were expressed as proportions of intracranial volume – obtained by summing the CSF, lateral ventricles, midbrain, cerebellum, subcortical grey matter and total grey and white matter. These ‘corrected’ proportions were used in the analyses.
To assess the reliability of these volumetric segmentations, intra-rater intra-class correlations (ICC) were performed between the final segmentations, and a repeat measurement of a randomly selected 20% of the automatically segmented images. For the intracranial volume, ICC = 0.998 (p < 0.001), indicating excellent reproducibility. Similar results from correlations were also achieved for the cerebellum (ICC = 0.948, p < 0.001) and subcortical grey matter (ICC = 0.923, p < 0.001). These ICCs were derived from the absolute measurements.
2.2.7. Statistical analysis
All data were analysed using SPSS software version 24.0 (IBM Corp., Armonk, NY). Preliminary analyses included descriptive statistics for the exposure (paternal sensitivity) and outcome (volumes of the subcortical grey matter and the cerebellum) variables. Bivariate correlations were also determined between potential covariates (maternal sensitivity, infant age and gender), and paternal sensitivity and brain volumes. Where a significant (i.e. p < 0.05) or moderate effect size (i.e. r > 0.3) correlation was observed, that covariate was controlled for in subsequent examination of paternal sensitivity and subcortical and cerebellar volumes; estimated via separate multiple linear regression models. Data were examined to confirm that these conformed to assumptions of normality. Effect sizes were calculated using Cohen’s f2 (Cohen, 1988) for multiple linear regression models.
Next, using the process macro tool (Hayes, 2013), we estimated whether the interaction term between sensitivity and infant communication (i.e. paternal sensitivity x infant communication) was associated with brain volume. PROCESS applies bias-corrected bootstrapping intervals to probe the interaction term and make inferences about indirect effects, rather than relying on the normality assumption. The number of bootstrap samples used to determine 95% bias-corrected bootstrap confidence intervals was 10,000. The conditional indirect effects of the independent variable at varying degrees of a continuous moderator (infant communication) – i.e. low (−1 SD below the mean), moderate (the mean), and high (1 SD above the mean) – were estimated.
3. Results
3.1. Descriptive analyses
Table 2 shows the means and standard deviations for the exposure and outcome variables and correlations with study covariates. Infant age was significantly correlated with subcortical grey matter (r = −0.39, p = .043) volumes; as well as with cerebellar (r = 0.33, p = 0.091) volumes and paternal sensitivity (r = 0.34, p = 0.074), at trend level. Infant sex was associated with cerebellar volumes (r = 0.32, p = 0.102) with a medium effect size, whereas maternal sensitivity was significantly associated with subcortical grey matter volumes (r = 0.50, p = 0.006).
Table 2.
Descriptive statistics and bivariate correlations for the main study variables (N = 28).
| Variable | Mean (SD) / N (%) | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Exposure | |||||||
| Paternal Sensitivity | 3.82 (0.52) | – | |||||
| Outcome | |||||||
| Subcortical grey matter volumes (cm3) | 35.50 (3.83) | −.13 | – | ||||
| Cerebellar Volumes (cm3) | 74.62 (8.99) | −.20 | −.07 | – | |||
| Potential confounders | |||||||
| Infant age at scan (days) | 144 (30) | .34 (p = 0.074) | −.39 (p = 0.043) | .33 (p = .091) | – | ||
| Infant sex (male) | 12 (43%) | .14 | −.21 | .32 | .20 | – | |
| Maternal Sensitivity | 3.67 (0.52) | .16 | .50 (p = 0.006) | −.07 | −.34 | .14 | – |
SD = Standard Deviation; Parental sensitivity scored on a scale from 1 to 5; low scores indicate poor interactions. Gender: 0 = male, 1 = female.
Consequently, we adjusted for infant age and maternal sensitivity in multivariate analyses including subcortical grey matter volumes, and infant age and gender for cerebellar volumes.
3.2. Relationship between paternal sensitivity and regional brain volumes in infants
Paternal sensitivity was not significantly associated with subcortical grey matter volumes (adjusted model: β = −0.15, p = 0.449), and there was no evidence of moderation by infant behaviour on this association, as the interaction term (sensitivity x infant communication) was not significant (p = 0.741).
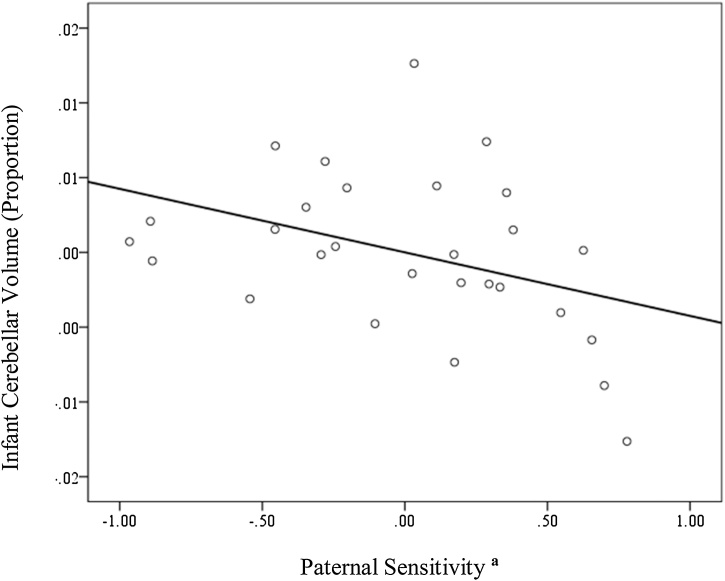
In contrast, paternal sensitivity was negatively associated with cerebellar volume (adjusted model: β = −0.38, p = 0.048), with a large effect size (Cohen’s f2 = 0.42) – implying a smaller cerebellum in infants interacting with more sensitive fathers (please see Fig. 1).
Fig. 1.
Partial regression plot of the association between paternal sensitivity and cerebellar volume, controlling for infant age at MRI scan and gender.
a Lower paternal sensitivity scores indicate inadequate interactions.
Furthermore, infant communication moderated the links between paternal sensitivity and cerebellar volume, as the interaction term (sensitivity x infant communication) was significant (B = −0.01, p = 0.025). Thus, smaller cerebellar volumes were observed in more communicative and engaged infants who are also exposed to more sensitive paternal behaviours (R2 increase due to the interaction = 0.15, F = 5.78, p = 0.025). While the conditional association between infant communication and paternal sensitivity was significant at high levels (i.e. 1SD above the mean) of infant communication ( = −0.01, p = 0.006); there was no such evidence at low levels (i.e. 1SD below the mean) of infant communication (B = 0.002, p = 0.526). Thus, the link between higher paternal sensitivity and smaller cerebellar volume was driven by the more communicative babies.
4. Discussion
In this cross-sectional study, the association between early father-infant interactions and infant brain volumes in typically developing 3-to-6-month-old infants was examined. We controlled for maternal sensitivity and tested whether infant communication moderated this association. We found that infants of more sensitive fathers had a smaller cerebellum, and this association was driven by more communicative babies; independent of infant age and gender. In contrast, there was no evidence of links between paternal sensitivity and subcortical grey matter volumes nor moderation by infant communication on this association.
Our findings of an association between paternal sensitivity and cerebellar volumes fits with evidence that the cerebellum is particularly susceptible to environmental experiences (Giedd et al., 2007). Moreover, the cerebellum contributes to the temporal processing of events (Schwartze and Kotz, 2016) and shows activation to auditory stimulation (Buckner, 2013), including speech processing by infants (Kuhl et al., 2014). These features of cerebellar function are relevant to the processing of stimuli during face-to-face father-infant interactions. Furthermore, the link between cerebellum and father-child interactions is not surprising given its role in allocating attentional resources in ‘real-time’ to guide or prepare behaviour (Schwartze and Kotz, 2016). For instance, the cerebellum is implicated in temporally specific behaviours, such as heightened autonomic arousal, excitatory stimulation and startle; features specific to paternal interactions that also enhance infant communication (Stgeorge and Freeman, 2017).
However, the direction of the association shown (i.e. higher levels of sensitivity with smaller cerebellar volumes) contradicts prior retrospective studies reporting smaller cerebellar volumes in older children exposed to early maltreatment (Bauer et al., 2009; De Bellis and Kuchibhatla, 2006). There are several possible explanations for this discrepancy. For example, since existing studies include older children it is not known whether brain regions were larger in infancy – that is the trajectory of brain growth for these populations remains unknown. It is also possible that many other confounders, including sub-optimal antenatal period and medication exposure (Belsky and de Haan, 2010), influence findings from extreme adversity. Furthermore, our measure of normative variation in sensitivity is defined so that the low end refers to the absence of sensitive (for example, warmth, acceptance, non-demanding) behaviours rather than the presence of extreme adversity, such as harsh or abusive behaviours. Moreover, we emphasize that our sample of infants was recruited from the community and assumed to be typically developing. The relationship between normative variation in sensitivity and normative variation in infant brain anatomy, caused for example by pruning during maturation (Lenroot and Giedd, 2006) is likely to be quite different from observations made in pathological settings. Taken together, in addition to different sampling frames, differences in specific forms of caregiving experienced, the timing of exposure and the timing of MRI assessment are likely to influence the direction of effects.
It is unlikely that parenting alone explains our findings – other factors, including genes, normal early variation in the development of neural structures and moderation by paternal stress may also modulate infant brain. For example, variability in cerebellar volumes may be influenced by genetic factors (Douet et al., 2014). Therefore, it is possible that shared genetic variants could explain the associations observed in this study – sensitive fathers could have had smaller cerebellar volumes in infancy and then have biological children with smaller volumes.
Finally, although we and others have reported that maternal sensitivity is linked with infant subcortical grey matter volumes (Kok et al., 2015; Sethna et al., 2017b) we did not find evidence for a similar relationship for fathers. It is possible that there are parental differences in the way their caregiving behaviours impact on infant brain development. For example, the association between fathers’ sensitivity and offspring sub-cortical brain development may perhaps be manifest in older children and not in infants. Some related evidence is a report linking father’s sensitive parenting in toddlerhood but not in infancy to children’s executive functioning (Towe-Goodman et al., 2014). Alternatively, it may be that we were not powered to detect this relationship in this modest initial study. Finally, the particular sample characteristics in our study may be important and other studies have shown that the sub-cortical biology of the parent varies alongside the child's expression of positive emotionality (Abraham et al., 2016). Thus, further research is needed to consider the impact of both parents jointly and/or examine a wider range of fathers and their paternal interaction dimensions (for example, paternal playfulness) and developmental time-points, in relation to subcortical volumes.
Overall, it is important to emphasize that our preliminary findings are correlational and do not indicate a causative link between early paternal caregiving and infant brain volumes. Importantly, our study is cross-sectional and therefore we cannot interpret the direction of our results, for example primary or secondary/compensatory. Hence, important associations remain unexplored. First, given the bidirectional associations between child temperament and parenting (Micalizzi et al., 2017), it remains unknown whether an infant with a smaller cerebellum volume elicits more positive parenting from their father or vice versa. Second, we did not have repeated measures of brain volume; hence, it is unknown whether the directions of the relationships reported are dependent on developmental stage. Neither can we be certain whether volumes reported have ‘positive’ or ‘negative’ developmental implications. Although increased cerebellar volumes have been reported in relation to autism spectrum disorder diagnosis (Traut et al., 2017), the volume of the cerebellum changes over childhood and into adolescence and adulthood (Tiemeier et al., 2009). To our best knowledge, no one has examined the cerebellum at this early age in infants who go on to receive an autism diagnosis. Third, considering the cerebellum’s sexually dimorphic developmental trajectory (Tiemeier et al., 2009), sex differences in the association between paternal caregiving and cerebellar volumes also require investigation. Fourth, fathers play a unique and distinctive role in their children’s lives and both the quality and amount of involvement fathers have with their children can influence development (Barker et al., 2017). Thus, in addition to the quality of care, the amount of contact the parent has with his/her child during the postpartum period is another avenue for future research when linking paternal caregiving to the offspring brain. Different aspects of father involvement could influence child development in different ways. Hence, we acknowledge that while sensitivity is one important aspect of the father-child relationship; future research on offspring brain would benefit from including paternal involvement by assessing both paternal availability (Lamb et al., 1985) and indirect care (Pleck, 2010). Additionally, other features of father-child interactions such as, reflective capacity and empathy – predictors of positive father-child relationships and child psychosocial outcomes (Arnott and Meins, 2007; Buttitta et al., 2019; McHarg et al., 2019) – require future consideration. Paternal psychopathology and attachment history may also be important considerations as they are linked to early interactions and child psychosocial outcomes (Madden et al., 2015; Mäntymaa et al., 2008). Finally, paternal stress may affect the brain development of his offspring, at least in part, by epigenetic factors that are inherited via the sperm (for a review on fathers see, Soubry, 2018). Taken together, the field has quite some way to go and further longitudinal and larger-scale research is therefore, required to examine several of these possibilities, which our work cannot address.
We acknowledge several limitations to our study. First, the scanning of very young infants is challenging and the structural sequences available for the majority of babies in our study were of low resolution. Hence, our overall volumetric measurement will have missed fine-grained structural differences that might be detectable with more sophisticated imaging procedures. Second, and in line with previous studies in early infancy (Hazlett et al., 2012), we were unable to differentiate between grey and white matter volumes. Third, our sample size was modest, and results need to be viewed cautiously until further replication. Fourth, our findings are based on cross-sectional data, and therefore, causation cannot be inferred. Fifth, we did not collect information about whether the children were in childcare or exposed to caregiving by other caregivers/family members.
In summary, we demonstrate for the first time that father-infant interactions are associated with variation in structural brain development at a very early age. If replicated in a larger sample, this work will suggest the importance of including fathers in research on the relation between early parental care and child brain development.
Declaration of Competing Interest
No conflicts declared.
Acknowledgements
This paper represents independent research part funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The work was conducted as part of EU-AIMS with support from the Innovative Medicines Initiative (IMI) Joint Undertaking (grant agreement No. 115300), and by a new IMI initiative- AIMS-2-Trials (grant agreement No. 777394). DGMM and GMM receive support from the Sackler Centre for Translational Neurodevelopment at King’s College London. The authors gratefully acknowledge parents and their infants for their invaluable contribution.
References
- Abraham E., Hendler T., Shapira-Lichter I., Kanat-Maymon Y., Zagoory-Sharon O., Feldman R. Father's brain is sensitive to childcare experiences. PNAS. 2014;111(27):9792–9797. doi: 10.1073/pnas.1402569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham E., Hendler T., Zagoory-Sharon O., Feldman R. Network integrity of the parental brain in infancy supports the development of children’s social competencies. Soc. Cogn. Affect Neurosci. 2016;11(11):1707–1718. doi: 10.1093/scan/nsw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Als H., Duffy F.H., McAnulty G.B., Rivkin M.J., Vajapeyam S., Mulkern R.V. Early experience alters brain function and structure. Pediatrics. 2004;113(4):846–857. doi: 10.1542/peds.113.4.846. [DOI] [PubMed] [Google Scholar]
- Arnott B., Meins E. Links among antenatal attachment representations, postnatal mind-mindedness, and infant attachment security: a preliminary study of mothers and fathers. Bull. Menninger Clin. 2007;71(2):132–149. doi: 10.1521/bumc.2007.71.2.132. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barker B., Iles J.E., Ramchandani P.G. Fathers, fathering and child psychopathology. Curr. Opin. Psychol. 2017;15:87–92. doi: 10.1016/j.copsyc.2017.02.015. [DOI] [PubMed] [Google Scholar]
- Bauer P.M., Hanson J.L., Pierson R.K., Davidson R.J., Pollak S.D. Cerebellar volume and cognitive functioning in children who experienced early deprivation. Biol. Psychiatry. 2009;66(12):1100–1106. doi: 10.1016/j.biopsych.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J., de Haan M. Annual research review: parenting and children’s brain development: the end of the beginning. J. Child Psychol. Psychiatry. 2010;52(4):409–428. doi: 10.1111/j.1469-7610.2010.02281.x. [DOI] [PubMed] [Google Scholar]
- Blasi A., Lloyd-Fox S., Sethna V., Brammer M.J., Mercure E., Murray L. Atypical processing of voice sounds in infants at risk for autism spectrum disorder. Cortex J Dev. Study Nerv. Syst. Behav. 2015;71:122–133. doi: 10.1016/j.cortex.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi A., Mercure E., Lloyd-Fox S., Thomson A., Brammer M., Sauter D. Early specialization for voice and emotion processing in the infant brain. Curr. Biol. 2011;21(14):1220–1224. doi: 10.1016/j.cub.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Buckner Randy L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Buttitta K.V., Smiley P.A., Kerr M.L., Rasmussen H.F., Querdasi F.R., Borelli J.L. In a father’s mind: paternal reflective functioning, sensitive parenting, and protection against socioeconomic risk. Attach. Hum. Dev. 2019:1–22. doi: 10.1080/14616734.2019.1582596. [DOI] [PubMed] [Google Scholar]
- Cabrera N.J., Fitzgerald H.E., Bradley R.H., Roggman L. The ecology of father-child relationships: an expanded model. J. Fam. Theory Rev. 2014;6(4):336–354. [Google Scholar]
- Cohen J. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- De Bellis M.D., Kuchibhatla M. Cerebellar volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol. Psychiatry. 2006;60(7):697–703. doi: 10.1016/j.biopsych.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Douet V., Chang L., Cloak C., Ernst T. Genetic influences on brain developmental trajectories on neuroimaging studies: from infancy to young adulthood. Brain Imaging Behav. 2014;8(2):234–250. doi: 10.1007/s11682-013-9260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvain M., Fagot B.I., Leve C., Kavanagh K. Instruction by mothers and fathers during problem solving with their young children. J. Fam. Psychol. 2002;16(1):81–90. doi: 10.1037//0893-3200.16.1.81. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Schmitt J.E., Neale M.C. Structural brain magnetic resonance imaging of pediatric twins. Hum. Brain Mapp. 2007;28(6):474–481. doi: 10.1002/hbm.20403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Zagoory-Sharon O., Leckman J.F., Feldman R. Oxytocin and the Development of Parenting in Humans. Biol. Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3943240/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning M., Conroy S., Valoriani V., Figueiredo B., Kammerer M.H., Muzik M. Measurement of mother-infant interactions and the home environment in a European setting: preliminary results from a cross-cultural study. Br. J. Psychiatry. 2018;184(S46):s38–s44. doi: 10.1192/bjp.184.46.s38. [DOI] [PubMed] [Google Scholar]
- Hayes A. Guilford; New York: 2013. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. [Google Scholar]
- Hazlett H.C., Poe M.D., Lightbody A.A., Styner M., MacFall J.R., Reiss A.L. Trajectories of early brain volume development in fragile X syndrome and autism. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(9):921–933. doi: 10.1016/j.jaac.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok R., Prinzie P., Bakermans-Kranenburg M.J., Verhulst F.C., White T., Tiemeier H. Socialization of prosocial behavior: gender differences in the mediating role of child brain volume. Child Neuropsychol. 2018;18(10):1–11. doi: 10.1080/09297049.2017.1338340. [DOI] [PubMed] [Google Scholar]
- Kok R., Thijssen S., Bakermans-Kranenburg M.J., Jaddoe V.W., Verhulst F.C., White T. Normal variation in early parental sensitivity predicts child structural brain development. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(10):824–831. doi: 10.1016/j.jaac.2015.07.009. e821. [DOI] [PubMed] [Google Scholar]
- Kuhl P.K., Ramírez R.R., Bosseler A., Lin J.-F.L., Imada T. Infants’ brain responses to speech suggest Analysis by Synthesis. Proc. Natl. Acad. Sci. U. S. A. 2014;111(31):11238–11245. doi: 10.1073/pnas.1410963111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklisova-Murgasova M., Aljabar P., Srinivasan L., Counsell S.J., Doria V., Serag A. A dynamic 4D probabilistic atlas of the developing brain. Neuroimage. 2011;54(4):2750–2763. doi: 10.1016/j.neuroimage.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Lamb M.E., Pleck J.H., Charnov E.L., Levine J.A. Paternal behavior in humans. Am. Zool. 1985;25(3):883–894. [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30(6):718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lewis C., Lamb M.E. Fathers’ influences on children’s development: the evidence from two-parent families. Eur. J. Psychol. Educ. 2003;18(2):211–228. [Google Scholar]
- Luby J.L., Barch D.M., Belden A., Gaffrey M.S., Tillman R., Babb C. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc. Natl. Acad. Sci. 2012;109(8):2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden V., Domoney J., Aumayer K., Sethna V., Iles J., Hubbard I. Intergenerational transmission of parenting: findings from a UK longitudinal study. Eur. J. Public Health. 2015;25(6):1030–1035. doi: 10.1093/eurpub/ckv093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntymaa M., Puura K., Luoma I., Kaukonen P., Salmelin R.K., Tamminen T. Infants’ social withdrawal and parents’ mental health. Infant Behav. Dev. 2008;31(4):606–613. doi: 10.1016/j.infbeh.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Mäntymaa M., Puura K., Luoma I., Salmelin R., Davis H., Tsiantis J. Infant–mother interaction as a predictor of child’s chronic health problems. Child Care Health Dev. 2003;29(3):181–191. doi: 10.1046/j.1365-2214.2003.00330.x. [DOI] [PubMed] [Google Scholar]
- McHarg G., Fink E., Hughes C. Crying babies, empathic toddlers, responsive mothers and fathers: exploring parent-toddler interactions in an empathy paradigm. J. Exp. Child Psychol. 2019;179:23–37. doi: 10.1016/j.jecp.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Micalizzi L., Wang M., Saudino K.J. Difficult temperament and negative parenting in early childhood: a genetically informed cross‐lagged analysis. Dev. Sci. 2017;20(2) doi: 10.1111/desc.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills-Koonce W.R., Willoughby M.T., Zvara B., Barnett M., Gustafsson H., Cox M.J. Mothers’ and fathers’ sensitivity and children’s cognitive development in low-income, rural families. J. Appl. Dev. Psychol. 2015;38:1–10. doi: 10.1016/j.appdev.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P., Card J., Fox N. EEG correlates of the development of infant joint attention skills. Dev. Psychobiol. 2000;36(4):325–338. [PubMed] [Google Scholar]
- Murray L., Fiori‐Cowley A., Hooper R., Cooper P. The impact of postnatal depression and associated adversity on early mother‐infant interactions and later infant outcome. Child Dev. 1996;67(5):2512–2526. [PubMed] [Google Scholar]
- Murray L., Hipwell A., Hooper R., Stein A., Cooper P. The cognitive development of 5-year-old children of postnatally depressed mothers. J. Child Psychol. Psychiatry. 1996;37(8):927–935. doi: 10.1111/j.1469-7610.1996.tb01490.x. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W., Jr, Helmeke C., Braun K. Lack of paternal care affects synaptic development in the anterior cingulate cortex. Brain Res. 2006;1116:58–63. doi: 10.1016/j.brainres.2006.07.106. https://www.ncbi.nlm.nih.gov/pubmed/16945352 [DOI] [PubMed] [Google Scholar]
- Pleck J.H. 5th ed. John Wiley & Sons Inc.; Hoboken, NJ, US: 2010. Paternal Involvement: Revised Conceptualization and Theoretical Linkages with Child Outcomes the Role of the Father in Child Development; pp. 58–93. [Google Scholar]
- Pluess M., Belsky J. Children’s differential susceptibility to effects of parenting. Fam. Sci. 2010;1(1):14–25. [Google Scholar]
- Rajhans P., Goin-Kochel R.P., Strathearn L., Kim S. It takes two! Exploring sex differences in parenting neurobiology and behavior. J. Neuroendocrinol. 2019 doi: 10.1111/jne.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani P.G., Ijzendoorn Mv., Bakermans-Kranenburg M.J. Differential susceptibility to fathers’ care and involvement: the moderating effect of infant reactivity. Fam. Sci. 2010;1(2):93–101. doi: 10.1080/19424621003599835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Kong L., Sim L.W., Sanmugam S., Broekman B.F., Chen H. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl. Psychiatry. 2015;5:e668. doi: 10.1038/tp.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkadi A., Kristiansson R., Oberklaid F., Bremberg S. Fathers’ involvement and children’s developmental outcomes: a systematic review of longitudinal studies. Acta Paediatr. 2007;97(2):153–158. doi: 10.1111/j.1651-2227.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- Schwartze M., Kotz S.A. Contributions of cerebellar event-based temporal processing and preparatory function to speech perception. Brain Lang. 2016;161:28–32. doi: 10.1016/j.bandl.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Sethna V., Perry E., Domoney J., Iles J., Psychogiou L., Rowbotham N.E.L. Father-child interactions at 3 months and 24 months: contributions to children’s cognitive development at 24 months. Infant Ment. Health J. 2017;38(3):378–390. doi: 10.1002/imhj.21642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna V., Pote I., Wang S., Gudbrandsen M., Blasi A., McCusker C. Mother-infant interactions and regional brain volumes in infancy: an MRI study. Brain Struct. Funct. 2017;222(5):2379–2388. doi: 10.1007/s00429-016-1347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout P.E., Fleiss J.L. Intraclass correlations: uses in assessing rater reliability. Psychol. Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Stgeorge J., Freeman E. Measurement of father–Child Rough-and-tumble play and its relations to child behavior. Infant Ment. Health J. 2017;38(6):709–725. doi: 10.1002/imhj.21676. [DOI] [PubMed] [Google Scholar]
- Tamis-LeMonda C.S., Shannon J.D., Cabrera N.J., Lamb M.E. Fathers and mothers at play with their 2- and 3-year-olds: contributions to language and cognitive development. Child Dev. 2004;75(6):1806–1820. doi: 10.1111/j.1467-8624.2004.00818.x. [DOI] [PubMed] [Google Scholar]
- Tiemeier H., Lenroot R.K., Greenstein D.K., Tran L., Pierson R., Giedd J.N. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2009;49(1):63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towe-Goodman N.R., Willoughby M., Blair C., Gustafsson H.C., Mills-Koonce W.R., Cox M.J. Family Life Project Key Investigators Fathers' sensitive parenting and the development of early executive functioning. J. Fam. Psychol. 2014;28(6):867–876. doi: 10.1037/a0038128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut N., Beggiato A., Bourgeron T., Delorme R., Rondi-Reig L., Paradis A.-L. Cerebellar volume in autism: literature meta-analysis and analysis of the autism brain imaging data exchange cohort. Biol. Psychiatry. 2017;83(7):579–588. doi: 10.1016/j.biopsych.2017.09.029. [DOI] [PubMed] [Google Scholar]