Abstract
Background:
Streptococcus mutans and Porphyromonas gingivalis are caries and periodontal disease-related bacteria. The mangosteen fruit (Garcinia mangostana L.) peel contains flavonoids, tannins, saponins, and xanthones that have antibacterial properties.
Aims:
The aim of this study is to analyze mangosteen peel extracts’ ability to inhibit S. mutans and P. gingivalis has biofilms growth in vitro.
Materials and Methods:
Mangosteen peel extract effects on the S. mutans ATCC-3198 and P. gingivalis ATCC-3327 in biofilms growth were evaluated by a crystal violet biofilm assay. Each bacterium was inoculated into a brain–heart infusion broth for 24 h at 37°C anaerobic conditions. A volume of 200 μL (107 colony-forming unit/mL) of bacterial suspension were distributed in microplate wells and incubated for 24 h. Mangosteen peel extracts with different concentrations were added into biofilm wells. Biofilm without treatment was used as negative control. Biofilm mass was calculated by 0.5% crystal violet staining, and optical density was measured at 600 nm using microplate reader. All obtained data were statistically analyzed using one-way analysis of variance test with P < 0.05 set as the level of significance.
Results:
The results showed that mangosteen peel extract could inhibit the growth of S. mutans and P. gingivalis in biofilms significantly compared to the negative control (P < 0.05). The most effective concentration and incubation time for inhibiting biofilm growth was 100% in 6 h for S. mutans and 100% in 24 h for P. gingivalis.
Conclusion:
Mangosteen peel extract is effective at inhibiting S. mutans and P. gingivalis biofilms, and this antibiofilm agent can be an alternative therapy in preventing caries and periodontal disease. Future studies are needed to explore this effect.
Keywords: Antibiofilm, biofilm, mangosteen peel extract, Porphyromonas gingivalis, Streptococcus mutans
Introduction
The national prevalence of dental caries in Indonesia is considered high. Based on a 2004 survey held by the Indonesian Household Health Survey, 71.2% of the population aged 15 and above had caries, and 52.3% of those cases were left untreated.[1] According to the basic health research conducted by the Indonesian government in 2007 and 2013, the decayed missing and filling teeth index was 4.9 and 4.6, respectively.[2] Other than caries, the main problems in the oral cavity are gingivitis and periodontitis. These diseases are caused mostly by bacteria. Most bacteria can naturally form biofilms as an act of survival.[3] Bacterial accumulation that forms plaque on the surfaces of teeth is commonly known as oral biofilm.[4] Oral biofilm is defined as a diverse community of microorganisms found on the surface of the tooth embedded in a matrix of extracellular polymeric substances (EPS).[5] Streptococcus mutans is the primary bacteria that forms oral biofilms is the main cause of dental caries.[6]
Oral biofilm is one of the key causes of caries and periodontal diseases, including gingivitis and periodontitis.[7] If left untreated, gingivitis may lead to periodontitis, which is defined as an inflammatory disease of the tissues supporting the teeth caused by a specific microorganism or group of microorganisms that lead to progressive destruction of periodontal ligament and alveolar bone. This disease can involve periodontal pocket formations, recession, or both, as well as tooth loss.[8]
Although many therapeutic agents to treat oral diseases are available, some of these agents may have side effects, including multiple drug resistance.[9] Therefore, the search for natural bioactive compounds that can treat oral diseases with few to no adverse effects continues. Many indigenous Indonesian cultures often use herbs to treat a variety of diseases in the oral cavity.[10,11] Native Indonesian plant species such as mangosteen fruit (Garcinia mangostana L.) are known to have medicinal potentials.[12] Mangosteen, especially the peel, contains abundant amounts of xanthones, a class of polyphenolic compounds that possess many significant biological activities in vitro,[13] and other bioactive compounds including flavonoids, tannins, and anthocyanins.[14] Several studies show that xanthone has antioxidant, anti-inflammatory, antiallergy, antibacterial, anticancer, and antifungal effects.[15] The purpose of this study was to analyze the antibiofilm properties of mangosteen peel extracts against S. mutans and Porphyromonas gingivalis biofilms in the oral cavity.
Materials and Methods
This study is an in vitro experimental design which was conducted at the Microbiology Center of Research and Education (MiCORE) Laboratory, Faculty of Dentistry, Trisakti University. In this study, we used crude forms of Indonesian mangosteen peel extract G. mangostana L. were collected from Bogor (Java Island, Indonesia). The microorganisms used in the study were standard strains of S. mutans ATCC 3198 and P. gingivalis ATCC 33277. They were obtained from the microbiology laboratory of Dipa Pharmalab Intersains (PT Dipa Heathcare) in Jakarta, Indonesia.
Extraction of Garcinia mangostana L. peels
G. mangostana L. peels were dried from 8 a.m. to 3 p.m. for 2 days. Then, dried G. mangostana peel was inserted into the tool grinder for 10 min. The results were obtained in the form of powder. Subsequently, the G. mangostana peel powder was extracted by the maceration method using 96% ethanol (Merck, Darmstadt, Germany) as a solvent. The mangosteen peel skin powder was diluted with 96% ethanol (1:5 ratio) for 1 day in a dark container, protected from light, and occasionally stirred. After 1 day, the solution was taken with filter paper. The filtered solution was then processed in the evaporator to evaporate the remaining ethanol solution. After the evaporation process, we obtained G. mangostana peel extract. The G. mangostana peel extract was then diluted with 5% dimethyl sulfoxide (Merck, Darmstadt, Germany) to obtain 100% concentration, and then serial dilution was performed using brain–heart infusion (BHI) broth to form an extract with concentrations of 50%, 25%, 12.5%, and 6.5%.
Phytochemical analysis assay
Phytochemical analysis assay is a series of rapid and simple qualitative methods to determine the presence of bioactive compounds in the plant extracts. In this study, each phytochemical analysis assay was performed in the Indonesian Medicinal and Aromatic Crops Research Institute (local acronyl BALITTRO) to detect the presence of saponins, tannins, alkaloids, phenolic compounds, flavonoids, triterpenoids, glycosides, and plant sterols. Saponins detection method: extracts were diluted in a tube containing the distilled water. The tube was shaken continuously in a shaker for 15 min. The formation of persistent foam layer at the top of the tube indicates the presence of saponins. Tannins detection method: 1% gelatin-sodium chloride solution was added into the tube containing the extract. The presence of tannins was indicated by the formation of white precipitate at the bottom of the tube. Alkaloids detection method: extracts were treated with hydrochloric acid (HCl) and filtered. A few drops of potassium mercuric iodide solution (Mayer's reagent) were added into the filtrate. The presence of alkaloid compounds was indicated by the formation of white creamy-colored precipitate. Phenol detection method: the ethanolic extract was spotted on a piece of filter paper, added with a drop of phosphomolybdic acid reagent and exposed to ammonia vapors. The presence of phenol was indicated by the formation of blue coloration of the spot. Triterpenoids detection method: detection was carried out using Liebermann–Burchard test. Mangosteen peel extract (100 mg) was shaken with chloroform in a tube. A few drops of acetic anhydride was added to the solution. The tube was boiled in a water bath and cooled into iced-water, followed by the addition of concentrated H2SO4 (2 mL) into the tube. The presence of triterpenoids was indicated by the formation of deep red color in the solution. Flavonoids detection method: a piece of magnesium ribbon was added into 2–3 mL of mangosteen peel ethanolic extract, followed by the addition of 1 mL of concentrated HCl. The presence of flavonoids was indicated by reddish-pink or red coloration in the solution. Glycosides detection method: HCl was added into the extract as a pretreatment to induce hydrolysis. Then, the extract was treated with ferric chloride solution and immersed in boiling water for 5 min. After cooling down, an equal volume of benzene was added into the extract. The benzene layer was separated and added with ammonia solution. The presence of plant glycosides was indicated by the formation of rose-pink color in the solution.[16,17,18]
Preparation and treatment of the biofilms
S. mutans ATCC 25175 and P. gingivalis ATCC 33277 were used in this study. S. mutans was inoculated into BHI broth (Thermo Scientific, Waltham, MA, USA) and then incubated at 37°C for 24 h in anaerobic conditions (CO2, H, and N). P. gingivalis was cultured in BHI broth and incubated in a GasPak Jar System (Becton Dickinson, Franklin Lakes, NJ, USA). The cultures that were incubated were then homogenized with a vortexer, and then their optical density (OD) was measured with a microplate reader on a wavelength of 600 nm. A volume of 200 μL bacterial suspension (1 × 107 colony-forming unit/mL) were distributed into the 96-well plates and incubated at 37°C for 24 h in anaerobic conditions to form biofilm. After 24 h, each well plate was rinsed with phosphate buffer solution (PBS) twice. The G. mangostana peel extracts with different concentrations (100%, 50%, 25%, 12.5%, 6.25%) were distributed into wells that were rinsed with PBS. As positive control of 0.2% chlorhexidine was distributed into the wells. Observations were made after 1 h, 3 h, 6 h, and 24 h of incubation time at 37°C in anaerobic conditions. The extracts were then removed, and the well plate was rinsed twice using PBS (AMRESCO, Solon, OH, USA), crystal violet (Merck, Darmstadt, Germany) (0.05%) were distributed into well plates and incubated for 15 min. The extraction of crystal violet in each well plate was calculated by adding 96 μL of 96% ethanol, while the amount of biofilm by absorbance was calculated at 600 nm with a microplate reader (AccuReader, Metertech, Taipei, Taiwan).[19] Chlorhexidine at 0.2% was used as a positive control. Biofilms without G. mangostana peel extracts were used as negative controls. All treatments were done in triplicate.
Statistical analysis
The Shapiro–Wilk test was used to test for normality, and Levene's test was used to test for homogeneity of variance. The normal data (P > 0.05) continued to be analyzed by a one-way analysis of variance test, and P < 0.05 was considered the level of significance. The significant difference between groups was analyzed with Tukey's post hoc least significant difference test. Statistical calculations were performed with IBM SPSS Statistics of Windows, Version 20.0. Armonk, NY: IBM Corp.
Results
Phytochemical analysis result
The result of phytochemical test shown in Table 1. These assays confirmed the presence of saponins, tannin, alkaloids, phenolic compounds, flavonoids, triterpenoids, glycosides, and plant sterols in Indonesian mangosteen peel crude extract.
Table 1.
Qualitative phytochemical analysis of ethanolic mangosteen pericarp crude extract
| Plant metabolites | Result |
|---|---|
| Saponins | + |
| Tannins | + |
| Alkaloids | + |
| Phenolics | + |
| Flavonoids | + |
| Triterpenoids | + |
| Steroids | − |
| Glycosides | + |
+: Metabolites were found in the mangosteen pericarp crude extract -: Metabolites were not found in the mangosteen pericarp crude extract
Garciana mangostana L. peel extract reduced the growth of Streptococcus mutans biofilm
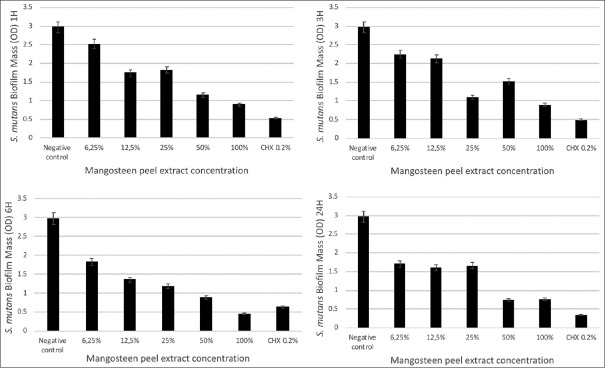
The results were achieved with 100% concentration of G. mangostana L. peel extract, which inhibited the growth of the S. mutans biofilm during an incubation period of 6 h. The statistical test results showed significant reduction of S. mutans biofilms after the treatment with G. mangostana L. peel extract in all concentrations at the 1 h incubation period compared to the negative control. All concentrations at the incubation period of 6 h and 24 h showed a significant reduction of S. mutans biofilms compared to the negative control (P = 0.000) [Figure 1].
Figure 1.
Concentration-response curves for treatment with mangosteen peel extract over different incubation times (1, 3, 6, and 24 h). The vertical axis indicates the Streptococcus mutans biofilm mass in optical density. The horizontal axis indicates the concentration of mangosteen peel extract (100%, 50%, 25%, 12.5%, and 6.25%). Chlorhexidine 0.2% was used as positive control and biofilm well without treatment was used as negative control
Garciana mangostana L. peel extract reduced the growth of Porphyromonas gingivalis biofilm
The results showed that the most effective concentration of G. mangostana L. peel extract for inhibiting the growth of the P. gingivalis biofilm was 100% over an incubation period of 24 h. The statistical analysis results showed a significant decrease in P. gingivalis biofilms after the G. mangostana L. extract treatment in all concentrations over a 1 h incubation period compared to the negative control (P = 0.000) [Figure 2].
Figure 2.
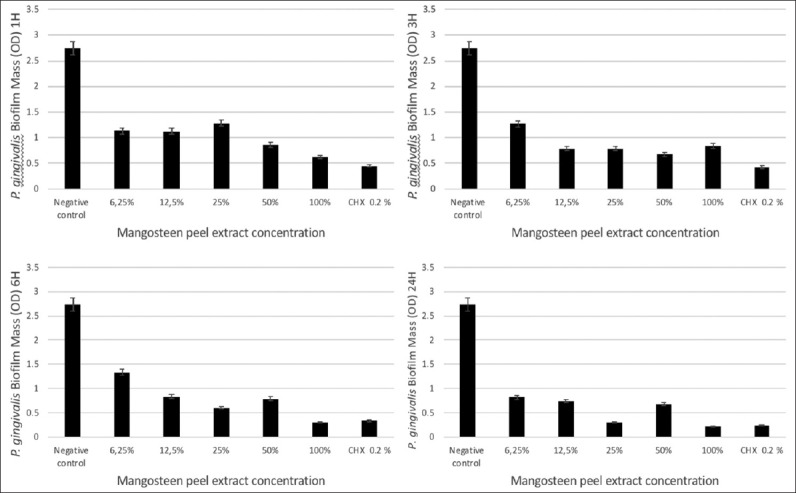
Concentration-response curves for treatment with mangosteen peel extract over different incubation times (1, 3, 6, and 24 h). The vertical axis indicates the Porphyromonas gingivalis biofilm mass in optical density. The horizontal axis indicates the concentration of mangosteen peel extract (100%, 50%, 25%, 12.5%, and 6.25%). Chlorhexidine 0.2% was used as positive control and biofilm well without treatment was used as negative control
Discussion
In this study, G. mangostana peel extract inhibited biofilm formation in S. mutans and P. gingivalis in a dose-dependent manner, with the best concentration being 100% over an incubation period of 6 h. The antimicrobial activity of mangosteen peel extracts was expressed better in S. mutans than in P. gingivalis. This difference may have been caused by the presence of one main active component in the mangosteen peel extracts, the α−mangostin, which possesses antimicrobial properties against Gram-positive oral streptococci that activate through many mechanisms, including the disruption of peptidoglycan that lead to membrane breakage and cell lysis.[20] Since P. gingivalis is a Gram-negative bacteria that contains a thinner layer of peptidoglycans,[21] this mechanism may not show the same effect as that of the Gram-positive bacteria.
Based on the results of this study, the authors also observed that achieving maximum antimicrobial activity from mangosteen peel extracts required longer incubation periods and higher concentrations. This preconclusional perspective was in agreement with a previous study of Janardhanan et al.[22] who stated that mangosteen peel crude extracts gradually showed better bactericidal effects as the extracts’ concentration increased. Mangosteen peel extracts have much potential to damage and kill bacteria, but bacteria in the biofilm states can be more resistant to antimicrobial agents than to suspension cells.[23]
Mangosteen peel extract has antioxidant, antibacterial, anticancer, antiviral, and antifungal properties.[15] Ethanol solvents were reported to be better than methanol solvents at extracting mangosteen peels in terms of the antioxidant activity content.[24] In the previous study, the toxicity of α-mangostin (4000 μg/mL) extracted using hexane and ethyl acetate was tested against gingival fibroblast cells, and no significant cytotoxicity effect was observed for 480 min.[20] Therefore, the α-mangostin was considered safe for the cells.
Previous studies showed that xanthone-containing mangosteen peel extract significantly inhibits the Candida albicans and S. mutans growth in vitro.[20,25] Oral biofilms are formed through several successive stages, starting with the formation of an acquired pellicle in the form of a thin layer of salivary glycoproteins attached to the tooth surface. Then, the primary bacteria begin to form colonies that can change the surrounding conditions to be more suitable for the growth of obligate anaerobic bacteria.[5] The bacterial colonies that have formed then synthesize EPS such as glucans, which lead to the formation of a biofilm matrix to help the bacteria remain attached to the acquired pellicle.[26] The bacteria inside the biofilm surroundings communicate to survive the environment. One form of communication is quorum sensing: the bacterial process that requires the production of a signal molecule to control bacterial attachment, production of extracellular matrices, and the virulence factor.[27] The biofilm structure reduces the effectiveness of the antimicrobial substance when compared to planktonic cells; the presence of enzyme modification and the efflux pump is associated with the higher resistance factor of biofilms.[28]
The presence of uncleansed oral biofilm can lead to chronic gingivitis and may even progress to periodontitis. Research shows that in the case of periodontitis, Gram-negative bacteria such as P. gingivalis are more common than Gram-positive bacteria.[29] The lypopolysaccharides components present in P. gingivalis will lead to inflammatory processes if interacting with host cell antibodies, which can lead to inflammation and recession of periodontal tissue.[4,30] Oral biofilm cleansing is one of the key treatments for gingivitis and periodontitis.[29]
A previous study showed that xanthones can damage the mechanical stability of S. mutans biofilm and inhibit glucosyltransferase enzyme activity, which results in the inhibition of biofilm growth.[31] The xanthone-derived compounds contained in the mangosteen peel include α-mangostin, gartanin, and γ-mangostin, garcinone B, garcinone E, and mangostin.[32,33] α-mangostin has antifungal properties, and studies show that α-mangostin is more effective at killing C. albicans than the same concentrations of clotrimazole and nystatin.[34] Further research proves that α-mangostin can also inhibit the growth of Mycobacterium tuberculosis with a minimal inhibitory concentration value of 6.25 μg/mL.[35] Flavonoids tend to bind proteins, thus interfering with bacterial metabolism.[36] Tannins are antibacterial because they destroy the permeability of cell walls, resulting in the inhibition of bacterial cell activity.[37] Saponins are also antibacterial because they increase membrane permeability, resulting in cell hemolysis.[38]
Conclusion
Mangosteen peel extract is effective at inhibiting the growth of S. mutans and P. gingivalis in biofilms. This study shows antibiofilm effect can be an alternative therapy in preventing caries and periodontal disease. However, future studies are needed to explore this effect with other oral pathogens.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank the Faculty of Dentistry, Trisakti University for their invaluable support for this study. Furthermore, we want to express appreciation to Stella Pranoto, SSI and Aradhea Monica Drestia, SSI for their laboratory assistance in Microbiology Center of Research and Education Laboratory (MiCORE Laboratory). Finally, the authors also would like to thank the Indonesian Medicinal and Aromatic Crops Research Institute (BALITTRO) for their help with phytochemical analysis assay.
References
- 1.Maharani DA. Inequity in dental care utilization in the Indonesian population with a self-assessed need for dental treatment. Tohoku J Exp Med. 2009;218:229–39. doi: 10.1620/tjem.218.229. [DOI] [PubMed] [Google Scholar]
- 2.Rahardjo A, Maharani DA. A review of Indonesia's dental health-past, present and future. Int J Clin Prev Dent. 2014;10:121–6. [Google Scholar]
- 3.Saini R, Saini S, Sharma S. Biofilm: A dental microbial infection. J Nat Sci Biol Med. 2011;2:71–5. doi: 10.4103/0976-9668.82317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen PT, Falsetta ML, Hwang G, Gonzalez-Begne M, Koo H. A-Mangostin disrupts the development of Streptococcus mutans biofilms and facilitates its mechanical removal. PLoS One. 2014;9:e111312. doi: 10.1371/journal.pone.0111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jakubovics NS. Talk of the town: Interspecies communication in oral biofilms. Mol Oral Microbiol. 2010;25:4–14. doi: 10.1111/j.2041-1014.2009.00563.x. [DOI] [PubMed] [Google Scholar]
- 6.Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis. 2014;33:499–515. doi: 10.1007/s10096-013-1993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manji F, Dahlen G, Fejerskov O. Caries and periodontitis: Contesting the conventional wisdom on their aetiology. Caries Res. 2018;52:548–64. doi: 10.1159/000488948. [DOI] [PubMed] [Google Scholar]
- 8.Saini R, Marawar PP, Shete S, Saini S. Periodontitis, a true infection. J Glob Infect Dis. 2009;1:149–50. doi: 10.4103/0974-777X.56251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedraza-Chaverri J, Cárdenas-Rodríguez N, Orozco-Ibarra M, Pérez-Rojas JM. Medicinal properties of mangosteen (Garcinia mangostana) Food Chem Toxicol. 2008;46:3227–39. doi: 10.1016/j.fct.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Widyarman AS, Widjaja SB, Idrus E. Strawberry extract's effects on Enterococcus faecalis and Porphyromonas gingivalis biofilms in vitro . Sci Dent J. 2017;1:1–5. [Google Scholar]
- 11.Liliany D, Widyarman AS, Erfan E, Sudiono J, Djamil MS. Enzymatic activity of bromelain isolated pineapple (Ananas comosus) hump and its antibacterial effect on Enterococcus faecalis. Sci Dent J. 2018;2:39–50. [Google Scholar]
- 12.Nugraha AS, Keller PA. Revealing indigenous Indonesian traditional medicine: Anti-infective agents. Nat Prod Commun. 2011;6:1953–66. [PubMed] [Google Scholar]
- 13.Obolskiy D, Pischel I, Siriwatanametanon N, Heinrich M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother Res. 2009;23:1047–65. doi: 10.1002/ptr.2730. [DOI] [PubMed] [Google Scholar]
- 14.Suttirak W, Manurakchinakorn S. In vitro antioxidant properties of mangosteen peel extract. J Food Sci Technol. 2014;51:3546–58. doi: 10.1007/s13197-012-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shan T, Ma Q, Guo K, Liu J, Li W, Wang F, et al. Xanthones from mangosteen extracts as natural chemopreventive agents: Potential anticancer drugs. Curr Mol Med. 2011;11:666–77. doi: 10.2174/156652411797536679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasidharan S, Chen Y, Saravanan D, Sundram KM, Yoga Latha L. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr J Tradit Complement Altern Med. 2011;8:1–0. [PMC free article] [PubMed] [Google Scholar]
- 17.Iqbal E, Salim KA, Lim LB. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J King Saud Univ Sci. 2015;27:224–32. [Google Scholar]
- 18.Banu KS, Cathrine DL. General techniques involved in phytochemical analysis. Int J Adv Res Chem Sci. 2015;2:25–32. [Google Scholar]
- 19.Witedja U, Suwartini T, Prahasti AE, Widyarman AS. Comparing the effectivities of chitosan citrate and chitosan acetate in eradicating Enterococcus faecalis biofilm. Sci Dent J. 2018;2:1–7. [Google Scholar]
- 20.Nguyen PT, Marquis RE. Antimicrobial actions of α-mangostin against oral streptococci. Can J Microbiol. 2011;57:217–25. doi: 10.1139/W10-122. [DOI] [PubMed] [Google Scholar]
- 21.How KY, Song KP, Chan KG. Porphyromonas gingivalis: An overview of periodontopathic pathogen below the gum line. Front Microbiol. 2016;7:53. doi: 10.3389/fmicb.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janardhanan S, Mahendra J, Girija AS, Mahendra L, Priyadharsini V. Antimicrobial effects of Garcinia mangostana on cariogenic microorganisms. J Clin Diagn Res. 2017;11:ZC19–22. doi: 10.7860/JCDR/2017/22143.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero-Lastra P, Sánchez MC, Ribeiro-Vidal H, Llama-Palacios A, Figuero E, Herrera D, et al. Comparative gene expression analysis of Porphyromonas gingivalis ATCC 33277 in planktonic and biofilms states. PLoS One. 2017;12:e0174669. doi: 10.1371/journal.pone.0174669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaomongkolgit R, Jamdee K, Chaisomboon N. Antifungal activity of alpha-mangostin against Candida albicans. J Oral Sci. 2009;51:401–6. doi: 10.2334/josnusd.51.401. [DOI] [PubMed] [Google Scholar]
- 25.Marsh PD. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38(Suppl 1):S11–5. doi: 10.1016/S0300-5712(10)70005-1. [DOI] [PubMed] [Google Scholar]
- 26.Zijnge V, van Leeuwen MB, Degener JE, Abbas F, Thurnheer T, Gmür R, et al. Oral biofilm architecture on natural teeth. PLoS One. 2010;5:e9321. doi: 10.1371/journal.pone.0009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li YH, Tian X. Quorum sensing and bacterial social interactions in biofilms. Sensors (Basel) 2012;12:2519–38. doi: 10.3390/s120302519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunsolley JC. A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J Am Dent Assoc. 2006;137:1649–57. doi: 10.14219/jada.archive.2006.0110. [DOI] [PubMed] [Google Scholar]
- 29.Buwitt-Beckmann U, Heine H, Wiesmüller KH, Jung G, Brock R, Akira S, et al. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur J Immunol. 2005;35:282–9. doi: 10.1002/eji.200424955. [DOI] [PubMed] [Google Scholar]
- 30.Widyarman AS, Drestia AM, Bachtiar EW, Bachtiar BM. The anti-inflammatory effects of glycerol-supplemented probiotic Lactobacillus reuteri on infected epithelial cells & in vitro . Contemp Clin Dent. 2018;9:298–303. doi: 10.4103/ccd.ccd_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z, Antalek M, Nguyen L, Li X, Tian X, Le A, et al. The effect of gartanin, a naturally occurring xanthone in mangosteen juice, on the mTOR pathway, autophagy, apoptosis, and the growth of human urinary bladder cancer cell lines. Nutr Cancer. 2013;65(Suppl 1):68–77. doi: 10.1080/01635581.2013.785011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinto MM, Sousa ME, Nascimento MS. Xanthone derivatives: New insights in biological activities. Curr Med Chem. 2005;12:2517–38. doi: 10.2174/092986705774370691. [DOI] [PubMed] [Google Scholar]
- 33.Shibata MA, Matoba Y, Tosa H, Iinuma M. Effects of mangosteen pericarp extracts against mammary cancer. Altern Integr Med. 2013;2:1–6. [Google Scholar]
- 34.Jung HA, Su BN, Keller WJ, Mehta RG, Kinghorn AD. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen) J Agric Food Chem. 2006;54:2077–82. doi: 10.1021/jf052649z. [DOI] [PubMed] [Google Scholar]
- 35.Hidalgo M, Sánchez-Moreno C, de Pascual-Teresa S. Flavonoid-flavonoid interaction and its effect on their antioxidant activity. Food Chem. 2010;121:691–6. [Google Scholar]
- 36.Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid Based Complement Alternat Med. 2011;2011:680354. doi: 10.1093/ecam/nep067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al Laham SA, Al Fadel FM. Antibacterial activity of various plants extracts against antibiotic-resistant Aeromonas hydrophila. Jundishapur J Microbiol. 2014;7:e11370. doi: 10.5812/jjm.11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arabski M, Węgierek-Ciuk A, Czerwonka G, Lankoff A, Kaca W. Effects of saponins against clinical E. coli strains and eukaryotic cell line. J Biomed Biotechnol. 2012;2012:286216. doi: 10.1155/2012/286216. [DOI] [PMC free article] [PubMed] [Google Scholar]