Abstract
Maternal care may predict limbic development, though relations may vary by age and type of assessment. Here, we examined maternal behavior during early infancy (i.e., six months postpartum) in relation to offspring hippocampal and amygdala volume and microstructure development between 4.5 (n = 99) and 6 (n = 111) years. In interaction with offspring sex, maternal sensitivity predicted left amygdala volume at 6.0 years (β=-0.214, p = 0.032, df = 89) and independently predicted predominately left lateralized aspects of amygdala and hippocampal microstructure at both time points (hippocampus: left FA at 4.5 years [β=-0.241, p = 0.043, df = 68], and, in interaction with sex, left [(β = 0.349, p = 0.022, df = 86) and right FA at 6 years (β = 0.357, p = 0.016, df = 86] and left MD growth [β = -0.517, p = 0.021, df = 37]; amygdala: left MD at 4.5 years [β = -0.319, p = 0.007, df = 69] and, in interaction with offspring sex, left MD growth [β = -0.546, p = 0.019, df = 37]). Results suggest exposure to non-extreme, early insensitive care impacts neuroanatomy important to learning and stress regulation, perhaps by accelerating development. This underscores the need to promote sensitive caregiving during early infancy within community samples.
Keywords: Maternal sensitivity, Infancy, Hippocampus, Amygdala, MRI, DTI, Accelerated development
1. Introduction
Early caregiving predicts children’s cognitive and socioemotional development, even within non-clinical samples (Deans, 2018). Differences in caregiving predict changes in early developing neuroanatomical regions like the hippocampus and amygdala, which are influenced by stress hormones exposure, as is common in the face of caregiving risk. In rodent research, stress associates with reduced hippocampal receptor expression, dendritic spine density, and cell number, increased amygdala dendritic length (McEwen, Nasca et al. 2016), and astrocytes, a type of glial cell important to supporting synaptic function. Still, the nature of relations may depend on both the timing and form of caregiving and neuroanatomical assessment.
1.1. Timing of the caregiving assessment
Exposure age may influence whether and how caregiving impacts the limbic system. In rats, certain forms of hippocampal cells with the ability to express Corticotrophin Releasing Hormone/Factor (CRH/CRF) are more common during early postnatal life than at later stages of development (Chen et al., 2004). Hippocampal neurons expressing the CRF receptor are also observed at early postnatal stages, with the receptor’s expression primarily occurring on dendrites at the earliest stages of development. Interestingly, CRF expressing cells may be relevant to dendritic branching and are also a component of stress responsivity. Taken together, such studies highlight ways in which stress exposure may have unique effects during earlier stages of life when such cells are more abundantly expressed than at later time points. As another example early, but not later, exposure to stress hormones may downregulate amygdala NMDA receptor gene expression.
Exposure timing may be of particular importance to amygdala volumetric development. The majority of studies examining variation in caregiving and/or related attachment relationships during infancy and amygdala volume in infancy (Rifkin-Graboi, Kong et al. 2015), childhood, and adulthood suggest more caregiving adversity predicts larger amygdalae. In contrast, caregiving, observed between four to seven and eight years, is not predictive of amygdala volumes in children aged six to twelve (2013), nor between ages 13–16.
1.2. Timing of neuroanatomical assessment
Because normative variation in early life sensitive caregiving may speed the pace of hippocampal (Rifkin-Graboi, Quan et al. 2018; Wang et al., 2019b) and amygdala (Thijssen, Muetzel et al. 2017) development, when outcomes are assessed becomes important. Hippocampal and amygdala volume increase rapidly through toddlerhood, and then start to stabilize, perhaps reaching their volumetric peaks at age 9–11 (Utsunomiya, Takano et al. 1999). Thus on average, pace-related differences in hippocampal volume may be most observable early in life, though the exact timing of periods of growth versus plateau are also influenced by sex, structure (amygdala versus hippocampus) and laterality.
Similarly, normative patterns of microstructure development may influence when pace related changes in Fractional Anisotropy (FA) and Mean Diffusivity (MD) can be observed. Examining FA and MD is important because they are sensitive to developmental processes, such as neuronal and axonal organization, membrane proliferation, and axonal myelination, which cannot be reflected by volumetric measurement (Qiu et al., 2015a,b).
FA and MD growth is not strictly linear, though FA tends to increase and MD to decrease across early development (Qiu et al., 2015a). More specifically, FA increases rapidly within the cerebral cortex from 0 to 24 months, after which point growth continues, but at a slower pace (Saksena, Husain et al. 2008). Likewise, MD rapidly declines between 0–24 months, before continuing to decline but at a slower pace (Saksena, Husain et al. 2008). Such changes are not consistent across neuroanatomical regions. For example, Uda and colleagues (Uda, 2015) observed FA growth in multiple cerebral regions to plateau around age 6, except within the right hippocampus (i.e., the cingulum) amongst girls.
The timing of neuroanatomical assessment, alongside differences in when and how caregiving is assessed, may explain mixed findings concerning hippocampal volume. Two groups have found negative associations between sensitivity in infancy (i.e,. 6 and 12 months) and hippocampal volume (i.e., during infancy (Rifkin-Graboi, Kong et al. 2015), as well as at age ten (Bernier et al., 2019) . However, no findings have also been reported between maternal behaviour and/or forms of insecure attachment assessed in later infancy (e.g., 12–18 months) and hippocampal volume in childhood and adulthood Furthermore, research focusing on caregiving quality during preschool reports negative relations to left hippocampal volume in early adolescence, and, alternatively, positive associations with hippocampal volume in late childhood/early adolescence. Finally, variation in caregiving during school age may have nil effects on late childhood/early adolescent volumes.
1.3. The current research
We examined maternal sensitivity six months postpartum in relation to offspring amygdala and hippocampal volume, FA, and MD between 4–6 years of age. Based on past work suggesting the special importance of early infancy, we expected maternal sensitivity assessed at six months to be associated with accelerated limbic development in early childhood. However, we also expected that the manner in which such relations manifest might depend on the structure (hippocampus versus amygdala) and neuroanatomical parameters (volume, FA, or MD). We additionally considered an interactive role of offspring sex; neuroanatomy may differential develop in girls versus boys. The results of this study provide, to our knowledge, the first analysis linking low maternal sensitivity during early infancy (i.e., at six months) and multiple early childhood neural outcomes within a general population.
2. Methods
2.1. Participants
Participants reflect subsamples of mother-child dyads participating in the prospective Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. GUSTO children were recruited for this neuroimaging study when they were 4.5 years and 6 years of age respectively. The GUSTO cohort initially recruited pregnant Singapore citizens or Permanent Residents of Chinese, Malay or Indian ethnic backgrounds from two major birthing hospitals in Singapore at the first antenatal visit (Soh, Lee et al. 2012) for further details). The GUSTO study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB) and the Sing Health Centralized Institutional Review Board (CIRB). Written informed consent was obtained from mothers.
Maternal education, ethnicity, age, and monthly household income were extracted from survey questionnaires at the 26th week of pregnancy. Birth outcomes, including gestational age, birth weight, APGAR score, and sex, were obtained from the hospital record.
Inclusion into the current study required gestational age ≥ 34 weeks, birth weight ≥ 2 kg and a 5-min APGAR score ≥ 8 (to avoid potential effects of birth complications on brain development) and available maternal depression data. The number of participating dyads in the T1 (volume) and DTI (FA/MD) 4.5-year, 6-year, and 4,5-to-6-year Growth subsamples are, respectively as follows: T1: 86, 99, and 57, ; DTI: 77, 95, and 46. Please see Supplementary Fig. 1 for additional details on the flow of the subject selection.
2.2. Maternal depressive symptoms
The Beck Depression Inventory-II (BDI-II) was administered to mothers at 3 months post-partum. The BDI-II is a widely used 21-item questionnaire assessing the existence and severity of depression symptoms (Beck, Ward et al. 1961). Each item of the BDI-II is scored on a four-point scale (0–3). Higher total scores indicate more severe depressive symptoms.
2.3. Maternal sensitivity
A 15-minute mother-child interaction was recorded as part of a three-hour laboratory visit when infants were six months of age (+/- 2 weeks), with toys/books only present during the last 10 min of recording. The mother was asked to “interact or play” with her six-month old infant “as she normally would at home.” Maternal sensitivity was assessed using the Revised Mini-A short form of the Maternal Behavioral Q-Sort-V (Mini-MBQS-V) (Tsotsi et al., 2018). The Mini-MBQS-V consists of 25-items, each representing different possible aspects of sensitive, and inversely, insensitive, maternal behaviour during interaction with an infant. Coders sort the 25 items into 5 piles of 5 cards each, ranging from 1 being "least like the mother", to 5 being "most like the mother." For example, if an observed mother’s behaviour is very sensitive coders might place cards such as: “Mother builds on the focus of the baby’s attention” and “Mother responds to the baby’s distress and non-distress signals even when engaged in some other activity.. . “in the “5″ pile. Likewise, when viewing a mother who is very sensitive, coders might put cards with descriptors such as, “Mother tends to tune out and not notice the infant’s bids for attention” and “The content and pace of the interaction is set by the mother rather than the baby’s response” into the “1″ pile. The pile assignment for the observed mother’s cards is then correlated with the a priori card rankings of a theoretically constructed prototypical sensitive mother. Thus, the observed mother is ultimately assigned a global sensitivity score that reflects the correlation with the prototype. Hence, the observed sensitivity score ranges from “-1″ (very much unlike a prototypical sensitive mother) to “1″ (very much similar to a prototypical sensitive mother). Southeast Asian coders, fluent in English and (individually) at least one of the predominant mother tongue languages of Singapore--Mandarin, Bahasu Melayu, and Mandarin achieved inter-coder reliability within the larger GUSTO singleton naturally conceived sample ranging In the larger sample 63 of 473 (13%) cases were independently coded by at least two of the three coders with good-to-excellent reliability. That is, 31 were coded by all three coders with an Absolute ICC (3,1) = 0.720, and individual pairs Absolute ICC (3,1)’s ranged from 0.661, 0.667, and 0.861 respectively across 35,31, and 59 person samples.
2.4. MRI acquisition and analysis
Children underwent MRI scans at the age of 4.5years (± 5month) and 6 years (± 7month) using a 3 T Siemens Skyra scanner with a 32-channel head coil at KK Women’s and Children’s hospital. Children were recruited during a 4-year and 6-year home visit. Children went through a MRI home training program prior to the MRI visit and on-site MRI training (see details in the Supplementary Material). The image protocols were: (i) high-resolution isotropic T1-weighted Magnetization Prepared Rapid Gradient Recalled Echo (MPRAGE; 192 slices, 1 mm thickness, in-plane resolution 1 mm, sagittal acquisition, field of view 192 × 192 mm, matrix = 192 × 192, repetition time = 2000 ms, echo time = 2.08 ms, inversion time = 877 ms, flip angle = 9°, scanning time = 3.5 min); (ii) isotropic axial diffusion weighted imaging protocol (single-shot echo-planar sequence, 69 slices of 2.0 mm thickness, with no inter-slice gaps, matrix 96 × 96, field of view 192 x 192 mm, repetition time = 8200 ms, echo time = 85 ms, flip angle = 90°, 30 diffusion weighted images with b = 1000s/mm2, 5 baseline images without diffusion weighting, GRAPPA = 3, scanning time = 5.5 min).
The image quality was verified immediately after the acquisition through visual inspection when children were still in the scanner. A scan was repeated when ring artefact on T1-weighted images and signal loss or check-board appearance on DTI (more than 5 volumes) were large. The image was removed from the study if no acceptable image was acquired after three repetitions.
To eliminate potential profound effects of head motion on our statistical results, we manually checked image quality based on the stringent criteria in (Ducharme et al., 2016). Disqualified images were excluded from this study. FreeSurfer was used to label each voxel in the T1-weighted image as cortical grey matter (GM), or white matter (WM), or cerebrospinal fluid (CSF), or subcortical structures (e.g., hippocampus, amygdala, thalamus, caudate, putamen, globus pallidus) (Fischl, Salat et al. 2002). FreeSurfer employed a Markov random field (MRF) model that requests for a prior probability obtained from a training dataset with T1-weighted images and their manual structural labels. In this study, we reconstructed the prior probability in the MRF model based on the manual segmentation of 30 Asian children and embedded it in FreeSurfer (replacing RB_all_2008-03-26.gca under freesurfer/average). FreeSurfer was then performed to each T1-weighted image in this study. Post-processing quality check was conducted following by the instruction on https://surfer.nmr.mgh.harvard.edu/fswiki/FsTutorial/TroubleshootingData.
For DTI data, we focused on FA and MD. FA expresses the degree to which water diffusion is restricted in one direction relative to others. It ranges from zero to one with zero being isotropic diffusion, and one being diffusion constrained in a single direction. Mean diffusivity (MD) corresponds to the magnitude of water diffusion. We conducted two quality check steps on diffusion weighted images (DWIs). First, we manually checked head motion of DWIs. If there were more than 5 DWI volumes with check-board appearance or signal loss, the dataset was removed from this study. Second, for the datasets passing through the first step, eddy current correction was applied to further correct for head motion (Huang, Ceritoglu et al. 2008). Using multivariate least-square fitting, six elements of the diffusion tensor were then determined, from which fractional anisotropy (FA) was calculated. The hippocampus mask in the T1-weighted image was then superimposed to the FA images through affine transformation obtained between the image without diffusion weighting and T1-weighted image. Mean FA values were computed for the hippocampus and used in the following statistical analysis.
2.5. Statistical analysis
Multiple regression analysis was used to investigate the interactive effects of maternal sensitivity and sex on left and right hippocampus/amygdala (a) volume, (b) FA and (c) MD at (1) 4.5 years of age, (2) 6 years of age and (3) growth from 4.5 years to 6 years of age. Growth was calculated by subtracting the 4.5 -year measure (i.e., volume, FA or MD) from the 6.0-year measure.
Variables that were previously found related to neuroanatomical measures and/or factors associated with maternal sensitivity were included as covariates. Covariates were entered into the first block of equations and included age at MRI, maternal ethnicity, maternal education, and postnatal maternal depressive symptoms. Even though prenatal maternal depressive symptoms also impact brain development of offspring (Gao et al., 2019; Lee et al., 2019; Lugo-Candelas et al., 2018; Ong et al., 2019; Qiu et al., 2015b, 2017; Rifkin-Graboi et al., 2013; Wang et al., 2018, 2019a; Wen et al., 2017), this study did not include prenatal maternal depressive symptoms as covariate. When considering limbic growth, the average age at both time points was included. In regressions predicting limbic volume, total brain volume (TBV) also served as a covariate, with TBV at both time points for growth analyses.
In the second block, mean-centered maternal sensitivity and sex were entered. The interaction term, the product of mean-centred maternal sensitivity and sex, was entered into the third block. When interactive effect was not significant, a reduced model, controlling for the same covariates and sex, examined maternal sensitivity in relation to the same outcome measures.
3. Results
3.1. Demographics
Maternal and child characteristics did not significantly differ across the 4.5, 6.0, and Growth Samples (See Supplementary Table 1).
Sex did not significantly associate with any of these characteristics, except for gestational age (See Supplementary Table 2).
Maternal sensitivity was not correlated with gestational age, birth weight, maternal ethnicity, and postnatal maternal depressive symptoms in both 4.5- and 6.0-year samples (all p > 0.05). Supplementary Tables 3 and 4 show no associations between maternal depression and the amygdala and hippocampus at these time points. However, maternal sensitivity was significantly associated with maternal education (r = 0.226, p = 0.025 for the 4.5-year sample; r = 0.204, p = 0.029 for the 6.0-year sample).
3.2. Relations between maternal sensitivity and the amygdala
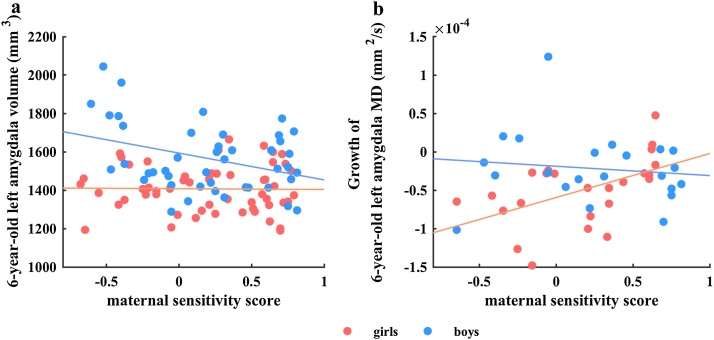
Neither maternal sensitivity alone, or in interaction with sex predicted amygdala volume at 4.5 years of age (Table 1). However, at 6.0 years of age, maternal sensitivity interacted with sex to predict left (β = -0.214, p = .032, df = 89) but not right (β = -150, p = .173, df = 89) amygdala volume. Post hoc analyses indicated that maternal sensitivity predicted smaller amygdala volumes in six year old boys (β = -0.331, p = 0.038, df = 47), with no significant associations in girls (β = -0.010, p = 0.995, df = 38; see Fig. 1a).
Table 1.
Maternal Sensitivity in Relation with Amygdala Volume, Fractional Anisotropy (FA), and Mean Diffusivity (MD).
| Standardized Beta (β) | Degrees of Freedom | P Value | Standardized Beta (β) | Degrees of Freedom | P Value | |
|---|---|---|---|---|---|---|
| Left Amygdala Volume | Right Amygdala Volume | |||||
| 4.5 Year Olds | ||||||
| sex-Sensitivity Interaction | 0.002 | 77 | 0.986 | 0.047 | 77 | 0.718 |
| Sensitivity | 0.046 | 78 | 0.640 | −0.032 | 78 | 0.766 |
| 6.0 Year Olds | ||||||
| sex-Sensitivity Interaction | −0.214 | 89 | 0.032 | −0.150 | 89 | 0.173 |
| Sensitivity | -- | −0.068 | 90 | 0.435 | ||
| Growth | ||||||
| sex-Sensitivity Interaction | −0.217 | 47 | 0.271 | −0.179 | 47 | 0.331 |
| Sensitivity | −0.001 | 48 | 0.993 | −0.219 | 48 | 0.140 |
| Left Amygdala FA | Right Amygdala FA | |||||
| 4.5 Year Olds | ||||||
| sex-Sensitivity Interaction | −0.008 | 68 | 0.962 | 0.198 | 68 | 0.244 |
| Sensitivity | −0.136 | 69 | 0.248 | −0.169 | 69 | 0.169 |
| 6.0 Year Olds | ||||||
| sex-Sensitivity Interaction | 0.217 | 86 | 0.161 | 0.183 | 86 | 0.235 |
| Sensitivity | −0.131 | 87 | 0.213 | −0.061 | 87 | 0.557 |
| Growth | ||||||
| sex-Sensitivity Interaction | 0.148 | 37 | 0.550 | 0.084 | 37 | 0.782 |
| Sensitivity | 0.028 | 38 | 0.862 | 0.195 | 38 | 0.219 |
| Left Amygdala MD | Right Amygdala MD | |||||
| 4.5 Year Olds | ||||||
| sex-Sensitivity Interaction | 0.280 | 68 | 0.082 | 0.039 | 68 | 0.826 |
| Sensitivity | −0.319 | 69 | 0.007 | 0.079 | 69 | 0.536 |
| 6.0 Year Olds | ||||||
| sex-Sensitivity Interaction | 0.007 | 86 | 0.960 | −0.040 | 86 | 0.799 |
| Sensitivity | −0.171 | 87 | 0.089 | −0.153 | 87 | 0.146 |
| Growth | ||||||
| sex-Sensitivity Interaction | −0.546 | 37 | 0.019 | −0.144 | 37 | 0.540 |
| Sensitivity | -- | 0.074 | 38 | 0.630 | ||
Interaction effects of maternal sensitivity with sex, and independent effects of maternal sensitivity, on left and right amygdala volume, fractional anisotropy (FA), and mean diffusivity (MD) as well as growth from 4.5 years to 6 years of age. Standardized β values are listed in the table. Note, *p < 0.05 level. The reduced model is not reported where the interaction model is significant.
Fig. 1.
Scatterplots of (a) interaction of maternal sensitivity and sex, with 6-year-old left amygdala volume, (b) interaction of maternal sensitivity and sex with growth of 6-year-old left amygdala MD. Standardized β values and p-values of the regression analysis in each sex group after adjustment for covariates reported in scatterplots (a–b). Abbreviations: MD, mean diffusivity.
In contrast, no associations between maternal sensitivity alone or in interaction with sex and amygdala FA were observed (Table 1).
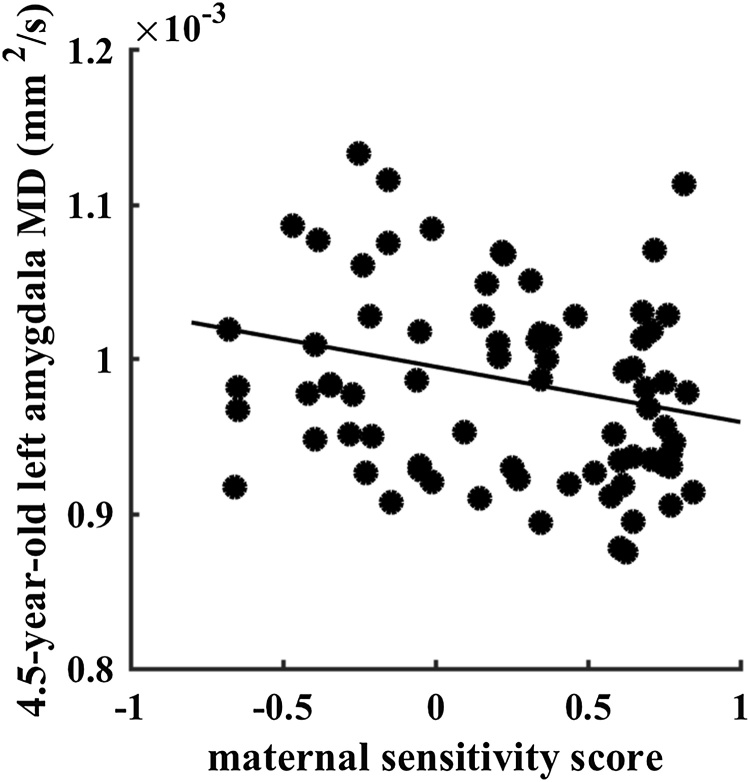
Still, higher maternal sensitivity was related to lower left (β= -0.319, p = 0.007, df = 69, see Fig. 2), but not right (β = 0.079, p = 0.536, df = 69), amygdala MD at 4.5 years of age. Though no significant effects on MD were observed at 6 years, maternal sensitivity in interaction with sex was predictive of MD growth from 4.5 to 6 years in the left (β = -0.546, p = 0.019, df = 37) but not right amygdala (β = -0.144, p = 0.540, df = 37). Post hoc analyses indicated that higher levels of maternal sensitivity associated with more left MD growth in girls (β = 0.570, p = 0.022, df = 17) but not boys (β = 0.014, p = 0.961, df = 15), see Fig. 1b.
Fig. 2.
Scatterplot of maternal sensitivity with 4.5-year-old left amygdala mean diffusivity (MD).
3.3. Relations between maternal sensitivity and the hippocampus
No significant associations between maternal sensitivity and hippocampal volume were observed, including when the interactive role of sex was considered (see Table 2).
Table 2.
Maternal Sensitivity in Relation with Hippocampal Volume, Fractional Anisotropy (FA), and Mean Diffusivity (MD).
| Standardized Beta (β) | Degrees of Freedom | P Value | Standardized Beta (β) | Degrees of Freedom | P Value | |
|---|---|---|---|---|---|---|
| Left Hippocampal Volume | Right Hippocampal Volume | |||||
| 4.5 Year Olds | ||||||
| Sex-Sensitivity Interaction | 0.076 | 77 | 0.540 | 0.036 | 77 | 0.766 |
| Sensitivity | −0.041 | 78 | 0.683 | 0.023 | 78 | 0.819 |
| 6.0 Year Olds | ||||||
| Sex-Sensitivity Interaction | −0.059 | 88 | 0.565 | −0.092 | 89 | 0.375 |
| Sensitivity | −0.125 | 89 | 0.126 | −0.089 | 90 | 0.277 |
| Growth | ||||||
| Sex-Sensitivity Interaction | −0.183 | 46 | 0.322 | −0.296 | 47 | 0.126 |
| Sensitivity | −0.011 | 47 | 0.939 | −0.071 | 48 | 0.646 |
| Left Hippocampal FA | Right Hippocampal FA | |||||
| 4.5 Year Olds | ||||||
| Sex-Sensitivity Interaction | −0.075 | 67 | 0.651 | 0.182 | 67 | 0.272 |
| Sensitivity | −0.241* | 68 | 0.043 | −0.168 | 68 | 0.157 |
| 6.0 Year Olds | ||||||
| Sex-Sensitivity Interaction | 0.349* | 86 | 0.022 | 0.357* | 86 | 0.016 |
| Growth | ||||||
| Sex-Sensitivity Interaction | 0.272 | 37 | 0.268 | −0.030 | 37 | 0.899 |
| Sensitivity | 0.100 | 38 | 0.533 | 0.129 | 38 | 0.403 |
| Left Hippocampal MD | Right Hippocampal MD | |||||
| 4.5 Year Olds | ||||||
| Sex-Sensitivity Interaction | −0.118 | 68 | 0.498 | −0.160 | 67 | 0.363 |
| Sensitivity | 0.115 | 69 | 0.358 | 0.066 | 68 | 0.597 |
| 6.0 Year Olds | ||||||
| Sex-Sensitivity Interaction | −0.199 | 85 | 0.218 | −0.097 | 84 | 0.549 |
| Sensitivity | −0.055 | 86 | 0.611 | 0.108 | 85 | 0.319 |
| Growth | ||||||
| Sex-Sensitivity Interaction | −0.517* | 37 | 0.021 | −0.083 | 36 | 0.734 |
| Sensitivity | --- | −0.026 | 37 | 0.866 | ||
Interaction effects of maternal sensitivity with sex, and independent effects of maternal sensitivity, on left and right hippocampal volume, fractional anisotropy (FA), and mean diffusivity (MD) as well as growth from 4.5 years to 6 years of age. Standardized β values are listed in the table. Note, *p < 0.05 level. The reduced model is not reported where the interaction model is significant.
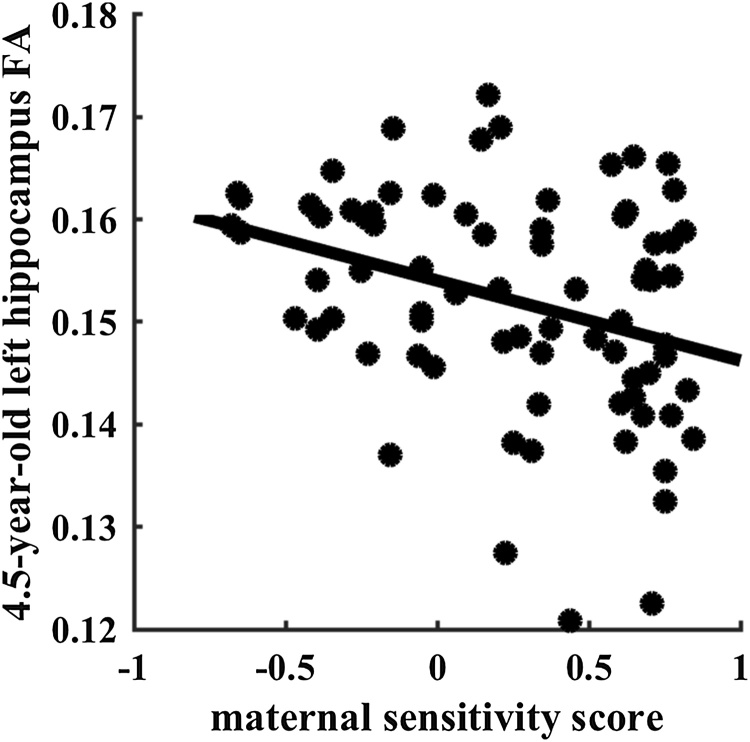
In general, higher levels of maternal sensitivity associated with lower hippocampal FA values at both time points (see Table 2). At 4.5 years the independent effect of maternal sensitivity was significant for 4.5-year-old left (β = -0.241, p = 0.043, df = 68; Fig. 3) but not right (β = -0.168, p = 0.157, df = 68) hippocampus FA.
Fig. 3.
Scatterplot of maternal sensitivity with 4.5-year-old left hippocampus fractional anisotropy (FA).
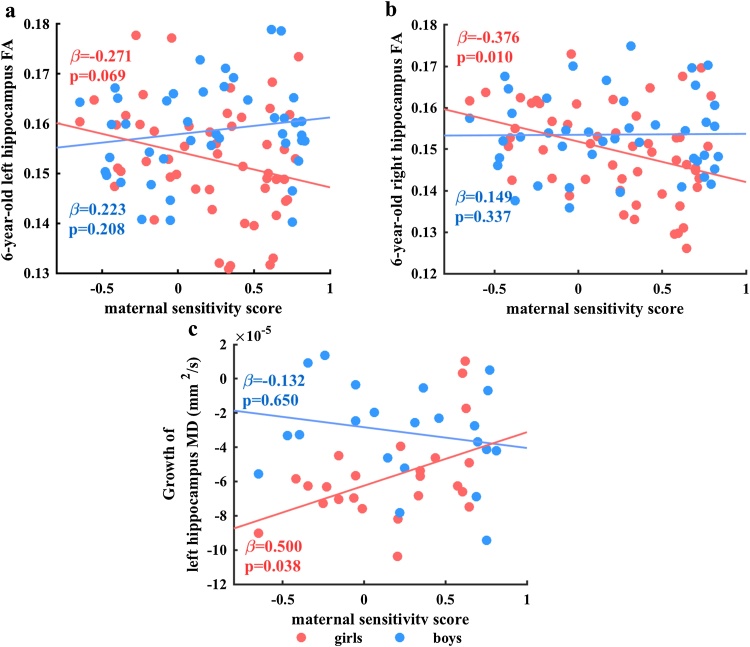
Higher maternal sensitivity was associated with lower 4.5-year-old left hippocampus FA. At 6 years maternal sensitivity and sex interacted to predict 6-year-old left (β = 0.349, p = 0.022, df = 86; Fig. 4a) and right (β = 0.357, p = 0.016, df = 86; Fig. 4b) hippocampus FA. Post hoc analysis revealed that maternal sensitivity showed a trend-level negative association with 6-year-old left hippocampal FA in girls (β = -0.271, p = 0.069, df = 45) that was not found in boys (β = 0.223, p = 0.208, df = 36). Similarly, maternal sensitivity showed a significant negative association with 6-year-old right hippocampus FA in girls (β = -0.376, p = 0.010, df = 45) that was not found in boys (β = 0.149, p = 0.337, df = 36). Sensitivity, alone and/or in interaction with sex, did not significantly predict the hippocampal growth.
Fig. 4.
Scatterplots of (a) interaction of maternal sensitivity and sex, with 6-year-old left hippocampus FA, (b) interaction of maternal sensitivity and sex, with 6-year-old right hippocampus FA, and (c) interaction of maternal sensitivity and sex, with growth of left hippocampus MD. Standardized β values and p-values of the regression analysis in each sex group after adjustment for covariates reported in scatterplots (a–c). Abbreviations: FA, fractional anisotropy; MD, mean diffusivity.
Maternal sensitivity did not predict MD values at either time point. However, the interaction between maternal sensitivity and sex significantly predicted the growth of left (β = -0.517, p = 0.021, df = 37; Fig. 4c) but not right (β = -0.083, p = 0.734, df = 36) hippocampus MD. Post hoc analysis revealed that maternal sensitivity showed a significant positive association with left hippocampal MD growth in girls (β = 0.500, p = 0.038, df = 17) but not boys (β = -0.132, p = 0.650, df = 15). The analysis on the independent effect of maternal sensitivity on the growth of the right (β = -0.026, p = 0.866, df = 37) hippocampus MD did not reveal a significant effect.
4. Discussion
Comparatively high levels of maternal insensitivity may represent a non-extreme, but still important source of information to the developing brain. In fact, cues important to the pace at which development unfolds need not be "extreme," so long as they are expectably linked to the environment of evolutionary adaptation and ultimately relevant for evolutionary fitness (Belsky, Steinberg et al. 1991). Maternal sensitivity is a proximal cue linked to distal aspects of the environment- and is frequently associated with socioeconomic status (Booth, Macdonald et al. 2018). Hence, maternal sensitivity may have historically been a reliable signal of expectable environmental conditions. As such, during early infancy, human infants may be especially sensitive to its variation as they commit to the form and pace of neural development, especially with regards to structures important to learning and emotional/stress regulation. Not surprisingly, then, here we found variation in maternal sensitivity, assessed during early infancy, associated with aspects of limbic neuroanatomy at four and six years of age, in a manner tentatively suggesting accelerated development.
As one example, here, we found less sensitive maternal care during infancy predictive of, amongst six year old boys, larger amygdalae, which are a neural correlate of self- but not necessarily parent- reported anxiety in children and adolescents. Our volumetric findings are in keeping with a previously observed relation between lower maternal accessibility at fifteen months and greater right amygdala volume at age 10 (Bernier et al., 2019). Given the expectable positive relation between age and amygdala volume, perhaps especially in boys (, our current findings may suggest that the pace of amygdala volumetric development is enhanced by insensitive maternal care. However, Lyons-Ruth and colleagues observed similar left lateralized effects, at a stage in development when amygdala volume is thought to have stabilized. Hence, it is also possible that a larger left amygdala either is, or becomes, a stable outcome of insensitive care at some point in early development, perhaps near to the onset of adrenarche, which occurs around 5–7 years and may precipitate sex differences in sequelae associated with early attachment and care. It is also unclear whether our findings concerning higher maternal sensitivity and lower left amygdala MD at 4.5 years, and higher growth at 6.0 years in girls suggest acceleration in the pace of microstructure development. In general, MD levels are expected to decrease with age (Qiu et al., 2015a).
Still, to the extent that differences reflect the rate of development, individual variation may be better captured during periods of growth rather than a plateau. Though hippocampal volume growth (Utsunomiya, Takano et al. 1999), may begin to plateau around age 2 (Saksena, Husain et al. 2008), some research suggests that microstructure growth follows a more extended time-course. FA, as well as aspects contributing to MD may plateau around six years of age, and FA development may differ across sex (Uda, Matsui et al. 2015). Here, we observed a main effect of maternal sensitivity upon left hippocampal FA at age 4, but moderation by sex upon bilateral hippocampal FA at age 6, a period closer to a sex-related difference in hippocampal FA plateaus on growth (Uda, Matsui et al. 2015).
In contrast to our findings concerning microstructure, and keeping with the idea that hippocampal volume has begun to plateau during the preschool years, here we did not observe relations between sensitivity and hippocampal volume at either 4.5 or 6 years of age. These nil volumetric effects are in keeping with work examining the relation between disrupted care, insensitive behaviour, and/or forms of insecure attachment during late infancy and hippocampal volume at school age or into adulthood. However, our former small scale (n = 20) volumetric study within the same cohort showed an inverse relation between sensitive maternal care during early infancy and bilateral hippocampal volume at six months of age. Similarly, Bernier and colleagues (Bernier et al., 2019), who also examined sensitivity via the MBQS (Moran, 2009; Moran, Pederson et al. 2009), observed an inverse relation between accessibility and availability at fifteen months and bilateral hippocampal volume at age 10. While these inconsistencies may indicate spurious past findings, taken together, they may also suggest that how and when parenting is measured (e.g., early versus late infancy, preschool age) and when hippocampal volume is measured (e.g., infancy, childhood, adulthood) matter in the ability to detect relations, and the nature (e.g., positive or negative) of the relations observed. Although here we measured neuroanatomy from 4.5 to 6.0 years, we were only able to assess maternal sensitivity at one-time point. Additional assessments of maternal sensitivity, as well as other aspects of caregiving, including positive verbal and nonverbal behaviour, aggressive behaviour (Whittle, Vijayakumar et al. 2016) and more extreme parenting behaviour including neglect, at multiple developmental stages will be necessary to more fully understand and describe sensitive windows.
When considering both the hippocampus and amygdala, all but one of our findings concerned the left hemisphere. Exact mechanisms related to our lateralized findings are unclear. One possibility is that differential growth patterns in the left and right hemisphere may have influenced our ability to detect change. Alternatively, adversity may have differential effects across hemispheres. Although findings from a meta-analysis examining trauma and volume reported effects on the bilateral hippocampi (Woon, Sood et al. 2010), some work does suggest childhood trauma exposure and low parental nurturance specifically relate to the left hippocampus. Moreover, the left hippocampus may influence sensitivity to familial relationships (Schriber, Anbari et al. 2017).
This study incorporated both structural and diffusion MRI datasets from early childhood, which is highly unique in its timing of acquisition and the sample size. Nevertheless, the statistical significance level of our findings was moderate. Hence, this study is best considered as exploratory. Moreover, DTI measures may be distorted because of partial volume effects that could vary according to the shape of the structure and sounding tissues (Vos, Jones et al. 2011). There is no literature that has discussed, in particular, a solution to partial volume effects on DTI measures of the amygdala and hippocampus. Hence, potential influences of partial volume effects on our DTI findings are unclear. Nevertheless, our study employed the same DTI analysis to all the datasets, which presumably produced comparable variation of DTI measures across all subjects. We therefore expect that partial volume effects concerning the DTI measures do not change the study’s conclusions.
This study is the first providing evidence on limbic neuroanatomy during the preschool years as a function of observed caregiving during early infancy, based on a longitudinal and relatively large dataset. These results demonstrate that even normative variation in early infancy care may influence neurodevelopment. An important area for future research, then, will be to determine the functional correlates of such differences with an eye towards intervention and prevention programs focusing upon sensitive caregiving within communities at large, and not just clinical groups.
Funding source
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore Ministry of Education (Academic research fund tier 1; NUHSRO/2017/052/T1-SRP-Partnership/01), and NUS Institute of Data Science, Singapore.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2019.100714.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Beck A.T., Ward C.H., Mendelson M.M., Mock J.J., Erbaugh J.J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4(6):561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Belsky J., Steinberg L., Draper P. Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Dev. 1991;62(4):647–670. doi: 10.1111/j.1467-8624.1991.tb01558.x. [DOI] [PubMed] [Google Scholar]
- Bernier A., Dégeilh F., Leblanc É., Daneault V., Bailey H.N., Beauchamp M.H. Mother–infant interaction and child brain morphology: a multidimensional approach to maternal sensitivity. Infancy. 2019;24(2):120–138. doi: 10.1111/infa.12270. [DOI] [PubMed] [Google Scholar]
- Booth A.T., Macdonald J.A., Youssef G.J. Contextual stress and maternal sensitivity: a meta-analytic review of stress associations with the Maternal Behavior Q-Sort in observational studies. Dev. Rev. 2018;48:145–177. [Google Scholar]
- Chen Y., Bender R.A., Brunson K.L., Pomper J.K., Grigoriadis D.E., Wurst W., Baram T.Z. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc. Natl. Acad. Sci. U. S. A. 2004;101(44):15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans C.L. Maternal sensitivity, its relationship with child outcomes, and interventions that address it: a systematic literature review. Early Child Dev. Care. 2018 [Google Scholar]
- Ducharme S., Albaugh M.D., Nguyen T.V., Hudziak J.J., Mateos-Perez J.M., Labbe A., Evans A.C., Karama S., G. Brain Development Cooperative Trajectories of cortical thickness maturation in normal brain development--The importance of quality control procedures. Neuroimage. 2016;125:267–279. doi: 10.1016/j.neuroimage.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Gao W., Grewen K., Knickmeyer R.C., Qiu A., Salzwedel A., Lin W., Gilmore J.H. A review on neuroimaging studies of genetic and environmental influences on early brain development. Neuroimage. 2019;185:802–812. doi: 10.1016/j.neuroimage.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Ceritoglu C., Li X., Qiu A., Miller M.I., van Zijl P.C., Mori S. Correction of B0 susceptibility induced distortion in diffusion-weighted images using large-deformation diffeomorphic metric mapping. Magn. Reson. Imaging. 2008;26(9):1294–1302. doi: 10.1016/j.mri.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A., Poh J.S., Wen D.J., Guillaume B., Chong Y.S., Shek L.P., Fortier M.V., Qiu A. Long-term influences of prenatal maternal depressive symptoms on the amygdala-prefrontal circuitry of the offspring from birth to early childhood. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019 doi: 10.1016/j.bpsc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Lugo-Candelas C., Cha J., Hong S., Bastidas V., Weissman M., Fifer W.P., Myers M., Talati A., Bansal R., Peterson B.S., Monk C., Gingrich J.A., Posner J. Associations between brain structure and connectivity in infants and exposure to selective serotonin reuptake inhibitors during pregnancy. JAMA Pediatr. 2018;172:525–533. doi: 10.1001/jamapediatrics.2017.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S., Nasca C., Gray J.D. Stress effects on neuronal structure: hippocampus, amygdala, and prefrontal cortex. Neuropsychopharmacology. 2016;41(1):3–23. doi: 10.1038/npp.2015.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran G. The Selected Works of Greg Moran. 2009. mini-MBQS-v revised mini-MBQS 25 item for video coding.http://works.bepress.com/gregmoran/49 [Google Scholar]
- Moran G., Pederson D.R., Bento S. Maternal behavior Q-Sort (MBQS) – overview, available materials and support. The Selected Works of Greg Moran. 2009 http://works.bepress.com/gregmoran/48 from. [Google Scholar]
- Ong M.L., Tuan T.A., Poh J., Teh A.L., Chen L., Pan H., MacIsaac J.L., Kobor M.S., Chong Y.S., Kwek K., Saw S.M., Godfrey K.M., Gluckman P.D., Fortier M.V., Karnani N., Meaney M.J., Qiu A., Holbrook J.D. Neonatal amygdalae and hippocampi are influenced by genotype and prenatal environment, and reflected in the neonatal DNA methylome. Genes Brain Behav. 2019 doi: 10.1111/gbb.12576. [DOI] [PubMed] [Google Scholar]
- Qiu A., Mori S., Miller M.I. Diffusion tensor imaging for understanding brain development in early life. Annu. Rev. Psychol. 2015;66:853–876. doi: 10.1146/annurev-psych-010814-015340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A., Anh T.T., Li Y., Chen H., Rifkin-Graboi A., Broekman B.F., Kwek K., Saw S.M., Chong Y.S., Gluckman P.D., Fortier M.V., Meaney M.J. Prenatal maternal depression alters amygdala functional connectivity in 6-month-old infants. Transl. Psychiatry. 2015;5:e508. doi: 10.1038/tp.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A., Shen M., Buss C., Chong Y.S., Kwek K., Saw S.M., Gluckman P.D., Wadhwa P.D., Entringer S., Styner M., Karnani N., Heim C.M., O’Donnell K.J., Holbrook J.D., Fortier M.V., Meaney M.J., the, G. s. g Effects of antenatal maternal depressive symptoms and socio-economic status on neonatal brain development are modulated by genetic risk. Cereb. Cortex. 2017;27:3080–3092. doi: 10.1093/cercor/bhx065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Kong L., Sim L., Sanmugam S., Broekman B., Chen H., Wong E., Kwek K., Saw S., Chong Y. Maternal sensitivity, infant limbic structure volume and functional connectivity: a preliminary study. Transl. Psychiatry. 2015;5(10):e668. doi: 10.1038/tp.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Bai J., Chen H., Hameed W.B., Sim L.W., Tint M.T., Leutscher-Broekman B., Chong Y.S., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. Prenatal maternal depression associates with microstructure of right amygdala in neonates at birth. Biol. Psychiatry. 2013;74:837–844. doi: 10.1016/j.biopsych.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Rifkin-Graboi A., Quan J., Richmond J., Goh S.K.Y., Sim L.W., Chong Y.S., Bureau J.F., Chen H., Qiu A. Greater caregiving risk, better infant memory performance? Hippocampus. 2018;28(7):497–511. doi: 10.1002/hipo.22949. [DOI] [PubMed] [Google Scholar]
- Saksena S., Husain N., Malik G.K., Trivedi R., Sarma M., Rathore R.S., Pandey C.M., Gupta R.K. Comparative evaluation of the cerebral and cerebellar white matter development in pediatric age group using quantitative diffusion tensor imaging. Cerebellum. 2008;7(3):392–400. doi: 10.1007/s12311-008-0041-0. [DOI] [PubMed] [Google Scholar]
- Schriber R.A., Anbari Z., Robins R.W., Conger R.D., Hastings P.D., Guyer A.E. Hippocampal volume as an amplifier of the effect of social context on adolescent depression. Clin. Psychol. Sci. 2017;5(4):632–649. doi: 10.1177/2167702617699277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh S.E., Lee S.S.M., Hoon S.W., Tan M.Y., Goh A., Lee B.W., Shek L.P.-C., Teoh O.H., Kwek K., Saw S.M., Godfrey K., Chong Y.S., Gluckman P., van Bever H.P.S. The methodology of the GUSTO cohort study: a novel approach in studying pediatric allergy. Asia Pac. Allergy. 2012;2(2):144–148. doi: 10.5415/apallergy.2012.2.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thijssen S., Muetzel R.L., Bakermans-Kranenburg M.J., Jaddoe V.W.V., Tiemeier H., Verhulst F.C., White T., Van Ijzendoorn M.H. Insensitive parenting may accelerate the development of the amygdala–medial prefrontal cortex circuit. Dev. Psychopathol. 2017;29(2):505–518. doi: 10.1017/S0954579417000141. [DOI] [PubMed] [Google Scholar]
- Tsotsi S., Borelli J.L., Abdulla N.B., Tan H.M., Sim L.W., Sanmugam S., Tan K.H., Chong Y.S., Qiu A., Chen H., Rifkin-Graboi A. Maternal sensitivity during infancy and the regulation of startle in preschoolers. Attach. Hum. Dev. 2018:1–18. doi: 10.1080/14616734.2018.1542737. [DOI] [PubMed] [Google Scholar]
- Uda S. Normal development of human brain white matter from infancy to early adulthood: A diffusion tensor imaging study. Dev. Neurosci. 2015 doi: 10.1159/000373885. [DOI] [PubMed] [Google Scholar]
- Uda S., Matsui M., Tanaka C., Uematsu A., Miura K., Kawana I., Noguchi K. Normal development of human brain white matter from infancy to early adulthood: a diffusion tensor imaging study. Dev. Neurosci. 2015;37(2):182–194. doi: 10.1159/000373885. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H., Takano K., Okazaki M., Mitsudome A. Development of the temporal lobe in infants and children: analysis by MR-based volumetry. AJNR Am. J. Neuroradiol. 1999;20(4):717–723. [PMC free article] [PubMed] [Google Scholar]
- Vos S.B., Jones D.K., Viergever M.A., Leemans A. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 2011;55(4):1566–1576. doi: 10.1016/j.neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Wang C., Shen M., Guillaume B., Chong Y.S., Chen H., Fortier M.V., Meaney M.J., Qiu A. FKBP5 moderates the association between antenatal maternal depressive symptoms and neonatal brain morphology. Neuropsychopharmacology. 2018;43:564–570. doi: 10.1038/npp.2017.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Zhang H., Poh J.S., Pecheva D., Broekman B.F.P., Chong Y.S., Shek L.P., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. Sex-dependent associations among maternal depressive symptoms, child reward network, and behaviors in early childhood. Cereb. Cortex. 2019 doi: 10.1093/cercor/bhz135. [DOI] [PubMed] [Google Scholar]
- Wang Q., Zhang H., Wee C.Y., Lee A., Poh J.S., Chong Y.S., Tan K.H., Gluckman P.D., Yap F., Fortier M.V., Rifkin-Graboi A., Qiu A. Maternal sensitivity predicts anterior hippocampal functional networks in early childhood. Brain Struct. Funct. 2019;224:1885–1895. doi: 10.1007/s00429-019-01882-0. [DOI] [PubMed] [Google Scholar]
- Wen D.J., Poh J.S., Ni S.N., Chong Y.S., Chen H., Kwek K., Shek L.P., Gluckman P.D., Fortier M.V., Meaney M.J., Qiu A. Influences of prenatal and postnatal maternal depression on amygdala volume and microstructure in young children. Transl. Psychiatry. 2017;7:e1103. doi: 10.1038/tp.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S., Vijayakumar N., Dennison M., Schwartz O., Simmons J.G., Sheeber L., Allen N.B. Observed measures of negative parenting predict brain development during adolescence. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0147774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woon F.L., Sood S., Hedges D.W. Hippocampal volume deficits associated with exposure to psychological trauma and posttraumatic stress disorder in adults: a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34(7):1181–1188. doi: 10.1016/j.pnpbp.2010.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.