Abstract
Poor quality of the early infant-parent bond predicts later child problems. Infant-parent attachment has been suggested to influence brain development, but this association has hardly been examined. In adults, larger amygdala volumes have been described in relation to early attachment disorganization; neuroimaging studies of attachment in children, however, are lacking.
We examined the association between infant-parent attachment and brain morphology in 551 children from a population-based cohort in the Netherlands. Infant-parent attachment was observed with the Strange-Situation Procedure at age 14 months and different brain measures were collected with magnetic resonance imaging at mean age 10 years.
Children with disorganized infant attachment had larger hippocampal volumes than those with organized attachment patterns. This finding was robust to the adjustment for confounders and consistent across hemispheres. The association was not explained by cognitive or emotional and behavioral problems. Disorganized attachment did not predict any other difference in brain morphology. Moreover, children with insecure organized infant attachment patterns did not differ from those who were securely attached in any brain outcome.
Causality cannot be inferred, but our findings in this large population-based study provide novel evidence for a long-term association between the quality of infant-parent attachment and specific brain differences in childhood.
Keywords: Hippocampus, Infant attachment, Brain morphology, Neuroimaging, Limbic system, Magnetic resonance imaging
1. Introduction
Infants have an innate tendency to seek parental protective proximity in stressful situations and this behavior is fostered by consistently available parents (Van IJzendoorn et al., 1999). If the caregiver is not consistently responsive, infants form an insecure, but organized attachment pattern (i.e. avoidant or resistant) (Van IJzendoorn et al., 1999). Some infants, however, develop a disorganized attachment, another typical variation of infant attachment. Infants with disorganized attachment display contradictory or stereotypical behavior when exposed to stress (Granqvist et al., 2017). This attachment pattern is considered to elevate the risk for later dissociative behavior and externalizing behavioral problems (Van IJzendoorn et al., 1999).
Infant attachment insecurity (i.e. avoidant, resistant or disorganized) has been hypothesized to influence brain development; in particular, amygdala and hippocampal morphology (Moutsiana et al., 2015). Although these limbic structures start developing in fetal life, a period of rapid growth occurs during infancy (Lupien et al., 2009). In addition, the development of the amygdala and the hippocampus is stress-sensitive. The stress hormone cortisol has a documented effect on the maturation and remodeling of axons and dendrites (Rinne-Albers et al., 2013), and the amygdala and the hippocampus have a high density of cortisol receptors, implying developmental vulnerability in conditions of sustained stress (Lupien et al., 2009). Animal research has shown that early psychosocial deprivation and poor caregiving conditions affect hypothalamic-pituitary-adrenal (HPA) axis functioning and cortisol production (Lupien et al., 2009). Moreover, an effect of early life stress on hippocampus-dependent memory functioning (Bonapersona et al., 2019) and amygdala and hippocampus morphology has been described (Bath et al., 2016; Coplan et al., 2014).
The association between highly adverse early caregiving conditions and brain morphology has repeatedly been examined in humans. Most studies in adults describe that the exposure to early life adversity is related to smaller hippocampal volumes (see for a meta-analysis, (Riem et al., 2015)), but not to amygdala volumetric differences (see for a meta-analysis, (Calem et al., 2017)). In children, however, the evidence is less consistent. Some studies reported no difference in the amygdala or hippocampal volumes between children with a history of maltreatment and those reporting no maltreatment (Riem et al., 2015; De Brito et al., 2013). Moreover, in the studies where differences were observed in the volume of these limbic structures, the direction of effect varied. (McLaughlin et al. (2016)) showed that the exposure to maltreatment was related to smaller amygdala and hippocampal volumes in a sample of 60 children aged 6 to 18 years old. Similar findings were described in relation to abuse and early life adversity (Hanson et al., 2015; Brooks et al., 2014). In contrast, Tupler and De Bellis (2006) observed larger hippocampal volumes in 4 to 17-year-old children with maltreatment-related PTSD, and Tottenham et al. (2010) found larger amygdala volumes in children after prolonged institutional rearing. Many factors may contribute to the heterogeneity across the studies on child maltreatment and limbic morphology, including the small size of most samples, unmeasured confounding by comorbid psychiatric disorders and additional stressors; and the variation in exposures and timing (Bick and Nelson, 2016). The type of adversity, the timing of the exposure occurrence and of the brain morphology measurement play a particularly important role. First, studies examining multiple types of adversity at the same time (such as physical abuse, sexual abuse and neglect) or traumatic events of great severity generally described smaller volumes of the amygdala and the hippocampus (McLaughlin et al. (2016); Brooks et al., 2014). Second, the timing of occurrence and the duration of the adversity are crucial because adversity occurring during different stages of brain development may affect it differently. In fact, Tottenham et al. (2010) found larger amygdala volumes only in children exposed to a longer period of institutional rearing, compared to those who were adopted early and those never institutionalized. Further, the child age at the brain morphology assessment may also affect results. The amygdala and hippocampus have non-linear developmental trajectories during childhood (Uematsu et al., 2012). Thus, the normal development may mask or change the association between adversity and the limbic volumes. Whittle et al. (2013) described seemingly contrasting findings in a longitudinal study that point to this explanation. Higher levels of childhood maltreatment were related to larger hippocampal volumes in early adolescence, but to a decrease in the normal hippocampal growth from early to mid-adolescence Whittle et al. (2013).
The association between the early child-parent relationship and brain morphology has also been examined in the general population. Although these studies are less confounded by factors that affect clinical samples, similarly inconsistent findings have been reported. Contrasting results can likely be attributed to the small sample sizes, differences in the sample characteristics, and the variation in the age of assessments. Most of these studies have focused on parental behavior, such as sensitivity or support. Two studies examined maternal sensitivity, observed during a non-stressful situation, in relation to brain structure in infancy. Rifkin-Graboi et al. (2015) described in 20 infant-mother dyads that reduced maternal sensitivity was related to larger hippocampal volumes, and Sethna et al. (2017) found an association between reduced sensitivity and smaller subcortical gray matter volumes (including the caudate, putamen, globus pallidus and thalamus) in a sample of 39 infants. A relation of early maternal sensitivity with brain volumes at later ages was documented by Bernier et al. (2019), who described that two dimensions of maternal sensitivity predicted smaller amygdala and hippocampal volumes in 33 10-year-old children. In contrast, higher levels of early parental sensitivity were not associated with the volume of these limbic structures, but predicted larger total brain and gray matter volumes in a subsample of 7-8-year-old children from the present cohort (N = 191) (Kok et al., 2015). Two studies examined other measures of maternal behavior and found similarly heterogeneous results. Luby et al. (2012) assessed maternal support in early childhood and found a positive relation with hippocampal volumes at ages 7–13 years (N = 92), whereas Rao et al. (2010) described that children receiving more parental nurturance, observed at age 4 years, had smaller hippocampal volumes at age 14 years (N = 49).
In contrast to this diverse literature documenting the neural correlates of early parental behavior in children from the general population, remarkably little is known regarding the association of infant-parent attachment, as a direct indicator of the child-parent relationship, and brain morphology. A few studies investigated this association in adults and only one focused on children. Leblanc et al. (2017) reported no association between early attachment security and amygdala volume in 33 10–11 year-old children, but larger gray matter volumes in regions of the temporal, frontal and parietal lobes in children who were securely attached in infancy. Moutsiana et al. (2015) examined 59 infant-parent dyads and observed that the insecurely attached infants had larger amygdala volumes as 22-year-old adults than those previously securely attached; no difference in hippocampal volumes was found. Lyons-Ruth et al. found in a sample of 18 29-year old adults from impoverished, highly-stressed families that the 12 adults with disorganized attachment at 18 months had greater amygdala volumes (Lyons-Ruth et al., 2016).
Early socioemotional deprivation and childhood trauma have also been described to influence the maturation of white matter microstructure in children (Daniels et al., 2013; Siehl et al., 2018). However, few studies have examined the association between infant-parent attachment and the white matter microstructure in the general population. A positive correlation between attachment security and fractional anisotropy of several tracts including the uncinate fasciculus and the hippocampal part of the cingulum was reported in an adult sample (Serra et al., 2015). Yet, in this study childhood attachment security was assessed with a retrospective self-reported measure, which could influence accuracy. Only one study has prospectively examined whether attachment security is related to white matter microstructure in children, and results were in the opposite direction compared to the adult sample. Dégeilh et al. (2019) found that lower attachment security at age 2 years predicted higher fractional anisotropy and lower mean diffusivity in a number of tracts at age 10 years, including the cingulum bundle. Given the scarcity and methodological limitations of the literature on the association between early attachment and later white matter microstructure, previous studies must be viewed as preliminary, thus precluding a hypothesis-driven approach when examining child white matter microstructure in relation to the early infant-parent bond.
We evaluated the association between infant attachment and brain morphology in middle childhood using a population-based sample (N = 551). We examined the hippocampal and amygdala volumes as regions of interest, based on theoretical and biological evidence for an association between adverse early caregiving experiences and the development of limbic structures. We hypothesized that insecure and especially disorganized patterns of infant-parent attachment are associated with differences in hippocampal and amygdala volumes in children. We additionally included the thalamus volume as a negative control sub-cortical structure, in which no effects were expected a-priori. Considering the scarcity of the existing literature regarding the association between early caregiving and brain regions other than the amygdala and hippocampus, we examined the relation between infant attachment and global brain structural metrics, vertex-wise cortical volume, and global white matter microstructural metrics with an exploratory approach. As we were particularly interested in the limbic structures, we additionally explored the association of infant attachment with white matter tracts related to the limbic system.
2. Methods and materials
2.1. Settings and population
This study was embedded in the Generation R Study, an ongoing population-based cohort in Rotterdam, the Netherlands (Kooijman et al., 2016). The Generation R Study follows children of mothers with a delivery date from April 2002 to January 2006 (61% response at baseline). From the children of the 9778 mothers enrolled in the study, a subsample with Dutch ethnic background (i.e. children whose parents and grandparents were born in the Netherlands) was randomly selected for detailed assessments, such as behavioral observations. The study was approved by the Medical Ethics Committee of the Erasmus Medical Center, Rotterdam, and informed consent was obtained from all participating parents and children.
Among the 1106 infant-parent dyads participating in the postnatal phase of this subgroup, 882 visited the research center at age 14 months, during which infant-parent attachment was assessed (Tharner et al., 2011). When one parent participated in the assessment of attachment with two children, we randomly excluded one (n = 24). We also excluded 29 children for whom attachment quality could not be coded because of technical or procedural problems. Brain MRI scans were obtained when children were 10 years old. Of the 829 children with attachment data, 588 (71%) had brain-imaging data. Children with poor image quality of the structural MRI data were excluded from the structural MRI analyses (n = 86), as were children with major incidental findings (n = 2). Similarly, 85 children with non-usable DTI data and 1 child with a major incidental finding were excluded from the DTI analyses. In total, 551 children were included in one or more analyses (500 with structural MRI and 502 with DTI data; Supplementary Fig. 1).
2.2. Measures
2.2.1. Attachment assessment
Infant-parent attachment was assessed in relation to the primary caregiver with the Strange Situation Procedure when infants were 14.6 (SD = 0.9) months old (Tharner et al., 2011). This validated procedure is designed to evoke mild stress in the infant and trigger attachment behavior (Ainsworth et al., 1978). It consists of eight 3 min-episodes in which the parent leaves the infant in a room twice; first with a female stranger, and later leaving the infant alone. After each separation, the parent reenters the room and the behavior of the child during these reunion episodes is observed. Due to limited time the pre-separation episodes were slightly shortened without impact on the validity of the measures (Tharner et al., 2011). Two reliable raters, trained and supervised, coded the attachment behavior from DVD-recordings, according to the (Ainsworth et al., 1978) and Main and Solomon (1990) coding systems. Inter-rater agreement was based on 70 cases independently coded by both raters. The inter-rater agreement on the ABCD attachment classification was 77% (kappa = 0.63), and the inter-rater agreement on disorganized versus non-disorganized attachment was 87% (kappa = 0.64) (Tharner et al., 2011). As previously described (Tharner et al., 2012), the distributions of attachment security and disorganization in our study cohort did not differ from those reported in a meta-analysis of normative non-US western samples.
2.2.2. Brain imaging
2.2.2.1. Acquisition
Magnetic resonance imaging was performed when children where 9 to 11 years old. Children were familiarized with the scanning environment in a mock scanning session, prior to the actual scanning session. Brain images were acquired on a 3 Tesla scanner (General Electric MR750w, Milwaukee, WI, USA) with an eight-channel head coil for signal reception. Details of the images acquisition are provided elsewhere (White et al., 2018). High-resolution T1-weighted images were obtained with an inversion recovery fast-spoiled gradient recalled sequence (sequence parameters: TR = 8.77 ms, TE = 3.4 ms, TI = 600 ms, Flip Angle = 10°, Field of View (FOV) = 220 x 220 mm, Acquisition Matrix = 220 × 220, slice thickness = 1 mm, number of slices = 230, Parallel Imaging Factor = 2). The diffusion weighted images were collected with an axial spin echo, echo-planar imaging sequence with 3 volumes with b = 0 s/mm2 (no diffusion weighting) and 35 diffusion-weighted images (sequence parameters: TR = 12,500 ms, TE = 72.8 ms, FOV = 240 x 240 mm, Acquisition Matrix = 120 × 120, slice thickness = 2 mm, number of slices = 65, Asset Acceleration Factor = 2, b = 900 s/mm2).
2.2.2.2. Image Processing
Cortical reconstruction and volumetric segmentation were conducted with the FreeSurfer image suite version 6.0 (http://surfer.nmr.mgh.harvard.edu/). In brief, removal of non-brain tissue, voxel intensity normalization, segmentation of subcortical structures, cortical reconstruction and definition of anatomic metrics were performed. FreeSurfer morphometric processes have shown good test-retest reliability (Han et al., 2006). The cortical volume-based map for each participant was smoothed with a 10 mm full width, half-maximum Gaussian kernel. The anatomical metrics included in analyses were total brain, total gray matter and cortical white matter volumes, average cortical thickness, and the mean volume (averaged over both hemispheres) of the amygdala, hippocampus and thalamus, and vertex-wise cortical volume.
The diffusion tensor imaging (DTI) data was processed with the FMRIB Software Library (FSL) (Jenkinson et al., 2012), and the Camino diffusion MRI toolkit (Cook et al., 2006). Non-brain tissue was removed and images were corrected for motion and eddy-current artifacts. The resulting transformation matrices were used to rotate the gradient direction table to account for rotations applied to the data. The diffusion tensor was fit at each voxel, and common scalar metrics (global fractional anisotropy (FA) and mean diffusivity (MD)) were computed. Fully-automated probabilistic tractography was run using a set of predefined seed and target masks, resulting in connectivity distributions for a number of large fiber bundles (de Groot et al., 2015). Mean FA and MD were extracted from each tract, and confirmatory factor analysis was used to generate latent FA and MD measures across 12 tracts which represent global white matter microstructure across the brain (cingulum bundle, corticospinal tract, forceps major, forceps minor, inferior longitudinal fasciculus, superior longitudinal fasciculus and the uncinate fasciculus) (Muetzel et al., 2018). (For more details on the probabilistic tractography, see the Supplementary Materials).
FreeSurfer image reconstructions of the T1 images were visually inspected for quality and all scans rated as unusable were excluded from statistical analyses (Muetzel et al., 2018). Diffusion image quality was assessed by manual and automated inspection. For more information on the image quality inspection see the Supplementary Materials.
2.3. Covariates
Potential confounders were selected a priori based on previous research (Moutsiana et al., 2015; Lyons-Ruth et al., 2016; Tharner et al., 2011). These included child sex, birthweight, total intracranial volume, age at the MRI scan, smoking and alcohol use during pregnancy, maternal education, maternal psychiatric symptoms and breastfeeding. Information on child sex and birthweight was obtained from midwives and hospital registries. Total intracranial volume was extracted from the processed structural imaging data. Child age at the MRI scan was based on the date of birth and date of the imaging data collection. Maternal self-reports of prenatal smoking and alcohol consumption were collected during pregnancy. Maternal education was self-reported in pregnancy and at two postnatal time points and was classified based on the highest completed education into: low (no bachelor), medium (university bachelor) and high education (further education) (Statistics Netherlands, 2004). Maternal psychiatric symptoms, assessed with the Brief Symptom Inventory (Derogatis, 1993), and current breastfeeding practices (exclusive breastfeeding, breast- and bottle-feeding, and bottle-feeding), were reported by mothers when children were 2 months old.
Traumatic life events, child IQ and children’s emotional and behavioral problems were included as covariates in sensitivity analyses. The information on traumatic life events was collected with an interview with the caregiver when children were 9 years old (previously described in Dunn et al. (2019)). In this assessment, caregivers were asked to indicate whether the children had experienced one or more of a list of 24 life events. A cumulative score was created by summing the occurrence of the events, with higher values representing more events. Child IQ was assessed in the research center when children were 5–7 years old, with a validated Dutch nonverbal intelligence test: Snijders-Oomen Niet-verbale intelligentie test, 2.5-7- revisie (SON-R 2.5–7) (Tellegen et al., 2005). When children were approximately 9 years old, mothers completed the Child Behavioral Checklist (CBCL) for ages 6-18. The CBCL is a standardized, valid instrument that measures behavioral and emotional problems in children (Achenbach and Rescorla, 2001). In our analyses, we included the Total Problems scale.
2.4. Statistical analysis
We examined two main dimensions of infant attachment. First, we compared children with disorganized infant attachment to those with an organized attachment (i.e. secure, resistant or avoidant). Then, we compared children with an insecure organized attachment pattern (i.e. avoidant or resistant) to those securely attached, excluding the children with disorganized attachment. The mean amygdala and hippocampal volumes were our primary outcomes. The volume of the thalamus was included as a control sub-cortical structure, to test the specificity of effects. Other brain structural measures (i.e. average cortical thickness and total brain, total gray matter and cortical white matter volumes, and vertex-wise cortical volume) and white matter metrics (global FA and MD) were examined in exploratory analyses. All brain measures were standardized to have a mean of zero and a standard deviation of one.
First, we explored the bivariate associations among the main variables in our study using Pearson’s and phi correlations. Then, we examined the association between infant-parent attachment and the brain outcomes with multiple linear regression models, adjusted for child sex, child age at MRI scan, maternal education, maternal psychiatric symptoms and alcohol consumption during pregnancy. Total intracranial volume was included as a covariate in the analyses with specific brain volumetric measures (i.e. amygdala, hippocampus and thalamus volumes) and white matter connectivity measures (Takao et al., 2011). We included in our models the covariates selected based on literature. As the theoretical evidence for a confounding effect of birthweight, breastfeeding, alcohol consumption and smoking during pregnancy is not very strong and can be debated, we tested the change-in-estimate criterion on our hypothesized associations (i.e. disorganized attachment with hippocampal and amygdala volumes) to decide whether to include them as confounders. Of these variables, only alcohol consumption changed the effect estimate in more than 10%, and thus was included as a confounder (Greenland, 1989; Walter and Tiemeier, 2009). We adjusted for confounders in two models. First, we controlled our analyses for child sex and child age at MRI scan (and total intracranial volume in specific analyses) to take into account brain maturation differences and to facilitate comparison with other studies. Second, we further adjusted the analyses for the confounding effect of the modifiable variables prenatal alcohol consumption, maternal education and maternal psychiatric symptoms.
The associations between attachment disorganization and insecurity with cortical volume were examined at each cortical vertex with similarly adjusted models, using the QdecR package version 2.0 (https://github.com/slamballais/QDECR). To account for multiple testing, cortical volume vertex-wise analyses were adjusted using Gaussian Monte Carlo Simulations (Hagler et al., 2006) with a cluster forming threshold (CFT) of p = 0.001 (Greve and Fischl, 2018) and a cluster-wise p-value of p < 0.025 (Bonferroni-corrected for two hemispheres).
Several sensitivity analyses were conducted. First, we examined the hemisphere-specific associations with the amygdala and hippocampus. Second, to examine the possibility of misclassification, we repeated our analyses excluding the children who had an attachment classification available that was rated as possibly problematic due to minor technical or procedural difficulties (n = 26 for structural, n = 23 for DTI). Third, we examined if the exclusion of children with minor incidental findings on the brain image such as asymmetric ventricles changed the results (White et al., 2018) (n = 30 for structural, n = 29 for DTI). Fourth, we excluded infant-father dyads (n = 69 for structural, n = 73 for DTI). And fifth, we tested the interaction between child sex and attachment security and disorganization on amygdala and hippocampal volumes.
We additionally adjusted our analyses in separate models for child traumatic life events, child IQ score and child emotional and behavioral problems. Disorganized attachment is more common among infants experiencing traumatic life events (such as maltreatment) (Van IJzendoorn et al., 1999), and such events are also related to hippocampal morphology (Tottenham and Sheridan, 2010). Similarly, the quality of attachment and brain development have been related to cognitive and psychological differences (Granqvist et al., 2017; Harris and Corriveau, 2011; Lenroot and Giedd, 2006). As these factors may confound the association and also represent proxies of the exposure (i.e. traumatic life events and infant attachment) or outcome (child cognition and behavior and child brain), controlling for these factors could represent overadjustment and bias our associations. We included these variables as covariates in sensitivity analyses, with a hypothesis-generating approach, in an attempt to examine whether they explain the associations between infant attachment and child brain morphology.
All analyses were conducted using the R statistical software (version 3.5.1) (R Core Team, 2018). Missing values (maximum percentage: maternal psychopathology = 17.2%) were imputed with the Multivariate Imputations by Chained Equations (MICE) package (version 3.3.0) (van Buuren and Groothuis-Oudshoorn, 2011) generating 20 imputed datasets.
2.5. Non-response analysis
We compared the children included in our study (n = 551) with the children who were lost to follow-up (n = 241) using t-tests and Mann-Whitney U tests for continuous and chi-square tests for categorical variables. We found no difference in child birth weight, sex (study sample: 49% girls, lost to follow-up: 50% girls, p = 0.79) or attachment classification (study sample: secure = 51%, avoidant = 12%, resistant = 15%, disorganized = 22%; lost to follow-up: secure = 51%, avoidant = 14%, resistant = 17%, disorganized = 18%. p = 0.59). Similarly, maternal psychopathology (p = 0.33) and maternal education (education in study sample: low: 27%, medium: 31%, high: 42%; in lost to follow-up: low: 31%, medium: 32%, high:37%, p = 0.42) did not substantially differ between the groups.
3. Results
The correlations between the main variables are shown in Supplementary Table 1. No strong correlations were observed between the attachment variables and the covariates. In total, 51% of the children had a secure, 15% a resistant, 12% an avoidant and 22% a disorganized attachment pattern. Table 1 presents the baseline characteristics of the sample for organized and disorganized attachment dyads. Of the children with organized attachment, 49% were girls, while this was 46% in the disorganized attachment group. A larger percentage of mothers had a high education in the organized attachment group (44%) compared to those in the disorganized attachment group (33%, p = 0.02). No difference was observed between the organized and disorganized attachment groups regarding child age at the MRI scan, birthweight, child IQ score, child behavioral and emotional problems and maternal psychiatric symptoms. Similarly, the main study variables did not differ when comparing secure and insecure dyads (Supplementary Table 2).
Table 1.
Sample characteristics by attachment disorganization.
| Organized | Disorganized | ||
|---|---|---|---|
| n = 431 | n = 120 | ||
| mean(SD) or %* | mean(SD) or %* | p | |
| Child characteristics | |||
| Sex, % girls | 49.2 | 45.8 | 0.58 |
| Age at the MRI scan, years | 10.1 (0.6) | 10.2 (0.6) | 0.22 |
| Birth weight, grams | 3524.3 (534.1) | 3515.5 (534.5) | 0.87 |
| Age at the Attachment assessment, months | 14.6 (0.9) | 14.6 (0.8) | 0.93 |
| Attachment classification (%) | – | ||
| Secure | 65.2 | 0 | |
| Avoidant | 16.0 | 0 | |
| Resistant | 18.8 | 0 | |
| Disorganized | 0 | 100 | |
| Child IQ score | 106.9 (13.0) | 107.2 (12.8) | 0.69 |
| Child Total Problems score, CBCL global scale, median (range) | 13.1 (0, 82.7) | 14.5 (0, 58) | 0.67 |
| Maternal characteristics | |||
| Education, % | 0.02 | ||
| Low | 27.9 | 25.9 | |
| Medium | 28.1 | 40.8 | |
| High | 44.0 | 33.3 | |
| Maternal Psychopathology, BSI score, median (range) | 0.1 (0, 2.3) | 0.1 (0, 0.7) | 0.34 |
Characteristics of the sample with available information for attachment and brain structural and/or DTI MRI data (n = 551).
Otherwise indicated. Groups were compared in the first imputed dataset with independent t-tests and Mann-Whitney U tests for continuous variables and chi-square tests for categorical variables.
Children with a disorganized infant attachment had, on average, 0.17 standard deviation larger amygdala volumes (SE = 0.08, p = 0.04) and 0.21 standard deviation larger hippocampal volumes (SE=0.08, p = 0.02) than children with organized attachment, accounting for total intracranial volume, child sex and age (Table 2) (see also Fig. 1). After additional adjustment for prenatal alcohol consumption, maternal education and psychiatric symptoms the association with mean hippocampal volume remained (b = 0.21, SE = 0.09, p = 0.02), but disorganized attachment was not significantly associated with the amygdala volume (b=0.16, SE=0.08, p = 0.06) anymore. No association was observed between disorganized attachment and any of the global brain measures, the thalamus, or the DTI metrics. In addition, we explored the association between attachment disorganization and the microstructure of the white matter tracts related to the limbic system, namely the uncinate fasciculus, the cingulum bundle and the parahippocampal part of the cingulum. We observed higher FA in the left uncinate fasciculus in children with disorganized attachment compared to those with an organized attachment pattern (b=0.22, SE=0.11, p = 0.04). However, this association did not survive multiple testing correction (False discovery rate (Benjamini and Hochberg, 1995) for 12 tests: 3 hemisphere-specific white matter tracts with FA and MD). Although the direction of the association is arguably consistent with that of the structural analysis of the hippocampus, this result should be interpreted with caution.
Table 2.
Attachment disorganization and brain morphology.
| Brain Outcomes |
|||||||
|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
||||||
| n | b | SE | P | b | SE | P | |
| Determinant | |||||||
| Disorganized Attachment, yes | |||||||
| Outcome | |||||||
| Global brain measures | |||||||
| Total brain volume | 500 | −0.08 | 0.09 | 0.37 | −0.07 | 0.09 | 0.47 |
| Total gray matter volume | 500 | −0.09 | 0.09 | 0.33 | −0.06 | 0.09 | 0.49 |
| Cortical white matter volume | 500 | −0.07 | 0.09 | 0.43 | −0.07 | 0.10 | 0.44 |
| Total cortical thickness, average | 500 | 0.16 | 0.11 | 0.14 | 0.16 | 0.11 | 0.15 |
| Global fractional anisotropy (DTI) | 502 | −0.04 | 0.10 | 0.71 | −0.03 | 0.11 | 0.80 |
| Global mean diffusivity (DTI) | 502 | −0.04 | 0.10 | 0.69 | −0.04 | 0.10 | 0.73 |
| Specific brain volumetric measures | |||||||
| Amygdala volume, average | 500 | 0.17 | 0.08 | 0.04 | 0.16 | 0.08 | 0.06 |
| Left Amygdala | 500 | 0.17 | 0.09 | 0.05 | 0.16 | 0.09 | 0.07 |
| Right Amygdala | 500 | 0.15 | 0.09 | 0.09 | 0.13 | 0.09 | 0.12 |
| Hippocampus volume, average | 500 | 0.21 | 0.08 | 0.02 | 0.21 | 0.09 | 0.02 |
| Left Hippocampus | 500 | 0.18 | 0.09 | 0.03 | 0.18 | 0.09 | 0.04 |
| Right Hippocampus | 500 | 0.21 | 0.09 | 0.02 | 0.22 | 0.09 | 0.02 |
| Thalamus volume, average | 500 | 0 | 0.07 | 0.95 | −0.01 | 0.07 | 0.90 |
Model 1 was adjusted for: total ICV (total intracranial volume), child age at brain MRI scan, child sex. Model 2 was additionally adjusted for: maternal education, maternal psychiatric symptoms and alcohol use during pregnancy. Global brain structural measures were not adjusted for total ICV. All outcomes were standardized.
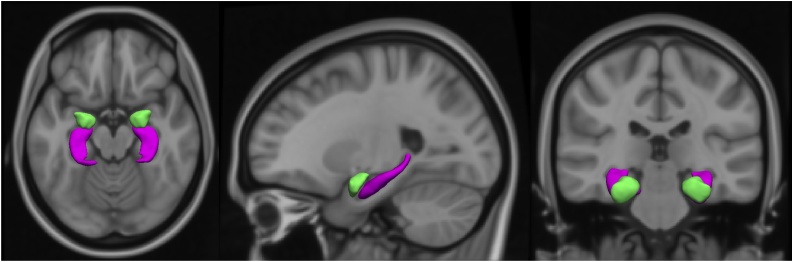
Fig. 1.
T1-weighted MRI scan (axial, sagittal and coronal view) showing the amygdala (in green) and hippocampus (in purple) segmentation (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
Table 3 shows that infants with an organized insecure attachment (i.e. avoidant or resistant) did not differ from those who were securely attached in any of the child brain measures (i.e. mean amygdala, hippocampus and thalamus volumes, average cortical thickness, total brain, total gray matter and cortical white matter volumes and global diffusion metrics).
Table 3.
Attachment security and brain morphology.
| Brain Outcomes | |||||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||
| n | b | SE | P | b | SE | P | |
| Determinant | |||||||
| Insecure Attachment, yes | |||||||
| Outcome | |||||||
| Global brain measures | |||||||
| Total brain volume | 390 | −0.02 | 0.09 | 0.82 | −0.02 | 0.09 | 0.80 |
| Total gray matter volume | 390 | 0.03 | 0.09 | 0.77 | 0.03 | 0.09 | 0.77 |
| Cortical white matter volume | 390 | −0.07 | 0.09 | 0.46 | −0.08 | 0.10 | 0.42 |
| Total cortical thickness, average | 390 | −0.02 | 0.11 | 0.86 | −0.03 | 0.11 | 0.77 |
| Global fractional anisotropy (DTI) | 392 | 0.01 | 0.10 | 0.90 | −0.01 | 0.10 | 0.94 |
| Global mean diffusivity (DTI) | 392 | −0.14 | 0.10 | 0.16 | −0.11 | 0.10 | 0.27 |
| Specific brain volumetric measures | |||||||
| Amygdala volume, average | 390 | −0.01 | 0.08 | 0.91 | −0.03 | 0.08 | 0.75 |
| Hippocampus volume, average | 390 | −0.05 | 0.08 | 0.53 | −0.05 | 0.09 | 0.60 |
| Thalamus volume, average | 390 | 0.07 | 0.07 | 0.31 | 0.08 | 0.07 | 0.27 |
Children with insecure organized attachment (avoidant or resistant attachment) were compared to children with secure attachment, excluding the children with disorganized attachment. Model 1 was adjusted for: total ICV (total intracranial volume), child age at brain MRI scan, child sex. Model 2 was additionally adjusted for: maternal education, maternal psychiatric symptoms and alcohol use during pregnancy. Global brain structural measures were not adjusted for total ICV. All outcomes were standardized.
Whole-brain exploratory analyses were performed to examine the associations of disorganized and insecure infant attachment with vertex-wise cortical volume. No associations were observed after adjusting for multiple testing.
3.1. Sensitivity analyses
The positive association of disorganized infant attachment with hippocampal volume was observed consistently in both hemispheres (adjusted left: b = 0.18, SE = 0.09, p = 0.04, adjusted right: b=0.22, SE=0.09, p = 0.02). After excluding cases with technical or procedural difficulties in the attachment assessment, disorganization of attachment was still related to larger hippocampal volumes (b=0.22, SE=0.09, p = 0.01). Similar results were also obtained after the exclusion of children who had minor incidental findings on MRI; the difference in hippocampal volume between children with and without disorganized infant attachment was, if anything, larger (b=0.23, SE=0.09, p = 0.01). The exclusion of infant-father dyads did not meaningfully change the results (disorganized attachment and hippocampal volume, adjusted model: b=0.23, SE=0.09, p = 0.01) (Supplementary Table 3 and 4). No interaction between disorganized infant attachment and child sex was found in the analyses with amygdala and hippocampal volumes.
The number of traumatic life events did not explain the association between disorganized attachment and hippocampal volume. After adjusting our analyses for traumatic life events, the association between disorganized attachment and hippocampal volume remained unchanged (mean hippocampal volume: b = 0.21, SE = 0.09, p = 0.02).
We also explored whether the association between disorganized attachment and hippocampal volume was explained by child IQ, assessed with a non-verbal test at 5–7 years of age, or by child emotional and behavioral problems, reported by the mothers with the Child Behavioral Checklist (CBCL) at age 9 years. We found no evidence for this explanation; the effect estimate did not change after additional adjustment for child IQ (mean hippocampal volume: b = 0.20, SE = 0.09, p = 0.02) nor after adjustment for the total CBCL score (mean hippocampal volume: b=0.21, SE=0.09, p = 0.02).
4. Discussion
In this population-based study, infants with disorganized attachment had larger hippocampal volumes in middle childhood. A similar association between disorganized attachment and amygdala volume did not reach significance. Disorganized attachment was not related to any other brain measure. Organized (in-)security of attachment did not predict any difference in specific or global brain measures.
Although often hypothesized based on biological insights, there is surprisingly little epidemiological evidence for the relation between the quality of the infant-parent attachment relationship and the development of limbic structures. Two small studies reported an association between insecure (including disorganized) infant-parent attachment and larger amygdala volume in adulthood (Moutsiana et al., 2015; Lyons-Ruth et al., 2016). In contrast to adult studies, infant attachment security did not predict any difference in the amygdala volume in a small developmental study (Leblanc et al., 2017).
To date, no study has examined the association between disorganized attachment and the limbic structures in childhood; previous studies broadly examined insecure infant-parent attachment, which likely included some infants with disorganized attachment. In contrast to the organized insecure attachment patterns (i.e. resistant and avoidant), disorganization of attachment is considered a major risk factor for later aggressive behavior and psychopathology (Van IJzendoorn et al., 1999). Additionally, most of the evidence on the neural correlates of the infant-parent relationship in the general population comes from studies of maternal sensitivity and support. Although the assessment of these maternal behaviors gives insight in the quality of the early caregiving, the infant-parent attachment offers a direct perspective on the infant-parent relationship (De Wolff and van IJzendoorn, 1997). Moreover, maternal sensitivity is known to predict the development of insecure attachment (De Wolff and van IJzendoorn, 1997) but it only weakly predicts the attachment disorganization (Van IJzendoorn et al., 1999). Typical antecedents of this attachment pattern are maltreatment and a parent’s unresolved loss or trauma (Granqvist et al., 2017). Thus, these issues need to be considered when interpreting our results in the light of findings on other measures of early caregiving. Moreover, studies on maternal sensitivity and the hippocampal volume are not consistent, with some reporting no difference, others a positive association, and some others a relation with a negative direction of effect. Whereas Kok et al. (2015) found no difference in the hippocampal volumes in a subset of the present cohort, Luby et al. (2012) described a positive relation of maternal support and larger hippocampal volumes in a study oversampled for child depression. Rao et al. (2010), in contrast, observed that less parental nurturance at age 4 years was related to larger hippocampal volume in adolescence, using data from a cohort that studies the prenatal use of cocaine.
We observed that disorganized infant-parent attachment is related to larger hippocampal volume in childhood. This finding may seem counterintuitive as larger volumes often indicate better functioning (Tupler and De Bellis, 2006). However, larger hippocampus and amygdala volumes must be understood within the rubric of developmental trajectories. Both structures undergo non-linear volumetric changes during childhood, develop rapidly during infancy and reach a peak volume during preadolescence (9–11 years) (Uematsu et al., 2012). Thus, the age period in which the brain structures are measured can influence the direction and strength of the association as differences may be masked or distorted by the developmental trajectories. Second, the severity of the adversity and additional co-occurring stressors may also influence results (Bick and Nelson, 2016). Children exposed to extreme adverse experiences such as maltreatment and institutional rearing are not only exposed to more severe adversities but also are likely to experience several other stressors, such as poverty and violence. It is possible that these events affect the brain developmental trajectories in a different way (Bick and Nelson, 2016). Finally, it has been suggested that some brain regions can have an initial accelerated development in response to stress, followed by a volumetric reduction when the exposure to the event is sustained (Callaghan and Tottenham, 2016). The larger hippocampal volume observed in children with disorganized infant attachment could reflect an initial response to stress, induced by disruptions in the infant-parent relationship. Disorganization of attachment is an indicator of stressful experiences, where the infant is confronted with a paradox: their caregiver is the source of fright and comfort at the same time (Van IJzendoorn et al., 1999; Granqvist et al., 2017). As the hippocampus is involved in the stress response and has large quantities of glucocorticoid receptors (Lupien et al., 2009), stress during infancy can influence its development. The exposure to early stress may induce an initial hypertrophy, increase in dendritic arborization and precocious myelination in the hippocampus, which might be followed by a volumetric reduction only if the exposure to stress continues throughout the life course (Tottenham and Sheridan, 2010). Our findings could be explained by an accelerated hippocampal development in response to challenging environmental factors. As suggested by animal and human studies, poor early caregiving may promote a precocious development of neural regions key in memory and emotion regulation (Bath et al., 2016; Thijssen et al., 2017). This accelerated development has been hypothesized to have evolutionary implications, as it may represent a biological strategy developed to increase survival and reproduction in unfavorable conditions (Belsky et al., 1991).
There are also other potential explanations for the relation between infant attachment and hippocampal volume in middle childhood. First, the hypothalamic hormone oxytocin has been shown to promote neurogenesis in the hippocampus (Sánchez-Vidaña et al., 2016) and to be involved in bonding behavior (Galbally et al., 2011). High oxytocin levels are related to a more stimulating and affective parenting behavior (Abraham and Feldman, 2018) and reduce the cortisol response to stress (Ditzen et al., 2009). In adverse early caregiving conditions, the low oxytocin levels may alter the hippocampal maturation. Although taken together these findings suggest a relation between oxytocin and child social and neural development, the possible role of oxytocin is yet to be elucidated (Galbally et al., 2011). Another possibility is that these limbic structural differences reflect a neurobiological predisposition to the formation of a disorganized infant-parent attachment. In fact, parental behavior only partly explains the etiology of a disorganized attachment, suggesting that other factors, such as genetics and biological infant characteristics, could play a role (Tharner et al., 2011). As described by Spangler et al. (1996), the status of disorganized attachment may be predicted by newborn emotional regulation and orientation to external stimuli, both of which are hippocampal-related tasks (Immordino-Yang and Singh, 2013; Bird and Burgess, 2008). However, most hypotheses trying to explain the association between infant attachment and limbic morphological differences are still highly speculative. First and foremost, these findings need to be replicated in similarly large population-based samples and the direction of the association needs to be examined with repeated MRI assessments.
In our study, the quality of attachment did not relate to differences in global brain volumetric measures, the vertex-wise cortical volume, or a non-limbic subcortical structure. This suggests that the associations pertain to the development of limbic structures, rather than a globally altered neurodevelopment. Also, although the quality of attachment and the hippocampal development are generally related to psychosocial adversity, child cognition and behavioral problems, the differences in hippocampal volume remained after these factors were accounted for in the analyses. Therefore, our findings appear to be specific for the disorganization of attachment, rather than explained by factors often related to the attachment quality. If replicated, the specificity of this association would underscore the importance of the early infant-parent attachment quality in the normative neurodevelopment of children.
Small effect sizes are expected for studies of parent-child interaction and subcortical brain structures after birth given that the development of subcortical structures, such as the hippocampus, occurs mostly prenatally and during infancy and less during childhood (Lupien et al., 2009). Thus, although we examined a relatively large sample of children, further population-based studies with large samples and repeated MRI and attachment measures are needed to examine the mechanism and direction of the association. Several limitations of our study should be considered. We cannot exclude reverse causality as disorganized attachment may be a marker of infant stress related to hippocampal development. Also, the sample of infant-father dyads in our study was rather small, precluding the evaluation of the specific relation between infant-father attachment and brain development.
In this study, disorganized early-life attachment was related to larger hippocampal volume in middle childhood. Our findings extend the knowledge on the relation between infant-parent attachment and limbic system morphology with evidence for an association between disorganized attachment and a subcortical structure key to emotional and cognitive processing. Causality cannot be inferred, but our results in a large prospective population-based sample suggest that disorganized infant attachment has a long-term relation with child neurological development.
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Acknowledgements
The authors gratefully acknowledge the contributions of the participating children and parents, general practitioners, hospitals, midwives, and pharmacies in Rotterdam, the Netherlands. The Generation R Study was conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation, and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond in Rotterdam.
Supercomputing resources were supported by NWO Physical Sciences Division (Exacte Wetenschappen) and SurfSara (Cartesius compute cluster).The work of Andrea P. Cortes Hidalgo and Marinus H. van IJzendoorn is supported by the Netherlands Organization for Scientific Research (Spinoza Prize to MvIJ). Marian J. Bakermans-Kranenburg is supported by the: European Research Council (ERC AdG, grant number 669249). Marian J. Bakermans-Kranenburg and Marinus H. van IJzendoorn are additionally supported by the Gravitation program of the Dutch Ministry of Education, Culture, and Science and the Netherlands Organization for Scientific Research (NWO grant number 024.001.003). Hanan El Marroun is supported by the European Union's Horizon 2020 research and innovation program [grant agreement No.633595 DynaHEALTH] and No.733206 LifeCycle. The neuroimaging and neuroimaging infrastructure is supported by the Netherlands Organization for Health Research and Development (ZonMw) TOP project number 91211021 awarded to Tonya White. The work of Henning Tiemeier is supported by the Netherlands Organization for Scientific Research (NWO-grant 016.VICI.170.200) and the European Union Seventh Framework Program (FP7/2007–2013): ACTION: Aggression in Children: Unravelling gene-environment interplay to inform Treatment and InterventiON strategies (grant number 602768). The general design of the Generation R Study was made possible by financial support from the Erasmus Medical Centre, the Erasmus University, the Dutch Ministry of Health, Welfare and Sport, and the Netherlands Organization for Health Research and Development.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.dcn.2019.100724.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Van IJzendoorn M.H., Schuengel C., Bakermans–Kranenburg M.J. Disorganized attachment in early childhood: Meta-analysis of precursors, concomitants, and sequelae. Dev. Psychopathol. 1999;11:225–250. doi: 10.1017/s0954579499002035. [DOI] [PubMed] [Google Scholar]
- Granqvist P., Sroufe L.A., Dozier M., Hesse E., Steele M., van IJzendoorn M. Disorganized attachment in infancy: a review of the phenomenon and its implications for clinicians and policy-makers. Attach. Hum. Dev. 2017;19:534–558. doi: 10.1080/14616734.2017.1354040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsiana C., Johnstone T., Murray L., Fearon P., Cooper P.J., Pliatsikas C. Insecure attachment during infancy predicts greater amygdala volumes in early adulthood. J. Child Psychol. Psychiatry. 2015;56:540–548. doi: 10.1111/jcpp.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10:434. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Rinne-Albers M.A.W., van der Wee N.J.A., Lamers-Winkelman F., Vermeiren R.R.J.M. Neuroimaging in children, adolescents and young adults with psychological trauma. Eur. Child Adolesc. Psychiatry. 2013;22:745–755. doi: 10.1007/s00787-013-0410-1. [DOI] [PubMed] [Google Scholar]
- Bonapersona V., Kentrop J., Van Lissa C.J., van der Veen R., Joëls M., Sarabdjitsingh R.A. The behavioral phenotype of early life adversity: a 3-level meta-analysis of rodent studies. Neurosci. Biobehav. Rev. 2019;102:299–307. doi: 10.1016/j.neubiorev.2019.04.021. [DOI] [PubMed] [Google Scholar]
- Bath K.G., Manzano-Nieves G., Goodwill H. Early life stress accelerates behavioral and neural maturation of the hippocampus in male mice. Horm. Behav. 2016;82:64–71. doi: 10.1016/j.yhbeh.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan J.D., Fathy H.M., Jackowski A.P., Tang C.Y., Perera T.D., Mathew S.J. Early life stress and macaque amygdala hypertrophy: preliminary evidence for a role for the serotonin transporter gene. Front. Behav. Neurosci. 2014:8. doi: 10.3389/fnbeh.2014.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem M.M.E., Alink L.R.A., Out D., Van IJzendoorn M.H., Bakermans-Kranenburg M.J. Beating the brain about abuse: empirical and meta-analytic studies of the association between maltreatment and hippocampal volume across childhood and adolescence. Dev. Psychopathol. 2015;27:507–520. doi: 10.1017/S0954579415000127. [DOI] [PubMed] [Google Scholar]
- Calem M., Bromis K., McGuire P., Morgan C., Kempton M.J. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2017;14:471–479. doi: 10.1016/j.nicl.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Brito S.A., Viding E., Sebastian C.L., Kelly P.A., Mechelli A., Maris H. Reduced orbitofrontal and temporal grey matter in a community sample of maltreated children. J. Child Psychol. Psychiatry. 2013;54:105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Gold A.L., Duys A., Lambert H.K., Peverill M. Maltreatment exposure, brain structure, and fear conditioning in children and adolescents. Neuropsychopharmacology. 2016;41:1956–1964. doi: 10.1038/npp.2015.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J.L., Nacewicz B.M., Sutterer M.J., Cayo A.A., Schaefer S.M., Rudolph K.D. Behavioral problems after early life stress: contributions of the Hippocampus and amygdala. Biol. Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.J., Dalvie S., Cuzen N.L., Cardenas V., Fein G., Stein D.J. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: a voxel-based morphometry study. Metab. Brain Dis. 2014;29:311–321. doi: 10.1007/s11011-014-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler L.A., De Bellis M.D. Segmented hippocampal volume in children and adolescents with posttraumatic stress disorder. Biol. Psychiatry. 2006;59:523–529. doi: 10.1016/j.biopsych.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Quinn B.T., McCarry T.W., Nurse M., Gilhooly T. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev. Sci. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bick J., Nelson C.A. Early adverse experiences and the developing brain. Neuropsychopharmacology. 2016;41:177–196. doi: 10.1038/npp.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A., Matsui M., Tanaka C., Takahashi T., Noguchi K., Suzuki M. Developmental trajectories of amygdala and Hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Dennison M., Vijayakumar N., Simmons J.G., Yücel M., Lubman D.I. Childhood maltreatment and psychopathology affect brain development during adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:940–952. doi: 10.1016/j.jaac.2013.06.007. e941. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Kong L., Sim L.W., Sanmugam S., Broekman B.F.P., Chen H. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl. Psychiatry. 2015;5:e668. doi: 10.1038/tp.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna V., Pote I., Wang S., Gudbrandsen M., Blasi A., McCusker C. Mother–infant interactions and regional brain volumes in infancy: an MRI study. Brain Struct. Funct. 2017;222:2379–2388. doi: 10.1007/s00429-016-1347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier A., Dégeilh F., Leblanc É, Daneault V., Bailey H.N., Beauchamp M.H. Mother–infant interaction and child brain morphology: a multidimensional approach to maternal sensitivity. Infancy. 2019;24:120–138. doi: 10.1111/infa.12270. [DOI] [PubMed] [Google Scholar]
- Kok R., Thijssen S., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Verhulst F.C., White T. Normal variation in early parental sensitivity predicts child structural brain development. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:824–831. doi: 10.1016/j.jaac.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Luby J.L., Barch D.M., Belden A., Gaffrey M.S., Tillman R., Babb C. Maternal support in early childhood predicts larger hippocampal volumes at school age. Proc. Natl. Acad. Sci. 2012;109:2854–2859. doi: 10.1073/pnas.1118003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H., Betancourt L., Giannetta J.M., Brodsky N.L., Korczykowski M., Avants B.B. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. NeuroImage. 2010;49:1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc É, Dégeilh F., Daneault V., Beauchamp M.H., Bernier A. Attachment security in infancy: a preliminary study of prospective links to brain morphometry in late childhood. Front. Psychol. 2017:8. doi: 10.3389/fpsyg.2017.02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K., Pechtel P., Yoon S.A., Anderson C.M., Teicher M.H. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav. Brain Res. 2016;308:83–93. doi: 10.1016/j.bbr.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels J.K., Lamke J.-P., Gaebler M., Walter H., Scheel M. White matter integrity and its relationship to PTSD and childhood trauma —a systematic review and meta-analysis. Depress. Anxiety. 2013;30:207–216. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- Siehl S., King J.A., Burgess N., Flor H., Nees F. Structural white matter changes in adults and children with posttraumatic stress disorder: a systematic review and meta-analysis. Neuroimage Clin. 2018;19:581–598. doi: 10.1016/j.nicl.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M., De Pisapia N., Rigo P., Papinutto N., Jager J., Bornstein M.H. Secure attachment status is associated with white matter integrity in healthy young adults. NeuroReport. 2015;26:1106–1111. doi: 10.1097/WNR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dégeilh F., Bernier A., Leblanc É, Daneault V., Beauchamp M.H. Early mother-child attachment as a predictor of child white matter microstructure: a 9-year longitudinal study. Poster session presented at the society for research in child development 2019. Biennial Meeting; Baltimore, Maryland; 2019. [Google Scholar]
- Kooijman M.N., Kruithof C.J., van Duijn C.M., Duijts L., Franco O.H., van IJzendoorn M.H. The Generation R Study: design and cohort update 2017. Eur. J. Epidemiol. 2016;31:1243–1264. doi: 10.1007/s10654-016-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharner A., Herba C.M., MPCM Luijk, van IJzendoorn M.H., Bakermans-Kranenburg M.J., Govaert P.P. Subcortical structures and the neurobiology of infant attachment disorganization: a longitudinal ultrasound imaging study. Soc. Neurosci. 2011;6:336–347. doi: 10.1080/17470919.2010.538219. [DOI] [PubMed] [Google Scholar]
- Ainsworth M.D.S., Blehar M.C., Waters E., Wall S. Erlbaum; Hillsdale, NJ: 1978. Patterns of Attachment: A Psychological Study of the Strange Situation. [Google Scholar]
- Main M., Solomon J. University of Chicago Press; Chicago, IL, US: 1990. Procedures for Identifying Infants As disorganized/disoriented During the Ainsworth Strange Situation. Attachment in the Preschool Years: Theory, Research, and Intervention; pp. 121–160. [Google Scholar]
- Tharner A., MPCM Luijk, van IJzendoorn M.H., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Hofman A. Maternal lifetime history of depression and depressive symptoms in the prenatal and early postnatal period do not predict infant–mother attachment quality in a large, population-based Dutch cohort study. Attach. Hum. Dev. 2012;14:63–81. doi: 10.1080/14616734.2012.636659. [DOI] [PubMed] [Google Scholar]
- White T., Muetzel R.L., El Marroun H., Blanken L.M.E., Jansen P., Bolhuis K. Paediatric population neuroimaging and the Generation R Study: the second wave. Eur. J. Epidemiol. 2018;33:99–125. doi: 10.1007/s10654-017-0319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X., Jovicich J., Salat D., van der Kouwe A., Quinn B., Czanner S. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E.J., Woolrich M.W., Smith S.M. Fsl. NeuroImage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Cook P.A., Bai Y., Nedjati-Gilani S., Seunarine K.K., Hall M.G., Parker G.J. Camino: Open-source diffusion-MRI reconstruction and processing. 14th Scientific Meeting of the International Society for Magnetic Resonance in Medicine; Seattle WA, USA, Pp 2759; 2006. [Google Scholar]
- de Groot M., Ikram M.A., Akoudad S., Krestin G.P., Hofman A., van der Lugt A. Tract-specific white matter degeneration in aging: the Rotterdam Study. Alzheimer’s & Dementia. 2015;11:321–330. doi: 10.1016/j.jalz.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Muetzel R.L., Blanken L.M.E., Jvd Ende, Marroun H.E., Shaw P., Sudre G. Tracking brain development and dimensional psychiatric symptoms in children: a longitudinal population-based neuroimaging study. Am. J. Psychiatry. 2018 doi: 10.1176/appi.ajp.2017.16070813. 175:appi.ajp.2017.16070813. [DOI] [PubMed] [Google Scholar]
- Statistics Netherlands . Statistics Netherlands (Centraal Bureau voor de Statistiek); Voorburg/Heerlen, the Netherlands: 2004. Standaard Onderwijsindeling 2003 (Dutch Standard Classification of Education 2003) [Google Scholar]
- Derogatis L.R. 3rd ed. National Computer Systems; Minneapolis, MN: 1993. Brief Symptom Inventory (BSI): Administration, Scoring and Procedures Manual. [Google Scholar]
- Dunn E.C., Nishimi K., Neumann A., Renaud A., Cecil C.A.M., Susser E.S. Time-dependent effects of exposure to physical and sexual violence on psychopathology symptoms in late childhood: in search of sensitive periods in development. J Am Acad Child Psy. 2019 doi: 10.1016/j.jaac.2019.02.022. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellegen P., Winkel M., Wijnberg-Williams B., Laros J.A. Boom Test Uitgevers.; Amsterdam: 2005. Snijders-Oomen Niet-Verbale Intelligentietest: SON-R 2 ½ -7. [Google Scholar]
- Achenbach T.M., Rescorla L.A. University of Vermont, Research Center for Children, Youth, & Families; Burlington, VT: 2001. Manual for the ASEBA School-age Forms & Profiles. [Google Scholar]
- Takao H., Hayashi N., Inano S., Ohtomo K. Effect of head size on diffusion tensor imaging. NeuroImage. 2011;57:958–967. doi: 10.1016/j.neuroimage.2011.05.019. [DOI] [PubMed] [Google Scholar]
- Greenland S. Modeling and variable selection in epidemiologic analysis. Am. J. Public Health. 1989;79:340–349. doi: 10.2105/ajph.79.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter S., Tiemeier H. Variable selection: current practice in epidemiological studies. Eur. J. Epidemiol. 2009;24:733. doi: 10.1007/s10654-009-9411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Saygin A.P., Sereno M.I. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. False positive rates in surface-based anatomical analysis. NeuroImage. 2018;171:6–14. doi: 10.1016/j.neuroimage.2017.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2010;3 doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.L., Corriveau K.H. Young children’s selective trust in informants. Philos. Trans. Biol. Sci. 2011;366:1179–1187. doi: 10.1098/rstb.2010.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot R.K., Giedd J.N. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing.; Vienna, Austria: 2018. R: a Language and Environment for Statistical Computing. [Google Scholar]
- van Buuren S., Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in r. J. Stat. Softw. 2011;45:67. [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- De Wolff M.S., van IJzendoorn M.H. Sensitivity and attachment: a meta-analysis on parental antecedents of infant attachment. Child Dev. 1997;68:571–591. [PubMed] [Google Scholar]
- Callaghan B.L., Tottenham N. The Stress Acceleration Hypothesis: effects of early-life adversity on emotion circuits and behavior. Curr. Opin. Behav. Sci. 2016;7:76–81. doi: 10.1016/j.cobeha.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Tiemeier H., Verhulst F.C. Insensitive parenting may accelerate the development of the amygdala–medial prefrontal cortex circuit. Dev. Psychopathol. 2017;29:505–518. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- Belsky J., Steinberg L., Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 1991;62:647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Vidaña D.I., N-MJ Chan, Chan A.H.L., Hui K.K.Y., Lee S., Chan H.-Y. Repeated treatment with oxytocin promotes hippocampal cell proliferation, dendritic maturation and affects socio-emotional behavior. Neuroscience. 2016;333:65–77. doi: 10.1016/j.neuroscience.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Galbally M., Lewis A.J., van IJzendoorn M., Permezel M. The role of oxytocin in mother-infant relations: a systematic review of human studies. Harv. Rev. Psychiatry. 2011;19:1–14. doi: 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- Abraham E., Feldman R. The neurobiology of human allomaternal care; implications for fathering, coparenting, and children’s social development. Physiol. Behav. 2018;193:25–34. doi: 10.1016/j.physbeh.2017.12.034. [DOI] [PubMed] [Google Scholar]
- Ditzen B., Schaer M., Gabriel B., Bodenmann G., Ehlert U., Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol. Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Spangler G., Fremmer-Bombik E., Grossmann K. Social and individual determinants of infant attachment security and disorganization. Infant Ment. Health J. 1996;17:127–139. [Google Scholar]
- Immordino-Yang M.H., Singh V. Hippocampal contributions to the processing of social emotions. Hum. Brain Mapp. 2013;34:945–955. doi: 10.1002/hbm.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird C.M., Burgess N. The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 2008;9:182. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.