Highlights
-
•
Severe pediatric obesity showed altered neural engagement during episodic encoding.
-
•
Severe pediatric obesity was associated with worse recollection but not recognition.
-
•
Lateral temporal activation during encoding mediated recollection deficits.
Keywords: fMRI, WRAML, Declarative memory, Visual memory
Abstract
Negative effects of obesity on memory and associated medial temporal circuitry have been noted in animal models, but the status in humans, particularly children, is not well established. Our study is the first to examine neural correlates of successful memory encoding of visual scenes and their associated context in adolescents with severe obesity (age 14–18 years, 43% male). Despite similar subsequent memory as adolescents without obesity (BMI for age and sex <95th percentile), those with severe obesity (BMI for age and sex 120% above the 95th percentile) showed reduced hippocampal, parahippocampal, frontal, and parietal engagement during encoding of remembered visual scenes and greater lateral temporal engagement during encoding of their associated context. Standardized testing revealed a trend level group difference in memory performance, with a larger magnitude of obesity-related difference in recollection-related memory that was mediated by individual differences in lateral temporal activation during contextual encoding. The observed widespread functional alterations are concerning in light of the importance of mnemonic processing for academic achievement and feeding behavior and underscore the need for prevention and intervention initiatives for pediatric obesity.
1. Introduction
One in five adolescents in the United States has obesity (body mass index—BMI—above the 95th percentile; Flegal and Ogden, 2010; Hales et al., 2018; Kuczmarski et al., 2002), with 8 % meeting criteria for severe obesity (BMI 120 % above the 95th percentile; Flegal et al., 2009; Hales et al., 2018; Kelly et al., 2013). Obesity poses increased risks for medical complications that have high morbidity (Kelly et al., 2013), poor quality of life (Zeller et al., 2015), cognitive deficits that are particularly relevant to adolescent development such as executive function (Pearce et al., 2018), and worse academic achievement (Kamijo et al., 2012). Less well understood is the effect of obesity on episodic memory, the encoding and explicit retrieval of past experience, which is pertinent to both academic achievement (Hassevoort et al., 2016) and feeding behavior (Stevenson and Francis, 2017). The hippocampus and surrounding tissue (termed medial temporal lobe – MTL) subserve episodic memory and its high vulnerability to the negative effects of obesity and high-fat diets are well established in animal models (Cordner and Tamashiro, 2015; Kanoski and Davidson, 2011). The MTL is posited to play a role in feeding behaviors through the integration of internal (e.g., hunger/fullness) and external (e.g., smell, food images) cues and information learned from previous experience (Kanoski and Grill, 2017; Stevenson and Francis, 2017). While parents report a higher number of memory problems in children with obesity relative to those with healthy weight (Hölcke et al., 2008), the extent to which the MTL and associated episodic memory functions are compromised in pediatric obesity has never been examined.
Animal studies suggest that the MTL may be more vulnerable to systemic and metabolic pathology associated with obesity due to its high potential for plasticity. In particular, obesity is associated with chronic, low-grade systemic inflammation, which contributes to elevated neuroinflammation (Miller and Spencer, 2014; Spyridaki et al., 2016) that is thought to be a primary mechanism for hippocampal dysfunction associated with obesity (Stranahan, 2015). Exposure to “Western Diets”, marked by high levels of saturated fats and sugar, has been shown to result in increased hippocampal cell death, with long-term exposure resulting in impaired long-term potentiation, a form of synaptic plasticity subserving learning and memory (Hargrave et al., 2016). Diet-induced obesity has also been associated with reductions in spatial learning and memory, contextual fear conditioning, and use of interoceptive food cues (Hargrave et al., 2016). Importantly, juvenile and adolescent rodents are particularly vulnerable to western diets as they show hippocampal dependent learning and memory deficits even prior to the development of diet-induced obesity (reviewed in Noble and Kanoski, 2016). Given the potential deleterious effects of high-fat/high-sugar diets and obesity on MTL function and the importance of episodic memory and learning for academic achievement (Hassevoort et al., 2016) and eating behavior (Kanoski and Grill, 2017; Stevenson and Francis, 2017), it is critical to understand whether severe obesity alters neural substrates of mnemonic processes in children.
Similar to the animal literature, there is consistent evidence for impaired episodic memory function in adults with obesity (Loprinzi and Frith, 2018), however, firm conclusions about pediatric obesity cannot be drawn as only two studies included children. These two studies differ on materials (e.g., visual or verbal), type of memory (e.g., item memory or associative memory), and retrieval mode (e.g., recognition or recall) of testing. While no deficits in verbal recall were observed (Gunstad et al., 2008), greater total abdominal adipose tissue was associated with worse memory for both item and associative visual material (Khan et al., 2015). This association appeared to be driven by children with more severe adiposity because performance did not differ when comparing children with overweight or obesity to those with healthy weight. Together, this limited past work suggests that children with severe obesity may be at a higher risk for deficits in episodic memory for visual material. Thus, as the first study to examine the neural correlates of episodic memory in pediatric obesity, we focused on item and associative memory for visual materials in children with severe obesity.
Using functional magnetic resonance imaging (fMRI), we tested whether differences in neural functioning between adolescents with severe obesity and those without obesity mediated behavioral deficits in item and associative memory performance for visual scenes. We focused on adolescents with severe obesity because they are at highest risk for neurocognitive and health comorbidities. In order to minimize the influence of pubertal differences, we included adolescents who were 14 years old or higher. The one previous study in adults showed reduced frontal, temporal, and parietal activation during encoding in those with obesity, suggesting alterations are not limited to MTL (Cheke et al., 2017). Thus, our experimental design aimed to address mnemonic processes tied to both MTL and widespread cortical regions. Specifically, we targeted visual item and associative memory in light of the Khan et al. (2015) findings showing deficits in children with obesity. Item and associative memory were assessed with two modalities—fMRI for identification of encoding-related neural correlates supporting subsequent memory and standardized behavioral testing using the Wide Range Assessment of Memory and Learning (WRAML-II; Adams and Reynolds, 2008). For both modalities, item memory was assessed with yes/no recognition, a retrieval mode that is posited to draw upon familiarity-based processes mediated by the perirhinal cortex in the MTL (Ranganath, 2010; Yonelinas, 2002). Associative memory was assessed via free and cued recall, retrieval modes that require retrieval of spatial-temporal contextual information known to draw upon recollection-based processes mediated by the hippocampus in the MTL and heteromodal cortical regions (Ranganath, 2010; Yonelinas, 2002). fMRI was performed during encoding while children made indoor/outdoor judgments for visual scenes. Upon exiting the scanner, children performed indoor/outdoor judgments for a new set of scenes. Therefore, the location associated with the encoded scenes (inside or outside the scanner) differed across items. A yes/no recognition test was administered for the scenes and recall of the associated encoding location (inside/outside scanner) was tested for each recognized scene. The Wide Range Assessment of Memory and Learning (WRAML-II; Adams and Reynolds, 2008) measures yes/no item recognition (termed Recognition Index) and cued and free recall (Memory Index) for abstract designs and complex scenes. Together, parallel behavioral and brain imaging measures allowed us to test which neural correlates mediated obesity-related behavioral differences.
2. Materials and methods
2.1. Participants
Thirty-five adolescents (M = 16.5, SD = 1.2 range 14–18 yrs) with severe obesity (BMI 120% above 95th percentile; n = 18) or without obesity (BMI < 95th percentile; n = 17) from Children’s National Health System (CNHS) and the Washington, DC area participated following informed consent and assent, obtained according to the guidelines of the Institutional Review Boards at Georgetown University and CNHS. This subsample only partially overlaps with studies examining neurocognitive outcomes of bariatric surgery (Mackey et al., 2018; Pearce et al., 2017) due to size restrictions of the scanner. In order to reduce variability due to differences in pubertal status, adolescents rather than pre-adolescents were selected for this study. Inclusion criteria included full-scale IQ > 75 (estimated from the vocabulary and matrix reasoning subtests of the Wechsler Abbreviated Intelligence Scale-II (WASI-II; Wechsler and Hsiao-pin, 2011), and parent-reported absence of past or current diagnosis of Type 2 diabetes, metabolic syndrome, neurological disorder, and prescription for psychotropic medication. BMI was higher in adolescents with severe obesity than healthy weight, however, age, gender, IQ, years of maternal education, ethnicity, race, and annual family income did not differ across groups (Table 1). Additionally, due to the partial overlap with the study examining impact of bariatric surgery, participants with severe obesity also met inclusion criteria for bariatric surgery.
Table 1.
Participant Characteristics.
| Obese | Non-Obese | |
|---|---|---|
| Sample (Male), N | 18 (6) | 17 (7) |
| Right Handed (Left), N | 15 (3) | 15 (2) |
| Age, yrs, mean (SD) | 16.57 (1.23) | 16.34 (1.26) |
| BMI, mean (SD) | 42.08 (5.78)*** | 21.53 (2.65)*** |
| IQ, mean (SD) | 98.24 (13.17) | 104.53 (12.76) |
| Maternal Ed, yrs,mean (SD | 14.00 (4.43) | 15.65 (2.57) |
| Ethnicity, N | ||
| Hispanic/Latino | 4 | 2 |
| Not Hispanic/Latino | 13 | 15 |
| Not Reported | 1 | 0 |
| Race, N | ||
| Black/African American | 9 | 6 |
| White/Caucasian | 5 | 9 |
| Other/Multiple | 4 | 2 |
| Not Reported | 0 | 0 |
| SES, N | ||
| >$80,000 | 8 | 8 |
| $50,000-$80,000 | 4 | 4 |
| <$50,000 | 6 | 5 |
| Not Reported | 0 | 0 |
Group differences tested with t-tests or Fisher's Exact test; ***p < 0.005; BMI: body mass index.
2.2. Procedure
Participants completed one fMRI session and a separate behavioral testing session, either on the same day or within a few days, during which a battery of neuropsychological tests was administered including the assessment of visual memory using the Wide Range Assessment of Memory and Learning-II (WRAML-II; Adams and Reynolds, 2008).
2.2.1. Anthropometrics
At the first session, weight and height were measured in triplicate using a digital scale (Health-O-Meter Professional 394KLX) and stadiometer (SECA 216 Wall-mount Mechanical measuring rod). The average of these measurements was used for calculation of BMI (m2/kg). Participants wore light clothing that would be comfortable in the scanner (i.e., no buttons, snaps, zippers) and no shoes while weight and height were collected. Participants’ weight status was characterized based on their BMI percentiles for age and sex. Participants with BMI under the 95th percentile were considered to not have obesity (Flegal and Ogden, 2010; Kuczmarski et al., 2002) while those with BMI 120% or more above the 95th percentile were considered to have severe obesity (Flegal et al., 2009; Kelly et al., 2013). BMI, rather than BMI percentile, is reported in Table 1 because all participants with severe obesity had a BMI >99th percentile. The participants with severe obesity were candidates for bariatric surgery at CNHS and therefore all had BMI > 32, which is the minimum BMI for consideration of weight loss surgery in children at CNHS.
2.2.2. Wide Range Assessment of Memory and Learning-II
The Design and Picture WRAML-II (Adams and Reynolds, 2008) subtests consisted of both free recall Memory and Recognition tests. For Design Memory participants viewed five abstract line drawings for 5 s and then were asked to draw what they could remember. Similarly, for Picture Memory participants viewed four complex scenes for 10 s and then were directed to mark parts of the scene that had been moved, changed, or added in a novel, similar picture. For Design and Picture Recognition subtests, participants indicated whether a series of line drawings or scene sections, respectively, were previously seen during the assessment or if they were new (i.e., not seen before). The WRAML-II provides scaled Memory and Recognition Indices, consisting of the combined scaled Design and Picture Memory and Recognition scores, respectively.
2.2.3. Subsequent memory paradigm
The subsequent memory paradigm consisted of three phases, an fMRI-encoding phase with 46 scenes, followed by an out-of-scanner encoding phase with another set of 46 scenes, and then an item recognition and associative context recall test for the encoded stimuli. In the two encoding phases, participants were instructed to indicate with a button press whether serially presented color photographs (1.5 s) depicted indoor (right hand) or outdoor (left hand) scenes. Participants were not informed about the subsequent memory test. All stimuli were presented using E-Prime (Psychology Software Tools, 2019). For the fMRI-encoding phase, trial presentation was event-related with jitter optimized for selective averaging using OPTSEQ2 (https://surfer.nmr.mgh.harvard.edu/optseq/), resulting in a scan time of 6.13 min. Stimuli were viewed through a mirror mounted on the head coil and presented using a magnet-compatible projector. After the participant exited the scanner, they performed the second encoding phase with a novel set of 46 scenes (1.5 s, 0.5 s inter-stimuli interval) on a computer. Together, the two encoding phases provided two encoding contexts for the 92 total scenes (46 inside and 46 outside the scanner), which enabled the subsequent assessment of associative recall of context. Item recognition and associative context recall was assessed at least 20 min after the in-scanner encoding task and immediately after the out-of-scanner encoding task with a self-paced recognition memory task. Participants were instructed to identify 184 serially presented scenes (92 encoded and 92 foils) as “New” (i.e., not previously presented) or “Old” (i.e., scene was previously presented). For scenes identified as “Old”, associative context recall was assessed by asking the participant whether they saw the scene while in the scanner or out of the scanner on the computer. The sets of scenes presented during the scan, outside the scanner, and as foils were counterbalanced across participants.
2.3. Acquisition parameters and preprocessing
Imaging was performed on a 3 T Trio Siemens scanner (Erlangen, Germany). A high resolution T1-weighted structural scan (MPRAGE) was acquired lasting 7.23 min with the parameters: TR/TE = 2300/2.94 ms, TI = 900 ms, 90-degree flip angle, 1 slab, 160 sagittal slices with a 1.0 mm thickness, FOV = 256 × 256mm2, resulting in an effective resolution of 1.03 mm isotropic voxels. The subsequent memory paradigm functional run was acquired using a T2*-sensitive gradient echo pulse sequence with parameters: TR/TE = 2000/30 ms, 90-degree flip angle, 43 interleaved slices (width = 2.5 mm, gap width = 0.5 mm, effective width = 3 mm) ascending in the transverse plane, FOV = 192 × 192mm2. Slice acquisition was angled in the plane of the hippocampus to optimize medial temporal lobe signal. Head movement was minimized with padding between the head and coil.
Functional images were analyzed using SPM12 (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included discarding the first 4 TRs for signal stabilization, correction for motion as suggested by Wilke (2012), slice-time correction, co-registration to each participant’s MPRAGE, and smoothing with an 8 mm FWHM Gaussian kernel. Responses were modeled using a canonical hemodynamic response function convolved with trial onset vectors. Task contrasts were specified for the two mnemonic processes of interest: 1) Scene recognition: subsequently recognized > forgotten scenes – this contrast targets item memory which reflects familiarity-based processing and 2) Inside/outside scanner context recall: subsequently recognized scene and correct context recall > subsequently recognized scene but forgotten context – this contrast targets associative memory which reflects recollection-based processes. For each subject, the general linear model included the task contrast and 7 regressors of no interest: 6 realignment parameters derived to estimate the effect of head motion on signal (Wilke, 2012) and 1 parameter which de-weighted volumes with greater than 1.5 mm motion scan-to-scan (STS). Participants were excluded from analyses if they had more than 10% of volumes with half a voxel (1.5 mm) or greater motion STS or had fewer than 5 scans per condition. The number of TRs included per condition (recognized: 11–41; forgotten: 5–35; recognized and context recalled: 8–35; recognized and context forgotten: 5–15) did not differ between groups, p’s > 0.21. These criteria resulted in retention of N = 15 per group for the item-recognition contrast and N = 10 per group for context-recall contrast. Deformation fields derived from participants’ MPRAGEs were applied to contrast maps to normalize into MNI standard stereotaxic space.
2.4. Analytic approach
All behavioral analyses were completed in R (R Core Team, 2014) and all group imaging analyses were conducted using GLM Flex Fast2 (http://mrtools.mgh.harvard.edu/). Behavioral analyses first assessed group differences in memory performance. Imaging analyses then assessed group differences in activation using general linear models controlling for mean motion STS and age. Multiple comparisons were controlled for at p < 0.05 with whole-brain Monte-Carlo simulations using 3dclustsim (2-sided, nearest neighbor 2; k = 121, p = 0.005; Cox et al., 2017). In order to determine if difference in activation for subsequently remembered scenes and context were associated with standardized assessment of memory function (i.e., WRAML-II), parameter estimates (i.e., beta values) were extracted from clusters showing significant group differences. In the presence of significant Pearson’s r correlation (multiple comparisons controlled using false discovery rate; FDR) between subsequent memory activation and WRAML-II performance, a brain as mediator model was tested. Mediation analyses used non-parametric bootstrapping and bias-corrected and accelerated confidence intervals (Tingley et al., 2014).
3. Results
3.1. WRAML-II
In order to test for group difference across both performance indices assessed by the WRAML-II, an Index (Recognition, Memory) X Group (Non-Obese, Severe Obesity) analysis of variance was conducted. There was a trend level effect of Group (F(1, 55) = 3.46, p = 0.067) but no effect of Index (p = 0.285) or interaction (p = 0.248; Table 2). As apparent in Table 2, effect size for group difference was larger in magnitude for the Memory (d = 0.81) than Recognition Index (d = 0.18), which suggests that adolescents with obesity had greater difficulty with visual recall that draws upon recollection-based mnemonic processes than item memory, which relies on familiarity. Indeed, this was confirmed with separate t-tests for each Index, which showed significantly lower scores in adolescents with severe obesity than without obesity on the Memory Index but not Recognition Index; Table 2).
Table 2.
Performance on the Subsequent Memory Task and the Wide Range Assessment of Memory and Learning-II.
| Obese | Non-Obese | |||
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | da | p | |
| Subsequent Memory Performance | ||||
| In-Scanner Scene Recognition Accuracyb, % | 53.38 (21.81) | 58.70 (15.66) | 0.28 | 0.412 |
| In-Scanner Scene Recognition Sensitivityc, d’ | 1.82 (0.80) | 1.93 (0.50) | 0.16 | 0.630 |
| Out-of-Scanner Scene Recognition Accuracyb, % | 64.93 (20.90) | 63.04 (16.09) | 0.10 | 0.782 |
| Out-of-Scanner Scene Recognition Sensitivityc, d’ | 2.14 (0.80) | 2.05 (0.60) | 0.13 | 0.720 |
| Context Recall Accuracyd, % | 74.24 (19.05) | 79.40 (8.79) | 0.42 | 0.267 |
| WRAML-II | ||||
| Memory Index | 86.83 (9.49) | 95.06 (10.71) | 0.81 | 0.022* |
| Recognition Index | 95.83 (12.53) | 98.65 (18.06) | 0.18 | 0.599 |
a: Cohen’s d. b: Corrected accuracy is reported for Scene Recognition: % correctly recognized - % False Alarms for scenes displayed in scanner. c: d’ is a measure of sensitivity to the difference between ‘old’ (target) and ‘new’ (distractor) images. d: Context Recall Accuracy: % of recognized scenes where location was also correctly recognized. WRAML-II: Wide Range Assessment of Memory and Learning-II; †p < 0.10.
3.2. Subsequent memory paradigm
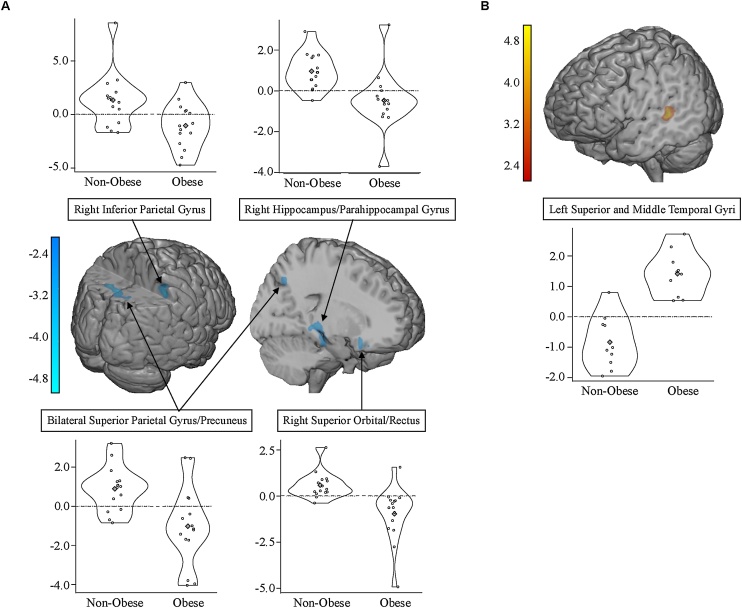
Groups did not differ significantly for item recognition memory (percent hits – percent false alarms for scenes encoded inside or outside the scanner), recognition sensitivity (d’), or associated context recall (percent correct recall of location of encoding—in/out scanner; Table 2). However, significant group differences in neural engagement were observed during encoding of subsequently recognized scenes and their associated context. During encoding of successfully recognized relative to forgotten scenes, adolescents with severe obesity showed reduced activation in frontal, MTL, and parietal regions relative to adolescents without obesity (Table 3; Fig. 1A). Clusters included right orbital frontal gyrus extending into the rectus, right hippocampal and parahippocampal gyri, and right inferior parietal lobule. The largest cluster consisted of bilateral superior parietal gyrus extending into right precuneus. It is important to note that subsequent recognition memory performance did not differ between adolescents with severe obesity and without obesity, suggesting that the observed neural engagement, albeit reduced, was adequate to support scene recognition subsequently.
Table 3.
Regions showing group differences in activation during encoding of subsequently remembered scenes and their associated encoding context (location) at p < .05, corrected.
| Scene Recognized > Forgotten | ||||||
|---|---|---|---|---|---|---|
| Region (BA) | H | Volumea | tb | x | y | z |
| Non-Obese > Obese | ||||||
| Superior Orbital Gyrus and Rectus (25, 11) | R | 278 | −4.78 | 12 | 20 | −18 |
| Parahippocampal Gyrus and Hippocampus (28, 35) | R | 210 | −4.44 | 28 | −34 | −10 |
| Inferior Parietal Lobule (40) | R | 171 | −4.06 | 46 | −40 | 52 |
| Superior Parietal Gyrus and Precuneus (7) | L/R | 613 | −4.85 | −14 | −62 | 47 |
| −3.91 | 6 | −66 | 62 | |||
| Location Recognized > Forgotten | ||||||
|---|---|---|---|---|---|---|
| Region (BA) | H | Volumea | tb | x | y | z |
| Obese > Non-Obese | ||||||
| Superior and Middle Temporal Gyrus (21/22) | L | 134 | 5.00 | −58 | −30 | 4 |
a: volume measured in mm3; b: peak t-value derived from the general linear model. BA: Brodmann's Area; H: hemisphere.
Fig. 1.
Activation differences during encoding of subsequently remembered scenes and their location context between adolescents with severe obesity and without obesity at p < 0.05, corrected. A) Non-Obese > Obese for remembered than forgotten scenes; B) Obese > Non-Obese for remembered scenes and location than forgotten location.
In contrast, during encoding of successfully recalled than forgotten associative context (i.e., where the scene was encoded, inside/outside the scanner) for successfully recognized scenes, adolescents with severe obesity showed greater activation in left superior and middle temporal gyrus relative to adolescents with without obesity (Table 3; Fig. 1B). Greater engagement of lateral temporal cortex during encoding of associative contextual information suggests that group differences in mnemonic processing for visual associative recall extend beyond the MTL. Together, these results indicate that severe pediatric obesity is associated with atypical neural engagement during encoding for visual items and context information in MTL and cortical regions in the absence of memory deficits for the material.
3.3. Mediation analyses
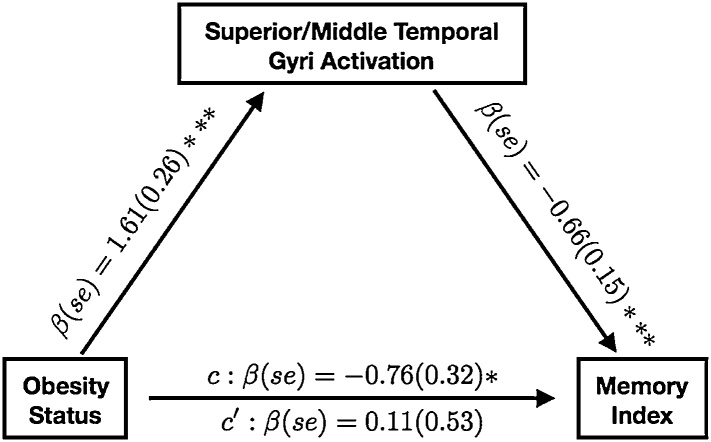
We tested whether observed group differences in Recognition and Memory WRAML-II indices were mediated by activation during scene and context encoding. Person’s correlations were conducted to test whether group differences in activation subserving subsequent scene recognition and associative context recall were related to standardized assessment of recognition (Recognition Index) and associative recall (Memory Index). Parameter estimates (i.e., beta values) from the four regions that differed between groups during encoding of recognized than forgotten scenes were not correlated with the Recognition or Memory Indices (FDR corrected p’s > 0.526). In contrast, parameter estimates from the left lateral temporal cortex that showed a group difference during encoding of recalled than forgotten associative context for recognized scenes were negatively associated with the Memory (r =−0.73, FDR corrected p = 0.002) but not Recognition Index (FDR corrected p = 0.251). When tested as a mediator, left lateral temporal activation fully accounted for the association between Memory Index scores and severe pediatric obesity (indirect effect (95% CI): −12.55 (−24.77, −2.56), p = 0.020), with the effect of obesity status on Memory Index scores becoming non-significant after its inclusion (Fig. 2). This suggests that differences in neural engagement subserving encoding of associative context accounts for obesity-related differences in standardized assessment of associative memory.
Fig. 2.
Models testing for mediation of the effect of obesity on recall performance (measured by the Wide Range Assessment of Memory and Learning) by left lateral temporal cortex engagement during encoding of subsequently remembered relative to forgotten context associated with visual scenes. Standardized beta coefficients and standard errors are reported; *p < 0.05 **p < 0.01, ***p < 0.005.
4. Discussion
Our study examined the neural correlates of episodic memory in pediatric obesity and revealed functional neural alterations associated with severe obesity in adolescents that extended beyond the MTL. In this study, familiarity-based processing was operationalized by recognition of items via yes/no assessments while recollection-based processing was operationalized by free and cued recall requiring retrieval of spatial-temporal contextual information associated with those items. Standardized memory testing on the WRAML-II revealed a marginally significant impairment in adolescents with severe obesity, which was driven by significant differences in recall but not item recognition. Impaired associative memory but intact item memory in adolescents with severe obesity suggests that obesity alters recollection-based memory processing but leaves intact familiarity-based processing. In contrast to the standardized memory assessment, behavioral performance during fMRI revealed that groups did not differ in recognition of encoded scenes or recall of their associated context (i.e., inside or outside scanner location of encoding). Despite similar memory performance, adolescents with severe obesity showed reduced neural engagement in multiple regions during encoding of subsequently recognized scenes but increased engagement while encoding scenes whose encoding context was also subsequently recalled. Thus, despite limited behavioral differences, the neural substrates supporting item and associative memory were sensitive to the effects of pediatric obesity.
Behaviorally, discrepant results between the two memory assessments are likely due to differences in the extent to which strategic processing of context details was engaged. While the WRAML-II tested associative recall of intentionally encoded visual information that required reproducing entire abstract designs and selecting features of pictures, the subsequent recall of encoding context tested recall of one incidentally encoded associative context (in vs. out-of-scanner encoding). The intentional encoding of visual information during the WRAML-II likely resulted in the use of organizational or other strategies to promote recollection. Thus, the observed poorer recall when required to intentionally, but not incidentally, encode visual information suggests differences in the extent to which adolescents with and without obesity engage in strategic processes during encoding. In addition, obesity-related differences may relate to the amount of to-be-recalled information, which was greater for the WRAML-II tests and therefore evoked more extensive associative processing than that required to recall encoding location for the fMRI assessment. Nonetheless, our results call for a more comprehensive assessment of episodic memory to pinpoint the processes most vulnerable to obesity.
Adolescents with severe obesity showed reduced frontal, hippocampal, and parietal engagement during encoding of subsequently recognized than forgotten visual scenes relative to adolescents without obesity. The previous studies in adults used a Where-What-When task (Cheke et al., 2017, 2016) that had participants ‘hide’ items on visual scenes and intentionally encode their location so they could later remember. Similar to our results, adults with obesity showed reduced frontal-parietal and hippocampal engagement than adults with healthy weight during the encoding phase (Cheke et al., 2017). The observed differences in parietal regions may be related to the use of vivid, colorful scenes during the task as these regions may impact episodic memory through the modulatory effect of attentional processing via direct projections to MTL and temporal and prefrontal cortices (Cabeza et al., 2008). Therefore, reduced engagement in parietal regions in adolescents with severe obesity may reflect altered visual-spatial attentional processing during encoding. Surprising, however, was the involvement of parahippocampal regions which are typically observed for encoding of contextual rather than item representations, (Mitchell and Johnson, 2009; Ranganath, 2010). Perhaps deciding whether the image depicted an indoor or outdoor scene during the scan evoked spatial processing, also known to engage parahippocampal regions (Davachi, 2006). The observed weaker neural engagement in adolescents with severe obesity was, however, sufficient to support subsequent recognition. It is possible that if memory for scenes was tested via free recall, deficits may have been observed. Additionally, weak activation on average may be a result of greater heterogeneity in activation loci among adolescents with severe obesity than among those without obesity. Together these findings show that despite preserved recognition memory for the encoded visual information, severe pediatric obesity was associated with under engagement of regions subserving mnemonic functioning.
In contrast to findings related to subsequent recognition, adolescents with severe obesity showed greater left lateral temporal engagement during encoding of subsequently recalled than forgotten associative context relative to adolescents without obesity, despite similar levels of performance. Although typically associated with processing of verbal and semantic information (Cabeza and Nyberg, 2000), a meta-analysis showed that left lateral temporal activation has been consistently associated with both successful encoding and retrieval across studies using verbal and visual stimuli (Spaniol et al., 2009). A study that aimed to examine encoding of item features (e.g., color, location presented on screen) showed that lateral temporal activation was related to subsequent memory for item locations but not color (Uncapher et al., 2006). Additionally, worse contextual recall and greater left lateral temporal engagement has been observed in elderly relative to young adults (Meulenbroek et al., 2010). Although there were no performance differences in associative context recall between groups in the present study, greater temporal engagement was associated with worse recollection-based performance during standardized testing. Whether the greater reliance on temporal cortex during encoding reflects more effortful encoding or suppression relative to baseline cannot be discerned and should be probed in future work. Although memory processes related to context are thought to be subserved by prefrontal cortex and parahippocampal and hippocampal gyri (Ranganath, 2010), we did not find any differences in encoding related to context in frontal or MTL regions. This may be due to the fact that the subsequent memory paradigm tested differences in incidental encoding of testing location rather than intentional or strategic encoding of contextual information related to the stimuli. Perhaps intentional encoding of encoding context may have engaged frontal and MTL regions. Greater engagement of lateral temporal cortex during encoding of contextual information suggests that differences in recollection-based memory processing extend beyond the MTL.
Obesity-related differences in recollection-based performance on standardized testing were related to obesity-related differences in neural engagement observed during encoding for associated context. Despite no differences in recollection-based memory for the subsequent memory paradigm, standardized testing revealed worse recollection-based recall but not familiarity-based recognition in adolescents with than without severe obesity. The lack of performance differences between groups in the subsequent memory paradigm was likely due to the fact that the subsequent recall task was designed to elicit roughly equal numbers of remember and forgotten stimuli to optimally model neural engagement rather than to challenge mnemonic ability. Importantly, neural engagement identified by modeling recalled than forgotten associative context was related to performance on the WRAML-II such that greater lateral temporal activation was associated with worse Memory Index scores. Together, these findings provide initial evidence that differences in functional neural systems subserving recollection-based processing can explain recollection-based deficits associated with pediatric obesity on standardized assessments.
Although our study provided initial evidence of altered neural processing and visual memory function in adolescents with severe obesity, some limitations should be noted when interpreting the results. First, all analyses were correlational in nature, thus, whether these results reflect causal factors or consequences of obesity is unknown. While this would be true of any fMRI study, designs that incorporate interventions for obesity are necessary to examine causal weight-related effects on brain function and behavior. Second, due to data loss due to head motion and performance criteria necessary for a subsequent memory design (e.g., comparable remembered and forgotten items), the small sample size of our study limits generalizability of the observed results. Therefore, these findings should be considered as preliminary evidence and may serve to generate hypothesis for future studies. Indeed, replication of the observed results is necessary in larger samples of adolescents with and without severe obesity. Participants with severe obesity pose a special challenge for neuroimaging studies due to constraints of magnet bore size because children with BMI greater than 50 could not fit within the bore. Third, we did not have measurements of diet and physical activity, which would have been useful to examine as secondary variables of interest or as mediators for the observed effects on brain activation. Further, studies have observed that other indices of adiposity such as waist and neck circumference may account for variance in metabolic function better than BMI (Rodríguez et al., 2004), and therefore, may have been more sensitive to variance in mnemonic functioning. We did not have these measures but urge their inclusion in future studies. Fourth, although the adolescents with severe obesity were not previously diagnosed with diabetes, it is possible that some adolescents with severe obesity had subclinical effects on organ systems. Obesity results in low-grade, systemic inflammation, which can lead to neuro-inflammation that has the potential of negatively impacting neural (Miller and Spencer, 2014) and cognitive functioning (Pistell et al., 2010). Indeed, when non-diabetic young adults were split into high and low fasting-insulin groups, those with relatively higher fasting-insulin showed decreased engagement in parietal and medial temporal regions during spatial-temporal encoding (Cheke et al., 2017). In order to disentangle causes and consequences of pediatric obesity related to mnemonic functioning, future studies should incorporate physiological measures (e.g., insulin, inflammation) in longitudinal or intervention (e.g., weight loss) designs. The extent to which episodic memory and the observed substrates respond to weight loss interventions is important to examine, in light of its critical contribution to educational achievement and adaptive functioning in adolescence.
5. Conclusion
This was the first study to examine the neural substrates of memory in adolescents with and without severe obesity. A subsequent memory design was employed which enables isolation of encoding-related neural substrates that lead to subsequent recognition of visual scenes and their encoding context. Results indicate that functional neural alterations in pediatric obesity extend beyond hippocampal regions, which have been strongly implicated in animal models of obesity, to include frontal, parietal, and lateral temporal cortices. Standardized memory testing suggested deficits of recollection-based mnemonic processing which were mediated by greater lateral temporal recruitment. Altered neural and memory function in severe pediatric obesity has the potential to impact not only scholastic and adaptive functioning, but also regulation of eating behavior. Further research is needed to elucidate the potential causes and consequences of neurocognitive deficits in pediatric obesity.
Funding
National Institue of Diabetes and Digestive and Kidney Diseases, United Stats of America, 1R56DK104644-01A1
Declaration of Competing Interest
None.
Acknowledgements
The authors have no acknowledgements.
References
- Adams W., Reynolds C.R. Wiley; Hoboken, NJ: 2008. Essentials of WRAML2 and TOMAL-2 Assessment. [Google Scholar]
- Cabeza R., Ciaramelli E., Olson I.R., Moscovitch M. vol. 9. Nature Publishing Group; 2008. pp. 613–625. (The parietal cortex and episodic memory: an attentional account). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J. Cogn. Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cheke L.G., Bonnici H.M., Clayton N.S., Simons J.S. Obesity and insulin resistance are associated with reduced activity in core memory regions of the brain. Neuropsychologia. 2017;96:137–149. doi: 10.1016/j.neuropsychologia.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheke L.G., Simons J.S., Clayton N.S. Higher body mass index is associated with episodic memory deficits in young adults. Q. J. Exp. Psychol. 2016;69:2305–2316. doi: 10.1080/17470218.2015.1099163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordner Z.A., Tamashiro K.L.K. Effects of high-fat diet exposure on learning & memory. Physiol. Behav. 2015;152:363–371. doi: 10.1016/j.physbeh.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false positive rates redux. Brain Connect. 2017:1–50. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Flegal K.M., Ogden C.L. Changes in terminology for childhood overweight and obesity. National Health Statistics Report. 2010 [PubMed] [Google Scholar]
- Flegal K.M., Wei R., Ogden C.L., Freedman D.S., Johnson C.L., Curtin L.R. Characterizing extreme values of body mass index–for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am. J. Clin. Nutr. 2009;90:1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- Gunstad J., Spitznagel M.B., Paul R.H., Cohen R.A., Kohn M., Luyster F.S., Clark R., Williams L.M., Gordon E. Body mass index and neuropsychological function in healthy children and adolescents. Appetite. 2008;50:246–251. doi: 10.1016/j.appet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Hales C.M., Fryar C.D., Carroll M.D., Freedman D.S., Ogden C.L. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007–2008 to 2015–2016. JAMA. 2018;319:1723–1725. doi: 10.1001/jama.2018.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave S.L., Jones S., Davidson T.L. The Outward Spiral: a vicious cycle model of obesity and cognitive dysfunction. Curr. Opin. Behav. Sci. 2016;9:40–46. doi: 10.1016/j.cobeha.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassevoort K.M., Khan N.A., Hillman C.H., Cohen N.J. Childhood markers of health behavior relate to hippocampal health, memory, and academic performance. Mind Brain Educ. 2016;10:162–170. [Google Scholar]
- Hölcke M., Marcus C., Gillberg C., Fernell E. Paediatric obesity: a neurodevelopmental perspective. Acta Paediatr. 2008;97:819–821. doi: 10.1111/j.1651-2227.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- Kamijo K., Khan N.A., Pontifex M.B., Scudder M.R., Drollette E.S., Raine L.B., Evans E.M., Castelli D.M., Hillman C.H. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity. 2012;20:2406–2411. doi: 10.1038/oby.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski S.E., Davidson T.L. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol. Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski S.E., Grill H.J. Hippocampus contributions to food intake control: mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry. 2017;81:748–756. doi: 10.1016/j.biopsych.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A., Barlow S.E., Rao G., Inge T.H., Hayman L.L., Steinberger J., Urbina E.M., Ewing L.J., Daniels S.R. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: a scientific statement from the American heart association. Circulation. 2013;128:1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- Khan N.A., Baym C.L., Monti J.M., Raine L.B., Drollette E.S., Scudder M.R., Moore R.D., Kramer A.F., Hillman C.H., Cohen N.J. Central adiposity is negatively associated with hippocampal-dependent relational memory among overweight and obese children. J. Pediatr. 2015;166:302–308. doi: 10.1016/j.jpeds.2014.10.008. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski R.J., Ogden C.L., Guo S.S., Grummer-Strawn L.M., Flegal K.M., Mei Z. 2000 CDC Growth Charts for the United States: Methods and Development. Vital Health Stat. 2002:1–203. [PubMed] [Google Scholar]
- Loprinzi P.D., Frith E. Obesity and episodic memory function. J. Physiol. Sci. 2018;68:321–331. doi: 10.1007/s12576-018-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey E.R., Jacobs M., Nadler E.P., Olson A., Pearce A.L., Cherry J.B.C., Magge S.N., Mietus-Snyer M., Vaidya C. Cognitive performance as predictor and outcome of adolescent bariatric surgery: a nonrandomized pilot study. J. Pediatr. Psychol. 2018;43:916–927. doi: 10.1093/jpepsy/jsy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenbroek O., Kessels R.P.C., de Rover M., Petersson K.M., Rikkert M.G.M.O., Rijpkema M., Fernández G. Age-effects on associative object-location memory. Brain Res. 2010;1315:100–110. doi: 10.1016/j.brainres.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Miller A.A., Spencer S.J. Obesity and neuroinflammation: a pathway to cognitive impairment. Brain Behav. Immun. 2014;42:10–21. doi: 10.1016/j.bbi.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Mitchell K.J., Johnson M.K. Source monitoring 15 years later: What have we learned from fMRI about the neural mechanisms of source memory? Psychol. Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble E.E., Kanoski S.E. Early life exposure to obesogenic diets and learning and memory dysfunction. Curr. Opin. Behav. Sci. 2016;9:7–14. doi: 10.1016/j.cobeha.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce A.L., Leonhardt C.A., Vaidya C.J. Executive and reward-related function in pediatric obesity: a meta-analysis. Child. Obes. 2018;14:265–279. doi: 10.1089/chi.2017.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce A.L., Mackey E., Cherry J.B.C., Olson A., You X., Magge S.N., Mietus-Snyder M., Nadler E.P., Vaidya C.J. Effect of adolescent bariatric surgery on the brain and cognition: a pilot study. Obesity. 2017;25:1852–1860. doi: 10.1002/oby.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell P.J., Morrison C.D., Gupta S., Knight A.G., Keller J.N., Ingram D.K., Bruce-Keller A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychology Software Tools, Inc., n.d. E-Prime 2.0.
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Ranganath C. Binding items and contexts: the cognitive neuroscience of episodic memory. Curr. Dir. Psychol. Sci. 2010;19:131–137. [Google Scholar]
- Rodríguez G., Moreno L.A., Blay M.G., Blay V.A., Garagorri J.M., Sarría A., Bueno M. Body composition in adolescents: measurements and metabolic aspects. Int. J. Obes. 2004;28:S54–S58. doi: 10.1038/sj.ijo.0802805. [DOI] [PubMed] [Google Scholar]
- Spaniol J., Davidson P.S.R., Kim A.S.N., Han H., Moscovitch M., Grady C.L. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47:1765–1779. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Spyridaki E.C., Avgoustinaki P.D., Margioris A.N. Obesity, inflammation and cognition. Curr. Opin. Behav. Sci. 2016;9:169–175. [Google Scholar]
- Stevenson R.J., Francis H.M. The hippocampus and the regulation of human food intake. Psychol. Bull. 2017;143:1011–1032. doi: 10.1037/bul0000109. [DOI] [PubMed] [Google Scholar]
- Stranahan A.M. Models and mechanisms for hippocampal dysfunction in obesity and diabetes. NSC. 2015;309:125–139. doi: 10.1016/j.neuroscience.2015.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D., Yamamoto T., Hirose K., Keele L., Imai K. Mediation: r package for causal mediation analysis. J. Stat. Softw. 2014:59. [Google Scholar]
- Uncapher M.R., Otten L.J., Rugg M.D. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D., Hsiao-pin C. Pearson; 2011. WASI-II: Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Wilke M. An alternative approach towards assessing and accounting for individual motion in fMRI timeseries. NeuroImage. 2012;59:2062–2072. doi: 10.1016/j.neuroimage.2011.10.043. [DOI] [PubMed] [Google Scholar]
- Yonelinas A.P. The nature of recollection and familiarity: a review of 30 years of research. J. Mem. Lang. 2002;46:441–517. [Google Scholar]
- Zeller M.H., Inge T.H., Modi A.C., Jenkins T.M., Michalsky M.P., Helmrath M., Courcoulas A., Harmon C.M., Rofey D., Baughcum A., Austin H., Price K., Xanthakos S.A., Brandt M.L., Horlick M., Buncher R. Severe obesity and comorbid condition impact on the weight-related quality of life of the adolescent patient. J. Pediatr. 2015;166:651–659. doi: 10.1016/j.jpeds.2014.11.022. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]