Version Changes
Updated. Changes from Version 1
We have updated Pathfinder in order to be more robust, easy to use, and more versatile. These updates include a new View Pane on the main window which displays the current user-defined parameters. This allows users to quickly see what they are changing in order to more accurately set desired classification limits. We have also added a ‘Define Software’ feature which allows users to import data that we have not explicitly implemented support for. Pathfinder now accepts data from any output as long as the .csv or .xlsx output contains columns for the x-coordinate, y-coordinate, and time. The new features were developed by Ricky Ma, who has therefore been added as an author in this updated version of the publication. Finally, we have added a relaxed focal search parameter, semi-focal search. This, like all previous parameters, is an optional Search Strategy for Pathfinder to classify trials into. Updates to Pathfinder are available on our GitHub https://github.com/MatthewBCooke/Pathfinder, where you can download the most up-to-date version of the software.
Abstract
Spatial navigation is a universal behavior that varies depending on goals, experience and available sensory stimuli. Spatial navigational tasks are routinely used to study learning, memory and goal-directed behavior, in both animals and humans. One popular paradigm for testing spatial memory is the Morris water maze, where subjects learn the location of a hidden platform that offers escape from a pool of water. Researchers typically express learning as a function of the latency to escape, though this reveals little about the underlying navigational strategies. Recently, a number of studies have begun to classify water maze search strategies in order to clarify the precise spatial and mnemonic functions of different brain regions, and to identify which aspects of spatial memory are disrupted in disease models. However, despite their usefulness, strategy analyses have not been widely adopted due to the lack of software to automate analyses. To address this need we developed Pathfinder, an open source application for analyzing spatial navigation behaviors. In a representative dataset, we show that Pathfinder effectively characterizes the development of highly-specific spatial search strategies as male and female mice learn a standard spatial water maze. Pathfinder can read data files from commercially- and freely-available software packages, is optimized for classifying search strategies in water maze paradigms, and can also be used to analyze 2D navigation by other species, and in other tasks, as long as timestamped xy coordinates are available. Pathfinder is simple to use, can automatically determine pool and platform geometry, generates heat maps, analyzes navigation with respect to multiple goal locations, and can be updated to accommodate future developments in spatial behavioral analyses. Given these features, Pathfinder may be a useful tool for studying how navigational strategies are regulated by the environment, depend on specific neural circuits, and are altered by pathology.
Keywords: search strategy, water maze, learning, memory, rodent, reversal, goal
Introduction
All living organisms move throughout space to survive. Amongst mammals, there is a diversity of spatial behaviors that depend on numerous factors such as anxiety 1, 2, learning 3, and the nature and pattern of stimuli that predict goals 4– 6. Given rodents’ natural propensity to explore stimuli and environments, an array of rodent navigational tasks have been developed to investigate how various brain regions interact to control goal-direct behavior 7. This has routinely been conducted using fixed-trajectory mazes such as the T-maze or radial maze. While these dry maze paradigms offer the convenience of fixed choice points that reduce ambiguity associated with classifying decisions and navigational responses, they cannot be used to study patterns of exploration in open environments.
A popular approach for studying free navigation in animals has been the water maze, where rodents learn the location of a hidden escape platform in a pool of water based on distal and/or local cue configurations 3 Early studies validated the usefulness of the water maze for studying spatial processing and described progressive stages of learning where a rodent searches for the platform with increasing spatial specificity 8, 9. The vast majority of studies have since used escape latency or path length as primary measures of spatial learning. However, water maze navigation is unconstrained and animals can solve the task using different strategies that may not always differ in terms of the time it takes to reach the platform 8, 9. Thus, while latency and path length measures are convenient, they discard a rich amount of behavioral data.
Over the years, a number of groups have described manual and automated methods for classifying search strategies used by animals and humans in water maze experiments 8, 10– 19. By mathematically relating the swim path to features of the maze environment one can identify and quantify the types of search strategies employed. Search strategy analyses have revealed that the ventral hippocampus is involved in coarse spatial goal-directed search 16, that adult neurogenesis promotes spatially precise search 20, and that spatially accurate search is reduced in humans with, and/or animal models of, Alzheimer’s disease 21, 22, autism 23, traumatic brain injury 22, 24 and aging 14, 25. Despite the utility of these analyses they have been relatively uncommon to date, likely because commercially-available software packages often do not perform these analyses and the analytic methods used in previous work are not typically available in the form of an easy-to-use software package.
To facilitate the study of navigational search strategies, whether in the water maze or other 2-dimensional navigational paradigms, we created a new software application called Pathfinder. Pathfinder is a Python-based, open source tool with an intuitive graphical user interface and adjustable parameters for conducting detailed analyses of spatial search patterns. We validate Pathfinder with a mouse water maze dataset, where we find that male and female mice develop increasingly specific and direct spatial search strategies with additional days of training.
Methods
Installation and dependencies
Pathfinder is freely available under the GNU General Public License version 3.0.
Detailed instructions on use and installation of the program can be found on Github at github.com/MatthewBCooke/Pathfinder. We recommend installing Anaconda for Python 3, as it includes all of the following packages that are needed to run Pathfinder: PIL ( https://pillow.readthedocs.io/en/latest/), xlrd ( https://xlrd.readthedocs.io/en/latest/), numpy ( https://www.numpy.org), pickle ( https://docs.python.org/3/library/pickle.html), scipy ( https://www.scipy.org), matplotlib ( https://matplotlib.org), and tkinter ( https://wiki.python.org/moin/TkInter). The MATLAB engine is optional and needs to be installed separately for entropy calculations (MATLAB and Statistics Toolbox Release 2018b, The MathWorks, Inc., Natick, Massachusetts, United States). Once Anaconda is installed, Pathfinder can be downloaded via Github or by typing “pip install jsl-pathfinder” in a shell window (i.e. Mac terminal or Windows command line). Pathfinder is then opened by typing “pathfinder” into the shell window and pressing return.
General usage
Pathfinder has a simple, user-friendly interface for extracting information from spatial navigation tracking files that contain xy coordinates over time ( Figure 1). While it can be used to analyze multiple types of 2D navigational data, it is optimized for rodent spatial water maze experiments and accepts inputs from commonly-used commercial tracking software, including Ethovison (Noldus), Anymaze (Stoelting) and WaterMaze (Actimetrics). Inputs can also be defined using the ‘Define..’ button. This allows the user to input files from other tracking systems or modified versions of supported output files. Pathfinder can also open files exported from the open source tracking software, ezTrack 26, enabling a cost-effective and fully open source workflow for detailed water maze behavioral analyses. Trial information from these programs are outputted in CSV or Excel format, which can then be inputted into Pathfinder through the File menu. The experimental setup is specified in the main window ( Figure 1a). Pathfinder can automatically calculate the position and size of the maze and the goal location (provided they are constant across trials), or these parameters can be entered manually.
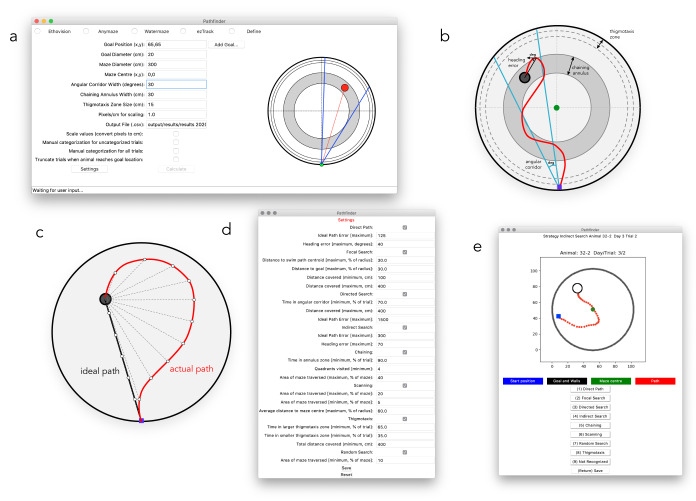
Figure 1. Graphical user interface and setting parameters.
a) Screenshot of the main application window, where maze geometry is defined, and input and output settings are established. On the right there is a live view of the parameters defined. b) Maze schematic and geometry for defining variables. The chaining corridor is centered on the goal platform and extends throughout all 4 quadrants; its width is specified in the main window. The larger thigmotaxis zone is specified in the main window; Pathfinder calculates the smaller thigmotaxis zone as half the width. Heading error is the angular distance between the actual path direction and a straight line to the goal (Pathfinder calculates average heading error at all points; only a single example shown). The angular corridor is used to define the directed search strategy, which depends on the accuracy of the animal’s trajectory as it approaches the platform. The width of the corridor (in degrees) is specified in the main window and is centered on the goal. c) Schematic of the Ideal Path Error (IPE) metric. The distance from the platform is measured at each timepoint provided by the tracking software (actual path; only a fraction of distances shown for clarity) to provide a cumulative distance measure. Assuming the same swim speed as the actual path, distances are similarly summed from the ideal path, to provide a cumulative ideal path measure. The ideal cumulative distance is subtracted from the actual cumulative distance to generate the IPE. d) Parameter bounds are entered in the settings window. e) The manual categorization window, for viewing trial paths and manually categorizing strategies.
Pathfinder relies on several variables that describe navigation relative to the pool and platform geometry: 1) Ideal Path Error (IPE): the summed error of the search path ( Figure 1c). It is conceptually similar to the Cumulative Search Error (CSE) since it also measures proximity to the goal throughout the trial 9, 27. An advantage of proximity measures is that they can distinguish two trials that have equivalent latencies/path lengths but differ in average distance to the platform. When calculating the IPE, the distance from the goal is measured at each time point in the trial and summed to generate a cumulative distance measure of the actual path (similar to CSE). In contrast to the CSE, the IPE is calculated by subtracting the cumulative ideal path distance from the cumulative actual path distance. The cumulative ideal path is simply the sum of all of the distances between the goal and the position of the animal if it swam along a straight line to escape, using the average velocity from the trial. 2) Heading error: the angular distance between the current path and a straight line to the goal location. The current path direction is defined by a line connecting two temporally-adjacent xy coordinates. The average heading error is an average of all of the heading error values for the trial and the initial heading error is the average of the heading error values for the first second of the trial.
Additional variables are user-defined on the main window: 3) Angular Corridor Width: the size of the angular navigational corridor (in degrees) that extends from the start location and widens towards the goal, centered on the goal location. 4) Chaining Annulus Width: the width of the chaining annulus, a donut-shaped zone that is centered on the goal and spans all areas of the maze at a fixed distance from the maze wall. 5) Thigmotaxis zone size: the width of a zone that spans the perimeter of the maze and extends inward from the maze wall. Pathfinder also defines a “small” thigmotaxic zone that is half the width of this value. 6) Add goal: Pathfinder will perform all calculations and strategy analyses with respect to an unlimited number of goal locations. All of these variables are plotted on our View Pane on the right of the main window. This can be used to measure performance and characterize strategies with respect to multiple goal locations (e.g. during spatial reversal, spatial choice). Selecting “truncate trials” will artificially end the trials if/when the subject reaches the additional goal locations. This is necessary, for example, to measure direct trajectories to a former goal location in a reversal paradigm (since the strategy will no longer meet direct path criteria if the former location in contacted and search continues elsewhere in the maze).
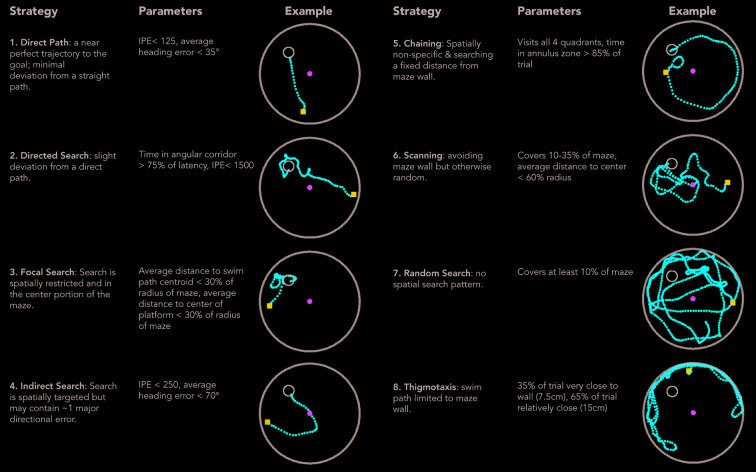
Once the variables are defined, boundaries must be set to establish the criteria for strategy categorization. Clicking “settings” will open up an additional window where strategy options can be selected and parameter bounds can be set ( Figure 1d). Upon clicking “calculate”, Pathfinder categorizes trials into one of eight search strategies that are ordered according to the degree of spatial specificity (high to low): 1) direct path, 2) focal search, 3) directed search, 4) indirect search, 5) semi-focal search, 6) chaining, 7) scanning, 8) random search, and 9) thigmotaxis. These categories are mutually exclusive and follow a defined order (1 to 9), but the user can opt to exclude strategies from the analysis. Thus, Pathfinder determines, in a stepwise fashion, whether a given trial fulfills the criteria for direct swim. If so, it moves on to categorize the next trial. If not, it determines whether the trial fits the subsequent strategy, and so on. The strategies and their parameters are shown in Figure 2. In the output file (.csv), each trial is categorized and the following additional metrics are provided: latency and distance travelled to reach the goal, average distance from the goal, percent of maze traversed, velocity, initial and average heading error and IPE. Pathfinder also has the ability to calculate the entropy for each trial, a measure of disorder in the path, relative to the goal location. The entropy calculation calls the MATLAB engine and requires a MATLAB license. Entropy measures the performance by looking at a shift from more disordered swimming (high entropy) to more spatially strategic paths (low entropy), and has been previously found to be highly sensitive to water maze search performance 28. Due to the manipulation of large matrices, calculating the entropy of trials is very slow.
Figure 2. Search strategies and associated parameters.
Pathfinder categorizes each trial according to 1 of 9 possible strategies. Categorization proceeds sequentially in the order shown (unless some strategies are excluded from the analysis). Semi-focal search (not-shown), a more relaxed focal-search, is classified 5 th after indirect search. For example, for a trial to be classified as Random Search, the path must cover a minimum proportion of the maze and not fit any of the criteria for strategies 1–7. In the examples shown, the blue square indicates the start point and the green circle indicates the middle of the pool. Parameter settings are those used in the present study and should be adjusted depending on changes to testing procedures and maze geometry.
Occasionally, some trials cannot be categorized. The user therefore has the option to manually categorize uncategorized trials, by selecting this option on the main window. Additionally, there is an option to manually categorize all trials. Here, Pathfinder provides an image of the trial as well as shortcut keys to select the appropriate strategy. The software will also display the strategy it had automatically categorized for the displayed trial. Manual categorization will not overwrite the automatic categorization but will be displayed separately in the output file. This allows for comparison between the automatically calculated and user-selected strategy.
In addition to strategy categorization, Pathfinder will also create heatmaps as a useful visual representation of groups of trials. This is accomplished by counting the number of times animal(s) visit each bin in a hexagonal array that is overlaid on the maze (bin size is user-defined). The range of colors (cool to warm) can be automatically set to occupy the full scale. Alternatively, the user can manually set the maximum, above which all bins will read the hottest.
Validation of Pathfinder
Animals. A group of 35 C57BL/6J mice (18 male, 17 female) were used in this experiment (Jackson Laboratories, Bar Harbor, Maine). Relative to commonly-used samples sizes of 8–10 mice/group, a large cohort was used to maximize reliability and detect potentially infrequent strategies. Mice were housed in same-sex groups (2–4/cage) in polyethylene cages (30 × 19 × 13cm) with pine chip bedding and a small tube and food and water available ad-libitum. Mice were housed under a reversed light-dark cycle (lights off 8:00am–8:00pm) and completed water-maze testing in the dark phase. Mice were first tested on the Barnes maze 29 and were 18 weeks old when tested on the water maze for the current experiment. All efforts were made to minimize animal suffering, and all procedures adhered to guidelines from the Canadian Council on Animal Care and were approved by the Dalhousie University Committee on Laboratory Animals.
Spatial water maze training. The water maze consisted of a plastic circular pool (110 cm diameter) painted black. The pool was filled with water (21–23 °C), which was made opaque with the addition of non-toxic white tempera paint (Schola). A circular escape platform (14 cm height, 9 cm diameter) was positioned 1 cm below the water. The water maze was placed in a diffusely lit room with many extra-maze visual cues (posters on walls, a desk, the experimenter, geometric layout of testing room etc.).
Animals were tested over a total of 15 days. They first completed 8 days of acquisition training (A1-A8) with a hidden escape platform (4 trials/day). Across trials, mice were released into the pool from four different locations, with the order differing across mice. They were given a maximum of 60 sec to locate the escape platform, after which they were guided to the platform by the experimenter. Mice remained on the platform for 15–20 seconds before being removed from the pool. During daily test sessions, mice were tested in squads of 4 and each mouse was held in separate cages filled with a bedding of paper towel. The inter-trial interval ranged from 2–8 minutes. The day following acquisition training, memory was assessed with a single 60-sec probe trial with no escape platform present. Mice then completed a single day of re-training (Retrain) to reduce extinction that may occur during the probe trial. During re-training the escape platform is returned to the same location used in acquisition training.
After acquisition re-training, reversal learning was assessed over 3 days (R1-R3) with the escape platform moved to the opposite side of the maze. A reversal probe trial (R probe) was then completed to assess memory for the location of the new escape platform location. Finally, a single day of visible platform training (Visible platform; 4 trials) was completed, where the escape platform was moved to a new location and made visible with the addition of a striped flag.
Behavior was recorded with the WaterMaze (Actimetrics) video tracking system (5 samples per second), via a camera placed directly above the pool.
Results
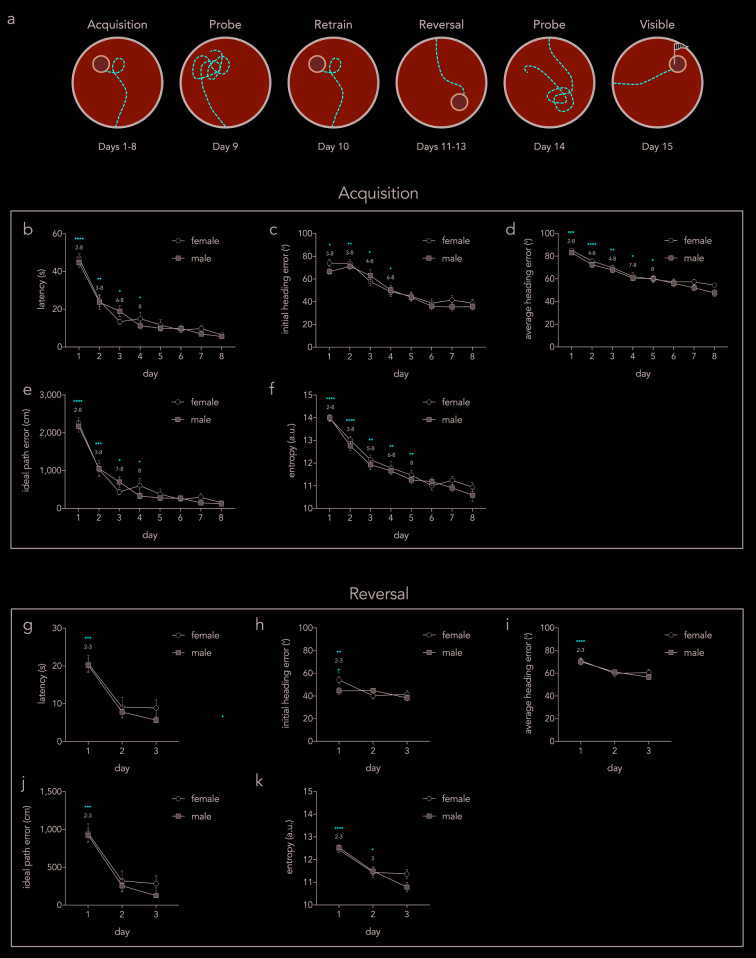
To validate Pathfinder, we trained mice for 8 days on a spatial water maze such that they achieved asymptotic performance according to standard metrics and should therefore have adopted distinct navigational strategies as they learned the procedural and spatial task demands. Following acquisition, mice received an unreinforced probe trial, 1 day of retraining, 3 days of reversal training (platform in opposite side of pool), another probe trial, and one day of visible platform training (outlined in Figure 3a).
Figure 3. Acquisition and reversal performance as assessed by individual parameters.
a) Schematic outline of full behavioral paradigm. Individual performance metrics were analyzed for acquisition ( b– f) and reversal ( g– k) stages of testing. b) Latency to reach the platform decreased across days (day effect F 7,231=75, P<0.0001; sex effect F 1,33=0.3, P=0.6; interaction F 7,231=0.9, P=0.5). Asterisks denote statistically significant differences from the subsequent days that are indicated by the numbers. c) Initial heading error decreased over days (day effect F 7,231=39, P<0.0001; sex effect F 1,33=0.4, P=0.6; interaction F 7,231=0.7, P=0.6). d) Average heading error decreased over days (day effect F 7,231=48, P<0.0001; sex effect F 1,33=2.5, P=0.12; interaction F 7,231=0.6, P=0.8). e) Idea path error decreased over days (day effect F 7,231=79, P<0.0001; sex effect F 1,33=0.3, P=0.6; interaction F 7,231=1.0, P=0.4). f) Entropy decreased over days (day effect F 7,231=75, P<0.0001; sex effect F 1,33=1.3, P=0.3; interaction F 7,231=0.5, P=0.8). g) Latency decreased over days (day effect F 2,66=69, P<0.0001; sex effect F 1,33=0.5, P=0.5; interaction F 2,66=0.7, P=0.5). h) Initial heading error decreased over days and was greater in females on day 1 (day effect F 2,66=9, P<0.001; sex effect F 1,33=1.0, P=0.3; interaction F 2,66=4.7, P=0.01). i) Average heading error decreased over days (day effect F 2,66=21, P<0.0001; sex effect F 1,33=0.2, P=0.7; interaction F 2,66=0.9, P=0.4). j) Ideal path error decreased over days (day effect F 2,66=98, P<0.0001; sex effect F 1,33=0.5, P=0.5; interaction F 2,66=0.7, P=0.5). k) Entropy decreased over days (day effect F 2,66=39, P<0.0001; sex effect F 1,33=0.6, P=0.4; interaction F 2,66=2.6, P=0.08). *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, †P<0.05 within day, male vs female comparison. Symbols = mean ± standard error.
To confirm that mice learned the task, we first analyzed performance using several metrics that indicate learning but do not reveal details about navigational strategies ( Figure 3). We focused on acquisition and reversal phases since they are the main focus of our subsequent strategy analyses. Over the 8 days of acquisition, mice reached the platform faster, increasingly swam in the direction of the platform as measured by heading angle error and had lower IPE and entropy scores. The greatest performance improvements occurred during the first 4 days and, while all measures revealed improvements beyond day 4, only average heading error and entropy analyses revealed improvements beyond day 5. There were no sex differences in acquisition performance.
Reversal learning performance improvements were mostly apparent after the first day of training, likely because mice had learned the procedural aspects of the task and the spatial environment, and only had to learn a new platform location ( Figure 3g–k) 30. Path entropy decreased from days 2–3, indicating continued learning. Females and males were equivalent in all performance measures except males had a lower initial heading error on day 1 of reversal training ( Figure 3h).
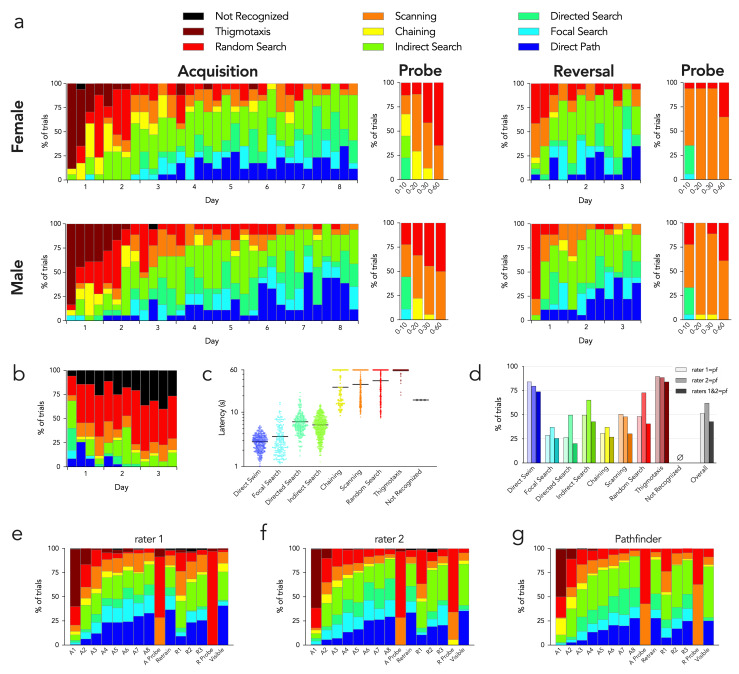
Pathfinder revealed clear differences in search strategies over days of training ( Figure 4). Over the first two days of acquisition, mice were initially thigmotaxic. After learning that the pool wall did not afford escape, they then transitioned to chaining, random and scanning search patterns, all of which indicate spatially non-specific search away from the pool wall. Over days 2–3 mice transitioned to spatially-specific forms of search, with ~30% performing indirect searches to locate the platform. A similar proportion of trials were indirect searches over days 2–8 of training. Mice increasingly displayed directed searches, focal searches and direct paths such that, by the end of training, search was spatially specific on over 80% of trials. There were no major sex differences in strategy. The usefulness of strategy analyses (at least with default settings) for long probe trials is limited since spatially-specific strategies rely on IPE, which rapidly increases with trial duration. Additionally, animals will change strategies as they learn that the escape platform is not available in the expected location. Indeed, when the probe trial analysis was restricted to the first 10s, mice displayed focal and directed search strategies, indicating perseveration at the former platform location. When the analysis was conducted on longer segments, chaining was common, indicating that mice adopted a procedural strategy of searching in similar regions throughout the pool. Finally, when examining the entire probe trial, scanning and random searches dominated, indicating that mice eventually abandoned strategies that were no longer successful. During reversal, spatial specificity was initially very poor; mice primarily scanned, indicating preserved knowledge of the procedural requirements but no knowledge of the platform location. By the end of day 2 mice displayed levels of spatially-specific search strategies that were comparable to those at the end of the acquisition phase. Using the “add goal” feature, we also analyzed reversal strategies with respect to the original goal location ( Figure 4b). This revealed a number of direct paths to the goal on the first day that quickly dissipated with additional trials as mice learning the new platform location. This analysis was performed without the semi-focal search strategy.
Figure 4. Pathfinder search strategy categorization of water maze performance.
a) Search strategies for male and female mice. Each set of stacked bars indicates strategies used for the 4 acquisition and reversal trials for each day. Probe strategies are shown for the entire trial (0–60s) and for the first 10, 20 and 30s. b) Reversal strategies relative to the original platform location (indirect search excluded from analyses, since short swims that bypass the old location but quickly go to the new location become incorrectly classified as indirect searches with current settings). c) Escape latencies for all 1888 trials varied by strategy. Symbols indicate individual trials, bars indicate means (Kruskal Wallis test, P<0.0001; Dunn’s tests: direct path vs all others except focal search, P<0.0001; focal search vs all others except direct path, P<0.0001; directed search vs all others except indirect search, P<0.0001; indirect search vs all others except directed search, P<0.0001; chaining vs all others except scanning and random, P<0.01; scanning vs all others except chaining and thigmotaxis, P<0.01; random vs all others except chaining, P<0.05; thigmotaxis vs all others except random search, P<0.05). d) Manual vs automatic categorization. For each strategy assigned by Pathfinder, the proportion that received the same classification (manually) by 2 raters is shown. “Raters 1+2” indicates the percentage of Pathfinder classified-trials that also received the same classification by both raters. e) Search strategy classification by rater 1 for each day of testing. f) Search strategy classification by rater 2 for each day of testing. g) Search strategy classification by Pathfinder for each day of testing.
To investigate possible relationships between strategy and conventional measures of water maze performance, we examined escape latencies for each strategy type, over all trials (1888 trials from all 15 days of testing; Figure 4c). Direct swim trials had the lowest latencies (2.9s on average) and was followed by the other spatially-specific strategies (focal search, 3.6s; directed search, 6.7s; indirect search, 5.8s). Non-specific strategies that avoided the pool wall were all significantly worse than the spatially specific strategies (chaining, 29s; scanning, 32s; random, 38s), and thigmotaxic trials were significantly worse than all other trial types (58s).
To determine how Pathfinder compared to subjective assessment of strategy, we compared Pathfinder categorization to manual scores generated by 2 independent raters (all trials). Rater 1 had experience in mouse behavior testing, but only brief training on water maze strategy classification. Rater 2 developed Pathfinder (MBC) and had extensive experience with strategy classification. Figure 4d shows the proportion of Pathfinder-categorized trials that received the same strategy classification via the manual raters. The greatest correspondence between automatic and manual categorization was seen for direct swims and thigmotaxis (~80% for both). Automatic-manual consistency was much lower for the other strategies, ranged from 25–75% and differed for the 2 raters. Overall consistency between the 2 manual raters was 65%. These data highlight the difficulty of intuitively differentiating complex search paths. Interestingly, when we averaged strategy analyses over all 15 days of testing, automatic and manual categorization resulted in similar patterns ( Figure 4e–g). Thus, manual scoring is unreliable at the level of an individual trial, and human error can be masked when data are averaged.
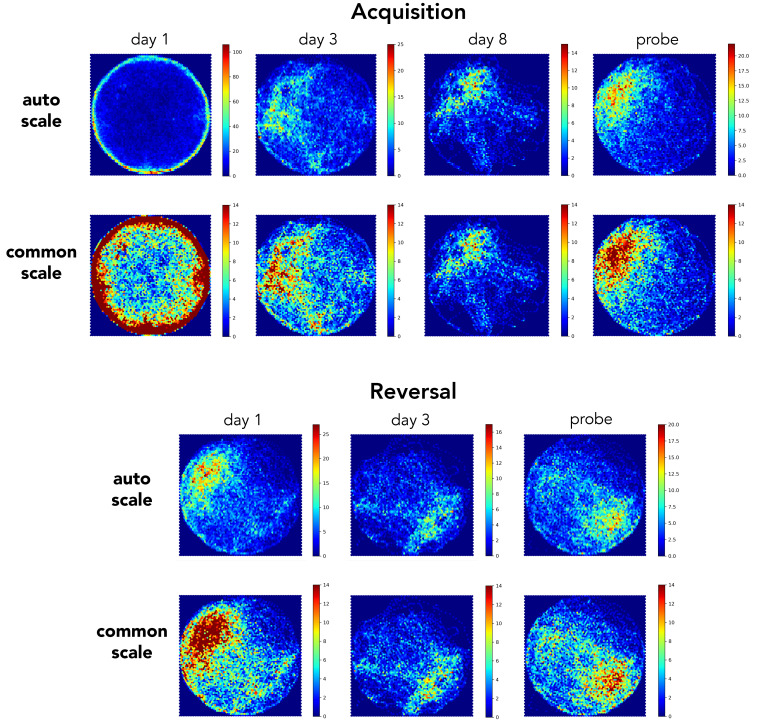
To provide an intuitive visual inspection of search performance, we used Pathfinder to generate heatmaps of spatial occupancy at stages of testing that differed in spatial search patterns ( Figure 5). Averaged over all trials and across sexes, mice swam in close proximity to the pool wall on day 1 of initial acquisition. By days 3 and 8 search was increasingly focused near the goal. Spatial preference was clearest on the probe trial, since these trials provided a longer temporal window to accumulate spatial occupancy samples. Day 1 of reversal testing resembled the probe trial, since mice spent the majority of time in the former platform location. By day 3, and on the probe trial, their spatial preference had shifted to the new, correct location. One set of heatmaps are presented using Pathfinder’s auto scale feature, which maximizes the color range within a trial and can be useful for visualizing within-trial details since it avoids saturation. However, by differentially scaling, it can also obscure or inflate differences across trials. We therefore include a second set of heatmaps that are all scaled equivalently.
Figure 5. Heatmap visualization of spatial occupancy.
Examples of heatmaps for various testing days (all trials from both sexes combined). Top rows: heatmaps were automatically scaled by Pathfinder, to occupy the full color spectrum and facilitate visualization of spatial occupancy within a given day. Bottom rows: heatmaps were set to a common scale, to facilitate comparison across days. Scale indicates number of samples within a spatial bin.
Discussion
Here we describe Pathfinder, an easy-to-use software package for analyzing patterns of spatial navigation. Pathfinder performs automatic classification of multiple search strategies that have been previously described in the rodent water maze, but it can also be used for analyzing navigational behavior in dry mazes, virtual mazes or any other environment where xy coordinates are provided. Currently, Pathfinder accepts inputs from three commonly-used, commercially-available tracking programs (Ethovision, Anymaze, Watermaze) and also the freely-available tracking software, ezTrack 26. It requires no programming knowledge, but is open source and can be expanded by developers in the future. Using a mouse water maze dataset, we validated Pathfinder’s performance and found that mice progressed through a series of search strategies that had increasing levels of spatial search specificity, consistent with earlier reports 16– 18, 20, 21, 25, 31. Mice initially displayed thigmotaxic, random and chaining search strategies as they learned the procedural components of the task. Pathfinder effectively demonstrated that mice transitioned to spatially-specific, presumably hippocampal-dependent, strategies during the later stages of training. Pathfinder also revealed the reverse transition from spatial to procedural to random strategies in the probe trial. By analyzing reversal performance with respect to multiple goal locations, Pathfinder showed that mice redirect their spatial search from the previously-reinforced platform location to the new location. Mice displayed a variety of search strategies on any given day, even after escape latency performance had plateaued. Since manual classification based on static images of swim paths was slow and inconsistent, Pathfinder may therefore be a useful tool for objectively characterizing swim strategies in the rodent water maze and 2D spatial navigation in other behavioral paradigms.
The water maze was initially described nearly 40 years ago and quickly became popular due to the ease of training, strong motivation for escape, and consistent reliance on hippocampal function 3, 32. While early work performed more comprehensive analyses and validated the water maze, escape latency and path length were quickly adopted as the primary measures of learning and, due to their simplicity and sufficiency for many experimental situations, they remain the most commonly-used metrics. However, they cannot always differentiate between behaviors that vary in the degree of spatial bias. For example, animals that employ a chaining strategy search nonspecifically in some cases can reach the platform as fast as animals that perform a directed spatial search ( Figure 4c). Latency and path length are also less capable of detecting age-related impairments in spatial learning, prompting development of measures of proximity to the goal location, which has proven to be highly sensitive to group differences in both training and probe trial performance 9, 27, 33. Our IPE proximity measure is similar to previous proximity measures with the exception that the cumulative ideal path distance is subtracted from the cumulative actual path distance to generate a path error measure. Finally, another recent metric that has been reported to be even more sensitive to group differences in spatial probe trial performance is entropy 28. Entropy is originally a measure of thermodynamic disorder in a system but, when applied to the distribution of sampled sites in a maze, can also be used to measure the transition from high to low disorder in navigation, as animals focus their search on the precise goal location. By default, Pathfinder applies equal weighting to the path and goal components of the entropy measure and, compared to standard metrics, entropy was slightly better at detecting performance changes during the later stages of water maze acquisition.
Despite their convenience, even the most precise individual measures cannot distinguish between multiple possible strategies that an animal might employ to reach a goal location. Thus, strategy analyses may be valuable for identifying the role that different circuits play in guiding behavior. Consistent with the lower spatial resolution of ventral hippocampal place cells 34, strategy analyses have found that the ventral hippocampus is particularly important for developing coarse, non-specific search patterns in the water maze and that increasingly spatially localized search depends on sequential recruitment of intermediate and then dorsal hippocampus 16. Adult-born neurons are believed to promote memory precision and, indeed, blocking neurogenesis greatly reduced the adoption of spatially-specific search strategies 20. Strategy analyses in animals have revealed spatial precision-related deficits in models of aging 25, stroke 35, traumatic brain injury 22, 24, autism 23 and Alzheimer’s pathology 21, 22. With the advent of virtual reality, it has also become possible to test whether rodent water maze findings generalize to humans 15. Indeed, hippocampal damage and CA1-specific lesions impair human water maze performance according to standard measures such as latency to reach the platform 36, 37. Human water maze experiments have also revealed superior spatial memory and greater spatial strategy use in younger individuals, and in males compared to females 14. Here, we did not observe any sex differences, consistent with a recent meta-analysis that revealed that male and female mice display broadly comparable performance in the spatial water maze 38.
Given the apparent utility of strategy classification, the question arises as to why it has not been used more extensively. One likely explanation is that it is not a standard feature of commercially-available software packages, therefore requiring time and programming experience to execute. Groups that have performed strategy analyses have developed their own software, using either a predefined parameter-based approach, like ours, or machine learning algorithms that classify based on user input 8, 11, 12, 15– 20, 25, 39. Since most previous approaches have not been developed into freely-available software packages, Pathfinder may enable more widespread adoption of strategy analyses. Moreover, in conjunction with freely-available tracking programs such as ezTrack (which is already supported) or others 39, users should be able to easily perform advanced navigation analyses at little cost.
It is worth noting that, with respect to water maze analyses, some behaviors (e.g. chaining and thigmotaxis) have been relatively well-described. In contrast, differences between spatially-specific search patterns (direct swim, directed search, focal search, indirect search) may be intuitive and quantifiable but the extent to which they are meaningful and result from distinct neural processes is less clear. Certainly, the fact that search strategies can now be easily quantified opens the door to future studies of the biology of complex navigation strategies. However, to some extent, strategy definitions are arbitrary, and it is therefore incumbent upon the user to determine which behaviors are relevant for their experimental paradigm.
Future developments and additional uses
One area where Pathfinder could be useful is for assessing spatial bias and choice behavior when there are multiple goal locations. Indeed, the water maze has been effectively used to study visuospatial goal discrimination 40, 41 and cue vs place-related choice behavior 42, 43. We have recently used Pathfinder to show that neurogenesis promotes spatial platform preference in a spatial alternation water maze, which was detected by a greater number of direct swims when the platform was in the rat’s preferred location than when it was in the non-preferred location 44. Neurogenesis-deficient rats often vacillated between the two platform locations, similar to vicarious trial and error behavior that has been described at choice points in dry mazes 45. Future software developments could possibly incorporate these types of movements between competing goal options to detect indecisiveness as animals refine goal-directed navigation behavior. Swim speeds are also currently not factored into Pathfinder’s classification scheme, but could provide useful additional information for strategies that incorporate goal expectancy 46, 47 or a transition between place- and cue-directed navigation 5. In the water maze, multiple platform locations (> 2) are typically only used in matching-to-place variants where it is expected that subjects quickly forget previous goal locations 30. However, there is evidence that search patterns can reflect memory for many individual goal locations as well as the overall distribution of goals, at recent and remote post-training intervals, respectively 6. Since Pathfinder can analyze navigation with respect to an unlimited number of goal locations, it may be useful for future investigations of how multiple spatial goals interact to guide search.
Spatial navigation and exploration have been studied in many paradigms and so it is worth reiterating that Pathfinder could be applied to study navigation by any species, in any open 2D environment, and not just the water maze. We have updated Pathfinder to allow users to define their own software inputs, allowing users to analyze data from species or tasks not originally supported. For example, it could be used to measure the spatial precision of homing behavior 48, 49, spatial preferences of mammals or invertebrates in novel environments 50– 52, or navigation with respect to other environmental features that are known to drive firing of select populations of neurons, such as local and distal cues 53, objects 54, 55 and environmental borders 56. An array of virtual environments also opens the door to similar analyses of spatial navigation in humans 36, 37, 57, 58. Finally, eye tracking data, as humans and nonhuman primates explore 2D scenes, provides a measure of navigation that is analogous to rodent spatial exploration 59. Indeed, hippocampal-damaged subjects display disorganized, inefficient search in a scene exploration task and are impaired according to several water maze-inspired metrics such as cumulative search error and heading angle error 60. As a user-friendly application that can be further developed to accommodate differences between these various paradigms, Pathfinder may be a useful tool for characterizing complex spatial behavior and bridging findings across humans and animal models.
Data availability
Underlying data
Figshare: Raw data for "Pathfinder: open source software for analyzing spatial navigation search strategies", https://doi.org/10.6084/m9.figshare.9686987.v1 61
This project contains the following underlying data:
-
-
Water maze output
-
-
Pathfinder output
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Software availability
Software source code available from: https://github.com/MatthewBCooke/Pathfinder
Archived source code at time of publication: https://doi.org/10.5281/zenodo.3882267 62
License: GNU General Public License 3.0.
Acknowledgements
The authors thank Sabri Snyder for contributing the name, Pathfinder, Kurt Stover for contributing initial work towards automatic strategy classification, Tian Rui Zhang for assistance with manual strategy classification, and Brie Dungate for help testing and revising software changes. A previous version of this work is available from: https://doi.org/10.1101/715961
Funding Statement
This work was funded by the Canadian Institutes of Health Research (JSS), the Natural Sciences and Engineering Research Council (JSS, REB), and the Michael Smith Foundation for Health Research (JSS, TPO).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Kjelstrup KG, Tuvnes FA, Steffenach HA, et al. : Reduced fear expression after lesions of the ventral hippocampus. Proc Natl Acad Sci U S A. 2002;99(16):10825–10830. 10.1073/pnas.152112399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bannerman DM, Deacon RM, Offen S, et al. : Double dissociation of function within the hippocampus: spatial memory and hyponeophagia. Behav Neurosci. 2002;116(5):884–901. 10.1037/0735-7044.116.5.884 [DOI] [PubMed] [Google Scholar]
- 3. Morris RGM: Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12(2):239–260. 10.1016/0023-9690(81)90020-5 [DOI] [Google Scholar]
- 4. McDonald RJ, White NM: A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107(1):3–22. 10.1037/0735-7044.107.1.3 [DOI] [PubMed] [Google Scholar]
- 5. Hamilton DA, Rosenfelt CS, Whishaw IQ: Sequential control of navigation by locale and taxon cues in the Morris water task. Behav Brain Res. 2004;154(2):385–397. 10.1016/j.bbr.2004.03.005 [DOI] [PubMed] [Google Scholar]
- 6. Richards BA, Xia F, Santoro A, et al. : Patterns across multiple memories are identified over time. Nat Neurosci. 2014;17(7):981–986. 10.1038/nn.3736 [DOI] [PubMed] [Google Scholar]
- 7. Penner MR, Mizumori SJ: Neural systems analysis of decision making during goal-directed navigation. Prog Neurobiol. 2012;96(1):96–135. 10.1016/j.pneurobio.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 8. Graziano A, Petrosini L, Bartoletti A: Automatic recognition of explorative strategies in the Morris water maze. J Neurosci Methods. 2003;130(1):33–44. 10.1016/S0165-0270(03)00187-0 [DOI] [PubMed] [Google Scholar]
- 9. Gallagher M, Burwell R, Burchinal M: Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107(4):618–626. 10.1037/0735-7044.107.4.618 [DOI] [PubMed] [Google Scholar]
- 10. Whishaw IQ, Jarrard LE: Similarities vs. differences in place learning and circadian activity in rats after fimbria-fornix section or ibotenate removal of hippocampal cells. Hippocampus. 1995;5(6):595–604. 10.1002/hipo.450050610 [DOI] [PubMed] [Google Scholar]
- 11. Wolfer DP, Madani R, Valenti P, et al. : Extended analysis of path data from mutant mice using the public domain software Wintrack. Physiol Behav. 2001;73(5):745–753. 10.1016/s0031-9384(01)00531-5 [DOI] [PubMed] [Google Scholar]
- 12. Dalm S, Grootendorst J, de Kloet ER, et al. : Quantification of swim patterns in the Morris water maze. Behav Res Methods Instrum Comput. 2000;32(1):134–139. 10.3758/BF03200795 [DOI] [PubMed] [Google Scholar]
- 13. Balschun D, Wolfer DP, Gass P, et al. : Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? J Neurosci. 2003;23(15):6304–6314. 10.1523/JNEUROSCI.23-15-06304.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schoenfeld R, Moenich N, Mueller FJ, et al. : Search strategies in a human water maze analogue analyzed with automatic classification methods. Behav Brain Res. 2010;208(1):169–177. 10.1016/j.bbr.2009.11.022 [DOI] [PubMed] [Google Scholar]
- 15. Schoenfeld R, Schiffelholz T, Beyer C, et al. : Variants of the Morris water maze task to comparatively assess human and rodent place navigation. Neurobiol Learn Mem. 2017;139:117–127. 10.1016/j.nlm.2016.12.022 [DOI] [PubMed] [Google Scholar]
- 16. Ruediger S, Spirig D, Donato F, et al. : Goal-oriented searching mediated by ventral hippocampus early in trial-and-error learning. Nat Neurosci. 2012;15(11):1563–1571. 10.1038/nn.3224 [DOI] [PubMed] [Google Scholar]
- 17. Vouros A, Gehring TV, Szydlowska K, et al. : A generalised framework for detailed classification of swimming paths inside the Morris Water Maze. Sci Rep. 2018;8(1):15089. 10.1038/s41598-018-33456-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Illouz T, Madar R, Louzon Y, et al. : Unraveling cognitive traits using the Morris water maze unbiased strategy classification (MUST-C) algorithm. Brain Behav Immun. 2016;52:132–144. 10.1016/j.bbi.2015.10.013 [DOI] [PubMed] [Google Scholar]
- 19. Rogers J, Churilov L, Hannan AJ, et al. : Search strategy selection in the Morris water maze indicates allocentric map formation during learning that underpins spatial memory formation. Neurobiol Learn Mem. 2017;139:37–49. 10.1016/j.nlm.2016.12.007 [DOI] [PubMed] [Google Scholar]
- 20. Garthe A, Behr J, Kempermann G: Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4(5):e5464. 10.1371/journal.pone.0005464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Janus C: Search strategies used by APP transgenic mice during navigation in the Morris water maze. Learn Mem. 2004;11(3):337–346. 10.1101/lm.70104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brody DL, Holtzman DM: Morris water maze search strategy analysis in PDAPP mice before and after experimental traumatic brain injury. Exp Neurol. 2006;197(2):330–340. 10.1016/j.expneurol.2005.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Faraji J, Karimi M, Lawrence C, et al. : Non-diagnostic symptoms in a mouse model of autism in relation to neuroanatomy: the BTBR strain reinvestigated. Transl Psychiatry. 2018;8(1):234. 10.1038/s41398-018-0280-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tucker LB, Velosky AG, McCabe JT: Applications of the Morris water maze in translational traumatic brain injury research. Neurosci Biobehav Rev. 2018;88:187–200. 10.1016/j.neubiorev.2018.03.010 [DOI] [PubMed] [Google Scholar]
- 25. Gil-Mohapel J, Brocardo PS, Choquette W, et al. : Hippocampal neurogenesis levels predict WATERMAZE search strategies in the aging brain. PLoS One. 2013;8(9):e75125. 10.1371/journal.pone.0075125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pennington ZT, Dong Z, Bowler R, et al. : ezTrack: An open-source video analysis pipeline for the investigation of animal behavior. bioRxiv. 2019. 10.1101/592592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tomás Pereira I, Burwell RD: Using the spatial learning index to evaluate performance on the water maze. Behav Neurosci. 2015;129(4):533–539. 10.1037/bne0000078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Maei H, Zaslavsky K, Wang AH, et al. : Development and validation of a sensitive entropy-based measure for the water maze. Front Integr Neurosci. 2009;3:33. 10.3389/neuro.07.033.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Leary TP, Brown RE: Optimization of apparatus design and behavioral measures for the assessment of visuo-spatial learning and memory of mice on the Barnes maze. Learn Mem. 2013;20(2):85–96. 10.1101/lm.028076.112 [DOI] [PubMed] [Google Scholar]
- 30. Steele RJ, Morris RG: Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9(2):118–136. [DOI] [PubMed] [Google Scholar]
- 31. Stone SS, Teixeira CM, Devito LM, et al. : Stimulation of Entorhinal Cortex Promotes Adult Neurogenesis and Facilitates Spatial Memory. J Neurosci. 2011;31(38):13469–13484. 10.1523/JNEUROSCI.3100-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Morris RGM: The Watermaze. In: The Maze Book. 2015;94:73–92(Humana Press, New York, NY). 10.1007/978-1-4939-2159-1_3 [DOI] [Google Scholar]
- 33. Maei HR, Zaslavsky K, Teixeira CM, et al. : What is the Most Sensitive Measure of Water Maze Probe Test Performance? Front Integr Neurosci. 2009;3:4. 10.3389/neuro.07.004.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kjelstrup KB, Solstad T, Brun VH, et al. : Finite scale of spatial representation in the hippocampus. Science. 2008;321(5885):140–143. 10.1126/science.1157086 [DOI] [PubMed] [Google Scholar]
- 35. Woitke F, Ceanga M, Rudolph M, et al. : Adult hippocampal neurogenesis poststroke: More new granule cells but aberrant morphology and impaired spatial memory. PLoS One. 2017;12(9):e0183463–16. 10.1371/journal.pone.0183463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, et al. : Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20(4):481–491. 10.1002/hipo.20651 [DOI] [PubMed] [Google Scholar]
- 37. Bartsch T, Schönfeld R, Müller FJ, et al. : Focal lesions of human hippocampal CA1 neurons in transient global amnesia impair place memory. Science. 2010;328(5984):1412–1415. 10.1126/science.1188160 [DOI] [PubMed] [Google Scholar]
- 38. Fritz AK, Amrein I, Wolfer DP: Similar reliability and equivalent performance of female and male mice in the open field and water-maze place navigation task. Am J Med Genet C Semin Med Genet. 2017;175(3):380–391. 10.1002/ajmg.c.31565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gulyás M, Bencsik N, Pusztai S, et al. : AnimalTracker: An ImageJ-Based Tracking API to Create a Customized Behaviour Analyser Program. Neuroinformatics. 2016;14(4):479–81. 10.1007/s12021-016-9303-z [DOI] [PubMed] [Google Scholar]
- 40. Bannerman DM, Bus T, Taylor A, et al. : Dissecting spatial knowledge from spatial choice by hippocampal NMDA receptor deletion. Nat Neurosci. 2012:15(8):1153–9. 10.1038/nn.3166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arruda-Carvalho M, Sakaguchi M, Akers KG, et al. : Posttraining ablation of adult-generated neurons degrades previously acquired memories. J Neurosci. 2011;31(42):15113–15127. 10.1523/JNEUROSCI.3432-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McDonald RJ, White NM: Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61(3):260–270. 10.1016/S0163-1047(05)80009-3 [DOI] [PubMed] [Google Scholar]
- 43. Snyder JS, Cahill SP, Frankland PW: Running promotes spatial bias independently of adult neurogenesis. Hippocampus. 2017;27(8):871–882. 10.1002/hipo.22737 [DOI] [PubMed] [Google Scholar]
- 44. Yu RQ, Cooke M, Zhao J, et al. : Adult neurogenesis promotes efficient, nonspecific search strategies in a spatial alternation water maze task. Behav Brain Res. 2019;376:112151. 10.1016/j.bbr.2019.112151/637462 [DOI] [PubMed] [Google Scholar]
- 45. Redish AD: Vicarious trial and error. Nat Rev Neurosci. 2016;17(3):147–159. 10.1038/nrn.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hollup SA, Kjelstrup KG, Hoff J, et al. : Impaired recognition of the goal location during spatial navigation in rats with hippocampal lesions. J Neurosci. 2001;21(12):4505–4513. 10.1523/JNEUROSCI.21-12-04505.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Whishaw IQ, Cassel JC, Jarrad LE: Rats with fimbria-fornix lesions display a place response in a swimming pool: a dissociation between getting there and knowing where. J Neurosci. 1995;15(8):5779–5788. 10.1523/JNEUROSCI.15-08-05779.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Schenk F: A homing procedure for studying spatial memory in immature and adult rodents. J Neurosci Methods. 1989;26(3):249–258. 10.1016/0165-0270(89)90123-4 [DOI] [PubMed] [Google Scholar]
- 49. Maaswinkel H, Jarrard LE, Whishaw IQ: Hippocampectomized rats are impaired in homing by path integration. Hippocampus. 1999;9(5):553–561. [DOI] [PubMed] [Google Scholar]
- 50. Valente D, Golani I, Mitra PP: Analysis of the trajectory of Drosophila melanogaster in a circular open field arena. PLoS One. 2007;2(10):e1083. 10.1371/journal.pone.0001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eilam D, Golani I: Home base behavior of rats ( Rattus norvegicus) exploring a novel environment. Behav Brain Res. 1989;34(3):199–211. 10.1016/S0166-4328(89)80102-0 [DOI] [PubMed] [Google Scholar]
- 52. Dvorkin A, Szechtman H, Golani I: Knots: attractive places with high path tortuosity in mouse open field exploration. PLoS Comput Biol. 2010;6(1):e1000638. 10.1371/journal.pcbi.1000638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Leutgeb S, Leutgeb JK, Barnes CA, et al. : Independent Codes for Spatial and Episodic Memory in Hippocampal Neuronal Ensembles. Science. 2005;309(5734):619–623. 10.1126/science.1114037 [DOI] [PubMed] [Google Scholar]
- 54. Tsao A, Moser MB, Moser EI: Traces of Experiencein the Lateral Entorhinal Cortex. Curr Biol. 2013;23(5):399–405. 10.1016/j.cub.2013.01.036 [DOI] [PubMed] [Google Scholar]
- 55. Deshmukh SS, Knierim JJ: Representation of non-spatial and spatial information in the lateral entorhinal cortex. Front Behav Neurosci. 2011;5:69. 10.3389/fnbeh.2011.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Solstad T, Boccara CN, Kropff E, et al. : Representation of geometric borders in the entorhinal cortex. Science. 2008;322(5909):1865–1868. 10.1126/science.1166466 [DOI] [PubMed] [Google Scholar]
- 57. Coutrot A, Silva R, Manley E, et al. : Global Determinants of Navigation Ability. Curr Biol. 2018;28(17):2861–2866.e4. 10.1016/j.cub.2018.06.009 [DOI] [PubMed] [Google Scholar]
- 58. Maguire EA, Nannery R, Spiers HJ: Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain. 2006;129(Pt 11):2894–2907. 10.1093/brain/awl286 [DOI] [PubMed] [Google Scholar]
- 59. Meister MLR, Buffalo EA: Getting directions from the hippocampus: The neural connection between looking and memory. Neurobiol Learn Mem. 2016;134(Pt A):135–144. 10.1016/j.nlm.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yee LTS, Warren DE, Voss JL, et al. : The hippocampus uses information just encountered to guide efficient ongoing behavior. Hippocampus. 2014;24(2):154–164. 10.1002/hipo.22211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Cooke M, O'Leary T, Harris P, et al. : Raw data for "Pathfinder: open source software for analyzing spatial navigation search strategies". figshare.Dataset.2019. 10.6084/m9.figshare.9686987.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cooke M, Harris P: MatthewBCooke/Pathfinder: Zenodo Release for DOI (Version 1.3.0). Zenodo. 2019. 10.5281/zenodo.3882267 [DOI] [Google Scholar]